- 1Department of Gastrointestinal Surgery, Peking University International Hospital, Beijing, China
- 2Department of Pathology, Peking University Cancer Hospital, Beijing, China
Epithelioid inflammatory myofibroblastic sarcoma (EIMS) is an extremely rare and aggressive form of inflammatory myofibroblastic tumor. Clinically, it has a high risk of relapse and peripheral organ infiltration, and it responds poorly to conventional chemotherapy. Anaplastic lymphoma kinase (ALK) inhibitors are currently the most effective targeted therapy for EIMS. This report discusses a typical case of abdominal EIMS in a 43-year-old woman. The tumors recurred rapidly within one month after surgery. Alectinib was promptly administered upon diagnosis. However, the patient developed a severe allergic reaction to the medication. After a comprehensive assessment and symptomatic treatment, her condition stabilized, leading to a favorable prognosis. This study summarizes cases of abdominal EIMS, highlights the successful use of Alectinib for treatment, and discusses the management of medication-related complications.
1 Introduction
Inflammatory myofibroblastic tumor (IMT) is a rare mesenchymal neoplasm with intermediate biological behavior and indolent clinical manifestations (1). It seldom results in distant metastasis and has a relatively low recurrence rate, ranging from 2% to 25% in various studies (2). Histologically, IMTs are primarily composed of proliferating spindle cells and infiltrating lymphocytes. Over 50% of IMTs exhibit rearrangements involving the anaplastic lymphoma kinase (ALK) gene located on chromosome 2p23, leading to abnormal activation of tyrosine kinase and transformation of cell phenotype (3, 4). When tyrosine kinases are activated at the nuclear membrane or in the perinuclear region, the tumor may transform into epithelioid inflammatory myofibroblastic sarcoma (EIMS), which consists mainly of round-to-epithelioid cells and exhibits more aggressive and malignant clinical features (5).
Since Marino et al. (6) first reported EIMS in 2011, dozens of similar cases have been documented over the past 13 years. EIMS has been identified in various organs, including the lung (7), omentum (8), skin (9), and uterus (10). According to existing literature, the recurrence rate after surgery for EIMS is extremely high, and the tumor shows resistance to conventional chemotherapy and immunotherapy. However, several studies have demonstrated that EIMS responds well to ALK inhibitors (6, 8, 12, 13, 15, 20–24, 33, 35). Therefore, we present this case of EIMS treated with the ALK inhibitor Alectinib and review relevant literature to provide guidance for future clinical diagnosis and treatment.
