- Department of Urology, Beijing Tiantan Hospital, Capital Medical University, Beijing, China
Background: Multiple clinical studies have demonstrated the numerous advantages of urine methylation test over cytology for monitoring patients with non-muscle invasive bladder cancer (NMIBC) following surgery. This research aims to provide a systematic review and meta-analysis to evaluate the efficacy and limits of urine methylation test in the clinical management of NMIBC.
Methods: This research was carried out by conducting a comprehensive search of clinical trials comparing cytology and urine methylation test for NMIBC follow-up using databases such as PubMed, Embase, Web of Science, and the Cochrane Library up until May 2023, including references from relevant articles. The study is registered on PROSPERO with ID CRD42023398969.
Result: This study comprised six studies with a total of 1676 patients. The analysis revealed that the AUC of urine methylation test had a greater AUC than that of the cytology examination (0.89 vs 0.71). In post-operative follow-up of patients with NMIBC, the urine methylation test demonstrated a significant sensitivity (0.69 vs 0.52), but with lower specificity (0.87 vs 0.93) than cytology examination.
Conclusion: The urine methylation test and cytology examination have both shown strong diagnostic performance in screening for NMIBC patients. However, urine methylation test outperforms cytology examination in terms of accuracy and sensitivity.
Systematic review registration: PROSPERO, identifier CRD42023398969.
Introduction
Bladder cancer (BC) is the sixth most prevalent malignancy and ninth leading cause of cancer death worldwide, with over 573,000 new cases and 213,000 deaths (1). According to GLOBOCAN 2020, cancer mortality rates are 9.5 per 100,000 males and 3.3 per 100,000 women, indicating that men have a nearly fourfold greater incidence than women. Smoking is the most important risk factor for BC, with a population attributable risk of approximately 50%. Epidemiology has indicated that while the incidence trend of BC has remained steady in males, from the 1990s to the 2020s, the incidence has grown with the rise of smoking prevalence in women (2). Since non-invasive tumors account for a considerable proportion of BC, early identification, timely intervention, and rigorous follow-up is critical to lowering the risk of recurrence and progression. The European Association of Urology (EAU) now recommends cystoscopy and urine cytology as the most frequently performed procedures for the diagnosis and follow-up of BC (3). Cystoscopy is an invasive test that might cause pain for BC patient as well as iatrogenic lesions and infections. Nevertheless, this technique remains significant in the diagnosis and monitoring of BC because to its remarkable specificity and sensitivity, particularly when performed by a trained surgeon (4). Urinary cytology, as the most often used non-invasive testing approach, nevertheless has limited accuracy, which is dependent on the expertise of pathologists (5, 6). Furthermore, the consistency and precision of the cytomorphological assessment may change throughout the therapy or development of BC (7).
The majority of newly diagnosed BC cases are non-muscle invasive bladder cancer (NMIBC), with most of these cases being limited to the mucosa (8).The recommended treatment options in the urology guidelines mainly include transurethral resection of bladder tumors, intravesical chemotherapy, and systemic chemotherapy for NMIBC (3). NMIBC, with its varied clinical histories, high risk of recurrence and propensity for advancement, need rigorous follow-up and continued monitoring after first therapy (9).
