- 1Department of Hematology and Rheumatology, The Affiliated Hospital of Putian University, Putian, Fujian, China
- 2Department of Oncology, The First Affiliated Hospital of Gannan Medical University, Jiangxi Clinical Research Center for Cancer, Ganzhou, Jiangxi, China
- 3Department of Hematology, The First School of Clinical Medicine, Guangdong Medical University, Dongguan, Guangdong, China
- 4Department of Hematology, Fujian Medical University Union Hospital, Fuzhou, Fujian, China
Background: Extranodal Non-Hodgkin lymphoma (NHL) is more prevalent in the gastrointestinal (GI) tract than in other sites. This study aimed to explore the clinical features and prognostic factors of primary intestinal diffuse large B-cell lymphoma (PI-DLBCL), in order to provide new references for basic research and clinical diagnosis and treatment of the rare extranodal malignant lymphoma.
Methods: The clinical data of 88 patients with PI-DLBCL admitted to Fujian Medical University Union Hospital from June 2011 to June 2022 were retrospectively studied, the clinical and pathological features, diagnosis and treatment process and prognosis of PI-DLBCL were analyzed, and univariate and multivariate analysis of prognostic factors was carried out. The Kaplan-Meier method was used for survival analysis. Meanwhile, the latest literature from PubMed was retrieved to systematically discuss the research progress in the diagnosis and treatment of PI-DLBCL.
Results: Among the 88 patients with PI-DLBCL included in this study, 60 cases were males (68.18%), 28 cases were females (31.82%), and 62 patients (70.45%) were complaining of abdominal pain, and the second most common clinical manifestation was changes in bowel habits in 16 (18.18%), with a median age of onset of 57 (17–82) years. The first-line treatment regimen was surgery combined with R-CHOP chemotherapy (56.82%). The median follow-up time was 72 (1–148) months, 51 (57.95%) of 88 patients with PI-DLBCL survived, 30 patients (34.09%) died, 7 patients (7.95%) were lost to follow-up, and the PFS rates of 1-year, 3-year and 5-year were 57.95%, 29.55% and 15.91%, and the OS rates of 1-year, 3-year and 5-year were 79.55%, 45.45% and 28.41%, respectively. The results of univariate Cox regression analysis showed that ECOG score, Lugano stage, B symptoms, IPI score, white blood cells, serum LDH, albumin, β2 microglobulin were the influencing factors of OS in PI-DLBCL patients, and ECOG score, Lugano stage, B symptoms, IPI score, white blood cells, serum LDH, albumin, β2 microglobulin were all the influencing factors of PFS in PI-DLBCL patients. The results of multivariate Cox analysis showed that Lugano stage may be an independent prognostic factor affecting OS and PFS in PI-DLBCL patients.
Conclusion: PI-DLBCL is more common in middle-aged and elderly men, clinical manifestations lack specificity, first-line treatment is mainly surgery combined with standard chemotherapy regimens. The Lugano stage may be an independent prognostic factor affecting OS and PFS in PI-DLBCL patients.
Introduction
Non-Hodgkin lymphoma (NHL) is a malignant tumor derived from immune cells. NHL is one of the most common hematolymphoid tumors worldwide, ranking eighth in the cause of death from cancer in the United States (1, 2). The gastrointestinal tract is the most frequent extranodal location of NHL. However, gastrointestinal lymphoma is a rare tumor, which accounts for only 10–15% of all NHL and 1–4% of gastrointestinal tumors (3, 4). Gastrointestinal lymphomas are mainly found in the stomach, followed by the small intestine and colorectum. Esophageal lymphomas are extremely rare, and the intestine is the second most commonly affected site after the stomach (5, 6). Primary intestinal lymphomas (PIL), including those of the small and large intestine, account for 20–30% of all gastrointestinal lymphomas, and the incidence is on the rise (1).
More than 50 different subtypes have been listed in the latest World Health Organization (WHO) Classification of Tumors of Hematopoietic and Lymphoid Tissues, follicular lymphoma is the most common indolent lymphoma, while diffuse large B-cell lymphoma is the most common aggressive NHL. Primary intestinal diffuse large B-cell lymphoma (PI-DLBCL) is malignancy that originates from the lymphocytes of the intestine. Diagnostic criteria of PI-DLBCL refer to Dawson’s diagnostic criteria (7). Most PI-DLBCL patients present with non-specific symptoms of the digestive system. The common diagnostic methods include ultrasound, contrast, CT, PET-CT, MRI, and endoscopy (8, 9). The treatment of PI-DLBCL include surgery, radiotherapy, chemotherapy and targeted therapy. Previous studies have shown that perforation, age ≥65 years, advanced stage, non-GCB type, EBER positivity, PD-L1 expression, and double expressor are prognostic factors in patients with PI-DLBCL (10, 11).
Nowadays, the academic research on PI-DLBCL is extremely limited, and there is no conclusion on the best treatment of PI-DLBCL. Therefore, the clinical data of 88 patients with PI-DLBCL were retrospectively analyzed in this study, and the latest literature was searched to systematically discuss the clinical characteristics, diagnosis and differentiation, treatment and prognosis of PI-DLBCL, so as to provide more basis for diagnosis and treatment of PI-DLBCL.
Materials and methods
Diagnostic method
In all patients included in this study pathological biopsy tissue was obtained at colonoscopy or abdominal surgery. All of them underwent bone marrow puncture biopsy after admission to determine the presence of bone marrow infiltration. Necessary laboratory tests were carried out. Comprehensive diagnosis of PI-DLBCL was performed after the above medical detection results were reported.
Diagnostic criteria
Diagnostic criteria refer to Dawson’s diagnostic criteria, including: (1) unobserved superficial lymph nodes; (2) No mediastinal lymph node enlargement was found on chest radiographs; (3) Normal total white blood cell count; (4) Gastrointestinal tract and local lymph nodes were mainly involved, and no other lymph nodes were involved; (5) There was no evidence of liver and spleen involvement. Patients with elevated white blood cell counts due to neutrophilia but who meet all other Dawson criteria are also classified as PI-DLBCL.
Inclusion criteria and exclusion criteria
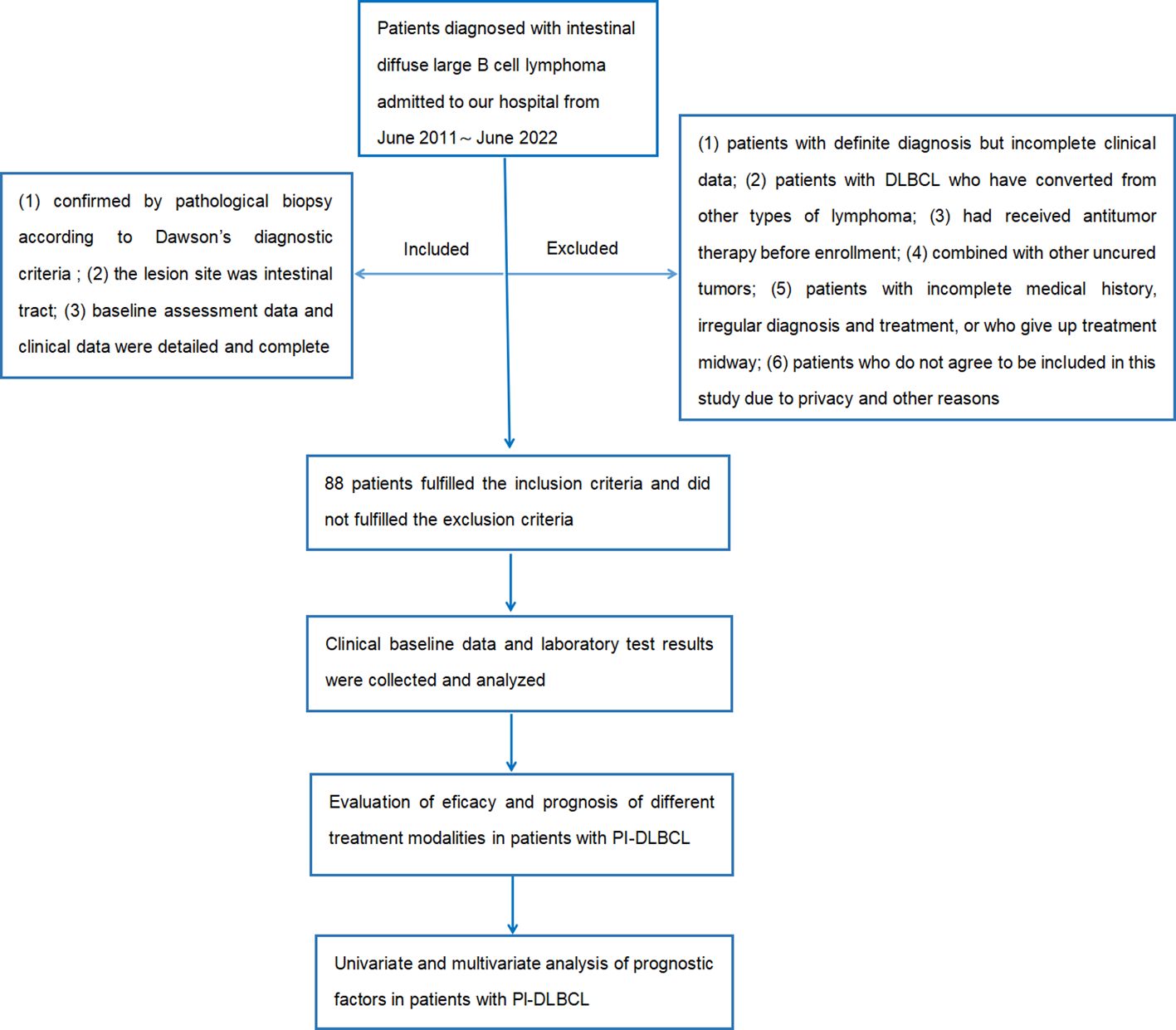
Inclusion criteria: (1) confirmed by pathological biopsy according to Dawson's diagnostic criteria; (2) the lesion site was intestinal tract; (3) baseline assessment data and clinical data were detailed and complete.
