
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Oncol. , 10 May 2024
Sec. Thoracic Oncology
Volume 14 - 2024 | https://doi.org/10.3389/fonc.2024.1402297
The co-occurrence of distinct lung cancer types within the same lobe is an exceedingly rare phenomenon. Here, we present a unique case wherein primary invasive squamous cell carcinoma and invasive adenocarcinoma concurrently manifested in the identical lung lobe. Additionally, we provide a comprehensive overview of the diagnosis and treatment approaches for multiple primary lung cancers, along with highlighting existing challenges based on the most recent guidelines. Our case underscores the importance of sampling each lesion individually, conducting separate diagnostic procedures, and determining the histological subtype for effective treatment planning irrespective of their location or size.
Multiple primary lung cancer (MPLC) is an infrequent form of primary lung malignancy, characterized by the simultaneous or sequential occurrence of two or more distinct lung cancers within the same individual in different regions of the lungs (1). These tumors may exhibit either similar or dissimilar histological types and are divided into simultaneous multiple primary lung cancer (sMPLC) and metachronous multiple primary lung cancer (mMPLC) based on the diagnosis interval exceeding 6 months (2). It has been reported that the incidence of MPLC in lung cancer is about 0.7% to 15%, and the incidence of sMPLC in MPLC in Chinese population is about 0.3% to 1.2% (3). In recent years the utilization of multislice spiral computed tomography (CT) and positron emission tomography (PET) scanning has significantly enhanced early detection rates for MPLC, prompting increased attention to this condition. Nevertheless, sMPLC and mMPLC are still relatively rare, especially within the same lobe, and most of MPLC are of the same histological type. Adenocarcinoma represents the predominant histological subtype observed in patients with MPLC (80%-99%), followed by squamous cell carcinoma (13%) (4). We report a rare case of sMPLC, characterized by simultaneous occurrence of two lung cancers with distinct histologic types within the same lobe. In this study, we aim to to summarize the latest advances in the diagnosis and treatment of MPLC based on the latest guidelines, and discuss the potential prognostic impact of two different histological types of cancer in the same lobe, in order to provide valuable insights for clinical practice.
A 65-year-old man was admitted to the hospital with intermittent hemoptysis lasting for 8 months. 8 months ago, he first developed blood-streaky sputum, and then 4 months ago his symptoms began to worsen significantly with hemoptysis, and underwent a high resolution CT at the local hospital, which revealed a nodule on the left upper lung of 2.2*2.6 cm. CT-guided aspiration biopsy was performed and pathology showed that there was fibrotic proliferation of the lung tissues, with widened alveolar septa, and a large number of lymphocytic infiltrates and lymphoid follicle deposits in the alveolar lumen. Hemoptysis improved after hemostatic and anti-infectious treatment. In the last two weeks, the hemoptysis aggravated, and the high-resolution CT in our hospital suggested that the left upper lung had a cavitary lung mass of 3.9*3cm, and a nodule of 1cm in the left upper lobe near the interlobar fissure (Figure 1), which was treated with symptomatic anti-infective and hemostasis treatment without significant improvement. Fiberoptic bronchoscopy reveals hemorrhage in the opening of the upper lobe of the left lung and it was recommended that re-excision biopsy and PET-CT again, but was forcefully refused, and he was given cerebral contrast enhanced magnetic resonance scan, thoracic and abdominal CT with i.v. contrast, cervical lymph nodes ultrasonography and radionuclide bone scan. There were no suspicious metastatic lesions. After MDT consultation and discussion with oncologists and pulmonary physicians, the patient underwent lobectomy of the left upper lung and mediastinal lymph node systematic clearance under single-port thoracoscopy (wedge resection was performed first, and then lobectomy of the lung was carried out after the frozen section pathology suggested squamous cell carcinoma during the operation).