2 Case report
In June 2023, a 43-year-old woman with a history of intermittent abdominal pain since April 2023 presented to our institution. Abdominal and pelvic enhanced CT scans revealed massive ascites and multiple soft tissue density shadows in the abdomen and pelvis. The tumor appeared irregular in shape with ill-defined margins and showed invasion into the bowel (Figure 1A). Following a multi-disciplinary team (MDT) discussion, the patient underwent exploratory laparotomy, abdominal mass resection, partial jejunal resection, jejunal anastomosis, ileocecal resection, and ileocolonic anastomosis (postoperative enhanced CT are shown in s).
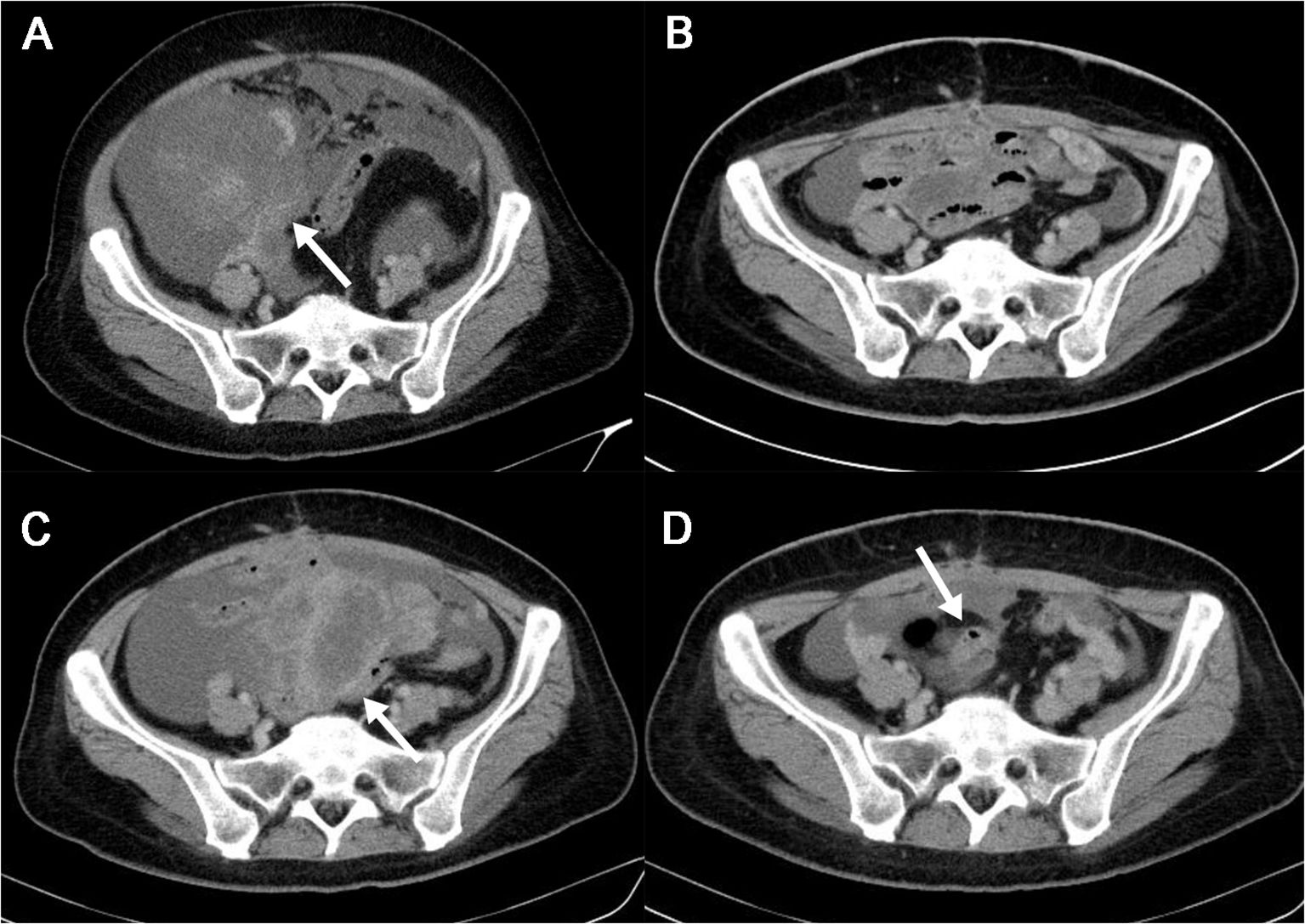
Figure 1. The patient’s imaging features during whole course of the disease in abdominal and pelvic enhanced CT. (A). Preoperative Abdominal and Pelvic Enhanced CT in June, 2023. Multiple soft tissue masses with blurred edge were observed in the abdominal cavity(white arrow). After contrast enhancement, the masses showed obvious uneven enhancement, the largest one was about 19.5*16.8cm. Massive ascites and multiple retroperitoneal enlarged lymph nodes were seen in the abdomen and pelvis. (B). Postoperative Abdominal and Pelvic Enhanced CT in August, 2023. Ascites was still in the pelvic cavity. (C). Abdominal and Pelvic Enhanced CT in September, 2023. Interim assessment of chemotherapy show disease progression(tumors recurred as shown by white arrow). (D). Abdominal and Pelvic Enhanced CT in November, 2023. One month after initiating ALKi treatment, assessment showed significant tumor shrinkage and reduction in ascites(white arrow). ALKi, anaplastic lymphoma kinase inhibitor.
Postoperative pathology (Figure 2A) revealed that the tumors originated from intra-abdominal mesenchymal tissue, with sizes of 35×25×13 cm, 14×11×9 cm, and 5×3×3 cm respectively. The tumor invaded the serosal layer of the intestinal wall to the muscularis mucosae and invaded the muscularis wall of the appendix, assessed as grade 2 in the FNCLCC (Fédération Nationale de Centres de Lutte Contre le Cancer) system. The tumors contained spindle cells and epithelioid cells simultaneously, with lamellar necrosis, mitotic figures of 2-3/10 HPF (high power field), and myxoid stroma (Figure 2B). Immunohistochemical (IHC) staining results (Figures 2C–E) were as follows: ALK, p16, p53, CDK4, desmin, SMA, S-100, CD34, CD30, CD68, WT1, Calretinin, D2-40, and Ki-67 (approximately 30%) were positive, while CK, MDM2, CD117, DOG-1, Myogenin, and EMA were negative. Based on the IHC results and the microscopic morphology of the tumor, a diagnosis of EIMS was considered.
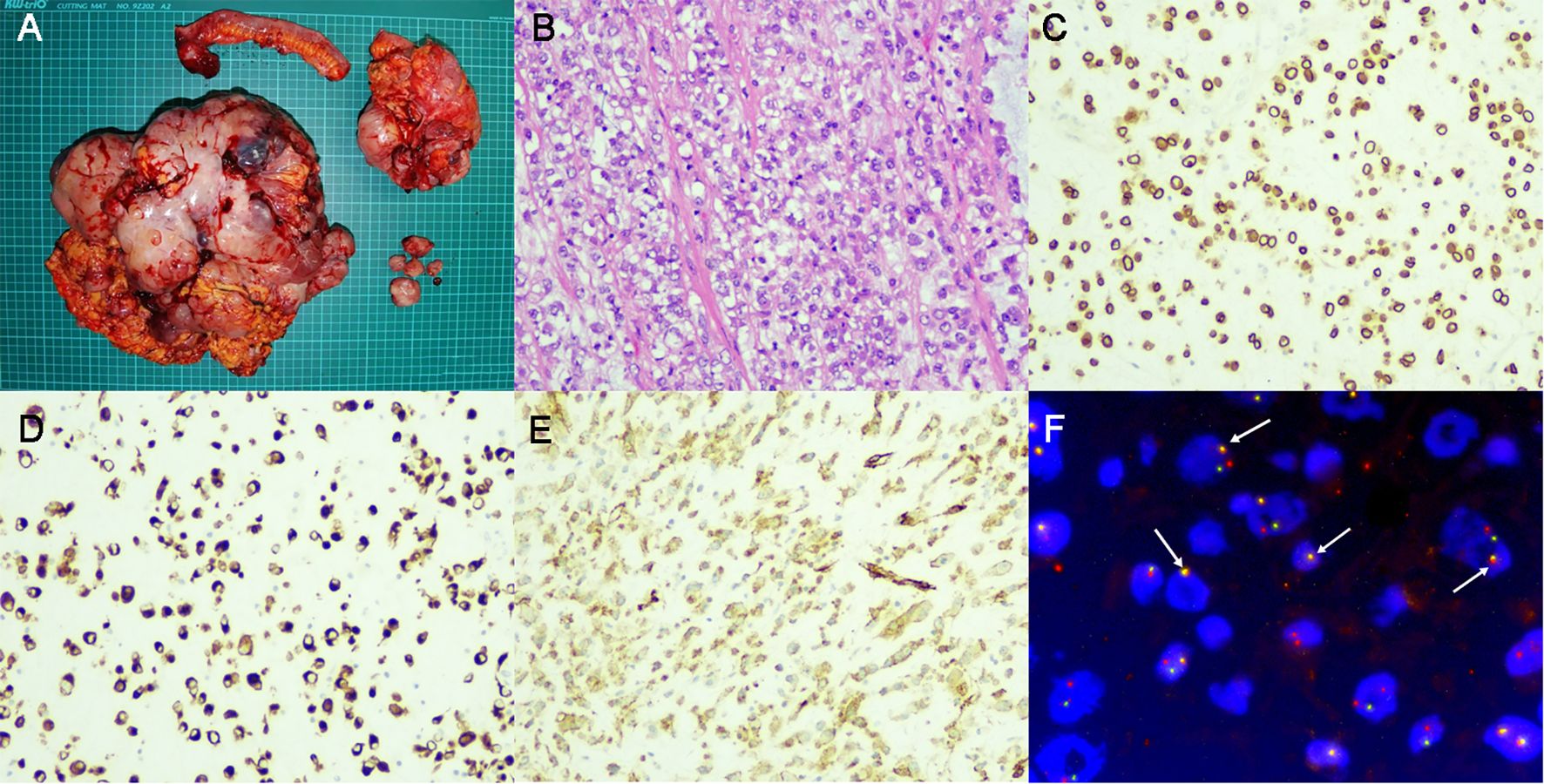
