- 1Department of Urology, Institute of Urology, West China Hospital, Sichuan University, Chengdu, China
- 2Department of Urology, West China Xiamen Hospital, Sichuan University, Xiamen, China
Background: Synchronous occurrence of prostate cancer (PCa) and renal cell carcinoma (RCC) is uncommon. RCC has a higher tendency to metastasize to the adrenal glands, renal hilar, and retroperitoneal lymph nodes compared to PCa. To date, there are no documented cases existing where metastatic tumors in these regions, observed in patients concurrently with PCa and RCC, have originated from the PCa rather than the RCC.
Case presentation: In this case report, we described a 67-year-old male presented with dysuria for two months and left lower extremity edema for three days. Percutaneous biopsies revealed synchronous primary RCC and PCa. However, the origin of the metastatic tumors, especially those involving the adrenal glands, renal hilum, and retroperitoneal regions, remained undetermined. Subsequent surgical procedures identified that the metastatic lesions originated from the PCa, while the RCC was localized. Ultimately, the patient with metastatic hormone-sensitive prostate cancer (mHSPC) received combination therapy with rezvilutamide and goserelin, which resulted in a satisfactory treatment response.
Conclusion: In patients with concurrent PCa and RCC, metastatic lesions in the adrenal glands, renal hilar, and retroperitoneal lymph nodes may also originate from the PCa. Accurate identification of the primary tumor and proper staging are critical for the appropriate management of patients with multiple primary malignancies with concurrent metastases.
1 Introduction
Prostate cancer (PCa) and renal cell carcinoma (RCC) are common tumors of the urinary system, with respective incidence rates of 7.3% and 2.2% in overall tumor landscape (1). However, the synchronous occurrence of RCC and PCa is rare (2). Most of the previous studies only reported the use of combined radical prostatectomy and partial nephrectomy as a treatment for synchronous, localized PCa and RCC (3). However, the metastatic patterns and treatment approaches for patients with coexisting PCa and RCC with metastases remain elusive owing to a lack of research. In patients with PCa, the predominant metastatic sites include the bone, lymph node, and liver (4), while RCC predominantly involves the lung, lymph node, and bone (5). In PCa, the metastatic potential to the kidney or adrenal gland is approximately 1.0%, with retroperitoneal lymph node involvement occurring in about 1.8% of cases (4). In RCC, the incidence of adrenal gland metastasis ranges from 6% to 10% (5), and the incidence of lymph node metastasis is 21.8%, with 6.8% occurring in the retroperitoneum (6). Notably, RCC exhibits a markedly higher frequency of retroperitoneal lymph node and adrenal gland metastases compared to PCa. Here, we present a case of an elderly male patient presenting with concurrent RCC and PCa. Notably, all metastatic lesions, including those in the adrenal gland and the renal hilar region, were determined to have originated from the PCa. To our knowledge, this unique coexistence has not been documented in prior reports.
2 Case presentation
2.1 Medical history and examinations
The patient was a 67-year-old male who presented with a two-month history of dysuria and a three-day history of edema in the left lower extremity. Upon admission, computed tomography (CT) scan (Figure 1A) revealed a 4.0 × 3.8 cm mass in the left kidney and a 4.8 × 4.0 cm mass in the left adrenal gland with irregular enhancement. Concurrently, there was evidence of increased and enlarged lymph nodes throughout multiple sites in the body, as well as irregular enhancement of the prostate and bilateral seminal vesicles. Subsequently, the 18F-FDG PET/CT scan (Figure 1B) identified areas of intense uptake in the seminal vesicles, prostate, left adrenal gland, skeletal regions, and lymph nodes of the cervical, axillary, mediastinal, and abdominal areas, which were indicative of malignancies. However, the precise location of the primary tumor could not be definitively identified. Additionally, the uptake level of the left renal mass was relatively lower than those of the aforementioned lesions but higher than that of normal tissues, suggesting that it might be a primary RCC. The digital rectal examination (DRE) revealed a firm, enlarged prostate with an indurated nodule. The patient’s prostate-specific antigen (PSA) level was 531 ng/ml, with no detected abnormalities in adrenal hormones. Physicians initially considered that the patient might have metastatic PCa (staged as T3bN1M1b), concurrently with RCC (staged as T4N1Mx) that had metastasized to the adrenal gland and retroperitoneal lymph nodes.