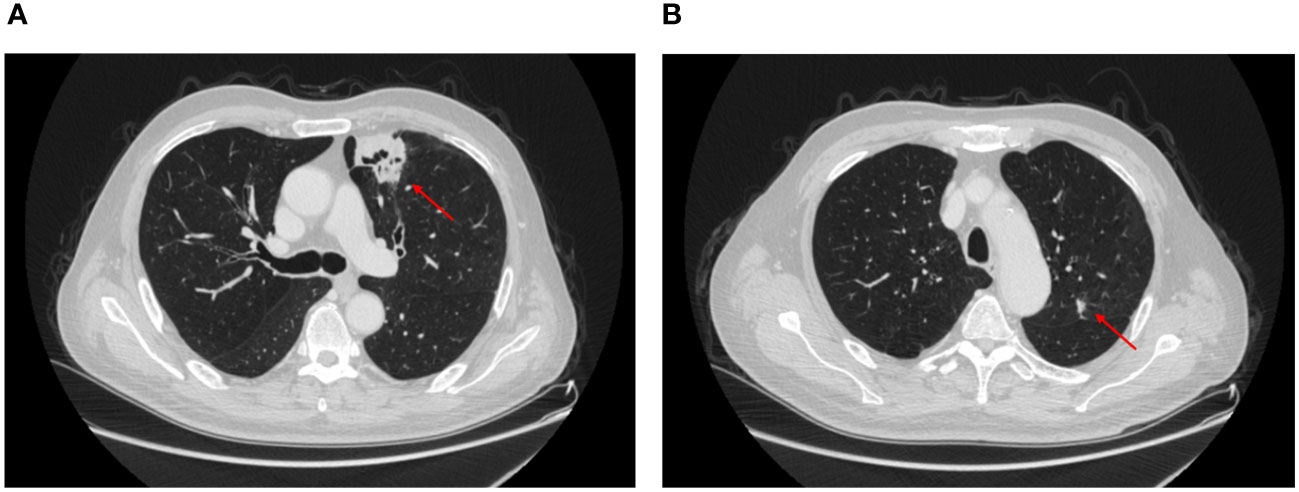
Figure 1 CT showed the location of the pulmonary nodule in the left upper lung. (A) squamous cell carcinoma, (B) adenocarcinoma.
Pathological examination of the left upper lobe revealed two lesions. Postoperative pathology suggests non-keratinizing invasive squamous cell carcinoma of moderate to poor differentiation without evidence of vascular or nerve invasion (Figure 2). Pleural invasion grade was categorized as PL1. Immunohistochemical findings demonstrated positivity for CK5/6 (+), P40 (+), P63 (+), Ki-67 (approximately 50% in hot spots), while Napsin A (-) and TTF-1 (-). Special staining results indicated presence of elastic fibers. The second tumor (anatomically superior) was revealed invasive adenocarcinoma. The proportions of tumor growth patterns of the papillary, acinar, micropapillary, cribriform parts were 40%, 30%, 20%, 10% respectively (Figure 3). The maximum diameter of invasion measured at 1.2cm, exhibiting poor differentiation and vascular invasion; STAS was poorly positive without any observed invasion of visceral pleura or nerves, lymph node (Group 5, 6, 7, 9, 10, 11, 12) without metastasis. Immunohistochemical results indicated CK7 (+), Ki-67 (+) at approximately 20%, Napsin A (+), TTF-1 (+), P53 weakly positive as wild type, and P40 (-).
