- 1School and Hospital of Stomatology, Fujian Medical University, Fuzhou, China
- 2Department of Stomatology, The First Affiliated Hospital of Anhui Medical University, Hefei, China
Objective: The margin status of oral squamous cell carcinoma patients is considered to be predictive of recurrence and long-term survival. Therefore, precise intraoperative margin assessment is crucial. This study investigated the feasibility of using near-infrared fluorescence imaging technology to guide margin design in oral squamous cell carcinoma patients.
Methods: In this retrospective study, indocyanine green solution was intravenously injected preoperatively into patients. Intraoperatively, the surgical area was illuminated using a near-infrared fluorescence imaging system, which caused the lesion to fluoresce in the surgical area. Surgery was performed with the assistance of fluorescence imaging. The fluorescence intensity of the lesion area and surrounding normal tissue was recorded during surgery. Intraoperative margins were sent for rapid pathology, and postoperative margin pathology results were documented.
Results: Sixteen patients were included in this study (7 males, 9 females), with an average age of 65.65 ± 12.37 years. Preoperative biopsy and postoperative pathology confirmed oral squamous cell carcinoma in all patients. No cancer cells were found in the margin pathology results. The average fluorescence intensity of the lesion area was 214 ± 4.70, and that of the surrounding normal tissue was 104.63 ± 3.14. There was no significant difference in the fluorescence intensity values of the lesion areas among all patients (F=0.38, P>0.05). There was a significant difference in fluorescence intensity between the lesion area and surrounding normal tissue (t=33.76, P<0.05).
Conclusion: Near-infrared fluorescence imaging technology can aid in real-time imaging differentiation of lesion areas based on differences in fluorescence intensity during surgery. The use of this technology can assist surgeons in assessing the safety margin and reliably guide surgery.
1 Introduction
Global cancer statistics for the year 2018 indicate that oral cancer is one of the most common lethal tumours worldwide, accounting for approximately 1.9% of global cancer-related deaths (1). Oral squamous cell carcinoma (OSCC) accounts for more than 90% of malignant tumours in the oral cavity (2). The 5-year overall survival (OS) rate for OSCC patients ranges from 50% to 64% (3). Surgical resection is the primary treatment for OSCC, and curative resection is a crucial principle in surgical treatment (4). Numerous studies have demonstrated that positive margins significantly increase the local recurrence rate after malignant tumour surgery (5). Therefore, providing objective guidance for surgeons regarding safe margins holds crucial clinical significance.
Rapid intraoperative pathology is a conventional method for assessing margin safety, but it is susceptible to sampling errors (6). Some scholars have proposed intraoperative MRI examination of excised tumours to evaluate margin safety, but effective assurance of margin safety remains challenging (7). Studies have indicated that optical coherence tomography can be used to assess the safety of tumour margins effectively, but further research is needed for detecting intratumoral margins (8). Near-infrared fluorescence (NIRF) agents, which have a high extinction coefficient and significant Stokes shift, can generate strong fluorescence emission within the range of 700 to 1000 nm (9). Additionally, biological tissues exhibit low spontaneous fluorescence in the NIR spectrum, ensuring the tissue penetration capability of NIRF and providing the potential for in vivo tumour diagnosis with live NIRF imaging technology (10). ICG, an NIRF imaging agent approved by the U.S. Food and Drug Administration for clinical use, rapidly binds to plasma proteins to form particles 6–8 nm in size after injection into the bloodstream. ICG selectively accumulates in tumour tissues through the enhanced permeability and retention effect (EPR), while diffuse ICG in normal tissues is quickly cleared through lymphatic drainage. Over time, concentration contrast between the tumour and normal tissues develops, resulting in fluorescence intensity (FI) contrast during tissue imaging (11). ICG has been applied for both clinical diagnosis and treatment, including sentinel lymph node biopsy (12) and tumour imaging (13). At the same time, some researchers have used ICG as a diagnostic tool for OSCC. Composition of drug delivery vehicles for targeted therapy (14).
The lesion resection margins used in oral cancer surgery can be divided into mucosal resection margins and basal resection margins. In 2013, the College of American Pathologists defined mucosal resection margins with normal and mild dysplasia as safe resection margins, while mucosal resection margins with moderate or severe dysplasia and residual cancer were defined as positive resection margins (15). The 2019 National Comprehensive Cancer Network treatment guidelines define the basal resection margin as a safe resection margin if the pathologically measured distance between the tumour infiltration front and the surgical resection margin on pathological sections is greater than 5 mm and the distance between the tumour infiltration front and the surgical resection margin is 0–5 mm. This type of margin is called a critical margin, and a margin with tumour cells is called a positive margin (16). According to statistics by Varvares et al (17), the postoperative local recurrence rate with positive margins is 3 times greater than that with safe margins. Therefore, correctly judging the safe tumour margin during surgery is closely related to complete tumour resection, reducing local and regional recurrence of oral cancer, and improving patient prognosis. This study is based on the fluorescence effect of ICG and the EPR effect in tumour tissue. By intravenously injecting ICG solution into the patient before surgery, near-infrared fluorescence imaging was performed on the surgical area of oral squamous cell patients. Due to the EPR effect of the tumour, the residence time of ICG macromolecules bound to haemoglobin in the tumour area is longer than that of the surrounding normal tissue. Intraoperative fluorescence imaging will result in differences in FI between the tumour and surrounding tissue. This study explored whether the difference in FI between the tumour and surrounding tissue can effectively achieve safe margins during tumour resection.
2 Materials and methods
This study included 16 patients with OSCC who visited the Oral and Maxillofacial Surgery Department of the First Affiliated Hospital of Anhui Medical University from June 2022 to June 2023. The study adhered to the principles of the Helsinki Declaration and was approved by the medical ethics committee of the First Affiliated Hospital of Anhui Medical University (PJ2024–03-42).
