
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol., 04 July 2024
Sec. Skin Cancer
Volume 14 - 2024 | https://doi.org/10.3389/fonc.2024.1406872
This article is part of the Research TopicManagement of Rare Oncological CasesView all 62 articles
 Danielle H. Tran1,2*
Danielle H. Tran1,2* Ryan Shanley3
Ryan Shanley3 Alessio Giubellino4
Alessio Giubellino4 Peter H. Tang5,6
Peter H. Tang5,6 Dara D. Koozekanani6
Dara D. Koozekanani6 Jianling Yuan7
Jianling Yuan7 Kathryn Dusenbery7
Kathryn Dusenbery7 Evidio Domingo-Musibay8,9*
Evidio Domingo-Musibay8,9*Introduction: Metastatic uveal melanoma (mUM) is a difficult to treat disease. The liver is the primary site of metastasis in most patients, though uveal melanoma spreads widely in advanced disease. The only FDA approved immunotherapy medication for metastatic uveal melanoma is the HLA-A02:01 restricted bispecific T cell engager drug, Tebentafusp. Checkpoint inhibitor strategies and combination approaches have been tried with some limited success. We describe our experience treating patients at the University of Minnesota.
Methods: Patients were included if they had biopsy-confirmed mUM. Twenty-five (25) patients meeting the criteria were identified. Medical records were reviewed and data extracted for patient baseline characteristics and response to treatments.
Results: Median time to metastasis from the time of local therapy to the eye was 14.2 months (IQR; 9.3-22.0), and first site of metastasis was liver in 92% of patients. Two patients (8%) did not receive systemic therapy or radiation therapy for metastatic disease. Twenty-three (92%) patients received systemic therapy, 13 patients (52%) received ipilimumab-nivolumab as the first-line, while 4 patients (16%) received pembrolizumab. Landmark survival analysis by receipt of systemic therapy and radiation therapy treatments within 6 months of biopsy confirmed diagnosis is shown. Twenty patients (80%) received systemic therapy within 6 months of mUM diagnosis. Thirteen patients (52%) received liver directed radiation therapy within 6 months of mUM diagnosis.
Discussion: Within our cohort, there was no overall survival benefit for patients receiving treatment of metastatic disease within 6 months of mUM diagnosis, versus those electing later or no treatment at all. There was remarkable clinical activity of ipilimumab and nivolumab in a subset of patients with mUM, in agreement with prior studies, and metastatic PD-L1 positive tumors were associated with a prolonged survival.
Uveal melanoma (UM) is the most common primary intraocular tumor found in adults and arises within the uveal tract of the eye (1). Melanocytes within the iris, ciliary body, and choroid can give rise to malignancy with a propensity for invasion and metastasis. Primary UM can be treated with enucleation or with various strategies involving radiotherapy, including plaque brachytherapy, gamma-knife stereotactic radiosurgery (GK-SRS), and proton beam therapy. While adjuvant therapy for high-risk UM has been tried with some additional benefit (2), early metastasis is common, and overall survival remains poor (3). Nearly half of UM ultimately metastasize, with the most common extraocular sites being the liver (95%), lungs (24%), bone (16%), and skin and soft tissue (11%) (4).
Due to the high rate of liver metastasis, targeted therapy to this organ is common. The selection of optimal therapeutic modality generally depends on the location and extent of metastasis. Liver-directed therapies include image-guided ablation (thermal ablation, radiofrequency ablation, or cryoablation), radioembolization [also known as selective internal radiation therapy (SIRT)], immunoembolization (IE), and transarterial chemoembolization (TACE). Benefits have also been seen with isolated hepatic perfusion and hepatic artery infusion (5). In select cases of oligometastatic disease, resection of metastatic nodules and/or stereotactic radiation therapy (SBRT) can also be considered.
The use of systemic chemotherapy (dacarbazine, fotemustine, temozolomide, cisplatin, or a combination thereof) for metastatic disease has yielded disappointing results (6). Response rates to chemotherapy were generally less than 10%, and neither single nor multiagent chemotherapy extended overall survival (OS) in patients with metastatic disease. In the last decade, immunotherapy using immune checkpoint blockade with inhibitors for programmed death 1 (PD-1) and cytotoxic T-lymphocyte-associated antigen (CTLA-4) has shown promise. While efficacy of checkpoint inhibition has radically changed the approach to treatment of cutaneous melanoma and many other solid tumors, its efficacy in metastatic UM has been less dramatic.