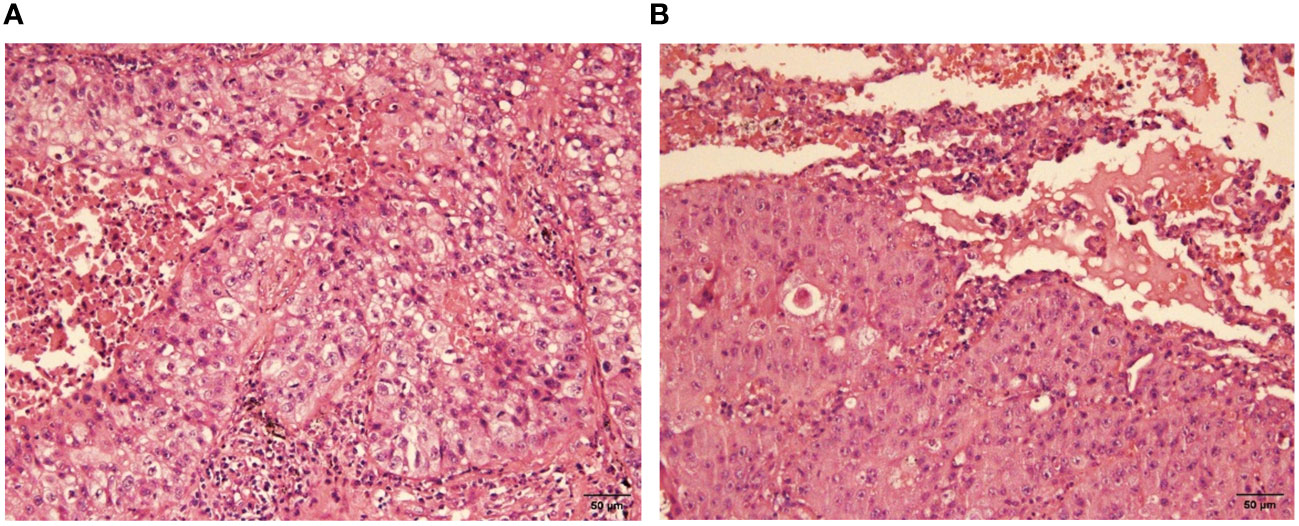
Figure 2 Moderately-poorly differentiated squamous cell carcinoma (H-E stain, x400), nonkeratinized. (A) the view of cancerous fields, (B) the view of both normal and cancerous fields.
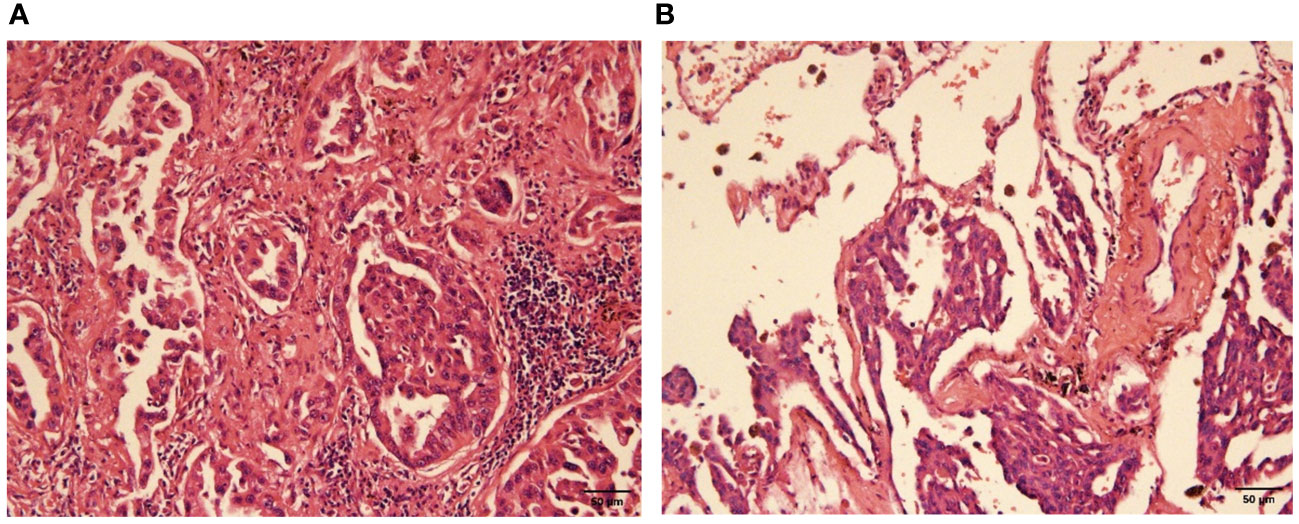
Figure 3 Poorly differentiated adenocarcinoma (H-E stain, x200). (A) the view of cancerous fields, (B) the view of both normal and cancerous fields.