The significance of DNA methylation in the transition from NMIBC to invasive BC has been substantiated by scientific investigations (10). After detecting BC based on methylation profiles, researchers discovered that these markers are expected to have a more major role in the diagnosis and subsequent surveillance of NMIBC (11). Several studies have demonstrated the unique benefits of bladder methylation markers in diagnosing disease progression as compared to urine cytology during follow-up of individuals with NMIBC (12–14).
The urine methylation test has been shown to be clinically valid in multiple investigations. However, evidence-based medicine does not yet include a comparison of urine methylation test and urine cytology in follow-up patients with NMIBC. To assess their effectiveness and accuracy, we conducted a meta-analysis of the included studies, as required by evidence-based medicine, to guide subsequent research and clinical decision making.
Materials and methods
Search strategy
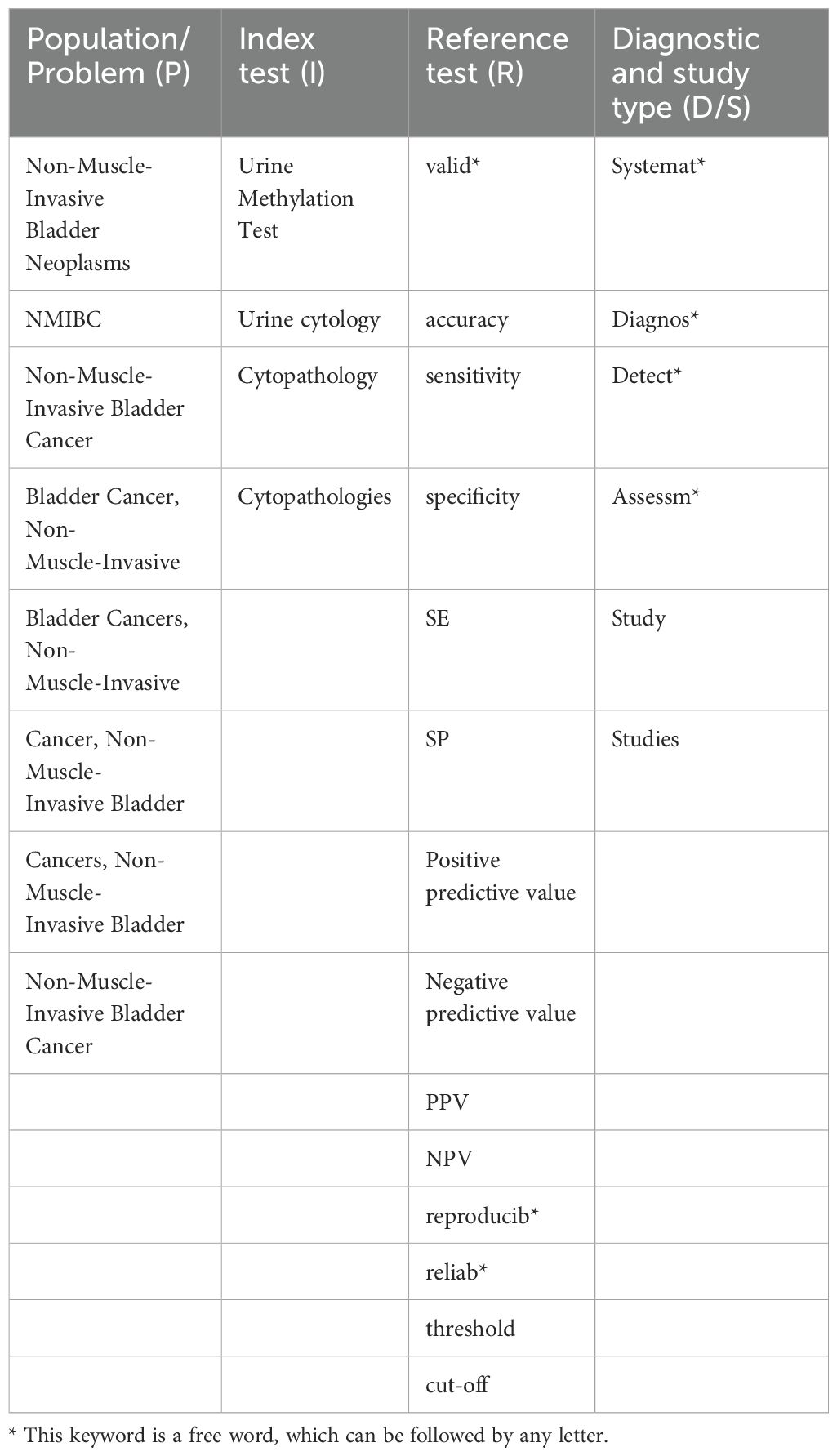
The systematic review was carried out in accordance with the requirements of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA). The study protocol is registered on PROSPERO with ID CRD42023398969. We searched the PubMed, Embase, Web of Science, and Cochrane Library databases for published English-language studies evaluating the diagnostic value of urine methylation test for NMIBC up to May 2023. The search strategy incorporated Medical Subject Headings (MeSH) and equivalent terms.
Inclusion criteria and exclusion criteria
We selected articles using the following inclusion criteria: (1) Pathological test findings were incorporated for BC classification; (2) Enrolled patients had been diagnosed and underwent initial treatment for NMIBC; (3) Patients must undergo both urinary cytology and urinary methylation test; (4) At least one of the following outcomes had to be assessed: diagnostic test accuracy expressed in terms of sensitivity (SE), specificity (SP), positive predictive value (PPV) and negative predictive value (NPV); (5) Published in English. The following were the exclusion criteria: (1) Case reports, letters, animal models, review articles, and meta-analyses; (2) Inadequate data, such as the number of patients based on the cut-off of urine methylation test, was not accessible.
Study selection and quality assessment
We utilized a modified version of the QUADAS-2 tool to assess the methodological quality of the selected studies. To identify relevant studies, two researchers independently assessed the title, abstract, and full text. The following information were extracted and recorded: first author’s name, date and place of publication, sample size, male to female ratio, age, and type of study. Two researchers extracted the data, while a third researcher settled any discrepancies.
Statistical analysis
All analyses were performed using the statistical software R (version 5.3.0, R Foundation for Statistical Computing, Vienna, Austria) in combination with the “mada” and “meta” packages. Statistical analysis was performed using a significance threshold of p < 0.05. The true positives (TP), false positives (FP), false negatives (FN), and true negatives (TN) were extracted from the selected studies. To evaluate the diagnostic effectiveness of urine methylation test, we performed a meta-analysis including sensitivity, specificity, diagnostic odds ratio, and patient manipulation characteristics. The chi-square test was performed to assess the heterogeneity of results. If I2 is less than 50%, a fixed-effect model is utilized because there is no detectable heterogeneity. Otherwise, a random effects model will be applied. To assess the methodological quality of the included articles, we employed the QUADAS-2 tool and data was analyzed using RevMan 5.2 software (15).
