
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Oncol. , 26 July 2024
Sec. Cancer Epidemiology and Prevention
Volume 14 - 2024 | https://doi.org/10.3389/fonc.2024.1405267
This article is part of the Research Topic Accelerating Cancer Genomics Research in Sub-Saharan Africa View all 4 articles
 Suleiman Zakari1,2,3*†
Suleiman Zakari1,2,3*† Nguedia K. Niels1,2,4*†
Nguedia K. Niels1,2,4*† Grace V. Olagunju5†
Grace V. Olagunju5† Precious C. Nnaji1
Precious C. Nnaji1 Oluwabusayo Ogunniyi1
Oluwabusayo Ogunniyi1 Mercy Tebamifor1,2
Mercy Tebamifor1,2 Emmanuel N. Israel1,2
Emmanuel N. Israel1,2 Sunday E. Atawodi6†
Sunday E. Atawodi6† Olubanke Olujoke Ogunlana1,2*†
Olubanke Olujoke Ogunlana1,2*†Cancer remains a global health challenge, necessitating continuous advancements in diagnostic and treatment strategies. This review focuses on the utility of non-invasive biomarkers in cancer diagnosis and treatment, their role in early detection, disease monitoring, and personalized therapeutic interventions. Through a systematic review of the literature, we identified 45 relevant studies that highlight the potential of these biomarkers across various cancer types, such as breast, prostate, lung, and colorectal cancers. The non-invasive biomarkers discussed include liquid biopsies, epigenetic markers, non-coding RNAs, exosomal cargo, and metabolites. Notably, liquid biopsies, particularly those based on circulating tumour DNA (ctDNA), have emerged as the most promising method for early, non-invasive cancer detection due to their ability to provide comprehensive genetic and epigenetic information from easily accessible blood samples. This review demonstrates how non-invasive biomarkers can facilitate early cancer detection, accurate subtyping, and tailored treatment strategies, thereby improving patient outcomes. It underscores the transformative potential of non-invasive biomarkers in oncology, highlighting their application for enhancing early detection, survival rates, and treatment precision in cancer care.
Systematic review registration: https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42023474749 PROSPERO, identifier CRD42023474749.
Cancer, a complex and multifaceted group of diseases, remains one of the most significant public health challenges worldwide (1, 2). Cancer remains a leading cause of morbidity and mortality worldwide, with an estimated 19.3 million new cases and 10 million cancer-related deaths in 2020 alone (3). The prevalence of various cancer types varies significantly, with breast, lung, colorectal, and prostate cancers being among the most common (4). Specifically, breast cancer accounted for 11.7% of new cases, while lung cancer was responsible for the highest number of cancer deaths at 18% (3, 5).
The development and implementation of biomarkers in cancer diagnosis and treatment have gained substantial traction in recent years (6). Biomarkers are biological molecules found in blood, other body fluids, or tissues, signaling an abnormal process, condition, or disease. They hold promise for early cancer detection, prognosis, and monitoring treatment response, thereby enhancing precision medicine. The quest for early diagnosis and effective treatment strategies is an enduring pursuit in oncology (6). While traditional approaches such as tissue biopsies have long served as cornerstones of cancer diagnosis and management, the emergence of non-invasive biomarkers is and revolutionizing the field. Non-invasive biomarkers encompass various molecules and analytes, ranging from circulating tumor DNA (ctDNA) and exosomes to microRNAs and metabolites (7, 8). These biomarkers promise early detection, real-time monitoring, and personalised treatment strategies. In an era of precision medicine, identifying and validating these biomarkers hold immense potential to transform the landscape of cancer care (9).
Significant progress has been made in the clinical trials for biomarker-based treatments. As of 2023, numerous clinical trials are actively investigating the efficacy of biomarkers in various cancers. For instance, trials for non-small cell lung cancer (NSCLC) focus on biomarkers like Programmed Death-Ligand 1 (PD-L1) and Epidermal Growth Factor Receptor (EGFR) mutations, with promising results leading to the approval of several targeted therapies (10). In breast cancer, HER2 and BRCA mutations are pivotal in guiding treatment decisions, with ongoing trials exploring new biomarker targets (11). Variations in the Androgen receptor and gene mutations of its coactivators have been studied extensively for various applications (12, 13). The utilization of non-invasive biomarkers is particularly noteworthy. Techniques such as liquid biopsies, which analyze biomarkers in body fluids like blood, urine, and saliva, offer a less invasive alternative to traditional tissue biopsies. This approach is beneficial for continuously monitoring disease progression and response to treatment, providing a dynamic view of the cancer’s evolution.
This review seeks to explore emerging biomarkers for non-invasive diagnosis and treatment of cancer. It delves into the evolving realm of non-invasive diagnostics, seeking to understand the latest trends, innovations, and future prospects. By scrutinizing the scientific literature and research developments, we aim to shed light on the groundbreaking potential of non-invasive biomarkers in the battle against cancer. Furthermore, we navigate through the intricacies of liquid biopsies, epigenetic markers, non-coding RNAs, exosomal cargo, and metabolites. Through a systematic lens, we embarked on an exercise to discern the critical role these emerging biomarkers play in advancing early detection, tailored therapies, and improved patient outcomes. It is hoped that this review will uncover the latest discoveries, innovations, and relentless efforts at improving the lives of those impacted by this unrelenting disease and consider the future possibilities in the ever-evolving field of oncology and cancer patient care.
The protocol was registered with the PROSPERO under the identification number CRD42023474749 (14). This research does not involve using humans or animals, so no institutional review board or ethics committee approval was deemed necessary for this review.
The search for relevant studies was conducted in multiple databases, including PubMed, Scopus, Web of Science, and Google Scholar. Keywords and search terms employed included “non-invasive biomarkers,” “cancer diagnosis,” “cancer treatment,” and variations thereof. Medical Subject Headings (MeSH) terms and Boolean operators were utilized to refine search results. The search encompassed articles published up to 2023. No language restrictions were applied, and non-English articles were considered. Grey literature sources, such as conference proceedings and preprint archives, were explored for potentially relevant studies. Manual searches included examining the reference lists of key articles and contacting experts in the field for additional studies.
Articles were included if they met the Population, Intervention, Comparators, Outcomes, and Study Design (PICOS) Criteria and provided relevant information on non-invasive biomarkers in cancer. Exclusion criteria included studies that did not pertain to cancer lacked full-text availability or were based on animal or in vitro models. No significant deviations from the PICOS framework were applied, ensuring a focused and comprehensive selection process. The PICOS framework employed to define inclusion and exclusion criteria are as follows:
i. Population: Studies involving human subjects with a confirmed or suspected cancer diagnosis.
ii. Intervention: Articles investigating non-invasive biomarkers for cancer diagnosis and treatment.
iii. Comparators: Studies comparing the effectiveness of different biomarkers or approaches.
iv. Outcomes: Articles reporting outcomes related to biomarker sensitivity, specificity, or clinical utility.
v. Study Design: Original research articles, systematic reviews, and meta-analyses.
The selection process involved the following steps: Initial screening of titles and abstracts to identify potentially relevant articles. This was followed by a full-text review of selected articles to assess eligibility based on inclusion and exclusion criteria. Discrepancies between reviewers during title and abstract screening were resolved through consensus, and any remaining disagreements during full-text review were addressed through discussion. A flow diagram (Figure 1) illustrates the study selection process.
The authors conducted individual literature reviews and documented their discoveries. Data extraction was conducted independently by two reviewers (SZ and NKN), and disagreements were resolved through discussion and consensus. To facilitate information extraction, a standard table containing characteristics of the studies included in the systematic study analysis was created. To mitigate selection bias, the authors cross-referenced their extracted data after the revision stage and addressed any discrepancies, while duplicate entries were eliminated. If disagreements persisted, a senior researcher (OOO) re-examined the data extraction process. Extracted data items included study characteristics (author, publication year), study design, population characteristics, biomarker types, outcomes, and key findings.
A search across various databases, including PubMed, Scopus, Google Scholar, and Web of Science, yielded 2,500 records. After an initial screening of titles and abstracts, 1,500 records were excluded due to irrelevance to the topic. The remaining 1,000 records underwent full-text assessment. After this thorough review, 925 articles were excluded for reasons including insufficient relevance, not meeting the inclusion criteria, and lack of full-text availability. After a meticulous screening process based on predefined inclusion and exclusion criteria, a further 30 articles were excluded for lack of relevance or inadequate methodology. Ultimately, 45 studies were included in the systematic review.
Table 1 presents a list of selected biomarkers categorized by cancer type, stage, and classification, as identified in the reviewed studies. The included studies exhibited diverse characteristics:
i. Study Design: The selected studies encompassed a variety of designs, including cohort studies, case-control studies, randomized controlled trials, and systematic reviews.
ii. Population: These studies investigated populations with confirmed or suspected cancer diagnoses, covering a wide spectrum of cancer types.
iii. Interventions: The primary focus of the studies was on developing and evaluating non-invasive biomarkers for cancer diagnosis and treatment.
iv. Outcomes: The studies reported outcomes related to biomarker sensitivity, specificity, clinical utility, and their potential impact on cancer management.
Early cancer detection is the key to improved quality of life and survival and to reducing the financial burden of cancer treatments, which are greater at later stage detection (29). Liquid biopsy (LB) is a term used to describe the analysis of body fluid such as saliva, blood, urine, and cerebrospinal fluid, to identify specific biomarkers associated with cancer development and progression, for example, identification of Circulating Tumor Cells (CTC) as well as circulating tumor cells DNA in blood (29). In the context of cancer management, especially diagnosis and treatment, liquid biopsy which is the analysis of body fluids for circulating tumors cells, cell free nucleic acids, proteins or any other tumor fragments has opened new direction for early tumor detection and precision medicine (20, 21).
The first liquid biopsy application in cancer analysis was circulating tumor cell detection which has different biomarkers depending on the type of cancer. Since many cancers are from epithelial cells there is a universal biomarker used for CTC detection epithelial cell adhesion molecules (EpCAM proteins). Its expression differs from one cancer type to another. It’s mostly used for breast and prostate cancer diagnosis (29). Liquid biopsy offers a non-invasive means for multiple clinical applications in cancer management including early cancer detection, staging and monitoring of localized cancer, predicting relapse and metastatic progression, assessing therapy efficacy, distinguishing early responders from non-responders, and tracking tumor evolution and resistance mechanisms, all of which can significantly enhance patient care and treatment outcomes (30). Therefore, liquid biopsies have a numerous advantages over the traditional biopsy (Table 2) as they help to obtain information from diagnosis to molecular profiling and response assessment without the need of tissue biopsy (31).
While liquid biopsies offer several advantages in cancer management compared to tissue biopsies, they are currently considered a secondary option in clinical applications. This is due to certain limitations (Table 3) that have hindered their full approval as the ‘gold standard’ for cancer diagnosis in clinical settings.
Blood, cerebrospinal fluid (CSF), bone marrow (BM), saliva, sputum, cyst fluid, urine, and saliva are biological fluids that are relevant for liquid biopsy. These fluids can be analyzed to determine whether circulating tumor cells (CTCs), circulating tumor DNA (ctDNA), and other cancer-related biomarkers are present (30). Circulating tumor cells (CTCs) in peripheral blood are one of the emergent topics in cancer research since they can be used as a “liquid biopsy” technique. Noninvasive biomarkers have a clinical potential in the management of solid malignancies, such as prostate, ovarian, and breast cancer (7), for instance fibronectin (FN) which is present on the surface of extra vesicles released from human breast cancer cell lines, was considered as a potential biomarker candidate (32). This liquid biopsy technique to find FN on circulating EVs shows promise as a means of identifying specific markers for early breast cancer diagnosis. Thus, microRNAs enclosed in exosomes that are circulating in biofluids are an intriguing prospective biomarker for cancer because of their expression characteristics specific to cancer (22). In fact, Zhai LY et al. (22) reports an in situ detection of microRNA-1246 (miR-1246) in human plasma exosomes as breast cancer biomarker by a nucleic acid functionalized Au nanoflare probe (22). Recent investigations have shown that genetic alterations or epigenetic modifications in ctDNA could be used for cancer detection with a liquid biopsy like blood (33). Review of the existing literature reveals that methylation biomarkers play a critical role for the diagnosis and prognosis of certain malignancies. Additionally, certain publications describe a “PanCancer” a panel cancer detection technique for the simultaneous identification of multiple cancer types, demonstrating the promise of DNA methylation-based biomarkers for cancer detection and management (23). The use of liquid biopsies for cancer management has become more popular in clinical research and has many uses (24), including diagnosis, treatment and therapeutic monitoring. Given that DNA methylation in plasma can be identified early in the development of cancer pathogenesis, blood-based epigenetic biomarkers have a great promise for early cancer detection. Under specific conditions, those markers can break out of their dormant state and stimulate proliferation, which can ultimately result in a distant relapse and cancer-related death (34). As shown in Table 4, different liquid biopsy approaches can be used to detect, characterize, and monitor minimal residual disease in breast cancer, prostate cancer, and melanoma (35).