In a retrospective analysis, 56 patients with metastatic UM refractory to prior lines of therapy were treated with anti-PD1 or anti-PDL1 therapy. Thirty-eight patients (68%) received pembrolizumab, 16 (29%) received nivolumab, and 2 (4%) received atezolizumab. Objective responses were seen in two patients for an overall response rate of 3.6% (7). In an analysis across 14 academic medical centers, the combination of nivolumab with ipilimumab yielded an overall response rate of 11.6%, median OS of 15 months, and median progression-free survival (PFS) of 2.7 months. Overall efficacy remains considerably lower than that seen for metastatic cutaneous melanoma (8). Similarly, in a recent prospective phase II study, patients received ipilimumab 3 mg/kg with nivolumab 1 mg/kg for four cycles, followed by nivolumab maintenance therapy for up to 2 years. The primary outcome of the study was overall response rate as determined by the Response Evaluation Criteria in Solid Tumors (RECIST) 1.1 criteria. Of 33 patients evaluated for efficacy, the overall response rate was 18%, including one confirmed complete response and five confirmed partial responses (9).
More recently, tebentafusp, a bispecific T-cell engager that redirects T cells to target glycoprotein 100-positive melanoma cells, has been approved for use in metastatic UM. Tebentafusp has been shown to produce longer OS than control therapy (either single-agent pembrolizumab, ipilimumab, or dacarbazine) with OS at 1 year of 73% in the tebentafusp group and 59% in the control group (10). Notably, the treatment is limited to patients that are HLA-A*02:01 positive and requires weekly IV infusions and initial blood pressure support and monitoring. There remains a critical unmet need for additional efficacious therapies in this patient population.
Here, we describe our recent experience in treating patients with metastatic UM at the University of Minnesota. Patients during this time period received several different treatments, including SBRT, Y90 radioembolization, chemotherapy, high-dose IL-2, dual- or single-agent checkpoint inhibitors, and cellular therapy. We report clinical data, response to radiation and systemic treatments, survival, and treatment-related adverse events in evaluable subjects.
We conducted a single-center retrospective study of patients with metastatic UM treated at the University of Minnesota between 2016 and 2022. Patients were included if they had biopsy-confirmed metastatic UM. Twenty-five patients meeting the criteria were identified. Medical records were reviewed, and data were extracted for patient baseline characteristics and response to therapy.
Biopsied metastatic lesions were assessed for expression of PD-L1 on the surface of the tumor cells at the University of Minnesota Central Pathology Laboratory or an external laboratory by means of immunohistochemical testing of formalin-fixed, paraffin-embedded tumor specimens using a monoclonal antihuman PD-L1 antibody. PD-L1 positivity was defined as having at least 1% of tumor cells showing PD-L1 staining of any intensity on the cell surface in 100 cells being evaluated. Seven patients in our study did not have PD-L1 results available.
Toxicity data were abstracted from clinical notes. Toxicity events were categorized. Imaging assessments were performed every 3 months using cross-sectional computed tomography (CT), combined positron emission tomography and CT (PET-CT), and magnetic resonance imaging (MRI) modalities. Response was evaluated by the treating oncologist and reported as the best overall response (BOR) using RECIST v1.1 criteria (11).
Survival was estimated using the Kaplan–Meier method and groups compared by log-rank test. Log-log confidence intervals are reported. OS of metastatic UM was measured from the date of biopsy-confirmed diagnosis of metastatic disease to the date of death or last known follow-up. Time to metastasis (TTM) was calculated from the date of local therapy to the eye to the date of biopsy-confirmed diagnosis of metastatic disease.
We used landmark analysis for overall survival through receipt of systemic therapy and radiation therapy. Six months after metastatic disease diagnosis was selected as the landmark time. Patients who experienced an event or were censored before the landmark were excluded from the analysis. For the analysis of OS by PD-L1 status, the full cohort was used, and the time of origin remained as the date of metastatic disease diagnosis. Statistical calculations were performed using R software, version 4.2.
We identified 25 patients with metastatic UM treated in our medical center with confirmed biopsy. Table 1 summarizes the baseline characteristics of the full cohort of patients. The median age at diagnosis of metastasis was 68.9 years old. All patients were Caucasian, and 13 patients (52%) were male. Median time to metastasis was 14.2 months (IQR; 9.3–22.0), and initial organ of spread was the liver in 92% of the patients. PD-L1 status was known for 18 patients; 4 were PD-L1+ and 14 were PD-L1-. PD-L1 status was not known for seven patients.
Two patients (2/25) did not receive systemic therapy or radiation therapy for metastatic disease. First-line treatment regimens are summarized in Table 2. Of the 23 patients that received systemic therapy, 13 patients (57%) received ipilimumab–nivolumab as a first-line, while 4 patients (17%) received pembrolizumab. Three patients received first-line sequential high dose IL-2 (6 weeks) and ipilimumab–nivolumab (13%), and 2 patients received first-line carboplatin–paclitaxel (9%). Nearly all patients (21/23, 95%) received ipilimumab and nivolumab at some point during their treatment. Of the patients receiving treatment, 13 (57%) received palliative intent radiation therapy within 6 months of metastasis diagnosis (Table 3).