Results
Screening research process
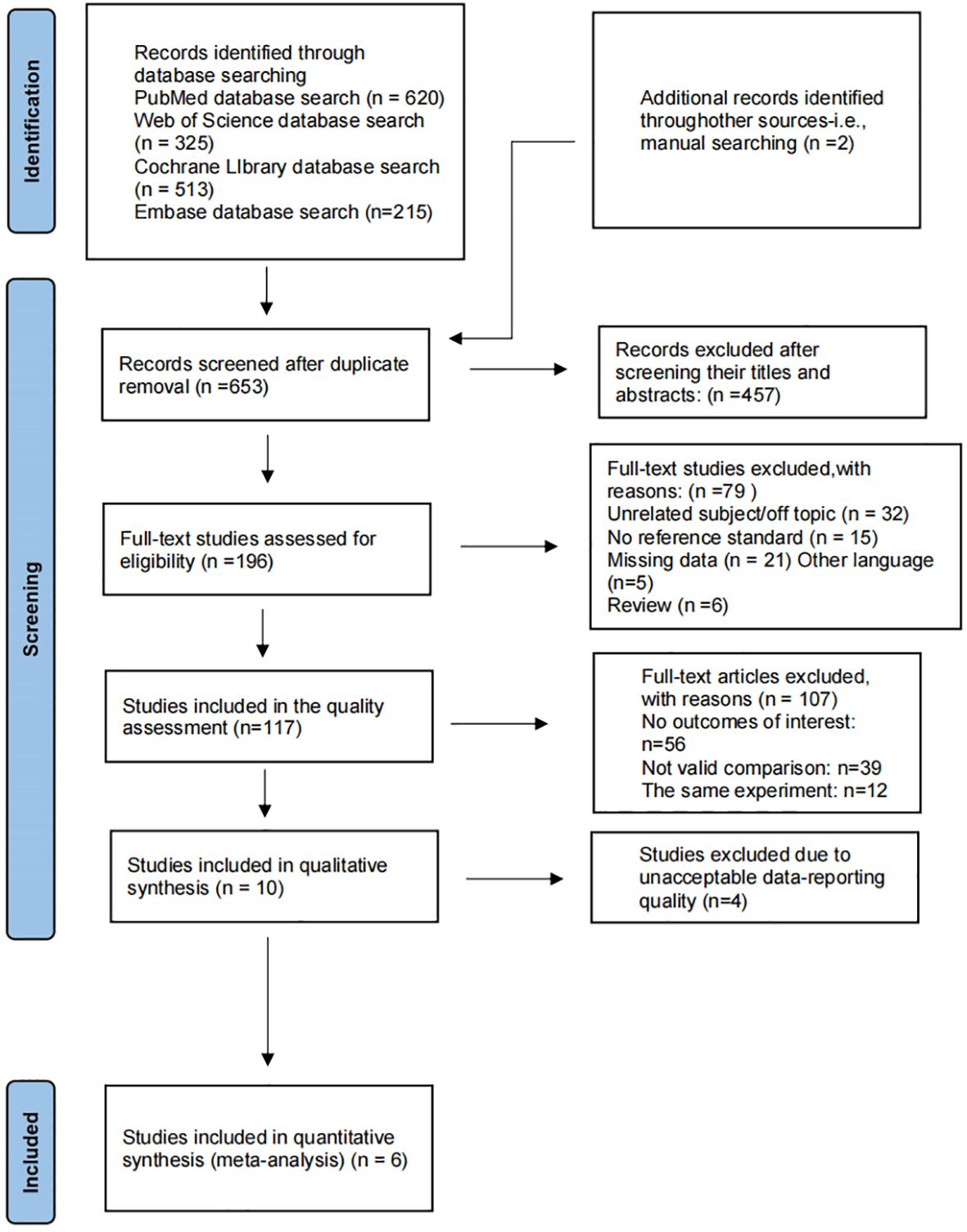
Table 1 presents the final consent search items based on the PIRD recommendations (Table 1) (16). We identified 620 records from PubMed, 215 from Embase, 325 from Web of Science, 513 from the Cochrane Library (Figure 1). The titles and abstracts of 653 records were screened after removing 1,020 duplicate records. Full texts for 196 studies were obtained. The quantitative analysis consists of ten articles. Four studies were removed because of poor quality. Finally, data is taken from 6 studies (17–22).
Characteristics included in the study
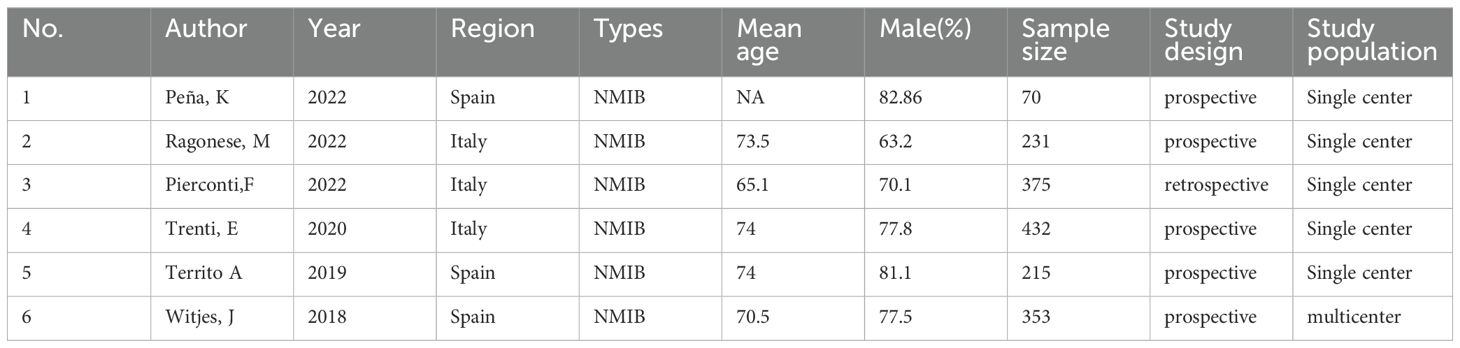
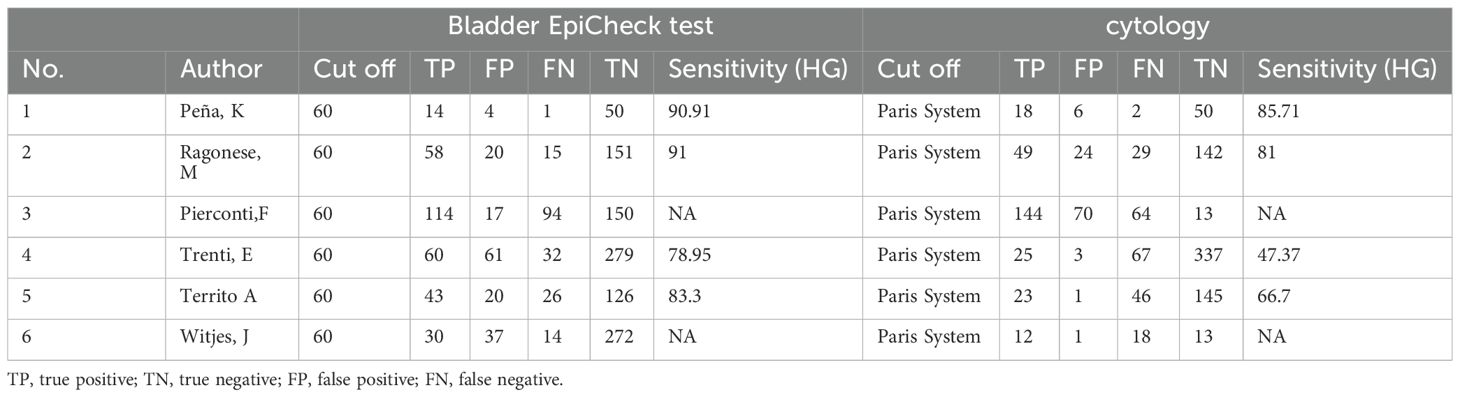
We have generated a summary of authors, publication years, sample sizes and other information from the included articles (Table 2). We have compiled the threshold values for cytology and urine methylation test, together with their diagnostic significance (Table 3). The six studies involved 1,676 participants, and a standard cut-off was used consistently across all research. In all investigations, PCR detection of bladder tissue samples was carried out using a urine methylation test kit, and the data were processed using urine manipulation software to generate an EpiScore ranging from 0-100. A positive EpiScore is ≥60, whereas a negative score < 60. Cytology was assessed by pasteurized staining, and Paris Urine Cytology Reporting System was used to make the diagnosis. Patients ranged in age from 65.1 to 74, with 63.2 percent to 82.86 percent being male.
