- 1Department of Clinical Oncology, Pamela Youde Nethersole Eastern Hospital, Hong Kong, Hong Kong SAR, China
- 2Department of Clinical Pathology, Pamela Youde Nethersole Eastern Hospital, Hong Kong, Hong Kong SAR, China
NUT carcinoma (NC) is an extremely rare, aggressive malignancy characterized by chromosomal rearrangements in the NUTM1 (nuclear protein in testis) gene. It usually affects younger patients with a median age of diagnosis at 23 years old. The mainstay of treatment consists of combination chemotherapy, surgical resection, and high dose radiation. However, prognosis remains dismal with reported median overall survival of 6.7 months. Literature reporting on use of immunotherapy in head and neck NC is limited. Prolonged remission without aggressive multimodality therapy is rare. We report a case of a 87-year-old woman with metastatic sinonasal NC treated with palliative radiotherapy and pembrolizumab who achieved sustained response 2 years from diagnosis.
Case presentation
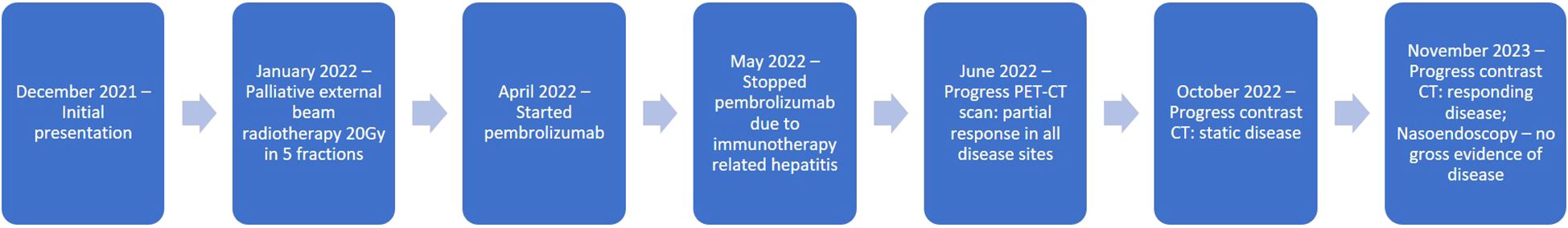
An 87-year-old woman presented to our hospital in December 2021 with 2 months’ history of an enlarging right upper alveolar mass and facial numbness. Apart from history of osteoporosis and lacunar infarct on aspirin, she had no other significant medical comorbidities and was a lifelong nonsmoker.
Clinical examination revealed a 2 cm fleshy tumor over the right upper alveolus and mild bulging over the right maxillary region. There was no clinical evidence of overlying skin infiltration. Cranial nerves were grossly intact except there was decreased sensation over right V2 dermatome. There were no palpable cervical lymph nodes. ECOG performance status was 2.
Whole Body Positron Emission Tomography (18 F FDG) showed a large hypermetabolic mass at the right maxillary sinus measuring 8 x 6.2cm with extension to the right orbit, parapharyngeal space, pterygoid muscles and upper molar regions (Figure 1A) and multiple hypermetabolic osseous metastases (Figures 1B, C).
