
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol., 21 May 2024
Sec. Thoracic Oncology
Volume 14 - 2024 | https://doi.org/10.3389/fonc.2024.1405683
This article is part of the Research TopicBiomarker-Guided Strategies in NSCLC ImmunotherapyView all 6 articles
Background: Programmed cell death ligand 1 (PD-L1) is more readily expressed in ROS proto-oncogene 1 (ROS1) rearranged non-small cell lung cancer (NSCLC) compared to NSCLC cases lacking driver gene mutations. Prior research has established a link between PD-L1 expression and reduced effectiveness of EGFR or ALK inhibitors in EGFR or ALK-positive NSCLC. Nonetheless, the relationship between initial PD-L1 levels and the clinical impact of first-line crizotinib therapy in ROS1-rearranged NSCLC is still uncertain.
Methods: From January 2016 to December 2021, a total of 246 patients with ROS1 positive tumors were collected. Out of these, 82 patients with advanced ROS1-rearranged NSCLC, who were treated with crizotinib as their initial therapy, were selected for the study. The study aimed primarily to evaluate the objective response rate (ORR) and progression-free survival (PFS), and secondarily to assess disease control rate (DCR) and overall survival (OS).
Results: Of the 82 advanced ROS1-rearranged NSCLC patients, 38 exhibited PD-L1 positivity, subdivided into 11 with high and 27 with low expression levels, while the remaining 44 showed no PD-L1 expression. The ORR for all included patients was 80.5%. No statistically significant variance in ORR was observed among ROS1-rearranged NSCLC patients across differing PD-L1 expression statuses. However, there was a statistically significant difference in DCR between PD-L1 negative group (100%) and high expression group (90.9%) (p=0.04). The median PFS spanned 26.4 months for the PD-L1 negative group, 16.6 for the low expression group, and 13.7 for the high expression group (p=0.001). Additionally, a notable statistical disparity was also observed in median PFS between the PD-L1 negative and positive groups (p=0.02). For the entire study population, the median OS was 53.0 months (95% CI 43.8 - 62.2). In the PD-L1-negative group, the median OS reached 57.2 months, compared to 53.0 months in the PD-L1-positive group, a difference lacking statistical significance (p=0.43).
Conclusions: Our results suggest that for ROS1-positive NSCLC patients receiving crizotinib as first-line therapy, PD-L1 expression may serve as a negative prognostic marker for PFS rather than OS.
Lung cancer is currently the most common cause of cancer death worldwide (1). Non-small cell lung cancer (NSCLC) accounts for 85% of all lung cancer cases, of which more than 50% of lung cancer at the time of diagnosis has been distant metastasis, and only 20% to 25% of NSCLC cancers have the opportunity for surgery (2). The evolution of molecular targeted therapy and immunotherapy has notably prolonged the overall survival in patients with advanced NSCLC (3, 4).
The ROS proto-oncogene 1 (ROS1) is encoded by the ROS1 gene, situated on chromosome 6q22.1, and belongs to the tyrosine kinase insulin receptor subfamily (5). ROS1 rearrangement have subsequently been identified in a variety of tumor types, including 1%-2% of NSCLCs (6, 7). Such rearrangements result in the fusion of a segment of ROS1, encompassing the entire tyrosine kinase domain, with one of several different partner proteins (8). Notably, tumors with ROS1 rearrangements and those with anaplastic lymphoma kinase (ALK) rearrangements in NSCLC share similar clinical characteristics (9). ROS1 rearrangements are more prevalent among light smokers or non-smokers (10). Although lung adenocarcinoma represents the most common histological type, ROS1 fusion has also been reported in large cell and squamous tissues (11). Crizotinib, endorsed by the U.S. Food and Drug Administration (FDA) in 2016, was the inaugural tyrosine kinase inhibitor (TKI) approved for first-line treatment of ROS1 fusion-positive NSCLC (12, 13). According to the PROFILE1001 study, the objective response rate (ORR) of crizotinib for ROS1-positive NSCLC was 72%, with the median progression free survival (PFS) and median overall survival (OS) were 19.3 months and 51.4 months, respectively. As of now, the FDA has approved three drugs demonstrating significant efficacy against ROS1 rearranged NSCLC: crizotinib, entrectinib and repotrectinib (14–17).
High expression of PD-L1 has been reported to be associated with ROS1 rearrangement, but negatively correlated with epidermal growth factor receptor (EGFR) mutations (18, 19). Previous studies have indicated that PD-L1 expression correlates with unfavorable treatment outcomes with EGFR-TKIs (20–22). Additionally, several investigations have highlighted that positive PD-L1 expression in ALK-positive NSCLC patients undergoing crizotinib treatment is associated with adverse clinical outcomes (23–25). However, the association between PD-L1 expression and crizotinib response in ROS1-positive NSCLC remains ambiguous. Clarifying the relationship between PD-L1 expression and crizotinib treatment could aid in precisely identifying which ROS1-positive patients would derive the greatest benefit from crizotinib therapy.
In this study, we enrolled advanced NSCLC patients harboring ROS1-positive mutations who underwent crizotinib treatment as first-line therapy. Our aim was to investigate the association between PD-L1 expression and clinical characteristics and to elucidate the impact of PD-L1 expression on the clinical outcomes of first-line crizotinib therapy.
In this retrospective study, patients with ROS1-rearranged NSCLC who were treated with crizotinib as first-line therapy at the First Affiliated Hospital of Zhengzhou University between January 2016 and December 2021, were included. The inclusion and exclusion criteria for this study were as follows: Inclusion criteria:1) Patients with pathologically confirmed advanced or metastatic NSCLC; 2) ROS1 gene fusion-positive; 3) Detection of PD-L1 expression at the time of diagnosis from primary tumor or from a metastatic lesion; 4) Crizotinib administered as the first-line treatment. Exclusion criteria:1) Patients with double or multiple primary neoplasms; 2) Presence of EGFR mutations or other sensitizing mutations.
The data of this study were collected from clinical records on medical history or phone contact. We collected baseline characteristics of patients through medical records, including age, sex, smoking status, pathological type, disease stage, metastatic site, ROS1 fusion partner, PD-L1 expression etc. The TNM staging adhered to the guidelines set forth by the International Association for the Study of Lung Cancer (8th edition). Owing to the retrospective nature of the study, obtaining written informed consent from participants was deemed unnecessary. Patients receiving crizotinib therapy were subject to chest computed tomography evaluations at minimum intervals of three months. The assessment of therapeutic response was conducted in accordance with the Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1. Individuals who had not experienced progression or death by the cut-off date of February 20, 2024, were categorized as censored at their most recent follow-up. PFS was defined as the duration from the initiation of crizotinib therapy to the inaugural documentation of tumor progression or mortality. OS was characterized as the interval from the onset of crizotinib treatment to the occurrence of death. ORR was calculated as the fraction of patients exhibiting either a complete or partial response to crizotinib, based on RECIST 1.1 criteria. The disease control rate (DCR) was defined as the percentage of patients achieving a complete response (CR), partial response (PR), or maintaining a stable disease (SD). The primary objectives of this study were to assess ORR and PFS, with a secondary objective to assess OS and DCR.