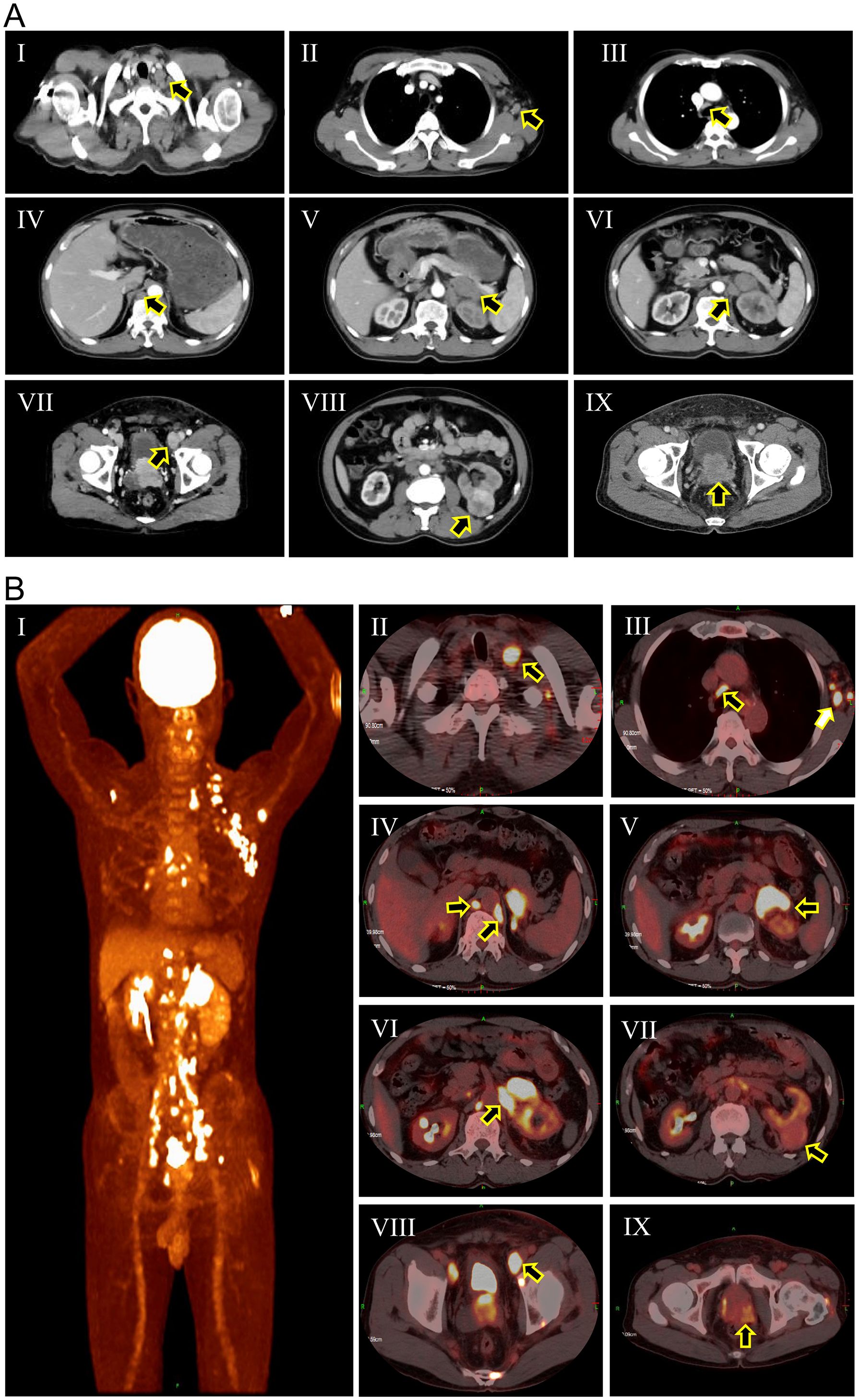
Figure 1. Computed tomography scan (A) and 18F-FDG PET/CT scan (B) of the patient at initial diagnosis. (A-I) Cervical lymph nodes (black arrow); (A-II) Axillary lymph nodes (black arrow); (A-III) Mediastinal lymph nodes (black arrow); (A-IV) Retroperitoneal lymph nodes (black arrow); (A-V) Adrenal metastasis (black arrow); (A-VI) Renal hilar lymph nodes (black arrow); (A-VII) External iliac lymph nodes (black arrow); (A-VIII) Renal mass (black arrow); (A-IX) Enlarged prostate; (B-I) Overview of pathological uptake; (B-II) Cervical lymph nodes (black arrow); (B-III) Axillary lymph nodes (white arrow) and mediastinal lymph nodes (black arrow); (B-IV) Retroperitoneal lymph nodes (black arrows). (B-V) Adrenal metastasis (black arrow); (B-VI) Renal hilar lymph nodes (black arrow); (B-VII) Renal mass (black arrow); (B-VIII) External iliac lymph nodes (black arrow); (B-IX) Prostatic mass (black arrow).
