
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Oncol., 29 July 2024
Sec. Cancer Epidemiology and Prevention
Volume 14 - 2024 | https://doi.org/10.3389/fonc.2024.1407008
This article is part of the Research TopicThe Future of Cancer Surveillance ResearchView all 25 articles
 Aya Hasan Alshammari1
Aya Hasan Alshammari1 Hideshi Ishii2
Hideshi Ishii2 Takaaki Hirotsu1
Takaaki Hirotsu1 Hideyuki Hatakeyama1
Hideyuki Hatakeyama1 Masayo Morishita1
Masayo Morishita1 Eric di Luccio1*
Eric di Luccio1*Cervical cancer screening is a critical public health measure, especially vital for underserved communities where disparities in access and outcomes are pronounced. Despite the life-saving potential of regular screening, numerous barriers—including geographical isolation, cultural and linguistic challenges, and socioeconomic factors—severely hinder accessibility for these populations. Multicancer early detection (MCED) tests emerge as a potentially effective intervention, offering a less invasive, more accessible approach that could transform how screenings are conducted. This paper explores the existing challenges in traditional cervical cancer screening methods, the potential of MCED tests to address these barriers, and the implications of these technologies for global health equity. Through a comprehensive review, we highlight the need for culturally sensitive, tailored interventions and the importance of effectively overcoming logistical and financial difficulties to implement MCED tests. Despite the promise shown by MCED tests, the paper acknowledges significant implementation challenges, including cost, logistical obstacles, and the need for cultural acceptance and validation studies. This study emphasizes the necessity for equitable MCED test implementation strategies, highlighting the potential of these innovative technologies to advance global health equity in cervical cancer prevention.
Despite its potential to save lives, cervical cancer screening remains inaccessible to individuals in underserved communities (1). This disparity is especially concerning as cervical cancer, though highly preventable through screening (2), disproportionately burdens underserved populations. Cervical cancer screening, a cornerstone of preventive healthcare, enables the early detection of malignancies and potentially life-saving interventions (3). Nevertheless, the efficacy of cervical cancer screening hinges on addressing the complex and interconnected barriers faced by diverse communities. Such barriers could include limited clinic access, cultural beliefs, language constraints, or socioeconomic factors (4). This literature review focuses on underserved communities, such as those in remote regions, minority groups facing cultural barriers, or areas with limited medical infrastructure.
These disparities are especially pronounced in preventive health measures like cancer screening, often impeded by cultural beliefs, language barriers, and religious constraints (5, 6). Understanding and addressing these disparities is vital to improving public health (7, 8). We will first survey existing screening modalities to explore potential solutions, emphasizing their importance and limitations. Our investigation then moves to the disparities in screening rates among underserved communities and how these may hinder the primary goal of reducing the cancer burden in these groups. While acknowledging these challenges, we also examine the potential of multicancer early detection (MCED) tests, which aim to detect multiple cancers with a single, non-invasive test. With their non-invasive nature, MCED tests could potentially address some traditional barriers (9, 10). However, successful implementation in underserved settings will require careful research into their efficacy within specific populations alongside culturally tailored approaches.
While the Pap smear has a proven track record in detecting cervical dysplasia, its effectiveness relies on accurate interpretation, which can be subjective (11, 12). Regular screenings with Pap smears can proactively identify and manage these abnormalities before they develop into cervical cancer, underscoring the importance of routine tests in cancer prevention (2, 13, 14). Pap smear accessibility and cost-effectiveness facilitate broader screening (15, 16). On the downside, Pap smears can sometimes produce false-positive results. These inaccuracies can lead to unnecessary follow-up tests, resulting in anxiety and possibly financial challenges for patients (14, 17). There is also a concern that some precancerous lesions might go undetected (18). These limitations are particularly concerning in low-resource settings, where expertise in cytologic interpretation may be limited, hindering the effectiveness of Pap smears as a primary screening method (19, 20).
Persistent infections with high-risk HPV types can lead to cellular changes in the cervix that may progress to cancer (21). Thus, HPV vaccination can play a vital role in preventing cervical cancer (22). HPV testing is a more recent addition to cervical cancer screening. It excels in identifying high-risk HPV types that have been linked to cervical cancer (14, 23). As a testament to its efficacy, some protocols have begun using HPV testing as the primary screening method, streamlining the process (23–25). An added advantage is the potential for patients to collect their own samples. This self-sampling can enhance accessibility, especially in under-resourced areas (20, 23, 24). However, HPV testing is not without limitations. It often necessitates greater infrastructure requirements than Pap smears, which can be challenging in areas with limited resources (23, 26). While self-sampling has its merits, the availability and awareness of such kits can be inconsistent, potentially limiting their reach (27–29). Existing disparities in healthcare access can further affect the adoption and follow-up processes associated with HPV testing (30, 31).
Visual inspection with acetic acid, commonly referred to as VIA, stands out as a screening technique due to its ability to yield immediate results (32). These immediate results facilitate real-time decision-making regarding any necessary treatment or further diagnostic tests, proving essential in many clinical settings (33). Financially, VIA is appealing as a low-cost method, offering an advantageous option for cervical cancer screening, especially in resource-constrained environments (32). Its simplicity and the absence of a requirement for any advanced technological equipment further enhance its appeal (32). However, VIA has its challenges. The accuracy of this method often depends on the proficiency and experience of the healthcare provider, introducing an element of subjectivity and potential variability in results (32).
Colposcopy serves as an indispensable tool in the detailed examination of the cervix. The procedure provides a magnified and well-illuminated view of the cervix, enabling healthcare providers to precisely detect and inspect any abnormalities (34). If, during the process, any suspicious areas are identified, colposcopy offers the advantage of facilitating targeted biopsies, ensuring a focused examination (34). With skilled practitioners, colposcopy possesses a high level of accuracy in detecting cervical abnormalities. On the downside, the procedure is more invasive than some of its counterparts, which might induce discomfort or anxiety in certain patients (35, 36). Moreover, false negatives exist, implying that despite the enhanced view, some precancerous lesions or concerning areas might escape detection (35). Cost and equipment considerations also limit its widespread availability (37).
The cervical biopsy procedure is the basis for a definitive diagnosis regarding cervical health (38, 39). It empowers medical professionals to make targeted treatment decisions based on the unambiguous presence or absence of precancerous or cancerous cells (38). The methodology behind a cervical biopsy is precise; the extracted tissue undergoes a thorough examination under a microscope, revealing detailed insights into cellular abnormalities (38). However, its invasive nature implies that it involves removing a tissue fragment from the cervix, which can also cause significant emotional strain for the patient. Additionally, there is an inherent risk of infection or bleeding post-procedure (40). Another concern revolves around the waiting period for the results. Unlike VIA, the outcomes from a cervical biopsy are not instantaneous, potentially leading to an unsettling period of anticipation for patients (40). Furthermore, depending on the location, there is a latent risk of missing the area with the most pronounced abnormality (40).
Despite the existence of these effective screening methods, significant disparities persist in who receives them. This uneven access to preventive care, particularly among underserved communities, is a critical public health concern that demands innovative solutions. The following section explores these disparities in detail, highlighting the geographical, cultural, linguistic, and social factors that create substantial barriers to cervical cancer screening.
This section underscores how access to life-saving cervical cancer screening varies substantially along lines of geography, culture, language, and religion. While disparities exist across nations, underserved communities within high-income countries also face barriers (41–43).