Sensitivity and specificity
We compared the sensitivity and specificity of the Urine Methylation Test with the cytology examination (Figure 2). In the urine methylation test, due to high heterogeneity (I2>50), the sensitivity was 0.69 (95% confidence interval (CI) 0.59 to 0.77, I2 = 77%) in the random effects model, while the pooled specificity was 0.87 (95% CI 0.84-0.90, I2 = 57%). In the cytology examination, the sensitivity was 0.52 (95% CI 0.35 to 0.68, I2 = 94%) in the random effects model, while the specificity was 0.93 (95% CI 0.64-0.99, I2 = 99%). The area under curve (AUC) for receiver operating characteristic (ROC) of urine methylation test was 0.89 (95% CI 0.85-0.91, I2 = 55%), and AUC was 0.71 (95% CI 0.67-0.75, I2 = 62%) for the cytology examination (Figure 3). Furthermore, as shown by Pena, Ragonese, Trenti, and Territo, urine methylation test has a higher sensitivity in detecting high-grade NMIBC, as indicated in Table 3.
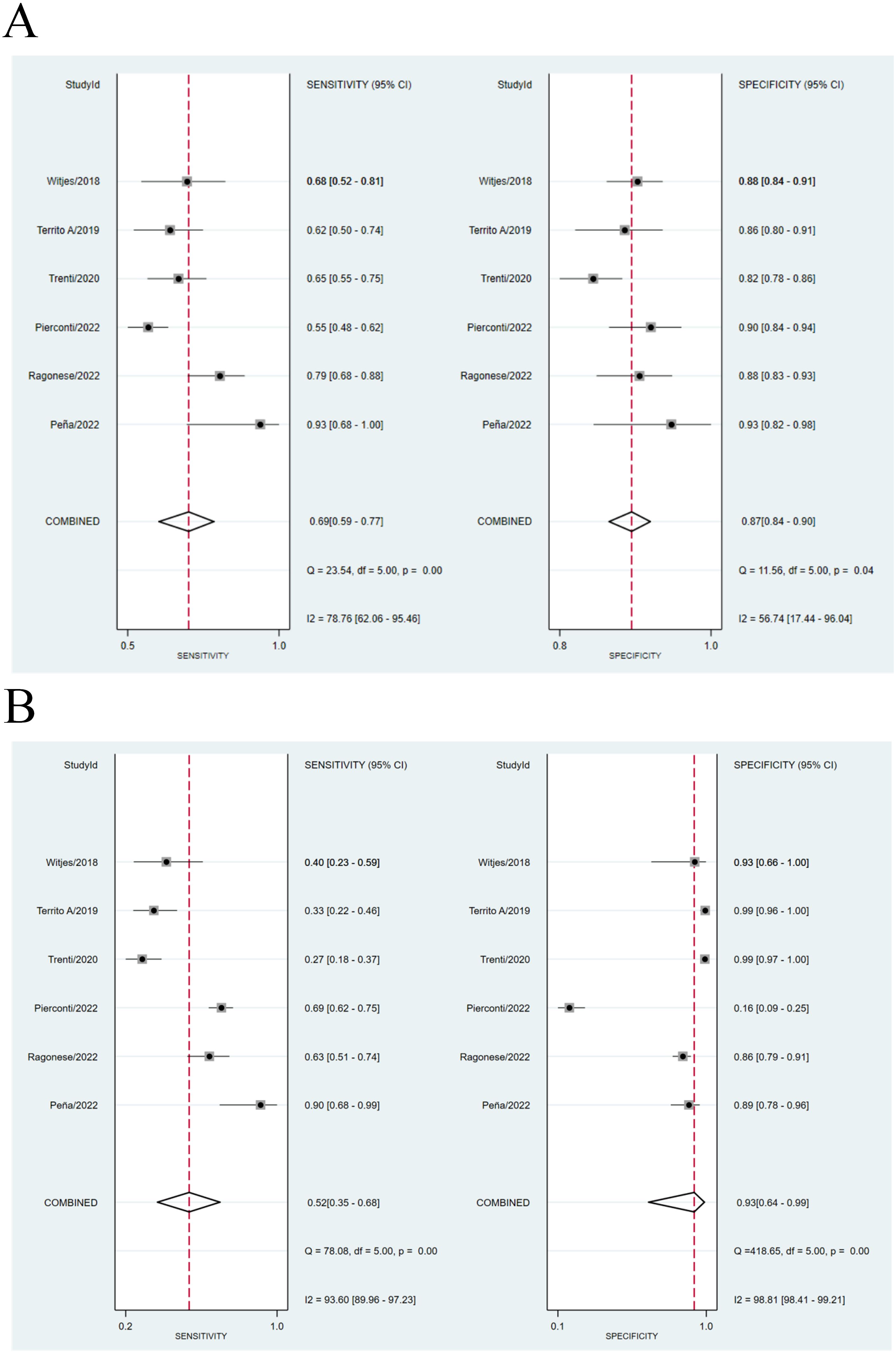
Figure 2. (A) Comparison of sensitivity between Urine Methylation Test (left) and cytology (right) of NMIBC. (B) Comparison of specificity between Urine Methylation Test (left) and cytology (right) of NMIBC.
