
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Oncol. , 19 June 2024
Sec. Genitourinary Oncology
Volume 14 - 2024 | https://doi.org/10.3389/fonc.2024.1404753
Objective: A novel systemic immune-inflammation index (SII), based on the neutrophils, lymphocytes, and platelet counts, is associated with the prognosis of several cancers, including non-metastatic renal cell carcinoma (RCC). In the present study, we evaluate the prognostic significance of SII in patients with metastatic RCC (mRCC) treated with systemic therapy.
Method: Relevant studies were searched comprehensively from Web of Science, PubMed, Embase and the Cochrane Library up to January 2024. The pooled hazard ratio (HR) and 95% confidence interval (CI) were extracted from each study to evaluate the prognostic value of SII in patients with mRCC treated with tyrosine kinase inhibitor (TKI) or immune checkpoint inhibitor (ICI).
Results: A total of 12 studies including 4,238 patients were included in the final analysis. High SII was significantly correlated to poor overall survival (OS, HR = 1.88; 95% CI 1.60–2.21; P < 0.001) and progression-free survival (PFS, HR = 1.66; 95% CI 1.39–1.99; P < 0.001). Stratified by therapy, high SII was also related to the poor OS (TKI: HR = 1.63, P < 0.001; ICI: HR = 2.27, P < 0.001) and PFS (TKI: HR = 1.67, P < 0.001; ICI: HR = 1.88, P = 0.002).
Conclusion: In conclusion, high SII could serve as an unfavorable factor in patients with mRCC treated with systemic therapy. Stratified by therapies, the elevated SII was also associated with worse prognosis. Whereas, more prospective and large-scale studies are warranted to validate our findings.
Systematic review registration: https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42024522831, identifier CRD42024522831.
Renal cell carcinoma (RCC) is one of the common urological cancers, accounts for approximately 2% of all malignancies, with an estimated 431,288 new cases and 179,368 deaths in 2020 worldwide (1). Although most patients are localized disease and could undergo surgical resection with curative intent, about one-third of patients will develop metastatic disease recurrence (2, 3). Furthermore, 30% of patients present regional or distant metastases at initial diagnosis (2). Over the last decades, advancements in the treatment of metastatic RCC (mRCC) improved patients’ prognosis dramatically, such as the tyrosine kinase inhibitors (TKI) and immune checkpoint inhibitors (ICIs) (4, 5).
With the progress of the management of mRCC, identification of predictive markers would be of great value to patients’ treatment and long-term outcomes. The International metastatic renal cell carcinoma Database Consortium (IMDC) risk model is widely used for mRCC patients’ stratification and treatment selection. Recently, other potential biomarkers have been investigated for their prognostic and predictive value, including programmed cell death ligand 1 (PD-L1) expression, tumor mutational burden (TMB), molecular and genomic signatures, and clinical factors (6).
Evidences suggested that host inflammation response plays an important role in cancer progression by enhancing tumor angiogenesis and metastasis (7, 8). Peripheral blood parameters might reflect the cancer-related inflammatory phenomena. Previous reported have reported that prognostic value of neutrophil-lymphocyte ratio (NLR), platelet-lymphocyte ratio (PLR), prognostic nutrition index (PNI), and systemic immune-inflammation index (SII) in many cancers (9–12).
The SII is defined as follows platelet count × neutrophil count/lymphocyte count and has been found to be associated with the prognosis of several cancers, such as urothelial carcinoma, hepatocellular carcinoma, and non-metastatic RCC (12–14). The prognostic value of SII in mRCC patients treated with systemic therapy remains unclear. Therefore, we summarized all relevant studies and investigated the prognostic significance of SII in mRCC.
The present study was performed based on the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) Statement (15). Moreover, present study has been registered in PROSPERO (CRD42024522831). We comprehensively searched Embase, Web of Science, PubMed, and the Cochrane Library up to January 2024. Two independent reviewers performed the study search based on the search strategy (SII, systemic inflammation index, systemic immune-inflammation index) and (kidney cancer, renal cancer). We also screened the references of eligible studies to avoid the omission.
