
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol. , 21 August 2024
Sec. Breast Cancer
Volume 14 - 2024 | https://doi.org/10.3389/fonc.2024.1412051
This article is part of the Research Topic The Exciting Opportunities and Challenges for Targeting “HER2 low” Breast Cancers and Beyond View all 8 articles
 Zhidong Lyu*
Zhidong Lyu* Linlin Gao
Linlin GaoBackground: This study aimed to retrospectively analyse the pathological response and safety of combining albumin-bound paclitaxel (nab-paclitaxel) or docetaxel with anti-HER2 therapy as a neoadjuvant treatment for HER2-positive breast cancer.
Methods: From June 2020 to August 2023, 225 HER2-positive breast cancer patients who underwent radical surgery following neoadjuvant treatment were enrolled in this study. The patients were divided into two groups based on the drugs they received: the nab-paclitaxel group (n=166, receiving nab-paclitaxel + platinum along with trastuzumab and pertuzumab) and the docetaxel group (n=59, receiving docetaxel + platinum along with trastuzumab and pertuzumab). The pathological response and adverse events related to the drugs were collected and evaluated in both groups.
Results: In the nab-paclitaxel group, the rates of breast and total pathological complete response (bpCR and tpCR) were significantly greater than those in the docetaxel group (69.27% vs. 47.45%, P=0.003; 68.67% vs. 45.76%, P=0.002). For patients who did not achieve pCR after chemotherapy, the pathological response of chemotherapy was analysed using MP grading and RCB grading. The results showed that there was a statistically significant difference between the two groups (P<0.05). Multivariate analysis revealed that therapeutic drugs, clinical stage, ER status, and Ki-67 level were independent predictors of pCR. The nab-paclitaxel group had a significantly greater proportion of patients with peripheral sensory neuropathy than did the docetaxel group (58.43% vs. 38.98%, P=0.035), while the docetaxel group had a greater proportion of patients with allergies and elevated ALT (31.93% vs. 69.49%, P=0.000; 23.49% vs. 40.68%, P=0.021).
Conclusions: Our real-world study revealed that nab-paclitaxel combined with anti-HER2 therapy was an effective neoadjuvant therapy for HER2-positive breast cancer. The multivariate analysis revealed that chemotherapy drugs, clinical stage, ER status, and Ki-67 level was the significant factor influencing treatment outcome. These findings offer a valuable reference for the neoadjuvant treatment of patients with HER2-positive breast cancer.
Neoadjuvant therapy is extensively utilized in the treatment of breast cancer, particularly in cases of human epidermal growth factor receptor 2 (HER2)-positive breast cancer and triple-negative breast cancer (1, 2). The advancement of anti-HER2 therapy in recent years has significantly enhanced the overall prognosis for patients with HER2-positive breast cancer (3). In the early NOAH study, a combination of chemotherapy and trastuzumab demonstrated a substantial 21% increase in the pathological complete response (pCR) rate, providing a crucial foundation for neoadjuvant treatment in HER2-positive breast cancer (4). Additionally, another study revealed that the pCR rates were 31%, 23%, and 49% when trastuzumab, pertuzumab, and their combinations were used, respectively (5). The Neosphere trial documented an 18% pCR rate with trastuzumab and pertuzumab, but when combined with docetaxel, the pCR rate increased to 49% (6). These results indicated that the inclusion and/or combination of chemotherapy and anti-HER2 therapy can improve the pCR rate.
Albumin-bound paclitaxel (nab-paclitaxel), a preparation of paclitaxel that binds to albumin, does not require a solvent (7). Studies have shown that when administered at the same dose, nab-paclitaxel reaches tumours at a concentration 1.3 times greater than that of solvent-based paclitaxel, leading to more powerful antitumour effects (8, 9). In a previous report, we observed that nab-paclitaxel was more effective at achieving a pCR rate than docetaxel in patients with HER2-negative breast cancer (10). That study revealed that the pCR rate of patients receiving nab-paclitaxel regimens was 36.71%, which exceeded the rate of 20% achieved with docetaxel regimens. With these positive results, nab-paclitaxel may be considered a new option for combination therapy with anti-HER2 agents.
Trastuzumab is a human monoclonal antibody targeting HER2 that induces antibody-dependent cell-mediated cytotoxicity and inhibits signal transduction (11, 12). Previous studies have shown that neoadjuvant therapy using reference trastuzumab (Herceptin®, Roche, USA) has a significant effect on the prognosis of early-stage breast cancer patients (13). Zercepac (HLX02, Fuhong Hanlin Pharmaceutical Co., Ltd., China) is the first Chinese monoclonal antibody highly similar to the reference trastuzumab and has good potential for reducing tumour cell proliferation and survival (14). Recently, Zercepac demonstrated effectiveness equivalent to that of reference trastuzumab for HER2-positive recurrent or metastatic breast cancer in a phase III multicentre clinical trial (15). However, more studies are needed to evaluate its potential effectiveness in neoadjuvant therapy.