Exclusion criteria: (1) patients with definite diagnosis but incomplete clinical data; (2) patients with DLBCL who have converted from other types of lymphoma; (3) had received antitumor therapy before enrollment; (4) combined with other uncured tumors; (5) patients with incomplete medical history, irregular diagnosis and treatment, or who give up treatment midway; (6) patients who do not agree to be included in this study due to privacy and other reasons.
Therapeutic schedule (regimen)
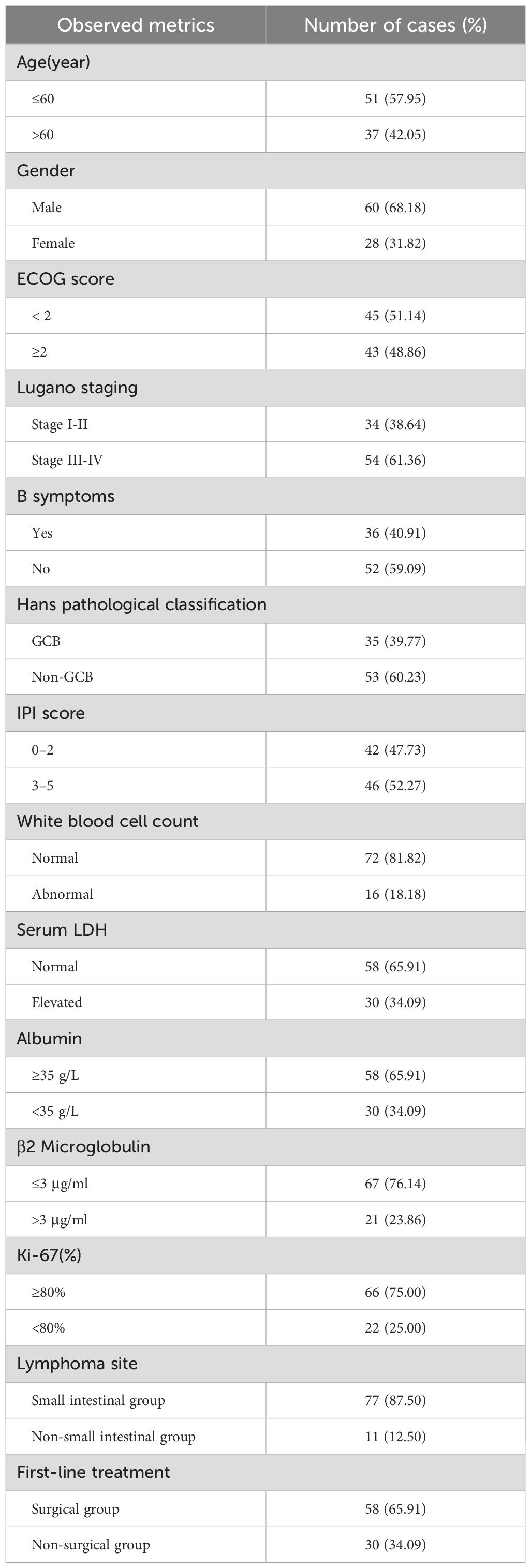
The main first-line treatment was operation combined with chemotherapy. 8 patients (9.09%) received operation alone, 30 patients (34.09%) received chemotherapy alone, and 50 patients (56.82%) received combination therapy. There are total 80 patients with PI-DLBCL received chemotherapy at the beginning, among them, 55 patients were treated with R-CHOP regimen, 7 patients received CHOP regimen, 5 patients were treated with R-miniCHOP regimen, 3 patients received R-DAEPOCH regimen, 2 patients were treated with Chidamide combined with R-CHOP regimen, 2 patients were treated with R2-CHOP regimen, 2 patients received R-COP regimen, 4 patients received E-CHOP, CP, RDHAP, PD-1 combined with R-miniCHOP regimen each. Details of specific chemotherapy regimen and usage of chemotherapy drugs are shown in Table 1.
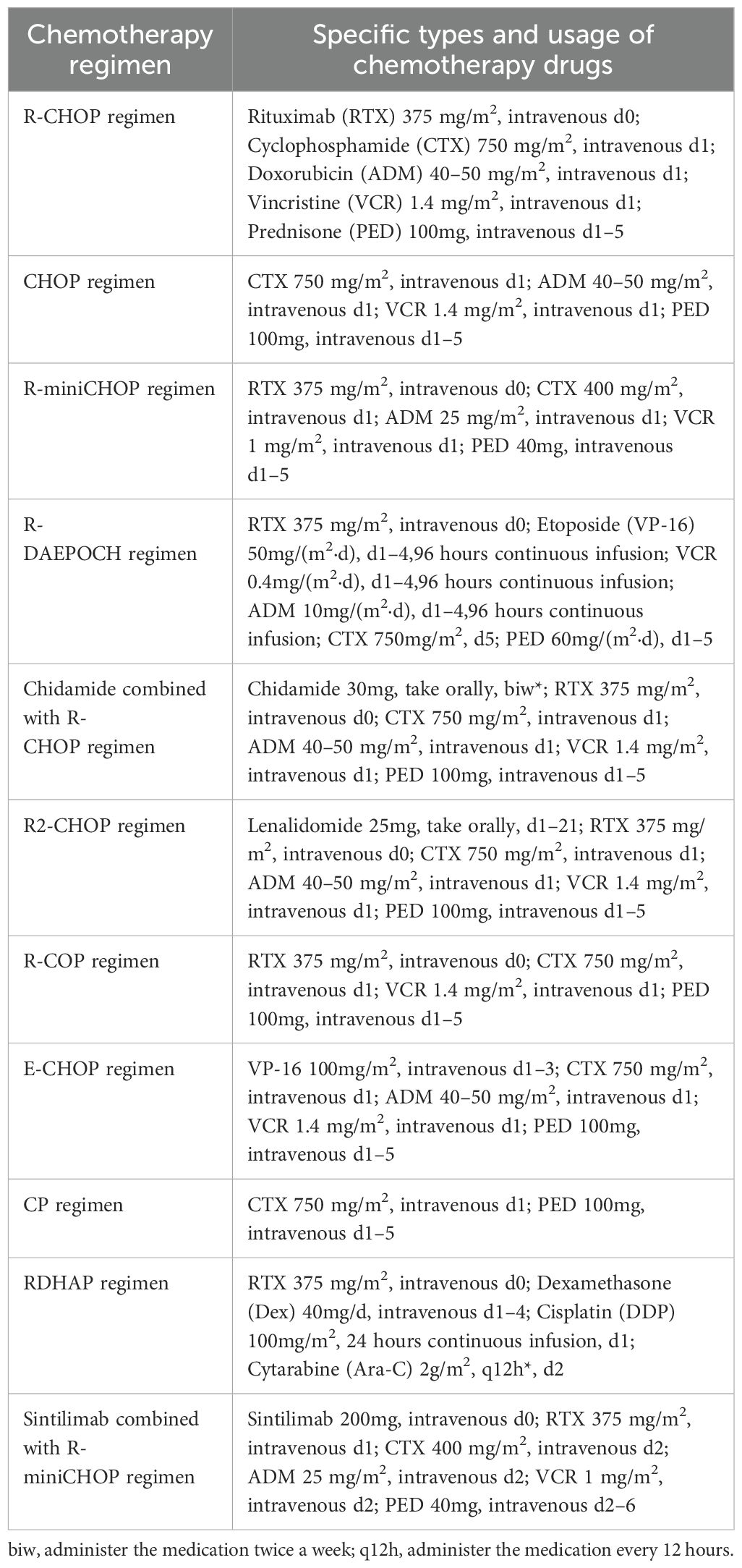
Table 1. Chemotherapy regimens and usage in 80 patients with PI-DLBCL received chemotherapy at the beginning.
Curative effect evaluation
The 2014 edition of Lugano evaluation criteria was adopted for evaluation. Firstly, complete remission (CR): PET-CT has a Deauville score of 1–3 or CT examination showed that the lesion diameter was less than 1.5 cm. Secondly, partial remission (PR): PET-CT has a Deauville score of 4–5 associated with decreased metabolism from baseline or CT examination showed that the total PPD (long diameter times short diameter) of up to 6 target lesions was reduced by ≥50%.Thirdly, stable disease (SD): PET-CT has a Deauville score of 4–5,and there was no significant change in metabolism from baseline, or CT examination showed that the total PPD (long diameter times short diameter) of up to 6 target lesions was increased by <50%.Finally, progression disease (PD): PET-CT has a Deauville score of 4–5 associated with increased metabolism from baseline or new lesions with increased metabolism appear, or CT examination showed that PPD was increased by ≥50% or the length of the lesion was more than 1.5 cm in at least one lesion, or the length or short diameter of the lesion was increased by 0.5 cm (lesion ≤ 2 cm) or 1 cm (lesion > 2 cm) when it was the smallest in at least one lesion.
Efficacy evaluation and follow-up
Follow-up data were obtained and recorded by consulting outpatient and inpatient medical records and telephone follow-up. The start time of observation was the date of the patient’s first diagnosis, and the end date of observation was set as May 30, 2023 or the patient’s death, and the end date of observation for the patients who were lost to follow-up was the date of the last follow-up. The follow-up frequency was to follow up the latest survival situation of patients once a month. Median follow-up was 72 (1–148) months. OS was the time from diagnosis to death or last follow-up, and PFS was the time from diagnosis to first recurrence or progression or death, both measured in months.
Statistical analysis
IBM SPSS 26.0 statistical software was used for statistical analysis of the collected clinical data. Chi-square test was used for comparison between the classification data groups, the Kaplan−Meier method was used for survival analysis, univariate and multivariate analysis of prognostic factors was carried out. Survival curves were drawn with GraphPad Prism 9.0 software. The significance level was set at P < 0.05.
The flow chart of the design and screening sources for this study is shown in Figure 1.