Geographical disparities drastically influence access to cervical cancer screening, leading to delays or complete absence of this vital preventive measure. This inequality significantly contributes to adverse health outcomes (44). Women in remote areas, low- and middle-income regions, and even specific locations within high-income countries experience substantial barriers due to distance from clinics, limited transportation, and inadequate healthcare facilities (45, 46). These disparities directly translate into higher cervical cancer incidence rates and poor survival outcomes in underserved areas (44).
The complexity of the issue is evident on both global and local scales. In China, geographical and socioeconomic inequities between regions fuel a high burden of cervical cancer (47). Even in the United States, despite targeted interventions like those in Maryland, significant geographical disparities in cancer mortality persist (48). This emphasizes how location, access to healthcare, and socioeconomic factors intertwine to influence individuals’ likelihood of receiving preventive screenings.
Certain populations face compounded challenges. Individuals with disabilities (49–51) and refugees (52) often encounter even greater difficulty accessing healthcare due to physical, linguistic, and systemic obstacles that are exacerbated by their geographical location. Addressing these complex issues requires a nuanced understanding of how different factors intersect to create barriers for specific groups.
Innovative approaches offer hope but necessitate careful implementation. HPV self-sampling holds promise for reaching underserved communities by reducing the need for in-person clinic visits (53). However, the sustained effectiveness of such interventions in improving outcomes must be assessed in the context of existing geographic disparities. Technologies like MCED, with their potential for less invasive sampling, could also help reduce geographical barriers; however, their efficacy in diverse real-world settings needs thorough investigation. While technology can be a powerful tool, it must be integrated with strategies that enhance healthcare infrastructure, address transportation issues, and provide tailored outreach to truly overcome geographic disparities in screening access.
In conclusion, geographical disparities play a major role in the preventable suffering caused by cervical cancer. Evidence underscores the urgent need for comprehensive strategies that address the root causes of these disparities (44, 45, 47, 48, 52, 53). These strategies must prioritize equity by improving healthcare infrastructure, tailoring education and awareness initiatives to specific regions, and leveraging technology to make screening more accessible for all.
Cultural beliefs and norms significantly influence how individuals perceive and approach healthcare decisions, particularly regarding sensitive topics like cervical cancer screening. Sociocultural factors, including religious beliefs, taboos around cancer, concerns about modesty, fear of familial judgment, and limitations on women’s decision-making autonomy, can all create substantial barriers to screening (54–56). These factors can lead to reluctance to seek care, even when symptoms are present.
This influence of culture on health behaviors is especially evident in low- and middle-income countries, where cultural values are deeply ingrained in how communities understand disease and interact with healthcare systems. In Uganda, for instance, cultural concerns about the HPV vaccine’s potential impact on fertility have hindered its acceptance, demonstrating how deeply held beliefs can supersede scientific evidence (57, 58).
Importantly, cultural barriers are not limited to low- and middle-income countries. Even within high-income nations like the United States, cultural and socioeconomic factors shape cervical cancer screening behaviors. Persistent misconceptions about the HPV vaccine’s safety, sometimes rooted in cultural beliefs, continue to impede preventive efforts (59–61). While innovative technologies like self-sampling or MCED might address concerns about privacy and invasiveness, overcoming deeply held misconceptions requires more than just technology.
Addressing the complexities of cultural barriers necessitates community-driven approaches. Utilizing community-based participatory research and developing culturally sensitive educational campaigns will be critical for increasing understanding and fostering trust within diverse communities. Achieving increased acceptance of cervical cancer screening requires acknowledging cultural differences and tailoring interventions accordingly.
Language barriers pose a significant obstacle to cervical cancer screening participation among culturally and linguistically diverse (CALD) populations. Challenges in accessing healthcare information and services due to language differences are a primary factor in the lower screening rates observed in these groups (62, 63). This underscores the broader challenges faced by CALD communities in accessing essential healthcare.
Research consistently demonstrates the impact of language barriers. In Hong Kong, limited language proficiency contributed to low screening rates among South Asian women, alongside broader informational gaps (62). Similarly, immigrant Muslim women face language-driven barriers in their decision-making about cervical cancer screening (64). This aligns with findings from other nations, where language is a recognized factor hindering screening access (65).
Healthcare providers are acutely aware of these challenges. General practitioners emphasize the need for interpreter services and culturally appropriate resources to better engage with CALD women during cancer screening programs (66). Studies from countries like the Netherlands further illustrate how language barriers lead to reduced screening participation and limit informed decision-making among Turkish and Moroccan women (67).
Language issues are recognized as health equity concerns even in countries like Canada, which have strong healthcare systems. Immigrants with limited fluency often encounter difficulties navigating the healthcare system, negatively impacting their access to preventive screenings (42, 68, 69).
To address these disparities, comprehensive solutions are needed. Healthcare systems must prioritize multilingual information, interpreter services, and culturally sensitive care. Technology, such as translation tools in MCED, could play a role but must be integrated with community outreach efforts that build trust and ensure information is tailored to the specific needs of CALD communities. Only by taking decisive action to overcome language barriers can we achieve equitable healthcare and improve cervical cancer screening outcomes for all.
Religious and social norms significantly influence cervical cancer screening uptake, particularly in culturally diverse settings. These norms shape women’s health behaviors and can result in screening delays or non-compliance. While innovative technologies like MCED offer the potential to reduce some barriers due to their less invasive nature, their successful implementation still requires a culturally sensitive approach.
Religious beliefs significantly influence attitudes toward healthcare interventions, including cervical cancer screening. In Zimbabwe, cultural and religious views, alongside insufficient knowledge and perceived stigma, hindered women’s participation in early screening (59). Similar challenges faced by immigrant Muslim women highlight the widespread impact of religious values on health-seeking behavior (64). However, integrating religious considerations into health messaging, as demonstrated by a faith-based intervention in Scotland, has the potential to mitigate these barriers and increase screening acceptance (70).
Social norms also play a powerful role. Women often underestimate the extent to which their peers engage in cervical cancer screening, suggesting that negative social perceptions can discourage individuals from seeking preventive care (71). Studies exploring norms related to cervical cancer screening, from Botswana to Singapore, further emphasize how gender roles, expectations, and broader social beliefs shape individual choices about screening (72, 73).
Targeted education interventions have shown promise in addressing the barriers posed by both religious and social norms. In Iran, theory-based education positively impacted knowledge and attitudes toward cervical cancer screening (74). Similarly, culturally tailored educational videos effectively improved understanding and screening intentions among Turkish- and Moroccan-Dutch women (75). Addressing cultural sensitivity and tailoring information is crucial, as seen in studies on breast and cervical cancer screening among immigrant populations in the United States (76).
In conclusion, religious and social norms significantly impact cervical cancer screening behaviors. Successful interventions must be designed with an understanding of the target population’s specific cultural, religious, and social context. These initiatives should focus on increasing knowledge, challenging harmful misconceptions, and leveraging positive social influences to encourage screening. By prioritizing community-driven approaches and tailoring healthcare solutions, we can make significant progress toward reducing cervical cancer disparities. Innovative technologies, such as MCED tests, offer the potential to address some of the traditional barriers faced by underserved communities. The following section will explore how MCED could substantially improve cervical cancer screening outcomes and propose strategies for overcoming the challenges in its implementation.
This proposal investigates how MCED tests have the potential to revolutionize cervical cancer screening outcomes, especially in underserved communities. These communities, including those in remote regions, recent immigrant populations, and areas with low socioeconomic status, face significant disparities due to limited clinic access, cultural barriers, and financial constraints (43, 65, 77–80). The disproportionate burden of cervical cancer in these communities underscores the urgent need for innovative solutions (81–84). Moreover, sample collection could potentially happen at home, further enhancing accessibility.