2.1 Patient inclusion and exclusion criteria
The inclusion criteria were as follows: 1) aged greater than 18 years, 2) had a preoperative biopsy confirming OSCC, and 3) voluntary participation in this research.
The exclusion criteria were as follows: 1) had an iodine allergy, thyroid disease, or liver dysfunction; 2) had altered consciousness, inability to cooperate, or unclear language expression; 3) had a history of radiotherapy or chemotherapy; and 4) refused to participate in the study.
2.2 Intraoperative application of the NIRF system
Approximately 7 ± 1 hours before surgery, patients received an intravenous injection of ICG solution (Dandong Yichuang Pharmaceutical Co., Ltd., China) prepared with pure water at a dosage of 0.75 mg/kg, which was administered for 30 minutes. During surgery, the laser unit of the real-time image-guided system (the FLI-108 real-time image-guided system, Nanjing Nuoyuan Medical Devices Co., Ltd., China) was aligned with the surgical area for imaging, maintaining a distance between 30 and 50 cm. The fluorescence images of the surgical area were displayed on the screen, revealing significant differences in FI between the lesion area and normal tissue (Figure 1). The maximum FI on the tumour surface was recorded, and numerous FIs on the tumour surface and surrounding tissues were randomly recorded. In the colour-coded fluorescence image, it can be seen that from the centre of the lesion to the safe tissue, the FI is arranged like a concentric circle. The FI intensity was recorded layer by layer from the highest FI value in the centre of the tumour to the edge (Figure 1D).
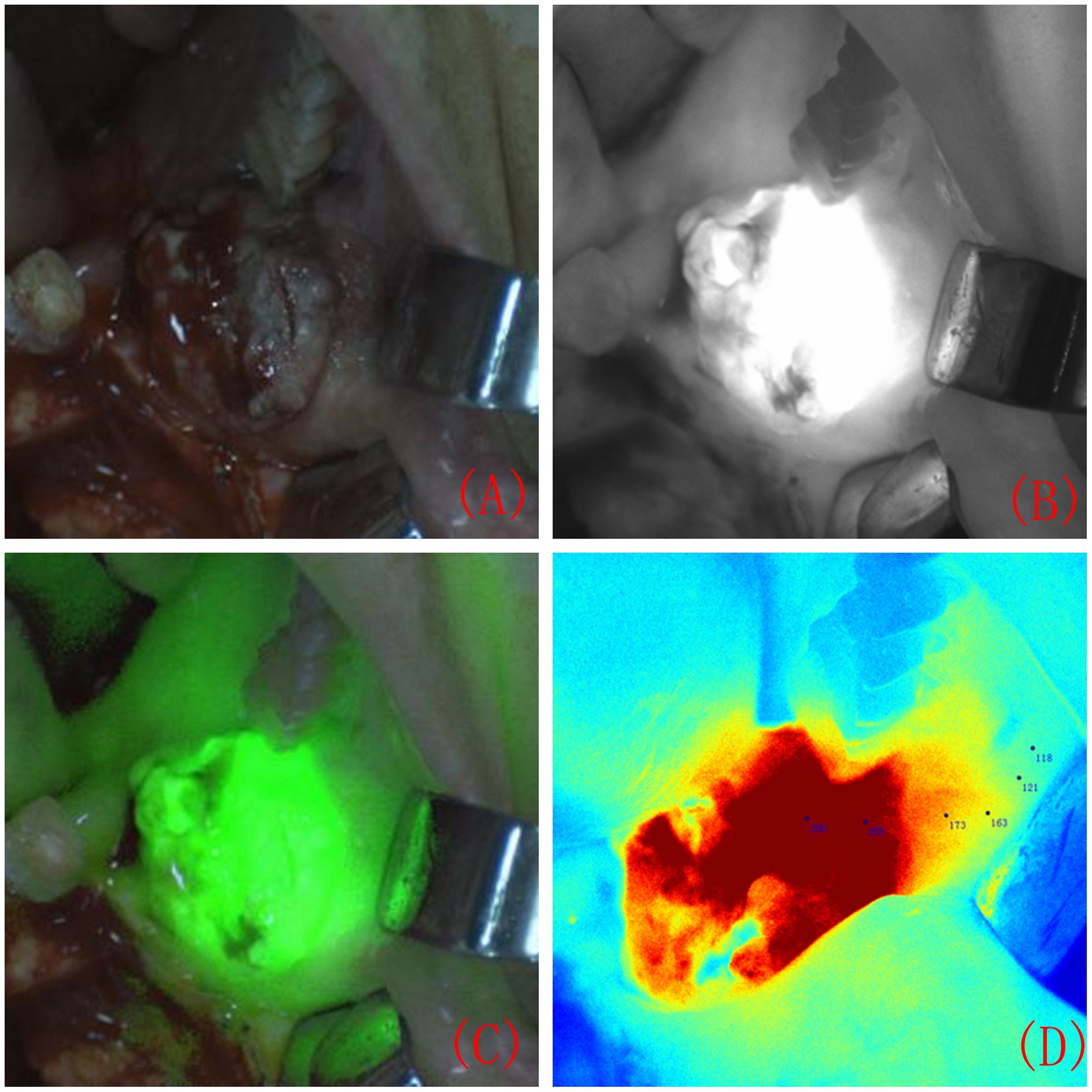
Figure 1 Surgical area during intraoperative near-infrared fluorescence system imaging. Surgical area under natural light imaging (A). Surgical area under black and white fluorescence imaging (B). Composite image of fluorescence and natural light (C). A colour-coded fluorescence image revealed that from the centre of the lesion to the safe tissue, the FI was arranged like a concentric circle. The FI intensity was recorded layer by layer from the highest FI value in the centre of the tumour to the edge (D).