Extracellular vesicles (EVs) are minute, lipid-bound particles secreted by cells under a variety of diseased and healthy conditions. They transport proteins and nucleic acid as part of their cargo (25). EVs from cancer cells aid in recruiting normal cells to promote tumor growth through proliferative signaling and apoptosis evasion. Research revealed that EVs containing RBM11 from glioblastoma cells induce oncogenic splicing in recipient cells, enhancing survival (36). In vivo studies on mice confirmed the malignancy-promoting potential of these EVs (37). Similarly, EVs from glioblastoma cells transfer CLIC1 protein to support tumor growth. Melanoma-derived EVs transfer PDGFR-β, activating PI3K/Akt pathway and inhibiting MAPK pathway in recipient cells, boosting proliferation. Bladder and gastric cancer cell EVs activate PI3K/AKT and MAP/ERK pathways, promoting proliferation and halting apoptosis in recipient cells. Extracellular vesicles may be used as circulating biomarkers for a variety of diseases, including various malignancies, there has been a notable upsurge in scientific interest in these molecules in recent years (Figure 2).
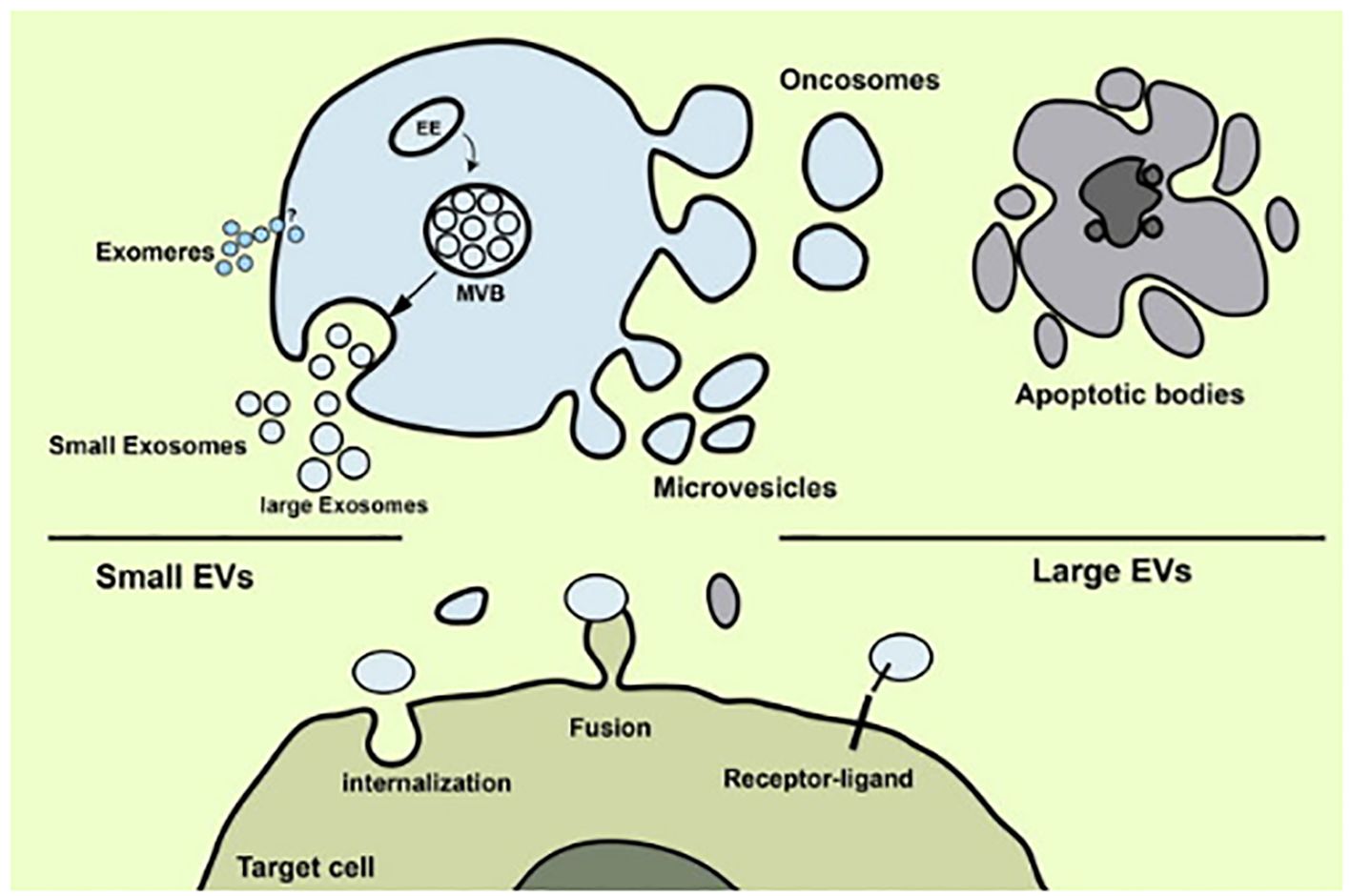
Figure 2 Release of extracellular vesicles of several types (Image inspired from Rezaie et al., 2022) (38).
Recent years have seen a significant increase in scientific interest in EVs because of their potential use as circulating biomarkers for a wide range of illnesses, including several cancers (39) (see Table 5). Studies have shown a significant correlation between the content of EVs, such as proteins, nucleic acids, and lipids, and various aspects of cancer biology, including tumor growth, angiogenesis, immune evasion, and drug resistance (45). The cargo carried by EVs can serve as potential biomarkers for cancer diagnosis, prognosis, and monitoring treatment response.
Exosomes: Serum exosomes from glioblastoma patients contain mutant EGFRvIII mRNA. Zhou and colleagues reported that the exosomal miR-15a-5p expression levels in endometrial cancer are 7–19 times higher than those in other cancer types (46). A poor prognosis was indicated by a substantial correlation between high exosomal miR-1247–3p expression and pulmonary metastases from liver cancer. Since exosomes are released by living cells and may reflect the pathophysiological condition of their parent cells, they are useful indicators for dynamic monitoring of disease progression (26). Increased miR-21 in circulating exosomes has been characterized as a potential biomarker in a number of malignancies, including liver, colorectal, gastric, breast, ovarian, and esophageal cancer. Higher levels of exosomal miR-21 found in urine have been correlated to bladder and prostate cancers (40).
Microvesicles: Microvesicles that developed on the cell surface of platelets were demonstrated to discharge lipid-rich molecules having procoagulant potential into their surroundings. The surface shedding, or “ectocytosis,” was later found to occur in a range of cell types, including tumor cells, the cells, neutrophils, and monocyte (47). Both benign as well as malignant colorectal tumor patients had significantly higher plasma concentrations of microvesicles. Microvesicles as biomarkers may improve the usefulness of colon cancer screening systems. Microvesicles are not only useful for detecting cancer, but also serve as biological markers that provide prognoses for many diseases. There are several neurological conditions such as Alzheimer’s disease, epilepsy that have been associated with increases in specific types of circulating microvesicles (48).
Ectosomes: Ectosomes are microscopic heterogeneous membrane vesicles that form when several cell types, most typically tumor cells, proliferate from the plasma membrane. They are characterized as a new form of intracellular interaction in which data is sent without physical touch between source and recipient cells. Beyond the plasma membrane, ectosomes are specialized, multipurpose carriers that expand the bounds of a cell. They establish communication networks that let cells share specific traits and information. Ectosomes as potential targets for biomarkers, diagnostic tools, and cancer therapy (41). The state of the living thing from which ectosomes emerge largely determines the specific composition of the substances they convey and their intended purpose. Because tumor-derived ectosomes are present in physiological fluids such as the blood and urine of cancer patients, they may prove to be useful prognostic and predictive biomarkers for breast and prostate cancers. Furthermore, a range of therapeutic modalities may target tumor-derived ectosomes (42).
Oncosomes: Extracellular vesicles called oncosomes are excessively large (1–10μm in diameter) and associated with severe disease. They are thought to have originated from malignancy. When membranes bleb shed, they are created. Fluorescence microscopy of large EVs revealed a morphology similar to oncosomes, indicating that these entities are oncosomes (43). One of the proteins localized in oncosomes, cytokeratin 18 (CK18), has been found to be highly prevalent (within the top fifth percentile) and was used in the creation of a test to detect oncosomes in tissues and circulatory of both human and mouse prostate cancer patients. These results imply that oncosomes are a distinct type of extracellular vesicles, or EVs, that can play a variety of roles in the growth of tumors and provide markers specific to malignancy. Potentially useful biomarkers for cancer diagnosis and prognosis are oncosomes. They are applied to prostate and breast cancer (49).
Prostasomes: Broadly expressed RNA, membrane, and cytosolic amino acids that are unique to the prostatectomy make up prostatesomes. Extracellular vesicles taken from people with prostate cancer have been shown to include altered levels of protein, mRNA, long non-coding RNA (lncRNA), and microRNA, both in regard to number and quality (27). RNA, which is membrane, and cytosolic amino acids that are unique to the prostatectomy and are broadly expressed make up prostatesomes. The extracellular vesicles taken from people with prostate cancer have been shown to include altered levels of protein, messenger RNA, long non-coding RNA (lncRNA), and microRNA, both in regard to number and quality (28).
Apoptotic bodies: Cells can perish by a variety of processes, including necrosis, autophagy, and catastrophic mitosis. Currently, however, it is believed that mediators of the death of apoptotic cells have tremendous potential as targets for cancer therapies. Morphological alterations include chromatin and its process of condensation, cell shrinkage, plasma membrane blebbing, and the creation of apoptotic bodies are indicative of apoptosis (50). Cytosolic cytochrome c activates the apoptosome complicated initiating caspase 9, and effector caspases. Apoptotic bodies are produced as a result of a sequence of irreversible events that include the fragmentation of cytokeratins (CKs) by caspase, poly (ADP-ribose) polymerase, with the reactivation of endonucleases to form nucleosomal DNA (nDNA) according to Ye et al, 2020 (Figure 3). Furthermore, they promote the exterior of the plasma membrane to become exposed to phosphatidylserine, which allows phagocytes to identify dying cells. Indicators of apoptosis in breast cancer include circulating soluble FASL, granzyme B, and cytochrome C, which increase following treatment. Both intact PCa cells and apoptotic particles made of PCa cells are seen in urine (52). Patients receiving medication can eventually release these biomarker molecules into their circulation as shown in Figure 2.
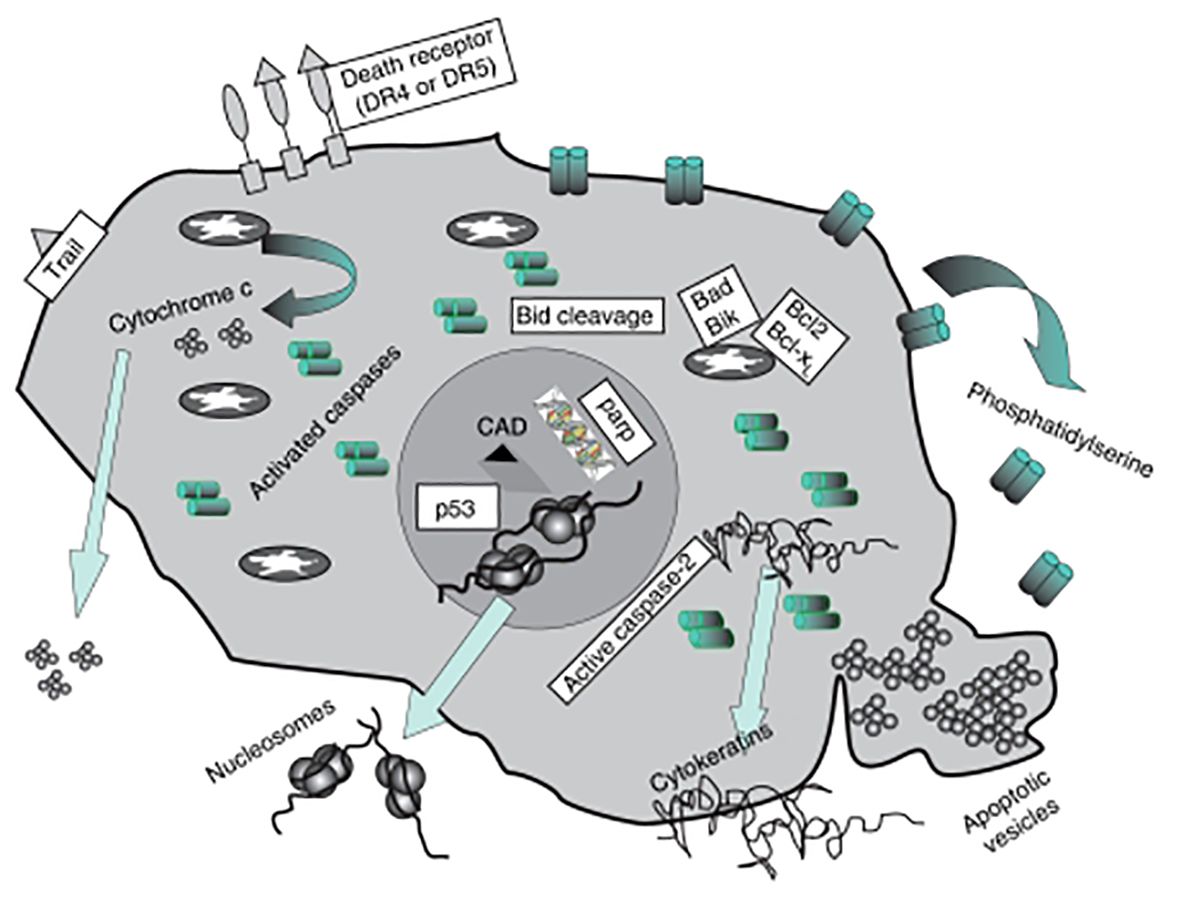
Figure 3 Diagram showing the subsequent protein buildup once apoptosis is induced. Patients receiving medication can eventually release these biomarker molecules into their circulation (Image inspired from Ward et al., 2008) (51).