MCED tests, which analyze blood, urine, or other bodily fluids for a variety of cancer biomarkers, hold promise for overcoming traditional screening barriers. Their non-invasive sampling methods reduce the need for in-person clinic visits, addressing significant obstacles for those in remote areas where travel is difficult (85–87).
Cultural sensitivities, such as the stigma surrounding invasive procedures like Pap smears, can also be addressed by the less invasive nature of MCED. This offers a potentially more culturally acceptable alternative, allowing tailored interventions within diverse communities (88, 89). Successful initiatives like the Pitt County Breast Wellness Initiative-Education highlight the effectiveness of combining non-invasive methods with community-led outreach to increase screening rates (90).
While the specific application of MCED technology to cervical cancer is still in its early stages, its potential is promising. The non-invasive nature of MCED sampling methods offers the possibility to overcome numerous barriers underserved communities face. Simulation studies suggest MCED’s potential to reduce cancer mortality (91), and its effectiveness in detecting multiple cancers is established (92). However, dedicated clinical validation studies are needed to confirm its specific efficacy for cervical cancer detection. Nonetheless, its potential to address challenges like stigma, cultural barriers, and accessibility warrants focused research in underserved settings. Pilot programs within specific communities will be crucial to determine if MCED can effectively reduce cervical cancer screening disparities. Should MCED prove effective, its multicancer detection capability (91) could significantly improve health outcomes in populations with limited healthcare access.
This proposal recognizes the complexities of implementing MCED in under-resourced areas. These include cost, logistical difficulties, and ensuring culturally competent approaches. Prioritizing validation studies, alongside focused pilot programs exploring implementation feasibility, is vital. Multisector collaboration, guided by community input, will be essential to ensure that MCED benefits underserved communities and promotes global health equity in cancer care. The following sections will explore these implementation challenges and outline strategies for addressing them.
While MCED tests offer significant potential for transforming cervical cancer screening in under-resourced areas, their implementation faces substantial challenges (93–96). These include cost, cultural acceptance, logistical complexities, and ethical considerations. In underserved communities, these challenges are amplified by limited resources, infrastructure constraints, and unique cultural and socioeconomic factors (94). Ensuring the effectiveness of MCED across diverse populations is crucial, with research highlighting the need for cancer screening approaches to be sensitive to cultural and genetic variations (97). Additionally, MCED tests raise ethical concerns, particularly in low-resource settings, regarding how to communicate results in a way that reduces anxiety and, importantly, how to ensure access to appropriate follow-up care (10).
Addressing cost is essential for MCED to be widely accessible in underserved communities. Government subsidies and volume guarantees are vital policy solutions. Alongside these, community-led initiatives could focus on awareness campaigns to address transportation barriers, culturally tailored outreach, and partnerships with local organizations for on-site screening or subsidy distribution (98). As unforeseen obstacles may arise, successful implementation will require flexible, iterative approaches developed in collaboration with communities.
Logistical difficulties, such as equipment distribution and maintenance, pose additional barriers to MCED adoption, especially in remote or under-resourced clinics. Emerging technologies, like blockchain and smart contracts, could potentially streamline supply chains and enhance trust (99). However, for these solutions to be effective, they must be adapted to the realities of clinics in underserved areas, ensuring compatibility and ease of use within existing infrastructure.
Addressing these multifaceted challenges requires a genuinely collaborative approach. Technological innovation, policy changes that specifically target the unique needs of underserved communities, and initiatives driven by community input and leadership must work in concert to ensure that the life-saving benefits of MCED reach those most in need. The following sections will propose strategies to overcome these obstacles, drawing inspiration from successful health initiatives in diverse contexts (Figure 1).
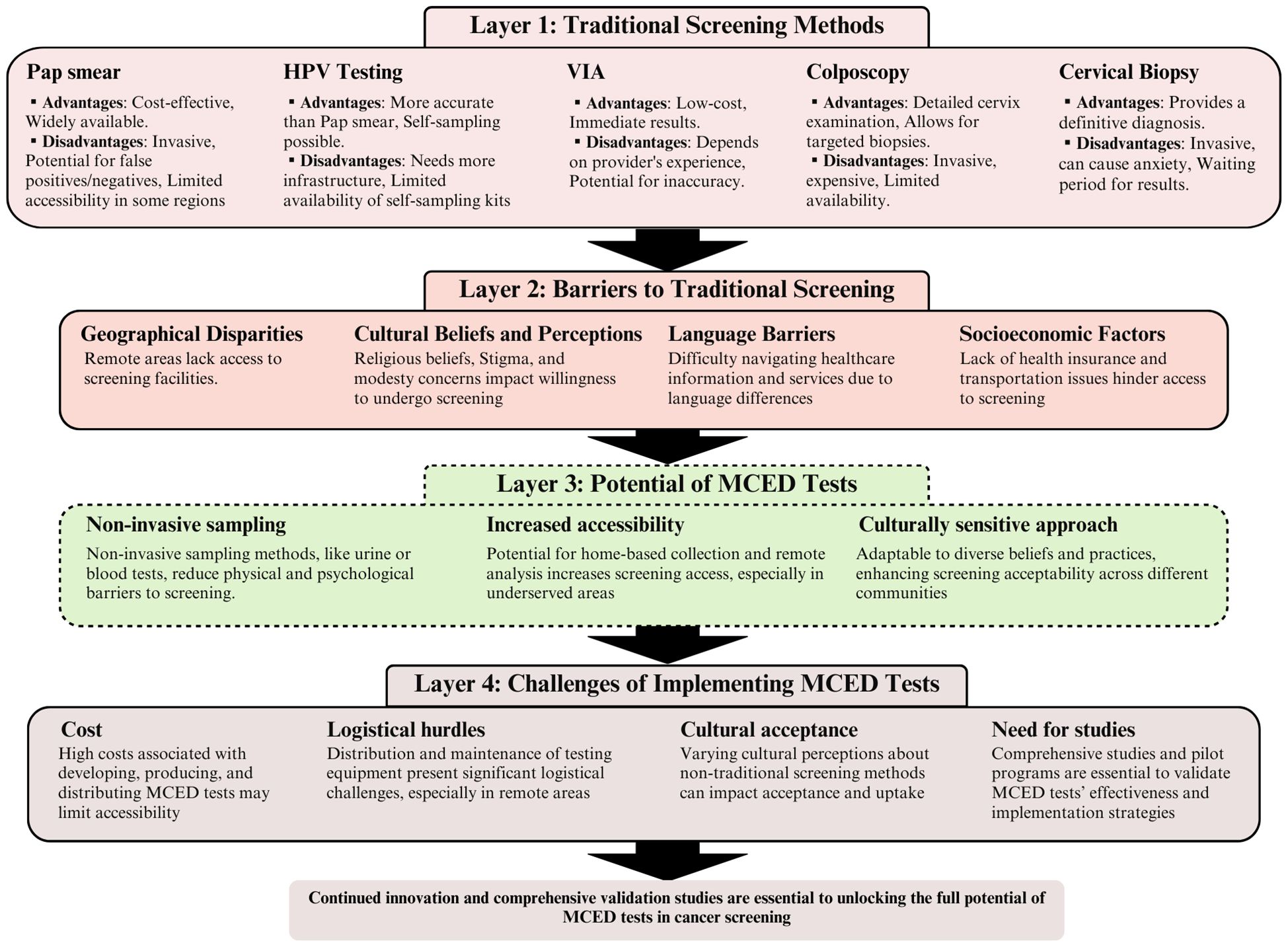
Figure 1 The evolution of cervical cancer screening: from traditional methods to multicancer early detection (MCED) innovations. This figure illustrates a four-layered analysis of cervical cancer screening strategies, beginning with traditional screening methods (layer 1), progressing through the barriers to these traditional screenings (layer 2), the potential benefits of MCED tests (layer 3), and concluding with the challenges associated with implementing MCED tests (layer 4). Each layer employs brief descriptions to represent key concepts, methods, barriers, and benefits, connected through a series of arrows to demonstrate the flow and relationships between layers. The figure concludes with a call to action, pointing toward the need for further research and pilot programs in the journey toward the effective implementation of MCED tests in cervical cancer screening.