2.3 Preparation of resection margins and removal of lesions
Under the guidance of FI differences, the lesion range was marked using methylene blue (Figure 2A). The surgical margin was 5 mm outside the marked tumour edge, and the FI value of the surgical margin was recorded. The lesion was completely removed, and the anterior, medial, lateral, posterior and bottom edges of the resection were removed. Based on the FI intensity displayed by the colour-coded fluorescence image, pathological resection margins were taken from areas with different FI intensities at the edge of the tumour. The lesion was excised under the guidance of a real-time image-guided system (Figure 2B). After complete tissue removal, there was no fluorescent area in the tissue surrounding the surgical area (Figure 2C).
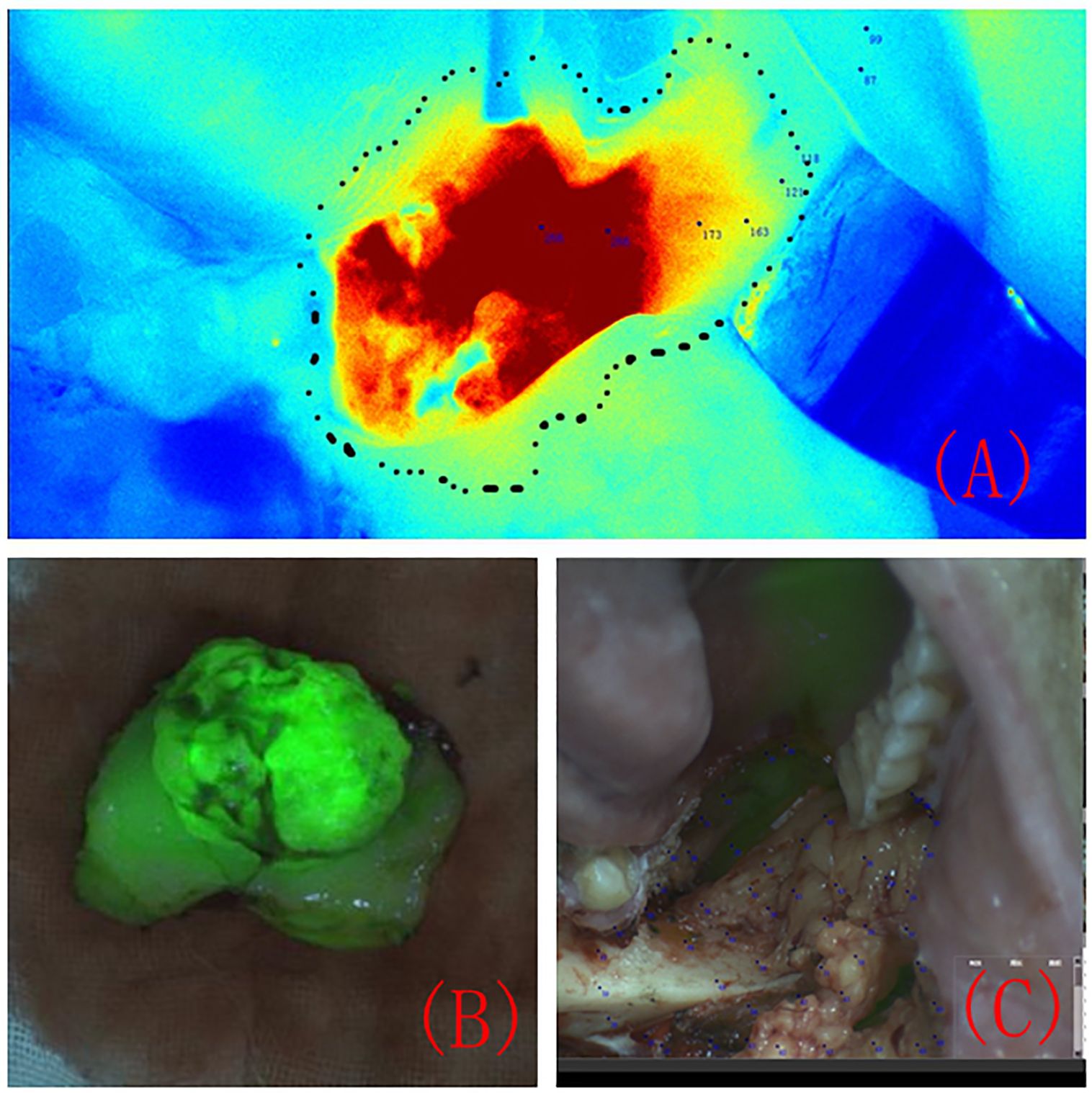
Figure 2 The tumour resection margin was determined under the guidance of colour-coded fluorescence imaging (A). Fluorescence image of the completely removed lesion (B). Fluorescence image of the surgical area after the lesion was completely removed (C).
2.4 Statistical methods
Statistical analysis was conducted using SPSS 23.0 software. Representative values (the P100, P75, P50, P25, P0) for each patient’s lesion area and safe margin measurement points were selected for statistical analysis. The difference in FI between the lesion area and normal tissue area in the 16 patients was compared using a two-sample t test, and P<0.05 was considered to indicate statistical significance. Analysis of variance (ANOVA) was used to compare the FI differences in the lesion area of each patient, with P<0.05 considered to indicate statistical significance.