Tumor PD-L1 expression was assessed through immunohistochemical (IHC) staining using the PD-L1 Clone 22C3 pharmDx Kit and the Automated Link 48 platform (Dako,Carpinteria, CA). The Tumor Proportion Score (TPS) quantifies PD-L1 expression, measuring the percentage of cells exhibiting positive membrane staining among 100 cancer cells. PD-L1 expression was detected in tumor samples prior to crizotinib treatment. Patients were stratified into three categories based on their TPS scores: a PD-L1 negative group (PD-L1 < 1%), a PD-L1 low expression group (1% ≤ PD-L1 < 50%), and a PD-L1 high expression group (PD-L1 ≥ 50%). The PD-L1 positive cohort was defined by a TPS ≥ 1%, whereas the PD-L1 negative group was characterized by a TPS < 1%. Detection of ROS1 rearrangements was performed using IHC, separation fluorescence in situ hybridization (FISH), reverse transcription-polymerase chain reaction (RT-PCR), or next-generation sequencing (NGS).
Statistical analyses were conducted using GraphPad Prism version 8.0.1 (GraphPad Software, Inc.) and IBM SPSS Statistics software (IBM Corporation). The Chi-square test or Fisher’s exact test was employed to compare clinical characteristics and response rates between PD-L1 cohorts. Kaplan-Meier survival curves were constructed to illustrate the impact of PD-L1 expression on PFS and OS. The log-rank test was utilized to analyze differences between the survival curves. HRs and 95% CIs were calculated using Cox regression analysis. A P-value of <0.05 was considered to indicate statistical significance.
Between January 2016 to December 2021, a total of 246 Chinese patients with ROS1-positive tumors were identified. Of these, 82 patients who met the inclusion criteria were ultimately enrolled in the study (Figure 1). All included patients were ROS1 fusion NSCLC, with 77 presenting with adenocarcinoma, 3 with squamous cell carcinoma, and 2 with adenosquamous carcinomas. The majority of the patients (87.8%) were classified as stage IV, and 10 patients were in stages IIIB or IIIC. The cohort comprised 52 females and 30 males, with a median age at diagnosis of 54 years (range: 30–78 years). A significant portion of the patients (76.8%) were never-smokers. ROS1 rearrangements were identified in 72 of the 82 patients (87.8%) through NGS, while 10 patients (12.2%) were detected by RT-PCR. Among the 72 cases with detectable ROS1 fusion variants, 10 different fusion partners were identified. As depicted in Figure 2A, the most prevalent ROS1 fusion partner was CD74, found in 43 of 82 samples (52.4%); other common partner genes included SDC4 (in 10 tumors), SLC34A2 (in 7 tumors), and EZR (in 6 tumors). Six rare fusion partners were also identified: GOPC, MPRIP, NUP210L, ZCCHC8, CCDC6, and CFAP53, each present in one tumor. Notably, NUP210L and CFAP53 have not been previously reported. The clinicopathological characteristics of the patients are summarized in Table 1.
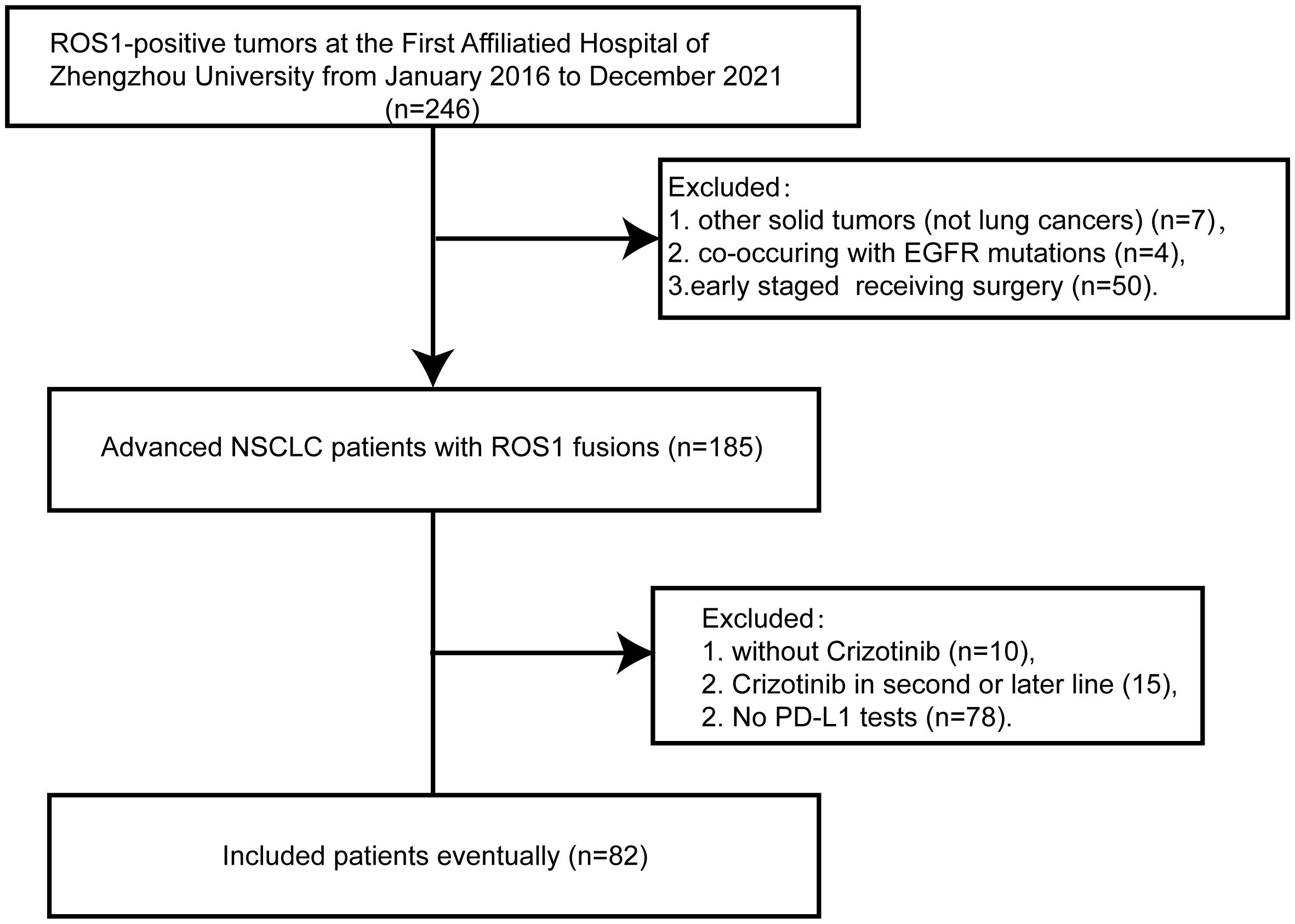
Figure 1 Flow chart of this study. ROS1, ROS proto-oncogene 1; EGFR, epidermal growth factor receptor;NSCLC, non-small-cell lung cancer; PD-L1, programmed death-ligand 1.