In China, nab-paclitaxel has not been used for a long time, and the effectiveness and safety of nab-paclitaxel combined with anti-HER2 agents for treating HER2-positive breast cancer patients need further clinical observation. Consequently, we conducted this real-world study to assess the pathological response and safety of nab-paclitaxel or docetaxel combined with anti-HER2 therapy for the treatment of HER2-positive breast cancer.
This retrospective study was conducted at the Affiliated Hospital of Qingdao University and focused on HER2-positive breast cancer patients who underwent neoadjuvant treatment followed by radical surgery. The study included patients who met the following criteria: (1) aged ≥18 years, (2) HER2-positive, (3) diagnosed with invasive breast cancer, (4) clinical stage II-III, (5) underwent radical surgery for breast cancer, and (6) received TCbHP therapy (taxane + platinum combined with trastuzumab and pertuzumab, 6 cycles) prior to surgery. Patients who had received any type of therapy before neoadjuvant treatment, had synchronous or previous in situ or invasive breast cancer, inflammatory breast cancer, male breast cancer, bilateral breast cancer, chronic or acute inflammatory disease, kidney insufficiency, mental disease, or autoimmune disease were excluded. This study enrolled consecutive patients who met the inclusion criteria from June 2020 to August 2023, resulting in the initial identification of 246 patients. Women who had received other treatments before therapy (n=4), had unavailable treatment records (n=5), had a history of other tumours (n=4), experienced severe complications (n=3), or were lost to follow-up (n=5) were excluded. Finally, the analysis focused on 225 HER2-positive breast cancer patients who were available for analysis (Figure 1). Patients were categorized into either the docetaxel group (n=59) or the nab-paclitaxel group (n=166) based on the medication they received. The treatment involved administering either nab-paclitaxel (Qilu Pharmaceutical Co., Ltd., China) at a dose of 260 mg/m2 or docetaxel (Kelun Pharmaceutical Co., Ltd., China) at a dose of 75 mg/m2, along with carboplatin (Qilu Pharmaceutical Co., Ltd., China) at the AUC5. Additionally, all patients received trastuzumab at a dose of 8 mg/kg in the first cycle, 6 mg/kg in subsequent cycles, and pertuzumab (Perjeta, Roche, USA) at a dose of 840 mg in the first cycle and 420 mg g in subsequent cycles every 3 weeks. The physicians determined the specific doses and schedules for the treatment based on the diagnosis and treatment guidelines. Subsequently, the patients underwent radical surgery 3-4 weeks after completing the last neoadjuvant treatment.
Before starting neoadjuvant treatment, a core needle biopsy was performed to obtain a pathological diagnosis. Immunohistochemistry was used to evaluate the levels of estrogen receptor (ER), progesterone receptor (PR), HER2, and Ki-67. A positive expression of ER or PR was defined as a rate of 1% or higher. According to the ASCO/CAP guidelines, HER2 positivity was defined as a score of 3+ on immunohistochemistry or a score of 2+ on FISH amplification. The Ki-67 level was calculated as the percentage of tumour cells showing positive nuclear staining. The classification of ki-67 in this article relies on St. Gallen consensus (16). This study was approved by the Medical Ethics Review Board of the Affiliated Hospital of Qingdao University (QYFY WZLL 28131). It should be noted that this study was a retrospective analysis, and the ethics committee waived the need for informed consent. Patient information has been anonymized in this article to protect privacy.
Evaluations of the effectiveness of neoadjuvant treatment were conducted every 2 cycles using ultrasonography or MR imaging. The pathological reports were independently reviewed by 2 pathologists to determine the category. The response to neoadjuvant treatment was assessed according to RECIST version 1.1. Total pathological complete response (tpCR) was defined as the absence of any pathological evidence of residual invasive carcinoma in both the breast and axillary lymph nodes (ypT0/isN0 status). A breast pathological complete response (bpCR) was defined as the absence of any pathological evidence of residual invasive carcinoma in the breast (ypT0/is status). The Miller-Payne (MP) system, which consists of 5 severity grades, was employed in the study to assess the degree of tumour reduction, with higher grades indicating a greater reduction. Grade 5 corresponds to a complete pathological response in breast cancer (17). The pathological response of treatment was also analysed using the residual cancer burden (RCB) grading system, which involves classification into levels 0-3 based on guidelines from the International Breast Collaboration Group (18). Adverse events were collected and graded using CTCAE 5.0.
The primary endpoint of this study was the PCR. The comparisons of clinical and pathological characteristics were made using Fisher’s exact test or Pearson’s chi-square test. Logistic regression models were used for both univariate and multivariate analyses. We first conducted a single factor analysis, and then conducted the multivariate logistic analysis based on the differences identified from the single factor analysis(P<0.05). Data analysis was performed using SPSS software version 19.0, with a significance level of P<0.05.
This study analysed a total of 225 patients, with 166 receiving nab-paclitaxel-based treatment and 59 receiving docetaxel-based regimens. The baseline characteristics of all patients are presented in Table 1. The average age of the patients in the nab-paclitaxel group was 49.49 ± 8.88 years, while that in the docetaxel group was 51.76 ± 12.83 years. The two groups had similar basic features, including age (P=0.213), sides (P=0.244), menstruation status (P=0.363), tumour size (P=0.555), histological grade (P=0.261), lymph node status (P=0.914), clinical stage (P=0.057), ER status (P=0.689), PR status (P=0.986), PR status (P=0.635), Ki-67 level (P=0.292), and targeted therapy (P=0.165).
Following neoadjuvant therapy, the bpCR rate in the nab-paclitaxel group was 69.27% (115/166), which was significantly greater than the 47.45% (28/59) in the docetaxel group (P=0.003). Similar findings were observed for tpCR, for which the tpCR rate was 68.67% (114/166) in the nab-paclitaxel group, which was significantly greater than the 45.76% (27/59) in the docetaxel group (P=0.002). Subgroup analysis revealed that among patients with a tumour size >3 cm, the nab-paclitaxel group achieved higher rates of bpCR and tpCR than did the docetaxel group (P<0.05). Additionally, subgroup analysis revealed that in the nab-paclitaxel group, patients with a positive lymph node grade, clinical stage III disease, ER-negative status, PR negative status, HER2 status, IHC 3+ status, and Ki67 ≤ 20% achieved a significantly greater pCR rate than did those receiving docetaxel (P<0.05) (Table 2). Furthermore, when comparing the pathological response of reference trastuzumab and Zercepac combined with taxane in treating HER2-positive breast cancer, both reference trastuzumab and Zercepac combined with nab-paclitaxel resulted in higher tpCR rates than did treatment combined with docetaxel alone (69.15% vs. 46.88%, P=0.040; 69.80% vs. 44.44%, P=0.021).
To assess the pathological response of chemotherapy in patients who did not achieve pCR, we utilized MP grading and RCB grading. In the nab-paclitaxel group, Grade 1 partial response occurred in 1.20% of patients, Grade 2 partial response in 6.63%, Grade 3 partial response in 10.24%, and Grade 4 partial response in 12.65%. For the docetaxel group, Grade 1 partial response was reported in 5.08% of patients, Grade 2 in 6.78%, Grade 3 in 22.03%, and Grade 4 in 18.64% among HER2-positive patients, and this difference was found to be statistically significant (P=0.039). Additionally, RCB analysis was conducted to evaluate the breast masses and axillary lymph nodes postchemotherapy. In the nab-paclitaxel group, RCB 1 partial response was observed in 9.64% of patients, RCB 2 in 16.87%, and RCB 3 in 4.82%. For the docetaxel group, RCB 1 partial response was reported in 15.25% of HER2-positive patients, RCB 2 in 28.81%, and RCB 3 in 10.17% (Figure 2), showing a statistically significant difference (P=0.023).
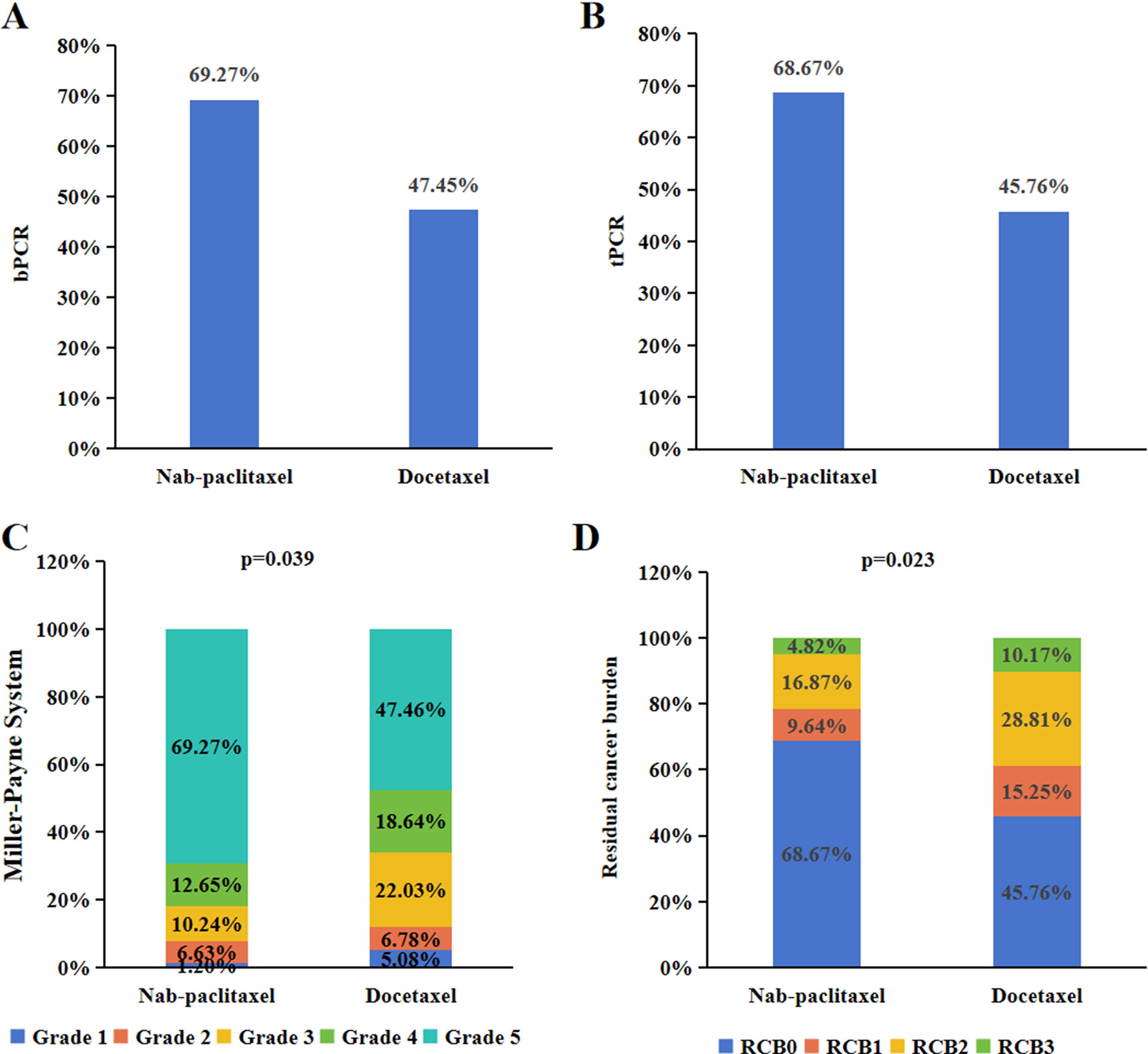
Figure 2. Pathological response of neoadjuvant treatment for HER2-positive breast cancer. The bpCR (A) and tpCR (B) of nab-paclitaxel or docetaxel combination with anti-HER2-therapy in HER2-positive breast cancer. The MP grading (C) and RCB grading (D) of nab-paclitaxel or docetaxel combination with anti-HER2-therapy in HER2-positive breast cancer.
The bpCR rate was 63.56% (143/225), and the tpCR rate was 62.67% (141/225). The univariate logistic analysis results showed that chemotherapy drugs, clinical stage, ER status, PR status and Ki-67 level were associated with bpCR and tpCR (all P<0.05, Tables 3, 4). We then conducted the multivariate logistic analysis based on the differences identified from the single factor analysis. The outcomes of the multivariate logistic regression analysis for bpCR and tpCR are shown in Tables 5, 6. Multivariate analysis revealed that the nab-paclitaxel group exhibited significantly improved bpCR and tpCR compared with the docetaxel group (P<0.05). The results indicated that chemotherapy drugs, clinical stage, ER status, PR status, and Ki-67 level were independent predictors of bpCR (all P<0.05). Furthermore, the multivariate analysis revealed that chemotherapy drugs, clinical stage, ER status, and Ki-67 level was the significant factor influencing tpCR (all P<0.05).
Safety as the second endpoint of this study. All patients enrolled in the study completed 6 cycles of TCbHP therapy within both groups. The drug-related adverse events were mild and are listed in Table 7. The most commonly observed adverse reaction during neoadjuvant treatment was fatigue, with an incidence of 68.67% in the nab-paclitaxel group and 59.32% in the docetaxel group. However, there was no statistically significant difference between the two groups (P=0.154). Peripheral sensory neuropathy was more prevalent in the nab-paclitaxel group (58.43% vs. 38.98%, P=0.035), whereas the incidence of allergies in this group was lower than that in the docetaxel group (31.93% vs. 69.49%, P=0.000). Additionally, the occurrence of elevated ALT was significantly lower in the nab-paclitaxel group than in the docetaxel group (23.49% vs. 40.68%, P=0.021). Other drug-related adverse events, such as diarrhoea, neutropenia, vomiting, dizziness, intermittent fever, infusion-related reactions, decreased appetite, elevated AST, anaemia, oral mucositis, and difficulty breathing were similar in both groups (P>0.05).
The standard neoadjuvant treatment option for HER2-positive breast cancer has become the combination of chemotherapy and anti-HER2 therapy (19, 20). Nab-paclitaxel shows superior effects in advanced breast cancer and advantages in neoadjuvant therapy (8). The objective of this retrospective study was to evaluate the pathological response and safety of nab-paclitaxel or docetaxel combined with anti-HER2 therapy for the treatment of HER2-positive breast cancer.
The GeparSepto trial demonstrated that weekly nab-paclitaxel is more effective than solvent-based paclitaxel followed by cyclophosphamide plus epirubicin as neoadjuvant therapy in breast cancer, as it significantly improves the pCR rate and disease-free survival (21, 22). It is believed that the albumin-mediated delivery of nab-paclitaxel may enhance its transportation to tumours, improve tolerability, reduce infusion time, and eliminate the need for preoperative prophylactic medication. In a trial involving metastatic breast cancer patients, patients treated with nab-paclitaxel had a greater response rate and longer progression time than patients treated with paclitaxel (23, 24). Previous studies have shown that the safety of nab-paclitaxel is acceptable, but there is a lack of direct comparisons between nab-paclitaxel and docetaxel. The aim of this study was to evaluate the clinical benefits and adverse events of nab-paclitaxel as a neoadjuvant therapy in HER2-positive breast cancer patients. In the present study, we found that nab-paclitaxel combined with anti-HER2 therapy resulted in higher pCR rates than docetaxel group. For all patients, the bpCR rate was 63.56%, and the tpCR rate was 62.67%. We also noticed that the PCR rate in the current study was lower than that in previous studies (TRAIN-2 and TRYPHAENA). In the TRAIN-2 study, a pCR rate of 68% was achieved after 9 cycles of TCbHP treatment (24). The TRYPHAENA study also confirmed a pCR rate of 66.2% after 6 cycles of TCbHP treatment (25). In the present study, the patients enrolled generally had late clinical stage disease, and 93.8% of patients had lymph node metastasis. The proportion of PCR products is also related to the number of cycles of chemotherapy. In this study, patients received 6 cycles of chemotherapy, while patients received 9 cycles of chemotherapy in the TRAIN-2 study (23). Neosphere and Peony utilized the THP regimen for HER2-positive breast cancer and reported a lower pCR rate of only 39.3% (6, 26). These studies further support the important role of platinum drugs in the treatment of HER2-positive breast cancer. Our findings additionally indicated that therapeutic drugs, clinical stage, ER status, and Ki-67 level were all independent predictors of pCR. Ki-67 is an indicator of tumour proliferation, and we found that Ki-67>20% resulted in a higher bPCR rate than Ki-67 ≤ 20% (68.0% vs. 48.0%) in this study. We further found that patients with Ki-67>20% benefitted more from nab-paclitaxel than those with Ki-67 ≤ 20% (71.2% vs. 61.76%). Furthermore, we compared the effectiveness of domestically produced Zercepac with that of the reference trastuzumab. Our data demonstrated that the pCR of HER2-positive breast cancer patients treated with nab-paclitaxel combined with reference trastuzumab was comparable to that of patients treated with Zercepac.
Previous studies have shown that nab-paclitaxel is associated with a greater incidence of peripheral sensory neuropathy, clinical safety and adverse reactions (27, 28). In contrast to docetaxel, nab-paclitaxel does not utilize nonionic surfactants to dissolve paclitaxel. These surfactants are known to cause toxicity and encapsulate paclitaxel in solvent-based micelles. This could be due to the unaffected delivery of nab-paclitaxel by solvents, as higher doses can be administered in comparison to docetaxel (29). TCbHP is a chemical treatment that is accompanied by severe side effects, with leukopenia being the most commonly observed adverse reaction. Furthermore, allergies and nausea are also frequent adverse reactions. The toxicity characteristics observed in this study closely resemble those found in the GeparSepto and ETNA studies (23). The nab-paclitaxel group had a greater incidence of peripheral sensory neuropathy, while the docetaxel group was more prone to allergies. In the GeparSepto study, adjusting the dose of nab-paclitaxel from 150 mg/m2 to 125 mg/m2 resulted in a decrease in the incidence of grade 3-4 peripheral sensory neuropathy from 15% to 8% in the nab-paclitaxel group (30). Moreover, other drug-related adverse events, such as nausea, joint pain, and difficulty breathing, were similar between the both groups.