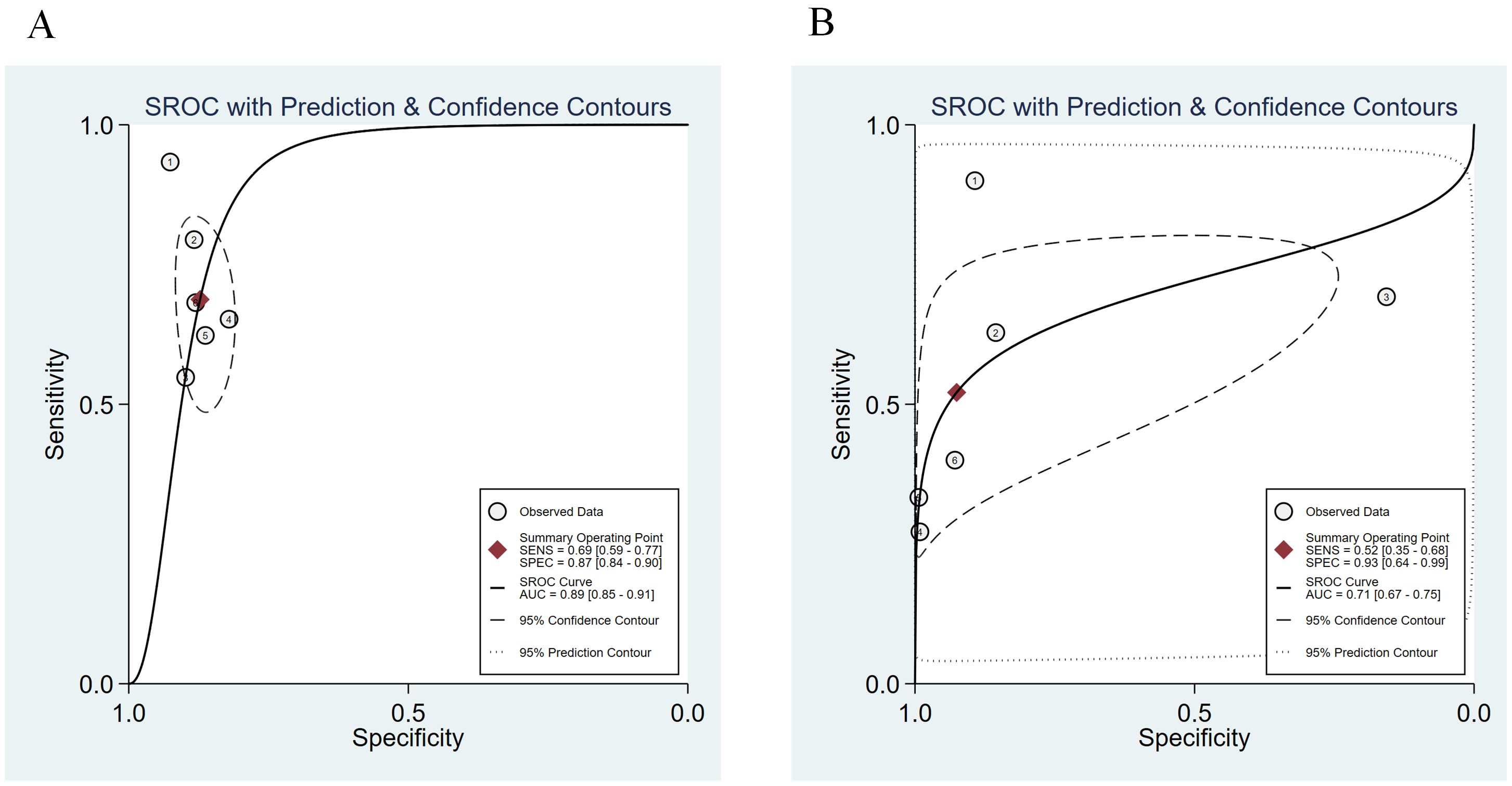
Figure 3. The summary receiver operating characteristic (SROC) curve of urine methylation test (A) and cytology (B) in the diagnosis of NMIBC.
Research quality assessment
Figure 4 illustrates the methodological quality of six studies. A study (22) examined the potential bias in patient selection, which was not clearly defined. Two studies (17, 20) reported the possibility for bias due to unclear reference criteria. One study (21) had a significant probability of bias in flow and timing. One study (22) had a substantial risk of bias, while another (17) had an unclear risk of bias in patient selection. One study (17) had a substantial risk of bias, whereas two studies (19, 23) had an uncertain risk of bias in the index test. One study (23) had an unclear risk of bias in reference standards.