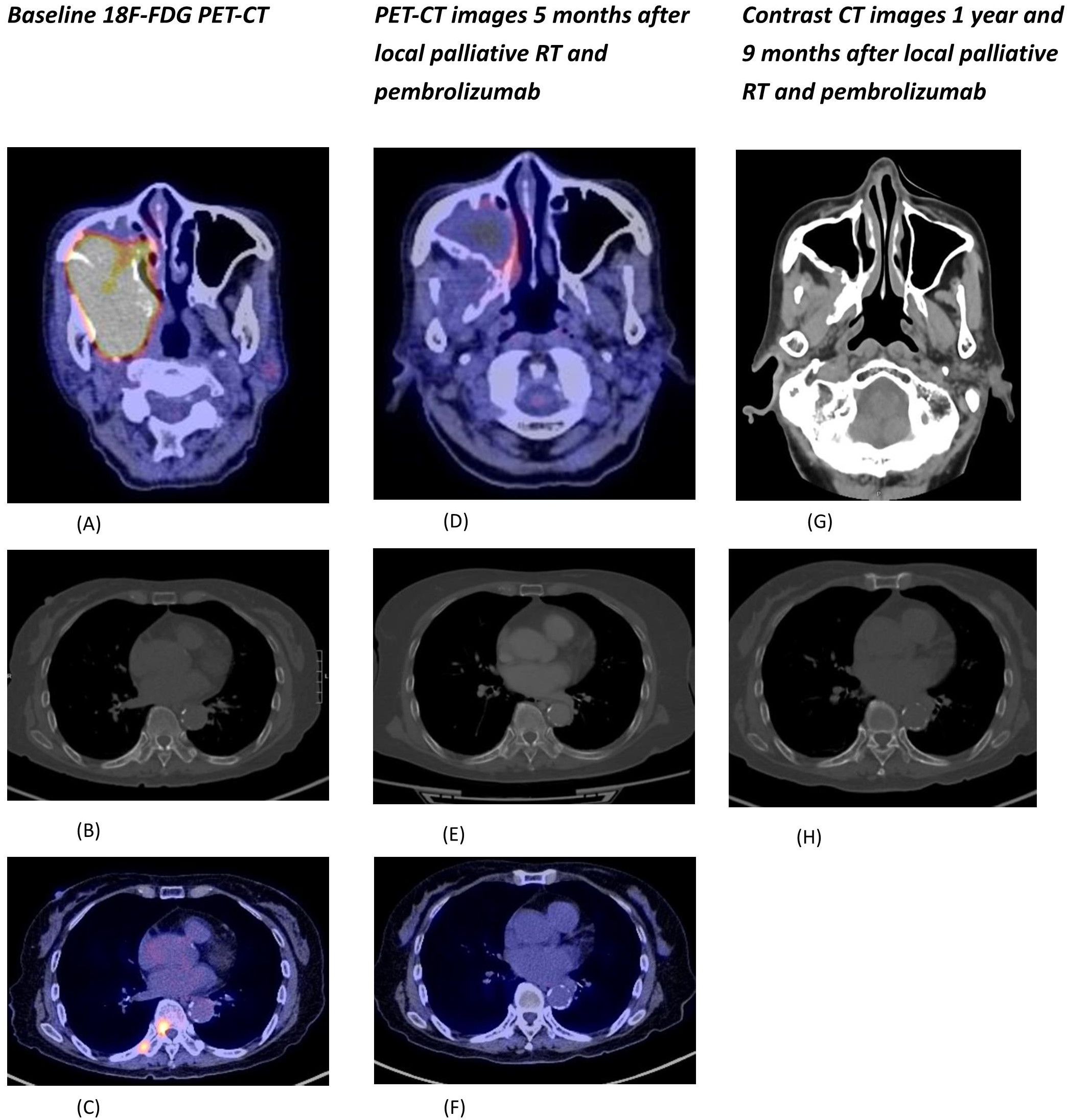
Figure 1. (A) Fusion PET-CT of the hypermetabolic primary tumor involving the right maxillary sinus and right parapharyngeal region. (B) CT (bone window) showing sclerotic bone metastases at right T9 and right 9th rib. (C) Fusion PET-CT of hypermetabolic bone metastases at right T9 and right 9th rib. (D) Fusion PET-CT of the primary tumor showing significant shrinkage and metabolic response. (E) CT (bone window) of lesions at right T9 and right 9th rib with radiological improvement. (F) Fusion PET-CT of lesions at right T9 and right 9th rib with metabolic response. (G) Contrast CT (soft tissue window) showing sustained tumor response with only residual mucosal thickening at the posterior wall of right maxillary sinus. (H) CT (bone window) of lesions at right T9 and right 9th rib with further radiological improvement.
Incisional biopsy of the mass revealed malignant cells exhibiting high N/C ratio, mitotic activity, necrosis with areas with abrupt keratinization. Immunohistochemical staining of the malignant cells were diffusely positive for p40 and NUT immunostains (Figure 2). The final pathological diagnosis was NC. PDL-1 CPS score was 3 and TPS score was 2.