The patient’s final diagnosis was lung squamous carcinoma combined with adenocarcinoma, T3N0M0, stage IIB according to the ninth edition of the tumor node metastasis (TNM) staging system and successfully discharged from the hospital after five days, and was proposed to receive adjuvant treatment after one month postoperatively.
Currently, the clinical diagnosis of MPLC is predominantly based on the Martini-Melamed diagnostic criteria (1), which primarily rely on histologic features, tumor location, presence or absence of carcinoma in situ, lymphatic invasion, metastasis, and other empirical characteristics. The American College of Chest Physicians (ACCP) has also emphasized the necessity for distinct molecular genetic characteristics among different lesions and recommended extending the tumor-free interval for mMPLC to 4 years (5, 6). The 2023 NCCN guidelines underscore that diagnosing MPLC necessitates multidisciplinary treatment involving thoracic surgery, pathology, radiology, and oncology while streamlining the diagnostic process to aid clinicians in making optimal medical decisions (7).
Accurate diagnosis of MPLC is essential. Various methods have been proposed to help the diagnosis, including DNA ploidy patterns and p53 gene mutation homology assays (8). However, clinical manifestations, imaging examination and pathological features are still the main factors for the comprehensive diagnosis of MPLC (9). Next-generation sequencing has emerged as a promising tool for precise diagnosis and prognostic assessment in lung cancer compared to traditional histopathological evaluation (10). Although EGFR and KRAS mutations can help to diagnose the synchronous occurrence of primary adenocarcinoma, combined immunohistochemistry may be a better choice due to intratumor heterogeneity limitations (11). Unfortunately, based on the patient’s wishes, only immunohistochemistry was performed in this case, and sequencing and other related examinations were not completed. However, we suggest that if the relevant conditions are met, further studies such as sequencing are necessary for accurate diagnosis and prognosis evaluation.
Accurate identification of MPLC is crucial for selecting appropriate treatment strategies. Patients with MPLC are at risk of receiving inappropriate treatment based solely on imaging and histopathological staging reports (12). In the past, multiple pulmonary nodules were often misinterpreted as metastatic lesions, leading to a lack of surgical intervention. Once diagnosed with MPLC, surgery should be promptly recommended if there are no clinical contraindications; subsequent radiotherapy or image-guided thermal ablation may follow suit. If local therapy is not feasible for the patient, palliative care or observation should be considered. Studies have demonstrated that surgical interventions can lead to favorable prognoses in patients with MPLC (13–15). Although the diagnostic criteria for MPLC have been established, there is still a lack of unified authoritative principles and guidelines for surgical treatment. Furthermore, microwave ablation, targeted therapy, immunotherapy and other technologies are increasingly utilized in patients with MPLC (16, 17). However, due to the multi-driver gene mutation characteristics of MPLC and the diversity of immune microenvironment, more prospective randomized trials are necessary to validate their efficacy. Additionally, positive driver genes-targeted therapy and immunotherapy have demonstrated promising outcomes in postoperative adjuvant phase (18, 19). The high frequency of gene mutations in MPLCs particularly EGFR mutations provides objective evidence for the effectiveness of targeted therapy (20). Nevertheless, differences in mutation between different lesions within the same patient (4) emphasize the importance of evaluating each lesion’s mutation status before considering targeted therapy for MPLC patients. Moreover, it is noteworthy that lymph node metastasis is an independent prognostic factor for MPLC; however, its characteristics and patterns remain unclear as there are limited relevant studies with differing perspectives. Given the future emergence of lung cancer screening programs and the rising incidence of MPLC cases, it is crucial to develop unified diagnostic criteria and treatment plans while standardizing post-treatment monitoring for optimal management.