2.2 Investigations
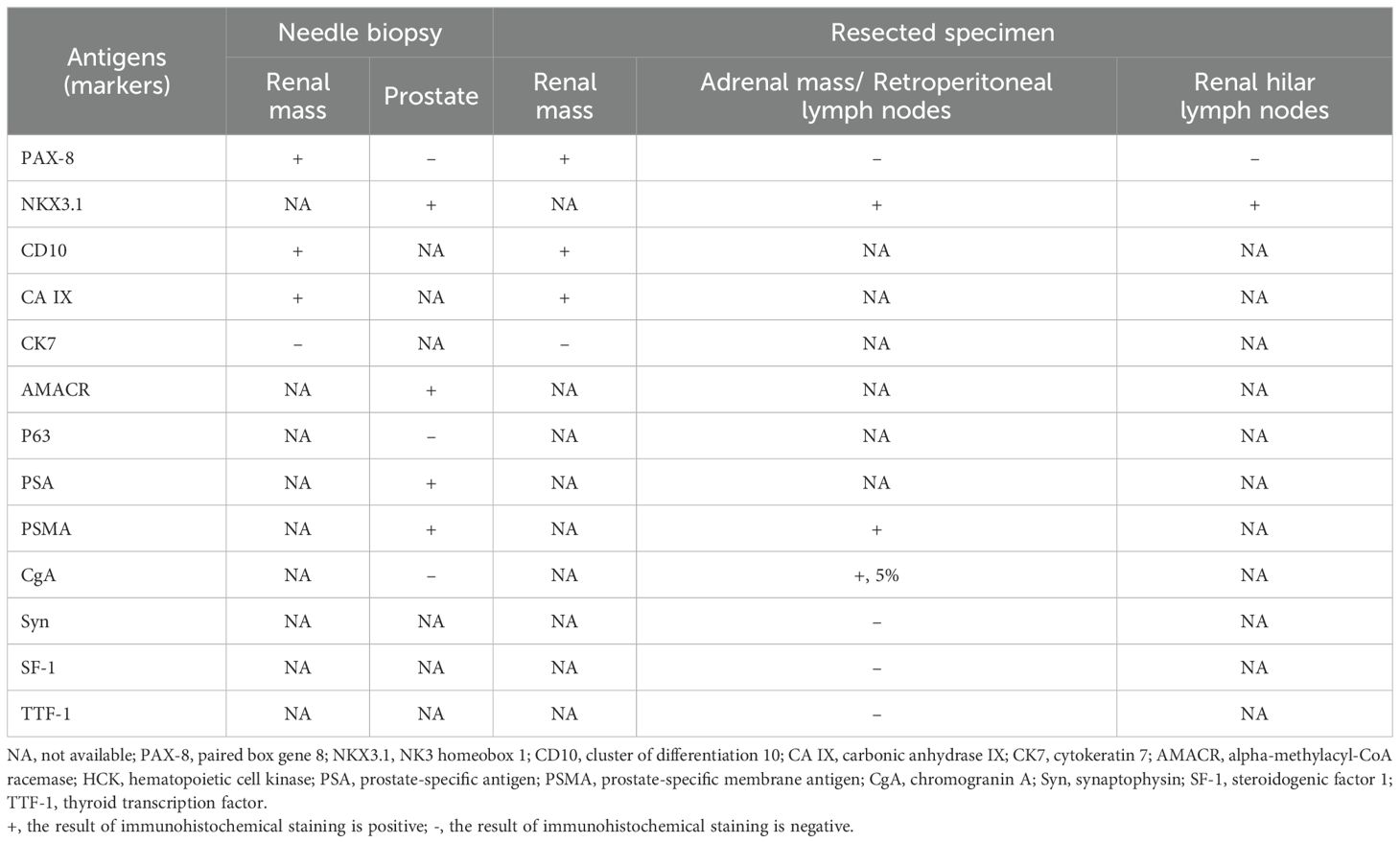
To further clarify the original tumors of this patient, we performed an ultrasound-guided percutaneous biopsy of the left renal mass and an ultrasound-guided transperineal biopsy of the prostate. The immunohistochemical results are summarized in Table 1, the pathologic diagnoses were primary clear cell renal cell carcinoma (ccRCC) and primary adenocarcinoma of prostate with a Gleason score of 4 + 4 = 8. Subsequently, the patient underwent a left nephrectomy, left adrenalectomy, and lymph node dissection of the left renal hilum and retroperitoneum region (Supplementary Figure 1). The immunohistochemical results of the resected specimens are summarized in Table 1. The renal mass was further confirmed as ccRCC with an International Society of Urological Pathology (ISUP) grade of 3, while the lesions of the adrenal glands, renal hilar lymph nodes and retroperitoneal lymph nodes were originated from PCa. The RCC was staged as T1N0M0, while the stage of PCa was determined to be T3bN1M1c.