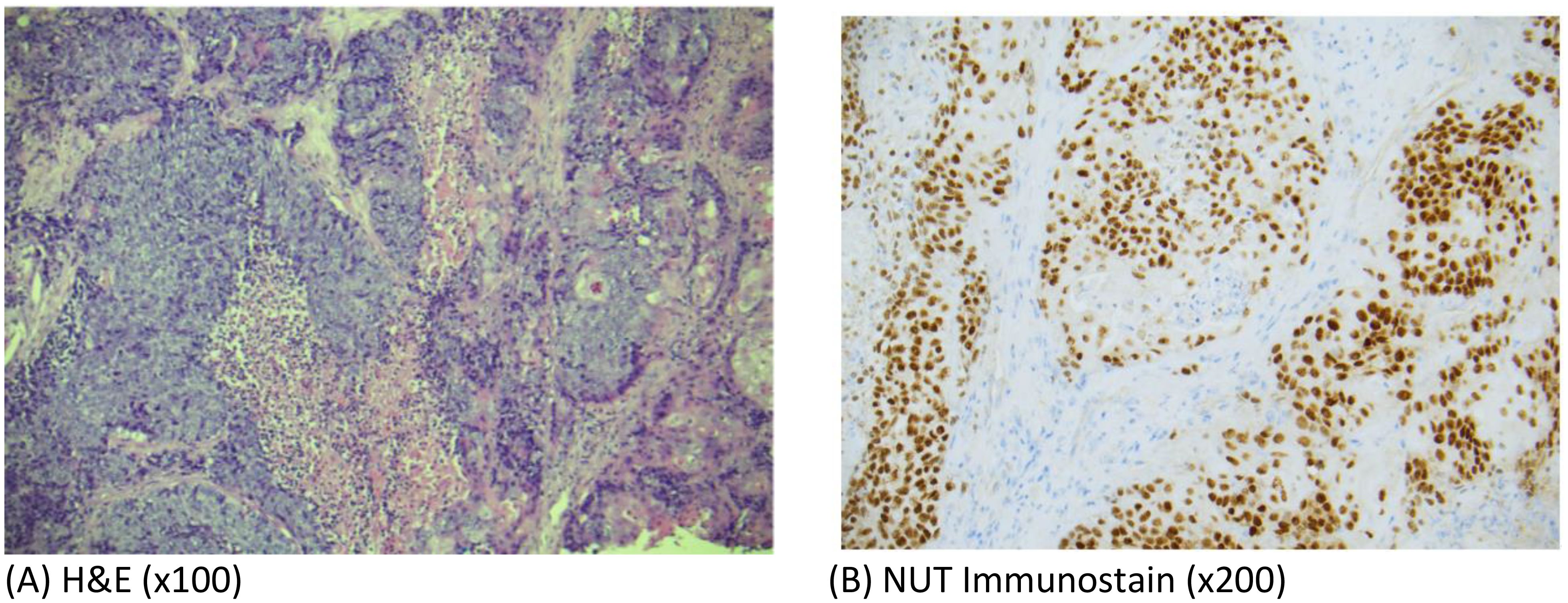
Figure 2. (A) Photomicrographs of hematoxylin-and-eosin (H&E)-stained sections (x100) from the tumor biopsy specimen showing sheets of malignant cells with high N/C ratio, mitotic activity and areas of abrupt keratinization consistent with poorly differentiated carcinoma. (B) Photomicrographs showing diffuse positivity of tumor cells with NUT immunostain (x200).
She was given palliative external beam radiotherapy 20Gy over 5 fractions to the maxilla tumor which was well tolerated with good clinical response, and followed by immunotherapy pembrolizumab (200mg IV Q3week). After one cycle of pembrolizumab, she developed derangement of liver function (ALT 335 ALP 336 total bilirubin 14). Baseline liver functions were all normal. Ultrasound of the hepatobiliary system showed no liver lesions. Liver function spontaneously normalized after 6 weeks of close observation without any additional medical treatment. Due to the occurrence of grade 2 immunotherapy related hepatitis, the patient opted not to resume further immunotherapy PET-CT scan 2 months after pembrolizumab administration showed significant shrinkage of the maxilla mass and both the primary and metastatic lesions became isometabolic (Figures 1D–F).