Figure 2. Surgical specimen and pathological examination. (A) Gross examination revealed a massive tumor weighing 8kg and adjacent organ involvement. (B). Hematoxylin and Eosin staining showed the existence of both spindle cells and epithelioid cells. (C). IHC staining for ALK revealed positive expression around the nuclei of tumor cells. (D, E). IHC staining showed positive for desmin (D) and SMA (E). (F). FISH revealed splitting signals inside tumor cells (As indicated by the white arrow), confirming the presence of ALK rearrangement. IHC, immunohistochemical; ALK, anaplastic lymphoma kinase; FISH, fluorescence in situ hybridization.
After the surgery, a Next-generation sequencing (NGS) test was immediately conducted, suggesting potential benefits from 5-fluorouracil, platinum, and PD-1/PD-L1 inhibitors for the patient. Consequently, we opted for the Xelox + sintilimab chemotherapy regimen. Following three cycles of chemotherapy, the patient returned to our institution with recurrent symptoms including abdominal distension, nausea, fever, and a perceived weight gain of 2.5 kg. Imaging examination revealed multiple nodules in the abdomen and pelvis, with sizes up to 2.2×1.7 cm (Figure 1C). Chemotherapy assessment indicated progressive disease (PD). Meanwhile, ALK rearrangement was confirmed via fluorescence in situ hybridization (FISH) as shown in Figure 2F. Based on our literature review and imaging assessments, the patient was initiated on Alectinib treatment (600 mg, BID).
On the 11th day of medication, the patient suddenly developed a high fever, rash, itchy, and other symptoms suggestive of a drug allergy. Following a comprehensive examination, we confirmed the presence of a drug allergy. Referring to the latest expert consensus on the management of adverse drug reactions associated with ALK inhibitors (ALKi) in 2023, we noted that the patient did not exhibit any signs of liver or kidney dysfunction, indicating no contraindication for treatment continuation. Therefore, we initiated symptomatic treatment (details provided in Table 1) and decided to proceed with Alectinib therapy. After 18 days of continuous Alectinib therapy, the patient’s abdominal circumference decreased from 100 cm to 92 cm, and all previous complaints resolved. Subsequent abdominal and pelvic enhanced CT scans in November 2023 revealed significant tumor shrinkage and reduction (the maximum tumor diameter decreased from 35.6cm to 5.1cm), along with a notable decrease in ascites (Figure 1D). According to the Response Evaluation Criteria in Solid Tumors (RECIST), the patient achieved a partial response (PR) and remained alive with the tumor without experiencing any significant adverse events at the time of manuscript preparation.