In underserved communities, addressing cultural barriers is crucial for successfully implementing MCED. Building trust through community-led education and addressing misconceptions are key components of this approach. Technology offers innovative ways to amplify these efforts.
Collaborating with organizations like the Partnership for Native American Cancer Prevention (NACP) highlights the importance of engaging community leaders. Their expertise in developing culturally sensitive messaging and outreach fosters trust and promotes participation (100). Additionally, targeted initiatives, such as student-led educational programs modeled on those used for skin cancer awareness, can increase overall health literacy and empower individuals from diverse backgrounds to make informed decisions about their health (101).
Technology may significantly expand the reach of these educational efforts. Adapting platforms like Shezlong, an online mental health platform offering virtual therapy services, can potentially deliver tailored MCED information to underserved populations and could address specific concerns and potential misconceptions (102). However, for these tools to be effective, they must be designed with an understanding of the cultural context, technological literacy, and the practical limitations faced by these communities.
Government subsidies integrated into national health schemes are essential for making MCED tests accessible to underserved communities. Even when diagnostics are nominally covered, out-of-pocket costs remain a substantial barrier for low-income individuals, as evidenced by analyses across several Asian countries (103). Successful subsidy programs must address the cost of MCED tests and associated logistical expenses, such as transportation and potential follow-up care, that can deter participation.
Streamlining reimbursement systems within subsidized schemes is crucial to avoid overburdening clinics serving underserved populations. Inefficient processes create additional barriers for healthcare providers (104). The long-term cost-effectiveness of preventative care, including cancer screening, has been well-documented (105), strengthening the argument for upfront investments in MCED accessibility.
Strategic partnerships with non-governmental organizations (NGOs) and international organizations with expertise in healthcare delivery, technology deployment, and community engagement are essential for the successful and equitable implementation of MCED in underserved settings. These partnerships offer resources and insights vital to addressing complex logistical, technological, and trust-related barriers.
NGOs specializing in healthcare provision in remote or low-resource areas possess significant knowledge of how to overcome access challenges. Their experience in tailoring technology deployment to the specific needs of underserved populations, as emphasized in child health initiatives (106), will be crucial for MCED. The design of MCED interfaces, result-reporting systems, and any associated technologies must be done in close collaboration with these NGOs to ensure compatibility and functionality within the constraints of rural or underfunded clinics.
Building community trust is essential for adopting novel screening technologies like MCED. Collaborations with NGOs that have strong community connections and a history of addressing health misconceptions, such as those combating cancer stigma in Pakistan (107), will be essential. These NGOs can lead targeted awareness campaigns and facilitate open dialogue between communities and healthcare providers. To ensure ethical deployment and rigorous evaluation of MCED in diverse contexts, partnering with NGOs specializing in community-engaged research is vital (10). Their expertise will ensure diverse patient perspectives are included throughout the process, from early-stage technology adaptations to long-term impact assessments.
The utilization of technology and telemedicine presents a promising solution to overcome geographical barriers that limit MCED access in underserved areas. Telemedicine, the practice of providing healthcare services remotely via telecommunications technology, may substantially alter how MCED results are delivered and how patients are monitored.
Research demonstrates the feasibility of remotely transmitting MCED sample results to centralized labs equipped with specialized staff (108). This addresses a critical challenge in areas where local clinics may lack the expertise to interpret complex test results. Furthermore, telemedicine is essential for continuous patient monitoring after MCED screening (109), ensuring timely follow-up care—a crucial component of effective cancer screening, especially in communities where traveling long distances for appointments is burdensome.
To maximize the reach of telemedicine in MCED implementation, user-friendly interfaces are critical, especially in populations with varying levels of technological literacy. Collaborating with community organizations can ensure these interfaces are designed with the specific needs of underserved populations in mind. Encouragingly, there is growing acceptance of telemedicine among healthcare providers, which is vital for successful MCED integration (110). However, alongside technological innovation, it is essential to proactively address concerns around data privacy and establish sustainable reimbursement models within healthcare systems to ensure equitable, long-term access.
This review underscores the urgent need to transform cervical cancer screening approaches, particularly within underserved communities. MCED tests hold the potential to revolutionize screening access due to their ability to overcome many traditional logistical and cultural barriers. However, successful implementation requires concerted efforts to address financial constraints, optimize deployment strategies, and ensure cultural sensitivity.
Future research must prioritize the rigorous clinical validation of MCED technologies, solidifying their efficacy in early cancer detection (111, 112). Simultaneously, investigations into cost-effective and contextually adaptable implementation strategies for low-resource settings are imperative (113, 114). To address potential reluctance and misinformation, community engagement in developing culturally appropriate outreach programs is essential (115).
Reducing cervical cancer’s global burden, with a focus on marginalized populations, demands an integrated approach. Technological innovation alone is insufficient. Concurrent policy changes promoting equitable distribution and access to MCED are essential. Research and policy efforts must collaborate to create an environment where all individuals, regardless of location or socioeconomic circumstances, have access to life-saving cancer screening services.
AA: Writing – review & editing, Writing – original draft, Visualization, Methodology, Investigation, Data curation, Conceptualization. HI: Writing – review & editing. TH: Writing – review & editing, Validation, Supervision. HH: Writing – review & editing. MM: Writing – review & editing. EL: Writing – review & editing, Validation, Supervision, Project administration.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
AA, MM, HH, EL, and TH CEO are employees of Hirotsu Bio Science, which develops and markets the N-NOSE, a technology for cancer detection. Although the N-NOSE is not directly mentioned in our manuscript, our affiliation with Hirotsu Bio Science could be perceived as a potential conflict of interest due to our involvement with commercially available cancer detection products.
The remaining author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Petersen Z, Jaca A, Ginindza TG, Maseko G, Takatshana S, Ndlovu P, et al. Barriers to uptake of cervical cancer screening services in low-and-middle-income countries: a systematic review. BMC Women’s Health. (2022) 22:486. doi: 10.1186/s12905-022-02043-y
2. Viveros-Carreño D, Fernandes A, Pareja R. Updates on cervical cancer prevention. Int J Gynecol Cancer. (2023) 33:394–402. doi: 10.1136/ijgc-2022-003703
3. Rezende GAS, Rezende MT, Carneiro CM. Low-cost interventions to improve cervical cancer screening: an integrative review. Oncol Nurs Forum. (2022) 50:59–78. doi: 10.1188/23.ONF.59-78
4. Ton M, Swami N, Germar MJV, Dee EC. HPV mRNA testing in cervical cancer screening: implications for low- and middle-income countries. Int J Gynecologic Cancer. (2022) 32(12):1632–3. doi: 10.1136/ijgc-2022-003959
5. Blumenthal D, Mort E, Edwards J. The efficacy of primary care for vulnerable population groups. Health Serv Res. (1995) 30:253–73.