Studies finally enrolled in the present study should meet the following criteria: (1) population-based studies, (2) involved patients with mRCC, (3) patients were treated with TKI or ICI, (4) SII was defined accurately and calculated based on the formula, (5) evaluate the prognostic value of SII, (6) available data such as hazard ratio (HR) and 95% confidence interval (CI) could be extracted. The following studies were excluded: (1) did not involve SII, (2) did not evaluate the prognostic value of SII, (3) insufficient data for HR and 95% CI, and (4) patients weren’t treated with systemic therapy. For the same cohort patients, we included the study with the largest and newest data.
Two reviewers extracted the relevant data from eligible studies independently based on the predefined items: publication year, participant, study design, disease, therapy, number and ages of patients, the cutoff value of SII, clinical outcomes, and duration of follow-up. The Newcastle-Ottawa Quality Assessment Scale (NOS) was used to evaluate the quality of included studies, incorporating three main aspects: selection, comparability, and exposure/outcome. Total scores ranged from 0 to 9, a score of no less than 7 was considered as high quality.
STATA (version 12, StataCorp, College Station, TX, USA) was applied to conduct all statistical analyses. We extracted and pooled HRs and 95% CIs through the inverse-variance method to investigated the prognostic value of SII in patients with mRCC. A random-effects approach was chosen over a fixed-effects approach, because using random effects is often preferred when performing a meta-analysis to guide patient treatment decision (16, 17). For the evaluation of heterogeneity across studies, the Cochran’s Q test and the Higgins’I2 statistic were calculated. If the I2 ≥ 50% or P < 0.10, the between-study heterogeneity was considered as significant (18). The sensitivity analyses were conducted to validate the stability of the final results by omitting each study in sequence. We also performed subgroup analysis and meta-regression to explore the potential source of heterogeneity. A two-sided P-value of < 0.05 was considered significant.
At first, 594 articles were identified based on the electronic database search. After excluding the 74 duplicated articles, the remaining 520 records were screened. According to the titles and abstracts, 66 studies were further reviewed detailedly. At last, a total of 12 studies incorporating 4,238 patients were included in the final analysis (19–30). The detailed information was illustrated in Figure 1 (Supplementary Table S1).
Most studies were retrospective, while two studies were prospective (19, 28). Eight of the studies were multicenter. Half of studies included mRCC patients treated with ICIs, and the other six studies involved mRCC patients treated with TKIs. Most studies have a quite large sample size, ranging from 49 to 1,034. SII was calculated based on the formula (platelet×neutrophil/lymphocyte). The cutoff value of SII in each study is not consistent. All studies reported the overall survival (OS), seven studies reported progression-free survival (PFS). All studies were regarded as high quality (Supplementary Table S2). The detailed information was summarized in Table 1.
All studies including 4,238 patients reported OS. We observed moderate heterogeneity among studies, so the random effect was applied (I2 = 51.2%; P = 0.021). A higher SII was significantly related to the worse OS compared with lower SII (HR = 1.88; 95% CI 1.60–2.21; P < 0.001; Figure 2A).