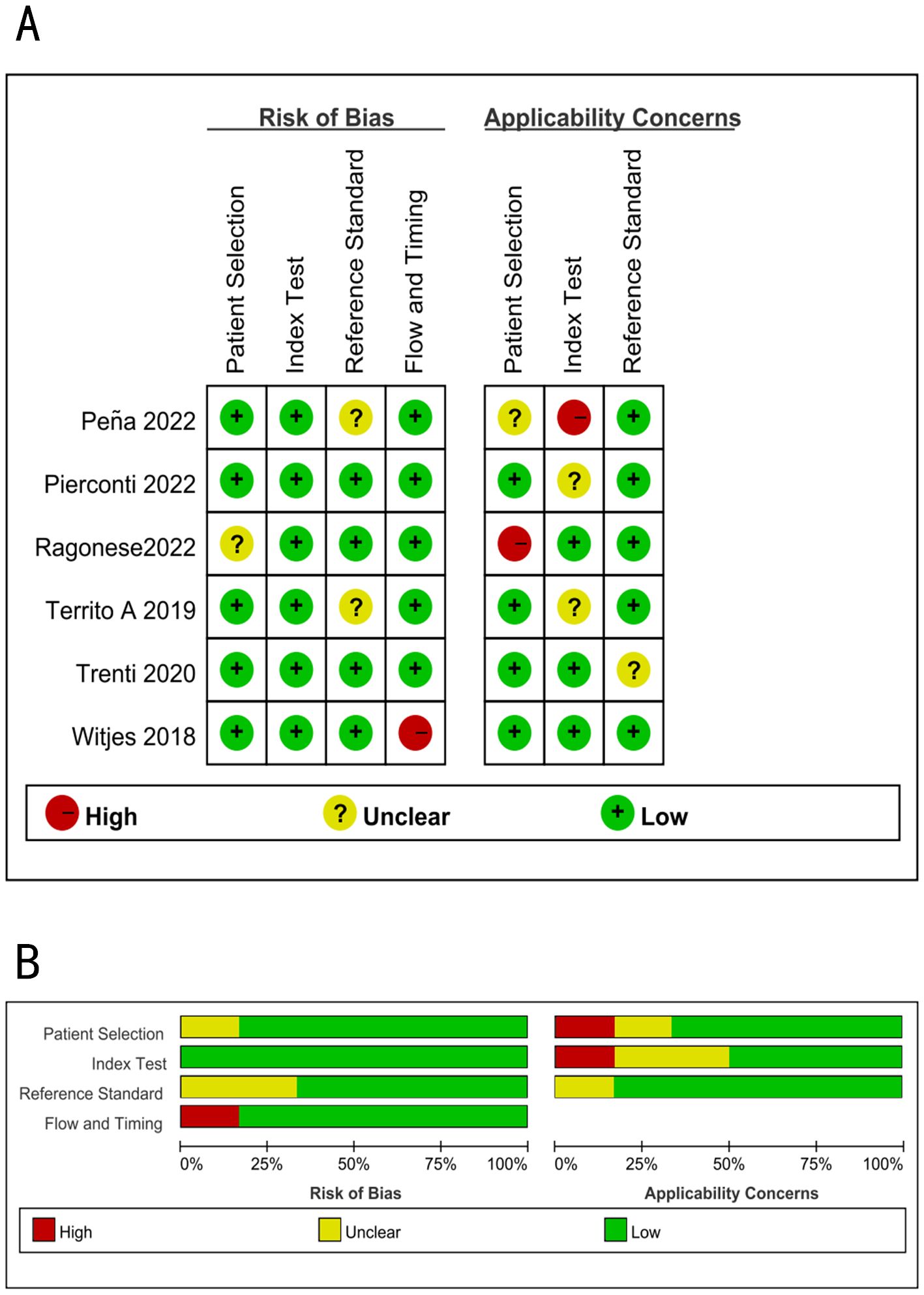
Figure 4. Summary of methodological quality of all studies using the QUADAS-2; (A) Risk of bias graph; (B) Risk of bias summary.
Discussion
DNA methylation is a fundamental epigenetic process that has a significant influence for gene expression and imprinting. It modifies genes without affecting their DNA sequence and performs crucial activities such as protecting against viral sequences, suppressing recombination, and facilitating the assembly of heterochromatin (24). Previously, DNA methylation research focused on the regulation of epigenetic genes in the central nervous system, as well as potential therapeutic targets for the treatment of neuropsychiatric diseases (25). Previous study has found that DNA methylation was associated with tumorigenesis and progression, with tumor suppressor genes often being hypermethylated and oncogenes being hypomethylated (26). As research has progressed, the use of DNA methylation in cancer screening and follow-up has gradually gained attention, especially in NMIBC.
Patients with NMIBC frequently experience multiple relapses throughout their lives, and the disease may progress in some cases. This illness presents severe challenges for patients and healthcare systems, resulting in a substantial burden (27). The statistical data reported that 1% to 45% of NMIBC can progress to a muscle-invasive tumor form within 5 years. The risk of recurrence and progression increases with the following factors: stage, grade of malignancy, size and number of lesions, and presence of carcinoma in situ (28). The quality of follow-up directly affects the mortality of NMIBC. Urine cytology, although being the most widely used non-invasive diagnostic test for BC, has a low sensitivity. As a result, cystoscopy and biopsy remain to be standard diagnostic methods, despite the discomfort and psychological stress they cause patients.