Median OS for the entire patient cohort was 24 months. Figure 1 depicts the OS as measured from time of pathologic diagnosis of metastatic disease for all 25 patients. Figure 2 depicts the landmark analysis of OS, stratified by systemic therapy treatment initiation within 6 months of metastasis diagnosis. Overall survival for the 20 treated patients was 48% at 24 months after diagnosis (95% CI: 23, 69) versus 75% (95% CI: 13, 96) for the 4 patients not treated before 6 months (p = 0.57). Two patients who began treatment after 6 months were included in the non-treated group for the landmark analysis. One patient who received systemic therapy but died within 6 months of diagnosis was not included in the landmark analysis.
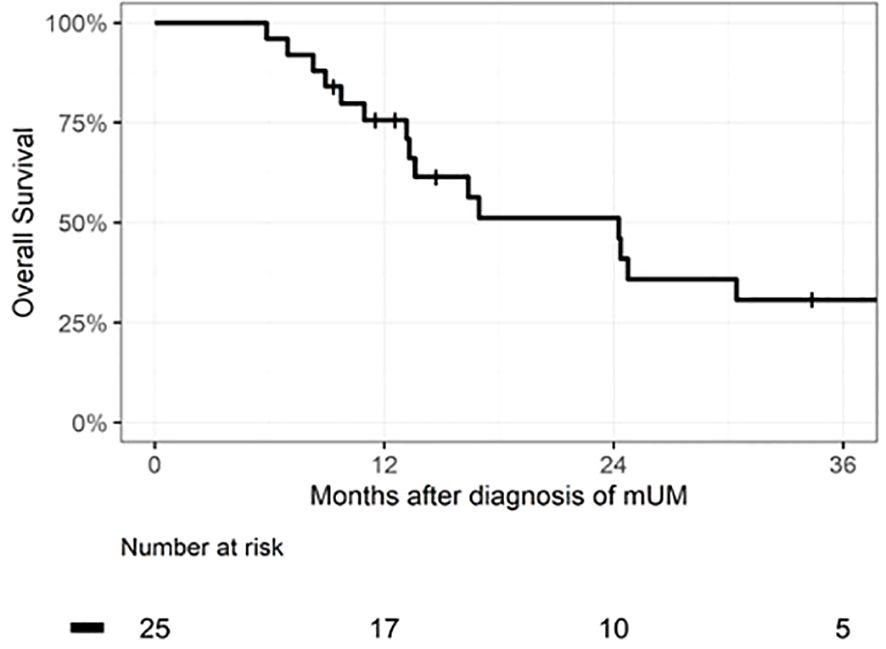
Figure 1 Kaplan–Meier curve of overall survival of the full cohort of patients diagnosed with metastatic uveal melanoma (mUM), n = 25 patients. Median survival time was 24 months (IQR 13–48).
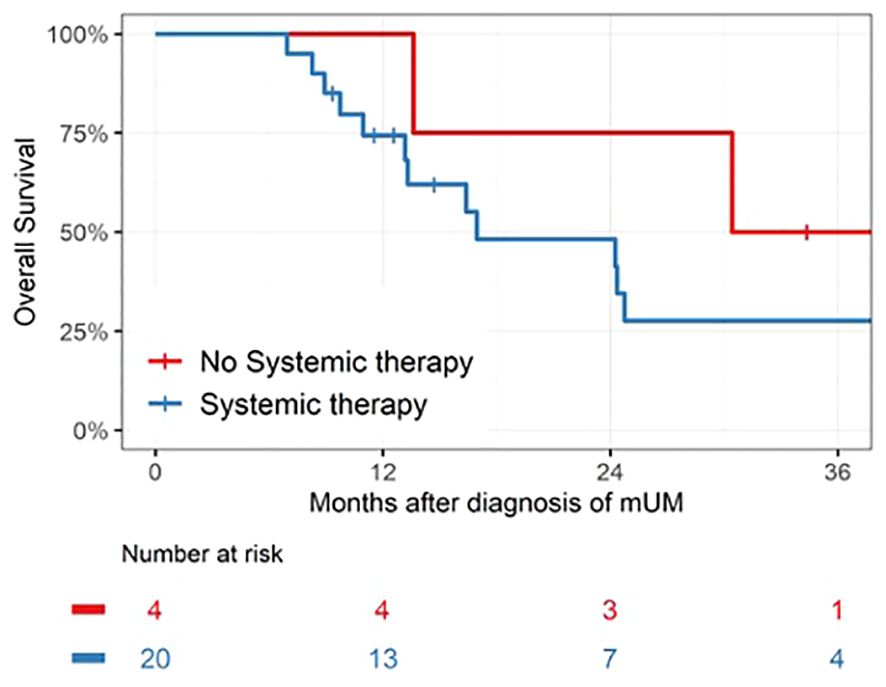
Figure 2 Landmark analysis of patients receiving systemic therapy within 6 months of mUM diagnosis. Median survival was 17 months (IQR 11 –50, n = 20) for the Systemic Therapy group versus 39 months (IQR 22–48, n = 4) for the No Systemic Therapy group, p = 0.57.