6. Weitz TA, Freund KM, Wright L. Identifying and caring for underserved populations: experience of the National Centers of Excellence in Women’s Health. J Womens Health Gend Based Med. (2001) 10:937–52. doi: 10.1089/152460901317193521
7. Brawley O, Berger M. Cancer and disparities in health: Perspectives on health statistics and research questions. Cancer. (2008) 113(7 Suppl):1744–54. doi: 10.1002/cncr.v113:7+
8. Yedjou C, Tchounwou P, Payton M, Miele L, Fonseca DD, Lowe L, et al. Assessing the racial and ethnic disparities in breast cancer mortality in the United States. Int J Environ Res Public Health. (2017) 14(5):486. doi: 10.3390/ijerph14050486
9. Etzioni R, Gulati R, Weiss NS. Multicancer early detection: learning from the past to meet the future. JNCI: J Natl Cancer Institute. (2022) 114:349–52. doi: 10.1093/jnci/djab168
10. Kessler L, Le Beau MM, Smith RA, Walter FM, Wender R. The modeling of multicancer early detection (MCED) tests’ residual risk and the challenges of MCED evaluation and implementation. Cancer. (2023) 129:1966–8. doi: 10.1002/cncr.34745
11. Bhattacharyya AK, Nath JD, Deka H. Comparative study between pap smear and visual inspection with acetic acid (via) in screening of CIN and early cervical cancer. J Midlife Health. (2015) 6:53–8. doi: 10.4103/0976-7800.158942
12. Nkwabong E, Laure Bessi Badjan I, Sando Z. Pap smear accuracy for the diagnosis of cervical precancerous lesions. Trop Doct. (2019) 49:34–9. doi: 10.1177/0049475518798532
13. Jansen EEL, Zielonke N, Gini A, Anttila A, Segnan N, Vokó Z, et al. Effect of organised cervical cancer screening on cervical cancer mortality in Europe: a systematic review. Eur J Cancer. (2020) 127:207–23. doi: 10.1016/j.ejca.2019.12.013
14. Updated Cervical Cancer Screening Guidelines . Available online at: https://www.acog.org/clinical/clinical-guidance/practice-advisory/articles/2021/04/updated-cervical-cancer-screening-guidelines (Accessed July 2, 2024).
15. Simms KT, Keane A, Nguyen DTN, Caruana M, Hall MT, Lui G, et al. Benefits, harms and cost-effectiveness of cervical screening, triage and treatment strategies for women in the general population. Nat Med. (2023) 29:3050–8. doi: 10.1038/s41591-023-02600-4
16. Cromwell I, Smith LW, van der Hoek K, Hedden L, Coldman AJ, Cook D, et al. Cost-effectiveness analysis of primary human papillomavirus testing in cervical cancer screening: Results from the HPV FOCAL Trial. Cancer Med. (2021) 10:2996–3003. doi: 10.1002/cam4.3864
17. Kyrgiou M, Athanasiou A, Paraskevaidi M, Mitra A, Kalliala I, Martin-Hirsch P, et al. Adverse obstetric outcomes after local treatment for cervical preinvasive and early invasive disease according to cone depth: systematic review and meta-analysis. BMJ. (2016) 354:i3633. doi: 10.1136/bmj.i3633
18. Basu P, Mittal S, Banerjee D, Singh P, Panda C, Dutta S, et al. Diagnostic accuracy of VIA and HPV detection as primary and sequential screening tests in a cervical cancer screening demonstration project in India. Int J Cancer. (2015) 137:859–67. doi: 10.1002/ijc.v137.4
19. Zhao F-H, Lewkowitz AK, Chen F, Lin MJ, Hu S-Y, Zhang X, et al. Pooled analysis of a self-sampling HPV DNA Test as a cervical cancer primary screening method. J Natl Cancer Inst. (2012) 104:178–88. doi: 10.1093/jnci/djr532
20. Bansil P, Wittet S, Lim JL, Winkler JL, Paul P, Jeronimo J. Acceptability of self-collection sampling for HPV-DNA testing in low-resource settings: a mixed methods approach. BMC Public Health. (2014) 14:596. doi: 10.1186/1471-2458-14-596
21. Castellsagué X, Díaz M, de Sanjosé S, Muñoz N, Herrero R, Franceschi S, et al. Worldwide human papillomavirus etiology of cervical adenocarcinoma and its cofactors: implications for screening and prevention. J Natl Cancer Inst. (2006) 98:303–15. doi: 10.1093/jnci/djj067
22. Zhu Y, Wang Y, Hirschhorn J, Welsh KJ, Zhao Z, Davis MR, et al. Human papillomavirus and its testing assays, cervical cancer screening, and vaccination. Adv Clin Chem. (2017) 81:135–92. doi: 10.1016/bs.acc.2017.01.004
23. Benevolo M, Vocaturo A, Caraceni D, French D, Rosini S, Zappacosta R, et al. Sensitivity, specificity, and clinical value of human papillomavirus (HPV) E6/E7 mRNA assay as a triage test for cervical cytology and HPV DNA test. J Clin Microbiol. (2011) 49(7):2643–50. doi: 10.1128/JCM.02570-10
24. Kusakabe M, Taguchi A, Sone K, Mori M, Osuga Y. Carcinogenesis and management of human papillomavirus-associated cervical cancer. Int J Clin Oncol. (2023) 28:965–74. doi: 10.1007/s10147-023-02337-7
25. Wentzensen N, von Knebel Doeberitz M. Biomarkers in cervical cancer screening. Dis Markers. (2007) 23:315–30. doi: 10.1155/2007/678793
26. Schiffman M, Wentzensen N, Wacholder S, Kinney W, Gage JC, Castle PE. Human papillomavirus testing in the prevention of cervical cancer. J Natl Cancer Inst. (2011) 103:368–83. doi: 10.1093/jnci/djq562
27. Bos AB, Rebolj M, Habbema JDF, van Ballegooijen M. Nonattendance is still the main limitation for the effectiveness of screening for cervical cancer in the Netherlands. Int J Cancer. (2006) 119:2372–5. doi: 10.1002/ijc.22114
28. Uppal S, Chapman C, Spencer RJ, Jolly S, Maturen K, Rauh-Hain JA, et al. Association of hospital volume with racial and ethnic disparities in locally advanced cervical cancer treatment. Obstet Gynecol. (2017) 129:295–304. doi: 10.1097/AOG.0000000000001819
29. Sabatino SA. Up-to-date breast, cervical, and colorectal cancer screening test use in the United States, 2021. Prev Chronic Dis. (2023) 20:230071. doi: 10.5888/pcd20.230071
30. Paskett ED, McLaughlin JM, Reiter PL, Lehman AM, Rhoda DA, Katz ML, et al. Psychosocial predictors of adherence to risk-appropriate cervical cancer screening guidelines: a cross sectional study of women in Ohio Appalachia participating in the Community Awareness Resources and Education (CARE) project. Prev Med. (2010) 50:74–80. doi: 10.1016/j.ypmed.2009.09.001
31. Cancino RS, Su Z, Mesa R, Tomlinson GE, Wang J. The impact of COVID-19 on cancer screening: challenges and opportunities. JMIR Cancer. (2020) 6:e21697. doi: 10.2196/21697
32. Lohiya A, Daniel RA, Kumar D, Varghese C, Rath RS, S AR, et al. Effectiveness of visual inspection with acetic acid (VIA) screening on cervical cancer mortality and incidence - A systematic review and meta-analysis. Asian Pac J Cancer Prev. (2022) 23:399–407. doi: 10.31557/APJCP.2022.23.2.399
33. Ardahan M, Temel AB. Visual inspection with acetic acid in cervical cancer screening. Cancer Nurs. (2011) 34:158. doi: 10.1097/NCC.0b013e3181efe69f