Autophagic EVS: Autophagy is one remarkably conserved method of cellular breakdown (53).Cancer cells’ autophagy stimulates the formation of tumors and the division of cancer cells, which inhibits the development of new cancers by killing cancer cells. The autophagic process is regulated by a number of proteins, including class III PtdIns3K complex, Bcl-2, Atg proteins. A degradative organelle known as the vacuole/lysosome receives portions of the cytosol and organelles that are sequestered into an autophagosome, a double-membrane vesicle, for eventual breakdown and recycling of the resultant macromolecules. Thus, autophagy both safeguards breast cancer cells and lessens their sensitivity to medicines. Consequently, autophagy may offer cytoprotection to breast cancer cells (19). Development of protocols to monitor autophagy could be useful in diagnosis and treatment monitoring.
The elements other than DNA sequence that influence gene expression and cellular phenotypes are referred to as epigenetics (54). The topic of how different phenotypes might be derived from the same genotype is addressed by epigenetics (18). The processes that add acetyl and methyl groups to histone tails, methylate DNA on cytosine residues, express non-coding RNA, and modify the structure of chromatin are the most well-understood epigenetic determinants of phenotype. To maintain the correct differentiation state, cells’ collective epigenetic status is strictly regulated (54–57). This precisely tuned genetic programming is upset in cancer, a process known as epimutation. This results in defective differentiation, unchecked cell division, and resistance to apoptosis (54). A heritable aberrant transcriptional suppression of gene activity that is unrelated to a DNA sequence is known as an epimutation. Although it can also occur in the germline, epimutation usually happens in somatic cells and shows up in the growth of tumors (18). Over the past forty years, epigenetic errors and their causes have become a prominent focus in cancer research when it was discovered that aberrant DNA methylation is associated with malignancy. It has been demonstrated that changes to the epigenome affect almost every stage of the development, growth, and management of tumors (54).
Histone alterations, non-coding DNA, and DNA methylation Since RNAs are found in all human cancer types and can manifest in the early stages of the disease, they make particularly appealing markers with a variety of diagnostic uses (58). Because of their notable stability over RNA and proteins, among other things, DNA methylation and microRNAs are more useful and feasible as biomarkers in therapeutic contexts (59). Specifically, great stability is provided by DNA methylation, microRNAs, and post-translational changes of histones in biofluids and low-quality materials like formalin-fixed paraffin embedded (FFPE) (60). Other advantages of epigenetic biomarkers over genetic or protein-based biomarkers are, that they are dynamic in nature, give more information about the function of the gene, thereby providing information about the specific genetic programs that alter during disease (60). By definition, an epigenetic biomarker is “any altered epigenetic mechanism or mark that is specifically stable and reproducible during sample processing and is generally used to evaluate health or disease status” (60).
The most extensively researched epigenetic modification in cancer is aberrant DNA methylation (61). In eukaryotic cells, aberrant hypermethylation of promoters can silence critical genes, including tumor suppressor genes, which in turn can cause illness. The reverse process can also have an impact on the development of cancer. Genes that are typically methylated, such as oncogenes, can have their expression elevated by hypomethylation (62). It is interesting to note that the first DNA methylation anomaly in human cancer to be discovered was hypomethylation (63). It has been found that 13% of sporadic colorectal cancer (CRC) show MLH1 hypermethylation, and a BRAF c.1799T>A, p.Val600Glu mutation has often also been identified in tumor DNA (64, 65). Though it is brought on by mutations in one of the DNA MMR genes, MSI and loss of MLS1 are both present in Lynch syndrome, the most frequent cause of hereditary colorectal cancer (66). It has been discovered that methylation of MGMT occurs in 40% of tumors in gliomas and CRC, but only in 25% of tumors in non-small cell lung carcinomas (NSCLCs), lymphomas, and head and neck carcinomas (67). When paired with IDH1 mutations, MGMT methylation status functions as a predictive biomarker. Patients with hypermethylated MGMT and the IDH1 p.R132H mutation had a better prognosis for their glioma (68). MGMT is a DNA repair gene that helps to eliminate harmful and mutagenic alkyl groups from O6-meG. Due to the fact that DNA alkylation causes mutations, MGMT shields cells from harm (67, 69).
The primary association of the RB1 gene with retinoblastoma is the loss of RB1 function. LOH or RB1 mutations are linked to the lack of expression of this gene in retinoblastoma and other malignancies, such as bladder carcinomas and malignant neuroendocrine lung carcinomas. However, methylation of RB1 might sometimes result in the suppression of its expression (70, 71). It has been said that RB1 methylation above the LOH and mutations are required for complete molecular diagnoses of retinoblastoma. It has been reported that 9% of spontaneous unilateral tumors are caused by RB1 hypermethylation, which is invariably acquired (72).
Circulating methylation SEPT9 DNA is one type of plasma epigenetic biomarker for colorectal cancer screening. SEPT9 is regarded as a tumor suppressor because it controls cell proliferation and inhibits unchecked cell division (73). Research has shown that SEPT9 methylation is linked to the pathophysiology of colorectal cancer (CRC), and that a decline in SEPT9 expression is connected with the advancement of neoplastic illness (74). SHOX2 hypermethylation has been noticed in the bronchial aspirates [43], pleural effusions [44], and blood plasma of patients with lung cancer (18, 75). DNA methylation analysis of SHOX2 combined with PTGER4 in blood plasma allows detection of lung cancer and differentiation of non-malignant diseases (75). In some cancer there are some epigenetic markers that has been discovered due to the fact that prostate cancers frequently contain methylation of the tumor suppressor genes GSTP1, RASSF1, and APC, these genes are regarded as cancer biomarkers (Table 6) (18).
Generally, chromatin can be divided into two categories: euchromatin, which is more loose and contains actively transcribed genes, and heterochromatin, which is heavily compacted and contains dormant genes (18). The arrangement and functionality of chromatin are changed by covalent alteration of the histones that make up nucleosomes, which has an impact on the regulation and expression of genes (90). The six main roles of chromatin are transcription, repression, enhancer, insulator, promoter, and inactive chromatin. Histone modification is a significant factor in determining the function of chromatin (91). Phosphorylation, acetylation, methylation (mostly of lysine and arginine residues), ubiquitylation, glycosylation, SUMOylation, ADP (adenosine diphosphate)-ribosylation, and carbonylation are examples of modifications to histone structure (90, 92). The primary indicators of active chromatin are histone acetylation and methylation, which are frequently linked to a more relaxed chromatin conformation. Conversely, chromatin condensation is frequently linked to histone deacetylation and phosphorylation, which are indicators of inactive chromatin (90).
Globally methylated or non-modified histones were linked to a poor prognosis, whereas individuals with NSCLC who had global histone acetylation had a better prognosis in survival analysis (93). Human tumor cells are typically characterized by a complete loss of H4 histone Lys16 monoacetylation and Lys20 trimethylation, which is linked to DNA hypomethylation. A lower level of H4Lys20 methylation and H4Lys16 acetylation in breast cancer is associated with a worse prognosis (94, 95). The propensity for cancer can be increased by different isoforms of histone proteins found in the nucleosome as well as covalent alterations. For instance, genitourinary malignancies, which include bladder and prostate tumors, and undifferentiated cancers have been shown to overexpress the H2A histone isoform H2A.Z. Moreover, H2A.Z may contribute to endocrine resistance in individuals with breast cancer. The association between H2A.Z levels and short overall patient survival suggests that H2A.Z may be a valuable biomarker for tumor progression (96). In terms of post-translational histone changes, CRC patients’ blood had lower levels of H3K9me3 and H4K20me3 than that of cancer-free people (97). Global patterns of histone H3 and H4 modification are significant because they may serve as indicators of both disease-free survival and tumor recurrence (Table 7).
The second most extensively researched epigenetic method of gene regulation, after methylation, is the interaction between microRNA (miRNA) and messenger RNA (mRNA). These are 18–25 nucleotide short, non-coding RNA molecules that are essential for controlling post-transcriptional gene expression. Translation is halted by miRNAs when they bind to the target mRNA molecule. It is believed that miRNA can regulate up to 60% of genes that encode proteins (18). Saliva, urine, serum, and plasma are among the bodily fluids into which tumor cells release miRNAs. Thus, the examination of circulating miRNAs in liquid biopsy samples offers potential biomarkers for non-invasive diagnostics in numerous human cancers, such as melanoma and rhabdomyosarcoma, as well as colorectal, lung, breast, prostate, gastric, pancreatic, esophageal, liver, thyroid, kidney, ovarian, endometrial, and cervical cancers (101). The control of numerous genes implicated in the genesis of cancer is greatly impacted by dysregulation of miRNA expression (18). The suppressor gene may be silenced as a result of overexpression of miRNA, which is implicated in the negative regulation of the suppressor gene. Conversely, overexpression of the oncogene occurs when the chromosomal loci encoding the miRNA that silences the oncogene are deleted. Therefore, oncogenes (oncomiRs) and miRNAs themselves can function as suppressors. It is also critical to keep in mind, that while a single miRNA molecule can control several genes, multiple miRNAs can target a single mRNA (18). Since miRNA activity and expression changes along the course of cancer development, miRNAs can function as biomarkers and be assessed in cancer patient blood and tumor tissue (Table 8) (18).
The combination of two miRNA biogenesis genes (DICER1 and DROSHA) and four miRNAs (miR-30d, miR-21, miR-17, and miR-155) is one of the putative predictive indicators in non-small cell lung cancer (114). Specifically, miR-30d has been discovered to function as an oncomiR in cancer, and a substantial decrease in lifespan is associated with an increased copy number of miR-30d in cancer tissue (gains or amplifications compared to others) (114, 115). miR-31–3p is an additional intriguing prognostic and predictive biomarker in metastatic colorectal cancer (116). Blood alterations are a reflection of the dysregulation of many miRNAs found in malignancies. Let-7a-1, 7a-2, 7a-3, 7b, 7c, 7d, 7e, 7f-1, 7f-2, 7g, 7i, miR-98, and miR-202 are a group of well-known miRNAs that have changed expression in malignancies (117). Since some members of the let-7 family are downregulated in melanoma, pancreatic cancer, prostate cancer, and sarcoma, the let-7 family is thought to be a tumor suppressor (118). However, they can also be upregulated in lymphoma, mesothelioma, and breast cancer (118). It was discovered that miR-21 acts as an oncogene by suppressing the expression of many tumor suppressors (119). For instance, the miRNA targets PDCD4, which is linked to the prevention of neoplastic transformation as well as the promotion, invasion, and advancement of cancer (118–120). Among the other targets of miR21 are BCL2, PTEN, RECK, RHOB, and TPM1 (121). This miRNA has been linked to hematological malignancies, breast cancer, gastric cancer, ovarian cancer, pancreatic cancer, colorectal cancer, lung cancer, and liver cancer in terms of its diagnostic, predictive, and/or prognostic properties (18).
In recent years, extensive research has elucidated the multifaceted roles of ncRNAs in cancer. Non-coding RNAs (ncRNAs) have emerged as pivotal players in the intricate landscape of cancer biology, contributing significantly to both the understanding of malignancies and the development of innovative therapeutic strategies (122, 123). These molecules, including microRNAs (miRNAs), long non-coding RNAs (lncRNAs), and circular RNAs (circRNAs), exert regulatory functions in gene expression and cellular processes (Figure 4). The dysregulation of ncRNAs has been linked to various malignancies, making them attractive candidates for diagnostic and therapeutic applications.