Contrast CT in November 2023 showed responding disease with non-enhancing soft tissue thickening at the right maxillary sinus wall and continuous radiological improvement in the bone metastases (Figures 1G, H). Nasal endoscopy in November 2023 showed no definite exophytic lesion in the maxillary sinuses. In her last clinic visit in September 2024 (33 months from initial diagnosis), she reported no active symptoms from her malignancy. A summary of our patient's treatment course is provided in Figure 3.
Discussion
First described in the 1990s, NUT carcinomas are poorly differentiated carcinomas defined by the presence of chromosomal rearrangements in the NUTM1 gene on chromosome 15q14. The most common rearrangement is the t(15:19) translocation with the BRD4 gene, accounting for around 67% of cases; less common alterations include BRD3::NUTM1 or NSD3::NUTM1 fusions. BRD::NUTM1 fusions are critical to the pathogenesis of NC; their presence alone has been shown to be sufficient to be sufficient to drive malignant transformation (1). The fusion proteins are hypothesized to cause carcinogenesis by blocking cellular differentiation via interference with several key genetic targets, including MYC, SOX2, MED24, and TP63 genes (2).
NC may arise from diverse organs, and most frequently originate from midline structures such as the sinonasal tract, lung, and thymus. Treatment pathways for NC usually follow that of the primary site. In head and neck NC, this consists of aggressive surgery and radiation for resectable cases. For unresectable or metastatic disease, the mainstay of treatment is combination platinum or ifosphamide based chemotherapy followed by surgical debulking and/or consolidation radiation.
Despite use of aggressive multimodality treatment regimens, the prognosis of head and neck NC remain poor. In two largest retrospective series conducted on head and neck NC (3, 4), median overall survival ranged from 9.7 to 14.6 months, and no patients with metastatic disease were reported surviving beyond two years.
Our patient, aged 87 at presentation, represents the oldest reported case of NC in the literature to date (5). Unlike younger NC patients, she was not a candidate for aggressive treatment, thus immunotherapy with immune checkpoint inhibitors (ICI) was considered.
Pembrolizumab has been approved for use in advanced HNSCC with high PDL 1 expression and confers long term overall survival benefit (6). In primary pulmonary NC, numerous case reports have shown encouraging responses to PD-1 inhibitors in pretreated disease (7, 8). Reported progression free survival ranged from 5 to 29 months and overall survival 19.5 to 79 months.