In recent studies, researchers investigated novel urinary tract tests that depend on identifying DNA alteration in urothelial cells. These tests aim to overcome the limitations of traditional BC screening methods and have the potential to decrease the need for cystoscopies in patients with low-grade tumors who are under active surveillance (29, 30). According to new research (18), urine methylation test performs well in the diagnosis of NMIBC. According to the meta-analysis, urine methylation test had higher sensitivity than cytology (0.69 vs. 0.52). In each included study, different investigators also found the same conclusions regarding the effectiveness of the two methods in diagnosing NMIBC. Furthermore, according to Pena, Ragonese, Trenti, and Territo et al, urine methylation test has a higher sensitivity in the diagnosis of high-grade NMIBC.
To better show the linear correlation between sensitivity and specificity, the researchers constructed the ROC curve based on the odds ratio (OR) weight from each study, referred to as the SROC curve. The SROC curve is unaffected by variations in the diagnostic threshold and may directly reflect the efficacy of diagnostic test using the Fig and Area (30). The AUC of the ROC curve represents the accuracy of diagnostic test. After analysis, the SROC curve of urine methylation test is compatible with the Fig that should be determined as the examination technique with high diagnostic value, with an AUC value of 0.89, which is larger than that of cytology (0.71). It should be mentioned that the specificity of urine methylation test is lower than that of the cytology test, which is a limitation of this technique.
It is critical to understand the limitations of this study. The papers included in the analysis had several limitations in terms of study design, patient characteristics, pathologist expertise, and incomplete data. In addition, selective and subjective factors should be considered. Therefore, the results should be interpreted with caution. The effectiveness of urine methylation test and cytology in the diagnosis of NMIBC must to be validated by investigations with larger sample size.
Conclusions
Urine methylation test have proven to be high sensitivity and accuracy in monitoring patients with NMIBC after surgery. However, their specificity is lower than cytology. There are a limited number of studies on the role of urine methylation test in monitoring NMIBC. More research is needed to conduct a meta-analysis.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
ZY: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Software, Writing – original draft. TW: Validation, Visualization, Writing – review & editing. YZ: Funding acquisition, Resources, Writing – review & editing. JL: Software, Supervision, Writing – review & editing. ZZ: Conceptualization, Funding acquisition, Investigation, Methodology, Project administration, Software, Validation, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
Thanks to the promotion plan of Beijing Municipal Hospital Administration, code: DFL20190502 for the support of this paper.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2021) 71:209–49. doi: 10.3322/caac.21660
2. Antoni S, Ferlay J, Soerjomataram I, Znaor A, Jemal A, Bray F. Bladder cancer incidence and mortality: A global overview and recent trends. Eur Urol. (2017) 71:96–108. doi: 10.1016/j.eururo.2016.06.010
3. Babjuk M, Böhle A, Burger M, Capoun O, Cohen D, Compérat EM, et al. EAU guidelines on non-muscle-invasive urothelial carcinoma of the bladder: update 2016. Eur Urol. (2017) 71:447–61. doi: 10.1016/j.eururo.2016.05.041
4. Wiener HG, Mian C, Haitel A, Pycha A, Schatzl G, Marberger M. Can urine bound diagnostic tests replace cystoscopy in the management of bladder cancer? J Urol. (1998) 159:1876–80. doi: 10.1016/S0022-5347(01)63184-7
5. Das S. Evaluation of urine cytology in urothelial carcinoma with respect to the Paris system for reporting urinary cytology. J Med Sci Clin Res. (2017) 5:70. doi: 10.18535/jmscr
6. Anbardar MH, Monjazeb R. Reclassification of urinary cytology regarding The Paris System for Reporting Urinary Cytology with cytohistological correlation demonstrates high sensitivity for high-grade urothelial carcinoma. Diagn Cytopathol. (2020) 48:446–52. doi: 10.1002/dc.24387
7. Messer J, Shariat SF, Brien JC, Herman MP, Ng CK, Scherr DS, et al. Urinary cytology has a poor performance for predicting invasive or high-grade upper-tract urothelial carcinoma. BJU Int. (2011) 108:701–5. doi: 10.1111/j.1464-410X.2010.09899.x
8. Woldu SL, Bagrodia A, Lotan Y. Guideline of guidelines: non-muscle-invasive bladder cancer. BJU Int. (2017) 119:371–80. doi: 10.1111/bju.13760
9. Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. (2015) 136:E359–86. doi: 10.1002/ijc.29210
10. Beukers W, Kandimalla R, Masius RG, Vermeij M, Kranse R, van Leenders GJ, et al. Stratification based on methylation of TBX2 and TBX3 into three molecular grades predicts progression in patients with pTa-bladder cancer. Mod Pathol. (2015) 28:515–22. doi: 10.1038/modpathol.2014.145
11. Thomas T. Bladder epiCheck for NMIBC. Nat Rev Urol. (2022) 19:67. doi: 10.1038/s41585-022-00564-7