In summary, MPLC exhibits distinct characteristics in imaging, pathology, and molecular genetics, necessitating multidisciplinary collaboration for the development of diagnosis and treatment strategies. Surgical intervention currently remains the primary therapeutic approach for MPLC patients with solitary lung lesions, offering a favorable prognosis when performed at an early stage (21).
Furthermore, despite the recent increase in the incidence of MPLC, there have been limited reports documenting synchronous adenocarcinoma and squamous cell carcinoma occurring in the same lobe (22–24),which are very old. Similar to our case, these reported patients were predominantly male smokers aged between 55 and 70 years. The cases of lung cancer with various histologic types that have been reported in recent years are summarized in Table 1. It remains unknown whether there are other undiagnosed and unreported cases, as well as the potential impact of gender, lifestyle factors, and other variables on this type of case. We speculate that the true incidence of MPLC may be higher than what is currently detected by CT and PET (28). Moreover, since the onset of the COVID-19 pandemic in 2019, there has been a significant rise in MPLC incidence due to increased utilization of CT scans among patients (29); however, no association between COVID-19 and MPLC has been reported. What’s more, most of the sMPLC reported at present are in the early stage, and the prognosis of surgery is good. The effect of related treatments such as chemotherapy and immunotherapy for patients with advanced sMPLC remains to be studied. The patient in this case was still in the early stage of the disease, and the prognosis of the operation was good. The two lesions recovered well after operation. We suggest that for patients with sMPLC with different histopathological features, after excluding intrapulmonary metastasis, multiple lesions should be treated as independent individuals for postoperative treatment and long-term follow-up.
In conclusion, the rarity of this case highlights the importance of obtaining separate diagnostic samples from each lesion in patients with sMPLC, irrespective of tumor location and size, to determine individual T, N, and M stages for each lesion. Furthermore, a comprehensive targeted treatment plan should be developed to enhance patient survival and improve their quality of life. It is crucial to emphasize that resectability should not be excluded for different lesions within the same lobe and they should be treated individually regardless of lesion location. Additionally, MPLC requires increased attention along with long-term follow-up; therefore, it is imperative to establish standardized guidelines for MPLC treatment at the earliest opportunity. With continuous advancements in molecular biomarkers and genetic analysis techniques, rapid and accurate diagnosis of MPLC as well as evaluation of treatment options will no longer pose significant challenges.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article. Written informed consent was obtained from the participant/patient(s) for the publication of this case report.
TZ: Writing – original draft. RH: Writing – original draft. YX: Writing – review & editing. QG: Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by grants from the National Natural Science Foundation of China (No. 8210082163 and 82300103), the Fundamental Research Funds for the Central Universities (No.2042021kf0081 and 2042023kf0011) and Science Fund for Creative Research Groups of the Natural Science Foundation of Hubei Province (No.2020CFA027).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
MPLC, multiple primary lung cancer; sMPLC, simultaneous multiple primary lung cancer; mMPLC, metachronous multiple primary lung cancer; CT, computed tomography; PET, positron emission tomography; TNM, tumor node metastasis; ACCP, The American College of Chest Physicians.