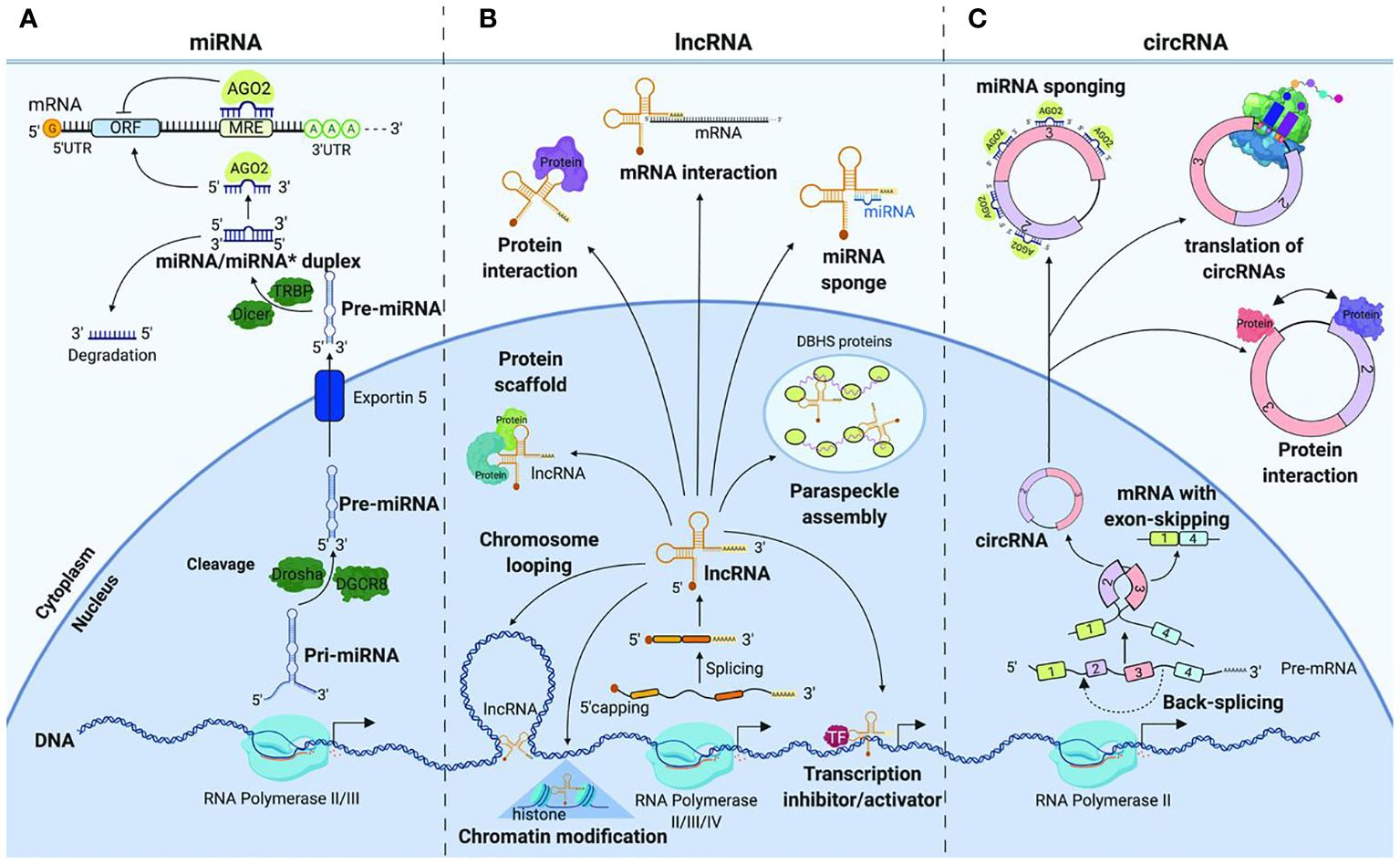
Figure 4 Non-coding RNAs in gastrointestinal cancer (Image inspired from Dragomir et al., 2019) (124).
MiRNAs: MiRNAs, small non-coding RNA molecules, have garnered attention for their involvement in cancer pathogenesis (125–129). Notably, elevated levels of Phosphatase and Tensin Homolog (PTEN) induced by specific miRNAs can inhibit AKT signaling, activate apoptosis, and prevent malignancies such as renal cell carcinoma (130, 131). MiRNAs, including miR-29a, have demonstrated a major impact on oncogenicity in various neoplasms by regulating key genes involved in cancer progression (128). These molecules play crucial roles in physiological and pathological processes, including viral replication, cell proliferation, differentiation, apoptosis, fibrosis, angiogenesis, tumorigenicity, metastasis, and drug resistance. Research has identified specific miRNA panels with distinct expression patterns in the serum of cancer patients, offering potential as diagnostic indicators (132). For instance, a combination of miR-145, miR-155, and miR-382 demonstrated improved sensitivity and specificity, suggesting the potential of miRNA profiling for breast cancer screening (132). The translational applications of miRNAs extend to the management and survival improvement of oral cancer patients (129).
LncRNAs: Sometimes referred to as versatile biomarkers across cancers, long non-coding RNAs (lncRNAs) have emerged as versatile biomarkers with diagnostic potential across various cancers (133). A systematic review and meta-analysis highlighted lncRNA AFAP1-AS1 as a novel biomarker in different cancers (134). Specific lncRNAs, such as MALAT-1, HOTAIR, LINC00152, and others, have shown diagnostic potential in prostate, lung, colorectal, hepatocellular, gastric, renal, and colorectal cancers (135). LncRNA GIHCG has been linked to the etiology of numerous malignancies, offering promise as a biomarker (136). Additionally, circulating lncRNAs, like PVT1 and UCA1, exhibit significant multicancer diagnostic potential (137). These findings underscore the diversity of lncRNAs as biomarkers and their potential application in clinical setting.
CircRNAs: Circular RNAs (circRNAs) represent a novel class of non-coding RNAs with emerging roles as biomarkers and therapeutic targets in cancer (138). The discovery of their involvement in the onset and progression of malignancies has opened new frontiers in cancer research. CircRNAs such as circ0001955 and circ-LDLRAD3 have shown promise as diagnostic and prognostic markers in cervical and pancreatic cancers, respectively (139). Notably, exosomal circ_0044516 was found to be highly elevated in prostate cancer patients, influencing cancer cell proliferation and metastasis by modulating miR-29a-3p expression (140). These findings underscore the potential of circRNAs as valuable biomarkers and therapeutic targets in diverse cancer types.
Metabolomic biomarkers refer to specific small molecule metabolites produced by the organism in biological fluids that can be identified and analyzed to provide insights into physiological or pathological states. These biomarkers, detected through nontargeted metabolomic analysis, serve as indicators of metabolic changes associated with various conditions, including diseases or responses to treatments. Essentially, metabolomic biomarkers are measurable metabolic features that can be used for diagnostic, prognostic, or therapeutic purposes, providing valuable information about the biochemical status of an organism (141). These compounds typically weigh ≤ 1500 Da and span a diverse range, including peptides, oligonucleotides, sugars, nucleosides, organic acids, ketones, aldehydes, amines, amino acids, lipids, steroids, alkaloids, and occasionally drugs or xenobiotics (141). Metabolomic biomarkers have emerged as promising tools for non-invasive diagnostic and treatment monitoring in oncology. Metabolomics technologies have advanced our understanding of cancer metabolism, particularly in the context of the “Warburg effect,” which elucidates how cancer cells utilize glycolysis to support tumor proliferation and vascularization (142, 143).
The tumor metabolome provides insights into the interconnectedness of metabolome, proteome, and genome within cancer cells. Cancer cells exhibit altered metabolic processes, such as glycolysis, which converts glucose into pyruvate and subsequently ferments it into lactate (Figure 5).
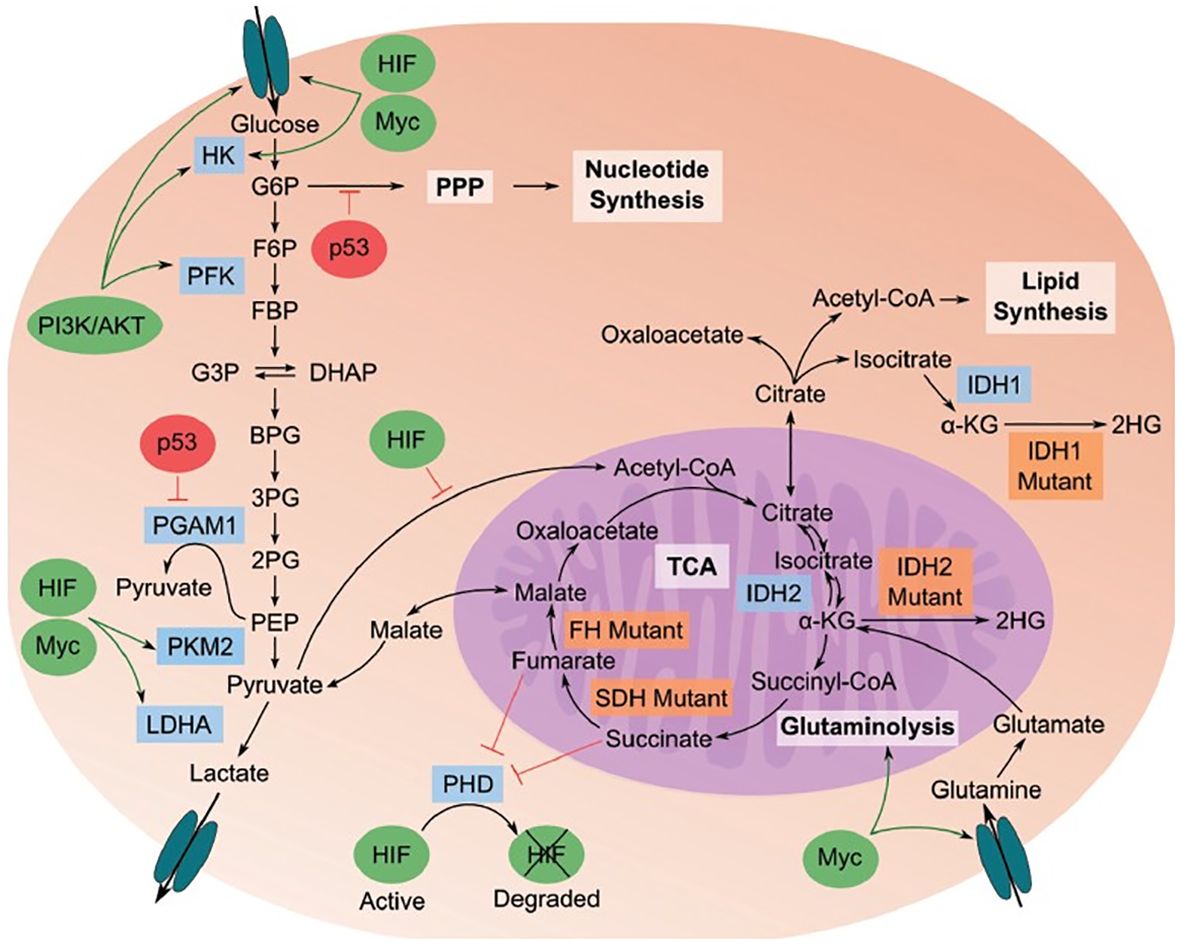
Figure 5 Cancer metabolome showing the relationships between metabolome, proteome, and genome in cancerous cells (Image inspired from Bhattacharjee et al., 2022) (144).
The flow of pyruvate through the TCA cycle is reduced in cancer cells. Additionally, pathways stemming from glycolysis, like the pentose phosphate pathway, generate essential building blocks to support the rapid growth of cancer cells. Specific genetic and enzyme-related behaviors play a role in this process. Enzymes highlighted in blue are crucial for the transition to a cancer metabolic phenotype, while those in orange indicate mutations found in cancer cells. Oncogenes, represented by green ovals, are up-regulated in cancer, whereas tumor suppressors, depicted by red ovals, are down-regulated.
In breast cancer, the integration of genomics, proteomics, and metabolomics has been proposed as a key approach for future biomarker discovery, highlighting the potential of metabolomics in this field (145). Furthermore, computational models applied to metabolomics data have hinted at the relevance of glutamine metabolism in breast cancer, emphasizing the potential of metabolomics in the development of new biomarkers for cancer (146). Metabolomics holds great promise for understanding the molecular determinants of cancer and advancing the development of new biomarkers for the diagnosis, prognosis, and treatment of neoplastic processes (147). Additionally, metabolomics offers a broad set of oncological applications, particularly in providing serum or imaging-based biomarkers for cancer (148). The utility of metabolomics in biomarker discovery for cancer has been demonstrated in various types of cancer, including colorectal cancer, where it has proven useful in early diagnostic biomarker discovery (149). The potential of metabolomics in cancer research extends to its application in precision medicine, as it can suggest new pathways and therapeutic targets for the targeted treatment of cancer (150).
Recent advances in metabolomics technologies have enabled a deeper investigation into cancer metabolism, providing a better understanding of how cancer cells utilize metabolic pathways for proliferation and vascularization (142), for instance, metabolic biomarkers for breast cancer, with a focus on glutamine metabolism (146). Additionally, metabolomics has been applied to urine and saliva for non-invasive cancer detection and biomarker discovery (151). Furthermore, metabolomics represents a potential strategy for the real-time selection and monitoring of patients treated with immunotherapy, indicating its relevance in treatment monitoring in oncology (152). The integration of metabolomic profiling with transcriptomics data has been proposed as a method for validating potential diagnostic biomarkers in cervical cancer, further emphasizing the potential of metabolomics in cancer research (153). Recent studies have shown the potential of metabolomics in identifying biomarkers for various cancers, such as endometrial cancer and primary glomerulonephritis sub-types (154) (Table 9).