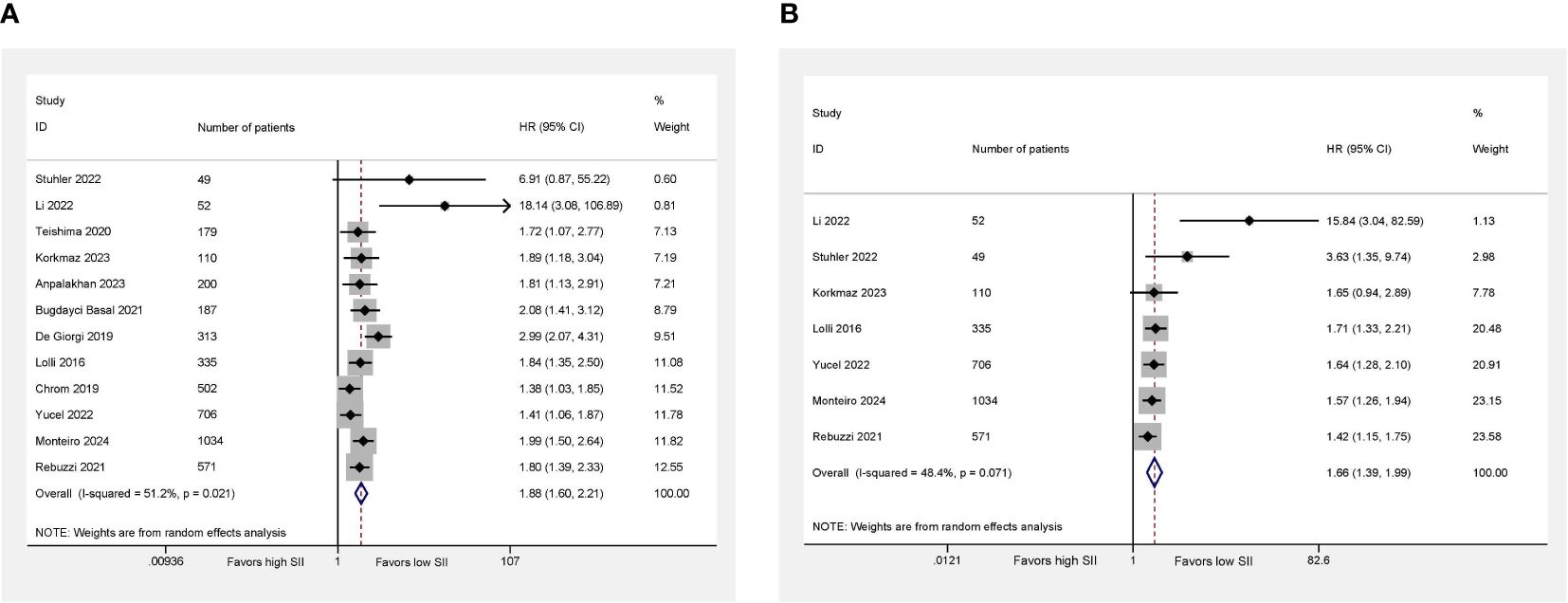
Figure 2 Higher SII was associated with worse OS (A) and PFS (B) in mRCC patients treated with systemic therapies. Right side (HR > 1) favors low SII, left side (HR < 1) favors high SII.
Regarding PFS, seven studies involving 2,857 patients revealed relevant data. We also observed the evidence of heterogeneity (I2 = 48.4%; P = 0.071). Using the random-effect model, we found that the patients with higher SII had a worse PFS compared with the patients with lower SII (HR = 1.66; 95% CI 1.39–1.99; P < 0.001; Figure 2B).
The sensitivity analysis for OS and PFS was conducted by eliminating each study to reflect the impact of the individual to overall. Consistently, we observed that removing any single study would not dramatically alter the trend of our results (Figure 3).
We performed subgroup analysis stratified by number of patients, study type, therapy, and enrollment. For the different types of studies, we both observed that high SII was significantly associated with poor OS. In the subgroup with large than 300 patients, high SII was also associated with the inferior OS and PFS, and in the <300 patients, we also detected the prognostic significance of SII. As for therapy, high SII was an unfavorable factor in patients treated with ICI or TKI. In addition, in studies from multicenter or single center, high SII was significantly related to the poor OS and PFS. Meta-regression revealed that none of these variables are significantly associated with the heterogeneity. The detailed information was summarized in Table 2.
