
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Oncol., 18 June 2024
Sec. Head and Neck Cancer
Volume 14 - 2024 | https://doi.org/10.3389/fonc.2024.1410057
A 54-year-old woman was admitted to the hospital with a left neck mass. Enhanced CT and ultrasound examinations revealed a lesion in the left sternocleidomastoid muscle. The patient undergone right thyroid lobe resection 8 years ago. Interestingly, the lesion on the sternocleidomastoid muscle, along with the left lobe of the patient’s thyroid, visually appears to form a displaced and complete thyroid in the early Tc-99m-MIBI parathyroid scintigraphy. Combined with Tc-99m-MIBI scintigraphy and abnormal PTH and blood calcium levels, the consideration was given to the lesion in the sternocleidomastoid muscle as an ectopic parathyroid adenoma. Subsequent surgical pathology confirmed this suspicion.
Primary hyperparathyroidism (PHPT) is a common endocrine disorder characterized by hypercalcemia and elevated parathyroid hormone levels. Hyperparathyroidism affects 20 to 30 people per 100,000 people each year, and most cases are closely associated with parathyroid adenomas (1). Ectopic parathyroid adenomas (EPA) are rare causes of PHPT, comprising less than 5% of cases (2). They can be located anywhere from the base of the tongue to the mediastinum, commonly found in the superior mediastinum or posterior to the esophagus (3). Here, we report a rare case of an ectopic parathyroid adenoma due to its unusual location and distinctive imaging features.
A 54-year-old female presented with a gradually enlarging left neck mass. Physical examination revealed a painless, firm, non-mobile 4 cm mass in the left neck. Axial (Figure 1A) and sagittal (Figure 1B) contrast enhanced CT images revealed a spindle-shaped soft tissue lesion with clear borders in the left sternocleidomastoid muscle, showing homogeneous hyperenhancement. Conventional ultrasound (Figure 1C) identified a well-defined slightly hyperechoic spindle-shaped lesion (measuring 40 mm × 17 mm × 9 mm) in the left sternocleidomastoid muscle with color Doppler displaying abundant blood flow signals, raising suspicion of a myogenic tumor. Subsequently, a Tc-99m-MIBI parathyroid scintigraphy was conducted. Early (Figure 1D, 15 minutes) and late (Figure 1E, 2 hours) images in Tc-99m-MIBI scintigraphy both exhibited well-defined, regularly shaped, cord-like high uptake in the projection area of the left sternocleidomastoid muscle. Interestingly, the lesion on the sternocleidomastoid muscle, along with the left lobe of the patient’s thyroid, visually appears to form a displaced and complete thyroid in the early Tc-99m scintigraphy. However, the patient had actually undergone right thyroid lobe resection 8 years ago due to nodular goiter. Blood tests revealed elevated serum calcium (2.92 mmol/L; reference range, 2.11–2.52 mmol/L) and parathyroid hormone (319 pg/mL; reference range, 15–65 pg/mL). And the serum creatinine levels (61 umol/L; reference range, 41–73 umol/L) were in the normal range. Urinary analyses revealed hypercalciuria, 15.44 mmol/24 hours (reference range, 2.5–7.5 mmol/24 hours). The patient has no history of osteoporosis or kidney stones. Based on imaging and laboratory examination results, there is a preoperative suspicion that the tumor of the sternocleidomastoid muscle originates from ectopic parathyroid tissue, with malignant potential not being excluded. Subsequently, the lesion measuring approximately 40mm× 20mm ×10 mm was successfully excised through surgery. Hematoxylin and eosin staining (Figures 2A, B) of the lesion showed the adenoma growing within the striated muscle, and conspicuous follicular structures, concomitantly with tumor cells exhibiting a palisade arrangement around blood vessels. Immunohistochemistry revealed a strong positive expression of parathyroid hormone (Figure 2C). Moreover, the expression of Ki-67 was less than 5%. Atypical features (4) such as cellular nests in a thickened connective tissue, trabecular growth, increased mitotic activity, atypical mitotic figures, coagulative necrosis, and a Ki-67 labeling index >5% were not identified in the pathology findings of our patient. Similarly, definitive criteria of malignancy (4) including angioinvasion, lymphatic invasion, perineural invasion, unequivocal invasion into adjacent structures, and histologically confirmed metastasis were absent. Therefore, the parathyroid adenoma ectopically located in the sternocleidomastoid muscle was confirmed by pathological findings. After surgery, the patient’s parathyroid hormone and blood calcium levels rapidly decreased to normal levels. Currently, the patient’s condition is good, with a favorable prognosis.