Cancer care in sub-Saharan Africa is challenged by several unique issues that contribute to worse outcomes compared to high-income countries (1). Most patients present with metastatic stage disease due to delayed diagnosis, secondary to low cancer awareness among both the population and healthcare workers. There are also cultural and economic barriers that hinder access to specialized care. Paradoxically, African populations are among the least studied in cancer genomics globally. Even though Africa is the most genetically diverse continent, it makes up only about 3% of the genetic data used in cancer genomics projects worldwide (2). Consequently, the genetic determinants of cancer risk and treatment response in African populations remain largely unknown. The limited cancer genomics research in Africa is also unevenly distributed, with studies primarily focused on North African populations, while sub-Saharan Africa remains vastly unexplored (3). This is problematic, as the genetic underpinnings of cancer can differ greatly across African subpopulations due to the continent’s immense genetic diversity. Another key issue is the tendency to treat Africans as a homogeneous group in cancer research, rather than disaggregating by ancestry, ethnicity or language (4). This obscures important within-group differences in cancer risk and biology, hindering the achievement of true equity in precision oncology.
Despite these challenges, recent cancer genomics studies in Africa have uncovered several “uninvited biomarkers” with potential clinical utility (5). For instance, inflammatory markers like C-reactive protein and certain cytokines have been linked to prognosis and cancer stage in African cohorts. Metabolic profiling has also revealed distinct patterns associated with common malignancies in the region. Notably, viral integrations, particularly from hepatitis B virus and human papillomavirus, have emerged as an intriguing category of biomarkers (6). These viral sequences integrated into the host genome can dysregulate critical cellular pathways, impacting cancer biology and behavior. Importantly, the foreign viral antigens expressed by these cancers offer opportunities for targeted therapies and immunotherapeutic strategies. Addressing the unique challenges in cancer care and genomics research in sub-Saharan Africa is crucial to improving outcomes and achieving equity in precision oncology. Sustained funding, multidisciplinary collaboration, and empowerment of African scientists are essential to drive progress in this field and ultimately reduce the devastating cancer burden in the region.
Non-invasive biomarkers, including liquid biopsies, epigenetic markers, non-coding RNAs, exosomal cargo, and metabolites, have emerged as promising tools in cancer diagnosis and treatment. The systematic review provides a comprehensive overview of the potential of these biomarkers in early detection, disease monitoring, and personalized treatment strategies across various cancer types. The ability to accurately detect cancer in its early stages and classify subtypes have significant implications for improving patient outcomes and advancing oncology. The studies reviewed in this article demonstrate the ability of non-invasive biomarkers such as liquid biopsies, epigenetic markers, non-coding RNAs, exosomal cargo, and metabolites to accurately detect cancer in its early stages, classify subtypes, and personalize treatment regimens. Understanding the roles of ncRNAs in cancer, not only provides insights into the intricate molecular mechanisms driving malignancies, but also paves the way for the development of targeted therapeutic interventions. Sorafenib, a multi-kinase inhibitor, stands out as an example of the successful translation of ncRNA research into cancer therapy, having been approved by U.S. Food and Drug Administration for the treatment of advanced renal cell carcinoma, hepatocellular carcinoma, and thyroid cancers. The development of these biomarkers represents a significant advancement in oncology, offering new avenues for improving patient outcomes and reducing the burden of cancer worldwide. Continued research and validation are necessary to establish these biomarkers as reliable tools in clinical practice, thereby, ultimately contributing to the development of more effective cancer management strategies.
While the studies reviewed in this article provide compelling evidence for the utility of non-invasive biomarkers in cancer medicine, there is still much work to be done in this field. Despite the advances in cancer biomarker research, several limitations hinder their clinical application. One significant issue is the lack of standardized protocols for biomarker detection and quantification, leading to variability in results across different studies and clinical settings (166). Additionally, many biomarkers currently lack sufficient validation in large, diverse patient populations, which raises concerns about their generalizability and reliability (167). Future research should focus on validating these biomarkers in larger patient cohorts, exploring their potential for use in combination with existing diagnostic and treatment modalities, and developing new technologies to enhance their sensitivity and specificity. Another limitation is the complexity of cancer biology, which makes it challenging to identify biomarkers that are both highly specific and sensitive for early detection and prognosis. Current biomarkers often fail to capture the heterogeneity of cancer, leading to false positives and negatives (168). There is need for more extensive research into the underlying mechanisms of the origin of these biomarkers and their significance in cancer biology. This knowledge will be crucial for developing more effective therapies that target the specific molecular pathways involved in cancer development and progression. Early detection is very important for the improvement of life quality, survival, and to reduce the financial burden of cancer treatments, which are greater at later stage detection. Moreover, the integration of biomarkers into clinical practice is complicated by the lack of robust bioinformatic tools to analyze and interpret large-scale biomarker data (169). To address these challenges, future research should focus on the following areas:
Standardization and Validation: Develop standardized protocols for biomarker detection and validation through multi-center studies involving diverse patient populations. This will help ensure the reliability and reproducibility of biomarker tests across different settings (166).
Multi-omics Approaches: Integrate genomic, transcriptomic, proteomic, and metabolomic data to identify composite biomarker signatures that better reflect the complexity of cancer. This holistic approach can improve the specificity and sensitivity of biomarkers (167).
Advanced Bioinformatics: Invest in the development of advanced bioinformatic tools and machine learning algorithms to analyze complex biomarker data. These tools can help uncover novel biomarker patterns and improve the interpretation of existing data (169).
Ethical and Safe Integration of AI: Explore the ethical and safe integration of artificial intelligence (AI) in cancer research to enhance biomarker discovery and application. AI can help in the rapid analysis of large datasets and the identification of potential biomarkers with high clinical relevance (170).
Combining Biomarkers with Existing Therapies: Investigate the potential of combining biomarkers with existing therapeutic strategies to enhance treatment efficacy and overcome resistance. This includes exploring the role of biomarkers in predicting and monitoring treatment response (171).
SZ: Conceptualization, Methodology, Writing – original draft, Writing – review & editing. NKN: Validation, Writing – review & editing. GVO: Conceptualization, Writing – original draft. OO: Visualization, Writing – review & editing. PCN: Resources, Writing – original draft. MT: Data curation, Writing – original draft. ENI: Investigation, Writing – original draft. SEA: Project administration, Writing – review & editing. OOO: Project administration, Writing – review & editing, Supervision.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
The authors appreciate Covenant University for publication fee of this article.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Abeshouse A, Ahn J, Akbani R, Ally A, Amin S, Andry CD, et al. The molecular taxonomy of primary prostate cancer. Cell. (2015) 163:1011–25. doi: 10.1016/j.cell.2015.10.025
2. Rotimi SO, Rotimi OA, Salhia B. A review of cancer genetics and genomics studies in Africa. Front Oncol. (2021) 10:606400. doi: 10.3389/fonc.2020.606400
3. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J Clin. (2021) 71:209–49. doi: 10.3322/caac.21660
4. W.H.O. Global cancer burden growing, amidst mounting need for services . Available online at: https://www.who.int/news/item/01–02-2024-global-cancer-burden-growing–amidst-mounting-need-for-services.
5. Bashar M, Begam N. Breast cancer surpasses lung cancer as the most commonly diagnosed cancer worldwide. Indian J Cancer. (2022) 59:438. doi: 10.4103/ijc.IJC_83_21
6. Sinobiologoical. Cancer Biomarker Discovery | Sino Biological . Available online at: https://www.sinobiological.com/resource/cancer-biomarker/discovery.
7. Lianidou ES, Strati A, Markou A. Circulating tumor cells as promising novel biomarkers in solid cancers. Crit Rev Clin Lab Sci. (2014) 51:160–71. doi: 10.3109/10408363.2014.896316
8. Marrugo-Ramírez J, Mir M, Samitier J. Blood-based cancer biomarkers in liquid biopsy: A promising non-invasive alternative to tissue biopsy. Int J Mol Sci. (2018) 19:2877. doi: 10.3390/ijms19102877
9. Zakari S, Ekenwaneze CC, Amadi EC, Ogunlana OO. Unlocking the secrets of androgen receptors in the susceptibility, progression, and treatment of prostate cancer. Biol Life Sci. (2023). doi: 10.20944/preprints202305.0720.v2
10. Li S, De Camargo Correia GS, Wang J, Manochakian R, Zhao Y, Lou Y. Emerging targeted therapies in advanced non-small-cell lung cancer. Cancers. (2023) 15:2899. doi: 10.3390/cancers15112899
11. Lopez-Gonzalez L, Sanchez Cendra A, Sanchez Cendra C, Roberts Cervantes ED, Espinosa JC, Pekarek T, et al. Exploring biomarkers in breast cancer: hallmarks of diagnosis, treatment, and follow-up in clinical practice. Medicina. (2024) 60:168. doi: 10.3390/medicina60010168
12. Zakari S, Ekenwaneze CC, Amadi EC, Abuhamdia A, Ogunlana OO. Unveiling the latest insights into androgen receptors in prostate cancer. Int J Med Biochem. (2024) 7(2):101–13. doi: 10.14744/ijmb.2024.93585
13. Zakari S, Cleanclay WD, Bella-Omunagbe M, Zakari H, Ogbu CO, Uti DE, et al. The role of SRC-3 in prostate cancer progression and implications for therapeutic targeting: A systematic review. J Pharm Pharmacogn Res. (2024) 12:994–1007. doi: 10.56499/jppres
14. Zakari S, Nguedia Kaze N, Nnaji P, Ogunniyi O, Tebamifor M, Israel EN, et al. Emerging Biomarkers for Non-Invasive Diagnosis and Treatment of Cancer: A systematic review. PROSPERO I.D: CRD42023474749 (2023). Available online at: https://www.crd.york.ac.uk/prospero/display_record.php?RecordID=474749.
15. Paleati N, Munirathinam G. Role of chromatin and epigenetic dysregulation in prostate cancer: from development to progression and therapeutic response. Cancers. (2023) 15:5638. doi: 10.3390/cancers15235638
16. Urbanucci A, Barfeld SJ, Kytölä V, Itkonen HM, Coleman IM, Vodák D, et al. Androgen receptor deregulation drives bromodomain-mediated chromatin alterations in prostate cancer. Cell Rep. (2017) 19:2045–59. doi: 10.1016/j.celrep.2017.05.049
17. Zakari S. SPOP gene mutations in prostate cancer: deciphering their role in disease characterization and targeted interventions on AR signaling. Biol Life Sci. (2023). doi: 10.20944/preprints202310.0345.v1
18. Kamińska K, Nalejska E, Kubiak M, Wojtysiak J, Żołna Ł, Kowalewski J, et al. Prognostic and predictive epigenetic biomarkers in oncology. Mol Diagnosis Ther. (2019) 23:83–95. doi: 10.1007/s40291-018-0371-7
19. Wu M, Wen L, Zhou Y, Wu W. Role of lncRNA AGAP2-AS1 in breast cancer cell resistance to apoptosis by the regulation of MTA1 promoter activity. Technol Cancer Res Treat. (2022) 21:153303382210853. doi: 10.1177/15330338221085361
20. Sokolenko AP, Imyanitov EN. Molecular diagnostics in clinical oncology. Front Mol Biosci. (2018) 5:76. doi: 10.3389/fmolb.2018.00076
21. Temilola DO, Wium M, Paccez J, Salukazana AS, Rotimi SO, Otu HH, et al. Detection of cancer-associated gene mutations in urinary cell-free DNA among prostate cancer patients in South Africa. Genes. (2023) 14:1884. doi: 10.3390/genes14101884
22. Zhai LY, Li MX, Pan WL, Chen Y, Li MM, Pang JX, et al. In situ detection of plasma exosomal microRNA-1246 for breast cancer diagnostics by a au nanoflare probe. ACS Appl Mater Interfaces. (2018) 10:39478–86. doi: 10.1021/acsami.8b12725
23. Constâncio V, Nunes SP, Henrique R, Jerónimo C. DNA methylation-based testing in liquid biopsies as detection and prognostic biomarkers for the four major cancer types. Cells. (2020) 9:624. doi: 10.3390/cells9030624
24. Yu W, Hurley J, Roberts D, Chakrabortty SK, Enderle D, Noerholm M, et al. Exosome-based liquid biopsies in cancer: opportunities and challenges. Ann Oncol. (2021) 32:466–77. doi: 10.1016/j.annonc.2021.01.074
25. Cambier L, Stachelek K, Triska M, Jubran R, Huang M, Li W, et al. Extracellular vesicle-associated repetitive element DNAs as candidate osteosarcoma biomarkers. Sci Rep. (2021) 11:94. doi: 10.1038/s41598-020-77398-z
26. Ye Z, Zheng Z, Peng L. Microrna profiling of serum exosomes in patients with osteosarcoma by high-throughput sequencing. J Invest Med. (2020) 68:893–901. doi: 10.1136/jim-2019-001196
27. Zijlstra C, Stoorvogel W. Prostasomes as a source of diagnostic biomarkers for prostate cancer. J Clin Invest. (2016) 126:1144–51. doi: 10.1172/JCI81128
28. Melo SA, Luecke LB, Kahlert C, Fernandez AF, Gammon ST, Kaye J, et al. Glypican-1 identifies cancer exosomes and detects early pancreatic cancer. Nature. (2015) 523:177–82. doi: 10.1038/nature14581
29. Connal S, Cameron JM, Sala A, Brennan PM, Palmer DS, Palmer JD, et al. Liquid biopsies: the future of cancer early detection. J Transl Med. (2023) 21:118. doi: 10.1186/s12967-023-03960-8
30. Alix-Panabières C, Pantel K. Liquid biopsy: from discovery to clinical application. Cancer Discovery. (2021) 11:858–73. doi: 10.1158/2159-8290.CD-20-1311
31. Pesapane F, Suter MB, Rotili A, Penco S, Nigro O, Cremonesi M, et al. Will traditional biopsy be substituted by radiomics and liquid biopsy for breast cancer diagnosis and characterisation? Med Oncol. (2020) 37:29.