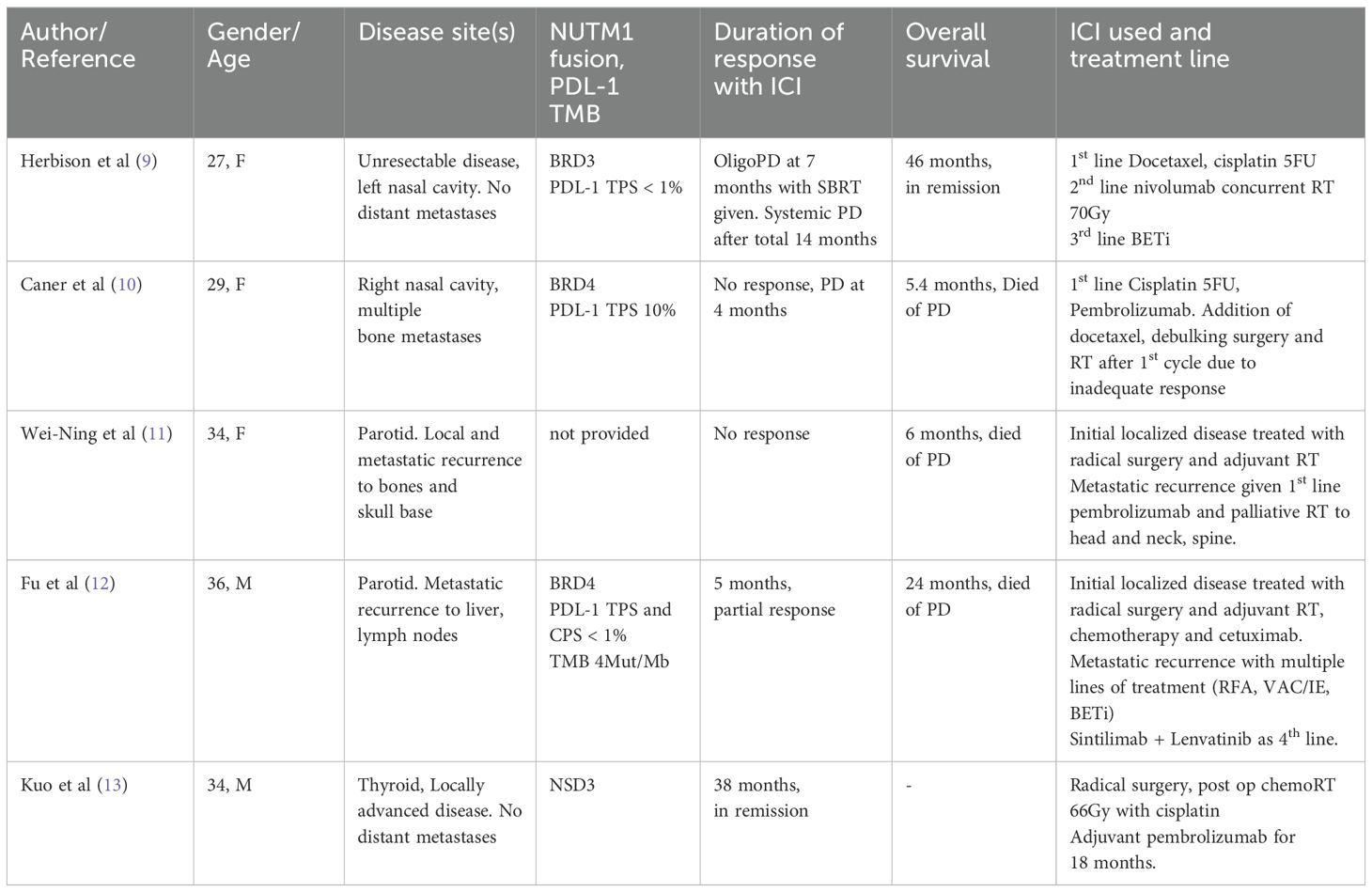
On the other hand, literature reporting on ICI use in head and neck NC is limited. There are 4 reported cases of ICI use in head and neck NC to date (see Table 1). The best responder was a 34-year-old male presenting with locally advanced NC of thyroid primary, with no evidence of disease 38 months from diagnosis. However, he underwent radical surgery and pembrolizumab given as adjuvant treatment. For cases with unresectable/metastatic disease, the best response occurred using nivolumab and radiotherapy to 70Gy, where 14 months disease control on ICI was achieved before further systemic progression. To our knowledge, our patient, in remission more than 2 years from diagnosis, has the longest disease control to date with ICI in metastatic head and neck NC.
Prior studies suggest NCs are immunologically cold tumors with low PDL-1 expression and tumor mutational burden (14). Our patient had PDL-1 TPS and CPS scores of 2 and 3 respectively, which predicts poor response to ICI monotherapy. Yet, prolonged disease control was achieved with pembrolizumab. Since ICI administration was preceded by palliative radiotherapy, radiation may have enhanced the immunotherapy response. This is supported by preclinical studies demonstrating radiation exerts a synergistic effect with immunotherapy via facilitating tumor associated antigen release, upregulating PDL-1 expression on tumor cells and increasing T cell infiltration (15, 16).
In non-small cell lung carcinoma (NSCLC), a phase II trial of pembrolizumab and stereotactic body radiotherapy doubled overall response rate compared to ICI alone, mainly in PDL-1 negative tumors; suggesting radiotherapy may activate non inflamed tumors to an inflamed microenvironment responsive to ICI (17). In pulmonary NC, a review of 12 cases treated with ICI revealed better outcomes in patients who also received radiation (18). The promising outcomes achieved with radiation and immunotherapy suggests this combination warrants further study in clinical trials for NC. Data on expression of immune markers (such as tumor infiltrating lymphocytes and circulating immune cells) before and after radiotherapy would be particularly valuable to delineate the role of radiation and immunotherapy for this disease.
In the 4 cases of head and neck NC identified in the literature, (Table 1) all received radiotherapy, yet outcomes were heterogenous. Thus, factors apart from radiation may have contributed to our patient’s exceptional response.