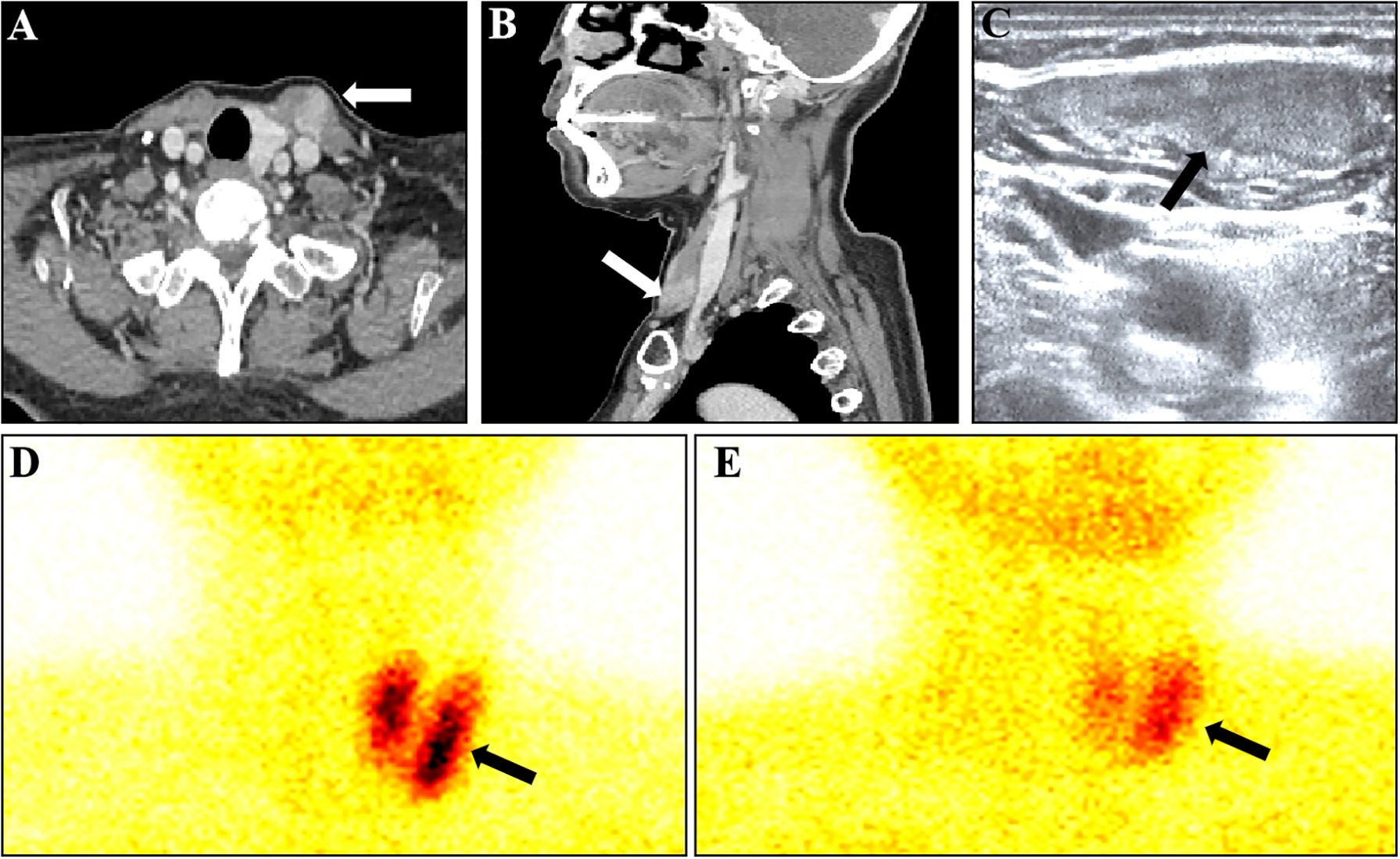
Figure 1 Various imaging findings of ectopic parathyroid adenoma in the sternocleidomastoid muscle. (A) Axial image in contrast-enhanced CT showed a soft tissue lesion (arrow). (B) Sagittal image in contrast-enhanced CT showed a soft tissue lesion (arrow). (C) Conventional ultrasound Showed a well-defined slightly hyperechoic mass. (D) Early (15 minutes) images in Tc-99m-MIBI scintigraphy showed a cord-like high uptake in the projection area of the left sternocleidomastoid muscle. (E) late (2 hours) images in Tc-99m-MIBI scintigraphy still showed a cord-like high uptake in the projection area of the left sternocleidomastoid muscle.