3 Discussion
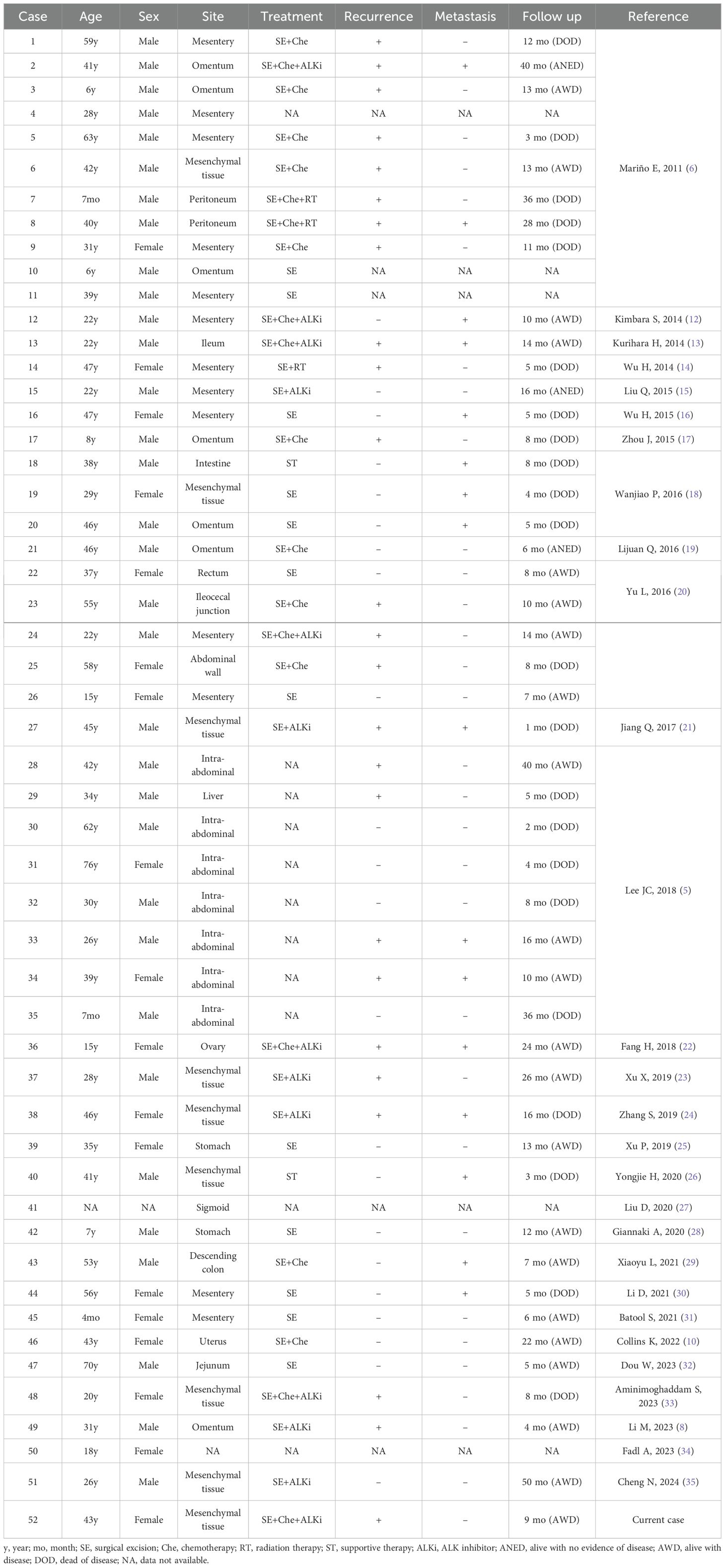
EIMS represents a highly aggressive and malignant variant of IMTs, characterized by epithelioid-to-round cell morphology. Unlike indolent tumors of mesenchymal origin, EIMS typically exhibits invasion into adjacent organs upon discovery by medical teams (3). Consistent with observations made by other researchers, our case demonstrated similar insensitivity to conventional chemotherapy and immunotherapy, posing significant treatment challenges initially. To comprehensively delineate the characteristics of EIMS, we conducted a literature search using PubMed and the China National Knowledge Infrastructure (CNKI), identifying a total of 51 cases of abdominal origin reported in 28 articles, as summarized in Table 2.
In summary, EIMS appears to be more prevalent in males (33/52) than females (18/52), with a wide age range of occurrence spanning from 7 months to 70 years. While EIMS can originate from various organs, it is most frequently observed within the abdominal cavity, particularly in the mesentery, omentum, and other mesenchymal tissues (38/52). Three-quarters of patients underwent surgical treatment (39/52), with 27 receiving adjuvant therapy and only 13 receiving ALKi treatment. Among the cases with available prognostic information (approximately 47 cases), 25 experienced postoperative recurrence, while 16 developed postoperative metastasis. Twenty-two patients ultimately succumbed to the disease, whereas 22 remained alive with tumors as documented in their respective articles. Remarkably, only three cases achieved complete recovery without recurrence or metastasis. Notably, among the 13 patients treated with ALKi, only three died, indicating a relatively favorable prognosis for the remaining ten patients.
Regarding IHC staining, our patient exhibited characteristic positivity for ALK, desmin, and SMA. Desmin (36) and SMA (37) are markers of fibroblasts, typically positive in the majority of EIMS cases. ALK positivity in the nuclear membrane or cytoplasm signifies the constitutive activation of ALK, a key factor in the transformation of IMT to EIMS (3, 38). The results of IHC staining for ALK are affected by many factors. For example, different ALK fusion partners can result in diverse expression levels and patterns of the ALK protein, which can affect the staining intensity and distribution observed in IHC staining. Takeuchi’s research found that EML4-ALK fusion showed stronger IHC staining compared to others like KIF5B-ALK or TFG-ALK, highlighting the variability in IHC results based on the fusion partner (39). Besides, the choice of antibody clone used for detecting ALK fusions also plays a crucial role in the sensitivity of IHC. Mino-Kenudson M’s study found the D5F3 clone demonstrated superior performance in detecting a broader range of ALK fusion variants, thus providing more reliable and consistent staining results (40). According to our findings, all cases with complete postoperative pathological information tested positive for ALK, which is quite different compared with the 50% positive rate of ALK in IMTs. This might attribute mainly to the difference of fusion partners between EIMS and IMTs. In addition, among other positive results of IHC staining in our case, p16 (41), p53 (42), CDK4 (43), WT1 (44) are all tumor suppressor genes, their mutation usually indicate strong proliferative activity of tumors. Positive expression of angiogenic marker CD34 (45), macrophage marker CD68 (46), and lymphatic vessel marker D2-40 (47) indicates active formation of tumor interstitial tissue, which usually mean the tumor has strong invasivity. Calretinin (48) and S-100 (49) are both calcium-binding proteins involved in various cellular functions. Their positivity is typically observed in soft tissue tumors. Of note, CD30 is reported positive in over half of cases, suggesting its potential as a diagnostic marker and treatment target. Despite the modest sensitivity and specificity of these markers in existing studies, IHC still holds significant diagnostic value for EIMS before FISH testing becomes available.