Results
Clinical data
In this study, the clinical data of 88 cases of patients with PI-DLBCL admitted to the Fujian Medical University Union Hospital from June 2011 to June 2022 were selected as the study objects. Among them, 60 were males and 28 were females. Age ranged from 18 to 82 years, with a median age of 57 years. Clinical baseline data such as age at first diagnosis, gender, ECOG score, Lugano stage, B symptoms, lymphoma site, pathological subtype, and IPI score of enrolled patients were collected and analyzed. Meanwhile, laboratory test results of leukocytes, serum lactate dehydrogenase, albumin, β2 microglobulin, Ki-67 and first-line treatment methods were collected and analyzed. The baseline data of all PI-DLBCL patients included in this study are shown in Table 2. This study was approved by the Medical Ethics Committee of Fujian Medical University Union Hospital (approval number: 2023KY060), and all patients and their families gave informed consent to this study.
According to inclusion and exclusion criteria, combined with follow-up, a total of 88 patients were included in this study, of which 51 patients (57.95%) survived, 30 patients (34.09%) died, and 7 patients (7.95%) were lost to follow-up. There were 60 cases of males (68.18%) and 28 cases of females (31.82%), with a male to female ratio of 2.1:1. According to the Lugano stage, the clinical stage was mostly advanced (stage III-IV), with 33 patients (37.50%) of stage I-II and 55 patients (62.50%) of stage III-IV. The main complaint was abdominal pain in 62 patients (70.45%), bowel habit change in 16 patients (18.18%) and other complaints in 10 patients (11.37%). The median age of onset was 57 (17–82) years old. FISH analysis suggested double-hit DLBCL in 2 patients and tripple-hit DLBCL in 2 patients. The main first-line treatment was operation combined with chemotherapy. 