34. Burness JV, Schroeder JM, Warren JB. Cervical colposcopy: indications and risk assessment. afp. (2020) 102:39–48.
35. Klam S, Arseneau J, Mansour N, Franco E, Ferenczy A. Comparison of endocervical curettage and endocervical brushing. Obstet Gynecol. (2000) 96:90–4. doi: 10.1016/S0029-7844(00)00836-X
36. Goksedef BPC, Api M, Kaya O, Gorgen H, Tarlaci A, Cetin A. Diagnostic accuracy of two endocervical sampling method: randomized controlled trial. Arch Gynecol Obstet. (2013) 287:117–22. doi: 10.1007/s00404-012-2542-9
37. Pleş L, Radosa J-C, Sima R-M, Chicea R, Olaru O-G, Poenaru M-O. The accuracy of cytology, colposcopy and pathology in evaluating precancerous cervical lesions. Diagnostics (Basel). (2022) 12:1947. doi: 10.3390/diagnostics12081947
38. Kamal M. Cervical pre-cancers: biopsy and immunohistochemistry. Cytojournal. (2022) 19:38. doi: 10.25259/CMAS_03_13_2021
39. Homesley HD, Jobson VW, Reish RL. Use of colposcopically directed, four-quadrant cervical biopsy by the colposcopy trainee. J Reprod Med. (1984) 29:311–6.
40. Elit LM. Pitfalls in the diagnosis of cervical intraepithelial neoplasia 1. J Lower Genital Tract Dis. (2004) 8:181. doi: 10.1097/00128360-200407000-00004
41. del Carmen MG, Findley M, Muzikansky A, Roche M, Verrill CL, Horowitz N, et al. Demographic, risk factor, and knowledge differences between Latinas and non-Latinas referred to colposcopy. Gynecol Oncol. (2007) 104:70–6. doi: 10.1016/j.ygyno.2006.07.008
42. Lofters A, Glazier RH, Agha MM, Creatore MI, Moineddin R. Inadequacy of cervical cancer screening among urban recent immigrants: A population-based study of physician and laboratory claims in Toronto, Canada. Prev Med. (2007) 44:536–42. doi: 10.1016/j.ypmed.2007.02.019
43. Lu M, Moritz S, Lorenzetti D, Sykes L, Straus S, Quan H. A systematic review of interventions to increase breast and cervical cancer screening uptake among Asian women. BMC Public Health. (2012) 12:413. doi: 10.1186/1471-2458-12-413
44. Melan K, Janky E, Macni J, Ulric-Gervaise S, Dorival M-J, Veronique-Baudin J, et al. Epidemiology and survival of cervical cancer in the French West-Indies: data from the Martinique Cancer Registry (2002–2011). Global Health Action. (2017) 10:1337341. doi: 10.1080/16549716.2017.1337341
45. Goodwin BC, Rowe AK, Crawford-Williams F, Baade P, Chambers SK, Ralph N, et al. Geographical disparities in screening and cancer-related health behaviour. Int J Environ Res Public Health. (2020) 17:1246. doi: 10.3390/ijerph17041246
46. Cox E, Awe M, Sabu S, Tumin D, Akpan US. Does greater distance from the hospital exacerbate socioeconomic barriers to neonatal intensive care unit clinic attendance? J Rural Med. (2023) 18:55–61. doi: 10.2185/jrm.2022-035
47. Di J, Rutherford S, Chu C. Review of the cervical cancer burden and population-based cervical cancer screening in China. Asian Pac J Cancer Prev. (2015) 16:7401–7. doi: 10.7314/APJCP.2015.16.17.7401
48. Bischoff CM, Kanarek NF. Abstract A01: No Maryland county left behind: Statewide intervention may reduce geographic disparities. Cancer Epidemiol Biomarkers Prev. (2014) 21:A01. doi: 10.1158/1055-9965.DISP12-A01
49. Orji AF, Sodeyi MY, Anoke CI, Cevasco KE, Orji BC. Disparities in cervical cancer screening by disability types: a systematic review. J Canc Educ. (2023) 38:752–60. doi: 10.1007/s13187-023-02280-1
50. Orji AF, Roess AA. Assessing disparities in cervical cancer screening with pap test by disability types. J Cancer Educ. (2024) 39:39–49. doi: 10.1007/s13187-023-02373-x
51. Tesfaye S, Litwin T, Schully S. Abstract A120: Disparities in cervical cancer screening rates and electronic health record completeness among All of Us Research Program participants. Cancer Epidemiol Biomarkers Prev. (2023) 32:A120. doi: 10.1158/1538-7755.DISP22-A120
52. Chen JJ, Sarkar IN, Hsu E, Dizon DS. An intersectional approach to cervical cancer screening disparities by race/ethnicity and immigrant status. J Clin Oncol. (2023) 41(16_suppl):6536. doi: 10.1200/JCO.2023.41.16_suppl.6536
53. Patel AV, Tong G, Jones BA, Hernandez-Ramirez RU, Tang W, Megiel S, et al. Abstract C124: Racial and ethnic disparities in follow-up of abnormal cervical cancer screening results. Cancer Epidemiol Biomarkers Prev. (2023) 32:C124. doi: 10.1158/1538-7755.DISP23-C124
54. Marlow LAV, Wardle J, Waller J. Understanding cervical screening non-attendance among ethnic minority women in England. Br J Cancer. (2015) 113:833–9. doi: 10.1038/bjc.2015.248
55. Chidyaonga-Maseko F, Chirwa ML, Muula AS. Underutilization of cervical cancer prevention services in low and middle income countries: a review of contributing factors. Pan Afr Med J. (2015) 21:231. doi: 10.11604/pamj.2015.21.231.6350
56. Alam Z, Deol H, Dean JA, Janda M. Reasons behind Low Cervical Screening Uptake among South Asian Immigrant Women: A Qualitative Exploration. Int J Environ Res Public Health. (2022) 19:1527. doi: 10.3390/ijerph19031527
57. Banura C, Mirembe FM, Katahoire AR, Namujju PB, Mbidde EK. Universal routine HPV vaccination for young girls in Uganda: a review of opportunities and potential obstacles. Infect Agent Cancer. (2012) 7:24. doi: 10.1186/1750-9378-7-24
58. LaMontagne DS, Barge S, Le NT, Mugisha E, Penny ME, Gandhi S, et al. Human papillomavirus vaccine delivery strategies that achieved high coverage in low- and middle-income countries. Bull World Health Organ. (2011) 89:821–830B. doi: 10.2471/BLT.11.089862
59. Gutusa F, Roets L. Early cervical cancer screening: The influence of culture and religion. Afr J Prim Health Care Fam Med. (2023) 15:3776. doi: 10.4102/phcfm.v15i1.3776
60. Qaderi K, Mirmolaei ST, Geranmayeh M, Farnam F, Sheikh Hasani S. ’Does HPV affect my fertility?’Reproductive concerns of HPV-positive women: a qualitative study. Reprod Health. (2021) 18:1–11. doi: 10.1186/s12978-021-01126-7
61. Fokom Domgue J, Cunningham SA, Yu RK, Shete S. Reasons for not receiving the HPV vaccine among eligible adults: Lack of knowledge and of provider recommendations contribute more than safety and insurance concerns. Cancer Med. (2020) 9:5281–90. doi: 10.1002/cam4.3192
62. Chan DNS, So WKW. Influential barriers perceived by South Asians in Hong Kong to undergoing cervical cancer screening. Eur J Cancer Care (Engl). (2022) 31:e13556. doi: 10.1111/ecc.13556
63. Cuevas R, Saini P, Roberts D, Beaver K, Chandrashekar M, Jain A, et al. A systematic review of barriers and enablers to South Asian women’s attendance for asymptomatic screening of breast and cervical cancers in emigrant countries. BMJ Open. (2018) 8:e020892. doi: 10.1136/bmjopen-2017-020892
64. Afsah YR, Kaneko N. Barriers to cervical cancer screening faced by immigrant Muslim women: a systematic scoping review. BMC Public Health. (2023) 23:2375. doi: 10.1186/s12889-023-17309-9
65. Srinath A, van Merode F, Rao SV, Pavlova M. Barriers to cervical cancer and breast cancer screening uptake in low-and middle-income countries: a systematic review. Health Policy Plann. (2023) 38:509–27. doi: 10.1093/heapol/czac104
66. Chandrakumar A, Hoon E, Benson J, Stocks N. Barriers and facilitators to cervical cancer screening for women from culturally and linguistically diverse backgrounds; a qualitative study of GPs. BMJ Open. (2022) 12:e062823. doi: 10.1136/bmjopen-2022-062823
67. Hamdiui N, Marchena E, Stein ML, van Steenbergen JE, Crutzen R, van Keulen HM, et al. Decision-making, barriers, and facilitators regarding cervical cancer screening participation among Turkish and Moroccan women in the Netherlands: a focus group study. Ethnicity Health. (2022) 27:1147–65. doi: 10.1080/13557858.2020.1863921
68. Hyman I, Cameron JI, Singh PM, Stewart DE. Physicians and Pap testing in the Chinese and Vietnamese communities in Toronto. J Health Care Poor Underserved. (2003) 14:489–502. doi: 10.1353/hpu.2010.0704
69. Ferdous M, Lee S, Goopy S, Yang H, Rumana N, Abedin T, et al. Barriers to cervical cancer screening faced by immigrant women in Canada: a systematic scoping review. BMC Womens Health. (2018) 18:165. doi: 10.1186/s12905-018-0654-5
70. Jong FC, Kotzur M, Amiri R, Ling J, Mooney JD, Robb KA. Qualitative evaluation of a codesigned faith-based intervention for Muslim women in Scotland to encourage uptake of breast, colorectal and cervical cancer screening. BMJ Open. (2022) 12:e058739. doi: 10.1136/bmjopen-2021-058739
71. Wilding S, O’Connor DB, Conner M. Social norms in cervical cancer screening. Psychol Rep. (2023) 4:00332941231219943. doi: 10.1177/00332941231219943
72. Ntsayagae E, Koyabe B, Major T, Molwane O, Monare B. Use of mixed method approach on the norms and beliefs related to cervical cancer screening among women aged 25-49 in Botswana: A pilot study. JGO. (2018) 4:31s–s. doi: 10.1200/jgo.18.38500
73. Surendran S, Foo CD, Tan DHY, Tan WH, Melody J, Ooi JLX, et al. Understanding barriers and facilitators of breast and cervical cancer screening among Singapore women: A qualitative approach. Asian Pac J Cancer Prev. (2023) 24:889–95. doi: 10.31557/APJCP.2023.24.3.889
74. Hosseini Z, Mohseni S, Momeni R, Aghamolaei T, Alavi A, Dadipoor S. Increasing cervical cancer screening in Iran: effectiveness of a theory-based educational intervention. Reprod Health. (2022) 19:186. doi: 10.1186/s12978-022-01489-5
75. Hamdiui N, Stein ML, van Steenbergen J, Crutzen R, Bouman M, Khan A, et al. Evaluation of a web-based culturally sensitive educational video to facilitate informed cervical cancer screening decisions among Turkish- and Moroccan-Dutch women aged 30 to 60 years: randomized intervention study. J Med Internet Res. (2022) 24:e35962. doi: 10.2196/35962
76. Orimadegun Boluwatito C, Oyerinde Oyewole O. Knowledge Perceived-risk and Screening-uptake for cervical cancer among Women in a Christian Religious Institution in Ibadan Oyo State Nigeria. Texila International Journal of Public Health (2019) 8(3):167–180. doi: 10.21522/TIJPH.2013.08.03.Art026
77. Nyblade L, Stockton M, Travasso S, Krishnan S. A qualitative exploration of cervical and breast cancer stigma in Karnataka, India. BMC Womens Health. (2017) 17:58. doi: 10.1186/s12905-017-0407-x
78. Zeno EE, Brewer NT, Spees LP, Marais ACD, Sanusi BO, Hudgens MG, et al. Racial and ethnic differences in cervical cancer screening barriers and intentions: The My Body My Test-3 HPV self-collection trial among under-screened, low-income women. PloS One. (2022) 17(10). doi: 10.1371/journal.pone.0274974
79. Nahvijou A, Hadji M, Marnani AB, Tourang F, Bayat N, Weiderpass E, et al. A systematic review of economic aspects of cervical cancer screening strategies worldwide: discrepancy between economic analysis and policymaking. Asian Pac J Cancer Prev. (2014) 15:8229–37. doi: 10.7314/APJCP.2014.15.19.8229
80. Sefuthi T, Nkonki L. A systematic review of economic evaluations of cervical cancer screening methods. Syst Rev. (2022) 11:162. doi: 10.1186/s13643-022-02017-z
81. Sokale IO, Oluyomi AO, Montealegre JR, Thrift AP. Racial/ethnic disparities in cervical cancer stage at diagnosis: mediating effects of neighborhood-level socioeconomic deprivation. Cancer Epidemiol Biomarkers Prev. (2023) 32:818–24. doi: 10.1158/1055-9965.EPI-23-0038
82. Rodney P, Rodney ZK, Nu S, Hemans-Richards JE. Cervical cancer and black women: an analysis of the disparity in prevalence of cervical cancer. J Health Care Poor Underserved. (2002) 13:24–37. doi: 10.1353/hpu.2010.0366
83. Powell TC, Dilley SE, Bae S, Straughn JM, Kim KH, Leath CA. The impact of racial, geographic and socioeconomic risk factors on the development of advanced stage cervical cancer. J Low Genit Tract Dis. (2018) 22:269–73. doi: 10.1097/LGT.0000000000000421
84. Buskwofie A, David-West G, Clare CA. A review of cervical cancer: incidence and disparities. J Natl Med Assoc. (2020) 112:229–32. doi: 10.1016/j.jnma.2020.03.002
85. Quercia K, Tran PL, Jinoro J, Herniainasolo JL, Viviano M, Vassilakos P, et al. A mobile health data collection system for remote areas to monitor women participating in a cervical cancer screening campaign. Telemed J E Health. (2018) 24:277–82. doi: 10.1089/tmj.2017.0146
86. Gizaw Z, Astale T, Kassie GM. What improves access to primary healthcare services in rural communities? A systematic review. BMC Primary Care. (2022) 23:313. doi: 10.1186/s12875-022-01919-0
87. Dalton H, Cosgrave C, MacKinnon D. Teen Clinic - An integrated primary healthcare model that improves access for young people in rural communities. Aust J Rural Health. (2023) 31:1050–9. doi: 10.1111/ajr.13003
88. Ferrari A, Neefs I, Hoeck S, Peeters M, Van Hal G. Towards novel non-invasive colorectal cancer screening methods: a comprehensive review. Cancers. (2021) 13:1820. doi: 10.3390/cancers13081820
89. Zhou C-B, Pan S-Y, Jin P, Deng J-W, Xue J-H, Ma X-Y, et al. Fecal signatures of Streptococcus anginosus and Streptococcus constellatus for noninvasive screening and early warning of gastric cancer. Gastroenterology. (2022) 162:1933–1947.e18. doi: 10.1053/j.gastro.2022.02.015