3 Results
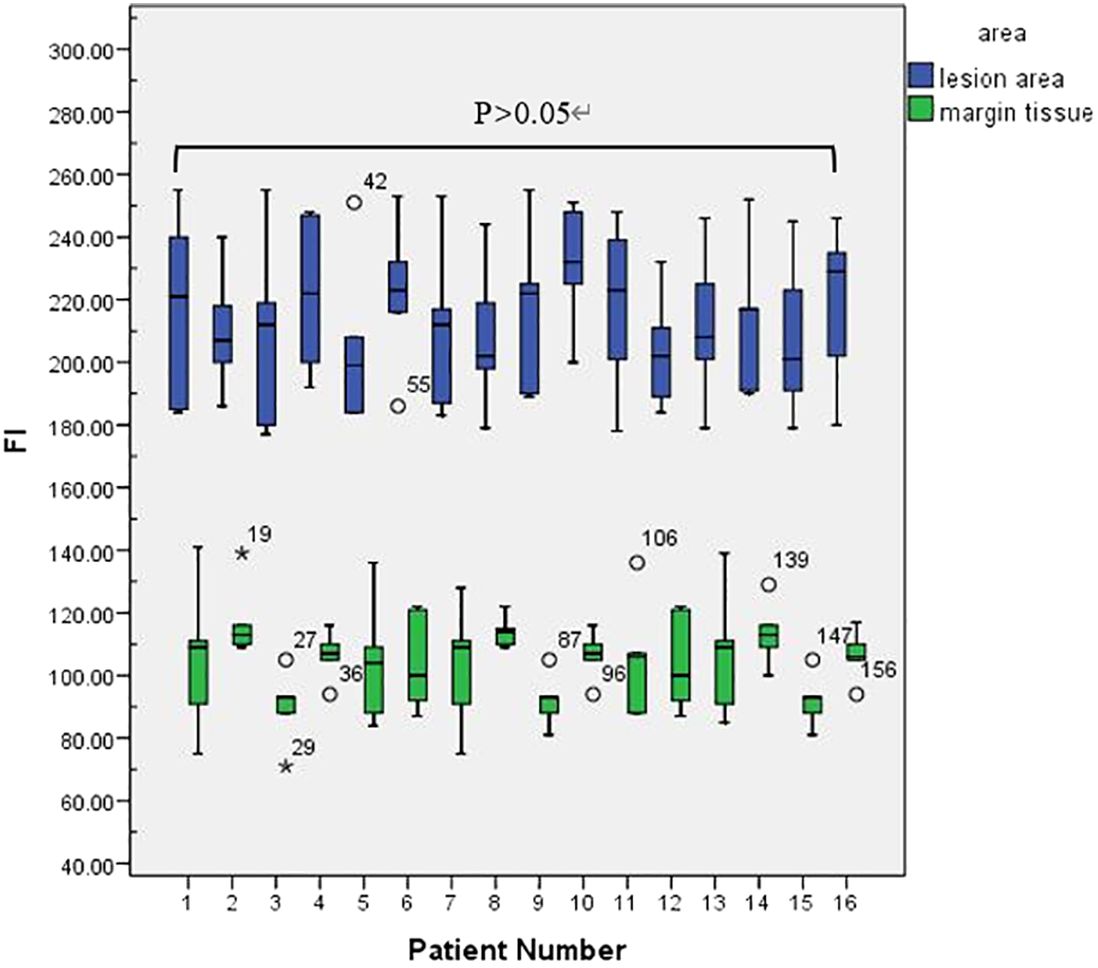
This retrospective study included a total of 16 patients—7 males and 9 females—with an average age of 65.65 ± 12.37 years. Preoperative biopsy and postoperative pathology confirmed OSCC in all patients. Patient details are shown in Table 1. In 16 patients, lesion regional FI intensity and surgical margin FI intensity were measured (Figure 3).
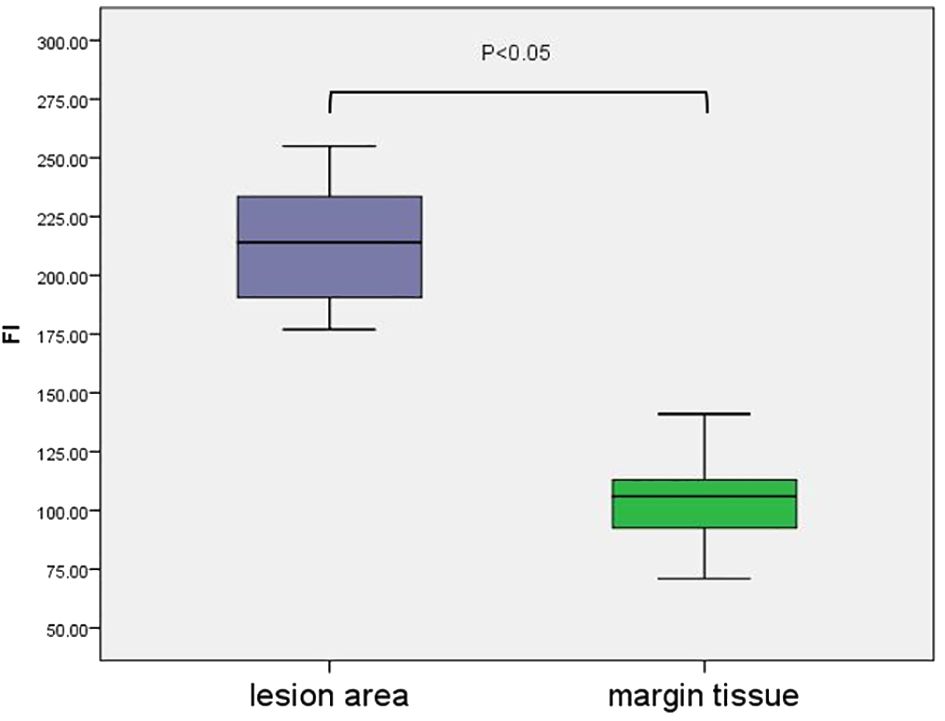
A colour-coded fluorescence image revealed that from the centre of the lesion to the safe tissue, the FI was arranged like a concentric circle, and the FI intensity ranged from strong to weak. The average FI of the lesion area was 214 ± 4.70. A colour-coded fluorescence image revealed that from the centre of the lesion to the safe tissue, the FI was arranged like a concentric circle, and the FI intensity ranged from strong to weak. The average FI of the lesion area was 214 ± 4.70. There was no significant difference in the fluorescence intensity values of the lesion areas among all patients (F=0.38, P>0.05) (Figure 3). The FI intensity of the surgical margin was 104.63 ± 3.14. There was a significant difference in the FI between the lesion area and tissue from the surgical margins (t=33.76, P<0.05) (Figure 4).