8 patients (9.09%) received operation alone, 30 patients (34.09%) received chemotherapy alone, and 50 patients (56.82%) received combination therapy.
According to different first-line treatment methods, the two groups were divided into operation group (including operation alone and operation combined with chemotherapy) and non-operation group. Evaluation of efficacy after at least 2 courses of treatment, for PI-DLBCL patients (P=0.789), there was no statistically significant difference in the efficacy of surgery, and the results were shown in Table 3.
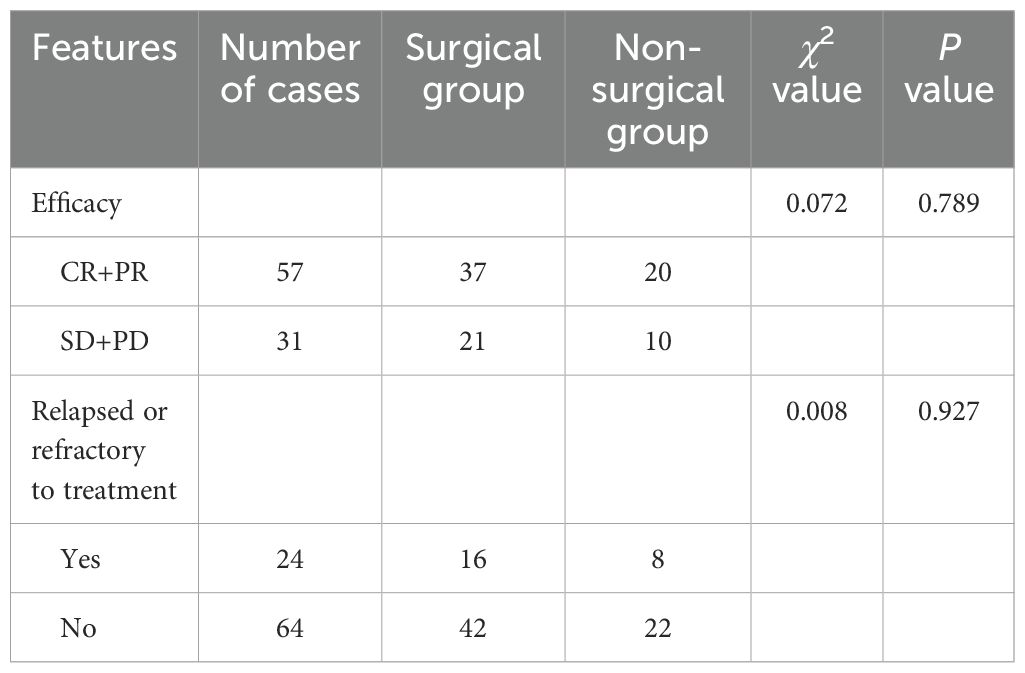
Table 3. Clinical efficacy and prognosis of different treatment modalities in patients with PI-DLBCL.
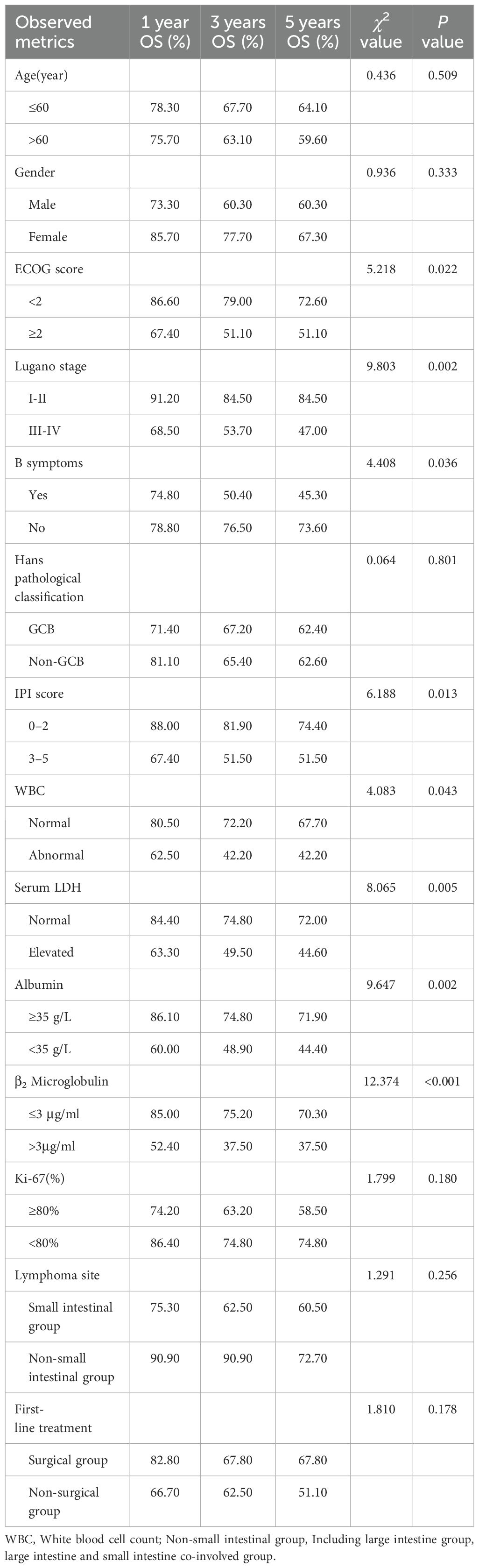
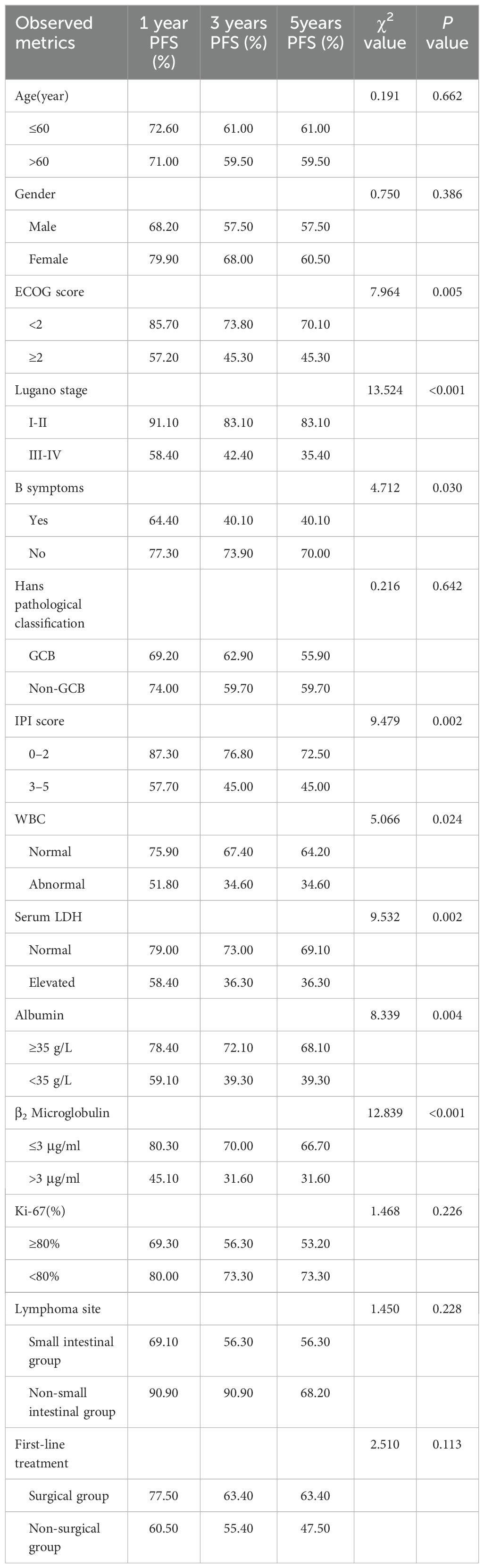
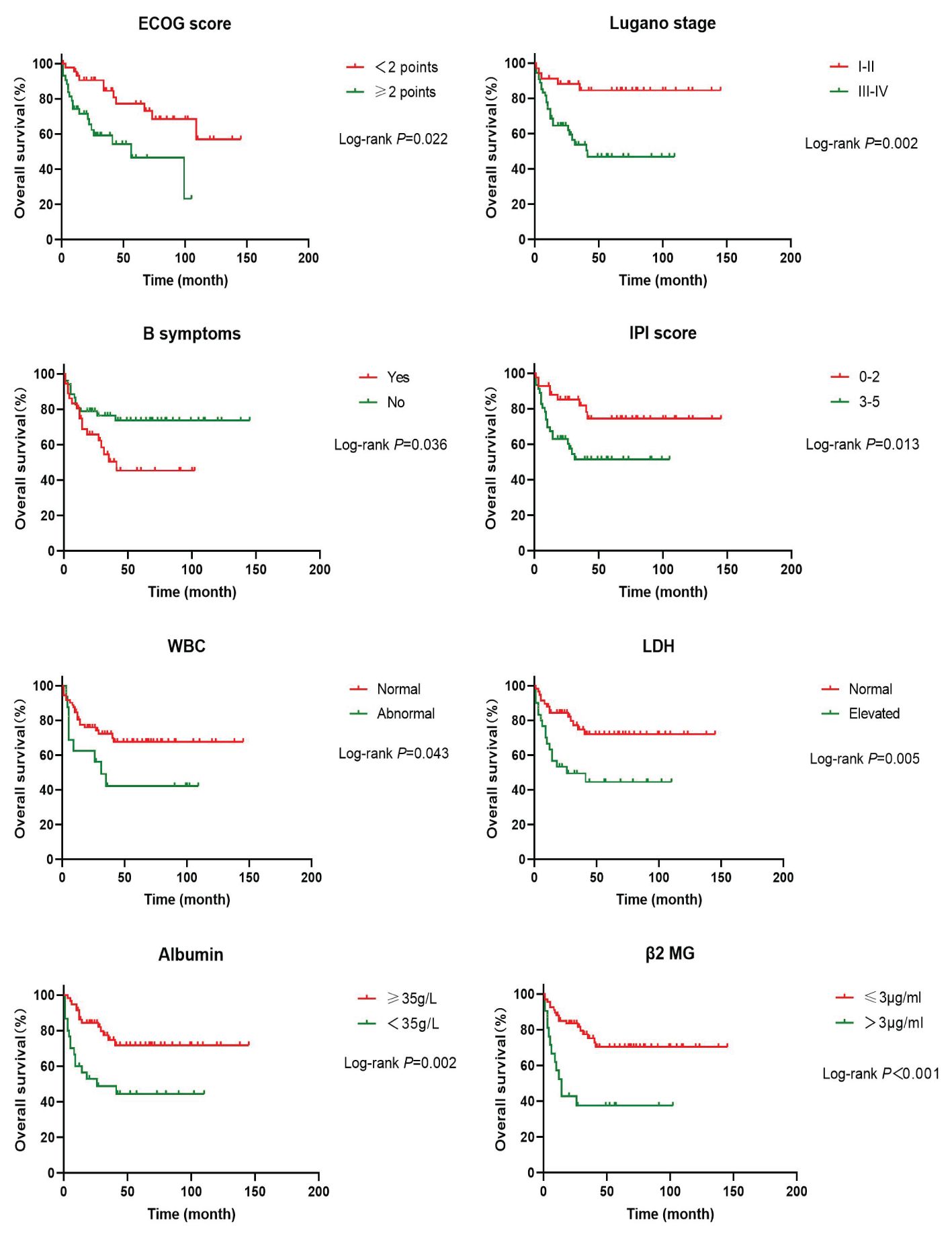
In order to investigate the effects of various influencing factors on the survival of 88 patients with PI-DLBCL, log-rank test was used for each indicator, and the results are shown in Tables 4, 5. The results in the table showed that ECOG score, Lugano stage, B symptoms, IPI score, white blood cells, serum LDH, albumin, and β2 microglobulin had an impact on the OS of patients. ECOG score, Lugano stage, B symptom, IPI score, leukocyte, serum LDH, albumin and β2 microglobulin had influence on PFS of patients. The survival curve was drawn by Kaplan-Meier method, and the results were shown in Figures 2, 3.
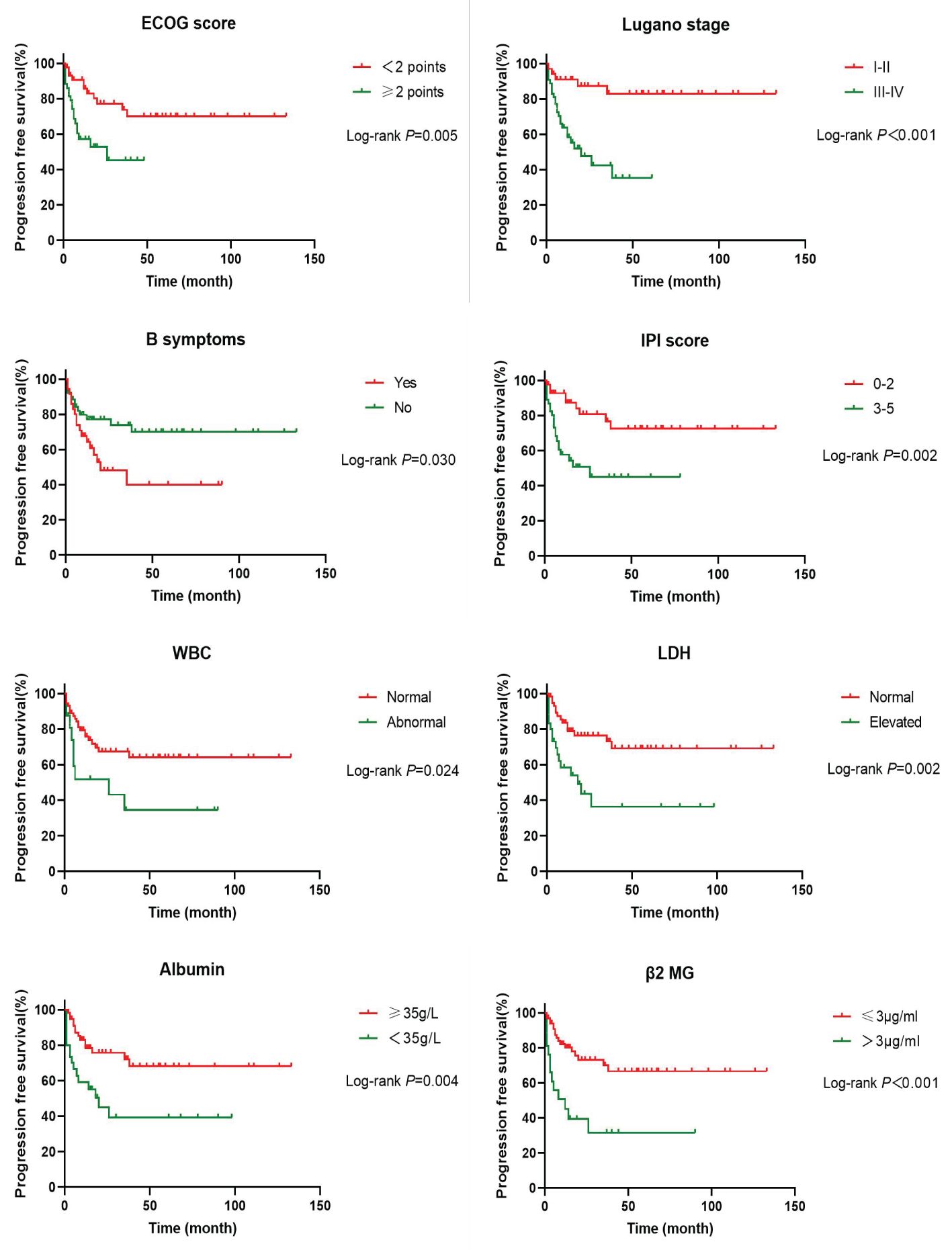
Figure 3. Kaplan-Meier progression free survival curves for prognostic factors in PI-DLBCL patients.