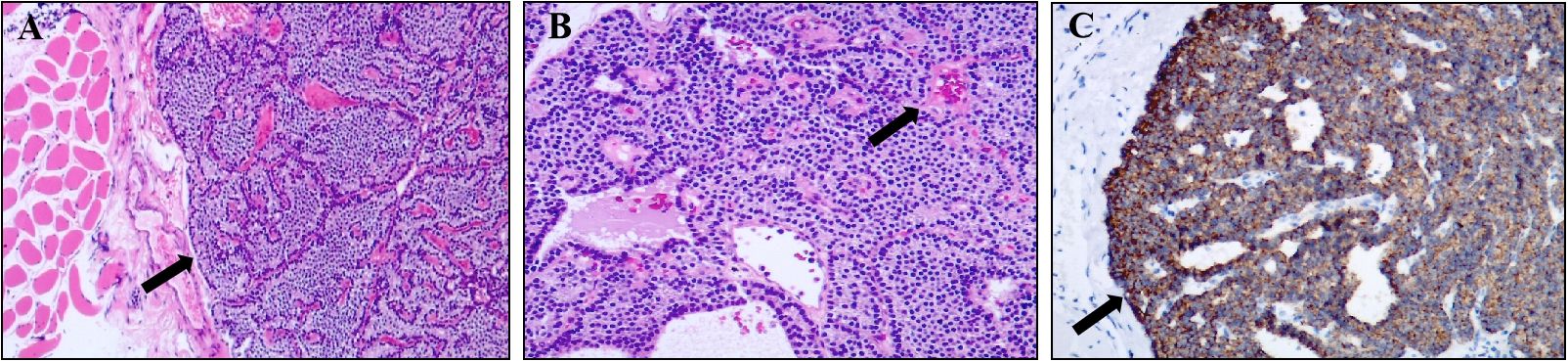
Figure 2 Pathological findings of ectopic parathyroid adenoma in the sternocleidomastoid muscle. (A) Image of hematoxylin-eosin (magnification, ×50) showed the mass occurred in striated muscle tissue. (B) Image of hematoxylin-eosin (magnification, ×100) showed conspicuous follicular structures, concomitantly with tumor cells exhibiting a palisade arrangement around blood vessels (arrow). (C) Immunohistochemistry result showed a strong positive expression of parathyroid hormone (magnification, ×100).
PHPT is a disease characterized by the excessive secretion of parathyroid hormone, leading to hypercalcemia, osteoporosis, and urinary tract stones (5). Both orthotopic parathyroid adenomas and EPA can cause PHPT. Parathyroid adenomas located in ectopic sites may present with different clinical manifestations than in situ parathyroid adenomas, which may be related to a higher incidence of multiglandular disease, more severe hypercalcemia, and missed imaging studies (6, 7). Nonetheless, ectopic parathyroid glands remain a diagnostic and surgical challenge for patients with PHPT, contributing to initial surgical failures and persistent or recurrent PHPT (8). Surgery remains the preferred treatment for PHPT caused by EPA. Preoperative localization is crucial for ensuring the safety and efficacy of surgery, particularly in the era of minimally invasive procedures.
EPA can be found in various locations ranging from the skull base to the mediastinum. Ectopic inferior parathyroid glands are more common than superior glands due to their longer and more variable embryological origins (9). Superior ectopic parathyroid glands are frequently located near the tracheoesophageal groove or the posterior mediastinum (10). Ectopic inferior parathyroid glands are predominantly found in the mediastinum, accounting for approximately 38% of cases. However, they may also be confined within the thyroid gland itself in approximately 18% of cases (11). Moreover, ectopic parathyroid glands may occur in the vicinity of the vagus nerve, the foramen lacerum, the retropharyngeal space, or even within pulmonary tissues, leading to persistent PHPT (12–14).