2.3 Treatment and outcome
Given the diagnosis of high-risk metastatic hormone-sensitive prostate cancer (mHSPC), the patient was treated with rezvilutamide and goserelin acetate sustained-release depot combination therapy. The CT scans performed at one and three months after treatment showed that all metastatic lesions were shrinking, with no signs of recurrence of RCC (Figure 2A; Supplementary Figure 2). Follow-up PSA levels, measured at one, three, and six months after treatment, were 144 ng/ml, 0.78 ng/ml, and 0.09 ng/ml, respectively (Figure 2B). Additionally, radionuclide bone scintigraphy did not detect any new lesions. The therapeutic outcomes substantiated that all metastatic lesions demonstrated a significant response to hormonal therapy.
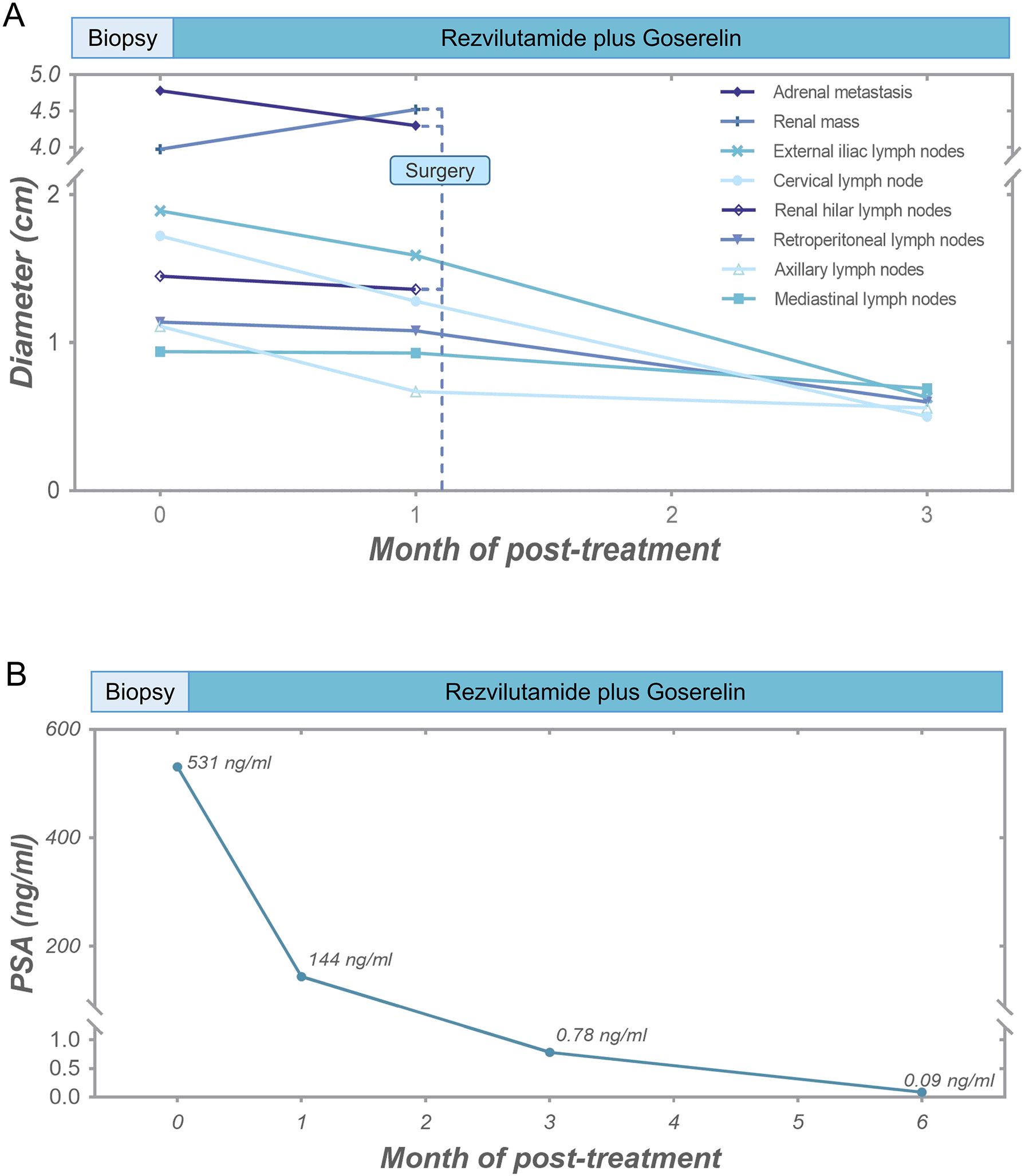
Figure 2. Tumor response of the patient. (A) Diameter alterations of tumors following anti-prostate cancer therapy and surgical procedure. (B) Post-treatment reductions in serum PSA levels.
3 Discussion
In this case report, we described for the first time a patient with concurrent PCa and RCC in whom the adrenal, renal hilar, and retroperitoneal lymph nodes, as well as other metastatic sites, were derived from PCa, not RCC. The patient was ultimately diagnosed with localized RCC and high-risk mHSPC with metastases to the left adrenal gland, bones, and multiple distant lymph nodes.
The synchronous occurrence of RCC and PCa is uncommon. Data from previous studies showed that the incidence of patients diagnosed with both PCa and RCC ranged from 0.83% to 2.10%, while the rate of synchronous occurrence was even lower (7–10). Meanwhile, metastasis from prostate cancer to the kidney has also been observed in case reports (11, 12), which is extremely rare but actually exists. Furthermore, two cases of retroperitoneal lymph node metastases containing both RCC and PCa, a phenomenon known as ‘collision metastasis’, have been reported in previous studies (13, 14). Therefore, the co-existence of