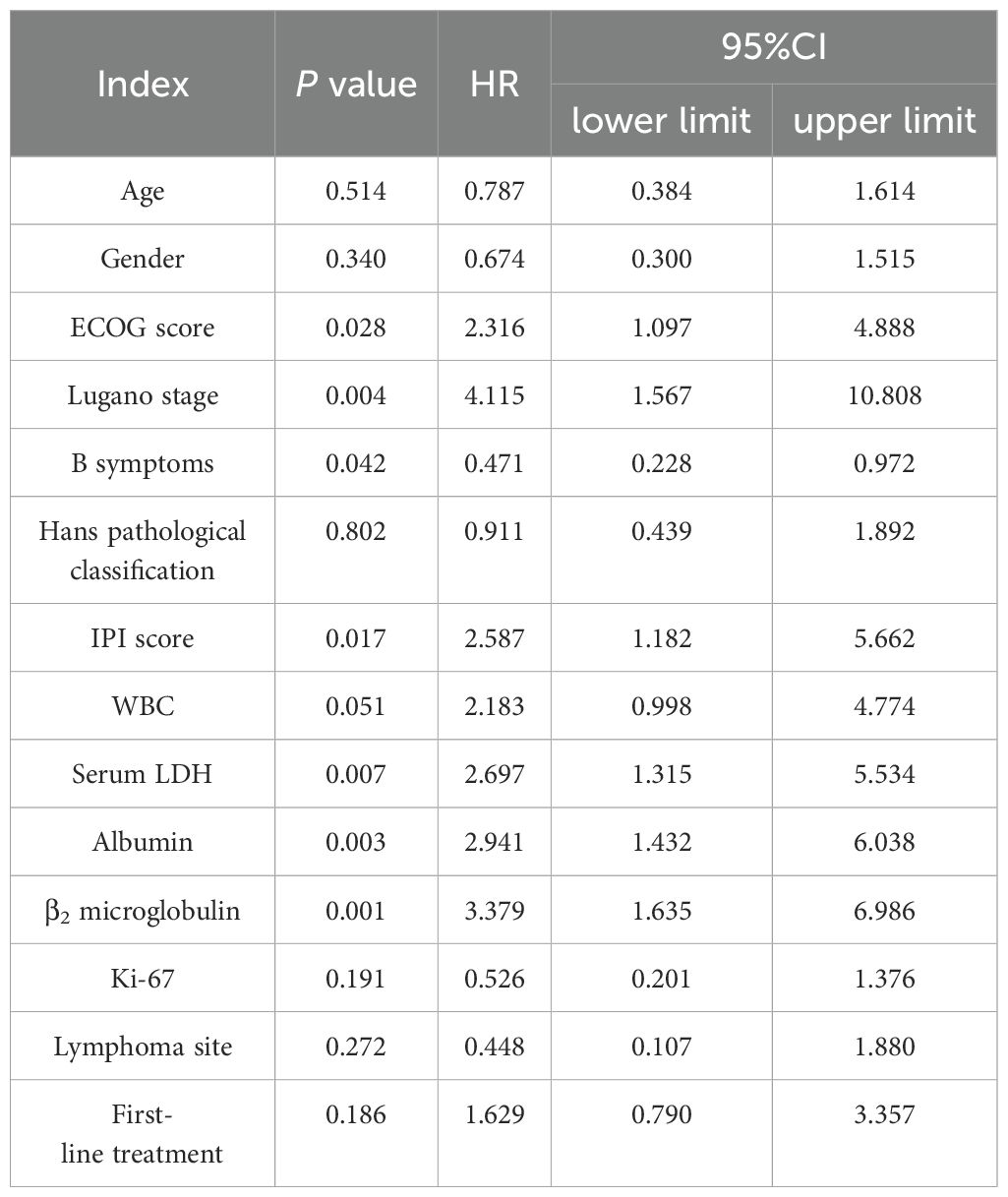
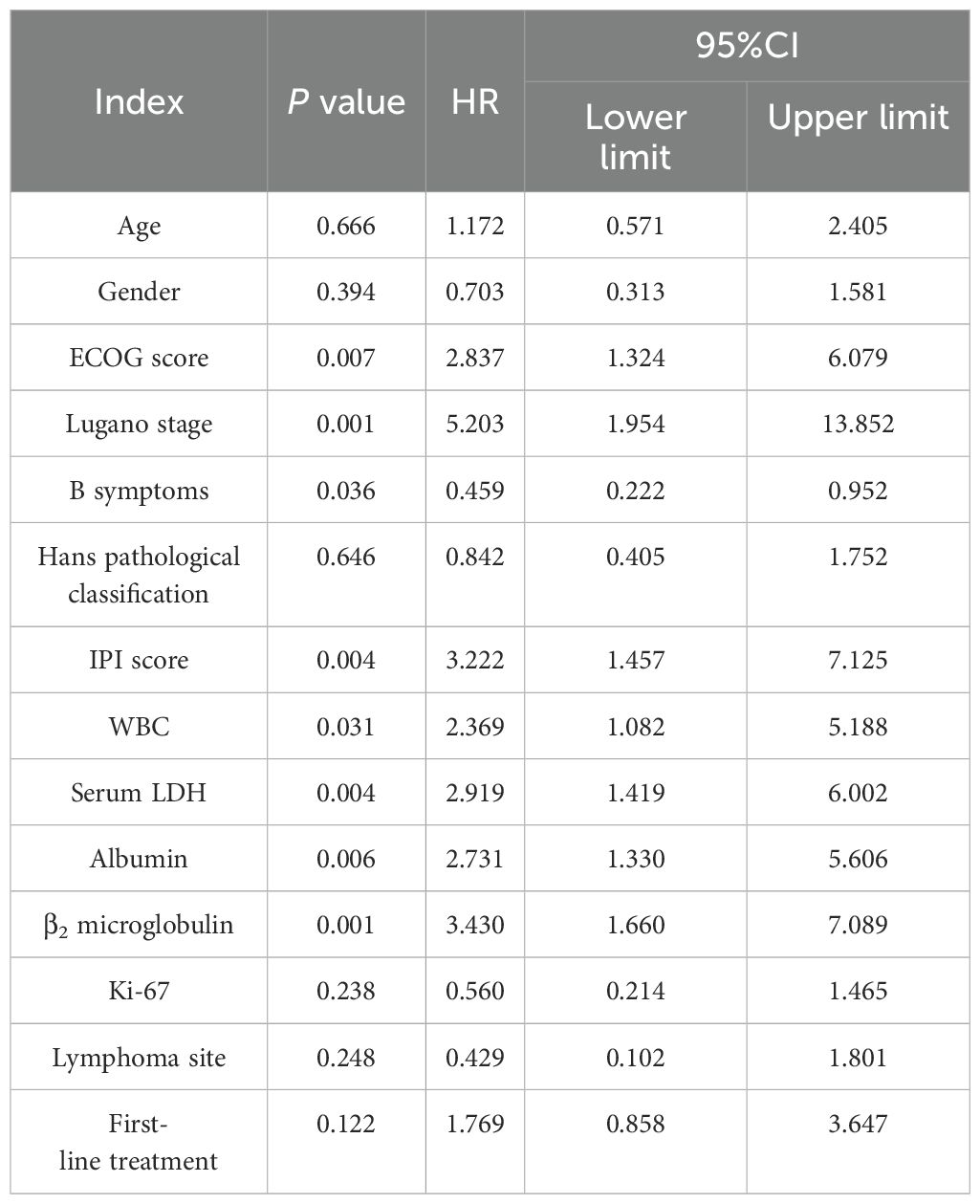
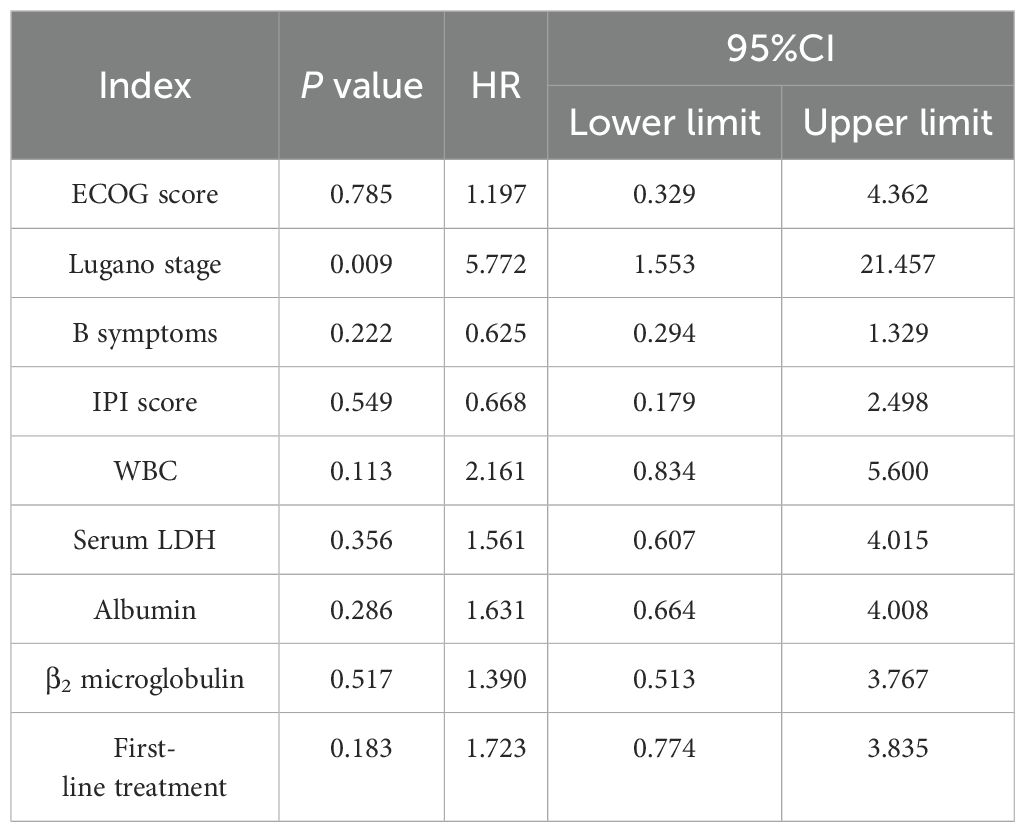
Age, sex, ECOG score, Lugano stage, B symptoms, pathological subtypes, IPI score, leukocyte, serum LDH, albumin, β2 microglobulin, Ki-67, lymphoma site, first-line treatment and other indicators of 88 PI-DLBCL patients were included in the COX regression model for OS univariate analysis. ECOG score, Lugano stage, B symptoms, IPI score, serum LDH, albumin, and β2 microglobulin were the influencing factors for OS in PI-DLBCL patients (Table 6). Variables with P < 0.20 in univariate COX regression analysis were included in the multivariate analysis model, and the results suggested that Lugano stage was an independent prognostic factor for OS in PI-DLBCL patients (Table 7).