Despite our patient’s advanced age, she was in relatively fit medical condition. Her basic activities of daily living her largely independent and she had very few significant medical comorbidities. This may have been a contributing factor to the immune response achieved, Prior studies on ICI use in head and neck cancer patients have also shown better survival and tumor response rates in patients without comorbidities (19). Ethnicity may have also played a role, as Asian origin has recently been suggested in meta analyses to confer better survival outcomes with ICI (20).
Our patient had immunotherapy stopped after one cycle due to suspected immune related hepatitis; this may have predicted for a stronger antitumor effect from pembrolizumab. The occurrence of immune related adverse events is increasingly recognized as a clinical biomarker for ICI response (21). In NSCLC and melanoma occurrence of immune related adverse events is associated with favorable treatment response despite early cessation of ICI (22, 23).
The exact underlying mechanisms are unclear. Studies have suggested tumor and organs with subclinical inflammation share common antigens, resulting in a common T cell response at both sites (24). Other studies have linked gut microbiome composition to higher rates of tumor response and IO colitis (25).
Another factor is the type of NUTM1 gene fusion, since studies have suggested atypical variant fusion partners (e.g BRD3/NSD3) carry better prognoses compared the commonest BRD4 translocation (26). Unfortunately, inadequate quality of the biopsy specimen prevented identification of gene fusion in our case.
Apart from immunotherapy, recent phase I/II clinical trials have tested use of BET inhibitors (BETi), which inhibits binding of BRD to chromatin, thereby disrupting activity of the NUTM1 fusion protein. So far only modest results have been achieved with BETi monotherapy; one of the larger phase I trials comprising of 19 patients reported PR in 4 patients and median PFS of 2.5 months (27). Given the favorable responses that have been achieved with immunotherapy and radiation in our case, using ICI and/or radiation therapy combined with BETi may represent a worthwhile strategy for further research. Preclinical studies have demonstrated BET inhibitors may combine synergistically with PD-1 inhibitors by downregulating T cell PD 1 expression and remodeling immunosuppressive tumor microenvironments (28, 29). BETi has also been shown in prostate cancer xenograft models to enhance the efficacy of radiotherapy and overcome radioresistance (30). In the two case reports where BET inhibitors were used before and after anti PD -1 therapy respectively for head and neck NC, partial tumor responses were achieved (refer to Table 1). Further clinical trials are awaited to explore the efficacy of BETi, radiotherapy and ICI combinations.
Conclusion
Although metastatic head and neck NC is characterized by poor prognosis, our case illustrates use of immunotherapy and radiation can produce a durable response. ICIs may still be considered in patients who are otherwise unfit for traditional aggressive multimodality treatments. More studies are warranted to explore the efficacy of immunotherapy and its potential interactions with other modalities such as radiation, chemotherapy and BET inhibition.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
JN: Conceptualization, Writing – original draft, Writing – review & editing. EW: Supervision, Writing – review & editing. TS: Supervision, Writing – review & editing. RW: Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Taylor Durall R, Huang J, Wojenski L, Huang Y, Gokhale PC, Leeper BA, et al. The BRD4-NUT fusion alone drives Malignant transformation of NUT carcinoma. Cancer Res. (2023) 83:3846–60. doi: 10.1158/0008-5472.CAN-23-2545