RCC and PCa presents a complex diagnostic and therapeutic challenge. Lymph nodes are a common site of metastasis for both PCa and RCC, but the involvement of retroperitoneal lymph nodes is an uncommon presentation in PCa (15). Metastases to the perirenal tissues are exceedingly rare (16, 17). However, in patients with concurrent RCC and PCa, the retroperitoneal lymph node metastases may not always originate from the RCC. Furthermore, although the incidence of adrenal metastasis in metastatic RCC (8.9%, 991/11157) (6) is higher than that in metastatic PCa (1.9%, 12/620) (18), both possibilities remain. Therefore, for patients presenting with synchronous RCC and PCa, biopsies are essential for determining the origin of metastatic lesions.
Previously, 16 cases of adrenal metastasis from PCa have been reported (19–32), which were summarized in Table 2. All patients had high Gleason scores. Adrenal metastases from prostate cancer occurred predominantly in patients with mCRPC, and only 2 patients reported adrenal metastases in patients with mHSPC (24, 26). In the reported cases, common specific symptoms were absent, though abdominal pain could be a possible exception in cases of large tumors. More than half of the patients (10/16) underwent adrenalectomy, with the majority experienced disease remission after surgery, especially those with isolated adrenal metastases. Among these cases, no patients had concomitant renal mass or renal hilar lymph node metastasis.
DRE and PSA screening are the most common prostate cancer screening methods. Despite PSA screening can lead to overdiagnosis and potential overtreatment in early low-risk prostate cancer, PSA screening remains crucial (33). Previous studies have revealed a low coverage of PSA screening in most Asian countries (34). This patient, for instance, had never undergone PSA screening or a DRE, presenting with widespread metastatic disease at initial diagnosis, which underscores the critical nature of prostate cancer screening.