To our best, only two cases of parathyroid adenomas located within the sternocleidomastoid muscle have been reported (15, 16). The two patients had a history of thyroidectomy due to goiter and medullary thyroid carcinoma respectively. Preoperative localization of lesion was performed by SPECT/CT imaging. And the localization of the lesion within the sternocleidomastoid muscle was confirmed by pathological examination. Two potential explanations have been proposed for this phenomenon. The first hypothesis suggests that parathyroid adenomas result from inadvertent seeding of parathyroid tissue during surgery. Research findings suggest that surgical relocation of the parathyroid gland into the sternocleidomastoid muscle may, albeit rarely, lead to EPA (15, 17). Therefore, we speculate that inadvertent damage to parathyroid tissue during thyroidectomy might result in its inadvertent implantation into the sternocleidomastoid muscle, potentially leading to the formation of EPA in this location. Wu et al. (8) reported a case of endoscopic parathyroidectomy performed via the axillary approach, which subsequently led to the emergence of an EPA within the ipsilateral pectoralis major muscle. Their case lends partial support to the theory implicating thyroidectomy in the genesis of EPA. The second hypothesis involves physiological stimuli inducing proliferation of embryologically pre-existing parathyroid tissue (13). In the present case, although the patient underwent partial resection of the right thyroid lobe due to nodular goiter, the ectopic parathyroid adenoma grew within the left sternocleidomastoid muscle. Consequently, the direct correlation between the ectopic parathyroid adenoma and the surgery performed eight years prior cannot be definitively established. Concerning the presence of parathyroid tissue within muscle, the topic of autotransplantation of parathyroid tissue is inevitable. Although muscle tissue is frequently utilized for autotransplantation of parathyroid tissue, the occurrence of adenomatous transformation in autotransplanted parathyroid tissue remains exceptionally uncommon.
Currently, several imaging modalities are also available to localize parathyroid adenomas preoperatively. While no method is perfect, most parathyroid adenomas are now localized preoperatively by a variety of imaging modalities, often using a combination of techniques to allow for successful resection with minimal harm to the patient (12). Presently, it is recommended that at least two diagnostic modalities be performed for patients who have developed persistent PHPT, achieving success rates as high as 95%. However, there is no consensus on the optimal combination of diagnostic modalities for these cases (18). Tc-99m-MIBI scintigraphy, ultrasound, and CT are the most commonly used methods for evaluating EPA. Tc-99m-MIBI scintigraphy is a highly mature imaging modality for the localization and diagnosis of parathyroid adenomas, particularly effective for adenomas smaller than 10 mm in diameter. In unexplored patients, Tc-99m-MIBI scintigraphy demonstrates a sensitivity of 70% to 89% and a specificity of 88% to 100% for localizing EPA (9). When identifying adenomas in patients undergoing repeat surgery, Tc-99m-MIBI scintigraphy exhibits a sensitivity of 65% to 67% and a specificity of 100% (9). Overall, Tc-99m-MIBI scintigraphy outperforms ultrasound and contrast-enhanced CT scanning (19). Ultrasound examination is cost-effective, widely available, and radiation-free, making it particularly suitable for assessing EPA. Generally, the sensitivity of ultrasound examination depends on the size and location of the enlarged gland. Ultrasound is accurate in identifying parathyroid adenomas adjacent to the inferior thyroid pole and the posterior aspect of the thyroid gland. Reported sensitivities of ultrasound for detecting EPA range from 11% to 59% in previously unexplored PHPT patients, with a specificity of up to 100% (20). The main reason for low ultrasound sensitivity is that many ectopic locations cannot be detected by routine ultrasound examination, especially in areas such as the thymus, the tracheoesophageal groove, etc. Additionally, the misidentification of thyroid nodules and lymph nodes as parathyroid lesions on ultrasound may also affect the specificity of ultrasound examination (21). Parathyroid adenomas appear as discrete soft tissue masses near or adjacent to the thyroid gland on CT scans. The sensitivity of CT scanning for parathyroid disease mainly depends on the size of the enlarged gland rather than its location. Therefore, CT scanning is better suited to evaluate the posterior esophageal, tracheal, and mediastinal regions compared to ultrasound examination (22). Since CT has lower diagnostic accuracy for smaller lesions and cases with multiple culprits, it is more commonly used when Tc-99m-MIBI scintigraphy results are negative (23). Finally, in cases where conventional imaging yields negative results, functional imaging modalities like 18F-choline PET/CT and 11C-choline PET/CT can aid in the detection of EPA. Their notable advantage lies in their heightened sensitivity and specificity, particularly in detecting minute tumors and precisely pinpointing their locations within the body (24, 25). Regrettably, our patient did not undergo preoperative PET/CT imaging, thus preventing us from obtaining PET/CT images of the EPA situated ectopically on the sternocleidomastoid muscle.