In our case, NGS did not detect the presence of genes with ALK rearrangement, which could be due to the detection methods (On the whole exome chip, there are only exon probes for the ALK gene, but the DNA fusion breakpoint is located in the intron). However, literature reports indicate that RANBP2 is the gene most commonly associated with ALK (8, 50). Besides RANBP2-ALK, rearrangement patterns involving RRBP1-ALK (5, 35), TPM3-ALK (5, 51), and EML4-ALK (21) have also been reported in some cases. RANBP1, RANBP2, and RRBP1 are localized to the nuclear membrane and play diverse roles in cell cycle activities, such as nucleocytoplasmic transport, centrosome assembly, microtubule polymerization, spindle assembly, and nuclear envelope remodeling (52–54). This may explain the significant changes in the morphological structure and biological behavior of tumor cells when ALK is rearranged with these genes. Some researchers suggest that RANBP2-ALK rearrangement may lead to ALK expression in the nuclear membrane, while RANBP1-ALK rearrangement may result in ALK expression in the cytoplasm (54–56). Both arrangements contribute to aggressive biological behavior, but further studies are necessary to explore the genetic mechanisms underlying EIMS.
During ALKi therapy, the patient experienced multiple allergic reactions to Alectinib, as outlined in Table 1. On October 3rd, 2023, the 11th day following the initiation of ALKi therapy, the patient revisited our institution due to fever, generalized rash, and itching, with a peak body temperature of 39.7°C. Following the diagnosis of drug allergy, immediate treatment was administered based on the Expert Consensus of Management of Adverse Drug Reactions with Anaplastic Lymphoma Kinase Tyrosine Kinase Inhibitors (referred to as “The Consensus” below) (11). Despite the severe fever, discontinuation of the drug wasn’t indicated as there were no signs of liver injury, as emphasized in The Consensus. Additionally, acute anemia was detected during routine laboratory tests on the third day of ALKi therapy, although no significant clinical symptoms were observed. Research indicates that subclinical drug-induced hemolytic anemia and the presence of acanthocytes are common in patients undergoing Alectinib treatment (57, 58). In line with The Consensus, the patient was prescribed iron supplements and regular blood tests. Currently, the patient’s hemoglobin level varies from 110g/L to 120g/L in regular blood tests, without any significant clinical symptoms.
Due to the inefficacy of conventional chemotherapy, there is a growing interest in experimental treatments for EIMS, both in clinical practice and laboratory research. Among these, ALKi has demonstrated promising clinical efficacy, as evidenced by our own clinical observations and literature review. Researchers are also exploring other potential targets. For instance, Li et al. identified a diffuse positive signal for PD-L1 in their EIMS case, suggesting a potential target for PD-1/PD-L1 inhibitors (8). However, initial adjuvant chemotherapy with sintilimab showed no response in our case, despite high PD-1/PD-L1 expression observed in NGS testing. In another study, Fordham AM’s experiments using animal models of allotransplantation revealed that combining a CD30 inhibitor with ALKi resulted in significant tumor shrinkage in both diagnostic and relapse xenograft model groups (59). Additionally, this combination therapy significantly prolonged tumor-free survival in the diagnostic group compared to the relapse group. Based on current experience, the optimal treatment approach for EIMS involves surgical intervention combined with ALKi administered in either adjuvant or neoadjuvant therapy. As more EIMS cases are identified, clinicians will have a broader range of targeted drug options. With ongoing research and a deeper understanding of EIMS pathogenesis, an optimal treatment regimen may gradually emerge, offering patients maximum benefits.
4 Conclusion
In conclusion, we presented a detailed case of EIMS characterized by round or epithelioid cell morphology, demonstrating aggressive biological behavior and a poor prognosis. To our knowledge, our report is the first to investigate the efficacy of Alectinib in treating EIMS, while also addressing severe drug-induced complications associated with its administration. Our literature review indicates that ALKi therapy is currently the most effective treatment for EIMS, although other targets may also hold promise. Further studies are warranted to elucidate the pathogenesis of EIMS.
Data availability statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author/s.