2. Moreno V, Saluja K, Pina-Oviedo S. NUT carcinoma: Clinicopathologic features, molecular genetics and epigenetics. Front Oncol. (2022) 12:860830. doi: 10.3389/fonc.2022.860830
3. Ramesh U, Contrera KJ, Shakibai N, Su SY, Brahimaj B, Roberts D, et al. Sinonasal NUT carcinoma: A consecutive case series and systematic review. Head Neck. (2024) 46:29–36. doi: 10.1002/hed.27553
4. Chau NG, Hurwitz S, Mitchell CM, Aserlind A, Grunfeld N, Kaplan L, et al. Intensive treatment and survival outcomes in NUT midline carcinoma of the head and neck. Cancer. (2016) 122:3632–40. doi: 10.1002/cncr.30242
5. Zhang K, Laxague F, MacMillan C, MacNeil SD, Fung K, Yoo J, et al. A sinonasal NUT midline carcinoma in an 84-year-old man undergoing radiation and proton therapy. Clin Case Rep. (2023) 11:e7262. doi: 10.1002/ccr3.7262
6. Harrington KJ, Burtness B, Greil R, Soulières D, Tahara M, de Castro G, et al. Pembrolizumab with or without chemotherapy in recurrent or metastatic head and neck squamous cell carcinoma: updated results of the phase III KEYNOTE-048 study. J Clin Oncol. (2023) 41:790–802. doi: 10.1200/JCO.21.02508
7. Xie X, Wang L, Qin Y, Lin X, Xie Z, Li M, et al. Clinical features, treatment, and survival outcome of primary pulmonary NUT midline carcinoma. Orphanet J Rare Dis. (2020) 15. doi: 10.1186/s13023-020-01449-x
8. Davis A, Mahar A, Wong K, Barnet M, Kao S. Prolonged disease control on nivolumab for primary pulmonary NUT carcinoma. Clin Lung Cancer. (2021) 22:e665–7. doi: 10.1016/j.cllc.2020.10.016
9. Herbison H, Davis S, Nickless D, Haydon A, Ameratunga M. Sustained clinical response to immunotherapy followed by BET inhibitor in a patient with unresectable sinonasal NUT carcinoma. J Immunother Precis Oncol. (2024) 7:67–72. doi: 10.36401/JIPO-23-19
10. Caner B, Orhan SO, Deligonul A, Evrensel T. Immunotherapy experience in sinonasal NUT midline carcinoma, case report. J Cancer Res Ther. (2024) 20:479–81. doi: 10.4103/jcrt.jcrt_1083_22
11. Saik W-N, Da Forno P, Thway K, Khurram SA. NUT carcinoma arising from the parotid gland: A case report and review of the literature. Head Neck Pathol. (2021) 15:1064–8. doi: 10.1007/s12105-020-01254-9
12. Fu S, Wang Z, Li C, Li Y, Zhang K, Zhong Z, et al. The whole treatment process and thinking of a patient with NUT carcinoma of the parotid gland: a case report. Front Oncol. (2023) 13:1094770. doi: 10.3389/fonc.2023.1094770
13. Kuo LE, Barletta J, Schoenfeld JD, White A, French CA, Wong KS, et al. NUT carcinoma of the thyroid: An unusual case with a complete response to treatment. Clin Thyroidol. (2021) 33:38–47. doi: 10.1089/ct.2021;33.38-47
14. He M, Chernock R, Zhou S, Gondim M, Dehner LP, Pfeifer JD. Tumor mutation burden and checkpoint immunotherapy markers in NUT Midline carcinoma. Appl Immunohistochem Mol Morphol. (2020) 28:495–500. doi: 10.1097/PAI.0000000000000781
15. Lin W, Xu Y, Chen X, Liu J, Weng Y, Zhuang Q, et al. Radiation-induced small extracellular vesicles as “carriages” promote tumor antigen release and trigger antitumor immunity. Theranostics. (2020) 10:4871–84. doi: 10.7150/thno.43539
16. Rompré-Brodeur A, Shinde-Jadhav S, Ayoub M, Piccirillo CA, Seuntjens J, Brimo F, et al. PD-1/PD-L1 immune checkpoint inhibition with radiation in bladder cancer: In situ and abscopal effects. Mol Cancer Ther. (2020) 19:211–20. doi: 10.1158/1535-7163.MCT-18-0986
17. Theelen WSME, Peulen HMU, Lalezari F, van der Noort V, de Vries JF, Aerts JGJV, et al. Effect of pembrolizumab after stereotactic body radiotherapy vs pembrolizumab alone on tumor response in patients with advanced non-small cell lung cancer: Results of the PEMBRO-RT phase 2 randomized clinical trial: Results of the PEMBRO-RT phase 2 randomized clinical trial. JAMA Oncol. (2019) 5:1276–82. doi: 10.1001/jamaoncol.2019.1478