In the present study, we evaluate the association between SII and mRCC patients, observing that high SII was associated with the poor prognosis of mRCC. When stratified by therapies, high SII also predicts an inferior OS and PFS in mRCC patients treated with TKI or ICI. Moreover, we performed subgroup analysis and found all subgroup results are consistent with overall results. We only included the studies that provided the largest and newest data, which may affect the totality of data. However, this would likely be non-differential given the prognostic nature of the studies.
Local recurrence or metastasis is highly likely to occur in the RCC, nearly 30% of patients will develop local or distant recurrence after surgery (3). Meanwhile, one-third of patients suffer from metastasis initially (2). Although systemic therapy achieved great improvement of mRCC patients’ survival, not all patients could respond to these therapies. Many efforts to explore predictive markers are continuing.
The association between inflammation and malignancy has been widely explored in the past decades. Lots of studies have revealed an immunogenic nature of RCC (31). This immunogenic microenvironment may explain the antitumor efficacy of immune-related therapy used in mRCC treatment. While, the tumor infiltrating cells and their secretions may play a role in tumorigenesis, progression and clinical outcomes (32). It has been revealed that some peripheral markers of inflammation, NLR, PLR, and CRP were associated with prognosis of mRCC patients (33). Yucel et al. collected 706 mRCC patients treated with first-line TKI from multicenter and observed that pre-treatment high SII was considered a predictor of poor OS (HR = 1.39; P = 0.01) and PFS (HR = 1.60; P < 0.001) (23). In addition, SII might provide the similar predictive value as the IMDC and MSKCC, with the similar C-index values for OS and PFS in SII, IMDC, and MSKCC risk scores (23). Bugdayci Basal et al. demonstrated that in different IMDC risk groups, the patients with higher SII had a significantly worse OS compared with those with lower SII. And the SII may increase the predictive value of IMDC risk model in mRCC patients treated with TKI (24). Chrom et al. demonstrated that the addition of the SII to the IMDC model in place of neutrophil and platelet counts increased the model’s prognostic performance (27). Moreover, for mRCC patients received ipilimumab plus nivolumab in the first-line setting, high SII was also an unfavorable factor for OS and PFS (22). A prospective cohort of patients with mRCC treated with nivolumab also revealed that SII independently predicted OS (HR = 2.99; P < 0.001) (28). Recently, a retrospective study of 1,034 mRCC patients from 56 centers also revealed that a high SII is associated with poor oncological outcomes in patients treated with first-line immune combinations therapy (30). Wang et al. performed a meta-analysis and explored the prognostic value of SII in cancer patients receiving ICI. They found SII could predictive OS and PFS irrespective the cancer type, ICIs type and cutoff value of SII (34). Based on the abovementioned evidence, high SII could be served as an unfavorable factor in mRCC patients treated with systemic therapies. However, more large-scale studies are required to verify our findings.
The potential mechanism for the prognostic significance of this combination might be explained by the functions of neutrophil, platelet, and lymphocyte. Neutrophils can promote cancer development through directly interacting with tumor cells. Neutrophils can secrete proinflammatory cytokine and chemokine related to the remodeling of the tumor microenvironment and have a tumor-promoting effect (35). Platelets have been reported to regulate tumor angiogenesis, protect tumor cells from cytolysis, and contribute to tumor metastasis (36). As a major cellular immunity component in humans, lymphocytes are implicated in killing the host cancer cells by cell-mediated immunization. Therefore, the decreased lymphocytes may cause a weak anti-tumor activity and lead to cancer progression (24).
SII had significance in clinical practice. SII was calculated based on the neutrophil, platelet, and lymphocyte, which is convenient, easily obtained and commonly tested before the treatment. SII could predict the prognosis of patients, which could be used for mRCC patients’ managements. However, the individual conditions should be considered during the treatment strategy decision.