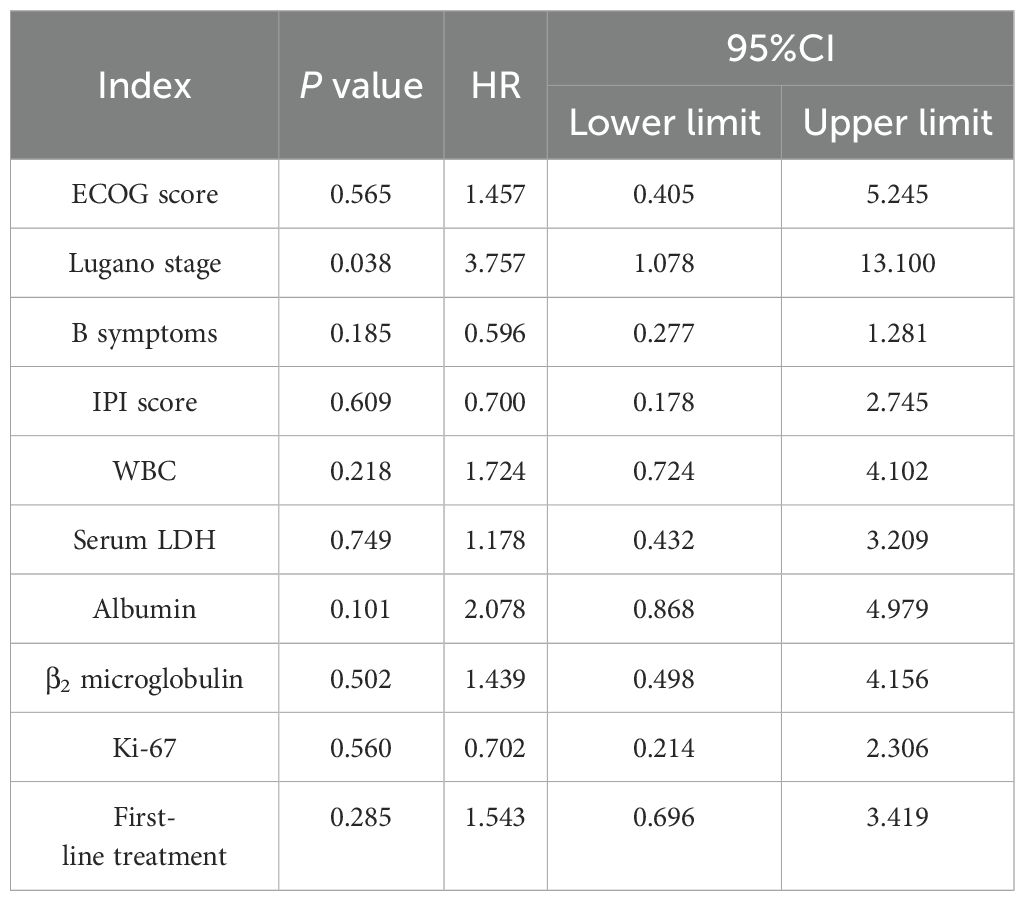
Age, sex, ECOG score, Lugano stage, B symptoms, pathological subtypes, IPI score, leukocyte, serum LDH, albumin, β2 microglobulin, Ki-67, lymphoma site, first-line treatment and other indicators of 88 PI-DLBCL patients were included in the COX regression model for PFS univariate analysis. ECOG score, Lugano stage, B symptoms, IPI score, leukocyte, serum LDH, albumin, and β2 microglobulin were the influencing factors for PFS in PI-DLBCL patients (Table 8). Variables with P < 0.20 in univariate COX regression analysis were included in the multivariate analysis model, and the results showed that Lugano stage was an independent prognostic factor affecting PFS in PI-DLBCL patients (Table 9).
Literature review
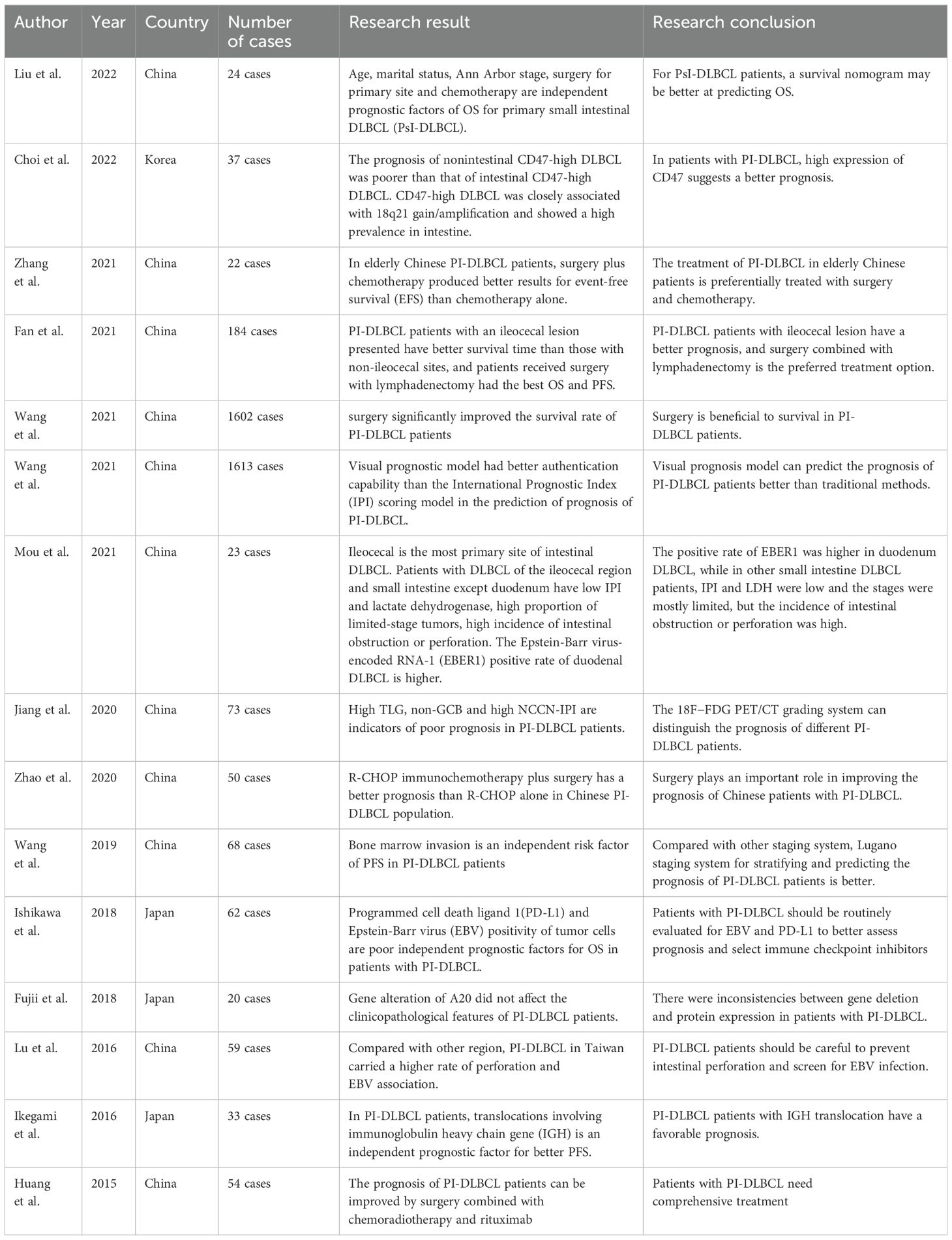
As shown in Table 10, the existing retrospective studies on PI-DLBCL from PubMed database in the past 10 years have been systematically listed and reviewed. The clinicopathological features and prognosis of PI-DLBCL patients in different regions were compared in various aspects. We can draw a conclusion that age, stage, treatment regimen, expression of certain genes, location of primary lesion and Epstein-Barr virus infection can seriously affect the prognosis of patients with PI-DLBCL. New prognostic models are emerging, and the best staging system is still under discussion.
Discussion
Primary gastrointestinal lymphoma is a common extranodal NHL, with an incidence of 0.5–1.1 per 100,000 people in developed countries. More than 60% of cases occur in the stomach, and the intestine is the second most commonly affected site (1, 2). As the most common type of NHL, DLBCL accounts for 30%-40% of all malignant lymphomas, and the median age of onset of DLBCL patients in China is about 10 years younger than patients in Japan and the United States (12). Most PI-DLBCL patients are not easy to diagnose clinically. At present, the research on PI-DLBCL is very limited. There are many prognostic factors for PI-DLBCL, but there is still no consensus on its treatment and prognosis assessment. In this study, the clinical data of 88 patients with PI-DLBCL was analyzed retrospectively and prognostic factors were identified in order to improve the understanding of PI-DLBCL.
As the etiology and pathogenesis of PI-DLBCL are still not yet clear, the existing research results suggest that the etiology of PI-DLBCL may be related to genetics, environmental factors, viral infection, immune deficiency, inflammatory bowel disease, organ transplantation and drugs (13–16). It is noteworthy that gut-associated lymphoid tissue (GALT) is mostly located in the ileocolonic region, which can produce specific immune responses, and repeated antigen stimulation of such lymphocytes can easily lead to monoclonal proliferation of this tissue and eventually it develops into lymphoma (16).
The results of our study showed that PI-DLBCL was more common in males (68.18%), and the median age of onset was 57 (17–82) years old. The most common and earliest clinical symptom was abdominal pain, which was consistent with the results reported in the literature (17, 18). The clinical manifestations of PI-DLBCL are often abdominal pain, ascites, hepatomegaly, splenomegaly, hemorrhage, general fatigue, anorexia, emaciation, and some patients may present with acute intestinal obstruction or perforation (19, 20). Since the lack of specificity of the above clinical manifestations, the diagnosis and treatment may be delayed and lead to poor prognosis. PI-DLBCL needs to be distinguished from intestinal ulcer, various inflammatory conditions, intestinal obstruction, intestinal carcinoma and other types of intestinal lymphoma (21–23). Although colon carcinoma is the most common intestinal malignancy, the possibility of intestinal lymphoma should still be considered in patients with recurrent abdominal pain. PI-DLBCL is rare, the initial symptoms are not obvious, the signs are not typical, and the patients are often in the advanced stage when diagnosed, so the early diagnosis is very challenging.
For the diagnosis of PI-DLBCL, the common imaging diagnostic methods include ultrasound, contrast, CT, PET-CT, MRI, and so on, as well as endoscopy such as gastroenteroscopy and capsule endoscopy. These methods should be used reasonably to improve the diagnosis rate of PI-DLBCL (24). Currently, one of the most recommended tests for lymphoma patients is PET-CT, which has irreplaceable advantages. In PI-DLBCL, PET-CT shows concentration of 18F-fluorodeoxyglucose metabolism (18F-FDG) at the lesion, and the SUV value fluctuate between 3.6–33.7, which could accurately locate the enteric and external lesions of PI-DLBCL. In addition to emergency surgery, endoscopy is an important tool for the diagnosis of intestinal lymphoma. Endoscopy can visually inspect the entire diseased intestine, locate the specific location of the lesion, and biopsy can be performed to enable the correct preoperative diagnosis of PI-DLBCL. In addition, it can visually compare the lesions in the same site before and after treatment. This method can produce less trauma and lower cost, and is worth advocating and applying (25). In PI-DLBCL patients, ESR, LDH, β2-MG and other laboratory test indicators can be elevated, and Ki-67 index >90% is seen in most PI-DLBCL patients. When the bone marrow of patients with PI-DLBCL is invaded, it can cause anemia, thrombocytopenia, leukopenia or abnormal elevation (26).