32. Moon PG, Lee JE, Cho YE, Lee SJ, Chae YS, Jung JH, et al. Fibronectin on circulating extracellular vesicles as a liquid biopsy to detect breast cancer. Oncotarget. (2016) 7:40189–99. doi: 10.18632/oncotarget.v7i26
33. Fiala C, Diamandis EP. Can a broad molecular screen based on circulating tumor DNA aid in early cancer detection? J Appl Lab Med. (2020) 5:1372–7.
34. Lianidou E. Detection and relevance of epigenetic markers on ctDNA: recent advances and future outlook. Mol Oncol. (2021) 15:1683–700. doi: 10.1002/1878-0261.12978
35. Heidrich I, Deitert B, Werner S, Pantel K. Liquid biopsy for monitoring of tumor dormancy and early detection of disease recurrence in solid tumors. Cancer Metastasis Rev. (2023) 42:161–82. doi: 10.1007/s10555-022-10075-x
36. Pavlyukov MS, Yu H, Bastola S, Minata M, Shender VO, Lee Y, et al. Apoptotic cell-derived extracellular vesicles promote Malignancy of glioblastoma via intercellular transfer of splicing factors. Cancer Cell. (2018) 34:119–135.e10. doi: 10.1016/j.ccell.2018.05.012
37. Kalimuthu S, Gangadaran P, Li XJ, Oh JM, Lee HW, Jeong SY, et al. In Vivo therapeutic potential of mesenchymal stem cell-derived extracellular vesicles with optical imaging reporter in tumor mice model. Sci Rep. (2016) 6:30418. doi: 10.1038/srep30418
38. Rezaie J, Ahmadi M, Ravanbakhsh R, Mojarad B, Mahbubfam S, Shaban SA, et al. Tumor-derived extracellular vesicles: The metastatic organotropism drivers. Life Sci. (2022) 289:120216. doi: 10.1016/j.lfs.2021.120216
39. Lak NSM, van der Kooi EJ, Enciso-Martinez A, Lozano-Andrés E, Otto C, Wauben MHM, et al. Extracellular vesicles: A new source of biomarkers in pediatric solid tumors? A systematic review. Front Oncol. (2022) 12:887210. doi: 10.3389/fonc.2022.887210
40. Gong L, Bao Q, Hu C, Wang J, Zhou Q, Wei L, et al. Exosomal miR-675 from metastatic osteosarcoma promotes cell migration and invasion by targeting CALN1. Biochem Biophys Res Commun. (2018) 500:170–6. doi: 10.1016/j.bbrc.2018.04.016
41. Minciacchi VR, Freeman MR, Di Vizio D. Extracellular vesicles in cancer: exosomes, microvesicles and the emerging role of large oncosomes. Semin Cell Dev Biol. (2015) 40:41–51. doi: 10.1016/j.semcdb.2015.02.010
42. Surman M, Stępień E, Hoja-Łukowicz D, Przybyło M. Deciphering the role of ectosomes in cancer development and progression: focus on the proteome. Clin Exp Metastasis. (2017) 34:273–89. doi: 10.1007/s10585-017-9844-z
43. Choi D, Kim D, Kim Y, Gho YS. Proteomics of extracellular vesicles: Exosomes and ectosomes. Mass Spectrometry Rev. (2015) 34:474–90. doi: 10.1002/mas.21420
44. Baglio SR, Lagerweij T, Pérez-Lanzón M, Ho XD, Léveillé N, Melo SA, et al. Blocking tumor-educated MSC paracrine activity halts osteosarcoma progression. Clin Cancer Res. (2017) 23:3721–33. doi: 10.1158/1078-0432.CCR-16-2726
45. Cleanclay WD, Zakari S, Adigun TO, Ayeni TO, Nnaji PO, Nnenna AD, et al. Cancer biology and therapeutics: navigating recent advances and charting future directions. TJNPR Trop J Natural Product Res. (2023) 7(12):5377–402. doi: 10.26538/tjnpr/v7i12.4
46. Al-Nedawi K, Meehan B, Rak J. Microvesicles: Messengers and mediators of tumor progression. Cell Cycle. (2009) 8:2014–8. doi: 10.4161/cc.8.13.8988
47. Safiri S, Sepanlou SG, Ikuta KS, Bisignano C, Salimzadeh H, Delavari A, et al. The global, regional, and national burden of colorectal cancer and its attributable risk factors in 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol Hepatol. (2019) 4:913–33. doi: 10.1016/S2468-1253(19)30345-0
48. Vuik FE, Nieuwenburg SA, Bardou M, Lansdorp-Vogelaar I, Dinis-Ribeiro M, Bento MJ, et al. Increasing incidence of colorectal cancer in young adults in Europe over the last 25 years. Gut. (2019) 68:1820–6. doi: 10.1136/gutjnl-2018-317592
49. Alexandru N, Badila E, Weiss E, Cochior D, Stępień E, Georgescu A. Vascular complications in diabetes: Microparticles and microparticle associated microRNAs as active players. Biochem Biophys Res Commun. (2016) 472:1–10. doi: 10.1016/j.bbrc.2016.02.038
50. Kadam CY, Abhang SA. Apoptosis markers in breast cancer therapy. Adv Clin Chem. (2016) 74:143–93. doi: 10.1016/bs.acc.2015.12.003
51. Ward TH, Cummings J, Dean E, Greystoke A, Hou JM, Backen A, et al. Biomarkers of apoptosis. Br J Cancer. (2008) 99:841–6. doi: 10.1038/sj.bjc.6604519
52. Kadam CY, Abhang SA. Serum levels of soluble Fas ligand, granzyme B and cytochrome c during adjuvant chemotherapy of breast cancer. Clinica Chimica Acta. (2015) 438:98–102. doi: 10.1016/j.cca.2014.08.012
53. Asrorov AM, Muhitdinov B, Tu B, Mirzaakhmedov S, Wang H, Huang Y. Advances on delivery of cytotoxic enzymes as anticancer agents. Molecules. (2022) 27:3836. doi: 10.3390/molecules27123836
54. Werner RJ, Kelly AD, Issa JPJ. Epigenetics and precision oncology. Cancer J (United States). (2017) 23:262–9. doi: 10.1097/PPO.0000000000000281
56. Sharma S, Kelly TK, Jones PA. Epigenetics in cancer. Carcinogenesis. (2009) 31:27–36. doi: 10.1093/carcin/bgp220
57. Banno K, Kisu I, Yanokura M, Tsuji K, Masuda K, Ueki A, et al. Epimutation and cancer: A new carcinogenic mechanism of Lynch syndrome (Review). Int J Oncol. (2012) 41:793–7. doi: 10.3892/ijo.2012.1528
58. Costa FF. Epigenomics in cancer management. Cancer Manage Res. (2010) 2:255–65. doi: 10.2147/CMAR
59. Hashimoto Y, Zumwalt TJ, Goel A. DNA methylation patterns as noninvasive biomarkers and targets of epigenetic therapies in colorectal cancer. Epigenomics. (2016) 8:685–703. doi: 10.2217/epi-2015-0013
60. Beltrán-García J, Osca-Verdegal R, Mena-Mollá S, García-Giménez JL. Epigenetic IVD tests for personalized precision medicine in cancer. Front Genet. (2019) 10:1–14. doi: 10.3389/fgene.2019.00621
61. Baylin SB. DNA methylation and gene silencing in cancer. Nat Clin Pract Oncol. (2005) 2:4–11. doi: 10.1038/ncponc0354
62. Goyette MA, Côté JF. NSCLC metastasis: going with ELMO3. Oncotarget. (2014) 5:5850–1. doi: 10.18632/oncotarget.v5i15
63. Feinberg AP, Vogelstein B. Hypomethylation distinguishes genes of some human cancers from their normal counterparts. Nature. (1983) 301:89–92. doi: 10.1038/301089a0
64. Weisenberger DJ, Siegmund KD, Campan M, Young J, Long TI, Faasse MA, et al. CpG island methylator phenotype underlies sporadic microsatellite instability and is tightly associated with BRAF mutation in colorectal cancer. Nat Genet. (2006) 38:787–93. doi: 10.1038/ng1834
65. Jass JR. Classification of colorectal cancer based on correlation of clinical, morphological and molecular features. Histopathology. (2007) 50:113–30. doi: 10.1111/j.1365-2559.2006.02549.x
66. Rustgi AK. The genetics of hereditary colon cancer. Genes Dev. (2007) 21:2525–38. doi: 10.1101/gad.1593107
67. Zhang L, Lu W, Miao X, Xing D, Tan W, Lin D. Inactivation of DNA repair gene O6-methylguanine-DNA methyltransferase by promoter hypermethylation and its relation to p53 mutations in esophageal squamous cell carcinoma. Carcinogenesis. (2003) 24:1039–44. doi: 10.1093/carcin/bgg062
68. Roszkowski K, Furtak J, Zurawski B, Szylberg T, Lewandowska MA. Potential role of methylation marker in Glioma supporting clinical decisions. Int J Mol Sci. (2016) 17:1–7. doi: 10.3390/ijms17111876
69. Coulondre C, Miller JH. Genetic studies of the lac repressor. IV. Mutagenic specificity in the lacI gene of Escherichia coli. J Mol Biol. (1977) 117:577–606. doi: 10.1016/0022-2836(77)90059-6
70. Choy KW, Pang CP, Yu CBO, Wong HL, Ng JSK, Fan DSP, et al. Loss of heterozygosity and mutations are the major mechanisms of RB1 gene inactivation in Chinese with sporadic retinoblastoma. Hum mutation. (2002) 20:408. doi: 10.1002/(ISSN)1098-1004
71. Greger V, Passarge E, Höpping W, Messmer E, Horsthemke B. Epigenetic changes may contribute to the formation and spontaneous regression of retinoblastoma. Hum Genet. (1989) 83:155–8. doi: 10.1007/BF00286709
72. Ohtani-Fujita N, Dryja TP, Rapaport JM, Fujita T, Matsumura S, Ozasa K, et al. Hypermethylation in the retinoblastoma gene is associated with unilateral, sporadic retinoblastoma. Cancer Genet Cytogenetics. (1997) 98:43–9. doi: 10.1016/S0165-4608(96)00395-0
73. Burrows JF, Chanduloy S, McIlhatton MA, Nagar H, Yeates K, Donaghy P, et al. Altered expression of the septin gene, SEPT9, in ovarian neoplasia. J Pathol. (2003) 201:581–8. doi: 10.1002/path.1484
74. Tóth K, Galamb O, Spisák S, Wichmann B, Sipos F, Valcz G, et al. The influence of methylated septin 9 gene on RNA and protein level in colorectal cancer. Pathol Oncol Res. (2011) 17:503–9. doi: 10.1007/s12253-010-9338-7
75. Weiss G, Schlegel A, Kottwitz D, König T, Tetzner R. Validation of the SHOX2/PTGER4 DNA methylation marker panel for plasma-based discrimination between patients with Malignant and nonmalignant lung disease. J Thorac Oncol. (2017) 12:77–84. doi: 10.1016/j.jtho.2016.08.123
76. Cocco E, Benhamida J, Middha S, Zehir A, Mullaney K, Shia J, et al. Colorectal carcinomas containing hypermethylated MLH1 promoter and wild-type BRAF/KRAS are enriched for targetable kinase fusions. Cancer Res. (2019) 79:1047–53. doi: 10.1158/0008-5472.CAN-18-3126
77. Jovanović N, Mitrović T, Cvetković VJ, Tošić S, Vitorović J, Stamenković S, et al. The impact of MGMT promoter methylation and temozolomide treatment in Serbian patients with primary glioblastoma. Medicina. (2019) 55:34. doi: 10.3390/medicina55020034
78. July J, Patricia D, Gunawan PY, Setiajaya H, Ginting TE, Putra TP, et al. Clinicopathological associations and prognostic values of IDH1 gene mutation, MGMT gene promoter methylation, and PD-L1 expressions in high-grade glioma treated with standard treatment. Pan Afr Med J. (2020) 36. doi: 10.11604/pamj.2020.36.309.24831
79. Li HT, Xu L, Weisenberger DJ, Li M, Zhou W, Peng CC, et al. Characterizing DNA methylation signatures of retinoblastoma using aqueous humor liquid biopsy. Nat Commun. (2022) 13:5523. doi: 10.1038/s41467-022-33248-2
80. Lam D, Clark S, Stirzaker C, Pidsley R. Advances in prognostic methylation biomarkers for prostate cancer. Cancers. (2020) 12:2993. doi: 10.3390/cancers12102993
81. Lu P, Zhu X, Song Y, Luo Y, Lin J, Zhang J, et al. Methylated septin 9 as a promising biomarker in the diagnosis and recurrence monitoring of colorectal cancer. Dis Markers. (2022) 2022:7087885. doi: 10.1155/2022/7087885
82. Powrózek T, Krawczyk P, Kucharczyk T, Milanowski J. Septin 9 promoter region methylation in free circulating DNA—potential role in noninvasive diagnosis of lung cancer: preliminary report. Med Oncol. (2014) 31:917.