The treatment methods for metastatic RCC and metastatic PCa are distinct. The common first-line treatments for advanced or metastatic RCC include single-agent targeted therapy or immune checkpoint inhibitor (ICI)-based combination therapy (35). In contrast, the treatment approaches for high-risk mHSPC typically involve combination therapies based on androgen deprivation therapy (ADT) (36). Hence, the accurate staging of both RCC and PCa as well as the identification of the primary tumor is of great importance. In this case, the RCC is small with the ISUP grade of 3, while the perinephric lymph nodes and adrenal metastases all originating from the prostate. Consequently, the patient was diagnosed with localized RCC coexisting with high-risk mHSPC. After receiving combination therapy based on ADT, there was a significant reduction in all metastatic lesions, resulting in stable disease management. Therefore, in cases with multiple primary malignancies, the accurate staging of different tumors is crucial for the success of the treatment.
4 Conclusion
In this case, conventional radiological and nuclear medicine examinations proved insufficient to determine the primary site of the tumor and accurately stage the malignancies. Percutaneous biopsies confirmed the presence of two synchronous tumors. Subsequent pathological analysis of the surgically removed tissues identified the origin of the metastasis and accurately staged the patient’s renal and prostate cancers, leading to a more favorable therapeutic response. This case highlights that metastatic lesions in the adrenal gland and renal hilar lymph nodes may not exclusively originate from the RCC but could also arise from the PCa in the context of concurrent RCC and PCa.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by Ethics Committee of West China Hospital, Sichuan University. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
YZ: Data curation, Formal analysis, Investigation, Writing – original draft. JC: Methodology, Project administration, Writing – original draft. LX: Data curation, Writing – original draft. XH: Conceptualization, Data curation, Writing – review & editing. HZ: Funding acquisition, Resources, Supervision, Writing – review & editing. ZL: Project administration, Supervision, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the Natural Science Foundation of China (NSFC 82172785 and 81974398).
Acknowledgments
The authors would like to express their sincere gratitude to the patient and their family for their trust, invaluable assistance, collaborative engagement, and consent to participate in this research.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2024.1412067/full#supplementary-material
Abbreviations
PCa, prostate cancer; RCC, renal cell carcinoma; ADT, androgen deprivation therapy; DRE, digital rectal examination; mHSPC, metastatic hormone-sensitive prostate cancer; CT, computed tomography; PSA, prostate-specific antigen; ccRCC, clear cell renal cell carcinoma; AR, androgen receptor; ICI, immune checkpoint inhibitor.
References
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J Clin. (2021) 71:209–49. doi: 10.3322/caac.21660
2. Li ZY, Ying CC, Wan ZH, Wang ZS, Li GH, Chen L, et al. Primary prostate cancer synchronous with renal cell carcinoma: clinical experience and literature review. Rom J Morphol Embryol. (2020) 61:555–61. doi: 10.47162/RJME
3. Kanehira M, Tamura D, Matsuura T, Maekawa S, Kato R, Kato Y, et al. Concurrent robot-assisted radical prostatectomy and robot-assisted partial nephrectomy for patients with synchronous prostate cancer and small renal tumor: A case series of five patients. Asian J Endosc Surg. (2022) 15:700–4. doi: 10.1111/ases.13060
4. Gandaglia G, Abdollah F, Schiffmann J, Trudeau V, Shariat SF, Kim SP, et al. Distribution of metastatic sites in patients with prostate cancer: A population-based analysis. Prostate. (2014) 74:210–6. doi: 10.1002/pros.22742