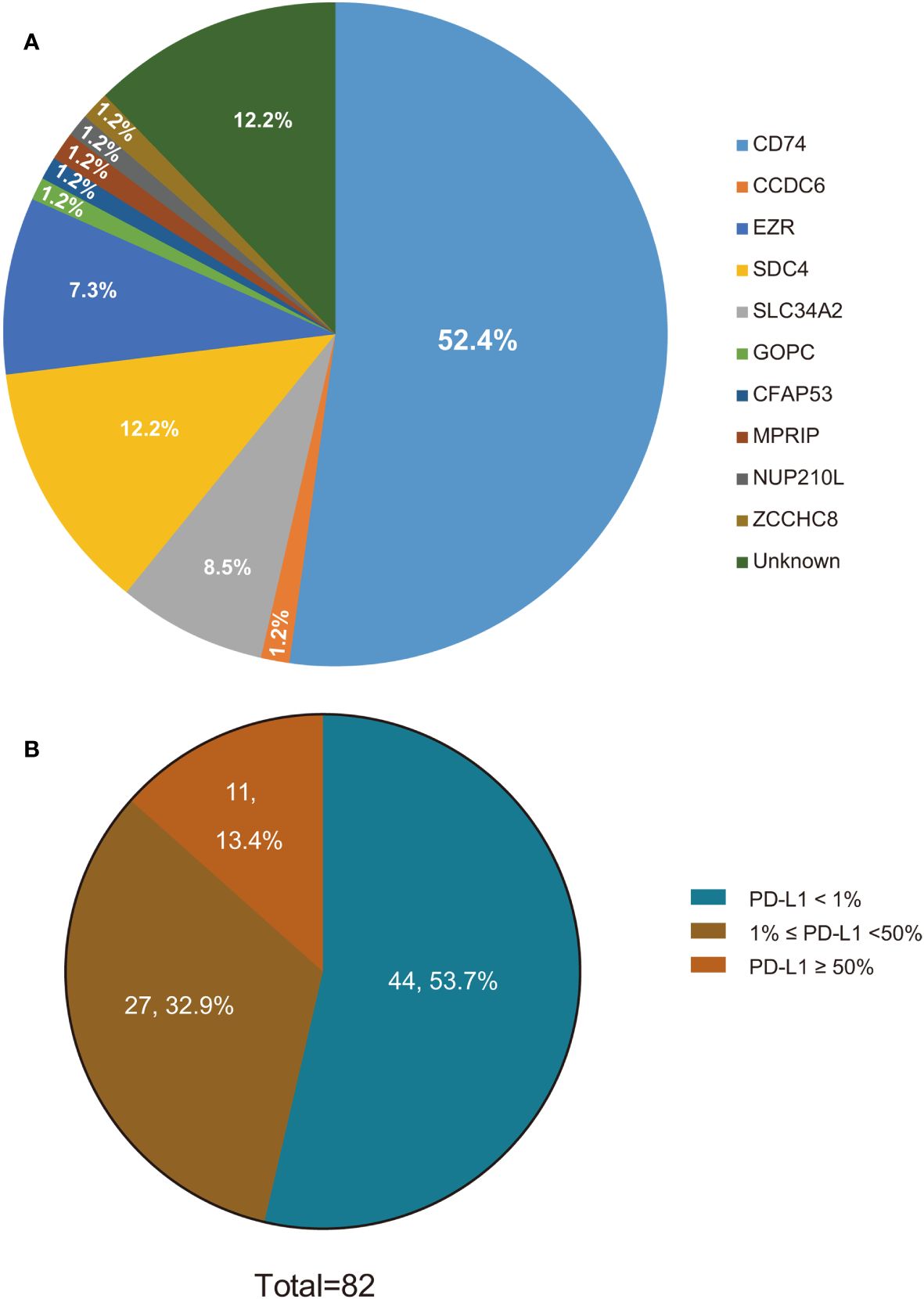
Figure 2 ROS1 fusion variants and PD-L1 status in ROS1-positive NSCLC patients. (A) ROS1 fusion variants in ROS1-positive NSCLC patients; (B) PD-L1 status in ROS1-positive NSCLC patients. ROS1, ROS proto-oncogene 1; PD-L1, programmed death-ligand 1; NSCLC, non-small-cell lung cancer.
We investigated the association between PD-L1 expression and various clinicopathological parameters, including gender, age, smoking status, TNM stage, and ROS1 fusion subtypes. As depicted in Figure 2B, PD-L1 expression was positive in 38 patients, with 11 patients exhibiting high PD-L1 expression, 27 patients displaying low PD-L1 expression, and the remaining 44 patients showing negative PD-L1 expression. Our study showed that PD-L1 expression was not affected by age, sex, histopathological type, tumor stage, or ROS1 fusion subtypes (Supplementary Table 1).
The ORR for all included patients was 80.5%. The ORR of the PD-L1 negative group was 84.1%, while the PD-L1 positive group had an ORR of 76.3% (p=0.38), as depicted in Supplementary Figure 1A. When patients were stratified into three groups based on TPS values, the ORR of the PD-L1 negative group and the PD-L1 low expression group were found to be similar, at 84.1% and 81.5%, respectively. However, the ORR of the PD-L1 high expression group was lower, at only 63.6% (p=0.31), as illustrated in Figure 3A. Consequently, no statistically significant difference in ORR was observed among ROS1 rearrangement NSCLC patients with varying PD-L1 expression statuses. The DCR of the PD-L1 positive and PD-L1 negative groups were 94.7% and 100%, respectively, as shown in Supplementary Figure 1B. Conversely, a significant difference in DCR was noted between the PD-L1 negative group and the PD-L1 high expression group, which were 100% and 90.9%, respectively (p=0.04), as presented in Figure 3B. Figure 3C illustrates the treatment response and PFS of each patient. Moreover, PD-L1 expression did not differ between responders (best response of PR) and non-responders (best response of SD and progressive disease (PD)), as demonstrated in Supplementary Figure 1C.