1. Martini N, Melamed MR. Multiple primary lung cancers. J Thorac Cardiovasc Surg. (1975) 70:606–12. doi: 10.1016/S0022-5223(19)40289-4
2. Liu Z, Wang L, Gao S, Xue Q, Tan F, Li Z, et al. Plasma metabolomics study in screening and differential diagnosis of multiple primary lung cancer. Int J Surg (London England). (2023) 109:297–312. doi: 10.1097/JS9.0000000000000006
3. Jiang L, Zheng X, Wu S, Zhang J, Ru G, Li Y. A rare case of synchronous multiple primary lung cancer: squamous cell cancer and small cell lung cancer. OncoTargets Ther. (2019) 12:8801–6. doi: 10.2147/OTT.S213259
4. Chen K, Chen W, Cai J, Yang F, Lou F, Wang X, et al. Favorable prognosis and high discrepancy of genetic features in surgical patients with multiple primary lung cancers. J Thorac Cardiovasc Surg. (2018) 155:371–379.e1. doi: 10.1016/j.jtcvs.2017.08.141
5. Detterbeck FC, Lewis SZ, Diekemper R, Addrizzo-Harris D, Alberts WM. Executive Summary: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. (2013) 143:7S–37S. doi: 10.1378/chest.12-2377
6. Shen KR, Meyers BF, Larner JM, Jones DR. & American College of Chest Physicians. Special treatment issues in lung cancer: ACCP evidence-based clinical practice guidelines (2nd edition). Chest. (2007) 132:290S–305S. doi: 10.1378/chest.07-1382
7. Ettinger DS, Wood DE, Aisner DL, Akerley W, Bauman JR, Bharat A, et al. NCCN guidelines® Insights: non-small cell lung cancer, version 2.2023. J Natl Compr Cancer Netw: JNCCN. (2023) 21:340–50. doi: 10.6004/jnccn.2023.0020
8. Zang R, Shi JF, Lerut TE, Wang L, Liu CC, Brunelli A, et al. Ten-year trends of clinicopathologic features and surgical treatment of lung cancer in China. Ann Thorac Surg. (2020) 109:389–95. doi: 10.1016/j.athoracsur.2019.08.017
9. Liu C, Li H, Xu K, Song S, He Y, Cai X, et al. Multiple primary lung cancer versus intrapulmonary metastatic cancer: A case of multiple pulmonary nodules. Thorac Cancer. (2019) 10:352–8. doi: 10.1111/1759-7714.12918
10. Goodwin D, Rathi V, Conron M, Wright GM. Genomic and clinical significance of multiple primary lung cancers as determined by next-generation sequencing. J Thorac Oncol. (2021) 16:1166–75. doi: 10.1016/j.jtho.2021.03.018
11. Liu M, He W, Yang J, Jiang G. Surgical treatment of synchronous multiple primary lung cancers: a retrospective analysis of 122 patients. J Thorac Dis. (2016) 8:1197–204. doi: 10.21037/jtd.2016.04.46
12. Tabrizi NS, Harris ES, Gallant BP, Fabian T. Clinical and pathologic staging accuracy in patients with synchronous multiple primary lung cancers. J Thorac Dis. (2024) 16:491–7. doi: 10.21037/jtd-23-1383
13. Ishikawa Y, Nakayama H, Ito H, Yokose T, Tsuboi M, Nishii T, et al. Surgical treatment for synchronous primary lung adenocarcinomas. Ann Thorac Surg. (2014) 98:1983–8. doi: 10.1016/j.athoracsur.2014.07.006
14. Qu R, Tu D, Ping W, Cai Y, Zhang N, Fu X. Surgical outcomes of one-stage resection for synchronous multiple primary lung adenocarcinomas with no less than three lesions. J Cardiothorac Surg. (2021) 16:265. doi: 10.1186/s13019-021-01647-z
15. Yang H, Sun Y, Yao F, Yu K, Gu H, Han B, et al. Surgical therapy for bilateral multiple primary lung cancer. Ann Thorac Surg. (2016) 101:1145–52. doi: 10.1016/j.athoracsur.2015.09.028
16. Lee D-S, LaChapelle C, Taioli E, Kaufman A, Wolf A, Nicastri D, et al. Second primary lung cancers demonstrate similar survival with wedge resection and lobectomy. Ann Thorac Surg. (2019) 108:1724–8. doi: 10.1016/j.athoracsur.2019.06.023