Due to the high clinical diversity of intestinal DLBCL patients, the gold standard for diagnosis is histopathological biopsy, and the treatment methods include surgery, chemotherapy and immunotherapy, among which the standard first-line chemotherapy regimen is R-CHOP (rituximab combined with cyclophosphamide, Epirubicin, vindesin, dexamethasone) (27). In our study, we found that surgery is a commonly used method in the diagnosis and treatment of PI-DLBCL. Complete resection of the lesion can not only relieve the symptoms of the patient, but also reduces the risk of complications such as perforation, bleeding, obstruction or intussusception. In addition, the early pathological diagnosis and clinical staging of the biopsy tissue obtained by surgery are of guiding significance for clinical diagnosis and treatment, and will not increase the risk of death of the patient (28). Wang et al. (29) found that patients with PI-DLBCL whose lesion site was in the small intestine had a reasonable survival prognosis after surgery, and there was no difference in treatment and prognosis. Since PI-DLBCL is usually highly malignant, surgical treatment alone is not recommended. Patients with PI-DLBCL may benefit from surgery combined with chemotherapy and immunotherapy. The results of this study suggest that surgery in PI-DLBCL patients had no statistical significance in OS and PFS, which was considered to be related to the small number of study cases and could not represent the whole population.
At present, the academic consensus for the treatment of PI-DLBCL is that surgery combined with adjuvant therapy, including radiotherapy, chemotherapy and targeted therapy, can result in a better prognosis (30). However, previous studies have found that the intestine is less suitable for radiation therapy than the stomach, and chemotherapy for primary small intestinal lymphoma appears to have a survival benefit over surgery alone, while colon lymphoma favors surgery plus chemotherapy (31). Chemotherapy for NHL in the small intestine occasionally causes perforation, which is life-threatening during chemotherapy. Perforation occurs in about 9% of patients with gastrointestinal lymphoma. The risk of perforation in aggressive lymphomas is more than 6 times greater than in indolent B-cell lymphomas, and perforation occurs due to transmural damage caused by tumor or tissue necrosis after chemotherapy. A 37-year retrospective study of patients with gastrointestinal lymphoma found that 9% developed perforation, while 55% of perforations occurred after chemotherapy. The most common perforation site was the small intestine (59%), followed by the large intestine (22%) and stomach (16%) (32). Last year, a study found that DLBCL in the ileocecal area showed a characteristic mutation pattern with the most frequent TP53 mutation (52.6%) and 18q21 gain (42.1%). This provides more evidence for targeted therapy of PI-DLBCL (33). In addition, studies have also shown that surgical treatment of primary intestinal NHL before chemotherapy can prevent perforation and reduce tumor. Wong et al. (34) confirmed that total parenteral nutrition (TPN) and fasting could not reduce the risk of perforation in PIL patients receiving chemotherapy. Clinically, this complication should be considered in advance and preventive measures should be taken according to the degree of tumor burden (35).
Studies have suggested that perforation, age ≥65 years, advanced stage, non-GCB type, EBER positivity, PD-L1 expression, and double expressor NHL are associated with poor prognosis in patients with PI-DLBCL (36, 37). The patients with EBV+ PI-DLBCL showed a progressive disease despite receiving R-containing chemotherapy and underwent an autologous peripheral blood stem cell transplantation (38). Double-hit and triple-hit genetics are associated with lower rates of complete remission (CR) and, consequently, in poor OS in DLBCL patients. Therefore, patients with double-hit and triple-hit NHL need aggressive interventions (39, 40). The results of univariate Cox regression analysis in this study showed that ECOG score, Lugano stage, B symptoms, IPI score, serum LDH, albumin, β2 microglobulin were the influencing factors of OS in PI-DLBCL patients, and ECOG score, Lugano stage, B symptoms, IPI score, white blood cells, serum LDH, albumin, β2 microglobulin were all the influencing factors of PFS in PI-DLBCL patients. It has been reported in the literature that the main affected sites and treatment methods do not affect the prognosis of patients with B-cell PIL (41), and the results of this study are consistent with those reported in the literature. Multivariate Cox analysis results of this study showed that Lugano stage is an independent prognostic factor affecting OS and PFS in PI-DLBCL patients, indicating that Lugano stage was an important prognostic factor, which was consistent with literature reports (41, 42). Some studies suggested that intestinal DLBCL with expression of CD47 have a better prognosis than that without CD47 expression (43). A study on adult PI-DLBCL showed that 3-, 5-, and 10-year survival rates in the whole cohort were 68.7%, 62.4% and 50.2% (29), while another study about intestinal FL showed that 3-year PFS and 3-year OS were 70% and 100% respectively, 5-year PFS and OS were 86.3% and 100% respectively (44).
To sum up, PI-DLBCL patients are mostly middle-aged and elderly men, which early clinical manifestations, symptoms and signs lack specificity. Imaging and laboratory examinations need to be comprehensive to identify the lesions and the extent of systemic involvement, and pathology is the gold standard for diagnosis. The first-line treatment of PI-DLBCL is mainly surgery combined with chemotherapy. Lugano stage is an independent prognostic factor affecting OS and PFS in PI-DLBCL patients. In the future, this research group will cooperate with multi-center units to further explore and study the clinical characteristics, diagnosis and treatment experience of rare extranodal lymphoma.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by Ethics Committee of Fujian Medical University Union Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements.
Author contributions
XC: Formal analysis, Writing – original draft, Writing – review & editing. JW: Formal analysis, Writing – original draft, Writing – review & editing. YL: Writing – review & editing. SL: Writing – review & editing. JS: Validation, Writing – review & editing. YY: Data curation, Writing – review & editing. YW: Formal analysis, Funding acquisition, Validation, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. The Science and Technology Foundation of Jiangxi Provincial Administration of Traditional Chinese Medicine (No.2023A0351), the National Clinical Key Specialty Construction Project (Min Medical Politics 2021-76),and the Foundation of Fujian Clinical Medical Research Center for Hematologic Malignancies (2020Y2006).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Cha RR, Baek DH, Lee GW, Park SJ, Lee JH, Park JH, et al. Clinical features and prognosis of patients with primary intestinal B-cell lymphoma treated with chemotherapy with or without surgery. Korean J Gastroenterol. (2021) 78:320–7. doi: 10.4166/kjg.2021.082
2. Thandra KC, Barsouk A, Saginala K, Padala SA, Barsouk A, Rawla P. Epidemiology of non-hodgkin's lymphoma. Med Sci (Basel). (2021) 9:5. doi: 10.3390/medsci9010005
3. Tran QT, Nguyen Duy T, Nguyen-Tran BS, Nguyen-Thanh T, Ngo QT, Tran Thi NP, et al. Endoscopic and histopathological characteristics of gastrointestinal lymphoma: A multicentric study. Diagnostics (Basel). (2023) 13:2767. doi: 10.3390/diagnostics13172767
4. Tian FY, Wang JX, Huang G, An W, Ai LS, Wang S, et al. Clinical and endoscopic features of primary small bowel lymphoma: a single-center experience from mainland China. Front Oncol. (2023) 13:1142133. doi: 10.3389/fonc.2023.1142133
5. Battah A, Abed H, Shamoon D, DaCosta T, Farouji I, Fedida A. A rare case of diffuse large B-cell lymphoma presenting as a Malignant mass in both duodenum and ascending colon. Radiol Case Rep. (2022) 17:3286–90. doi: 10.1016/j.radcr.2022.06.030
6. Singh V, Gor D, Gupta V, Jacob A, Du D, Eltoukhy H, et al. Epidemiology and determinants of survival for primary intestinal non-hodgkin lymphoma: A population-based study. World J Oncol. (2022) 13:159–71. doi: 10.14740/wjon1504
7. Senyondo G, Bella S, Saleem A, Mehdi SA, Sidhu J. Ileo-colonic fistula due to diffuse large B cell lymphoma: unusual presentation of a rare disease. Cureus. (2021) 13:e12956. doi: 10.7759/cureus.12956
8. Dias E, Medas R, Marques M, Andrade P, Cardoso H, Macedo G. Clinicopathological characteristics and prognostic factors of small bowel lymphomas: a retrospective single-center study. Porto BioMed J. (2023) 8:e217. doi: 10.1097/j.pbj.0000000000000217