Patients receiving systemic immunotherapy were also included in the best overall response analysis to check-point blockade. In Figure 3, BOR for ipilimumab/nivolumab as first-line treatment was 15% versus 0% for pembrolizumab. There were two complete responses (CR) and three stable diseases (SD) with the combination of ipilimumab/nivolumab, while only one patient treated with pembrolizumab maintained stable disease.

Figure 3 Patients were evaluated for Best Overall Response (BOR) with (A) first-line ipilimumab/nivolumab (n = 13), compared with (B) first-line pembrolizumab (n = 4). CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease.
A total of 20 patients received radiation therapy for disease palliation, described in Table 3. Fourteen patients received liver-directed therapy (either SBRT or Y-90 radioemoblization), with one patient initially receiving SBRT to the liver followed by Y90 radioembolization at a later time. Two patients received GK SRS for brain metastases, three patients received palliative dose radiation therapy for bone metastases, and one patient received SBRT for lung metastasis.
A total of 13 patients received radiation therapy for metastases within 6 months of metastasis diagnosis: four received SBRT to dominant hepatic masses, eight received Y90-radioembolization to the liver (generally as two separate treatments), and one received palliative radiation to a painful bony vertebral metastasis. For the purpose of the landmark analysis, patients who initiated radiotherapy within 6 months were included in the Radiation Therapy group (n = 13). Patients who did not initiate radiotherapy within 6 months (n = 11) were counted in the No Radiation Therapy group, which included six patients who later initiated radiotherapy. There was no survival benefit seen with radiotherapy initiated within 6 months of diagnosis. OS at 24 months was 37% (95% CI: 11, 65) for the Radiation Therapy group versus 70% (95% CI: 33, 89) for the No Radiation Therapy group (p = 0.02; Figure 4).
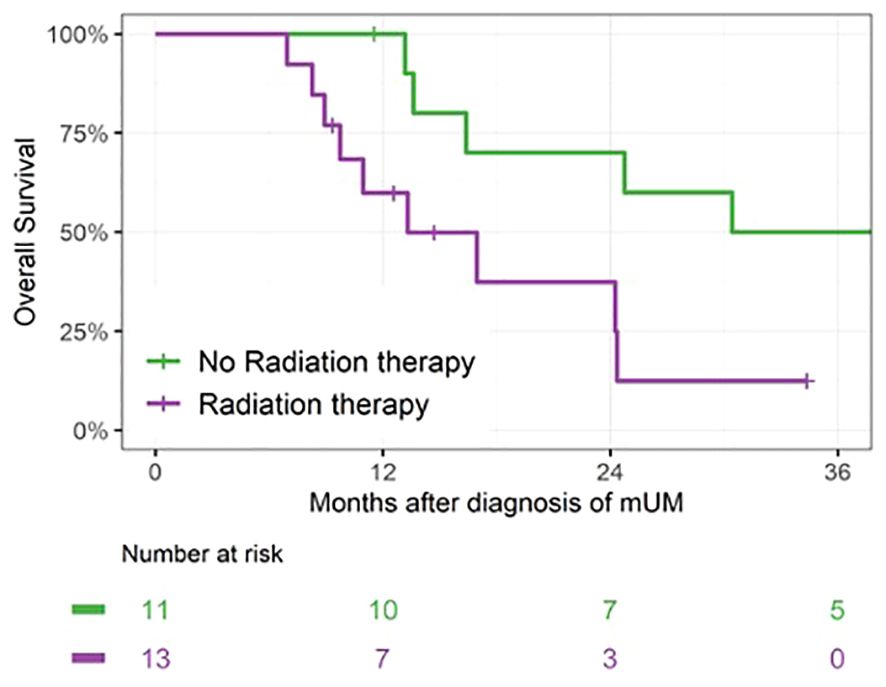
Figure 4 Landmark analysis of patients receiving radiation therapy within 6 months of mUM diagnosis. For patients in the Radiation Therapy group, median survival was 13 months (IQR 10–24, n = 13) versus 39 months (IQR 16–56, n = 11) for patients in the No Radiation Therapy group, p = 0.02.
We also assessed the potential impact of PD-L1 expression on survival. The median survival for PD- L1-negative cases (14/25, 56%) was 17 months and for PD-L1+ cases (4/25, 16%), it was 56 months (p = 0.04; Figure 5). All PD-L1+ patients received systemic therapy. None of the PD-L1+ received radiotherapy within 6 months.
Of the 23 patients who opted to receive systemic therapy, 16 reported immune-related adverse events (irAEs) during treatment with either pembrolizumab, ipilimumab/nivolumab, or maintenance nivolumab (Table 4). The median number of reported irAEs were 2 per patient (range: 0–6). The severity of irAEs correlated with response. The median reported irAEs of the PD- L1-positive patients (n = 4) was 3.5 (range: 2–6), while those of the PD- L1-negative patients (n = 13) was 1 (range: 0–5), and of the unknown PD-L1 status patients (n = 3) was 2 (range: 0–2).