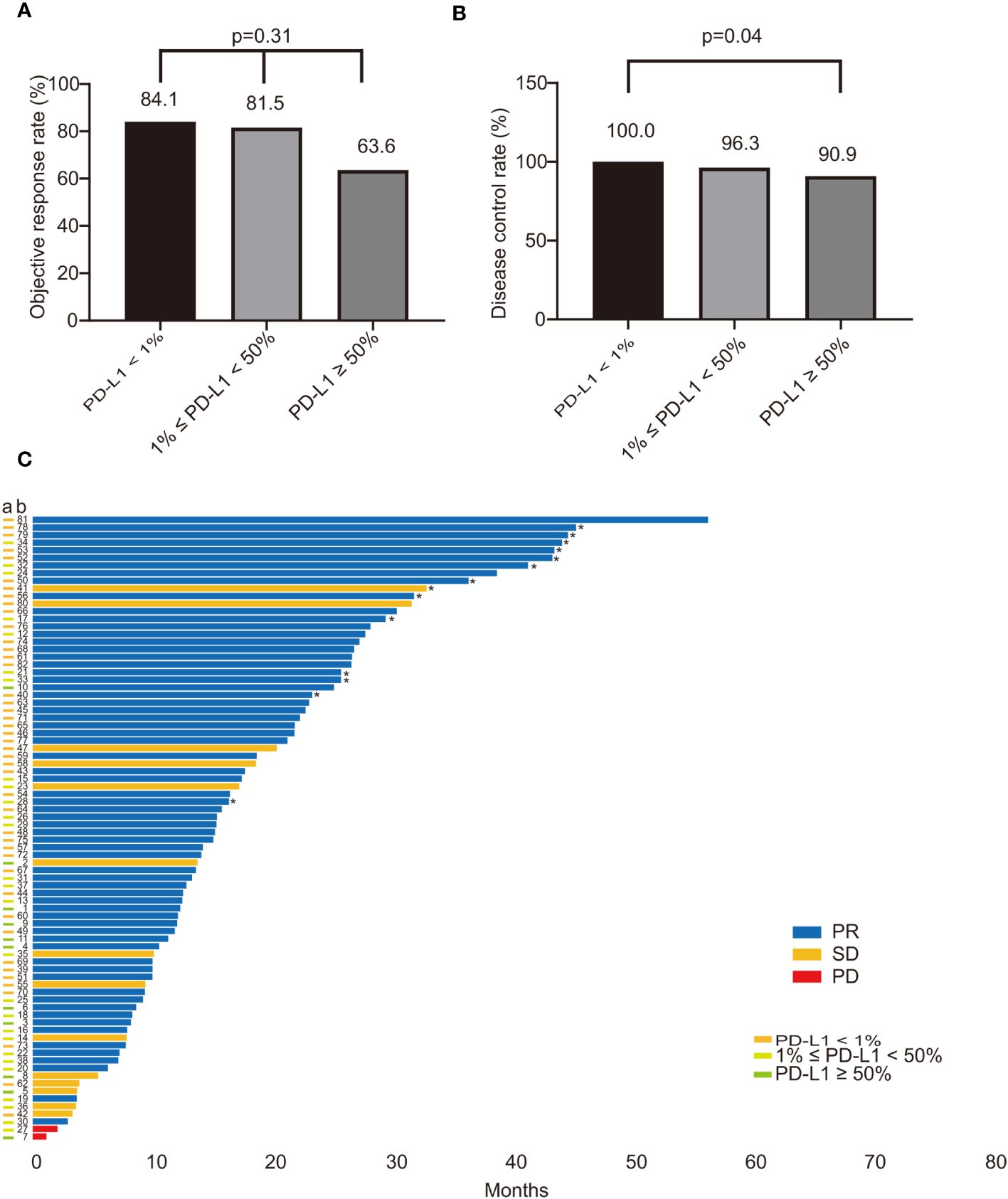
Figure 3 The impact of PD-L1 on the treatment response of ROS1-positive NSCLC patients treated with crizotinib. (A) The ORR in patients with different PD-L1 status (PD-L1 < 1% vs. 1% ≤ PD-L1 < 50% vs. PD-L1 ≥ 50%) was 84.1%, 81.5%, and 63.6%, respectively (p=0.31); (B) The DCR of patients compared with PD-L1 status (PD-L1 < 1% vs. 1% ≤ PD-L1 < 50% vs. PD-L1 ≥ 50%); There was a statistical difference in DCR between the PD-L1 negative group and the PD-L1 high expression group, which were 100% and 90.9%, respectively (p=0.04); (C) The survival plot illustrating the treatment response and PFS of each patient. The bar colors of blue, yellow, and red refer to PR, SD, PD. The different colors of the “a” column on the vertical axis represent the PD-L1 expression status. "*" indicates that the patient was not resistant to crizotinib treatment at the last follow-up. PD-L1, programmed death-ligand 1; ROS1, ROS proto-oncogene 1; NSCLC, non-small-cell lung cancer; ORR, objective response rate; DCR, disease control rate; PFS, progression free survival; PR, partial response; SD, stable disease; PD, progressive disease.
The median PFS of patients treated with crizotinib was 19.7 months (95% confidence interval [CI], 15.6 - 23.8). The survival plots depicting PFS related to crizotinib treatment are illustrated in Figure 4A. Kaplan-Meier analysis revealed that the median PFS of patients in the PD-L1 negative group, PD-L1 low expression group, and PD-L1 high expression group were 26.4, 16.6, and 13.7 months, respectively (p=0.001) (Figure 4C). The median PFS in the PD-L1-negative group was 26.4 months, compared to 14.7 months in the PD-L1-positive group, with a statistically significant difference between these two groups (p=0.02) (Figure 4D). In univariate analysis, brain metastases (p=0.06) and non-adenocarcinoma pathologic types (p=0.09) tended to be associated with shorter PFS, but did not achieve a statistical difference, as shown in Table 2. In the multivariate Cox regression analysis model, which included all factors with p<0.1 in the univariate analysis, PD-L1 expression status (p=0.02) and brain metastasis (p=0.04) showed a significant association with PFS (Table 2). Overall, our findings indicate that in ROS1-positive NSCLC patients treated with crizotinib, those with positive PD-L1 expression experienced shorter PFS compared to patients with negative PD-L1 expression.