Ethics statement
The studies involving humans were approved by the Ethics Committee of Peking University International Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
XW: Writing – original draft. JZ: Data curation, Writing – original draft. YY: Formal Analysis, Writing – original draft. DN: Methodology, Writing – original draft. LC: Resources, Writing – review & editing. NN: Project administration, Writing – review & editing. YZ: Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was supported by Youth fund of Peking University International Hospital Research Grant: YN2020QN08 and Peking University International Hospital Research Grant: YN2023ZD05.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Surabhi VR, Chua S, Patel RP, Takahashi N, Lalwani N, Prasad SR. Inflammatory myofibroblastic tumors: current update. Radiol Clin North Am. (2016) 54:553–63. doi: 10.1016/j.rcl.2015.12.005
2. Gleason BC, Hornick JL. Inflammatory myofibroblastic tumours: where are we now? J Clin Pathol. (2008) 61:428–37. doi: 10.1136/jcp.2007.049387
3. Coffin CM, Hornick JL, Fletcher CD. Inflammatory myofibroblastic tumor: comparison of clinicopathologic, histologic, and immunohistochemical features including ALK expression in atypical and aggressive cases. Am J Surg Pathol. (2007) 31:509–20. doi: 10.1097/01.pas.0000213393.57322.c7
4. Wang QA, Chen HW, Wu RC, Wu CE. Update of diagnosis and targeted therapy for ALK+ Inflammation myofibroblastic tumor. Curr Treat Options Oncol. (2023) 24:1683–702. doi: 10.1007/s11864-023-01144-6
5. Lee JC, Li CF, Huang HY, Zhu MJ, Mariño-Enríquez A, Lee CT, et al. ALK oncoproteins in atypical inflammatory myofibroblastic tumours: novel RRBP1-ALK fusions in epithelioid inflammatory myofibroblastic sarcoma. J Pathol. (2017) 241:316–23. doi: 10.1002/path.4836
6. Mariño-Enríquez A, Wang WL, Roy A, Lopez-Terrada D, Lazar AJ, Fletcher CD, et al. Epithelioid inflammatory myofibroblastic sarcoma: An aggressive intra-abdominal variant of inflammatory myofibroblastic tumor with nuclear membrane or perinuclear ALK. Am J Surg Pathol. (2011) 35:135–44. doi: 10.1097/PAS.0b013e318200cfd5
7. Fu X, Jiang J, Tian XY, Li Z. Pulmonary epithelioid inflammatory myofibroblastic sarcoma with multiple bone metastases: case report and review of literature. Diagn Pathol. (2015) 10:106. doi: 10.1186/s13000-015-0358-1
8. Li M, Xing R, Huang J, Shi C, Wei C, Wang H. Case report: Epithelioid inflammatory myofibroblastic sarcoma treated with an ALK TKI ensartinib. Front Oncol. (2023) 13:1084456. doi: 10.3389/fonc.2023.1084456
9. Gadeyne L, Creytens D, Dekeyser S, van der Meulen J, Haspeslagh M. Primary cutaneous epithelioid inflammatory myofibroblastic sarcoma harboring RANBP2-ALK fusion: report of an exceptional case. Am J Dermatopathol. (2022) 44:302–5. doi: 10.1097/DAD.0000000000002096
10. Collins K, Ramalingam P, Euscher ED, Reques Llanos A, García A, Malpica A. Uterine inflammatory myofibroblastic neoplasms with aggressive behavior, including an epithelioid inflammatory myofibroblastic sarcoma: A clinicopathologic study of 9 cases. Am J Surg Pathol. (2022) 46:105–17. doi: 10.1097/PAS.0000000000001756
11. Zhou F, Yang Y, Zhang L, Cheng Y, Han B, Lu Y, et al. Expert consensus of management of adverse drug reactions with anaplastic lymphoma kinase tyrosine kinase inhibitors. ESMO Open. (2023) 8:101560. doi: 10.1016/j.esmoop.2023.101560
12. Kimbara S, Takeda K, Fukushima H, Inoue T, Okada H, Shibata Y, et al. A case report of epithelioid inflammatory myofibroblastic sarcoma with RANBP2-ALK fusion gene treated with the ALK inhibitor, crizotinib. Jpn J Clin Oncol. (2014) 44:868–71. doi: 10.1093/jjco/hyu069
13. Kurihara-Hosokawa K, Kawasaki I, Tamai A, Yoshida Y, Yakushiji Y, Ueno H, et al. Epithelioid inflammatory myofibroblastic sarcoma responsive to surgery and an ALK inhibitor in a patient with panhypopituitarism. Intern Med. (2014) 53:2211–4. doi: 10.2169/internalmedicine.53.2546
14. Hui W, Yuhong M, Haoyong N, Ping L, Liu H, Xiaoling K, et al. Abdominal epithelioid inflammatory myofibroblastic sarcoma: a clinicopathological study. Chin J Diagn Pathol. (2014) 21:680–4.
15. Liu Q, Kan Y, Zhao Y, He H, Kong L. Epithelioid inflammatory myofibroblastic sarcoma treated with ALK inhibitor: a case report and review of literature. Int J Clin Exp Pathol. (2015) 8:15328–32.
16. Wu H, Meng YH, Lu P, Ning HY, Hong L, Kang XL, et al. Epithelioid inflammatory myofibroblastic sarcoma in abdominal cavity: a case report and review of literature. Int J Clin Exp Pathol. (2015) 8:4213–9.
17. Zhou J, Jiang G, Zhang D, Zhang L, Xu J, Li S, et al. Epithelioid inflammatory myofibroblastic sarcoma with recurrence after extensive resection: significant clinicopathologic characteristics of a rare aggressive soft tissue neoplasm. Int J Clin Exp Pathol. (2015) 8:5803–7.
18. Wanjiao P, Weifeng Z, Jianping L, Chunwei X, Gang C. Abdominal epithelioid inflammatory myofibroblastic sarcoma: a clinicopathological analysis and literature review. J Clin Pathol. (2016) 36:1589–93.
19. Lijuan Q, Huibin Z, Jie G, Xiaoxia G, Xuzhou W. Clinicopathological features of abdominal epithelioid inflammatory myofibroblastic sarcoma. World Chin J Digestion. (2016) 24:2438–44.
20. Yu L, Liu J, Lao IW, Luo Z, Wang J. Epithelioid inflammatory myofibroblastic sarcoma: a clinicopathological, immunohistochemical and molecular cytogenetic analysis of five additional cases and review of the literature. Diagn Pathol. (2016) 11:67. doi: 10.1186/s13000-016-0517-z
21. Jiang Q, Tong HX, Hou YY, Zhang Y, Li JL, Zhou YH, et al. Identification of EML4-ALK as an alternative fusion gene in epithelioid inflammatory myofibroblastic sarcoma. Orphanet J Rare Dis. (2017) 12:97. doi: 10.1186/s13023-017-0647-8
22. Fang H, Langstraat CL, Visscher DW, Folpe AL, Schoolmeester JK. Epithelioid inflammatory myofibroblastic sarcoma of the ovary with RANB2-ALK fusion: report of a case. Int J Gynecol Pathol. (2018) 37:468–72. doi: 10.1097/PGP.0000000000000431
23. Xu X, Li H, Peng K, Yu Y, Chen L, Fang Y, et al. ALK-G1269A mutation in epithelioid inflammatory myofibroblastic sarcoma after progression on crizotinib: A case report. Oncol Lett. (2019) 17:2370–6. doi: 10.3892/ol.2018.9865
24. Zhang S, Wang Z. A case report on epithelioid inflammatory myofibroblastic sarcoma in the abdominal cavity. Int J Clin Exp Pathol. (2019) 12:3934–9.