In this study of 25 patients with metastatic UM, median overall survival of the entire patient cohort was 24 months. This fares well with historical accounts of median survival of 6–10 months (12, 13). Although patient numbers are small, median OS was not statistically improved in patients receiving systemic therapy within 6 months of diagnosis of metastatic disease. Similarly, no apparent survival benefit was seen with (early) radiation therapy within 6 months of diagnosis. In fact, there was a statistically significant difference in survival favoring no radiation, though later radiation therapy administration in six patients and inclusion of all four PD- L1-positive patients in the No Radiation Therapy group may have skewed results. Nonetheless, our results suggest that early systemic and radiation therapy treatments may not seemingly confer significant survival benefit for most patients. The data appear to agree with results of the meta-analysis by Rantala et al. suggesting there is no evidence for longer median OS for patients with metastatic UM by any treatment modality (3).
We did, however, note objective responses with immunotherapy and radiotherapy treatments. Systemic immunotherapy responses were highest with ipilimumab/nivolumab. BOR to ipilimumab/nivolumab as a first-line treatment was 15% versus 0% for pembrolizumab. The BOR to ipilimumab/nivolumab, irrespective of treatment line, was 24%. Our analysis showed a statistically significant difference in survival when patients were stratified by PD-L1 status. While only a small number of patients expressed PD-L1 on their tumors, survival appears improved in this small group of patients treated with checkpoint inhibition.
PD-L1 expression on metastatic UM biopsies was associated with a higher likelihood of complete responses to first-line ipilimumab/nivolumab supporting the notion that PD-L1 expression may help predict response to dual checkpoint blockade. Of the four PD- L1-positive patients in this study, there were two CRs, one PR, and one PD with ipilimumab/nivolumab. On the other hand, in the PD-L1-negative majority of patients, there were no CRs and only two PRs seen. The PD- L1-positive responses are consistent with our current understanding that high PD-L1 expression in the tumor cell population correlates positively with response to anti-PD-1 antibodies (14, 15). Unfortunately, only about 10% of primary UM tumors and just 5% of metastatic UM cells at distant sites express PD-L1 in the microenvironment (16).
PD1 checkpoint blockade in metastatic UM is less efficacious than in cutaneous melanoma likely due to differences in biology, including differences in PD-L1 and immune checkpoint molecule expression, and low mutational burden compared to cutaneous melanoma (14). However, patients with low or negative PD-L1 expression may still respond to immunotherapy, and treatment based solely on PD-L1 expression may exclude potential patient responders. For example, there is a reported case of a complete response after treatment with pembrolizumab in a patient with UM with metastases to the liver, lung, and bones (17). Another metastatic UM patient received ipilimumab plus pembrolizumab and maintained stable disease for 10 months and experienced a prolonged survival for 2 years, twice the median survival length (18). Instructively, the tumors of these two patients had a high mutational burden, and both harbored germline mutations of methyl-CpG-binding domain protein (MBD4) found in approximately 2% of UM patients (17, 18). Their exceptional response may be explained by prior studies showing that immunotherapy may be more effective in tumors with a high mutational burden and that these tumors are more likely to be recognized by CTLs due to a higher expression of recognizable neoantigens (14, 19, 20). However, UM is a tumor with a relatively low mutational burden, with an average of 0.5 per Mb sequence (21). Therefore, the probability of recognizable neoantigens is generally low in UM, likely contributing to the disappointing overall response to checkpoint inhibition compared with UV radiation-associated cutaneous melanomas. Intriguingly, one of our best overall responders had a low tumor mutational burden (TMB) of 2 Muts/Mb.
We also note that one patient initially negative for PD-L1 expression, after two cycles of high dose IL-2, had an increase in tumor cell PD-L1 expression. More than 7 months after the diagnosis of metastasis, biopsy of the patient’s liver tumor after IL-2 therapy demonstrated PD-L1 expression >5%. She was subsequently treated with two cycles of combination ipilimumab plus nivolumab and had a partial response (PR) to treatment, though complicated by severe autoimmune colitis. The patient was treated with high-dose corticosteroids, infliximab, and mycophenolate, but ultimately transitioned to hospice and received no additional cancer-directed therapy.
Our findings also suggested higher median irAEs among PD- L1-positive patients versus PD- L1-negative patients. This is consistent with the literature that supports the notion of irAEs to reflect the response to immunotherapy. In a retrospective analysis of 148 melanoma patients treated with nivolumab monotherapy, cutaneous irAEs were associated with an improved survival (22). Another meta-analysis of 48 clinical trials investigated the incidence rates of irAEs and their correlations with objective response rate (ORR) in patients with advanced solid tumors treated with nivolumab or nivolumab plus ipilimumab (23). The authors found that the ORR of nivolumab positively correlated with the incidence rate of the skin, gastrointestinal, and endocrine irAEs, but not hepatic, pulmonary, and renal irAEs. Similarly, the ORR of nivolumab plus ipilimumab was positively correlated with the incidence rate of the skin and gastrointestinal irAEs, but not endocrine, hepatic, pulmonary, and renal irAEs (23). There were higher rates of irAEs in complete responders compared to patients who did not achieve a complete response. In our patient population, patients receiving systemic treatment (n = 20) reported a range of 1 to 6 irAEs per patient. PD- L1-positive patients (n = 4) had a higher median number of 3.5 irAEs compared to the PD- L1-negative patients (n = 13) with a median of 1 irAE per patient.