The fluorescence intensity of the surrounding normal tissue at the edge of the tumour was mainly orange, yellow, yellow−green or blue−green (Figure 5A). Pathology was performed on the tissue edges corresponding to the four colours. Pathology revealed cancer cells in the orange area (Figure 5B), and pathology at the resection edge of the yellow area revealed low-grade intraepithelial neoplasia (Figure 5C). Pathology of the resection margin in the blue–green area revealed normal tissue (Figure 5D). No cancer cells were found on the surgical margin.
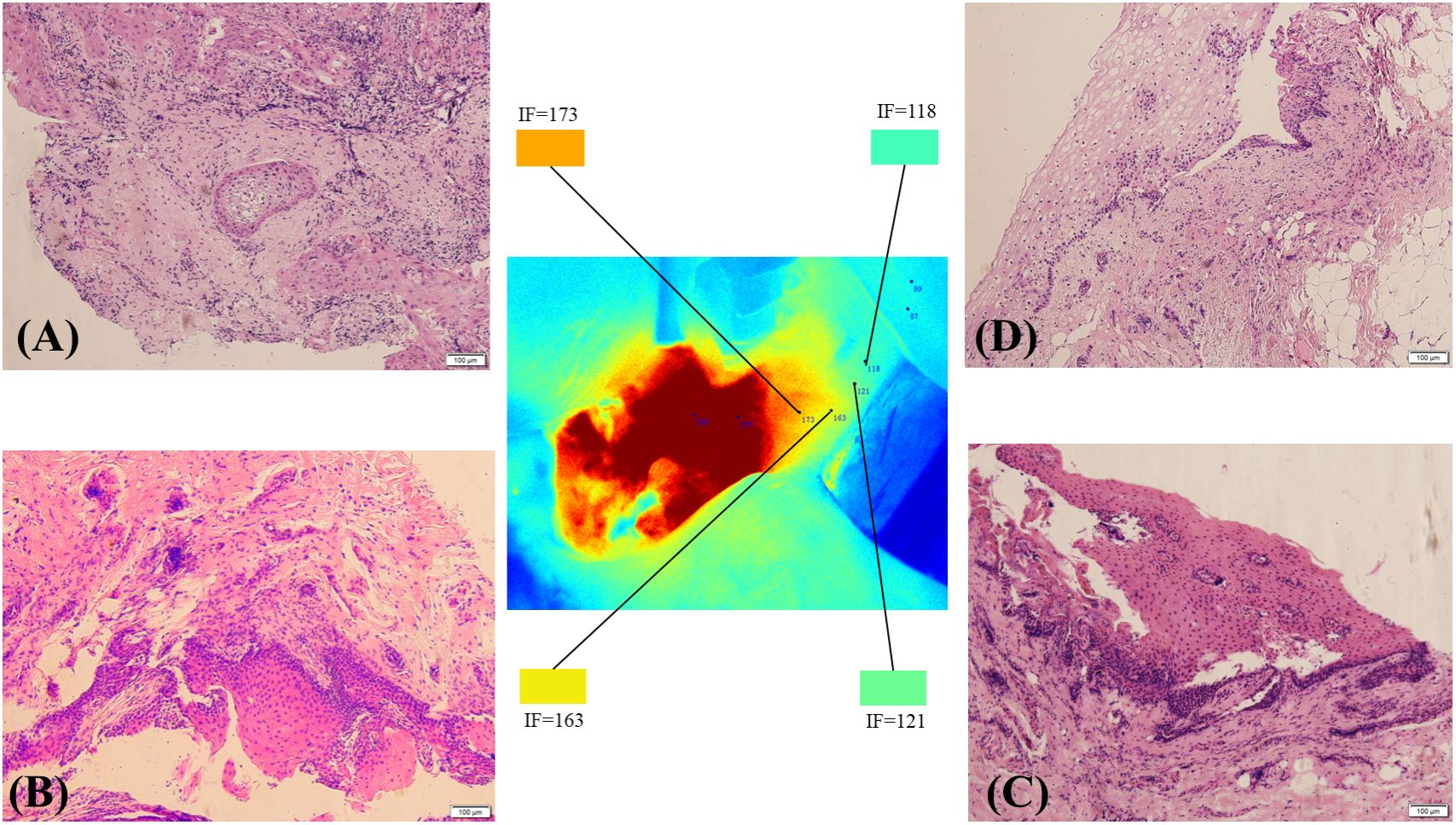
Figure 5 The tumour margin and pathological conditions of the resection margin under different FI intensities. Pathology revealed cancer cells in the orange area (A). Pathology of the resection margin in the yellow area showing low-grade intraepithelial neoplasia (B). Pathological sections at the edge of the yellow−green area show possible intramutation of low-grade epithelial tumours (C). Pathology of the resection margin in the blue–green area showing normal tissue (D).