83. Nakasone ES, Zemla TJ, Yu M, Lin SY, Ou FS, Carter K, et al. Evaluating the utility of ZNF331 promoter methylation as a prognostic and predictive marker in stage III colon cancer: results from CALGB 89803 (Alliance). Epigenetics. (2024) 19:2349980. doi: 10.1080/15592294.2024.2349980
84. van den Bent MJ, Erdem-Eraslan L, Idbaih A, de Rooi J, Eilers PHC, Spliet WGM, et al. MGMT-STP27 methylation status as predictive marker for response to PCV in anaplastic Oligodendrogliomas and Oligoastrocytomas. A report from EORTC study 26951. Clin Cancer Res. (2013) 19:5513–22. doi: 10.1158/1078-0432.CCR-13-1157
85. Bady P, Delorenzi M, Hegi ME. Sensitivity analysis of the MGMT-STP27 model and impact of genetic and epigenetic context to predict the MGMT methylation status in gliomas and other tumors. J Mol Diagnostics. (2016) 18:350–61. doi: 10.1016/j.jmoldx.2015.11.009
86. Abdel-hamid NR, Mohammed EA, Toraih EA, Kamel MM, Abdelhafiz AS, Badr FM. Circulating ESR1, long non-coding RNA HOTAIR and microRNA-130a gene expression as biomarkers for breast cancer stage and metastasis. Sci Rep. (2023) 13:22654. doi: 10.1038/s41598-023-50007-5
87. Śledzińska P, Bebyn MG, Furtak J, Kowalewski J, Lewandowska MA. Prognostic and predictive biomarkers in gliomas. Int J Mol Sci. (2021) 22:10373. doi: 10.3390/ijms221910373
88. Gaździcka J, Gołąbek K, Strzelczyk JK, Ostrowska Z. Epigenetic modifications in head and neck cancer. Biochem Genet. (2020) 58:213–44. doi: 10.1007/s10528-019-09941-1
89. Misawa K, Misawa Y, Imai A, Mochizuki D, Endo S, Mima M, et al. Epigenetic modification of SALL1 as a novel biomarker for the prognosis of early stage head and neck cancer. J Cancer. (2018) 9:941–9. doi: 10.7150/jca.23527
90. Dawson MA, Kouzarides T. Cancer epigenetics: From mechanism to therapy. Cell. (2012) 150:12–27. doi: 10.1016/j.cell.2012.06.013
91. Ernst J, Kheradpour P, Mikkelsen TS, Shoresh N, Ward LD, Epstein CB, et al. Mapping and analysis of chromatin state dynamics in nine human cell types. Nature. (2011) 473:43–9. doi: 10.1038/nature09906
92. Kurdistani SK. Histone modifications as markers of cancer prognosis: A cellular view. Br J Cancer. (2007) 97:1–5. doi: 10.1038/sj.bjc.6603844
93. Song JS, Kim YS, Kim DK, Park S, Jang SJ. Global histone modification pattern associated with recurrence and disease-free survival in non-small cell lung cancer patients. Pathol Int. (2012) 62:182–90. doi: 10.1111/j.1440-1827.2011.02776.x
94. Fraga MF, Ballestar E, Paz MF, Ropero S, Setien F, Ballestar ML, et al. Epigenetic differences arise during the lifetime of monozygotic twins. Proc Natl Acad Sci United States America. (2005) 102:10604–9. doi: 10.1073/pnas.0500398102
95. Elsheikh SE, Green AR, Rakha EA, Powe DG, Ahmed RA, Collins HM, et al. Global histone modifications in breast cancer correlate with tumor phenotypes, prognostic factors, and patient outcome. Cancer Res. (2009) 69:3802–9. doi: 10.1158/0008-5472.CAN-08-3907
96. Monteiro FL, Baptista T, Amado F, Vitorino R, Jerónimo C, Helguero LA. Oncotarget 3428 www.impactjournals.com/oncotarget Expression and functionality of histone H2A variants in cancer. Oncotarget. (2003) 5:497–508.
97. Gezer U, Yörüker EE, Keskin M, Kulle CB, Dharuman Y, Holdenrieder S. Histone methylation marks on circulating nucleosomes as novel blood-based biomarker in colorectal cancer. Int J Mol Sci. (2015) 16:29654–62. doi: 10.3390/ijms161226180
98. Thålin C, Lundström S, Seignez C, Daleskog M, Lundström A, Henriksson P, et al. Citrullinated histone H3 as a novel prognostic blood marker in patients with advanced cancer. PloS One. (2018) 13:e0191231. doi: 10.1371/journal.pone.0191231
99. Rahier JF, Druez A, Faugeras L, Martinet JP, Géhénot M, Josseaux E, et al. Circulating nucleosomes as new blood-based biomarkers for detection of colorectal cancer. Clin Epigenetics. (2017) 9:53. doi: 10.1186/s13148-017-0351-5
100. Liu H, Li Y, Li J, Liu Y, Cui B. H3K4me3 and Wdr82 are associated with tumor progression and a favorable prognosis in human colorectal cancer. Oncol Lett. (2018) 16(2):2125–34. doi: 10.3892/ol.2018.8902
101. Roi A, Boia S, Rusu LC, Roi CI, Boia ER, Riviş M. Circulating miRNA as a biomarker in oral cancer liquid biopsy. Biomedicines. (2023) 11. doi: 10.3390/biomedicines11030965
102. Rhim J, Baek W, Seo Y, Kim JH. From molecular mechanisms to therapeutics: understanding microRNA-21 in cancer. Cells. (2022) 11:2791. doi: 10.3390/cells11182791
103. Zhao Q, Yuan X, Zheng L, Xue M. miR-30d-5p: A non-coding RNA with potential diagnostic, prognostic and therapeutic applications. Front Cell Dev Biol. (2022) 10:829435. doi: 10.3389/fcell.2022.829435
104. Lin X, Wu W, Ying Y, Luo J, Xu X, Zheng L, et al. MicroRNA-31: a pivotal oncogenic factor in oral squamous cell carcinoma. Cell Death Discovery. (2022) 8:140. doi: 10.1038/s41420-022-00948-z
105. Gilad S, Lithwick-Yanai G, Barshack I, Benjamin S, Krivitsky I, Edmonston TB, et al. Classification of the four main types of lung cancer using a microRNA-based diagnostic assay. J Mol Diagnostics. (2012) 14:510–7. doi: 10.1016/j.jmoldx.2012.03.004
106. Wang Jy, Zhang Q, Wang Dd, Yan W, Sha Hh, Zhao Jh, et al. MiR-29a : a potential therapeutic target and promising biomarker in tumors. Biosci Rep. (2018) 38:BSR20171265. doi: 10.1042/BSR20171265
107. Yang X, Zeng Z, Hou Y, Yuan T, Gao C, Jia W, et al. MicroRNA-92a as a potential biomarker in diagnosis of colorectal cancer: A systematic review and meta-analysis. PloS One. (2014) 9:e88745. doi: 10.1371/journal.pone.0088745
108. Krawczyk P, Powrózek T, Olesiński T, Dmitruk A, Dziwota J, Kowalski D, et al. Evaluation of miR-506 and miR-4316 expression in early and non-invasive diagnosis of colorectal cancer. Int J Colorectal Dis. (2017) 32:1057–60. doi: 10.1007/s00384-017-2814-8
109. Fogli S, Polini B, Carpi S, Pardini B, Naccarati A, Dubbini N, et al. Identification of plasma microRNAs as new potential biomarkers with high diagnostic power in human cutaneous melanoma. Tumour Biol. (2017) 39:101042831770164. doi: 10.1177/1010428317701646
110. Choi PW, Bahrampour A, Ng SK, Liu SK, Qiu W, Xie F, et al. Characterization of miR-200 family members as blood biomarkers for human and laying hen ovarian cancer. Sci Rep. (2020) 10:20071. doi: 10.1038/s41598-020-77068-0
111. Lv S, Wang Y, Xu W, Dong X. Serum exosomal miR-17–5p as a promising biomarker diagnostic biomarker for breast cancer. Clin Lab. (2020) 66. doi: 10.7754/Clin.Lab.2020.200127
112. Cui ZJ, Xie XL, Qi W, Yang YC, Bai Y, Han J, et al. Cell-free miR-17–5p as a diagnostic biomarker for gastric cancer inhibits dendritic cell maturation. OTT Onco Targets Ther. (2019) 12:2661–75. doi: 10.2147/OTT
113. Shao C, Yang F, Qin Z, Jing X, Shu Y, Shen H. The value of miR-155 as a biomarker for the diagnosis and prognosis of lung cancer: a systematic review with meta-analysis. BMC Cancer. (2019) 19:1103. doi: 10.1186/s12885-019-6297-6
114. Czubak K, Lewandowska MA, Klonowska K, Roszkowski K, Kowalewski J, Figlerowicz M, et al. High copy number variation of cancer-related microRNA genes and frequent amplification of DICER1 and DROSHA in lung cancer. Oncotarget. (2015) 6:23399–416. doi: 10.18632/oncotarget.v6i27
115. Li N, Kaur S, Greshock J, Lassus H, Zhong X, Wang Y, et al. A combined array-based comparative genomic hybridization and functional library screening approach identifies mir-30d as an oncomir in cancer. Cancer Res. (2012) 72:154–64. doi: 10.1158/0008-5472.CAN-11-2484
116. Manceau G, Imbeaud S, Thiébaut R, Liébaert F, Fontaine K, Rousseau F, et al. Hsa-miR-31–3p expression is linked to progression-free survival in patients with KRAS wild-type metastatic colorectal cancer treated with anti-EGFR therapy. Clin Cancer Res. (2014) 20:3338–47. doi: 10.1158/1078-0432.CCR-13-2750
117. Roush S, Slack FJ. The let-7 family of microRNAs. Trends Cell Biol. (2008) 18:505–16. doi: 10.1016/j.tcb.2008.07.007
118. Boyerinas B, Park SM, Hau A, Murmann AE, Peter ME. The role of let-7 in cell differentiation and cancer. Endocrine-Related Cancer. (2010) 17:19–36. doi: 10.1677/ERC-09-0184
119. Buscaglia LEB, Li Y. Apoptosis and the target genes of microRNA-21. Chin J Cancer. (2011) 30:371–80. doi: 10.5732/cjc
120. Leupold JH, Yang HS, Colburn NH, Asangani I, Post S, Allgayer H. Tumor suppressor Pdcd4 inhibits invasion/intravasation and regulates urokinase receptor (u-PAR) gene expression via Sp-transcription factors. Oncogene. (2007) 26:4550–62. doi: 10.1038/sj.onc.1210234
121. Larrea E, Sole C, Manterola L, Goicoechea I, Armesto M, Arestin M, et al. New concepts in cancer biomarkers: Circulating miRNAs in liquid biopsies. Int J Mol Sci. (2016) 17. doi: 10.3390/ijms17050627
122. Chen B, Dragomir MP, Yang C, Li Q, Horst D, Calin GA. Targeting non-coding RNAs to overcome cancer therapy resistance. Sig Transduct Target Ther. (2022) 7:121. doi: 10.1038/s41392-022-00975-3
123. Toden S, Goel A. Non-coding RNAs as liquid biopsy biomarkers in cancer. Br J Cancer. (2022) 126:351–60. doi: 10.1038/s41416-021-01672-8
124. Dragomir MP, Kopetz S, Ajani JA, Calin GA. Non-coding RNAs in GI cancers: from cancer hallmarks to clinical utility. Gut. (2020) 69:748–63. doi: 10.1136/gutjnl-2019-318279
125. Flatmark K, Høye E, Fromm B. microRNAs as cancer biomarkers. Scandinavian J Clin Lab Invest. (2016) 76:S80–3. doi: 10.1080/00365513.2016.1210330
126. Peng Y, Croce CM. The role of MicroRNAs in human cancer. Sig Transduct Target Ther. (2016) 1:15004. doi: 10.1038/sigtrans.2015.4
127. Uzuner E, Ulu GT, Gürler SB, Baran Y. The role of miRNA in cancer: pathogenesis, diagnosis, and treatment. Methods Mol Biol. (2022) 2257:375–422.