Beyond PD1, alternative checkpoints may contribute to the susceptibility of metastatic UM to immune checkpoint inhibition strategies. T-cell immunoglobulin and ITIM domain (TIGIT) inhibition is being investigated in clinical trials (24, 25). Chauviin et al. originally found an upregulation of TIGIT and a co-expression of PD-1 in patients with melanoma (26). Stalhammer et al. discovered that metastatic primary UM has a higher number of TIGIT-positive cells/mm2 than non-metastatic UM (27). Several monoclonal antibodies have been synthesized to target TIGIT, with clinical trials on TIGIT inhibition in several cancer types underway, but further investigation for activity against metastatic UM is needed (28, 29). On the other hand, lymphocyte activation gene 3 (LAG-3) inhibition has been focused on recently as an immune checkpoint in solid tumors, including both cutaneous and UM (30–32). Opdualag (combination of relatlimab and nivolumab) has become the first FDA-approved immunotherapy to target LAG-3, with relatlimab blocking the activity of LAG-3. The RELATIVITY-047 trial compared the combination of nivolumab and relatlimab versus nivolumab alone, and showed that inhibition of two immune checkpoints (LAG-3 and PD-1) provided greater progression-free survival compared to PD-1 inhibition alone (10.1 vs. 4.6 months) in patients with advanced cutaneous melanoma (33). Ongoing trials with Opdualag in metastatic UM are currently underway (NCT04552223 and NCT05077280).
There are key limitations of this study, which are inherent to its retrospective nature, including a small number of patients and selection bias of a single-center series. There was also variability in data reporting due to patients receiving some of their care at other institutions. It was also not possible to correlate PD-L1 expression and response to radiotherapy as there were no PD- L1-positive patients treated with radiotherapy. PD-L1-positive skewing of the No Radiation Therapy group may have contributed to a measured survival difference favoring this cohort, and larger numbers of patients balanced for PD-L1 expression are needed for further verification. Adverse events were based on patient charts and descriptions, and therefore likely underreported patient-subjective adverse events (e.g., fatigue, myalgias, pruritus). Furthermore, mild toxicities and symptoms occurring between clinical visits may not have been reported.
There is an unmet need for efficacious treatment of metastatic UM. Therapeutic options are limited, and it is crucial to understand the impact of immunotherapy in combination strategies involving checkpoint inhibitors, chemotherapy, cytokines, and radiation therapy approaches.
UM is a distinct subset of melanoma with decreased response to checkpoint inhibition versus cutaneous melanoma. Due to the rarity of the diagnosis and poor outcomes, there is a need for studies aimed at understanding the response to systemic and local treatments for metastatic disease. Our study showed no significant difference in survival for patients electing early treatment of their disease. There was remarkable clinical activity of ipilimumab and nivolumab in a subset of patients with UM, in agreement with prior studies. In our cohort, there were two complete responses to ipilimumab–nivolumab. Our data suggests that PD- L1-positive tumors may respond better to checkpoint blockade, and these tumors were associated with a prolonged survival. However, larger datasets are necessary to characterize the biomarkers of response to checkpoint blockade and identify molecular signatures of responders and non-responders in UM. Our data supports anti-PD-1/anti-CTLA-4 therapy as a viable treatment approach for metastatic UM patients particularly in those with PD- L1-positive tumors.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving humans were approved by University of Minnesota Institutional Review Board. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements.