17. Hirsch FR, Scagliotti GV, Mulshine JL, Kwon R, Curran WJ, Wu YL, et al. Lung cancer: current therapies and new targeted treatments. Lancet. (2017) 389:299–311. doi: 10.1016/S0140-6736(16)30958-8
18. Cheng B, Deng H, Zhao Y, Zhu F, Liang H, Li C, et al. Management for residual ground-glass opacity lesions after resection of main tumor in multifocal lung cancer: A case report and literature review. Cancer Manage Res. (2021) 13:977–85. doi: 10.2147/CMAR.S290830
19. Wu S, Li D, Chen J, Chen W, Ren F. Tailing effect of PD-1 antibody results in the eradication of unresectable multiple primary lung cancer presenting as ground-glass opacities: a case report. Ann Palliative Med. (2021) 10:778–84. doi: 10.21037/apm-20-2132
20. Wang M, Herbst RS, Boshoff C. Toward personalized treatment approaches for non-small-cell lung cancer. Nat Med. (2021) 27:1345–56. doi: 10.1038/s41591-021-01450-2
21. Nie Y, Wang X, Yang F, Zhou Z, Wang J, Chen K. Surgical prognosis of synchronous multiple primary lung cancer: systematic review and meta-analysis. Clin Lung Cancer. (2021) 22:341–350.e3. doi: 10.1016/j.cllc.2020.10.022
22. Kitamura K, Mitsudomi T, Ishida T, Kaneko S, Sugimachi K. Adenocarcinoma and squamous cell carcinoma in the same lobe of the lung. Respiration. (1991) 58:226–8. doi: 10.1159/000195933
23. Bacalja J, Tomasović Lončarić Č, Kukulj S, Nikolić I. The case of synchronous occurrence of primary adenocarcinoma and squamous cell carcinoma in the same lobe of the lung. Acta Clin Belg. (2017) 72:289–92. doi: 10.1080/17843286.2016.1237697
24. Wu L, Kang P, Tao S, Zhao Z, Chen L, Xiao Y, et al. Genomic profiles and transcriptomic microenvironments in 2 patients with synchronous lung adenocarcinoma and lung squamous cell carcinoma: a case report. BMC Med Genomics. (2020) 13:15. doi: 10.1186/s12920-020-0663-8
25. Diffalha AL, Hasan S, Tahmasbi M. & Khalil, F. Rare case of combined small cell lung cancer with adenocarcinoma and squamous cell carcinoma. Hum Pathol: Case Rep. (2017) 7:31–4. doi: 10.1016/j.ehpc.2016.05.002
26. Liu Y, Kang L, Hao H, Zhang X, Zheng G, Guo X, et al. Primary synchronous colloid adenocarcinoma and squamous cell carcinoma in the same lung: A rare case report. Med (Baltimore). (2021) 100:e24700. doi: 10.1097/MD.0000000000024700
27. Xu J, Wang J, Li C, Yao J, Liu P, Yang X. Multiple primary lung cancer comprised of adenocarcinoma and adenoid cystic carcinoma: a case report. Trans Cancer Res. (2022) 11:591–6. doi: 10.21037/tcr-22-166
28. Samadzadeh Tabrizi N, Gallant B, Harris E, Arnold BN, Fabian T. Contemporary incidence of synchronous multiple primary lung cancers and survival in the era of lung cancer screening. Innovations. (2024) 19:23–9. doi: 10.1177/15569845231210242
Keywords: lung squamous cell carcinoma, lung adenocarcinoma, same lobe, multiple primary lung cancer, simultaneous multiple primary lung cancer
Citation: Zhang T, He R, Xiao Y and Geng Q (2024) Primary squamous cell carcinoma and adenocarcinoma simultaneously occurring in the same lung lobe: a case report and literature review. Front. Oncol. 14:1402297. doi: 10.3389/fonc.2024.1402297
Received: 17 March 2024; Accepted: 18 April 2024;
Published: 10 May 2024.
Edited by:
Mohamed Rahouma, NewYork-Presbyterian, United StatesReviewed by:
Wenzheng Guo, University of Kentucky, United StatesCopyright © 2024 Zhang, He, