- 1Huaihe Hospital of Henan University, Kaifeng, Henan, China
- 2Institute of Nursing and Health, Henan University, Kaifeng, Henan, China
With the development of gene testing technology, we have found many different genes, and lncRNA is one of them. LncRNAs refer to a non-protein coding RNA molecule with a length of more than 200bp, which is one of the focuses of research on human malignant diseases such as LUAD. LncRNAs act as an oncogene or inhibitor to regulate the occurrence and progression of tumors. The differential expression of LncRNAs promotes or inhibits the progression of lung adenocarcinoma by affecting cell proliferation, metastasis, invasion, and apoptosis, thus affecting the prognosis and survival rate of patients. Therefore, LncRNAs can be used as a potential target for diagnosis and treatment of cancer. The early diagnosis of the disease was made through the detection of tumor markers. Because lung adenocarcinoma is not easy to diagnose in the early stage and tumor markers are easy to ignore, LncRNAs play an important role in the diagnosis and treatment of lung adenocarcinoma. The main purpose of this article is to summarize the known effects of LncRNAs on lung adenocarcinoma, the effect of differential expression of LncRNAs on the progression of lung adenocarcinoma, and related signal transduction pathways. And to provide a new idea for the future research of lung adenocarcinoma-related LncRNAs.
1 Introduction
In the past, much of the non-protein-coding portion of the human genome has historically been regarded as useless DNA. Through examination, we found different classes of non-coding RNAs. Non-coding RNAs are broadly categorized based on their size: small non-coding RNAs represent noncoding transcripts less than 200 nucleotides in length, such as miRNAs, siRNAs, and piRNAs (1). Among the various types of non-protein-coding transcripts, a class referred to as long noncoding RNAs (lncRNAs) has attracted increasing attention. LncRNAs are defined as transcripts of more than 200 nucleotides that are not translated into proteins (2). LncRNAs are often master regulators of gene expression and exert their functions through transcriptional, post-transcriptional, and epigenetic gene-regulatory mechanisms (3). For the past few years, LncRNAs have become one of the hotspots of tumor research, and the dysfunction of LncRNAs is considered to be one of the main factors leading to a variety of diseases, including cancer, cardiovascular disease, fibrosis, obesity, etc. (4). LncRNAs are involved in oncogenes and tumor suppressor genes by regulating target genes or signal pathways at epigenetic, transcriptional and post-transcriptional levels (5).
Increasing studies demonstrated the involvement of thousands of LncRNAs in almost all life processes and played an indispensable regulatory role. Studies have found that there are two broad mechanisms by which LncRNAs exert their function. One mode of function involves LncRNA associations with promoters and regulatory regions of genes, where they recruit transcriptionally-activating or repressive protein complexes that are either directly involved in transcriptional initiation, or alternatively chromatin-remodeling complexes that once recruited by LncRNAs render chromatin transcriptionally repressive. Another mode of function involves LncRNAs that act by sequestration of regulatory factors. One example is that of “sponge” LncRNAs (ceRNAs) that bind microRNAs, thus protecting their target mRNAs from the effects of microRNAs. Since microRNAs typically repress translation or destabilize mRNAs, LncRNA sponge action typically increases protein production. A second example of sequestration-acting LncRNAs is that of decoy LncRNAs, which sequester gene regulatory proteins (2). Some LncRNAs were spliced into mRNA-like PolyA tail and promoter structures, which could be dynamically expressed and spliced in different ways during tissue and organ differentiation, thus showing time and space specificity.
Meantime, about LncRNA functions, many studies have found that LncRNAs have multiple biological functions: they are involved in chromatin modification, genomic imprinting, intranuclear transportation, and other regulations. Protecting protein-coding genes in multiple modes; containing highly efficient key sequences, and more sensitive structural and functional constraints than proteins; having conserved secondary structure, splicing form, and precise subcellular localization; having cis and trans regulation. The function of LncRNAs cannot be determined by its simple classification but is usually determined by its localization, sequence, and/or secondary structure.
In LncRNA expression, further development of genomic technologies has proved that LncRNAs are important in complex organisms by regulating chromatin remodeling and transcriptional regulation, etc., and participate in the pluripotency and differentiation of stem cells, which correlate to the progression of a variety of human diseases like cancer and neurological disorders. Different LncRNAs play different roles in cellular processes. Some LncRNAs participate in the imprinting process, affecting the single allelic expression of genes. Some LncRNAs perform epigenetic modification by introducing chromatin remodeling complexes into specific chromatin sites (6).
2 LUAD overview
Lung cancer is the second most common malignancy in the world and the death rate is increasing year by year (7, 8). Lung cancer is mainly divided into small cell (SCLC) and non-small cell (NSCLC). Approximately 80–85% of lung patients are diagnosed with non-small cell lung cancer (NSCLC) in pathological diagnosis, of which lung adenocarcinoma (LUAD) is the most common subtype (9, 10). Lung adenocarcinoma (LUAD) is the most common histologic type of lung cancer with a high mortality rate. LUAD is one of the deadliest cancers in the world. The main treatment for LUAD is surgery, but the prognosis is poor due to Histological heterogeneity (11). LUADs originate from cells that secrete surfactant components. Morphological patterns of LUADs include acinar, papillary, solid, micropapillary, and invasive mucinous types. Lepidic components or pure lepidic patterns are found in noninvasive forms, previously classified as bronchoalveolar carcinoma, which can be associated with invasive mucinous or acinar LUAD (12). As for clinicopathological features of lung cancer, the most common is smoking. However, LUAD can occur in both smokers and non-smokers. LUADs are the most common type of lung cancer seen in non-smokers. It is more common in women than in men and is more likely to occur in younger people than other types of lung cancer and to present at more advanced stages of the disease (13).
Early symptoms of lung cancer are relatively benign, and this contributes to low detection rates in early disease stages. And because the lung adenocarcinoma is not sensitive to the treatment of anticancer drugs, the drug resistance increases gradually, and the drug resistance is not obvious. Therefore, the metastatic rate of advanced lung cancer is high and the overall prognosis is poor (14). For the past few years, despite the advancement of the treatment for LUAD, it still has some limitations on the whole. The early diagnosis of LUAD is difficult, and it tends to relapse and metastasis, leading to poor prognosis for LUAD patients. Although great progress has been made in diagnosis and treatment recently, the long-term clinical prognosis and overall survival of lung adenocarcinoma (LUAD) patients remain unsatisfactory (15). The inadequate comprehension of LUAD-related biological mechanisms limits the improvement in therapeutic efficacies. Thus, it is urgent to further elucidate the involved mechanisms of cancer progression to improve the clinical outcome of LUAD patients (16).
3 The mechanism of LncRNAs in LUAD
With the development of next-generation sequencing technology, the in-depth study of non-coding genes has attracted more and more attention from researchers. Many studies have shown that the treatment of tumors is related to LncRNAs. Aberrantly expressed LncRNAs play pivotal roles in the progression of LUAD. In the meantime, the aberrant expression and mutation patterns are tightly related to tumor proliferation, apoptosis, invasion, metastasis, TNM stage, drug resistance, lymphatic metastasis, and shorter prognosis. For example, Zhang et al (17) found that the up-regulate of CRNDE was significantly correlated with poor differentiation, TNM stage, lymph node metastasis, radiotherapy response, and significantly shorter overall survival. In addition to the abnormal expression of LncRNAs has an impact on lung adenocarcinoma, some LncRNAs can also act as oncogenes to interact with miRNA, thus acting with certain proteins to affect the function of lung adenocarcinoma cells. MicroRNAs (miRNAs) are conserved, non-coding short RNA molecules. MiRNAs can regulate numerous cellular functions such as cell growth, migration, invasion, and EMT (18). Significant evidence shows that abnormal regulation of miRNAs is related to the malignant progression of many cancers, and miRNAs are potential therapeutic targets for lung cancer (19). Yang et al (14) found that LncRNA PCAT6 can act as a molecular sponge for miR-545-3p and that this promotes LUAD development.
4 The differential expression of lncRNA in LUAD
Long non-coding RNAs (LncRNAs) are a kind of transcriptional RNA containing over 200 nucleotides, which are encoded by the genome and lack protein-coding function. It is related to a variety of cellular processes, including the regulation of gene expression (including epigenetics, pre-transcription, and post-transcription), tissue metabolism, cell growth, differentiation, and development (20). The abnormal expression of LncRNAs is significant in multiple cancers.
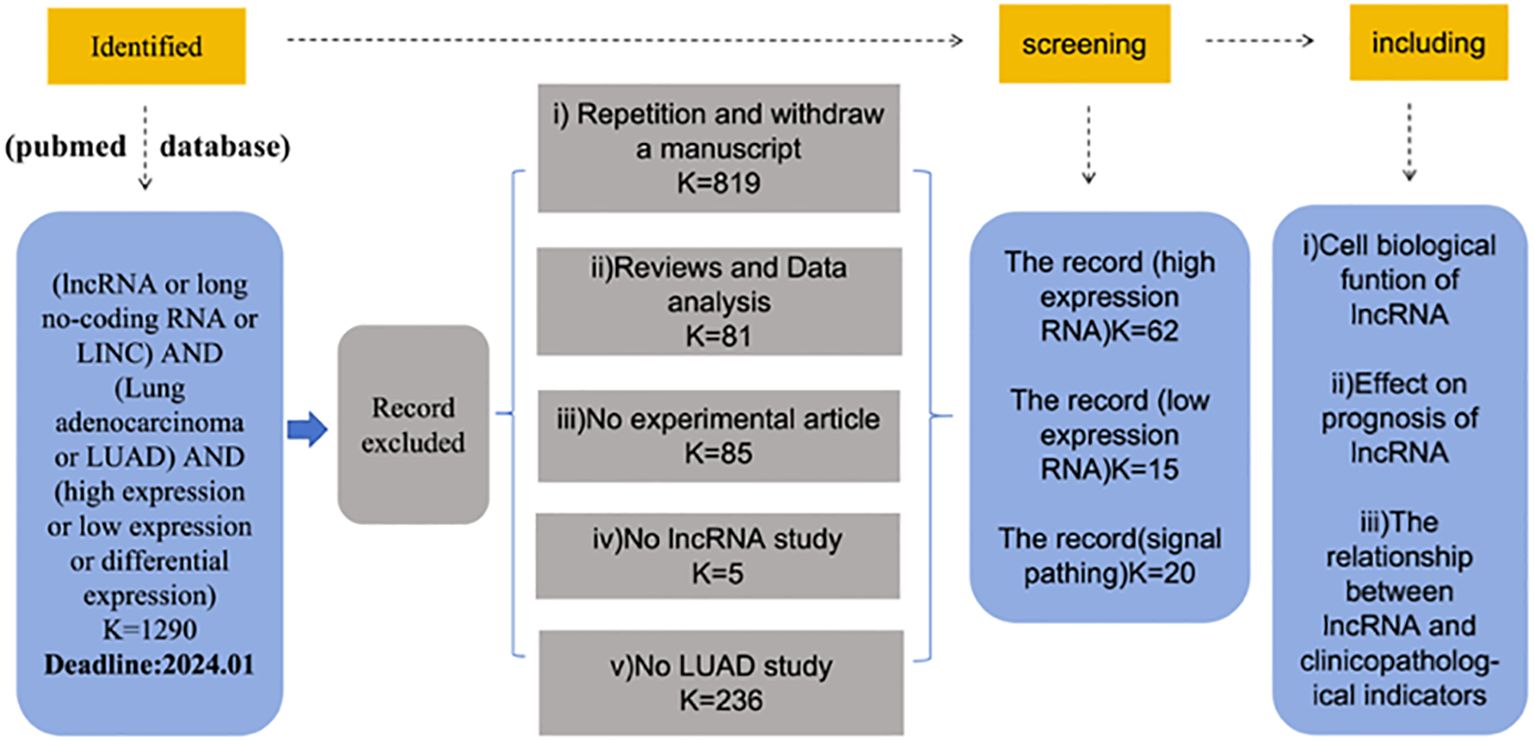
To distinguish the published LncRNAs biomarkers for diagnosis or prognosis in lung adenocarcinoma patients, entry terms “(LncRNA OR long no-coding RNA) AND (lung adenocarcinoma OR LUAD) AND (high expression OR low expression)” were firstly seek in the NCBI PubMed database. Finally,62 up-regulated LncRNAs and 15 down-regulated LncRNAs in lung adenocarcinoma were identified from these studies, as shown in Figure 1.
4.1 High-level expressed lncRNA in LUAD and clinical significance
During the data retrieval, we found that 62 articles demonstrated the up-regulation of LncRNAs. When analyzing these data, we found that the up-regulation of LncRNAs is often related to the poor prognosis of lung adenocarcinoma, but also affects the function of lung adenocarcinoma cells. At the same time, we also found that the clinicopathology of most LncRNAs is related to tumor size, TNM stage, and lymph node metastasis. The data are shown in Table 1.
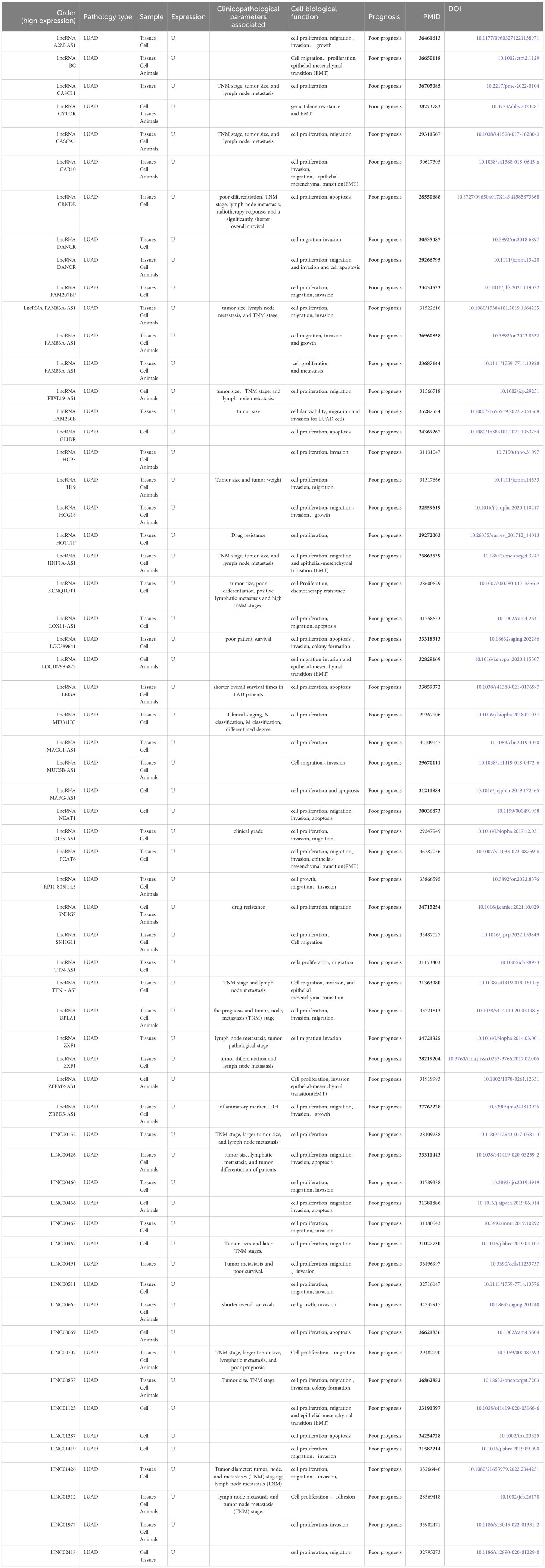
Table 1 Biological function and clinical significance of up-regulated lncRNA in lung adenocarcinoma.
4.2 Low-level expressed lncRNA in LUAD and clinical significance
We also analyzed the down-regulation of LncRNAs. In the retrieved data, we found that most of the down-regulation was related to the poor prognosis of patients with lung adenocarcinoma, but the down-regulation of LncRNAs suggested a better prognosis. For example, Liu et al. (21) found that LncRNA SGMS1-AS1 was down-regulated in LUAD tissue as well as cells, which was related to good prognosis of patients with lung adenocarcinoma. The same down-regulation has an effect on cell function. The data are shown in Table 2.
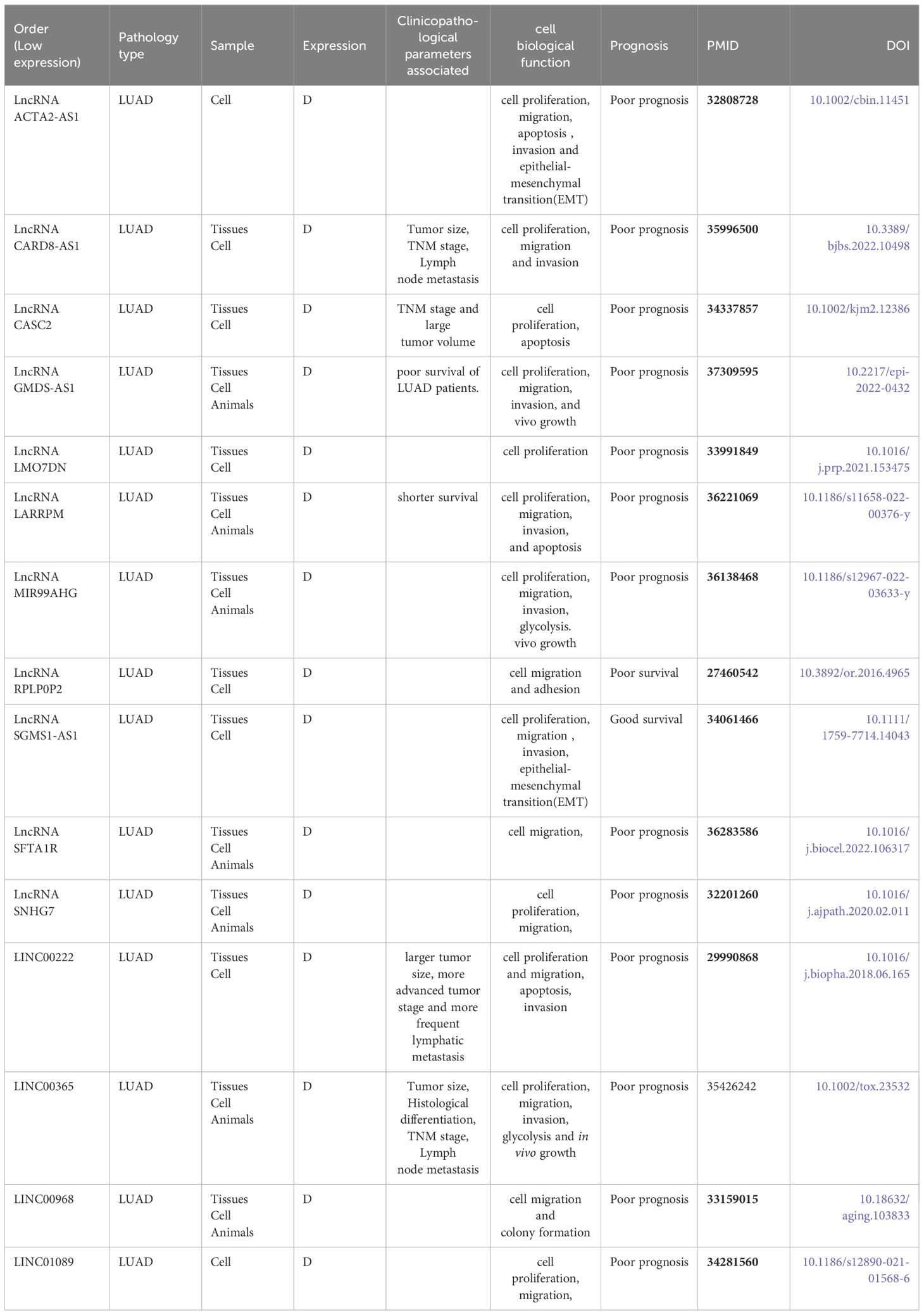
Table 2 Biological function and clinical significance of down-regulated lncRNA in lung adenocarcinoma.
4.3 High-level expression and low-level expressed LncRNA in LUAD and clinical significance of LncRNA
In the summary, we found that a LncRNA is expressed differently through different pathways in lung adenocarcinoma. It can be either high expression or low expression. However, both high and low expression lead to poor prognosis in patients with lung adenocarcinoma. This lncRNA is SNHG7. Pei et al. (22) found that LncRNA SNHG7 was found to be down-regulated in LUAD tissues compared with normal tissues. And it is related to the poor prognosis of patients. Zhang et al. (23) found that LncRNA SNHG7 was found to be the high-level expression in LUAD. It is related to the poor prognosis of patients and the enhancement of paclitaxel resistance.
5 The regulation of signaling pathway that lncRNA participated in LUAD
A large number of studies have proved that LncRNAs are an important regulator of gene regulation, and they can play a key role in a variety of biological functions and disease processes. The LncRNA-mediated gene expression involves various mechanisms, such as regulation of transcription, translation, protein modification, and the formation of RNA-protein or protein-protein complexes (24). Cellular signal transduction plays a key role in various cellular processes that respond to intracellular or extracellular stimuli. Many signaling pathways have been found in cells, which are usually essential for cascading biological responses and gene expression (24). Most studies have shown that LncRNAs are involved in various pathways, and the common signal pathways are P53, Notch, JATT, STAT, PI3K, Akt, Wnt/β-catenin (beta-catenin), and so on.
5.1 JAK/STAT signaling pathway
Han et al (25) found that LncRNA ZFPM2-AS1 negative regulation of ZFPM2 expression and positive regulation of PD-L1 expression via the JAK-STAT and AKT pathways. Xiao et al. (26)found that LncRNA LOC389641 is a potential oncogene that acts by influencing MET, EGFR, and STAT3, thereby regulating multiple cell survival/death signals including cell proliferation, colony formation, cell invasion, apoptosis, and autophagy. Wu et al. (27)found that LncRNA LEISA promotes the progression of lung adenocarcinoma via enhancing interaction between STAT3 and IL-6 promoters, as shown in Figure 2.
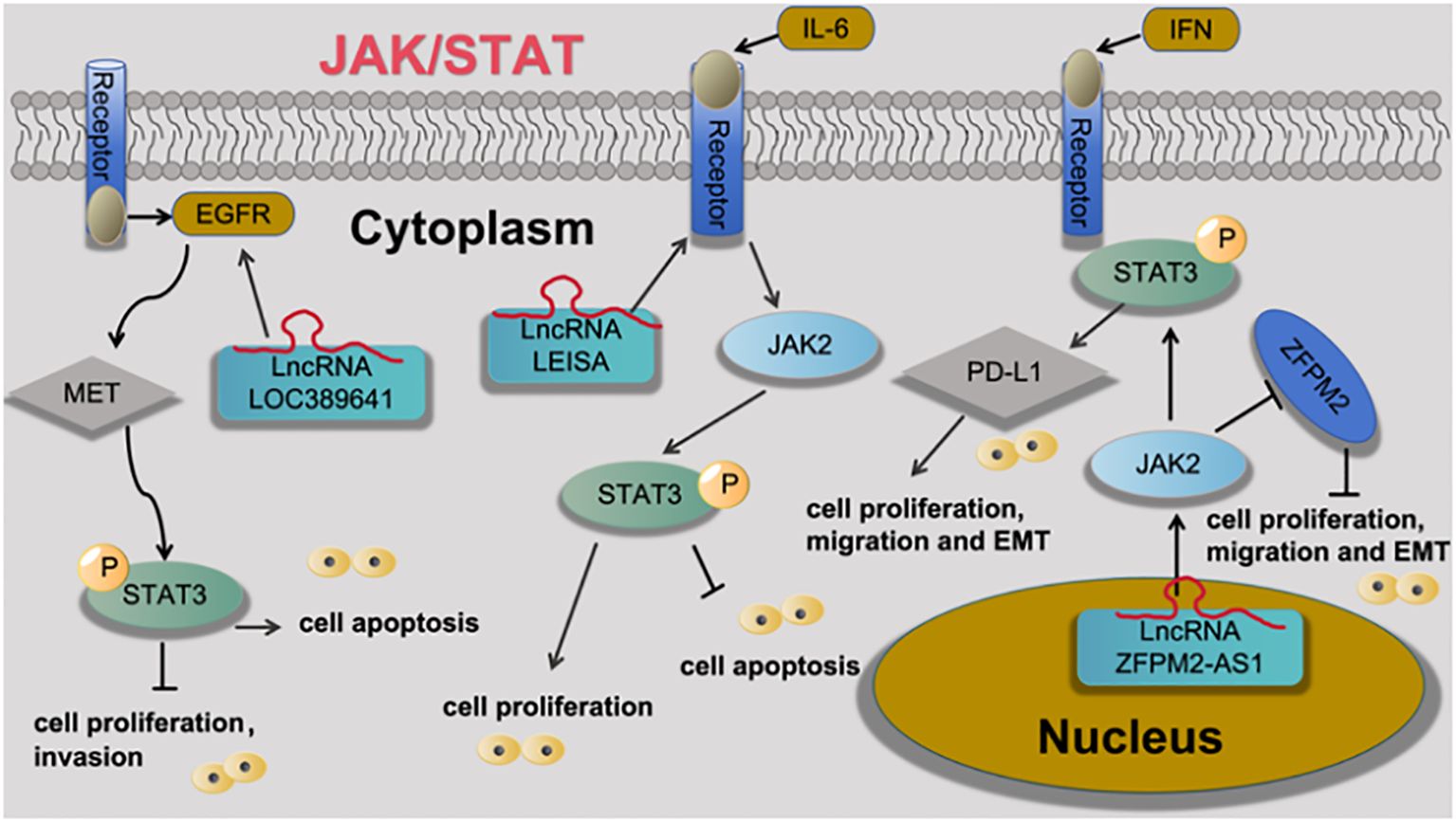
Figure 2 LncRNA is involved in the processes of lung adenocarcinoma cell proliferation, apoptosis, metastasis, invasion and EMT through the regulation of JAK/STAT signaling pathway.
5.2 PI3K/Akt/mTOR signaling pathway
Zhang et al. (23) found that LncRNA SNHG7 activates the PI3K/AKT pathway through CUL4A and PTEN, thus enhancing the resistance of LUAD cells to docetaxel resistance. Qin et al. (28) found that LncRNA MIR31HG expression increases gefitinib resistance in non-small cell lung cancer cell lines through the EGFR/PI3K/AKT signaling pathway. Zhang et al. (29) found that overexpression of LncRNA HOTTIP promotes proliferation and drug resistance of lung adenocarcinoma by regulating the AKT signaling pathway. Lu et al. (30) found that LncRNA DANCR contributes to lung adenocarcinoma progression by sponging miR-496 to modulate mTOR expression. as shown in Figure 3.
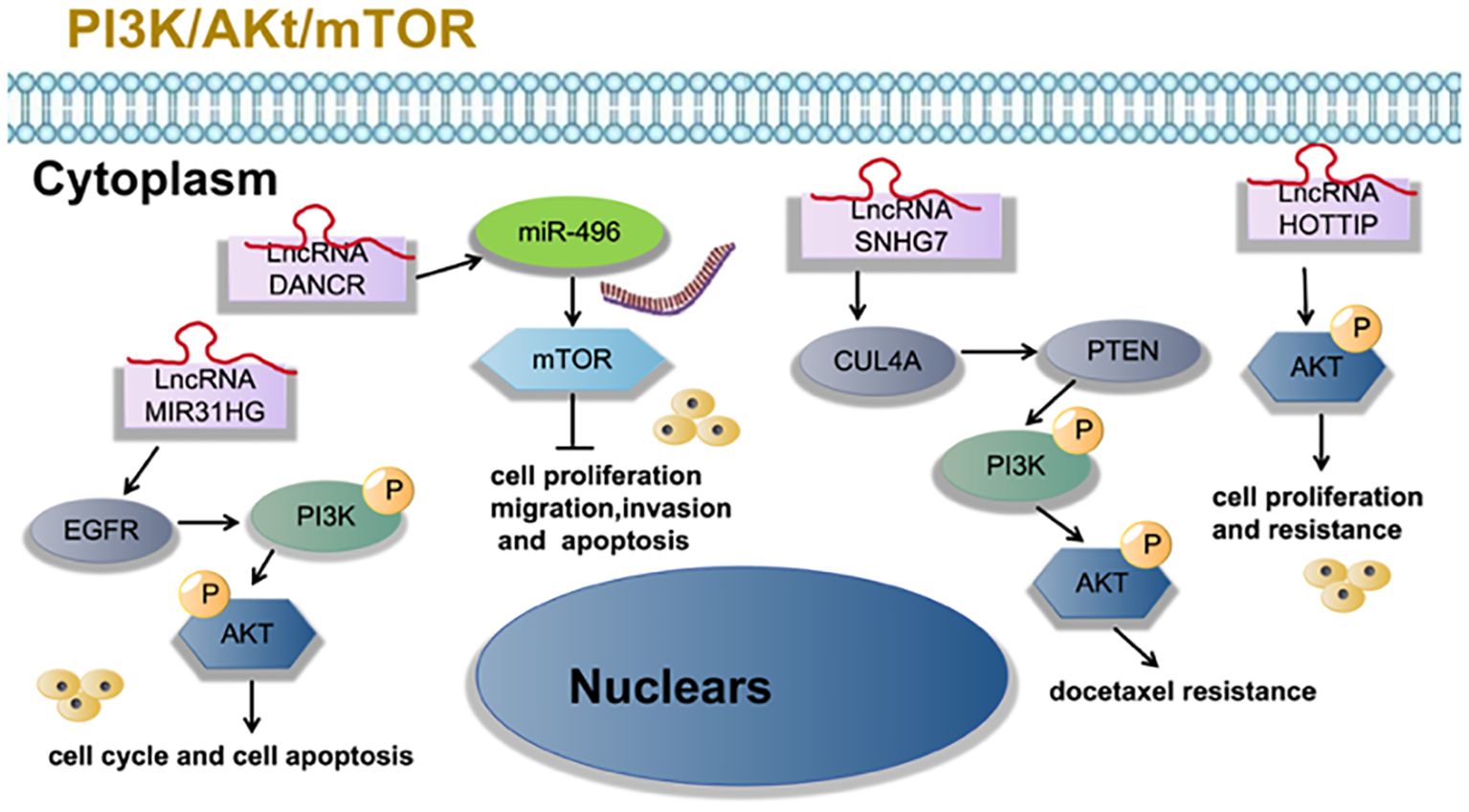
Figure 3 LncRNA is involved in tumor cell proliferation, migration, invasion, apoptosis and other processes of lung adenocarcinoma through PI3K/Akt/mTOR signaling pathway.
5.3 Wnt/β-catenin (beta-catenin) signaling pathway
Han et al. (31) found that LncRNA UPLA1 was mainly located in the nucleus using FISH assay and that it promoted Wnt/β-catenin signaling by binding to desmoplakin using RNA pulldown assay and mass spectrometry. Pei et al. (22) found that LncRNA SNHG7 interacted with microRNA mir-181 and sequentially up-regulated cbx7. cbx7, which suppresses the Wnt/β-catenin pathway in LUAD, was found to be a direct target of mir-181. Yang et al. (32) found that STAT1-induced upregulation of LINC00467 promoted LUAD progression by epigenetically silencing DKK1 to activate the Wnt/β-catenin signaling pathway. Wan et al. (33) found that LINC00491 promotes MTSS1 degradation via the ubiquitin-proteasome degradation through the Wnt/β-Catenin-signaling pathway, and promotes LUAD proliferation and metastasis. Zhu et al. (34) found that LINC00669 promotes lung adenocarcinoma growth by stimulating the Wnt/β-catenin signaling pathway, as shown in Figure 4.
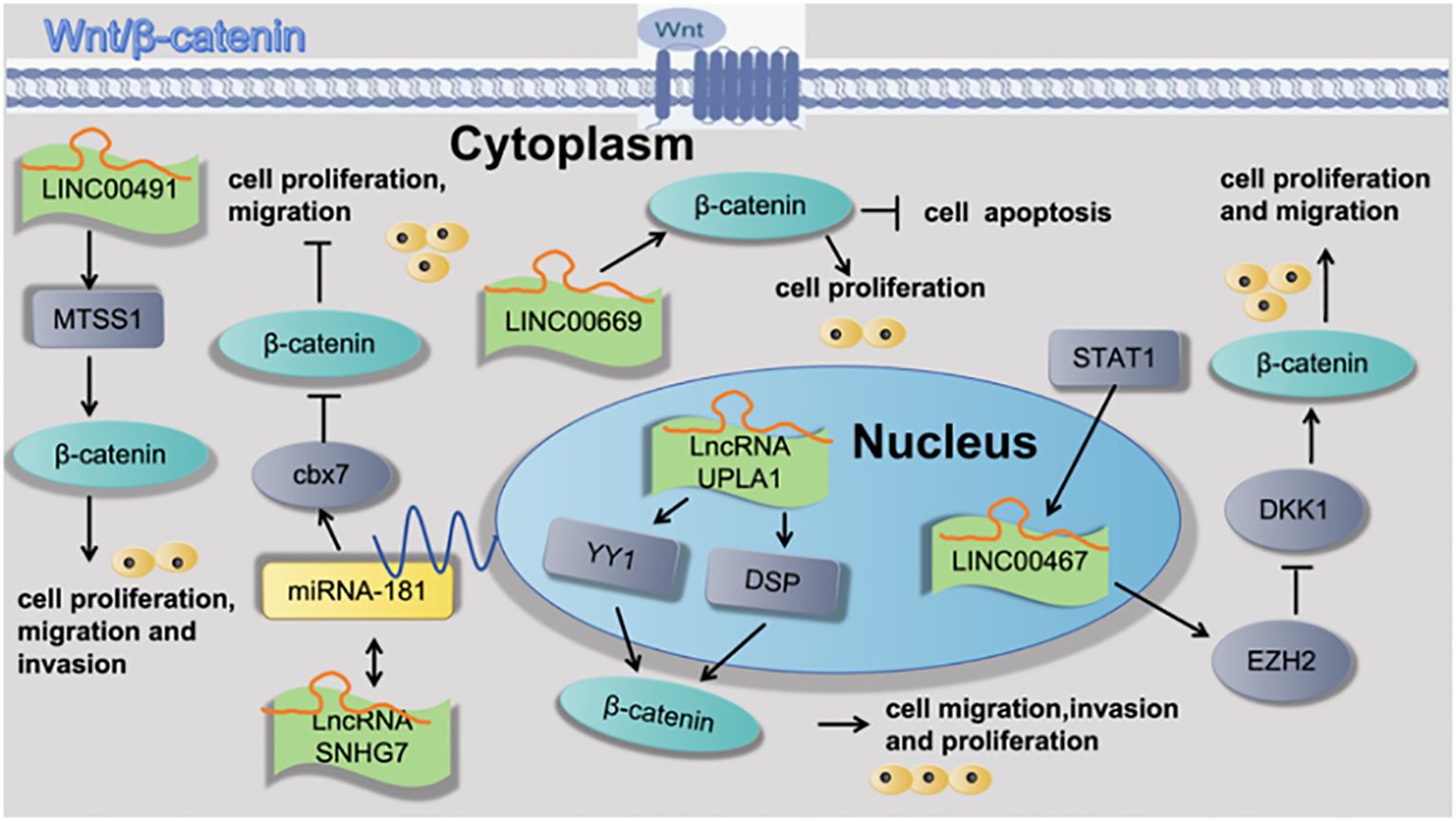
Figure 4 Cell functions of lncRNAs is regulated through the Wnt/β-catenin signaling pathway attributed to the pathogenesis of lung adenocarcinoma.
5.4 P53 signaling pathway
Wu et al. (35) found that LncRNA CASC2/miR-21/p53 forms a positive feedback loop to mediate cell proliferation and apoptosis in LUAD, which may provide new insight into the pathological mechanisms of LUAD. As shown in Figure 5.
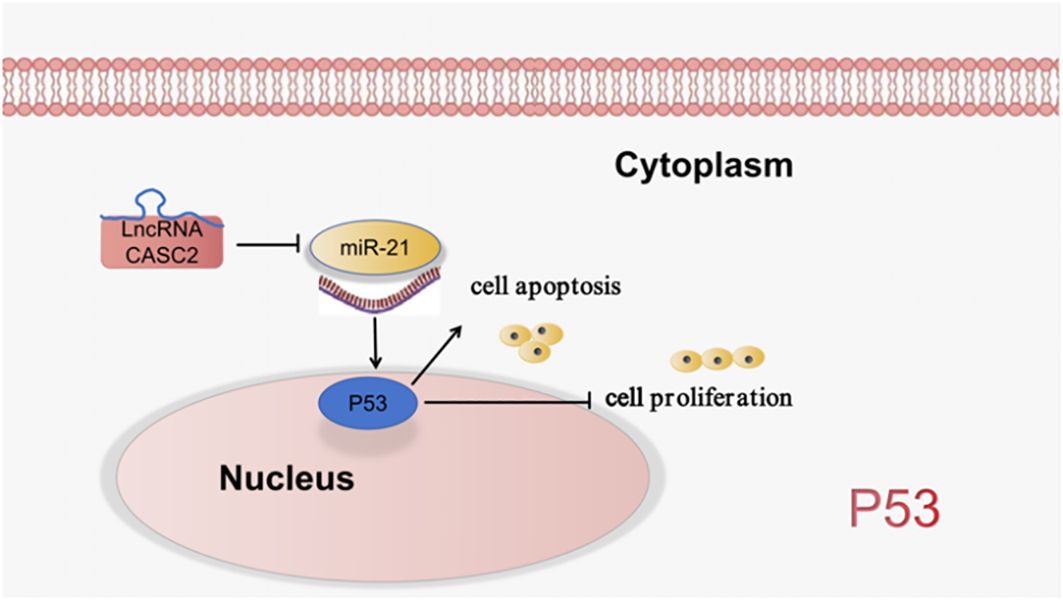
Figure 5 LncRNA is involved in the processes of lung cancer cell proliferation, apoptosis through the regulation of P53 signaling pathway.
5.5 TGF-3/SMAD signaling pathway
Wang et al. (36) found that LncRNA MIR99AHG acted as a sponge of miR-136-5p to inhibit miR-136-5p expression, upregulated the expression of USP4, and enhanced the stability of ACE2 protein, thereby inhibiting EMT in pulmonary fibrosis. Jiang et al. (37) found that LncRNA HCP5 is induced by TGF-β and is directly regulated by SMAD3, which, in turn, is a positive regulator of the TGF-β/SMAD signaling via the miR-203/SNAI axis. This study demonstrates that overexpression of HCP5 in LUAD is important for promoting EMT and metastasis and may be a potential therapeutic target for LUAD treatment (37). Zhang et al. (38) found that LINC01977, a cancer-testis LncRNA, was hijacked by SE, which promoted proliferation and invasion both in vitro and in vivo. Activating SMAD3 to bind the promoter and the SE of LINC01977, which up-regulated LINC01977 expression. LINC01977 also promoted malignancy via the canonical TGF-β/SMAD3 pathway. as shown in Figure 6.

Figure 6 LncRNA regulates tumor motility, invasiveness and other functions of LUAD cells via regulation the TGF-3/SMAD signaling pathway.
5.6 Notch signaling pathway
Guo et al. (39) found that LncRNA LOC107985872 promotes lung adenocarcinoma progression via the notch1 signaling pathway with exposure to traffic- originated PM2.5 organic extract. Zhang et al. (40) found that ZEB1-induced LINC01123 mediates LUAD development via miR-449b- 5p/NOTCH1 axis-activated NOTCH1 signaling. Deng et al. (41) found that LncRNA SNHG11 promotes malignant behaviors of LUAD cells by sponging miR-193a-5p and elevating Notch3 expression. as shown in Figure 7.
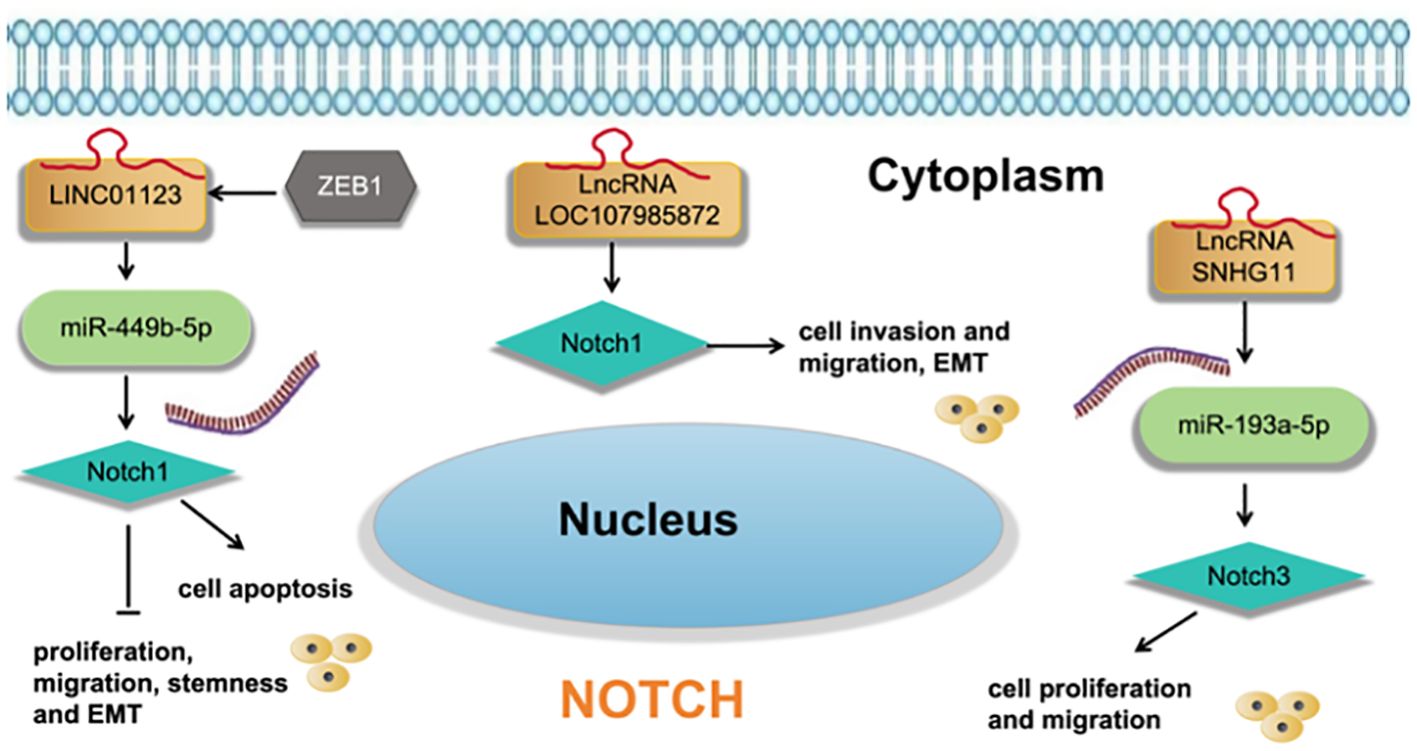
Figure 7 LncRNA regulates cell functions and EMT of LUAD cells via regulation the Notch signaling pathway.
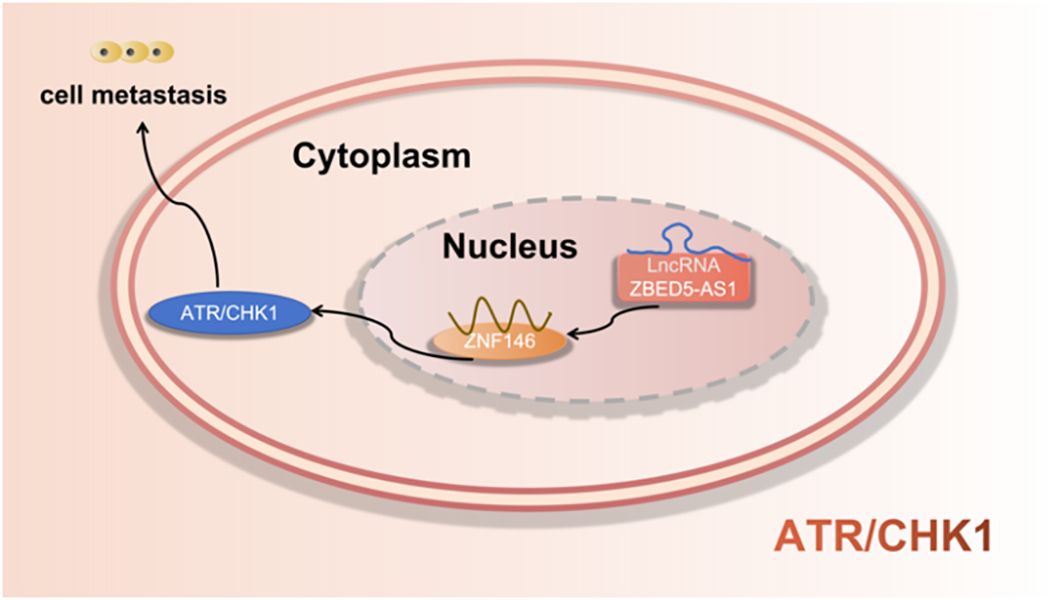
5.7 ATR/CHK1 signaling pathway
Jiang et al. (42) found that LncRNA ZBED5-AS1 enhanced the metastatic potential of LUAD cells through the EMT pathway by activating ZNF146/ATR/CHK1 axis. as shown in Figure 8.
5.8 miRNA signaling axis
5.8.1 miRNA-3p
Cheng et al. (43) found that LINC01419 promotes cell proliferation and metastasis in lung adenocarcinoma via sponging miR-519b-3p to up-regulate RCCD1. Wang et al. (44) found that LncRNA FBXL19-AS1 induces tumor growth and metastasis by sponging miR-203a-3p in lung adenocarcinoma. Yang et al. (45) found that LncRNA PCAT6 promotes proliferation, migration, invasion, and epithelial-mesenchymal transition of lung adenocarcinoma cells by targeting miR-545-3p. Zhong et al. (46) found that LncRNA TTN-AS1 drives the invasion and migration of lung adenocarcinoma cells via modulation of miR-4677-3p/ZEB1 axis. Wang et al. (47) found that LINC02418 promotes malignant behaviors in lung adenocarcinoma cells by sponging miR-4677-3p to upregulate KNL1 expression. Qian et al. (48) found that LINC01089 suppresses lung adenocarcinoma cell proliferation and migration via miR-301b-3p/STARD13 axis. Xiong et al. (49) found that LncRNA NEAT1 may compete with USF1 for binding to miR-193a-3p as a ceRNA and accelerates lung adenocarcinoma deterioration. Ying et al. (50) found that LncRNA ACTA2-AS1 suppresses the malignant processes of LUAD cells by sequestering miR-378a-3p and miR-4428 to augment SOX7 expression. Xiong et al. (51) found that LncRNA FAM83A-AS1 promoted cell migration, invasion, and growth of LUAD cancer cells. Mechanistically, FAM83A-AS1 sponged miR-202-3p to regulate the expression of hexokinase II (HK2) in post-transcription, which facilitated the malignancy and glycolysis. as shown in Figure 9.
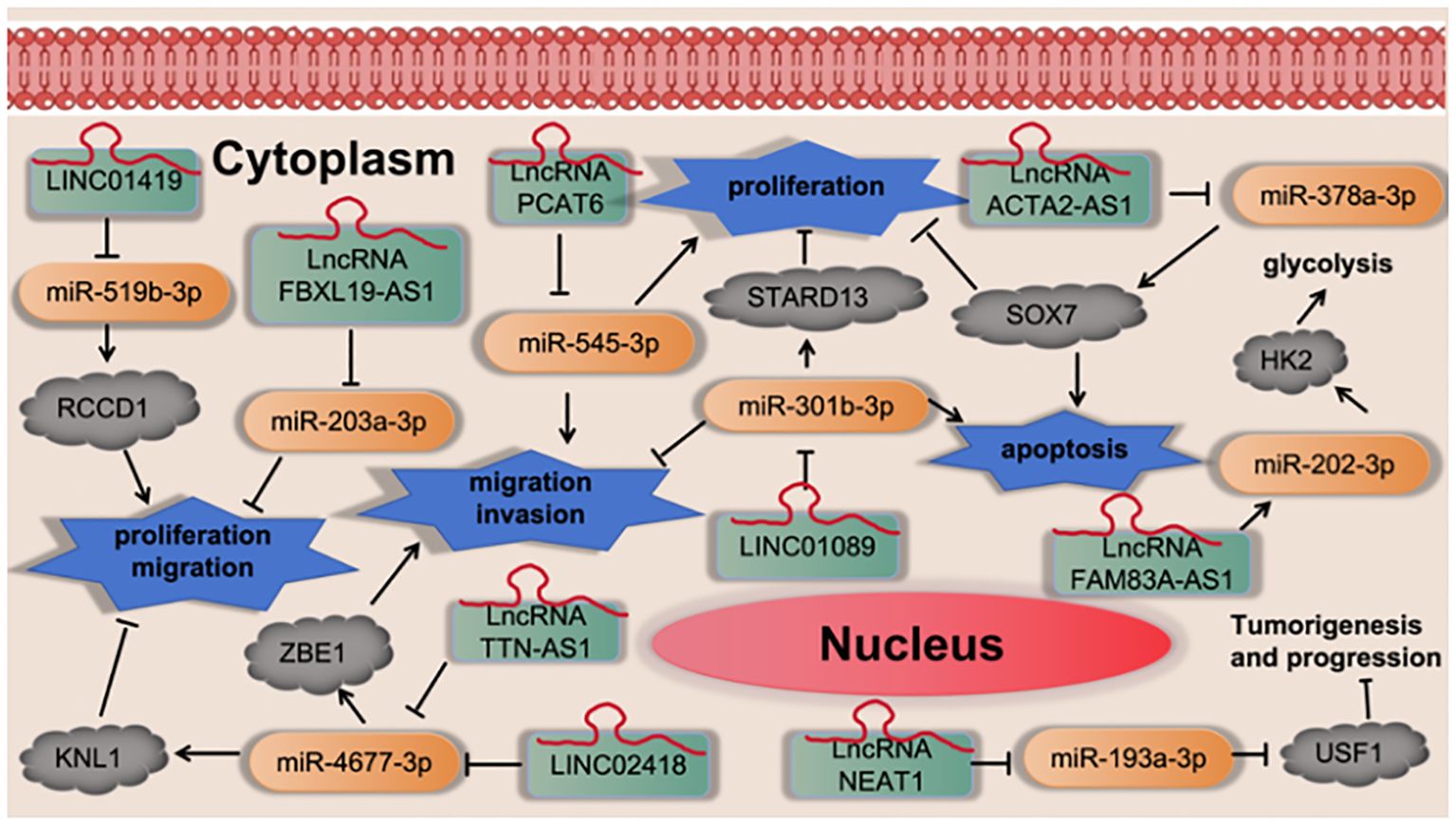
Figure 9 LncRNA regulates cell proliferation, migration, invasion and apoptosis via regulation the miRNA-3p signaling axis.
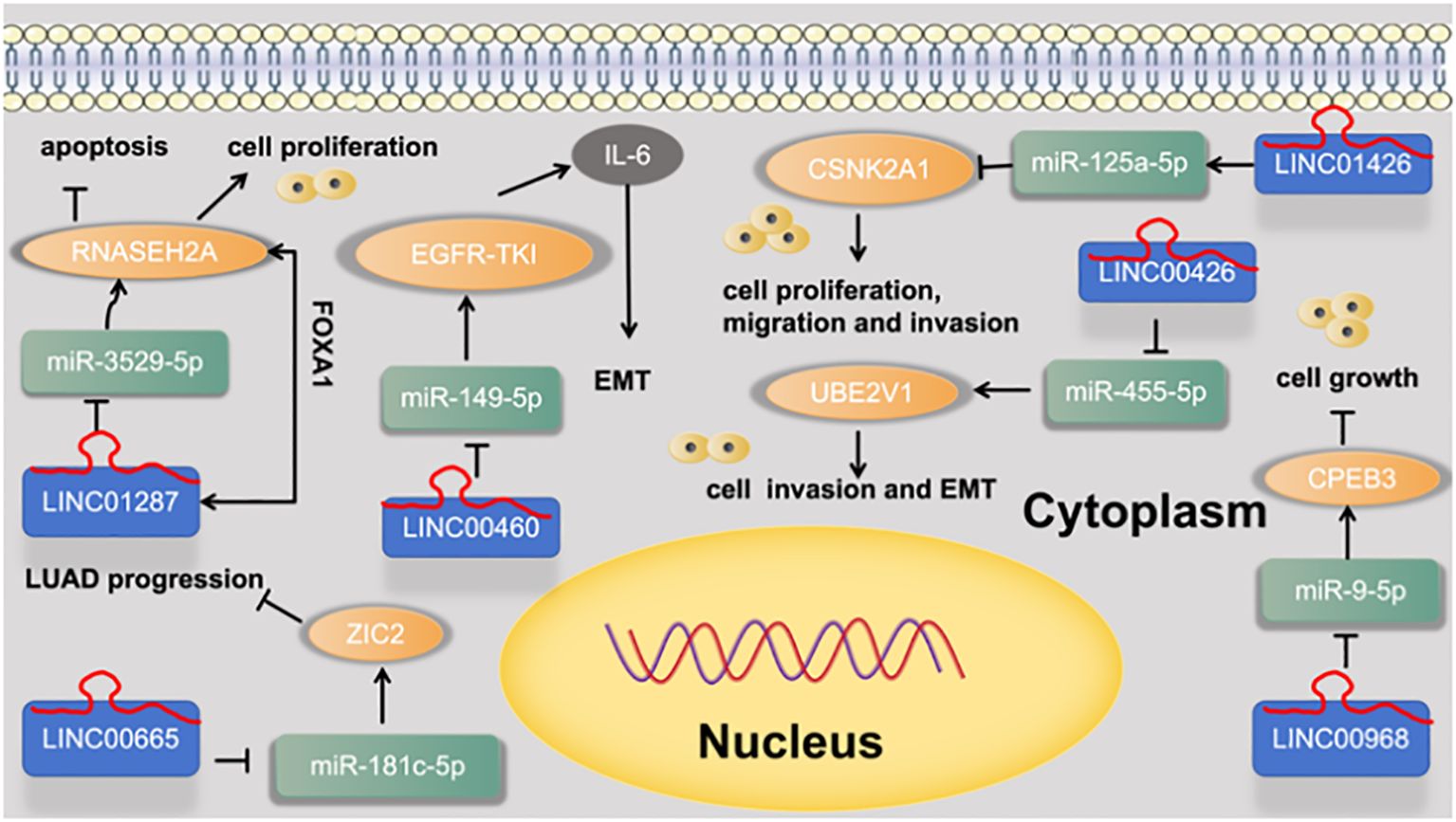
5.8.2 miRNA-5p of LINC
Zhang et al. (52) found that FOXA1-induced LINC01287 serves as a competing endogenous RNA to promote proliferation and inhibit apoptosis of LUAD cells via upregulation of RNASEH2A expression at the posttranscriptional level by competitively combining with miR-3529-5p. Wei et al. (53) found that silencing of LINC00665 suppresses LUAD progression by targeting miR-181c-5p/ZIC2 axis. Yuta Nakano et al. (54) found that LINC00460 was identified as a novel marker of a poor response to EGFR-TKI and prognosis. In lung cancer cells, LINC00460 promoted EGFR-TKI resistance as a competitive decoy for miR-149-5p, thereby facilitating interleukin (IL)-6 expression and inducing EMT-like phenotypes. Li et al. (55) found that LINC00426 accelerates LUAD progression by acting as a molecular sponge to regulate miR-455-5p. Zhu et al. (56) found that LINC01426 promotes the progression of lung adenocarcinoma via regulating miRNA-125a-5p/casein kinase 2 alpha 1 axis. Tang et al. (57) found that LINC00968 inhibits the tumorigenesis and metastasis of lung adenocarcinoma via serving as a ceRNA against miR-9-5p and increasing CPEB3. as shown in Figure 10.
5.8.3 miRNA-5p of LncRNA
Xiao et al. (58) found that LncRNA FAM83A-AS1 promotes lung adenocarcinoma cell migration and invasion by targeting miR-150-5p and modifying MMP14. Li et al. (59) found that miR-34a-5p could directly bind to LncRNA HCG18 and miR-34a-5p could directly bind to HCG18. At last, rescue assays proved the carcinogenic role of HCG18/miR-34a-5p/HMMR axis in LUAD cell growth. Liu et al. (21) found that LncRNA SGMS1-AS1 regulates lung adenocarcinoma cell proliferation, migration, invasion, and EMT progression via miR-106a-5p/MYLI9 axis. Jia et al. (60) found that LncRNA TTN-AS1 promotes migration, invasion, and epithelial- mesenchymal transition of lung adenocarcinoma via sponging miR-142-5p to regulate CDK5. Zhang et al. (61) found that LncRNA SFTA1P activates hypoxic exosome-delivered miR-4766-5p through modulating LATS1/YAP pathway, thereby suppressing LUAD cell metastasis, which may serve as a suitable target for the LUAD therapy. Sui et al. (62) found that LncRNA MAFG-AS1 served as a molecular sponge of miR-744-5p to upregulate its nearby gene MAFbZIP transcription factor G (MAFG) in LUAD cells. Finally, LncRNA MAFG-AS1 boosts the proliferation of lung adenocarcinoma cells via regulating miR-744-5p/MAFG axis. Cao et al. (63) found that LncRNA CYTOR upregulates ANLN and RRM2 proteins by inhibiting miR-125a-5p, thus promoting gemcitabine resistance and EMT in lung adenocarcinoma. Li et al. (64) found that LncRNA LOXL1‐AS1 functioned as an oncogene in LUAD, and regulated LUAD development through sponging miR‐423‐5p and targeting MYBL2. as shown in Figure 11.
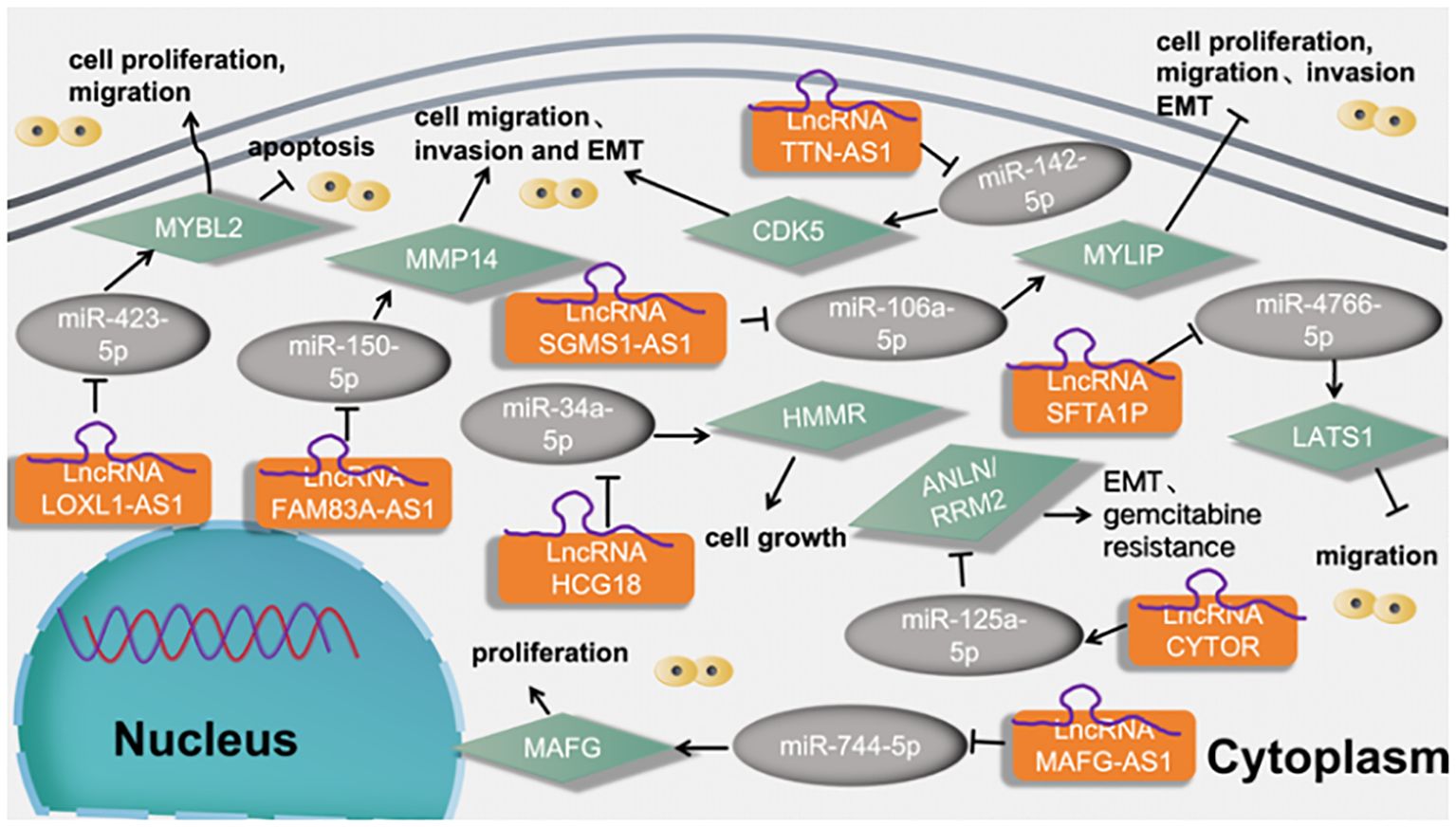
Figure 11 LncRNAs regulates tumor motility and functions of LUAD cells via regulation the miRNA-5p signaling axis.
6 Conclusions
Lung cancer ranks second among health problems and is the major cause of cancer-associated mortality worldwide. LUAD is considered to be the most common subtype in nonsmokers. The prevalence of LUAD is rapidly increasing with the development of anti-smoking movements. Although headway has been made in cancer treatment, the overall survival of LUAD remains disappointing due to a lack of reliable early prognostic indicators (65). LncRNAs, over the last decade, have been recognized as a diverse class of macromolecules in terms of function and mechanism. Many LncRNAs have demonstrated functional activity in a wide range of cancers. While the LncRNAs transcriptome is far from complete, we now have an appreciation of their diversity due to cell-type specificity. This characteristic can aid in the development of early detection methods and targeted therapies for multiple cancer types. Alongside these immediate applications, understanding the mechanism(s) by which these transcripts are regulated will shed light on the etiology of cancer development, allowing clinicians to implement better treatment strategies and improve overall survival rates. Given the effect of differential expression of LncRNAs on the progression of lung adenocarcinoma, we looked up a large number of literature and analyzed it. We found that the high and low expression of LncRNAs can promote or inhibit lung adenocarcinoma cells, mainly affecting tumor proliferation, migration, apoptosis, and invasion. At the same time, for the treatment of anticancer drugs, LncRNAs will affect the drug resistance of patients with lung adenocarcinoma to anticancer drugs. At present, lung adenocarcinoma is one of the major cancers threatening the lives of Chinese people. We need to pay a lot of attention to the study of lung adenocarcinoma to improve the prognosis and survival rate of patients. It has important clinical significance for the detection of tumor markers of lung adenocarcinoma and the improvement of early detection, early diagnosis, and early treatment of patients with lung adenocarcinoma. The main purpose of this paper is to summarize the effects of LncRNAs on the occurrence and development of lung adenocarcinoma and to provide a new idea for future research on lung adenocarcinoma-related LncRNAs. About the study of LncRNAs in lung adenocarcinoma, there are still many deficiencies in our research, which will be further studied in the future.
Author contributions
HW: Conceptualization, Writing – review & editing, Funding acquisition. SZ: Writing – original draft. XL: Data curation, Formal analysis, Writing – review & editing. RF: Data curation, Formal analysis, Writing – review & editing. LL: Conceptualization, Formal analysis, Writing – review & editing, Funding acquisition.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work is supported by the science and technology research project of Henan Provincial Science and Technology Department. Grant numbers: 242102310280; 242102310100.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. McCabe EM, Rasmussen TP. lncRNA involvement in cancer stem cell function and epithelial-mesenchymal transitions. Semin Cancer Biol. (2021) 75:38–48. doi: 10.1016/j.semcancer.2020.12.012
2. Kopp F, Mendell JT. Functional classification and experimental dissection of long noncoding RNAs. Cell. (2018) 172:393–407. doi: 10.1016/j.cell.2018.01.011
3. Castro-Oropeza R, Melendez-Zajgla J, Maldonado V, Vazquez-Santillan K. The emerging role of lncRNAs in the regulation of cancer stem cells. Cell Oncol Dordr. (2018) 41:585–603. doi: 10.1007/s13402-018-0406-4
4. Li C, Liang G, Yang S, Sui J, Wu W, Xu S, et al. LncRNA-LOC101928316 contributes to gastric cancer progression through regulating PI3K-Akt-mTOR signaling pathway. Cancer Med. (2019) 8:4428–40. doi: 10.1002/cam4.2165
5. Minami A, Ogino M, Nakano N, Ichimura M, Nakanishi A, Murai T, et al. Roles of oncogenes and tumor-suppressor genes in osteoclastogenesis (Review). Int J Mol Med. (2017) 39:261–7. doi: 10.3892/ijmm.2017.2847
6. Nguyen H, Sokpor G, Pham L, Rosenbusch J, Stoykova A, Staiger JF, et al. Epigenetic regulation by BAF (mSWI/SNF) chromatin remodeling complexes is indispensable for embryonic development. Cell Cycle Georget Tex. (2016) 15:1317–24. doi: 10.1080/15384101.2016.1160984
7. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2021) 71:209–49. doi: 10.3322/caac.21660
8. Cortes J, Perez-García JM, Llombart-Cussac A, Curigliano G, El Saghir NS, Cardoso F, et al. Enhancing global access to cancer medicines. CA Cancer J Clin. (2020) 70:105–24. doi: 10.3322/caac.21597
9. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2021. CA Cancer J Clin. (2021) 71:7–33. doi: 10.3322/caac.21654
10. Bai Y, Xiong L, Zhu M, Yang Z, Zhao J, Tang H. Co-expression network analysis identified KIF2C in association with progression and prognosis in lung adenocarcinoma. Cancer biomark Sect Dis Markers. (2019) 24:371–82. doi: 10.3233/CBM-181512
11. Ding Q, Chen D, Wang W, Chen Y. [Progress in research on the cribriform component in lung adenocarcinoma]. Zhongguo Fei Ai Za Zhi Chin J Lung Cancer. (2020) 23:621–5. doi: 10.3779/j.issn.1009-3419.2020.101.19
13. de Groot P, Munden RF. Lung cancer epidemiology, risk factors, and prevention. Radiol Clin North Am. (2012) 50:863–76. doi: 10.1016/j.rcl.2012.06.006
14. LncRNA PCAT6 promotes proliferation, migration, invasion, and epithelial-mesenchymal transition of lung adenocarcinoma cell by targeting miR-545-3p. PubMed (Accessed February 25, 2024).
15. BIN1 reverses PD-L1-mediated immune escape by inactivating the c-MYC and EGFR/MAPK signaling pathways in non-small cell lung cancer. PubMed (Accessed February 25, 2024).
16. LncRNA TTN-AS1 promotes migration, invasion, and epithelial mesenchymal transition of lung adenocarcinoma via sponging miR-142-5p to regulate CDK5. PubMed (Accessed February 25, 2024).
17. Zhang M, Gao C, Yang Y, Li G, Dong J, Ai Y, et al. Long noncoding RNA CRNDE/PRC2 participated in the radiotherapy resistance of human lung adenocarcinoma through targeting p21 expression. Oncol Res. (2018) 26:1245–55. doi: 10.3727/096504017X14944585873668
19. Exploring miRNA based approaches in cancer diagnostics and therapeutics. - Search Results . PubMed (Accessed February 25, 2024).
20. Overexpression of Long Noncoding RNA Uc.187 Induces Preeclampsia-Like Symptoms in Pregnancy Rats. PubMed (Accessed February 25, 2024).
21. Liu T, Yang C, Wang W, Liu C. LncRNA SGMS1-AS1 regulates lung adenocarcinoma cell proliferation, migration, invasion, and EMT progression via miR-106a-5p/MYLI9 axis. Thorac Cancer. (2021) 12:2104–12. doi: 10.1111/1759-7714.14043
22. Pei Y-F, He Y, Hu L-Z, Zhou B, Xu H-Y, Liu X-Q. The crosstalk between lncRNA-SNHG7/miRNA-181/cbx7 modulates Malignant character in lung adenocarcinoma. Am J Pathol. (2020) 190:1343–54. doi: 10.1016/j.ajpath.2020.02.011
23. Zhang K, Chen J, Li C, Yuan Y, Fang S, Liu W, et al. Exosome-mediated transfer of SNHG7 enhances docetaxel resistance in lung adenocarcinoma. Cancer Lett. (2022) 526:142–54. doi: 10.1016/j.canlet.2021.10.029
24. LncRNA-mediated regulation of cell signaling in cancer - Search Results . PubMed (Accessed February 25, 2024).
25. Han S, Cao D, Sha J, Zhu X, Chen D. LncRNA ZFPM2-AS1 promotes lung adenocarcinoma progression by interacting with UPF1 to destabilize ZFPM2. Mol Oncol. (2020) 14:1074–88. doi: 10.1002/1878-0261.12631
26. Xiao L, Li Y, Zeng X, Zhou Z, Hu S, Zhang S, et al. Silencing of LOC389641 impairs cell proliferation and induces autophagy via EGFR/MET signaling in lung adenocarcinoma. Aging. (2020) 13:2539–52. doi: 10.18632/aging.202286
27. Wu S, Liu B, Zhang Y, Hong R, Liu S, Xiang T, et al. Long non-coding RNA LEISA promotes progression of lung adenocarcinoma via enhancing interaction between STAT3 and IL-6 promoter. Oncogene. (2021) 40:3449–59. doi: 10.1038/s41388-021-01769-7
28. Qin J, Ning H, Zhou Y, Hu Y, Yang L, Huang R. LncRNA MIR31HG overexpression serves as poor prognostic biomarker and promotes cells proliferation in lung adenocarcinoma. BioMed Pharmacother Biomedecine Pharmacother. (2018) 99:363–8. doi: 10.1016/j.biopha.2018.01.037
29. Zhang G-J, Song W, Song Y. Overexpression of HOTTIP promotes proliferation and drug resistance of lung adenocarcinoma by regulating AKT signaling pathway. Eur Rev Med Pharmacol Sci. (2017) 21:5683–90. doi: 10.26355/eurrev_201712_14013
30. Lu Q-C, Rui Z-H, Guo Z-L, Xie W, Shan S, Ren T. LncRNA-DANCR contributes to lung adenocarcinoma progression by sponging miR-496 to modulate mTOR expression. J Cell Mol Med. (2018) 22:1527–37. doi: 10.1111/jcmm.13420
31. Han X, Jiang H, Qi J, Li J, Yang J, Tian Y, et al. Novel lncRNA UPLA1 mediates tumorigenesis and prognosis in lung adenocarcinoma. Cell Death Dis. (2020) 11:999. doi: 10.1038/s41419-020-03198-y