DT: Writing – review & editing, Writing – original draft, Methodology, Investigation, Formal analysis, Data curation, Conceptualization. RS: Writing – review & editing, Writing – original draft, Software, Methodology, Formal analysis, Data curation. AG: Writing – review & editing, Writing – original draft, Methodology, Data curation. PT: Writing – review & editing, Writing – original draft, Formal analysis, Data curation. DK: Writing – review & editing, Writing – original draft. JY: Writing – review & editing, Writing – original draft, Data curation. KD: Writing – review & editing, Writing – original draft. ED-M: Writing – review & editing, Writing – original draft, Supervision, Software, Resources, Project administration, Methodology, Investigation, Formal analysis, Data curation, Conceptualization.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. Research reported in this publication was supported by NIH grant P30CA077598 utilizing the Biostatistics and Bioinformatics Core shared resource of the Masonic Cancer Center, University of Minnesota and by the National Center for Advancing Translational Sciences of the National Institutes of Health Award Number UM1TR004405.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
1. Kaliki S, Shields CL. Uveal melanoma: relatively rare but deadly cancer. Eye (Lond). (2017) 31:241–57. doi: 10.1038/eye.2016.275
2. Valsecchi ME, Orloff M, Sato R, Chervoneva I, Shields CL, Shields JA, et al. Adjuvant sunitinib in high-risk patients with uveal melanoma: comparison with institutional controls. Ophthalmology. (2018) 125:210–7. doi: 10.1016/j.ophtha.2017.08.017
3. Rantala ES, Hernberg M, Kivelä TT. Overall survival after treatment for metastatic uveal melanoma: a systematic review and meta-analysis. Melanoma Res. (2019) 29:561–8. doi: 10.1097/CMR.0000000000000575
4. Collaborative Ocular Melanoma Study Group. Assessment of metastatic disease status at death in 435 patients with large choroidal melanoma in the Collaborative Ocular Melanoma Study (COMS): COMS report no. 15. Arch Ophthalmol. (2001) 119:670–6. doi: 10.1001/archopht.119.5.670
5. Rowcroft A, Loveday BPT, Thomson BNJ, Banting S, Knowles B. Systematic review of liver directed therapy for uveal melanoma hepatic metastases. HPB (Oxford). (2020) 22:497–505. doi: 10.1016/j.hpb.2019.11.002
6. Pereira PR, Odashiro AN, Lim LA, Miyamoto C, Blanco PL, Odashiro M, et al. Current and emerging treatment options for uveal melanoma. Clin Ophthalmol. (2013) 7:1669–82. doi: 10.2147/OPTH.S28863
7. Algazi AP, Tsai KK, Shoushtari AN, Munhoz RR, Eroglu Z, Piulats JM, et al. Clinical outcomes in metastatic uveal melanoma treated with PD-1 and PD-L1 antibodies. Cancer. (2016) 122:3344–53. doi: 10.1002/cncr.30258
8. Najjar YG, Navrazhina K, Ding F, Bhatia R, Tsai K, Abbate K, et al. Ipilimumab plus nivolumab for patients with metastatic uveal melanoma: a multicenter, retrospective study [published correction appears in J Immunother Cancer. J Immunother Cancer. (2020) 8:e000331. doi: 10.1136/jitc-2019-000331. 2020.
9. Pelster MS, Gruschkus SK, Bassett R, Gombos DS, Shephard M, Posada L, et al. Nivolumab and ipilimumab in metastatic uveal melanoma: results from a single-arm phase II study. J Clin Oncol. (2021) 39:599–607. doi: 10.1200/JCO.20.00605
10. Nathan P, Hassel JC, Rutkowski P, Baurain JF, Butler MO, Schlaak M, et al. Overall survival benefit with tebentafusp in metastatic uveal melanoma. N Engl J Med. (2021) 385:1196–206. doi: 10.1056/NEJMoa2103485
11. Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. (2009) 45:228–47. doi: 10.1016/j.ejca.2008.10.026
12. Damato B, Eleuteri A, Taktak AF, Coupland SE. Estimating prognosis for survival after treatment of choroidal melanoma. Prog Retin Eye Res. (2011) 30:285–95. doi: 10.1016/j.preteyeres.2011.05.003
13. Krantz BA, Dave N, Komatsubara KM, Marr BP, Carvajal RD. Uveal melanoma: epidemiology, etiology, and treatment of primary disease. Clin Ophthalmol. (2017) 11:279–89. doi: 10.2147/OPTH.S89591
14. Yarchoan M, Hopkins A, Jaffee EM. Tumor mutational burden and response rate to PD-1 inhibition. N Engl J Med. (2017) 377:2500–1. doi: 10.1056/NEJMc1713444
15. Shen X, Zhao B. Efficacy of PD-1 or PD-L1 inhibitors and PD-L1 expression status in cancer: meta-analysis. BMJ. (2018) 362:k3529. doi: 10.1136/bmj.k3529
16. Wessely A, Steeb T, Erdmann M, Heinzerling L, Vera J, Schlaak M, et al. The role of immune checkpoint blockade in uveal melanoma. Int J Mol Sci. (2020) 21:879. doi: 10.3390/ijms21030879
17. Rodrigues M, Mobuchon L, Houy A, Fievet A, Gardrat S, Barnhill RL, et al. Outlier response to anti-PD1 in uveal melanoma reveals germline MBD4 mutations in hypermutated tumors. Nat Commun. (2018) 9:1866. doi: 10.1038/s41467-018-04322-5
18. Johansson PA, Stark A, Palmer JM, Bigby K, Brooks K, Rolfe O, et al. Prolonged stable disease in a uveal melanoma patient with germline MBD4 nonsense mutation treated with pembrolizumab and ipilimumab [published correction appears in Immunogenetics. 2019 Jul;71(7):511]. Immunogenetics. (2019) 71:433–6. doi: 10.1007/s00251-019-01108-x
19. Rizvi NA, Hellmann MD, Snyder A, et al. Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. . Science. (2015) 348:124–8. doi: 10.1126/science.aaa1348
20. Schumacher TN, Schreiber RD. Neoantigens in cancer immunotherapy. Science. (2015) 348:69–74. doi: 10.1126/science.aaa4971
21. Johansson P, Aoude LG, Wadt K, Glasson WJ, Warrier SK, Hewitt AW, et al. Deep sequencing of uveal melanoma identifies a recurrent mutation in PLCB4. Oncotarget. (2016) 7:4624–31. doi: 10.18632/oncotarget.6614
22. Freeman-Keller M, Kim Y, Cronin H, Richards A, Gibney G, Weber JS. Nivolumab in resected and unresectable metastatic melanoma: characteristics of immune-related adverse events and association with outcomes. Clin Cancer Res. (2016) 22:886–94. doi: 10.1158/1078-0432.CCR-15-1136
23. Xing P, Zhang F, Wang G, Xu Y, Li C, Wang S, et al. Incidence rates of immune-related adverse events and their correlation with response in advanced solid tumours treated with NIVO or NIVO+IPI: a systematic review and meta-analysis [published correction appears in J Immunother Cancer. J Immunother Cancer. (2019) 7:341. doi: 10.1186/s40425-019-0779-6. 2020.