4 Discussion
The absence of residual cancer in surgical margins is crucial for the success of curative surgery for various solid malignant tumours. Currently, surgeons rely primarily on preoperative clinical assessments, imaging studies, and intraoperative visual-tactile judgement to determine the extent of tumour resection, lacking objective guidance. NIRF imaging technology effectively addresses this issue, providing surgeons with real-time intraoperative guidance for achieving safe margins. Yokoyama et al. (18) first utilized ICG-based NIRF imaging technology to detect head and neck tumours, achieving excellent tumour edge imaging results. In the present study, six OSCC patients were observed intraoperatively using a surgical fluorescence imaging system, which revealed good imaging results, with clear fluorescence in the tumour tissues. The boundaries between the tumour and surrounding normal tissues were distinctly visible, aiding in the assessment of safe margins. No residual fluorescence was observed in the surgical field, assisting in the evaluation of surgical field margins, consistent with previous research results.
Most patients with OSCC have ulcers on the inner surface of the oral cavity. The surgeon can design the surgical margin based on the ulcer surface and MRI images and determine the safety of the surgical margin through intraoperative freezing of the tissue at the surgical margin. However, squamous cell carcinoma has the characteristic of invasive growth. The abovementioned resection margin design plan relies on the clinical experience of surgeons, and the selection of surgical margins is subjective. Near-infrared fluorescence imaging can be used to visualize cancer tissue in the entire surgical area, enabling clinicians to detect infiltrating and growing cancer tissue. When squamous cell carcinoma displays invasive growth and disconnected lesions appear in local areas (Figure 6), the near-infrared fluorescence system can give the surgeons intraoperative prompts and ensure the complete removal of lesions.
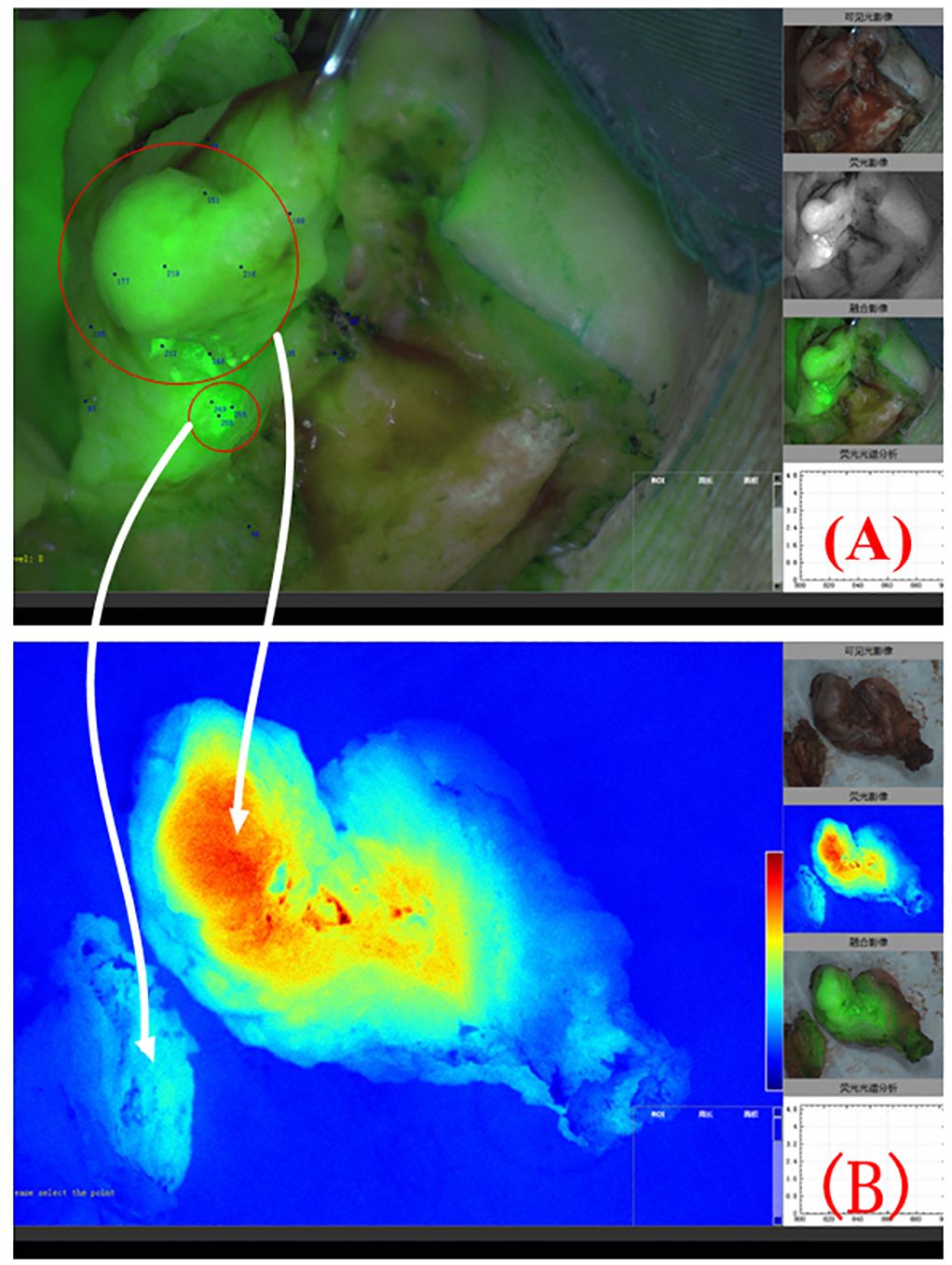
Figure 6 Composite image of fluorescence and natural light during surgery for right lower gingival cancer. Two disconnected lesion areas can be seen in the red circle area (A). The white arrow indicates the colour-coded fluorescence image corresponding to the 2 lesion areas (B).