32. Yang J, Liu Y, Mai X, Lu S, Jin L, Tai X. STAT1-induced upregulation of LINC00467 promotes the proliferation migration of lung adenocarcinoma cells by epigenetically silencing DKK1 to activate Wnt/β-catenin signaling pathway. Biochem Biophys Res Commun. (2019) 514:118–26. doi: 10.1016/j.bbrc.2019.04.107
33. Wan H, Lin T, Shan M, Lu J, Guo Z. LINC00491 facilitates tumor progression of lung adenocarcinoma via wnt/β-catenin-signaling pathway by regulating MTSS1 ubiquitination. Cells. (2022) 11:3737. doi: 10.3390/cells11233737
34. Zhu J, Cao K, Zhang P, Ma J. LINC00669 promotes lung adenocarcinoma growth by stimulating the Wnt/β-catenin signaling pathway. Cancer Med. (2023) 12:9005–23. doi: 10.1002/cam4.5604
35. Wu Z-H, Zhou J, Hu G-H, Liu J, Li W-C, Lai X-H, et al. LncRNA CASC2 inhibits lung adenocarcinoma progression through forming feedback loop with miR-21/p53 axis. Kaohsiung J Med Sci. (2021) 37:675–85. doi: 10.1002/kjm2.12386
36. Wang J, Xiang Y, Yang S-X, Zhang H-M, Li H, Zong Q-B, et al. MIR99AHG inhibits EMT in pulmonary fibrosis via the miR-136-5p/USP4/ACE2 axis. J Transl Med. (2022) 20:426. doi: 10.1186/s12967-022-03633-y
37. Jiang L, Wang R, Fang L, Ge X, Chen L, Zhou M, et al. HCP5 is a SMAD3-responsive long non-coding RNA that promotes lung adenocarcinoma metastasis via miR-203/SNAI axis. Theranostics. (2019) 9:2460–74. doi: 10.7150/thno.31097
38. Zhang T, Xia W, Song X, Mao Q, Huang X, Chen B, et al. Super-enhancer hijacking LINC01977 promotes Malignancy of early-stage lung adenocarcinoma addicted to the canonical TGF-β/SMAD3 pathway. J Hematol OncolJ Hematol Oncol. (2022) 15:114. doi: 10.1186/s13045-022-01331-2
39. Guo H, Feng Y, Yu H, Xie Y, Luo F, Wang Y. A novel lncRNA, loc107985872, promotes lung adenocarcinoma progression via the notch1 signaling pathway with exposure to traffic-originated PM2.5 organic extract. Environ pollut Barking Essex 1987. (2020) 266:115307. doi: 10.1016/j.envpol.2020.115307
40. Zhang M, Han Y, Zheng Y, Zhang Y, Zhao X, Gao Z, et al. ZEB1-activated LINC01123 accelerates the Malignancy in lung adenocarcinoma through NOTCH signaling pathway. Cell Death Dis. (2020) 11:981. doi: 10.1038/s41419-020-03166-6
41. Deng Y, Zhang L. LncRNA SNHG11 accelerates the progression of lung adenocarcinoma via activating Notch pathways. Pathol Res Pract. (2022) 234:153849. doi: 10.1016/j.prp.2022.153849
42. Jiang F, Huang X, Ling L, Tang S, Zhou H, Cai X, et al. Long noncoding RNA ZBED5-AS1 facilitates tumor progression and metastasis in lung adenocarcinoma via ZNF146/ATR/chk1 axis. Int J Mol Sci. (2023) 24:13925. doi: 10.3390/ijms241813925
43. Cheng Z, Hou S, Wu Y, Wang X, Sun Y, Liu B, et al. LINC01419 promotes cell proliferation and metastasis in lung adenocarcinoma via sponging miR-519b-3p to up-regulate RCCD1. Biochem Biophys Res Commun. (2019) 520:107–14. doi: 10.1016/j.bbrc.2019.09.090
44. Wang L, Zhang X, Liu Y, Xu S. Long noncoding RNA FBXL19-AS1 induces tumor growth and metastasis by sponging miR-203a-3p in lung adenocarcinoma. J Cell Physiol. (2020) 235:3612–25. doi: 10.1002/jcp.29251
45. Yang C, Huang H, Li Y, Zhuo T, Zhu L, Luo C, et al. LncRNA PCAT6 promotes proliferation, migration, invasion, and epithelial-mesenchymal transition of lung adenocarcinoma cell by targeting miR-545-3p. Mol Biol Rep. (2023) 50:3557–68. doi: 10.1007/s11033-023-08259-x
46. Zhong Y, Wang J, Lv W, Xu J, Mei S, Shan A. LncRNA TTN-AS1 drives invasion and migration of lung adenocarcinoma cells via modulation of miR-4677-3p/ZEB1 axis. J Cell Biochem. (2019) 120:17131–41. doi: 10.1002/jcb.28973
47. Wang T, Zhai R, Lv X, Wang K, Xu J. LINC02418 promotes Malignant behaviors in lung adenocarcinoma cells by sponging miR-4677-3p to upregulate KNL1 expression. BMC Pulm Med. (2020) 20:217. doi: 10.1186/s12890-020-01229-0
48. Qian Y, Zhang Y, Ji H, Shen Y, Zheng L, Cheng S, et al. LINC01089 suppresses lung adenocarcinoma cell proliferation and migration via miR-301b-3p/STARD13 axis. BMC Pulm Med. (2021) 21:242. doi: 10.1186/s12890-021-01568-6
49. Xiong D-D, Li Z-Y, Liang L, He R-Q, Ma F-C, Luo D-Z, et al. The lncRNA NEAT1 accelerates lung adenocarcinoma deterioration and binds to mir-193a-3p as a competitive endogenous RNA. Cell Physiol Biochem Int J Exp Cell Physiol Biochem Pharmacol. (2018) 48:905–18. doi: 10.1159/000491958
50. Ying K, Wang L, Long G, Lian C, Chen Z, Lin W. ACTA2-AS1 suppresses lung adenocarcinoma progression via sequestering miR-378a-3p and miR-4428 to elevate SOX7 expression. Cell Biol Int. (2020) 44:2438–49. doi: 10.1002/cbin.11451
51. Xiong X, Zhang L, Zang B, Xu D, Chen C, Dong G, et al. High−risk pathological subtype associated FAM83A−AS1 promotes Malignancy and glycolysis of lung adenocarcinoma via miR−202−3p/HK2 axis. Oncol Rep. (2023) 49:95. doi: 10.3892/or.2023.8532
52. Zhang J, Ma D, Kang H, Zhao J, Yang M. Long noncoding RNA LINC01287 promotes proliferation and inhibits apoptosis of lung adenocarcinoma cells via the miR-3529-5p/RNASEH2A axis under the competitive endogenous RNA pattern. Environ Toxicol. (2021) 36:2093–104. doi: 10.1002/tox.23325
53. Wei W, Zhao X, Liu J, Zhang Z. Downregulation of LINC00665 suppresses the progression of lung adenocarcinoma via regulating miR-181c-5p/ZIC2 axis. Aging. (2021) 13:17499–515. doi: 10.18632/aging.203240
54. Nakano Y, Isobe K, Kobayashi H, Kaburaki K, Isshiki T, Sakamoto S, et al. Clinical importance of long non−coding RNA LINC00460 expression in EGFR−mutant lung adenocarcinoma. Int J Oncol. (2020) 56:243–57. doi: 10.3892/ijo.2019.4919
55. Li H, Mu Q, Zhang G, Shen Z, Zhang Y, Bai J, et al. Linc00426 accelerates lung adenocarcinoma progression by regulating miR-455-5p as a molecular sponge. Cell Death Dis. (2020) 11:1051. doi: 10.1038/s41419-020-03259-2
56. Zhu X, Zhao J, Xu J. Long noncoding RNA LINC01426 promotes the progression of lung adenocarcinoma via regulating miRNA-125a-5p/casein kinase 2 alpha 1 axis. Bioengineered. (2022) 13:7020–33. doi: 10.1080/21655979.2022.2044251
57. Tang H, Han X, Feng Y, Hao Y. linc00968 inhibits the tumorigenesis and metastasis of lung adenocarcinoma via serving as a ceRNA against miR-9-5p and increasing CPEB3. Aging. (2020) 12:22582–98. doi: 10.18632/aging.103833
58. Xiao G, Wang P, Zheng X, Liu D, Sun X. FAM83A-AS1 promotes lung adenocarcinoma cell migration and invasion by targeting miR-150-5p and modifying MMP14. Cell Cycle Georget Tex. (2019) 18:2972–85. doi: 10.1080/15384101.2019.1664225
59. Li W, Pan T, Jiang W, Zhao H. HCG18/miR-34a-5p/HMMR axis accelerates the progression of lung adenocarcinoma. BioMed Pharmacother Biomedecine Pharmacother. (2020) 129:110217. doi: 10.1016/j.biopha.2020.110217
60. Jia Y, Duan Y, Liu T, Wang X, Lv W, Wang M, et al. LncRNA TTN-AS1 promotes migration, invasion, and epithelial mesenchymal transition of lung adenocarcinoma via sponging miR-142-5p to regulate CDK5. Cell Death Dis. (2019) 10:573. doi: 10.1038/s41419-019-1811-y
61. Zhang W, Bai M, Liu K, Tan J, Ma J, Zhao J, et al. LncRNA surfactant associated 1 activates large tumor suppressor kinase 1/Yes-associated protein pathway via modulating hypoxic exosome-delivered miR-4766-5p to inhibit lung adenocarcinoma metastasis. Int J Biochem Cell Biol. (2022) 153:106317. doi: 10.1016/j.biocel.2022.106317
62. Sui Y, Lin G, Zheng Y, Huang W. LncRNA MAFG-AS1 boosts the proliferation of lung adenocarcinoma cells via regulating miR-744-5p/MAFG axis. Eur J Pharmacol. (2019) 859:172465. doi: 10.1016/j.ejphar.2019.172465
63. Cao Q, Wang H, Zhu J, Qi C, Huang H, Chu X. lncRNA CYTOR promotes lung adenocarcinoma gemcitabine resistance and epithelial-mesenchymal transition by sponging miR-125a-5p and upregulating ANLN and RRM2. Acta Biochim Biophys Sin. (2024) 56:210–22. doi: 10.3724/abbs.2023287
64. Li W, Zhang B, Jia Y, Shi H, Wang H, Guo Q, et al. LncRNA LOXL1-AS1 regulates the tumorigenesis and development of lung adenocarcinoma through sponging miR-423-5p and targeting MYBL2. Cancer Med. (2020) 9:689–99. doi: 10.1002/cam4.2641
Keywords: long non-coding RNAs, lung adenocarcinoma, differential expression, diagnostic markers, prognostic marker, clinical application, signal pathways
Citation: Wei H, Zhang S, Lin X, Fang R and Li L (2024) Differential expression and clinical significance of long non-coding RNAs in the development and progression of lung adenocarcinoma. Front. Oncol. 14:1411672. doi: 10.3389/fonc.2024.1411672
Received: 03 April 2024; Accepted: 15 May 2024;
Published: 06 June 2024.
Edited by:
Yitao Qi, Shaanxi Normal University, ChinaReviewed by:
Chao Huang, Kunming University of Science and Technology, ChinaKenichi Takayama, Tokyo Metropolitan Institute of Gerontology, Japan
Copyright © 2024 Wei, Zhang, Lin, Fang and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Li Li, MTAyMTAwNTFAdmlwLmhlbnUuZWR1LmNu
†These authors have contributed equally to this work and share first authorship
 Haitao Wei1†
Haitao Wei1† Sa Zhang
Sa Zhang Li Li
Li Li