5. Dudani S, De Velasco G, Wells JC, Gan CL, Donskov F, Porta C, et al. Evaluation of clear cell, papillary, and chromophobe renal cell carcinoma metastasis sites and association with survival. JAMA Netw Open. (2021) 4:e2021869. doi: 10.1001/jamanetworkopen.2020.21869
6. Bianchi M, Sun M, Jeldres C, Shariat SF, Trinh QD, Briganti A, et al. Distribution of metastatic sites in renal cell carcinoma: a population-based analysis. Ann Oncol. (2012) 23:973–80. doi: 10.1093/annonc/mdr362
7. Lim JH, You D, Jeong IG, Hong JH, Ahn H, Kim CS. Prognosis of prostate cancer with other primary Malignancies. Korean J Urol. (2014) 55:327. doi: 10.4111/kju.2014.55.5.327
8. Beisland C, Talleraas O, Bakke A, Norstein J. Multiple primary Malignancies in patients with renal cell carcinoma: a national population-based cohort study. BJU Int. (2006) 97:698–702. doi: 10.1111/j.1464-410X.2006.06004.x
9. Cochetti G, Cocca D, Maddonni S, Paladini A, Sarti E, Stivalini D, et al. Combined robotic surgery for double renal masses and prostate cancer: myth or reality? Medicina. (2020) 56:318. doi: 10.3390/medicina56060318
10. Ozsoy O, Fioretta G, Ares C, Miralbell R. Incidental detection of synchronous primary tumours during staging workup for prostate cancer. Swiss Med Wkly. (2010) 140(15-16):233–6. doi: 10.4414/smw.2010.12976
11. Chen C, He H, Yu Z, Qiu Y, Wang X. Renal and retroperitoneal metastasis from prostate adenocarcinoma: a case report. World J Surg Onc. (2016) 14:74. doi: 10.1186/s12957-016-0834-4
12. Denti F, Wisard M, Guillou L, Francke ML, Leisinger HJ. Renal metastasis from prostatic adenocarcinoma: A potential diagnostic pitfall. Urol Int. (1999) 62:171–3. doi: 10.1159/000030384
13. Morton M, Omar N, Madi R, Terris M, Powell M. Collision metastasis: Renal cell carcinoma and prostatic adenocarcinoma to a retroperitoneal lymph node. Urol Case Rep. (2022) 40:101884. doi: 10.1016/j.eucr.2021.101884
14. Vyas M, Menon S, Desai SB. Collision tumor of kidney: A case of renal cell carcinoma with metastases of prostatic adenocarcinoma. Indian J Med Paediatr Oncol. (2013) 34(1):21–3. doi: 10.4103/0971-5851.113409
15. Moussa M, Chakra MA. Metastatic prostate cancer presenting as a retroperitoneal mass: a case report and review of literature. J Surg Case Rep. (2019) 2019:rjz291. doi: 10.1093/jscr/rjz291
16. Chandekar KR, Satapathy S, Panaiyadiyan S, Kumar R. Perirenal fascia — an uncommon site of metastases in prostate cancer detected on 68Ga-PSMA-11 PET/CT. Nucl Med Mol Imaging. (2023) 57:254–5. doi: 10.1007/s13139-023-00801-w
17. Wakita T, Yamanaka K, Yoshimura A, Fukae S, Yoshida T, Kishikawa H. Perirenal fat metastasis of prostate cancer. Urol Case Rep. (2021) 39:101778. doi: 10.1016/j.eucr.2021.101778
18. Vinjamoori AH, Jagannathan JP, Shinagare AB, Taplin ME, Oh WK, Van Den Abbeele AD, et al. Atypical metastases from prostate cancer: 10-year experience at a single institution. Am J Roentgenol. (2012) 199:367–72. doi: 10.2214/AJR.11.7533
19. Topal E, Has Simsek D, Vatansever S, Sanli Y, Kuyumcu S. Isolated adrenal metastases of castration-resistant prostate cancer: excellent response to 177Lu-PSMA with no adrenal insufficiency. Clin Nucl Med. (2024) 49:e8–9. doi: 10.1097/RLU.0000000000004964
20. Sakellakis MJ, Hahn AW, Ramachandran S, Zhang M, Hoang A, Song JH, et al. Characterization of prostate cancer adrenal metastases: dependence upon androgen receptor signaling and steroid hormones. Prostate Cancer Prostatic Dis. (2023) 26:751–8. doi: 10.1038/s41391-022-00590-x
21. Kanatsız O, Özülker F, Aydın T, Özülker T. Isolated castrate-resistant prostate cancer metastasis to both adrenal glands detected on 68Ga PSMA PET/CT. Mol Imaging Radionucl Ther. (2023) 32(3):244–6. doi: 10.4274/mirt.galenos.2023.03780
22. Ayala Soriano C, Benitez Barzaga M, Chhina A, Jain M, Nava VE. Hepatoid prostatic carcinoma with adrenal metastasis and novel genetic alterations. Diagn Cytopathol. (2022) 50(11):E310–4. doi: 10.1002/dc.25006