A previous study revealed that for OSCC patients, the half-life of ICG in normal tissues (3.863 hours) was approximately 1 hour shorter than that in tumours (4.63 hours), confirming the role of the EPR effect in the accumulation of ICG in OSCC tissue (19). In this study, involving six OSCC patients, there was a significant difference in the intraoperative FI between the lesion area and surrounding normal tissue, consistent with the aforementioned research. Notably, there was no statistically significant difference in the intraoperative FI of the lesion area among the six patients. Given the differences in metabolic efficiency among individual livers, under similar objective conditions, the FI displayed during imaging of the same tissue may vary among different patients. Onda et al. (20) suggested that, in addition to the EPR effect, the preferential uptake of ICG by tumour cells is another reason for its accumulation in tumours. Whether the FI of OSCC tissue in different patients falls within a certain range under identical conditions is worthy of further clinical research.
ICG, a commonly used fluorescent dye, has been widely applied due to its mechanism of plasma protein binding, visualization of the lymphatic system and vascular circulation, and tissue perfusion. In recent years, its application in tumour surgery has gradually gained increasing attention (21, 22). However, ICG lacks tumour-targeting specificity, and its accumulation in tumour cells primarily depends on the EPR effect rather than active targeting characteristics (23). Therefore, the efficacy of ICG in identifying different tumour types via NIRF imaging requires extensive clinical verification. Recently, heptamethine cyanine dyes, NIRF compounds with tumour-targeting specificity, including IR780, IR808, IR820, IR783, and MHI-148, have been reported to be able to specifically recognize and aggregate in tumour cells (24). With nanomodification, better imaging results can be obtained (25). Thus, targeted fluorescent agents are also a future research focus.
In conclusion, NIRF imaging technology can provide precise and safe margin guidance for surgery for OSCC patients, potentially reducing the risk of positive margins. While ICG is useful, it has inherent limitations. Its functionality relies on the enhanced permeability and retention (EPR) effect, and it cannot be used to pinpoint and enhance characteristic tumour tissue sites. Additionally, it cannot be used to achieve a pathological diagnosis. Further investigation is necessary to ascertain if tissue pathology assessments can be conducted based on discrepancies in FI revealed by ICG.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving humans were approved by the medical ethics committee of the First Affiliated Hospital of Anhui Medical University. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
HW: Conceptualization, Data curation, Formal Analysis, Investigation, Validation, Visualization, Writing – original draft. TL: Data curation, Formal Analysis, Investigation, Methodology, Project administration, Writing – original draft. YC: Data curation, Formal Analysis, Investigation, Writing – original draft. MY: Data curation, Investigation, Writing – original draft. LZ: Supervision, Validation, Writing – review & editing. JH: Conceptualization, Supervision, Validation, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was supported by the Clinical Research Project of the First Affiliated Hospital of Anhui Medical University (LCJY2021YB001).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Bray F, Ferlay J, Soerjomataram I, RL S, LA T, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. Ca-Cancer J Clin. (2018) 68:394–424. doi: 10.3322/caac.21492
2. Neville BW, Day TA. Oral cancer and precancerous lesions. Ca-Cancer J Clin. (2002) 52:195–215. doi: 10.3322/canjclin.52.4.195
3. Zanoni DK, Montero PH, Migliacci JC, Shah JP, Wong RJ, Ganly I, et al. Survival outcomes after treatment of cancer of the oral cavity (1985-2015). Oral Oncol. (2019) 90:115–21. doi: 10.1016/j.oraloncology.2019.02.001
4. Alicandri-Ciufelli M, Bonali M, Piccinini A, Marra L, Ghidini A, Cunsolo EM, et al. Surgical margins in head and neck squamous cell carcinoma: what is 'close'? Eur Arch Oto-Rhino-L. (2013) 270:2603–9. doi: 10.1007/s00405-012-2317-8
5. Haque R, Contreras R, McNicoll MP, Eckberg EC, Petitti DB. Surgical margins and survival after head and neck cancer surgery. BMC Ear Nose Throat Disord. (2006) 6:2. doi: 10.1186/1472-6815-6-2
6. Gerber S, Gengler C, Gratz KW, Kruse AL. The impact of frozen sections on final surgical margins in squamous cell carcinoma of the oral cavity and lips: a retrospective analysis over an 11 years period. Head Neck Oncol. (2011) 3:56. doi: 10.1186/1758-3284-3-56
7. Heidkamp J, Weijs W, van Engen-van GA, de Laak-de VI, Maas MC, Rovers MM, et al. Assessment of surgical tumor-free resection margins in fresh squamous-cell carcinoma resection specimens of the tongue using a clinical MRI system. Head Neck-J Sci Spec. (2020) 42:2039–49. doi: 10.1002/hed.26125