25. Xu P, Shen P, Jin Y, Wang L, Wu W. Epithelioid inflammatory myofibroblastic sarcoma of stomach: diagnostic pitfalls and clinical characteristics. Int J Clin Exp Pathol. (2019) 12:1738–44.
26. Yongjie H, Sihong Z. Abdominal epithelioid inflammatory myofibroblastic sarcoma: a case report and literature review. Chin J Pract Med Technol. (2020) 27:785–7. doi: 10.19522/j.cnki.1671-5098.2020.06.053
27. Liu D, Luo R, Tang H, Li T. Sigmoid epithelioid inflammatory myofibroblastic sarcoma with high white blood cell count: A case report. Asian J Surg. (2020) 43:838–9. doi: 10.1016/j.asjsur.2020.03.010
28. Giannaki A, Doganis D, Giamarelou P, Konidari A. Epithelioid inflammatory myofibroblastic sarcoma presenting as gastrointestinal bleed: case report and literature review. JPGN Rep. (2020) 2:e019. doi: 10.1097/PG9.0000000000000019
29. Xiaoyu L, Qingxin X, Dongmei Z, Jianbo Z. Clinicopathological study of pelvic and abdominal epithelioid inflammatory myofibroblastic sarcoma. Modern Oncol Med. (2021) 29:2700–4.
30. Li D, Jie Z, Lan R, Yiming H, Rong T, Wan Y. Epithelioid inflammatory myofibroblastic sarcoma: a clinicopathological study of one case. Chin J Diagn Pathol. (2021) 28:24–7.
31. Batool S, Ahuja A, Chauhan DS, Bhardwaj M, Meena AK. Epithelioid inflammatory myofibroblastic sarcoma: the youngest case reported. Autops Case Rep. (2021) 11:e2021288. doi: 10.4322/acr.2021.288
32. Dou W, Guan Y, Liu T, Zheng H, Feng S, Wu Y, et al. Epithelioid inflammatory myofibroblastic sarcoma: a case report and brief literature review. Front Oncol. (2023) 13:1212529. doi: 10.3389/fonc.2023.1212529
33. Aminimoghaddam S, Pourali R. Epithelioid inflammatory myofibroblastic sarcoma with poor response to crizotinib: A case report. Clin Med Insights Case Rep. (2023) 16:11795476231163954. doi: 10.1177/11795476231163954
34. Fadl A, Feldman AL. Epithelioid inflammatory myofibroblastic sarcoma: a pitfall in the differential diagnosis of ALK-positive anaplastic large cell lymphoma. J Hematop. (2023) 16:125–6. doi: 10.1007/s12308-023-00537-8
35. Cheng N, Xue L. A case report of RRBP1-ALK fusion gene-positive epithelioid inflammatory myofibroblastic sarcoma with collagenous stroma and good prognosis. Asian J Surg. (2024) 47:617–9. doi: 10.1016/j.asjsur.2023.09.135
36. Li Z, Dranoff JA, Chan EP, Uemura M, Sévigny J, Wells RG. Transforming growth factor-beta and substrate stiffness regulate portal fibroblast activation in culture. Hepatology. (2007) 46:1246–56. doi: 10.1002/hep.21792
37. Shinde AV, Humeres C, Frangogiannis NG. The role of α-smooth muscle actin in fibroblast-mediated matrix contraction and remodeling. Biochim Biophys Acta Mol Basis Dis. (2017) 1863:298–309. doi: 10.1016/j.bbadis.2016.11.006
38. Kertowidjojo EC, Bennett JA. Update on uterine mesenchymal neoplasms. Surg Pathol Clin. (2022) 15:315–40. doi: 10.1016/j.path.2022.02.008
39. Takeuchi K, Choi YL, Togashi Y, Soda M, Hatano S, Inamura K, et al. KIF5B-ALK, a novel fusion oncokinase identified by an immunohistochemistry-based diagnostic system for ALK-positive lung cancer. Clin Cancer Res. (2009) 15:3143–9. doi: 10.1158/1078-0432.CCR-08-3248
40. Mino-Kenudson M, Chirieac LR, Law K, Hornick JL, Lindeman N, Mark EJ, et al. A novel, highly sensitive antibody allows for the routine detection of ALK-rearranged lung adenocarcinomas by standard immunohistochemistry. Clin Cancer Res. (2010) 16:1561–71. doi: 10.1158/1078-0432.CCR-09-2845
41. Begum S, Gillison ML, Ansari-Lari MA, Shah K, Westra WH. Detection of human papillomavirus in cervical lymph nodes: a highly effective strategy for localizing site of tumor origin. Clin Cancer Res. (2003) 9:6469–75.