This study has several potential limitations that should be considered. First, this was a retrospective study within a single institution, and the sample size was relatively small. We have predicted the power of the analysis using STATA software. When the α value is set to 0.1, the power of the analysis can reach 0.8. We believe this can meet the sample size required for the study. Second, due to its expensive price, nab-paclitaxel did not receive widespread application in the early stage. After the launch of domestically produced drugs in China, nab-paclitaxel have been widely used, our cases were mainly included in 2022 and 2023, this study did not include follow-up data or analyse long-term survival rates. In the future, larger samples and longer follow-up periods are needed to verify these results.
In conclusion, our real-world study revealed that nab-paclitaxel combined with anti-HER2 therapy was an effective neoadjuvant therapy for HER2-positive breast cancer. The multivariate analysis revealed that clinical stage, ER status, and Ki-67 status was the significant factor influencing treatment outcome. The results provide a valuable reference for the neoadjuvant treatment of patients with HER2-positive breast cancer.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving humans were approved by The Affiliated Hospital of Qingdao University. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin because this is a retrospective study conducted at the Affiliated Hospital of Qingdao University.
ZL: Conceptualization, Formal analysis, Funding acquisition, Investigation, Methodology, Resources, Software, Supervision, Validation, Writing – original draft. LG: Conceptualization, Data curation, Formal analysis, Investigation, Resources, Writing – original draft.
The author(s) declare financial support was received for theresearch, authorship, and/or publication of this article. The Qilu Health Outstanding Young Talents Project of Shandong Province provided financial support for this study.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
pCR, pathological complete response; nab-paclitaxel, albumin-bound paclitaxel; ER, estrogen receptor; PR, progesterone receptor; HER2, human epidermal growth factor receptor 2; OR, odds ratio; CIs, confidence intervals.
1. Van Bockstal MR, Dano H, Benhaddi N, Dubois D, Vanderveken J, Van Marcke C, et al. Predictive markers for pathological complete response (pCR) after neo-adjuvant chemotherapy in HER2-positive breast carcinoma. Histol Histopathol. (2024) 39:153–64. doi: 10.14670/HH-18-626
2. Provenzano E, Shaaban AM. Pathology of neoadjuvant therapy and immunotherapy testing for breast cancer. Histopathology. (2023) 82:170–88. doi: 10.1111/his.14771
3. Xiao Y, Ding J, Ma D, Chen S, Li X, Yu K. Predicting pathological complete response in neoadjuvant dual blockade with trastuzumab and pertuzumab in HER2 gene amplified breast cancer. Front Immunol. (2022) 13:877825. doi: 10.3389/fimmu.2022.877825
4. Gianni L, Eiermann W, Semiglazov V, Manikhas A, Lluch A, Tjulandin S, et al. Neoadjuvant chemotherapy with trastuzumab followed by adjuvant trastuzumab versus neoadjuvant chemotherapy alone, in patients with HER2-positive locally advanced breast cancer (the NOAH trial): a randomised controlled superiority trial with a parallel HER2-negative cohort. Lancet. (2010) 375:377–84. doi: 10.1016/S0140-6736(09)61964-4
5. Swain SM, Ewer MS, Viale G, Delaloge S, Ferrero JM, Verrill M, et al. Pertuzumab, trastuzumab, and standard anthracycline- and taxane-based chemotherapy for the neoadjuvant treatment of patients with HER2-positive localized breast cancer (BERENICE): a phase II, open-label, multicenter, multinational cardiac safety study. Ann Oncol. (2018) 29:646–53. doi: 10.1093/annonc/mdx773
6. Gianni L, Pienkowski T, Im YH, Tseng LM, Liu MC, Lluch A, et al. 5-year analysis of neoadjuvant pertuzumab and trastuzumab in patients with locally advanced, inflammatory, or early-stage HER2-positive breast cancer (NeoSphere): a multicentre, open-label, phase 2 randomised trial. Lancet Oncol. (2016) 17:791–800. doi: 10.1016/S1470-2045(16)00163-7