Our study is not devoid of shortcomings. First of all, total 12 studies consisting of 4,238 patients were included, which is not a relatively large sample and may limit the power of final results. Next, almost all studies were retrospective studies with the potential inherent bias, resulting heterogeneity. Therefore, we conducted sensitivity analysis and subgroup analysis. We only included the studies that provided the largest and newest data, which may affect the totality of data. But this would likely be non-differential given the prognostic nature of the studies. At last, although we performed subgroup analyses and meta-regression, there are several factors that are not available and may result heterogeneity such as detailed treatments and comorbidity, so we could not conduct additional analyses.
In conclusion, high SII could serve as an unfavorable factor in patients with mRCC treated with systemic therapy. Stratified by therapies, the elevated SII was also associated with worse prognosis. Whereas, more prospective and large-scale studies are warranted to validate our findings.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
JX: Writing – original draft, Methodology, Formal analysis, Data curation. PC: Writing – original draft, Methodology, Formal analysis, Data curation. SC: Writing – original draft, Methodology, Formal analysis, Data curation. XH: Writing – original draft, Data curation. XL: Writing – review & editing, Supervision, Investigation, Conceptualization.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2024.1404753/full#supplementary-material
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: Cancer J Clin. (2021) 71:209–49. doi: 10.3322/caac.21660
2. Siegel RL, Giaquinto AN, Jemal A. Cancer statistics, 2024. CA: Cancer J Clin. (2024) 74:12–49. doi: 10.3322/caac.21820
3. Brookman-May SD, May M, Shariat SF, Novara G, Zigeuner R, Cindolo L, et al. Time to recurrence is a significant predictor of cancer-specific survival after recurrence in patients with recurrent renal cell carcinoma–results from a comprehensive multi-centre database (Corona/Saturn-project). BJU Int. (2013) 112:909–16. doi: 10.1111/bju.12246
4. Lyskjær I, Iisager L, Axelsen CT, Nielsen TK, Dyrskjøt L, Fristrup N. Management of renal cell carcinoma: promising biomarkers and the challenges to reach the clinic. Clin Cancer Res. (2024) 30:663–72. doi: 10.1158/1078–0432.Ccr-23–1892
5. Ljungberg B, Albiges L, Abu-Ghanem Y, Bedke J, Capitanio U, Dabestani S, et al. European association of urology guidelines on renal cell carcinoma: the 2022 update. Eur Urol. (2022) 82:399–410. doi: 10.1016/j.eururo.2022.03.006
6. Fotia G, Stellato M, Guadalupi V, Sepe P, Claps M, Giannatempo P, et al. Current status of predictive biomarker development in metastatic renal cell carcinoma. Curr Oncol Rep. (2023) 25:671–7. doi: 10.1007/s11912–023-01395–4
7. Diakos CI, Charles KA, McMillan DC, Clarke SJ. Cancer-related inflammation and treatment effectiveness. Lancet Oncol. (2014) 15:e493–503. doi: 10.1016/s1470–2045(14)70263–3
8. Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. (2008) 454:436–44. doi: 10.1038/nature07205
9. Xu C, Wu F, Du L, Dong Y, Lin S. Significant association between high neutrophil-lymphocyte ratio and poor prognosis in patients with hepatocellular carcinoma: A systematic review and meta-analysis. Front Immunol. (2023) 14:1211399. doi: 10.3389/fimmu.2023.1211399
10. Zhu M, Feng M, He F, Han B, Ma K, Zeng X, et al. Pretreatment neutrophil-lymphocyte and platelet-lymphocyte ratio predict clinical outcome and prognosis for cervical cancer. Clinica chimica Acta. (2018) 483:296–302. doi: 10.1016/j.cca.2018.05.025