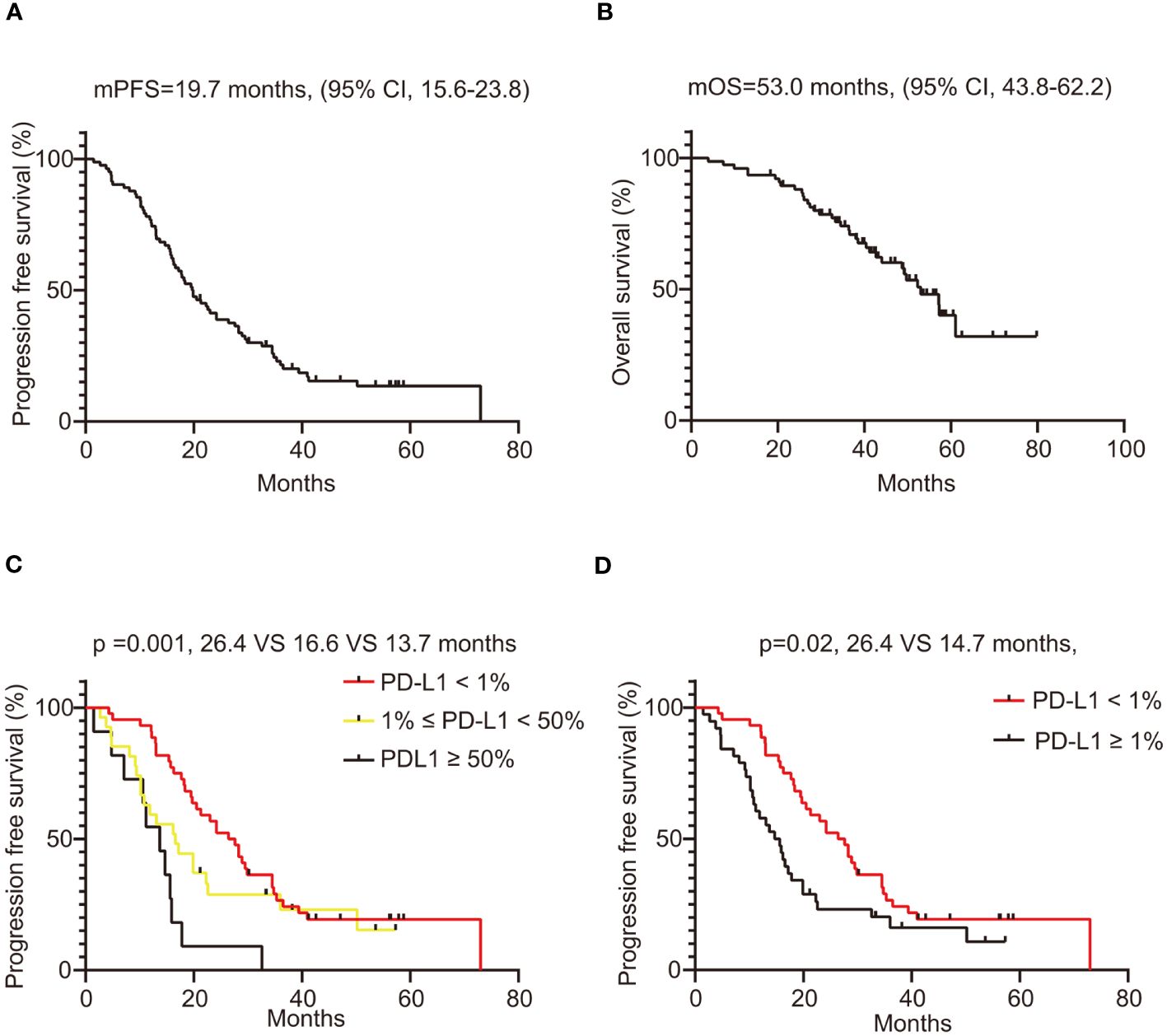
Figure 4 Survival analysis of ROS1-positive NSCLC patients treated with crizotinib. (A) PFS of all included patients, with 95% confidence intervals indicated; (B) OS of all included patients, with 95% confidence intervals indicated; (C) The PFS of ROS1-positive lung cancer patients compared by PD-L1 status (PD-L1 < 1% vs. 1% ≤ PD-L1 < 50% vs. PD-L1 ≥ 50%); (D) The PFS of ROS1-positive lung cancer patients compared by PD-L1 status (negative vs. positive). ROS1, ROS proto-oncogene 1; NSCLC, non-small-cell lung cancer; PFS, progression free survival; OS, overall survival; PD-L1, programmed death-ligand 1.

Table 2 Univariate analysis and multivariate analysis of PFS of ROS1-positive NSCLC patients receiving crizotinib.
We further analyzed the association between PD-L1 expression and OS in ROS1-positive patients treated with crizotinib. The survival plots depicting OS related to crizotinib treatment are illustrated in Figure 4B. The median OS of the entire population was 53.0 months (95% CI, 43.8 - 62.2). The median OS of the PD-L1 negative group was longer than that of the PD-L1 positive group, at 57.2 months and 53.0 months, respectively, but the difference was not statistically significant (p=0.43), as presented in Supplementary Figure 2B. Furthermore, when patients were stratified into PD-L1 negative, PD-L1 low expression, and PD-L1 high expression groups, there was still no statistically significant difference in OS among the three groups (p = 0.71) (Supplementary Figure 2A). Overall, patients with positive PD-L1 expression tended to have shorter OS compared to those with negative PD-L1 expression, but this difference was not statistically significant.
The management of advanced lung cancer has evolved into the era of precision medicine (26, 27). The selection of an appropriate treatment regimen is determined not merely by the histological subtype but also by the presence of specific driver mutations and the level of PD-L1 expression (28, 29). Consequently, the precise detection of biomarkers is essential for the customization of therapy on an individual basis.
Accumulating evidence suggests that the regulation of PD-L1 expression is intricately linked to oncogenic mutations driving NSCLC (30). Evans et al. (2018) conducted PD-L1 detection with the 22C3 Assay on 10005 NSCLC patients, and the results showed that poorly-differentiated tumor histology and ALK translocation were significantly associated with PD-L1 expression (31). Another study examined PD-L1 in 130 consecutive NSCLC samples and found that PD-L1 expression (TPS≥1%) was significantly associated with wild-type EGFR, and ROS1 rearrangement was associated with high PD-L1 expression (19). The above studies suggest that mutations in the EGFR gene are associated with diminished PD-L1 expression, whereas higher incidences of PD-L1 positivity are observed in NSCLC patients harboring ALK or ROS1 rearrangements (31–33). The latter, through the activation of receptor tyrosine kinases, significantly enhances NSCLC cell proliferation (8). Furthermore, the escalation of PD-L1 expression in NSCLC has been correlated with the activation of the RAS signaling pathway, a process in which the ROS1 receptor tyrosine kinase plays a pivotal role (34). Our research findings indicate that 46.3% of NSCLC patients with ROS1 rearrangements exhibit PD-L1 positivity, with 13.4% displaying high levels of PD-L1 expression and 32.9% showing lower levels. These observations underscore the association between ROS1 rearrangement and elevated PD-L1 expression in NSCLC, aligning with existing literature that points to the abnormal activation of oncogenes, including ROS1 and ALK, as a driving force behind PD-L1 expression in this disease context (18, 35).