90. Richman AR, Torres E, Wu Q, Kampschroeder AP. Evaluating a community-based breast cancer prevention program for rural underserved latina and black women. J Community Health. (2020) 45:1205–10. doi: 10.1007/s10900-020-00856-2
91. Hubbell E, Clarke CA, Aravanis AM, Berg CD. Modeled reductions in late-stage cancer with a multi-cancer early detection test. Cancer Epidemiol Biomarkers Prev. (2021) 30:460–8. doi: 10.1158/1055-9965.EPI-20-1134
92. Brito-Rocha T, Constâncio V, Henrique R, Jerónimo C. Shifting the cancer screening paradigm: the rising potential of blood-based multi-cancer early detection tests. Cells. (2023) 12:935. doi: 10.3390/cells12060935
93. Betancourt JR, Green AR, Carrillo JE, Ananeh-Firempong O. Defining cultural competence: a practical framework for addressing racial/ethnic disparities in health and health care. Public Health Rep. (2003) 118:293–302. doi: 10.1016/S0033-3549(04)50253-4
94. Gupta S, Sussman DA, Doubeni CA, Anderson DS, Day L, Deshpande AR, et al. Challenges and possible solutions to colorectal cancer screening for the underserved. J Natl Cancer Inst. (2014) 106:dju032. doi: 10.1093/jnci/dju032
95. Sankaré IC, Bross R, Brown AF, del Pino HE, Jones LF, Morris DM, et al. Strategies to build trust and recruit African American and Latino community residents for health research: A cohort study. Clin Trans Sci. (2015) 8:412–20. doi: 10.1111/cts.12273
96. Dang JH, Rodriguez EM, Luque JS, Erwin DO, Meade CD, Chen MS. Engaging diverse populations about biospecimen donation for cancer research. J Community Genet. (2014) 5:313–27. doi: 10.1007/s12687-014-0186-0
97. Venn O, Bredno J, Thornton A, Hubbell E, Kurtzman K, Beausang J, et al. Abstract C139: The robustness of a targeted methylation-based multi-cancer early detection (MCED) test to population differences in self-reported ethnicity. Cancer Epidemiol Biomarkers Prev. (2023) 32:C139. doi: 10.1158/1538-7755.DISP23-C139
98. Van Hal G, Zeeb H, de Koning HJ. Editorial: social inequality in cancer screening. Front Public Health. (2022) 10:854659. doi: 10.3389/fpubh.2022.854659
99. Kumar NM, Chopra SS. Leveraging blockchain and smart contract technologies to overcome circular economy implementation challenges. Sustainability. (2022) 14:9492. doi: 10.3390/su14159492
100. Sanderson PR, Monroy F, Brown H, Harris R. Abstract IA008: Weaving university and community-based partnership. Cancer Epidemiol Biomarkers Prev. (2023) 32:IA008–8. doi: 10.1158/1538-7755.DISP23-IA008
101. Abdelwahab R, Abdou M, Newman C. Piloting a community education skin cancer program coordinated by medical students. JMIR Dermatol. (2022) 5:e36793. doi: 10.2196/36793
102. Elsafty A, Shawky M. How online psychotherapy platforms facilitate accessibility to mental health services in Cairo; the case of Shezlong mental therapy platform. Int J Soc Sci Stud. (2023) 11:9–29. doi: 10.11114/ijsss.v11i6.6564
103. Bigio J, Hannay E, Pai M, Alisjahbana B, Das R, Huynh HB, et al. The inclusion of diagnostics in national health insurance schemes in Cambodia, India, Indonesia, Nepal, Pakistan, Philippines and Viet Nam. BMJ Global Health. (2023) 8:e012512. doi: 10.1136/bmjgh-2023-012512
104. Okunna N, Ezeama NN, Ezeama CO, Munala L. Exploring physicians′ experiences under the national health insurance scheme in Southeastern Nigeria. Int J Health Plann Manage. (2023) 38:398–415. doi: 10.1002/hpm.3591
105. Marchiori L, Pierrard O. Health subsidies, prevention and welfare. J Public Economic Theory. (2023) 25:1304–36. doi: 10.1111/jpet.12583
106. Mills N, Howsley P, Bartlett CM, Olubajo L, Dimitri P. Overcoming challenges to develop technology for child health. J Med Eng Technol. (2022) 46:547–57. doi: 10.1080/03091902.2022.2089254
107. Rani B. Illuminating the shadows: exploring the causes, overcoming challenges, and advancing solutions for skin cancers in Pakistan. Isra Med J. (2023) 15:35–6. doi: 10.55282/imj.ltr35
108. Bekaroo G, Santokhee A, Augusto JC. 5G smart and innovative healthcare services: Opportunities, challenges, and prospective solutions. In: 5G Multimedia Communication. CRC Press, Boca Raton, Florida (2020). p. 279–97.
109. Deng N, Chen J, Liu Y, Wei S, Sheng L, Lu R, et al. Using mobile health technology to deliver a community-based closed-loop management system for chronic obstructive pulmonary disease patients in remote areas of China: development and prospective observational study. JMIR mHealth uHealth. (2020) 8:e15978. doi: 10.2196/15978
110. Andronic O, Petrescu GED, Artamonov AR, Bolocan A, Rădăvoi D, Bran M, et al. Healthcare professionals’ Specialists’ Perception of telemedicine in Romania—A quantitative study of beliefs, practices, and expectations. Healthcare (Basel). (2023) 11:1552. doi: 10.3390/healthcare11111552
111. Saslow D, Solomon D, Lawson HW, Killackey M, Kulasingam SL, Cain J, et al. American Cancer Society, American Society for Colposcopy and Cervical Pathology, and American Society for Clinical Pathology screening guidelines for the prevention and early detection of cervical cancer. Am J Clin Pathol. (2012) 137:516–42. doi: 10.1309/AJCPTGD94EVRSJCG
112. Schiffman M, Doorbar J, Wentzensen N, De Sanjosé S, Fakhry C, Monk BJ, et al. Carcinogenic human papillomavirus infection. Nat Rev Dis Primers. (2016) 2:1–20. doi: 10.1038/nrdp.2016.86
113. Arbyn M, Weiderpass E, Bruni L, de Sanjosé S, Saraiya M, Ferlay J, et al. Estimates of incidence and mortality of cervical cancer in 2018: a worldwide analysis. Lancet Global Health. (2020) 8:e191–203. doi: 10.1016/S2214-109X(19)30482-6
114. Pham M-A, Benkortbi K, Kenfack B, Tincho Foguem E, Sormani J, Wisniak A, et al. Recruitment strategies to promote uptake of cervical cancer screening in the West Region of Cameroon. BMC Public Health. (2022) 22:548. doi: 10.1186/s12889-022-12951-1
Keywords: screening, MCED tests, disparities, early detection, underserved communities, cervical cancer
Citation: Alshammari AH, Ishii H, Hirotsu T, Hatakeyama H, Morishita M and di Luccio E (2024) Bridging the gap in cervical cancer screening for underserved communities: MCED and the promise of future technologies. Front. Oncol. 14:1407008. doi: 10.3389/fonc.2024.1407008
Received: 26 March 2024; Accepted: 09 July 2024;
Published: 29 July 2024.
Edited by:
Philip Rosenberg, National Cancer Institute (NIH), United StatesReviewed by:
Olugbenga Akindele Silas, University of Jos, NigeriaCopyright © 2024 Alshammari, Ishii, Hirotsu, Hatakeyama, Morishita and di Luccio. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Eric di Luccio, ZS5kaWx1Y2Npb0BoYmlvLmpw
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.