128. Wang C, Jing Q. Non-coding RNAs as biomarkers for acute myocardial infarction. Acta Pharmacol Sin. (2018) 39:1110–9. doi: 10.1038/aps.2017.205
129. Yete S, Saranath D. MicroRNAs in oral cancer: Biomarkers with clinical potential. Oral Oncol. (2020) 110:105002. doi: 10.1016/j.oraloncology.2020.105002
130. Ghafouri-Fard S, Abak A, Shoorei H, Mohaqiq M, Majidpoor J, Sayad A, et al. Regulatory role of microRNAs on PTEN signaling. Biomed Pharmacother. (2021) 133:110986. doi: 10.1016/j.biopha.2020.110986
131. Wang Q, Wang J, Xiang H, Ding P, Wu T, Ji G. The biochemical and clinical implications of phosphatase and tensin homolog deleted on chromosome ten in different cancers. Am J Cancer Res. (2021) 11:5833–55.
132. Mar-Aguilar F, Mendoza-Ramírez JA, Malagón-Santiago I, Espino-Silva PK, Santuario-Facio SK, Ruiz-Flores P, et al. Serum circulating microRNA profiling for identification of potential breast cancer biomarkers. Dis Markers. (2013) 34:163–9. doi: 10.1155/2013/259454
133. Beylerli O, Gareev I, Sufianov A, Ilyasova T, Guang Y. Long noncoding RNAs as promising biomarkers in cancer. Non-coding RNA Res. (2022) 7:66–70. doi: 10.1016/j.ncrna.2022.02.004
134. Wang Y, Mo Y, Yang X, Zhou R, Wu Z, He Y, et al. Long non-coding RNA AFAP1-AS1 is a novel biomarker in various cancers: a systematic review and meta-analysis based on the literature and GEO datasets. Oncotarget. (2017) 8:102346–60. doi: 10.18632/oncotarget.v8i60
135. Badowski C, He B, Garmire LX. Blood-derived lncRNAs as biomarkers for cancer diagnosis: the Good, the Bad and the Beauty. NPJ Precis Onc. (2022) 6:40. doi: 10.1038/s41698-022-00283-7
136. Zhao W, Huang Z, Liu H, Wang C. LncRNA GIHCG promotes the development of esophageal cancer by modulating miR-29b-3p/ANO1 axis. OTT Onco Targets Ther. (2020) 13:13387–400. doi: 10.2147/OTT.S282348
137. Wang M, Zhang Z, Pan D, Xin Z, Bu F, Zhang Y, et al. Circulating lncRNA UCA1 and lncRNA PGM5-AS1 act as potential diagnostic biomarkers for early-stage colorectal cancer. Biosci Rep. (2021) 41:BSR20211115. doi: 10.1042/BSR20211115
138. Beilerli A, Gareev I, Beylerli O, Yang G, Pavlov V, Aliev G, et al. Circular RNAs as biomarkers and therapeutic targets in cancer. Semin Cancer Biol. (2022) 83:242–52. doi: 10.1016/j.semcancer.2020.12.026
139. Wang W, Luo H, Chang J, Yang X, Zhang X, Zhang Q, et al. Circular RNA circ0001955 promotes cervical cancer tumorigenesis and metastasis via the miR-188–3p/NCAPG2 axis. J Transl Med. (2023) 21:356. doi: 10.1186/s12967-023-04194-4
140. Li T, Sun X, Chen L. Exosome circ_0044516 promotes prostate cancer cell proliferation and metastasis as a potential biomarker. J Cell Biochem. (2020) 121:2118–26. doi: 10.1002/jcb.28239
141. Aboud OA, Weiss RH. New opportunities from the cancer metabolome. Clin Chem. (2013) 59:138–46. doi: 10.1373/clinchem.2012.184598
142. Beger RD. A review of applications of metabolomics in cancer. Metabolites. (2013) 3:552–74. doi: 10.3390/metabo3030552
143. Iheagwam FN, Iheagwam OT, Odiba JK, Ogunlana OO, Chinedu SN. Cancer and glucose metabolism: A review on warburg mechanisms. Trop J Natural Product Res. (2022) 6.
144. Bhattacharjee R, Dey T, Kumar L, Kar S, Sarkar R, Ghorai M, et al. Cellular landscaping of cisplatin resistance in cervical cancer. Biomed Pharmacother. (2022) 153:113345. doi: 10.1016/j.biopha.2022.113345
145. Hadi NI. Onic” tumor markers for breast cancer- a review. Pak J Med Sci. (2015) 31. doi: 10.12669/pjms.315.7627
146. Trilla-Fuertes L, Gámez-Pozo A, López-Camacho E, Prado-Vázquez G, Zapater-Moros A, López-Vacas R, et al. Computational models applied to metabolomics data hints at the relevance of glutamine metabolism in breast cancer. BMC Cancer. (2020) 20:307. doi: 10.1186/s12885-020-06764-x
147. Puchades-Carrasco L, Pineda-Lucena A. Metabolomics applications in precision medicine: an oncological perspective. Curr Top Med Chem. (2017) 17(24):2740–51. doi: 10.2174/1568026617666170707120034
148. Ashrafian H, Sounderajah V, Glen R, Ebbels T, Blaise BJ, Kalra D, et al. Metabolomics: the stethoscope for the twenty-first century. Med Principles Practice. (2020) 30:301–10. doi: 10.1159/000513545
149. Gu J, Xiao Y, Shu D, Liang X, Hu X, Xie Y, et al. Metabolomics analysis in serum from patients with colorectal polyp and colorectal cancer by 1 H-NMR spectrometry. Dis Markers. (2019) 2019:1–14. doi: 10.1155/2019/3491852
150. Jafari A, Babajani A, Rezaei-Tavirani M. Multiple sclerosis biomarker discoveries by proteomics and metabolomics approaches. biomark Insights. (2021) 16:11772719211013352. doi: 10.1177/11772719211013352
151. Panneerselvam K, Ishikawa S, Krishnan R, Sugimoto M. Salivary metabolomics for oral cancer detection: A narrative review. Metabolites. (2022) 12:436. doi: 10.3390/metabo12050436
152. Ghini V, Laera L, Fantechi B, del Monte F, Benelli M, McCartney A, et al. Metabolomics to assess response to immune checkpoint inhibitors in patients with non-small-cell lung cancer. Cancers. (2020) 12:3574. doi: 10.3390/cancers12123574
153. Yang K, Xia B, Wang W, Cheng J, Yin M, Xie H, et al. A comprehensive analysis of metabolomics and transcriptomics in cervical cancer. Sci Rep. (2017) 7:43353. doi: 10.1038/srep43353
154. Roointan A. Metabolome panels as potential noninvasive biomarkers for Primary Glomerulonephritis Sub-types: Meta-analysis of Profiling Metabolomics Studies. (2023). doi: 10.21203/rs.3.rs-2686981/v1
155. Lima AR, Pinto J, Amaro F, Bastos M de L, Carvalho M, Guedes de Pinho P. Advances and perspectives in prostate cancer biomarker discovery in the last 5 years through tissue and urine metabolomics. Metabolites. (2021) 11:181. doi: 10.3390/metabo11030181
156. Dereziński P, Klupczynska A, Sawicki W, Pałka JA, Kokot ZJ. Amino acid profiles of serum and urine in search for prostate cancer biomarkers: a pilot study. Int J Med Sci. (2017) 14:1–12. doi: 10.7150/ijms.15783
157. Spur EM, Decelle EA, Cheng LL. Metabolomic imaging of prostate cancer with magnetic resonance spectroscopy and mass spectrometry. Eur J Nucl Med Mol Imaging. (2013) 40:60–71. doi: 10.1007/s00259-013-2379-x
158. Das S, Jackson WP, Prasain JK, Hanna A, Bailey SK, Tucker JA, et al. Loss of Merlin induces metabolomic adaptation that engages dependence on Hedgehog signaling. Sci Rep. (2017) 7:40773. doi: 10.1038/srep40773
159. Subramani R, Poudel S, Smith KD, Estrada A, Lakshmanaswamy R. Metabolomics of Breast Cancer: A Review. (2022). doi: 10.3390/metabo12070643
160. Denkert C, Bucher E, Hilvo M, Salek R, Orešič M, Griffin J, et al. Metabolomics of human breast cancer: new approaches for tumor typing and biomarker discovery. Genome Med. (2012) 4:37. doi: 10.1186/gm336
161. Di Meo A, Bartlett J, Cheng Y, Pasic MD, Yousef GM. Liquid biopsy: a step forward towards precision medicine in urologic Malignancies. Mol Cancer. (2017) 16:80. doi: 10.1186/s12943-017-0644-5
162. Wan L, Liu Q, Liang D, Guo Y, Liu G, Ren J, et al. Circulating tumor cell and metabolites as novel biomarkers for early-stage lung cancer diagnosis. Front Oncol. (2021) 11:630672. doi: 10.3389/fonc.2021.630672
163. Ahmed N, Kidane B, Wang L, Qing G, Tan L, Buduhan G, et al. Non-invasive exploration of metabolic profile of lung cancer with Magnetic Resonance Spectroscopy and Mass Spectrometry. Contemp Clin Trials Commun. (2019) 16:100445. doi: 10.1016/j.conctc.2019.100445
164. Villéger R, Lopès A, Veziant J, Gagnière J, Barnich N, Billard E, et al. Microbial markers in colorectal cancer detection and/or prognosis. World J Gastroenterol. (2018) 24:2327–47. doi: 10.3748/wjg.v24.i22.2327
165. Łuczykowski K, Warmuzińska N, Operacz S, Stryjak I, Bogusiewicz J, Jacyna J, et al. Metabolic evaluation of urine from patients diagnosed with high grade (HG) bladder cancer by SPME-LC-MS method. Molecules. (2021) 26:2194. doi: 10.3390/molecules26082194
166. D′Avó Luís AB, Seo MK. Has the development of cancer biomarkers to guide treatment improved health outcomes? Eur J Health Econ. (2021) 22:789–810.
167. Jain KK. Cancer biomarkers: current issues and future directions. Curr Opin Mol Ther. (2007) 9:563–71.
168. Mayeux R. Biomarkers: Potential uses and limitations. Neurotherapeutics. (2004) 1:182–8. doi: 10.1602/neurorx.1.2.182
169. Lever J, Jones MR, Danos AM, Krysiak K, Bonakdar M, Grewal JK, et al. Text-mining clinically relevant cancer biomarkers for curation into the CIViC database. Genome Med. (2019) 11:78. doi: 10.1186/s13073-019-0686-y
170. Sebastian AM, Peter D. Artificial intelligence in cancer research: trends, challenges and future directions. Life. (2022) 12:1991. doi: 10.3390/life12121991
Keywords: biomarkers, cancer diagnosis, non-invasive biomarkers, cancer treatment, biomarker sensitivity, specificity, or clinical utility
Citation: Zakari S, Niels NK, Olagunju GV, Nnaji PC, Ogunniyi O, Tebamifor M, Israel EN, Atawodi SE and Ogunlana OO (2024) Emerging biomarkers for non-invasive diagnosis and treatment of cancer: a systematic review. Front. Oncol. 14:1405267. doi: 10.3389/fonc.2024.1405267
Received: 22 March 2024; Accepted: 05 July 2024;
Published: 26 July 2024.
Edited by:
Abdou Azaque Zoure, Institut de recherche en sciences de la santé (IRSS), Burkina FasoReviewed by:
Prasanth Kumar, St. Jude Children’s Research Hospital, United StatesCopyright © 2024 Zakari, Niels, Olagunju, Nnaji, Ogunniyi, Tebamifor, Israel, Atawodi and Ogunlana. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Suleiman Zakari, c3VsZWltYW4uemFrYXJpcGdzQHN0dS5jdS5lZHUubmc=; Nguedia K. Niels, a2F6ZS5uZ3VlZGlhcGdzQHN0dS5jdS5lZHUubmc=; Olubanke Olujoke Ogunlana, YmFua2Uub2d1bmxhbmFAY292ZW5hbnR1bml2ZXJzaXR5LmVkdS5uZw==
†ORCID: Suleiman Zakari, orcid.org/0000-0003-2130-0937
Nguedia K. Niels, orcid.org/0009-0006-4311-259X
Grace V. Olagunju, orcid.org/0009-0008-6783-3601
Sunday E. Atawodi, orcid.org/0000-0001-9786-8591
Olubanke Olujoke Ogunlana, orcid.org/0000-0001-5781-592X
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.