The diagnosis of PHPT caused by EPA remains challenging. Multimodal imaging examinations aid in the localization and diagnosis of EPA. In this case, we report a very rare instance of an EPA arising within the sternocleidomastoid muscle, and the unusual presentation on early Tc-99m-MIBI scintigraphy adds an intriguing aspect to the case.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
The studies involving humans were approved by Guangzhou First People’s Hospital Ethics Committee. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
HS: Writing – original draft. ZJ: Writing – original draft. JX: Writing – original draft. JL: Writing – original draft. XZ: Writing – review & editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Daruwalla J, Sachithanandan N, Andrews D, Miller JA. Ectopic intravagal parathyroid adenoma. Head Neck. (2015) 37:E200–4. doi: 10.1002/hed.24068
2. Ruda JM, Hollenbeak CS, Stack BC. A systematic review of the diagnosis and treatment of primary hyperparathyroidism from 1995 to 2003. Otolaryngol–Head Neck Surg. (2016) 132:359–72. doi: 10.1016/j.otohns.2004.10.005
3. Chen M, Chen S, Li X, Li Y. An ectopic parathyroid adenoma mimicking submandibular gland on 99mtc-mibi parathyroid scintigraphy. Clin Nucl Med. (2022) 47:916–7. doi: 10.1097/rlu.0000000000004277
4. Erickson LA, Mete O, Juhlin CC, Perren A, Gill AJ. Overview of the 2022 who classification of parathyroid tumors. Endocrine Pathol. (2022) 33:64–89. doi: 10.1007/s12022–022-09709–1
5. He Y, Liu R-x, Zhu M-t, Shen W-b, Xie J, Zhang Z-y, et al. The browning of white adipose tissue and body weight loss in primary hyperparathyroidism. EBioMed. (2019) 40:56–66. doi: 10.1016/j.ebiom.2018.11.057
6. Johnson NA, Tublin ME, Ogilvie JB. Parathyroid imaging: technique and role in the preoperative evaluation of primary hyperparathyroidism. Am J Roentgenol. (2007) 188:1706–15. doi: 10.2214/ajr.06.0938
7. Durmuş ET, Atmaca A, Kefeli M, Çolak R, Durmuş B, Polat C. Clinical predictors of ectopic parathyroid adenomas: experience with 421 confirmed parathyroid adenoma localizations. J Endocrinol Invest. (2022) 46:1197–203. doi: 10.1007/s40618–022-01986–1
8. Wu M, Chen L, Huo L, Jing H. Ectopic parathyroid adenoma on pectoralis major after endoscopic parathyroidectomy revealed by 99mtc-mibi spect/ct. Clin Nucl Med. (2021) 46:933–4. doi: 10.1097/rlu.0000000000003701
9. Parikh AM, Suliburk JW, Morón FE. Imaging localization and surgical approach in the management of ectopic parathyroid adenomas. Endocrine Pract. (2018) 24:589–98. doi: 10.4158/ep-2018–0003
10. Hemead HM, Abdellatif AA, Abdel Rahman MA. Ectopic pure mediastinal parathyroid adenoma: A case report. Int J Surg Case Rep. (2022) 90:106598. doi: 10.1016/j.ijscr.2021.106598
11. Lee Y, Baek W, Cho J, Oh J. An ectopic parathyroid adenoma of the retropharynx in a patient with primary hyperparathyroidism and papillary thyroid cancer—a rare case. Diagnostics. (2024) 14:110. doi: 10.3390/diagnostics14010110
12. Morris MA, Saboury B, Ahlman M, Malayeri AA, Jones EC, Chen CC, et al. Parathyroid imaging: past, present, and future. Front Endocrinol. (2022) 12:760419. doi: 10.3389/fendo.2021.760419