24. Rousseau A, Parisi C, Barlesi F. Anti-TIGIT therapies for solid tumors: a systematic review. ESMO Open. (2023) 8:101184. doi: 10.1016/j.esmoop.2023.101184
25. Banta KL, Xu X, Chitre AS, Au-Yeung A, Takahashi C, O'Gorman WE, et al. Mechanistic convergence of the TIGIT and PD-1 inhibitory pathways necessitates co-blockade to optimize anti-tumor CD8+ T cell responses. Immunity. (2022) 55:512–526.e9. doi: 10.1016/j.immuni.2022.02.005
26. Chauvin JM, Pagliano O, Fourcade J, Sun Z, Wang H, Sander C, et al. TIGIT and PD-1 impair tumor antigen-specific CD8+ T cells in melanoma patients. J Clin Invest. (2015) 125:2046–58. doi: 10.1172/JCI80445
27. Stålhammar G, Seregard S, Grossniklaus HE. Expression of immune checkpoint receptors Indoleamine 2,3-dioxygenase and T cell Ig and ITIM domain in metastatic versus nonmetastatic choroidal melanoma. Cancer Med. (2019) 8:2784–92. doi: 10.1002/cam4.2167
28. Masaoutis C, Kokkali S, Theocharis S. Immunotherapy in uveal melanoma: novel strategies and opportunities for personalized treatment. Expert Opin Investig Drugs. (2021) 30:555–69. doi: 10.1080/13543784.2021.1898587
29. Kim TW, Bedard PL, LoRusso P, Gordon MS, Bendell J, Oh D, et al. Anti-TIGIT antibody tiragolumab alone or with atezolizumab in patients with advanced solid tumors: A phase 1a/1b nonrandomized controlled trial. JAMA Oncol. (2023) 9:1574–82. doi: 10.1001/jamaoncol.2023.3867
30. Triebel F, Jitsukawa S, Baixeras E, Roman-Roman S, Genevee C, Viegas-Pequignot E, et al. LAG-3, a novel lymphocyte activation gene closely related to CD4. J Exp Med. (1990) 171:1393–405. doi: 10.1084/jem.171.5.1393
31. Souri Z, Wierenga APA, Kroes WGM, van der Velden PA, Verdijk RM, Eikmans M, et al. LAG3 and its ligands show increased expression in high-risk uveal melanoma. Cancers (Basel). (2021) 13:4445. doi: 10.3390/cancers13174445
32. Woo SR, Turnis ME, Goldberg MV, Bankoti J, Selby M, Nirschl CJ, et al. Immune inhibitory molecules LAG-3 and PD-1 synergistically regulate T-cell function to promote tumoral immune escape. Cancer Res. (2012) 72:917–27. doi: 10.1158/0008-5472.CAN-11-1620
Keywords: metastatic uveal melanoma, immunotherapy, radiation therapy (radiotherapy), retrospective study, ipilimumab, nivolumab, PD-L1
Citation: Tran DH, Shanley R, Giubellino A, Tang PH, Koozekanani DD, Yuan J, Dusenbery K and Domingo-Musibay E (2024) Radiation and systemic immunotherapy for metastatic uveal melanoma: a clinical retrospective review. Front. Oncol. 14:1406872. doi: 10.3389/fonc.2024.1406872
Received: 25 March 2024; Accepted: 27 May 2024;
Published: 04 July 2024.
Edited by:
Dragos Eugen Georgescu, Carol Davila University of Medicine and Pharmacy, RomaniaReviewed by:
Jessica Crystal, University of Miami, United StatesCopyright © 2024 Tran, Shanley, Giubellino, Tang, Koozekanani, Yuan, Dusenbery and Domingo-Musibay. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Danielle H. Tran, ZHRyYW44NUB1dy5lZHU=; Evidio Domingo-Musibay, bXVzaWIwMjRAdW1uLmVkdQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.