With the elucidation of the complex interaction between the immune system and the activation of oncogenic driver genes, the role of PD-L1 expression as a predictive biomarker for targeted therapies has received increasing attention. Accumulating evidence has identified PD-L1 expression as an adverse prognostic indicator in patients harboring EGFR mutations, irrespective of the application of targeted drug therapies (20, 21). Historically, the influence of PD-L1 on the effectiveness of targeted treatments in EGFR-mutant NSCLC has been a focal point of research. Notably, recent comprehensive analyses reveal that elevated PD-L1 levels correlate with diminished efficacy of EGFR-TKIs in EGFR-mutated NSCLC (36). A study on patients with ALK-positive NSCLC treated with crizotinib reported that the absence of PD-L1 expression is linked to prolonged PFS (24). However, divergent findings from another investigation suggest that initial PD-L1 levels do not significantly forecast the success of alectinib therapy in individuals with ALK-positive lung cancer (25).
To our knowledge, this constitutes the inaugural investigation into the correlation between PD-L1 expression and crizotinib’s therapeutic efficacy. Our study demonstrates a significant association between PD-L1 expression and reduced PFS in patients with advanced NSCLC harboring ROS1 fusions. Conversely, although patients with positive PD-L1 expression exhibited a trend towards decreased OS compared to those with negative PD-L1 expression, this observation did not reach statistical significance. These findings propose that PD-L1 may serve more effectively as a predictive biomarker for crizotinib-induced PFS, rather than as a prognostic biomarker for OS. Investigating the immune mechanisms underlying TKI resistance and exploring potential synergies with combination immunotherapy could enhance crizotinib’s therapeutic impact on NSCLC patients with ROS1 fusion.
The underlying mechanism of immune microenvironment-mediated TKI resistance in oncogene-driven lung cancer is still under investigation. Recent research indicates that in melanoma driven by B-type Raf kinase (BRAF) mutations, resistance to inhibitors of the mitogen-activated protein kinases (MAPK) pathway may arise through macrophage-secreted Tumor Necrosis Factor alpha (TNF-α) (37). Concurrently, elevated expression of PD-L1 has been observed in cell lines resistant to BRAF inhibitors as well as in samples from patients, hinting at a potential role of PD-L1 in conferring resistance to targeted therapies (38–40). Furthermore, lung cancer specimens and cell lines that developed resistance to ALK inhibitors exhibited increased PD-L1 expression, linking the PD-1/PD-L1 pathway to ALK inhibitor resistance in cases of ALK rearrangement (41). Liu et al. (2020) demonstrated that in NSCLC resistant to crizotinib, ROS1 rearrangements lead to an upregulation of PD-L1 expression via the mitogen-activated protein kinase kinase (MEK) - extracellular signal-regulated kinases (ERK) signaling pathway (18). This evidence supports the hypothesis that blocking the PD-L1/PD-1 pathway could represent a promising approach for treating crizotinib-resistant NSCLC with ROS1 rearrangement. However, The efficacy of immunotherapy in ROS1 fusion NSCLC patients remains to be further studied. We have reported a ROS1 positive NSCLC patient, who achieved a continuous PR to immunotherapy plus chemotherapy and a more than 35 months PFS (42). Nevertheless, another study indicated that ROS1 fusion NSCLC patients treated with immunotherapy alone displayed an unsatisfactory ORR of 16.7% (43). Chemotherapy combined with immunotherapy with or without bevacizumab may be one of the treatment options for ROS1 positive NSCLC patients after TKI failure (44). A large number of studies have shown that NSCLC patients with high PD-L1 expression are more likely to benefit from immunotherapy (45). Immunotherapy combined with chemotherapy after TKI resistance may be a reason why PD-L1 expression was not associated with the OS of the crizotinib treatment in our study.
Several limitations warrant mention within the context of this study. Initially, the retrospective nature of this study inherently introduces selection bias. Additionally, the relatively rare occurrence of ROS1 fusion in NSCLC constrained the size of the patient cohort, potentially limiting the representativeness of our sample for the broader ROS1 fusion-positive population. Moreover, patients treated with other TKIs, such as entrectinib or ceritinib, were excluded from our analysis, leaving the relationship between PD-L1 expression and the prognosis with these other TKIs an open question for future research. Consequently, expansive studies with larger cohorts are essential to substantiate our findings.
In summary, our investigation reveals that among ROS1-positive NSCLC patients treated with crizotinib as an initial therapeutic approach, PD-L1 expression may serve as an adverse prognostic marker for PFS rather than OS. Consequently, assessing PD-L1 expression in patients with ROS1-positive NSCLC could offer valuable insights for predicting clinical outcomes.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
The studies involving humans were approved by Ethics Committee of the First Affiliated Hospital of Zhengzhou University (2024-KY-0231-002). The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants’ legal guardians/next of kin because our study was a retrospective study.