7. Futamura M, Ishihara K, Nagao Y, Ogiso A, Niwa Y, Nakada T, et al. Neoadjuvant chemotherapy using nanoparticle albumin-bound paclitaxel plus trastuzumab and pertuzumab followed by epirubicin and cyclophosphamide for operable HER2-positive primary breast cancer: a multicenter phase II clinical trial (PerSeUS-BC04). Breast Cancer. (2023) 30:293–301. doi: 10.1007/s12282-022-01425-2
8. Matsumoto A, Jinno H, Naruse S, Isono Y, Maeda Y, Sato A, et al. Efficacy and safety of dose-dense neoadjuvant chemotherapy with nab-paclitaxel followed by epirubicin and cyclophosphamide for operable breast cancer. Jpn J Clin Oncol. (2023) 53:1119–24. doi: 10.1093/jjco/hyad112
9. Liu Y, Zhao F, Wang Q, Zhao Q, Hou G, Meng Q. Current perspectives on paclitaxel: focus on its production, delivery and combination therapy. Mini Rev Med Chem. (2023) 23:1780–96. doi: 10.2174/1389557523666230210145150
10. Lv ZD, Song HM, Niu ZH, Nie G, Zheng S, Xu YY, et al. Efficacy and safety of albumin-bound paclitaxel compared to docetaxel as neoadjuvant chemotherapy for HER2-negative breast cancer. Front Oncol. (2022) 11:760655. doi: 10.3389/fonc.2021.760655
11. von Arx C, De Placido P, Caltavituro A, Di Rienzo R, Buonaiuto R, De Laurentiis M, et al. The evolving therapeutic landscape of trastuzumab-drug conjugates: Future perspectives beyond HER2-positive breast cancer. Cancer Treat Rev. (2023) 113:102500. doi: 10.1016/j.ctrv.2022.102500
12. Houvenaeghel G, Cohen M, Gonçalves A, Berthelot A, Chauvet MP, Faure C, et al. Triple-negative and Her2-positive breast cancer in women aged 70 and over: prognostic impact of age according to treatment. Front Oncol. (2023) 13:1287253. doi: 10.3389/fonc.2023.1287253
13. Hurvitz SA, Martin M, Jung KH, Huang CS, Harbeck N, Valero V, et al. Neoadjuvant trastuzumab emtansine and pertuzumab in human epidermal growth factor receptor 2-positive breast cancer: three-year outcomes from the phase III KRISTINE study. J Clin Oncol. (2019) 37:2206–16. doi: 10.1200/JCO.19.00882
14. Zhu X, Ding Y, Yu Y, Wang M, Zhou W, Wang J, et al. A Phase 1 randomized study compare the pharmacokinetics, safety and immunogenicity of HLX02 to reference CN- and EU-sourced trastuzumab in healthy subjects. Cancer Chemother Pharmacol. (2021) 87:349–59. doi: 10.1007/s00280-020-04196-9
15. Xu B, Zhang Q, Sun T, Li W, Teng Y, Hu X, et al. Efficacy, safety, and immunogenicity of HLX02 compared with reference trastuzumab in patients with recurrent or metastatic HER2-positive breast cancer:A randomized phase III equivalence trial. BioDrugs. (2021) 35:337–50. doi: 10.1007/s40259-021-00475-w
16. Vasconcelos I, Hussainzada A, Berger S, Fietze E, Linke Jörg, Siedentopf F, et al. The St. Gallen surrogate classification for breast cancer subtypes successfully predicts tumor presenting features, nodal involvement, recurrence patterns and disease free survival. Breast. (2016) 29:181–5. doi: 10.1016/j.breast.2016.07.016
17. Wang W, Liu Y, Zhang H, Zhang S, Duan X, Ye J, et al. Prognostic value of residual cancer burden and Miller-Payne system after neoadjuvant chemotherapy for breast cancer. Gland Surg. (2021) 10:3211–21. doi: 10.21037/gs
18. Symmans WF, Yau C, Chen YY, Balassanian R, Klein ME, Pusztai L, et al. Assessment of residual cancer burden and event-free survival in neoadjuvant treatment for high-risk breast cancer: an analysis of data from the I-SPY2 randomized clinical trial. JAMA Oncol. (2021) 7:1654–63. doi: 10.1001/jamaoncol.2021.3690
19. Korde LA, Somerfield MR, Carey LA, Crews JR, Denduluri N, Hwang ES, et al. Neoadjuvant chemotherapy, endocrine therapy, and targeted therapy for breast cancer: ASCO guideline. J Clin Oncol. (2021) 39:1485–505. doi: 10.1200/JCO.20.03399
20. Untch M, Jackisch C, Schneeweiss A, Conrad B, Aktas B, Denkert C, et al. Nab-paclitaxel versus solvent-based paclitaxel in neoadjuvant chemotherapy for early breast cancer (GeparSepto-GBG 69): a randomised, phase 3 trial. Lancet Oncol. (2016) 17:345–56. doi: 10.1016/S1470-2045(15)00542-2