11. Okadome K, Baba Y, Yagi T, Kiyozumi Y, Ishimoto T, Iwatsuki M, et al. Prognostic nutritional index, tumor-infiltrating lymphocytes, and prognosis in patients with esophageal cancer. Ann Surg. (2020) 271:693–700. doi: 10.1097/sla.0000000000002985
12. Hu B, Yang XR, Xu Y, Sun YF, Sun C, Guo W, et al. Systemic immune-inflammation index predicts prognosis of patients after curative resection for hepatocellular carcinoma. Clin Cancer Res. (2014) 20:6212–22. doi: 10.1158/1078–0432.Ccr-14–0442
13. Zheng J, Peng L, Zhang S, Liao H, Hao J, Wu S, et al. Preoperative systemic immune-inflammation index as a prognostic indicator for patients with urothelial carcinoma. Front Immunol. (2023) 14:1275033. doi: 10.3389/fimmu.2023.1275033
14. Hu X, Shao YX, Yang ZQ, Dou WC, Xiong SC, Li X. Preoperative systemic immune-inflammation index predicts prognosis of patients with non-metastatic renal cell carcinoma: A propensity score-matched analysis. Cancer Cell Int. (2020) 20:222. doi: 10.1186/s12935-020-01320-w
15. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the Prisma statement. PLoS Med. (2009) 6:e1000097. doi: 10.1371/journal.pmed.1000097
16. Ades AE, Lu G, Higgins JP. The interpretation of random-effects meta-analysis in decision models. Med decision making. (2005) 25:646–54. doi: 10.1177/0272989x05282643
17. Lehrer EJ, Singh R, Wang M, Chinchilli VM, Trifiletti DM, Ost P, et al. Safety and survival rates associated with ablative stereotactic radiotherapy for patients with oligometastatic cancer: A systematic review and meta-analysis. JAMA Oncol. (2021) 7:92–106. doi: 10.1001/jamaoncol.2020.6146
18. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ (Clinical Res ed). (2003) 327:557–60. doi: 10.1136/bmj.327.7414.557
19. Anpalakhan S, Signori A, Cortellini A, Verzoni E, Giusti R, Aprile G, et al. Using peripheral immune-inflammatory blood markers in tumors treated with immune checkpoint inhibitors: an invidia-2 study sub-analysis. iScience. (2023) 26:107970. doi: 10.1016/j.isci.2023.107970
20. Korkmaz M, Erylmaz MK. Systemic inflammatory markers predicting the overall survival of patients using tyrosine kinase inhibitors in the first-line treatment of metastatic renal cell carcinoma. J Coll Physicians Surgeons–Pakistan: JCPSP. (2023) 33:653–8. doi: 10.29271/jcpsp.2023.06.653
21. Li PH, Lyu JL, Yue JB. Relationship between platelet-to-lymphocyte ratio and systemic immune-inflammation index and prognostic outcomes in patients with advanced renal cell carcinoma treated with immune checkpoint inhibitors. Chin J Cancer Prev Treat. (2022) 29:523–30.
22. Stuhler V, Herrmann L, Rausch S, Stenzl A, Bedke J. Role of the systemic immune-inflammation index in patients with metastatic renal cell carcinoma treated with first-line ipilimumab plus nivolumab. Cancers. (2022) 14. doi: 10.3390/cancers14122972
23. Yucel KB, Yekeduz E, Karakaya S, Tural D, Erturk I, Erol C, et al. The relationship between systemic immune inflammation index and survival in patients with metastatic renal cell carcinomatreated withtyrosine kinase inhibitors. Sci Rep. (2022) 12:16559. doi: 10.1038/s41598-022-20056-3
24. Bugdayci Basal F, Karacin C, Bilgetekin I, Oksuzoglu OB. Can systemic immune-inflammation index create a new perspective for the Imdc scoring system in patients with metastatic renal cell carcinoma? Urologia internationalis. (2021) 105:666–73. doi: 10.1159/000513456