18. Li X, Shi H, Zhang W, Bai C, He M, Ta N, et al. Immunotherapy and targeting the tumor microenvironment: Current place and new insights in primary pulmonary NUT carcinoma. Front Oncol. (2021) 11:690115. doi: 10.3389/fonc.2021.690115
19. Guller M, Cooper DJ, Alkhatib H, Suru A, Blancaflor A, Maroun CA, et al. Impact of comorbidities on outcomes in patients with advanced head and neck cancer undergoing immunotherapy. Head Neck. (2023) 45:2789–97. doi: 10.1002/hed.27502
20. Kim CM, Lee JB, Shin SJ, Ahn JB, Lee M, Kim HS. The efficacy of immune checkpoint inhibitors in elderly patients: a meta-analysis and meta-regression. ESMO Open. (2022) 7:100577. doi: 10.1016/j.esmoop.2022.100577
21. Das S, Johnson DB. Immune-related adverse events and anti-tumor efficacy of immune checkpoint inhibitors. J Immunother Cancer. (2019) 7:306. doi: 10.1186/s40425-019-0805-8
22. Lievense LA, Heukels P, van Walree NC, van der Leest CH. Clinical outcomes of patients with metastatic NSCLC after discontinuation of immunotherapy because of immune-related adverse effects. JTO Clin Res Rep. (2023) 4:100441. doi: 10.1016/j.jtocrr.2022.100441
23. Ksienski D, Truong PT, Wai ES, Croteau NS, Chan A, Patterson T, et al. Survival outcomes following discontinuation of ipilimumab and nivolumab for advanced melanoma in a population-based cohort. Clin Oncol. (2021) 33:e561–9. doi: 10.1016/j.clon.2021.06.009
24. Johnson DB, Balko JM, Compton ML, Chalkias S, Gorham J, Xu Y, et al. Fulminant myocarditis with combination immune checkpoint blockade. N Engl J Med. (2016) 375:1749–55. doi: 10.1056/nejmoa1609214
25. Chaput N, Lepage P, Coutzac C, Soularue E, Asvatourian V, Lanoy E, et al. Baseline gut microbiota in metastatic melanoma patients treated with ipilimumab: Relation with clinical response and colitis. Ann Oncol. (2017) 28:v28–9. doi: 10.1093/annonc/mdx363.021
26. Chau NG, Ma C, Danga K, Al-Sayegh H, Nardi V, Barrette R, et al. An anatomical site and genetic-based prognostic model for patients with nuclear protein in testis (NUT) Midline carcinoma: Analysis of 124 patients. JNCI Cancer Spectr. (2020) 4:kz094. doi: 10.1093/jncics/pkz094
27. Piha-Paul SA, Hann CL, French CA, Cousin S, Braña I, Cassier PA, et al. Phase 1 study of molibresib (GSK525762), a bromodomain and extra-terminal domain protein inhibitor, in NUT carcinoma and other solid tumors. JNCI Cancer Spectr. (2020) 4:kz093. doi: 10.1093/jncics/pkz093
28. Zhong M, Gao R, Zhao R, Huang Y, Chen C, Li K, et al. BET bromodomain inhibition rescues PD-1-mediated T-cell exhaustion in acute myeloid leukemia. Cell Death Dis. (2022) 13:743. doi: 10.1038/s41419-022-05204-x
29. Wang H, Liu G, Jin X, Song S, Chen S, Zhou P, et al. BET inhibitor JQ1 enhances anti-tumor immunity and synergizes with PD-1 blockade in CRC. J Cancer. (2022) 13:2126–37. doi: 10.7150/jca.69375
Keywords: sinonasal malignances, NUT carcinoma (NC), radiotherapy, immunotherapy, NUTM1 gene rearrangement
Citation: Ng JKW, Wong ECY, So TCY and Wong RTS (2025) Case report: Long term remission of metastatic sinonasal NUT carcinoma after palliative radiotherapy and immunotherapy in an elderly patient. Front. Oncol. 14:1412070. doi: 10.3389/fonc.2024.1412070
Received: 04 April 2024; Accepted: 13 December 2024;
Published: 07 January 2025.
Edited by:
Jan Baptist Vermorken, University of Antwerp, BelgiumReviewed by:
Tianshun Zhang, University of Minnesota Twin Cities, United StatesChristopher McEvoy, Peter MacCallum Cancer Centre, Australia
Copyright © 2025 Ng, Wong, So and Wong. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Justin K. W. Ng, anVzdGlua3duZzY1QGdtYWlsLmNvbQ==
†ORCID: Tommy C. Y. So, orcid.org/0000-0003-2598-2525
 Justin K. W. Ng
Justin K. W. Ng Edwin C. Y. Wong1
Edwin C. Y. Wong1