9. Chiang SW, Chiang TW. Colo-enteric fistula associated with diffuse large B cell lymphoma that resulted in gastrointestinal bleeding: A case report and literature review. Int J Surg Case Rep. (2022) 91:106798. doi: 10.1016/j.ijscr.2022.106798
10. Owattanapanich W, Ruchutrakool T, Pongpruttipan T, Maneerattanaporn M. A 10-year cohort study of 175 primary gastrointestinal lymphoma cases in Thailand: clinical features and outcomes in the immunochemotherapy era. Hematology. (2021) 26:249–55. doi: 10.1080/16078454.2021.1889160
11. Wang Y, Song J, Wen S, Zhang X. A visual model for prognostic estimation in patients with primary diffuse large B-cell lymphoma of small intestine and colon: analysis of 1,613 cases from the SEER database. Transl Cancer Res. (2021) 10:1842–55. doi: 10.21037/tcr-20-3086
12. Kanas G, Ge W, Quek RGW, Keeven K, Nersesyan K, Arnason JE. Epidemiology of diffuse large B-cell lymphoma (DLBCL) and follicular lymphoma (FL) in the United States and Western Europe: population-level projections for 2020-2025. Leuk Lymphoma. (2022) 63:54–63. doi: 10.1080/10428194.2021.1975188
13. Dias E, Marques M, Fonseca E, Macedo G. Clinical and pathological characterization of colon lymphomas. Dig Liver Dis. (2021) 53:672–4. doi: 10.1016/j.dld.2021.01.001
14. Small S, Barnea Slonim L, Williams C, Karmali R. B cell lymphomas of the GI tract. Curr Gastroenterol Rep. (2021) 23:9. doi: 10.1007/s11894-021-00811-8
15. Ma WL, Yeh KH, Yao M, Tang JL, Lin CW, Wang YT, et al. Comparison of clinicopathological features and treatment outcomes in aggressive primary intestinal B- and T/NK-cell lymphomas. J Formos Med Assoc. (2021) 120:293–302. doi: 10.1016/j.jfma.2020.10.001
16. Phillips F, Verstockt B, Ribaldone DG, Guerra I, Teich N, Katsanos K, et al. Diagnosis and outcome of extranodal primary intestinal lymphoma in inflammatory bowel disease: an ECCO CONFER case series. J Crohns Colitis. (2022) 16:500–5. doi: 10.1093/ecco-jcc/jjab164
17. Malipatel R, Patil M, Rout P, Correa M, Devarbhavi H. Primary intestinal lymphoma: clinicopathological characteristics of 55 patients. Euroasian J Hepatogastroenterol. (2021) 11:71–5. doi: 10.5005/jp-journals-10018-1345
18. Albrijawy R, Al Laham O, Shaheen J, Atia F, Alshiekh A. A rare case of primary non-metastatic Non-Hodgkin's diffuse large B-cell lymphoma in the ileum in a 19-year-old male manifested as intestinal obstruction- A case report. Int J Surg Case Rep. (2022) 90:106748. doi: 10.1016/j.ijscr.2021.106748
19. Hu C, Zhang H. Primary small intestinal lymphoma: A rare cause of small intestinal perforation. Asian J Surg. (2021) 44:1443–4. doi: 10.1016/j.asjsur.2021.07.020
20. Ota K, Nishida S, Akutagawa H, Imai Y, Tani K, Shinohara Y, et al. Massive ascites caused by primary diffuse large B-cell lymphoma of the jejunum: A case of a rare initial symptom. Intern Med. (2021) 60:397–402. doi: 10.2169/internalmedicine.5516-20
21. Jia N, Tang Y, Li Y. Rare Malignant ulcer related to primary intestinal diffuse large B-cell lymphoma: A case report. Med (Baltimore). (2020) 99:e18590. doi: 10.1097/MD.0000000000018590
22. Oh CH, Kim HS DO, Na K. Gastrointestinal follicular lymphoma: A single-institutional experience of 22 cases with emphasis on the comprehensive clinicopathological analysis and diagnostic re-classification. Anticancer Res. (2023) 43:4089–96. doi: 10.21873/anticanres.16598
23. Iwamuro M, Tanaka T, Ennishi D, Matsueda K, Yoshioka M, Miyahara K, et al. Long-term outcomes of patients with primary intestinal follicular lymphoma managed with watch-and-wait strategy. Sci Rep. (2023) 13:5858. doi: 10.1038/s41598-023-32736-9
24. Watanabe T. Recent advances in treatment of nodal and gastrointestinal follicular lymphoma. World J Gastroenterol. (2023) 29:3574–94. doi: 10.3748/wjg.v29.i23.3574
25. Yachida T, Matsuda T, Sakamoto T, Nakajima T, Kakugawa Y, Maeshima AM, et al. Endoscopic features of colorectal lymphoma according to histological type. JGH Open. (2022) 6:257–62. doi: 10.1002/jgh3.12738
26. Ishibashi H, Imakiire S, Goto M, Nomaru R, Shibata M, Matsuoka H, et al. Epstein-barr virus-positive intestinal diffuse large B-cell lymphoma in a Japanese patient with celiac disease: first reported case and a literature review. Intern Med. (2022) 61:329–34. doi: 10.2169/internalmedicine.7876-21
27. Bobillo S, Joffe E, Lavery JA, Sermer D, Ghione P, Noy A, et al. Clinical characteristics and outcomes of extranodal stage I diffuse large B-cell lymphoma in the rituximab era. Blood. (2021) 137:39–48. doi: 10.1182/blood.2020005112
28. Liu X, Lv T, Zhang X, Li J. Extensive small intestinal diffuse large B cell lymphoma. Rev Esp Enferm Dig. (2023) 115:267. doi: 10.17235/reed.2022.9100/2022
29. Wang M, Ma S, Shi W, Zhang Y, Luo S, Hu Y. Surgery shows survival benefit in patients with primary intestinal diffuse large B-cell lymphoma: A population-based study. Cancer Med. (2021) 10:3474–85. doi: 10.1002/cam4.3882
30. Lu PW, Fields AC, Yoo J, Irani J, Goldberg JE, Bleday R, et al. Surgical management of small bowel lymphoma. J Gastrointest Surg. (2021) 25:757–65. doi: 10.1007/s11605-020-04730-3
31. Gupta V, Singh V, Bajwa R, Meghal T, Sen S, Greenberg D, et al. Site-specific survival of extra nodal diffuse large B-cell lymphoma and comparison with gastrointestinal diffuse large B-cell lymphoma. J Hematol. (2022) 11:45–54. doi: 10.14740/jh984
32. Matsumoto M, Kasahara K, Nagakawa Y, Katsumata K, Tsuchida A. [Multiple intestinal perforations caused by diffuse large B-cell lymphoma-A case report]. Gan To Kagaku Ryoho. (2022) 49:1377–9.
33. Koh HH, Yoon SE, Kim SJ, Kim WS, Cho J. Differences in mutational signature of diffuse large B-cell lymphomas according to the primary organ. Cancer Med. (2023) 12:19732–43. doi: 10.1002/cam4.6533
34. Wong YJ, Lum HM, Fook-Chong S, Lim ST, Salazar E. Do total parenteral nutrition and bowel rest reduce the risk for perforation in patients with gastrointestinal tract lymphoma receiving chemotherapy? Nutrition. (2019) 67-68:110515. doi: 10.1016/j.nut.2019.03.023
35. Modemann F, Ahmadi P, von Kroge PH, Weidemann S, Bokemeyer C, Dierlamm J, et al. The prognostic impact of lymphoma perforation in patients with primary gastrointestinal lymphoma - a single-center analysis. Leuk Lymphoma. (2023) 64:1801–10. doi: 10.1080/10428194.2023.2240921
36. Konstantinidou S, Cosgrove C, Campbell W. FOLLICULAR LYMPHOMA OF THE RECTUM. Ulster Med J. (2020) 89:125–6.
37. Suzuki T, Iwamoto K, Nozaki R, Saiki Y, Tanaka M, Fukunaga M, et al. Diffuse large B-cell lymphoma originating from the rectum and diagnosed after rectal perforation during the treatment of ulcerative colitis: a case report. BMC Surg. (2021) 21:50. doi: 10.1186/s12893-021-01060-2
38. Ishikawa E, Nakamura M, Shimada K, Tanaka T, Satou A, Kohno K, et al. Prognostic impact of PD-L1 expression in primary gastric and intestinal diffuse large B-cell lymphoma. J Gastroenterol. (2020) 55:39–50. doi: 10.1007/s00535-019-01616-3
39. Wu CH, Gau JP, Teng CJ, Shih YH, Su YC, Wang RC, et al. Clinical features and immunophenotypes of double-hit diffuse large B-cell lymphoma. Diagnostics (Basel). (2022) 12:1106. doi: 10.3390/diagnostics12051106
40. Mehta A, Verma A, Gupta G, Tripathi R, Sharma A. Double hit and double expresser diffuse large B cell lymphoma subtypes: discrete subtypes and major predictors of overall survival. Indian J Hematol Blood Transfus. (2020) 36:627–34. doi: 10.1007/s12288-019-01248-w
41. Erkut M, Erkut N, Bektaş Ö, Fidan S, Coşar AM, Sönmez M. Effect of clinical, endoscopic, radiological findings, and complications on survival in patients with primary gastrointestinal lymphoma. Turk J Gastroenterol. (2022) 33:909–17. doi: 10.5152/tjg.2022.211003
42. Tran T, Vu TH, Vo HQ, Thi Nguyen H, Van Nguyen H. Primary gastrointestinal non-Hodgkin lymphoma: a retrospective study in Vietnam. Ann Med Surg (Lond). (2023) 85:2390–4. doi: 10.1097/MS9.0000000000000858
43. Cho J, Yoon SE, Kim SJ, Ko YH, Kim WS. CD47 overexpression is common in intestinal non-GCB type diffuse large B-cell lymphoma and associated with 18q21 gain. Blood Adv. (2022) 6:6120–30. doi: 10.1182/bloodadvances.2021006305
Keywords: diffuse large B-cell lymphoma, intestinal lymphoma, extranodal lymphoma, gastrointestinal tract, prognosis
Citation: Chen X, Wang J, Liu Y, Lin S, Shen J, Yin Y and Wang Y (2024) Primary intestinal diffuse large B-cell lymphoma: novel insights and clinical perception. Front. Oncol. 14:1404298. doi: 10.3389/fonc.2024.1404298
Received: 20 March 2024; Accepted: 29 July 2024;
Published: 15 August 2024.
Edited by:
Nina Zidar, University of Ljubljana, SloveniaReviewed by:
Mallikarjun Patil, St. John’s Medical College Hospital, IndiaMagda Zanelli, Azienda USL-IRCCS di Reggio Emilia, Italy
Copyright © 2024 Chen, Wang, Liu, Lin, Shen, Yin and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yili Wang, d3lsbHp0QGFsaXl1bi5jb20=
†These authors have contributed equally to this work and share first authorship
 Xiaojun Chen
Xiaojun Chen Jing Wang2†
Jing Wang2† Yanquan Liu
Yanquan Liu