HZ: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. ZZ: Conceptualization, Data curation, Investigation, Methodology, Supervision, Writing – original draft, Writing – review & editing. NY: Conceptualization, Formal analysis, Investigation, Methodology, Project administration, Software, Supervision, Validation, Writing – original draft, Writing – review & editing. XL: Conceptualization, Funding acquisition, Investigation, Methodology, Resources, Visualization, Writing – original draft, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was supported by a grant of the Henan Provincial Science and Technology Research Project (Grant N. 2008020081).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2024.1405683/full#supplementary-material
1. Siegel RL, Miller KD, Wagle NS, Jemal A. Cancer statistics, 2023. CA: A Cancer J Clin. (2023) 73:17–48. doi: 10.3322/caac.21763
2. Liang Y, Wakelee HA. Adjuvant chemotherapy of completely resected early stage non-small cell lung cancer (Nsclc). Transl Lung Cancer Res. (2013) 2:403–10. doi: 10.3978/j.issn.2218–6751.2013.07.01
3. Paz-Ares LG, Ramalingam SS, Ciuleanu TE, Lee JS, Urban L, Caro RB, et al. First-line nivolumab plus ipilimumab in advanced nsclc: 4-year outcomes from the randomized, open-label, phase 3 checkmate 227 part 1 trial. J Thorac Oncol. (2022) 17:289–308. doi: 10.1016/j.jtho.2021.09.010
4. Wu YL, Tsuboi M, He J, John T, Grohe C, Majem M, et al. Osimertinib in resected egfr-mutated non-small-cell lung cancer. N Engl J Med. (2020) 383:1711–23. doi: 10.1056/NEJMoa2027071
5. D’Angelo A, Sobhani N, Chapman R, Bagby S, Bortoletti C, Traversini M, et al. Focus on ros1-positive non-small cell lung cancer (Nsclc): crizotinib, resistance mechanisms and the newer generation of targeted therapies. Cancers (Basel). (2020) 12:3293. doi: 10.3390/cancers12113293
6. Bergethon K, Shaw AT, Ou SH, Katayama R, Lovly CM, McDonald NT, et al. Ros1 rearrangements define a unique molecular class of lung cancers. J Clin Oncol. (2012) 30:863–70. doi: 10.1200/JCO.2011.35.6345
7. Davies KD, Le AT, Theodoro MF, Skokan MC, Aisner DL, Berge EM, et al. Identifying and targeting ros1 gene fusions in non-small cell lung cancer. Clin Cancer Res. (2012) 18:4570–9. doi: 10.1158/1078–0432.Ccr-12–0550
8. Davies KD, Doebele RC. Molecular pathways: ros1 fusion proteins in cancer. Clin Cancer Res. (2013) 19:4040–5. doi: 10.1158/1078–0432.Ccr-12–2851
9. Wu J, Lin Z. Non-small cell lung cancer targeted therapy: drugs and mechanisms of drug resistance. Int J Mol Sci. (2022) 23:15056. doi: 10.3390/ijms232315056
10. Gainor JF, Shaw AT. Novel targets in non-small cell lung cancer: ros1 and ret fusions. Oncol. (2013) 18:865–75. doi: 10.1634/theoncologist.2013–0095
11. Yu ZQ, Wang M, Zhou W, Mao MX, Chen YY, Li N, et al. Ros1-positive non-small cell lung cancer (Nsclc): biology, diagnostics, therapeutics and resistance. J Drug Target. (2022) 30:845–57. doi: 10.1080/1061186x.2022.2085730
12. Shaw AT, Riely GJ, Bang YJ, Kim DW, Camidge DR, Solomon BJ, et al. Crizotinib in ros1-rearranged advanced non-small-cell lung cancer (Nsclc): updated results, including overall survival, from profile 1001. Ann Oncol. (2019) 30:1121–6. doi: 10.1093/annonc/mdz131
13. Shaw AT, Ou SH, Bang YJ, Camidge DR, Solomon BJ, Salgia R, et al. Crizotinib in ros1-rearranged non-small-cell lung cancer. N Engl J Med. (2014) 371:1963–71. doi: 10.1056/NEJMoa1406766
14. Lim SM, Kim HR, Lee J-S, Lee KH, Lee Y-G, Min YJ, et al. Open-label, multicenter, phase ii study of ceritinib in patients with non–small-cell lung cancer harboring ros1 rearrangement. J Clin Oncol. (2017) 35:2613–8. doi: 10.1200/JCO.2016.71.3701
15. Dziadziuszko R, Krebs MG, De Braud F, Siena S, Drilon A, Doebele RC, et al. Updated integrated analysis of the efficacy and safety of entrectinib in locally advanced or metastatic ros1 fusion-positive non-small-cell lung cancer. J Clin Oncol. (2021) 39:1253–63. doi: 10.1200/jco.20.03025
16. Shaw AT, Solomon BJ, Chiari R, Riely GJ, Besse B, Soo RA, et al. Lorlatinib in advanced ros1-positive non-small-cell lung cancer: A multicentre, open-label, single-arm, phase 1–2 trial. Lancet Oncol. (2019) 20:1691–701. doi: 10.1016/s1470–2045(19)30655–2
17. Dhillon S. Repotrectinib: first approval. Drugs. (2024) 84:239–46. doi: 10.1007/s40265–023-01990–6
18. Liu Z, Zhao K, Wei S, Liu C, Zhou J, Gou Q, et al. Ros1-fusion protein induces pd-L1 expression via mek-erk activation in non-small cell lung cancer. OncoImmunology. (2020) 9:1758003. doi: 10.1080/2162402x.2020.1758003
19. Lee J, Park CK, Yoon HK, Sa YJ, Woo IS, Kim HR, et al. Pd-L1 expression in ros1-rearranged non-small cell lung cancer: A study using simultaneous genotypic screening of egfr, alk, and ros1. Thorac Cancer. (2018) 10:103–10. doi: 10.1111/1759–7714.12917
20. Hsu K-H, Huang Y-H, Tseng J-S, Chen K-C, Ku W-H, Su K-Y, et al. High Pd-L1 Expression Correlates with Primary Resistance to Egfr-Tkis in Treatment naïve advanced EGFR-Mutant Lung Adenocarcinoma Patients. Lung Cancer. (2019) 127:37–43. doi: 10.1016/j.lungcan.2018.11.021
21. Yang C-Y, Liao W-Y, Ho C-C, Chen K-Y, Tsai T-H, Hsu C-L, et al. Association between programmed death-ligand 1 expression, immune microenvironments, and clinical outcomes in epidermal growth factor receptor mutant lung adenocarcinoma patients treated with tyrosine kinase inhibitors. Eur J Cancer. (2020) 124:110–22. doi: 10.1016/j.ejca.2019.10.019
22. Kim T, Cha YJ, Chang YS. Correlation of pd-L1 expression tested by 22c3 and sp263 in non-small cell lung cancer and its prognostic effect onegfrmutation–positive lung adenocarcinoma. Tuberculosis Respir Dis. (2020) 83:51–60. doi: 10.4046/trd.2019.0026
23. Chang G-C, Yang T-Y, Chen K-C, Hsu K-H, Huang Y-H, Su K-Y, et al. Alk variants, pd-L1 expression, and their association with outcomes in alk-positive nsclc patients. Sci Rep. (2020) 10:21063. doi: 10.1038/s41598–020-78152–1