42. Olivier M, Hollstein M, Hainaut P. TP53 mutations in human cancers: origins, consequences, and clinical use. Cold Spring Harb Perspect Biol. (2010) 2:a001008. doi: 10.1101/cshperspect.a001008
43. Liu J, Cheng M, Xu J, Liang Y, Yin B, Liang J. Effect of CDK4/6 inhibitors on tumor immune microenvironment. Immunol Invest. (2024) 53:437–49. doi: 10.1080/08820139.2024.2304565
44. Hastie ND. Wilms' tumour 1 (WT1) in development, homeostasis and disease. Development. (2017) 144:2862–72. doi: 10.1242/dev.153163
45. Radu P, Zurzu M, Paic V, Bratucu M, Garofil D, Tigora A, et al. CD34-structure, functions and relationship with cancer stem cells. Medicina (Kaunas). (2023) 59:938. doi: 10.3390/medicina59050938
46. Yan WL, Shen KY, Tien CY, Chen YA, Liu SJ. Recent progress in GM-CSF-based cancer immunotherapy. Immunotherapy. (2017) 9:347–60. doi: 10.2217/imt-2016-0141
47. Kalof AN, Cooper K. D2-40 immunohistochemistry–so far! Adv Anat Pathol. (2009) 16:62–4. doi: 10.1097/PAP.0b013e3181915e94
48. Schwaller B. Calretinin: from a "simple" Ca(2+) buffer to a multifunctional protein implicated in many biological processes. Front Neuroanat. (2014) 8:3. doi: 10.3389/fnana.2014.00003
49. Chen Y, Ouyang Y, Li Z, Wang X, Ma J. S100A8 and S100A9 in cancer. Biochim Biophys Acta Rev Cancer. (2023) 1878:188891. doi: 10.1016/j.bbcan.2023.18889
50. Lee JC, Wu JM, Liau JY, Huang HY, Lo CY, Jan IS, et al. Cytopathologic features of epithelioid inflammatory myofibroblastic sarcoma with correlation of histopathology, immunohistochemistry, and molecular cytogenetic analysis. Cancer Cytopathol. (2015) 123:495–504. doi: 10.1002/cncy.21558
51. Mariño-Enríquez A, Dal Cin P. ALK as a paradigm of oncogenic promiscuity: different mechanisms of activation and different fusion partners drive tumors of different lineages. Cancer Genet. (2013) 206:357–73. doi: 10.1016/j.cancergen.2013.07.001
52. Ogawa-Goto K, Tanaka K, Ueno T, Tanaka K, Kurata T, Sata T, et al. p180 is involved in the interaction between the endoplasmic reticulum and microtubules through a novel microtubule-binding and bundling domain. Mol Biol Cell. (2007) 18:3741–51. doi: 10.1091/mbc.e06-12-1125
53. Diefenbach RJ, Diefenbach E, Douglas MW, Cunningham AL. The ribosome receptor, p180, interacts with kinesin heavy chain, KIF5B. Biochem Biophys Res Commun. (2004) 319:987–92. doi: 10.1016/j.bbrc.2004.05.069
54. Cai Y, Singh BB, Aslanukov A, Zhao H, Ferreira PA. The docking of kinesins, KIF5B and KIF5C, to Ran-binding protein 2 (RanBP2) is mediated via a novel RanBP2 domain. J Biol Chem. (2001) 276:41594–602. doi: 10.1074/jbc.M104514200
55. Chopra S, Maloney N, Wang WL. Epithelioid inflammatory myofibroblastic sarcoma with VCL-ALK fusion of central nervous system: case report and brief review of the literature. Brain Tumor Pathol. (2022) 39:35–42. doi: 10.1007/s10014-021-00416-z
56. Alshammari HK, Alzamami HF, Ashoor M, Almarzouq WF, Kussaibi H. A rare presentation of inflammatory myofibroblastic tumor in the nasolabial fold. Case Rep Otolaryngol. (2019) 2019:3257697. doi: 10.1155/2019/3257697
57. Kuzich JA, Heynemann S, Geoghegan N, Evelyn C, O'Mahoney S, Wilson S, et al. Alectinib induces marked red cell spheroacanthocytosis in a near-ubiquitous fashion and is associated with reduced eosin-5-maleimide binding. Pathology. (2021) 53:608–12. doi: 10.1016/j.pathol.2020.10.023
58. Kunz J, Wiedemann C, Grosch H, Kriegsmann K, Gryzik S, Felden J, et al. Early development of ubiquitous acanthocytosis and extravascular hemolysis in lung cancer patients receiving alectinib. Cancers (Basel). (2022) 14:2720. doi: 10.3390/cancers14112720
Keywords: epithelioid inflammatory myofibroblastic sarcoma, inflammatory myofibroblastic tumor, anaplastic lymphoma kinase, Alectinib, drug allergy
Citation: Wu X, Zhu J, Yan Y, Niu D, Chen L, Ning N and Zhang Y (2024) Epithelioid inflammatory myofibroblastic sarcoma treated with Alectinib: a case report and literature review. Front. Oncol. 14:1412225. doi: 10.3389/fonc.2024.1412225
Received: 04 April 2024; Accepted: 13 August 2024;
Published: 30 August 2024.
Edited by:
Matiullah Khan, AIMST University, MalaysiaReviewed by:
José Manuel Lopes, University of Porto, PortugalWaleed Kian, The Institute of Oncology, Assuta Ashdod, Israel
Hongying Zhang, Sichuan University, China
Copyright © 2024 Wu, Zhu, Yan, Niu, Chen, Ning and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ning Ning, bmluZ25pbmczMDFAMTI2LmNvbQ==; Yankai Zhang, emhhbmd5YW5rYWkwMzlAMTYzLmNvbQ==
†These authors have contributed equally to this work and share first authorship
‡These authors have contributed equally to this work and shared last authorship
 Xinchun Wu
Xinchun Wu Junxi Zhu1†
Junxi Zhu1†