21. Untch M, Jackisch C, Schneeweiss A, Schmatloch S, Aktas B, Denkert C, et al. NAB-paclitaxel improves disease-free survival in early breast cancer: GBG 69-geparSepto. J Clin Oncol. (2019) 37:2226–34. doi: 10.1200/JCO.18.01842
22. Gianni L, Mansutti M, Anton A, Calvo L, Bisagni G, Bermejo B, et al. Comparing neoadjuvant nab-paclitaxel vs paclitaxel both followed by anthracycline regimens in women with ERBB2/HER2-negative breast cancer-the evaluating treatment with neoadjuvant abraxane (ETNA) trial: A randomized phase 3 clinical trial. JAMA Oncol. (2018) 4:302–8. doi: 10.1001/jamaoncol.2017.4612
23. Loibl S, Treue D, Budczies J, Weber K, Stenzinger A, Schmitt WD, et al. Mutational diversity and therapy response in breast cancer: A sequencing analysis in the neoadjuvant geparSepto trial. Clin Cancer Res. (2019) 25:3986–95. doi: 10.1158/1078-0432.CCR-18-3258
24. van Ramshorst MS, van der Voort A, van Werkhoven ED, Mandjes IA, Kemper I, Dezentjé VO, et al. Neoadjuvant chemotherapy with or without anthracyclines in the presence of dual HER2 blockade for HER2-positive breast cancer (TRAIN-2): a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. (2018) 19:1630–40. doi: 10.1016/S1470-2045(18)30570-9
25. Schneeweiss A, Chia S, Hickish T, Harvey V, Eniu A, Hegg R, et al. Pertuzumab plus trastuzumab in combination with standard neoadjuvant anthracycline-containing and anthracycline-free chemotherapy regimens in patients with HER2-positive early breast cancer: a randomized phase II cardiac safety study (TRYPHAENA). Ann Oncol. (2013) 24:2278–84. doi: 10.1093/annonc/mdt182
26. Shao Z, Pang D, Yang H, Li W, Wang S, Cui S, et al. Efficacy, safety, and tolerability of pertuzumab, trastuzumab, and docetaxel for patients with early or locally advanced ERBB2-positive breast cancer in Asia: the PEONY phase 3 randomized clinical trial. JAMA Oncol. (2020) 6:e193692. doi: 10.1001/jamaoncol.2019.3692
27. Klein-Brill A, Amar-Farkash S, Lawrence G, Collisson EA, Aran D. Comparison of FOLFIRINOX vs gemcitabine plus nab-paclitaxel as first-line chemotherapy for metastatic pancreatic ductal adenocarcinoma. JAMA Netw Open. (2022) 5:e2216199. doi: 10.1001/jamanetworkopen.2022.16199
28. Kogure Y, Iwasawa S, Saka H, Hamamoto Y, Kada A, Hashimoto H, et al. Efficacy and safety of carboplatin with nab-paclitaxel versus docetaxel in older patients with squamous non-small-cell lung cancer (CAPITAL): a randomised, multicentre, open-label, phase 3 trial. Lancet Healthy Longev. (2021) 2:e791–800. doi: 10.1016/S2666-7568(21)00255-5
29. Yang X, Fu C. The potential feasibility of nab-paclitaxel as the first-line chemotherapy for ovarian cancer: clinical development and future perspectives. Arch Gynecol Obstet. (2022) 306:1417–29. doi: 10.1007/s00404-022-06425-3
30. Furlanetto J, Jackisch C, Untch M, Schneeweiss A, Schmatloch S, Aktas B, et al. Efficacy and safetyof nab-paclitaxel 125 mg/m2 and nab-paclitaxel 150 mg/m2 compared to paclitaxel in early high-risk breast cancer. Results from the neoadjuvant randomized GeparSepto study (GBG 69). Breast Cancer Res Treat. (2017) 163:495–506. doi: 10.1007/s10549-017-4200-1
Keywords: HER2-positive breast cancer, neoadjuvant treatment, albumin-bound paclitaxel, docetaxel, pathological complete response
Citation: Lyu Z and Gao L (2024) Pathological response and safety of albumin-bound paclitaxel as a neoadjuvant treatment for HER2-positive breast cancer compared to docetaxel combined with anti-HER2 therapy: a real-world study. Front. Oncol. 14:1412051. doi: 10.3389/fonc.2024.1412051
Received: 04 April 2024; Accepted: 05 August 2024;
Published: 21 August 2024.
Edited by:
Alessandro De Vita, Scientific Institute of Romagna for the Study and Treatment of Tumors (IRCCS), ItalyReviewed by:
Takeo Fujii, National Institutes of Health (NIH), United StatesCopyright © 2024 Lyu and Gao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhidong Lyu, emhpZG9uZ2x2QHFkdS5lZHUuY24=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.