24. Yang C-Y, Liao W-Y, Ho C-C, Chen K-Y, Tsai T-H, Hsu C-L, et al. Association of programmed death-ligand 1 expression with fusion variants and clinical outcomes in patients with anaplastic lymphoma kinase-positive lung adenocarcinoma receiving crizotinib. Oncol. (2020) 25:702–11. doi: 10.1634/theoncologist.2020–0088
25. Pan Y, Liu X, Zhang W, Wang W, Wang H, Luo L, et al. Association of pd-L1 expression with efficacy of alectinib in advanced nsclc patients with alk fusion. Lung Cancer. (2023) 181:107233. doi: 10.1016/j.lungcan.2023.107233
26. Wang Q, Su C, Zhou C. Recent advances in immunotherapy for lung cancer. Cancer Innovation. (2023) 2:18–24. doi: 10.1002/cai2.55
27. Li MSC, Mok KKS, Mok TSK. Developments in targeted therapy & Immunotherapy—How non-small cell lung cancer management will change in the next decade: A narrative review. Ann Trans Med. (2023) 11:358–. doi: 10.21037/atm-22–4444
28. Herrera-Juárez M, Serrano-Gómez C, Bote-de-Cabo H, Paz-Ares L. Targeted therapy for lung cancer: beyond egfr and alk. Cancer. (2023) 129:1803–20. doi: 10.1002/cncr.34757
29. Brody R, Zhang Y, Ballas M, Siddiqui MK, Gupta P, Barker C, et al. Pd-L1 expression in advanced nsclc: insights into risk stratification and treatment selection from a systematic literature review. Lung Cancer. (2017) 112:200–15. doi: 10.1016/j.lungcan.2017.08.005
30. Saigi M, Alburquerque-Bejar JJ, Mc Leer-Florin A, Pereira C, Pros E, Romero OA, et al. Met-oncogenic and jak2-inactivating alterations are independent factors that affect regulation of pd-L1 expression in lung cancer. Clin Cancer Res. (2018) 24:4579–87. doi: 10.1158/1078–0432.Ccr-18–0267
31. Evans M, O’Sullivan B, Hughes F, Mullis T, Smith M, Trim N, et al. The clinicopathological and molecular associations of pd-L1 expression in non-small cell lung cancer: analysis of a series of 10,005 cases tested with the 22c3 assay. Pathol Oncol Res. (2018) 26:79–89. doi: 10.1007/s12253–018-0469–6
32. Azuma K, Ota K, Kawahara A, Hattori S, Iwama E, Harada T, et al. Association of pd-L1 overexpression with activating egfr mutations in surgically resected nonsmall-cell lung cancer. Ann Oncol. (2014) 25:1935–40. doi: 10.1093/annonc/mdu242
33. Lastwika KJ, Wilson W, Li QK, Norris J, Xu H, Ghazarian SR, et al. Control of pd-L1 expression by oncogenic activation of the akt–mtor pathway in non–small cell lung cancer. Cancer Res. (2016) 76:227–38. doi: 10.1158/0008–5472.Can-14–3362
34. Coelho MA, de Carné Trécesson S, Rana S, Zecchin D, Moore C, Molina-Arcas M, et al. Oncogenic ras signaling promotes tumor immunoresistance by stabilizing pd-L1 mRNA. Immunity. (2017) 47:1083–1099.e6. doi: 10.1016/j.immuni.2017.11.016
35. Cai L, Duan J, Qian L, Wang Z, Wang S, Li S, et al. Ros1 fusion mediates immunogenicity by upregulation of pd-L1 after the activation of ros1–shp2 signaling pathway in non-small cell lung cancer. Front Immunol. (2020) 11:527750. doi: 10.3389/fimmu.2020.527750
36. Yoneshima Y, Ijichi K, Anai S, Ota K, Otsubo K, Iwama E, et al. Pd-L1 expression in lung adenocarcinoma harboring <Em>Egfr</em> mutations or <Em>Alk</em> rearrangements. Lung Cancer. (2018) 118:36–40. doi: 10.1016/j.lungcan.2018.01.024
37. Smith MP, Sanchez-Laorden B, O’Brien K, Brunton H, Ferguson J, Young H, et al. The immune microenvironment confers resistance to mapk pathway inhibitors through macrophage-derived tnfα. Cancer Discovery. (2014) 4:1214–29. doi: 10.1158/2159–8290.Cd-13–1007
38. Kakavand H, Rawson RV, Pupo GM, Yang JYH, Menzies AM, Carlino MS, et al. Pd-L1 expression and immune escape in melanoma resistance to mapk inhibitors. Clin Cancer Res. (2017) 23:6054–61. doi: 10.1158/1078–0432.Ccr-16–1688
39. Jiang X, Zhou J, Giobbie-Hurder A, Wargo J, Hodi FS. The activation of mapk in melanoma cells resistant to braf inhibition promotes pd-L1 expression that is reversible by mek and pi3k inhibition. Clin Cancer Res. (2013) 19:598–609. doi: 10.1158/1078–0432.Ccr-12–2731
40. Kim MH, Kim CG, Kim S-K, Shin SJ, Choe EA, Park S-H, et al. Yap-induced pd-L1 expression drives immune evasion in brafi-resistant melanoma. Cancer Immunol Res. (2018) 6:255–66. doi: 10.1158/2326–6066.Cir-17–0320
41. Kim S-J, Kim S, Kim D-W, Kim M, Keam B, Kim TM, et al. Alterations in pd-L1 expression associated with acquisition of resistance to alk inhibitors in alk-rearranged lung cancer. Cancer Res Treat. (2019) 51:1231–40. doi: 10.4143/crt.2018.486
42. Yan N, Huang S, Li L, Guo Q, Geng D, Zhang H, et al. Case report: durable response to immuno-chemotherapy in a case of ros1 fusion-positive advanced lung adenocarcinoma: A case report. Front Pharmacol. (2022) 13:898623. doi: 10.3389/fphar.2022.898623
43. Choudhury NJ, Schneider JL, Patil T, Zhu VW, Goldman DA, Yang SR, et al. Response to immune checkpoint inhibition as monotherapy or in combination with chemotherapy in metastatic ros1-rearranged lung cancers. JTO Clin Res Rep. (2021) 2:100187. doi: 10.1016/j.jtocrr.2021.100187
44. Bylicki O, Tomasini P, Radj G, Guisier F, Monnet I, Ricordel C, et al. Atezolizumab with or without Bevacizumab and Platinum-Pemetrexed in Patients with Stage Iiib/Iv Non-Squamous Non-Small Cell Lung Cancer with Egfr Mutation, Alk Rearrangement or Ros1 Fusion Progressing after Targeted Therapies: A Multicentre Phase Ii Open-Label Non-Randomised Study Gfpc 06–2018. Eur J Cancer. (2023) 183:38–48. doi: 10.1016/j.ejca.2023.01.014
Keywords: PD-L1, clinical outcomes, ROS1, NSCLC, crizotinib
Citation: Zhang H, Zhang Z, Yan N and Li X (2024) Association of PD-L1 expression and clinical outcomes in ROS1 - rearranged advanced non-small cell lung cancer treated with crizotinib. Front. Oncol. 14:1405683. doi: 10.3389/fonc.2024.1405683
Received: 23 March 2024; Accepted: 30 April 2024;
Published: 21 May 2024.
Edited by:
Myrto K. Moutafi, University General Hospital Attikon, GreeceReviewed by:
Evangelos Zazas, University General Hospital Attikon, GreeceCopyright © 2024 Zhang, Zhang, Yan and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xingya Li, bGl4aW5neWEwOTA1QHZpcC4xNjMuY29t; Ningning Yan, eWFubmluZ3JqQDE2My5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.