12. van Rhijn BW, van der Poel HG, van der Kwast TH. Urine markers for bladder cancer surveillance: a systematic review. Eur Urol. (2005) 47:736–48. doi: 10.1016/j.eururo.2005.03.014
13. Pierconti F, Martini M, Cenci T, Fiorentino V, Gianfrancesco LD, Ragonese M, et al. The bladder epicheck test and cytology in the follow-up of patients with non-muscle-invasive high grade bladder carcinoma. Urol Oncol. (2022) 40:108.e19–108.e25. doi: 10.1016/j.urolonc.2021.11.013
14. Pierconti F, Rossi ED, Fiorentino V, Bakacs A, Carlino A, Navarra E, et al. Methylation analysis of urinary sample in non-muscle-invasive bladder carcinoma: frequency and management of invalid result. Biomedicines. (2023) 11:3288. doi: 10.3390/biomedicines11123288
15. Shim SR, Kim SJ, Lee J. Diagnostic test accuracy: application and practice using R software. Epidemiol Health. (2019) 41:7:e201900. doi: 10.4178/epih.e2019007
16. Campbell JM, Klugar M, Ding S, Carmody DP, Hakonsen SJ, Jadotte YT, et al. Diagnostic test accuracy: methods for systematic review and meta-analysis. Int J Evid Based Healthc. (2015) 13:154–62. doi: 10.1097/XEB.0000000000000061
17. Peña KB, Riu F, Hernandez A, Guilarte C, Badia J, Parada D. Usefulness of the urine methylation test (Bladder epiCheck(®)) in follow-up patients with non-muscle invasive bladder cancer and cytological diagnosis of atypical urothelial cells-an institutional study. J Clin Med. (2022) 11:3855. doi: 10.3390/jcm11133855
18. Cochetti G, Rossi de Vermandois JA, Maulà V, Cari L, Cagnani R, Suvieri C, et al. Diagnostic performance of the Bladder EpiCheck methylation test and photodynamic diagnosis-guided cystoscopy in the surveillance of high-risk non-muscle invasive bladder cancer: A single centre, prospective, blinded clinical trial. Urol Oncol. (2022) 40:105.e11–105.e18. doi: 10.1016/j.urolonc.2021.11.001
19. Pierconti F, Martini M, Fiorentino V, Cenci T, Capodimonti S, Straccia P, et al. The combination cytology/epichek test in non muscle invasive bladder carcinoma follow-up: Effective tool or useless expence? Urol Oncol. (2021) 39:131.e17–131.e21. doi: 10.1016/j.urolonc.2020.06.018
20. Territo A, Gallioli A, Diana P, Boissier R, Fontana M, Gaya JM, et al. DNA methylation urine biomarkers test in the diagnosis of upper tract urothelial carcinoma: results from a single-center prospective clinical trial. J Urol. (2022) 208:570–9. doi: 10.1097/JU.0000000000002748
21. Witjes JA, Morote J, Cornel EB, Gakis G, van Valenberg FJP, Lozano F, et al. Performance of the bladder epiCheck™ Methylation test for patients under surveillance for non-muscle-invasive bladder cancer: results of a multicenter, prospective, blinded clinical trial. Eur Urol Oncol. (2018) 1:307–13. doi: 10.1016/j.euo.2018.06.011
22. Ragonese M, Gianfrancesco LD, Palermo G, Pierconti F, Martini M, Foti M, et al. The role of bladder epicheck test in follow-up of patients with non-muscle invasive bladder cancer. Clin Genitourin Cancer. (2022) 20:e271–5. doi: 10.1016/j.clgc.2022.03.009
23. Trenti E, Pycha S, Mian C, Schwienbacher C, Hanspeter E, Kafka M, et al. Comparison of 2 new real-time polymerase chain reaction-based urinary markers in the follow-up of patients with non-muscle-invasive bladder cancer. Cancer Cytopathol. (2020) 128:341–7. doi: 10.1002/cncy.22246
24. Bird A. DNA methylation patterns and epigenetic memory. Genes Dev. (2002) 16:6–21. doi: 10.1101/gad.947102
25. Moore LD, Le T, Fan G. DNA methylation and its basic function. Neuropsychopharmacology. (2013) 38:23–38. doi: 10.1038/npp.2012.112
26. Ilango S, Paital B, Jayachandran P, Padma PR, Nirmaladevi R. Epigenetic alterations in cancer. Front Biosci (Landmark Ed). (2020) 25:1058–109. doi: 10.2741/4847
27. Leal J, Luengo-Fernandez R, Sullivan R, Witjes JA. Economic burden of bladder cancer across the European union. Eur Urol. (2016) 69:438–47. doi: 10.1016/j.eururo.2015.10.024
28. Poli G, Brancorsini S, Cochetti G, Barillaro F, Egidi MG, Mearini E. Expression of inflammasome-related genes in bladder cancer and their association with cytokeratin 20 messenger RNA. Urol Oncol. (2015) 33:505.e1–7. doi: 10.1016/j.urolonc.2015.07.012
29. Fasulo V, Paciotti M, Lazzeri M, Contieri R, Casale P, Saita A, et al. Xpert bladder cancer monitor may avoid cystoscopies in patients under “Active surveillance” for recurrent bladder cancer (BIAS project): longitudinal cohort study. Front Oncol. (2022) 12:832835. doi: 10.3389/fonc.2022.832835
Keywords: NMIBC, urine methylation test, receiver operating characteristic, meta-analysis, diagnose
Citation: Yao Z, Wang T, Liu J, Zhou Z and Zhang Y (2024) Diagnostic accuracy of cytology and urine methylation test in patients with non-muscle invasive bladder cancer: a systematic review and meta-analysis. Front. Oncol. 14:1412346. doi: 10.3389/fonc.2024.1412346
Received: 21 May 2024; Accepted: 13 August 2024;
Published: 03 September 2024.
Edited by:
Badrinath Konety, Allina Health, United StatesReviewed by:
Maurizio Martini, University of Messina, ItalyRoberto Contieri, Humanitas University, Italy
Vincenzo Fiorentino, University of Messina, Italy
Copyright © 2024 Yao, Wang, Liu, Zhou and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhongbao Zhou, MzQ2ODk0MzgzQHFxLmNvbQ==; Yong Zhang, enkxOTgzMTE1Nzc1MUAxNjMuY29t
 Zhuoyue Yao
Zhuoyue Yao Tao Wang
Tao Wang Yong Zhang
Yong Zhang