25. Rebuzzi SE, Signori A, Banna GL, Maruzzo M, De Giorgi U, Pedrazzoli P, et al. Inflammatory indices and clinical factors in metastatic renal cell carcinoma patients treated with nivolumab: the development of a novel prognostic score (Meet-Uro 15 study). Ther Adv Med Oncol. (2021) 13. doi: 10.1177/17588359211019642
26. Teishima J, Inoue S, Hayashi T, Matsubara A, Mita K, Hasegawa Y, et al. Impact of the systemic immune-inflammation index for the prediction of prognosis and modification of the risk model in patients with metastatic renal cell carcinoma treated with first-line tyrosine kinase inhibitors. Can Urological Assoc J. (2020) 14. doi: 10.5489/cuaj.6413
27. Chrom P, Zolnierek J, Bodnar L, Stec R, Szczylik C. External validation of the systemic immune-inflammation index as a prognostic factor in metastatic renal cell carcinoma and its implementation within the international metastatic renal cell carcinoma database consortium model. Int J Clin Oncol. (2019) 24:526–32. doi: 10.1007/s10147-018-01390-x
28. De Giorgi U, Procopio G, Giannarelli D, Sabbatini R, Bearz A, Buti S, et al. Association of systemic inflammation index and body mass index with survival in patients with renal cell cancer treated with nivolumab. Clin Cancer Res. (2019) 25:3839–46. doi: 10.1158/1078–0432.Ccr-18–3661
29. Lolli C, Basso U, Derosa L, Scarpi E, Sava T, Santoni M, et al. Systemic immune-inflammation index predicts the clinical outcome in patients with metastatic renal cell cancer treated with sunitinib. Oncotarget. (2016) 7:54564–71. doi: 10.18632/oncotarget.10515
30. Monteiro FSM, Fiala O, Massari F, Myint ZW, Kopecky J, Kucharz J, et al. Systemic immune-inflammation index in patients treated with first-line immune combinations for metastatic renal cell carcinoma: insights from the Aron-1 study. Clin genitourinary Cancer. (2024) 22:305–14.e3. doi: 10.1016/j.clgc.2023.11.013
31. Chevrier S, Levine JH, Zanotelli VRT, Silina K, Schulz D, Bacac M, et al. An immune atlas of clear cell renal cell carcinoma. Cell. (2017) 169:736–49.e18. doi: 10.1016/j.cell.2017.04.016
32. Díaz-Montero CM, Rini BI, Finke JH. The immunology of renal cell carcinoma. Nat Rev Nephrol. (2020) 16:721–35. doi: 10.1038/s41581–020-0316–3
33. Teishima J, Inoue S, Hayashi T, Matsubara A. Current status of prognostic factors in patients with metastatic renal cell carcinoma. Int J Urol. (2019) 26:608–17. doi: 10.1111/iju.13956
34. Wang Y, Ni Q. Prognostic and clinicopathological significance of systemic immune-inflammation index in cancer patients receiving immune checkpoint inhibitors: A meta-analysis. Ann Med. (2023) 55:808–19. doi: 10.1080/07853890.2023.2181983
35. Jaillon S, Ponzetta A, Di Mitri D, Santoni A, Bonecchi R, Mantovani A. Neutrophil diversity and plasticity in tumour progression and therapy. Nat Rev Cancer. (2020) 20:485–503. doi: 10.1038/s41568-020-0281-y
Keywords: prognostic value, systemic immune-inflammation index, metastatic renal cell carcinoma, systemic therapy, meta-analysis
Citation: Xu J, Chen P, Cao S, Hu X and Li X (2024) Prognostic value of systemic immune-inflammation index in patients with metastatic renal cell carcinoma treated with systemic therapy: a meta-analysis. Front. Oncol. 14:1404753. doi: 10.3389/fonc.2024.1404753
Received: 21 March 2024; Accepted: 05 June 2024;
Published: 19 June 2024.
Edited by:
Walter J. Storkus, University of Pittsburgh, United StatesReviewed by:
Leonard Joseph Appleman, University of Pittsburgh, United StatesCopyright © 2024 Xu, Chen, Cao, Hu and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiang Li, eGlhbmdsaS44N0AxNjMuY29t
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.