13. Valizadeh M, Ebadinejad A, Amouzegar A, Zakeri A. Persistent hyperparathyroidism secondary to ectopic parathyroid adenoma in lung: case report. Front Endocrinol. (2022) 13:988035. doi: 10.3389/fendo.2022.988035
14. Karvounaris DC, Symeonidis N, Triantafyllou A, Flaris N, Sakadamis A. Ectopic parathyroid adenoma located inside the hypoglossal nerve. Head Neck. (2010) 32:1273–6. doi: 10.1002/hed.21215
15. Touska P, Srikanthan A, Amarasinghe K, Jawad S. Parathyroid adenoma arising within the sternocleidomastoid muscle: A rare complication of autotransplantation. BMJ Case Rep. (2016) 2016:bcr2015213184. doi: 10.1136/bcr-2015–213184
16. Thumerel M, Belaroussi Y, McSweeney J, Haissaguerre M. Parathyroid adenoma in the sternocleidomastoid muscle 30 years after thyroidectomy. Annales d'Endocrinol. (2023) 84:764–6. doi: 10.1016/j.ando.2023.10.002
17. Chou FF, Lee Ch Fau - Chen H-Y, Chen Hy Fau - Chen J-B, Chen Jb Fau - Hsu K-T, Hsu Kt Fau - Sheen-Chen S-M, Sheen-Chen SM. Persistent and recurrent hyperparathyroidism after total parathyroidectomy with autotransplantation. Ann Surg. (2002) 235:99–104. doi: 10.1097/00000658–200201000–00013
18. Walker MD, Silverberg SJ. Primary hyperparathyroidism. Nat Rev Endocrinol. (2017) 14:115–25. doi: 10.1038/nrendo.2017.104
19. Silberfein EJ. Reoperative parathyroidectomy location of missed glands based on contemporary nomenclature system. Arch Surg. (2010) 145:1065–8. doi: 10.1001/archsurg.2010.230
20. Zerizer I, Parsaï A, Win Z, Al-Nahhas A. Anatomical and functional localization of ectopic parathyroid adenomas. Nucl Med Commun. (2011) 32:496–502. doi: 10.1097/MNM.0b013e32834557a3
21. Sugg SL, Krzywda EA, Demeure MJ, Wilson SD. Detection of multiple gland primary hyperparathyroidism in the era of minimally invasive parathyroidectomy. Surgery. (2004) 136:1303–9. doi: 10.1016/j.surg.2004.06.062
22. Itani M, Middleton WD. Parathyroid imaging. Radiol Clinics North America. (2020) 58:1071–83. doi: 10.1016/j.rcl.2020.07.006
23. Kedarisetty S, Fundakowski C, Ramakrishnan K, Dadparvar S. Clinical value of tc99m-mibi spect/ct versus 4d-ct or us in management of patients with hyperparathyroidism. Ear Nose Throat J. (2019) 98:149–57. doi: 10.1177/0145561319828668
24. Broos WAM, van der Zant FM, Knol RJJ, Wondergem M. Choline pet/ct in parathyroid imaging. Nucl Med Commun. (2019) 40:96–105. doi: 10.1097/mnm.0000000000000952
Keywords: primary hyperparathyroidism, ectopic parathyroid adenoma, Tc-99m-MIBI, SPECT, sternocleidomastoid muscle
Citation: Shan H, Jiang Z, Xu J, Li J and Zhu X (2024) Ectopic parathyroid adenoma on sternocleidomastoid muscle: a case report. Front. Oncol. 14:1410057. doi: 10.3389/fonc.2024.1410057
Received: 31 March 2024; Accepted: 28 May 2024;
Published: 18 June 2024.
Edited by:
Mitali Dandekar, Paras Cancer Centre, IndiaReviewed by:
Coskun Meric, University of Health Sciences, TürkiyeCopyright © 2024 Shan, Jiang, Xu, Li and Zhu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: XuSheng Zhu, MTM3MjUxNTI3MDVAMTYzLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.