23. Zhao Q, Yang B, Dong A, Zuo C. 68Ga-PSMA-11 PET/CT in isolated bilateral adrenal metastases from prostate adenocarcinoma. Clin Nucl Med. (2022) 47:e101–2. doi: 10.1097/RLU.0000000000003759
24. Ribeiro AMB, Lima ENP, Garcia D, Bezerra SM. Single adrenal metastasis from prostate cancer detected by 68Ga-PSMA PET/CT and confirmed by biopsy: A case report. Clin Nucl Med. (2022) 47:e61–2. doi: 10.1097/RLU.0000000000003829
25. Muñoz López AY, Domínguez Castillo RE, Rodenas Gil EA, Navarro Ruesga I, Morales Montor JG, Pacheco Gahbler C. Primary neuroendocrine prostate cancer with adrenal gland metastasis. Urol Case Rep. (2022) 40:101896. doi: 10.1016/j.eucr.2021.101896
26. McGeorge S, Phillips R, Rukin NJ, Reynolds J, Roberts MJ. Solitary castrate-resistant prostate cancer metastasis to adrenal gland with concordant intense avidity on PSMA and FDG PET. Urol Case Rep. (2021) 38:101696. doi: 10.1016/j.eucr.2021.101696
27. Ashrafi AN, Fay C, Yip W, De Freitas DM, Nabhani J, Aron M. Durable biochemical response following adrenal metastasectomy for oligometastatic castrate-resistant prostate cancer. Urol Case Rep. (2020) 32:101229. doi: 10.1016/j.eucr.2020.101229
28. Matrone F, Sivolella S, Bellavita R, Casciola L, Cristallini EG, Aristei C. Use of 18 F-choline positron emission tomography/CT in high-risk prostate cancer: a case of solitary adrenal metastasis. Tumori. (2015) 101:e21–3. doi: 10.5301/tj.5000260
29. Subhawong AP, Subhawong TK, Li QK. Fine needle aspiration of metastatic prostate carcinoma simulating a primary adrenal cortical neoplasm: A case report and review of the literature. Diagn Cytopathol. (2010) 38:147–53. doi: 10.1002/dc.21165
30. Barrisford GW, Sartor O, Richie JP. Solitary adrenal metastatic lesion in a patient with a history of prostate cancer. Clin Genitourin Cancer. (2009) 7:64–6. doi: 10.3816/CGC.2009.n.012
31. Kawahara T, Taguchi H, Yamagishi T, Udagawa K, Ouchi H, Misaki H. A case of bilateral adrenal and pleural metastases from prostate cancer. Case Rep Oncol. (2009) 2:217–9. doi: 10.1159/000258964
32. Sakamoto N, Eto M, Ando S. Surgical excision of a metastatic adrenal lesion in a patient with prostatic cancer. Int J Urol. (1999) 6:38–40. doi: 10.1046/j.1442-2042.1999.06125.x
33. Wu X, Ko ICH, Hong CYL, Yee SCH, Teoh JYC, Chan SYS, et al. A prospective cohort of men with localized prostate cancer on active surveillance protocol in Hong Kong, China: what did we learn? Asian J Androl. (2024) 26:245–9. doi: 10.4103/aja202373
34. Ruan X, Zhang N, Wang D, Huang J, Huang J, Huang D, et al. The impact of prostate-specific antigen screening on prostate cancer incidence and mortality in China: 13-year prospective population-based cohort study. JMIR Public Health Surveill. (2024) 10:e47161. doi: 10.2196/47161
35. Motzer RJ, Jonasch E, Agarwal N, Alva A, Bagshaw H, Baine M, et al. NCCN guidelines® Insights: kidney cancer, version 2.2024: featured updates to the NCCN 3guidelines. J Natl Compr Cancer Netw. (2024) 22:4–16. doi: 10.6004/jnccn.2024.0008
Keywords: multiple primary cancers, renal cell carcinoma, prostate cancer, metastatic tumor, adrenal metastasis, lymph node metastasis, surgical diagnostic procedures
Citation: Zhang Y, Chen J, Xu L, Hu X, Zeng H and Liu Z (2024) Case report: Synchronous prostate cancer and renal cell carcinoma with prostate cancer-origin metastases to adrenal and renal hilar lymph nodes. Front. Oncol. 14:1412067. doi: 10.3389/fonc.2024.1412067
Received: 08 April 2024; Accepted: 18 September 2024;
Published: 07 October 2024.
Edited by:
Ronald M. Bukowski, Cleveland Clinic, United StatesReviewed by:
Xiaobo Wu, The Chinese University of Hong Kong, ChinaChunguang Yang, Huazhong University of Science and Technology, China
Copyright © 2024 Zhang, Chen, Xu, Hu, Zeng and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhenhua Liu, emhsaXVAc2N1LmVkdS5jbg==
†These authors have contributed equally to this work and share first authorship
 Yaowen Zhang
Yaowen Zhang Junru Chen1†
Junru Chen1† Xu Hu
Xu Hu Hao Zeng
Hao Zeng Zhenhua Liu
Zhenhua Liu