8. Hamdoon Z, Jerjes W, McKenzie G, Jay A, Hopper C. Optical coherence tomography in the assessment of oral squamous cell carcinoma resection margins. Photodiagn Photodyn. (2016) 13:211–7. doi: 10.1016/j.pdpdt.2015.07.170
9. Shi C, JB Wu, Pan D. Review on near-infrared heptamethine cyanine dyes as theranostic agents for tumor imaging, targeting, and photodynamic therapy. J BioMed Opt. (2016) 21:50901. doi: 10.1117/1.JBO.21.5.050901
10. Yang X, Shi C, Tong R, Qian W, Zhau HE, Wang R, et al. Near IR heptamethine cyanine dye-mediated cancer imaging. Clin Cancer Res. (2010) 16:2833–44. doi: 10.1158/1078-0432.CCR-10-0059
11. DeLong JC, Chakedis JM, Hosseini A, Kelly KJ, Horgan S, Bouvet M. Indocyanine green (ICG) fluorescence-guided laparoscopic adrenalectomy. J Surg Oncol. (2015) 112:650–3. doi: 10.1002/jso.24057
12. Xia C, Zhou Q, Zhang Q, Hu S, Meacci E, Matsuura Y, et al. Comparative study on the diagnostic value of intravenous/peritumoral injection of indocyanine green for metastatic lymph node location in patients with head and neck squamous cell carcinoma (HNSCC). Ann Transl Med. (2021) 9:507. doi: 10.21037/atm-21-392
13. Kusada T, Yogi A, Hirakawa H, Yasutomi Y, Aoyama H, Matsuo Y, et al. Different indocyanine green fluorescence patterns of two skin metastases of hypopharyngeal squamous carcinoma: A case report. Photodiagn Photodyn. (2021) 34:102211. doi: 10.1016/j.pdpdt.2021.102211
14. Wang Y, Xie D, Pan J, Xia C, Fan L, Pu Y, et al. A near infrared light-triggered human serum albumin drug delivery system with coordination bonding of indocyanine green and cisplatin for targeting photochemistry therapy against oral squamous cell cancer. Biomater Sci-Uk. (2019) 7:5270–82. doi: 10.1039/C9BM01192G
15. Dias FL, Lima RA, Kligerman J, Farias TP, Soares JR, Manfro G, et al. Relevance of skip metastases for squamous cell carcinoma of the oral tongue and the floor of the mouth. Otolaryng Head Neck. (2006) 134:460–5. doi: 10.1016/j.otohns.2005.09.025
16. Bargon CA, Huibers A, Young-Afat DA, Jansen B, Borel-Rinkes I, Lavalaye J, et al. Sentinel lymph node mapping in breast cancer patients through fluorescent imaging using indocyanine green: The INFLUENCE trial. Ann Surg. (2022) 276:913–20. doi: 10.1097/SLA.0000000000005633
17. van den Berg NS, Brouwer OR, Klop WM, Karakullukcu B, Zuur CL, Tan IB, et al. Concomitant radio- and fluorescence-guided sentinel lymph node biopsy in squamous cell carcinoma of the oral cavity using ICG-(99m)Tc-nanocolloid. Eur J Nucl Med Mol I. (2012) 39:1128–36. doi: 10.1007/s00259-012-2129-5
18. Yokoyama J, Ohba S, Fujimaki M, Kojima M, Suzuki M, Ikeda K. Significant improvement in superselective intra-arterial chemotherapy for advanced paranasal sinus cancer by using indocyanine green fluorescence. Eur Arch Oto-Rhino-L. (2014) 271:2795–801. doi: 10.1007/s00405-013-2846-9
19. Wang Y, Xie D, Wang Z, Zhang X, Zhang Q, Wang Y, et al. Kinetics of indocyanine green: Optimizing tumor to normal tissue fluorescence in image-guided oral cancer surgery applications. Head Neck-J Sci Spec. (2019) 41:1032–8. doi: 10.1002/hed.25541
20. Onda N, Kimura M, Yoshida T, Shibutani M. Preferential tumor cellular uptake and retention of indocyanine green for in vivo tumor imaging. Int J Cancer. (2016) 139:673–82. doi: 10.1002/ijc.30102
21. Keating J, Judy R, Newton A, Singhal S. Near-infrared operating lamp for intraoperative molecular imaging of a mediastinal tumor. BMC Med Imaging. (2016) 16:15. doi: 10.1186/s12880-016-0120-5
22. Sun LF, Wang CX, Cao ZY, Han W, Guo SS, Wang YZ, et al. Evaluation of autofluorescence visualization system in the delineation of oral squamous cell carcinoma surgical margins. Photodiagn Photodyn. (2021) 36:102487. doi: 10.1016/j.pdpdt.2021.102487
23. Jiang JX, Keating JJ, Jesus EM, Judy RP, Madajewski B, Venegas O, et al. Optimization of the enhanced permeability and retention effect for near-infrared imaging of solid tumors with indocyanine green. Am J Nucl Med Molec. (2015) 5:390–400.
24. Thomas RG, Jeong YY. NIRF heptamethine cyanine dye nanocomplexes for multi modal theranosis of tumors. Chonnam Med J. (2017) 53:83–94. doi: 10.4068/cmj.2017.53.2.83
Keywords: near-infrared fluorescence imaging technology, oral squamous cell carcinoma, surgical margin, indocyanine green, oral surgery
Citation: Wang H, Li T, Chi Y, Yang M, Zhao L and Hou J (2024) Near-infrared fluorescence imaging technology guided margin design in oral squamous cell carcinoma: a single-centre retrospective study. Front. Oncol. 14:1406595. doi: 10.3389/fonc.2024.1406595
Received: 25 March 2024; Accepted: 23 May 2024;
Published: 06 June 2024.
Edited by:
Rainer Lutz, University of Erlangen Nuremberg, GermanyReviewed by:
Chuanhui Song, Nanjing Drum Tower Hospital, ChinaJoão Figueira Scarini, Albert Einstein Israelite Hospital, Brazil
Copyright © 2024 Wang, Li, Chi, Yang, Zhao and Hou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jun Hou, aG91anVuQGFobXUuZWR1LmNu; Li Zhao, Njc0MDI5MTc1QHFxLmNvbQ==
†These authors have contributed equally to this work and share first authorship
 Honghao Wang
Honghao Wang Tingyu Li2†
Tingyu Li2† Jun Hou
Jun Hou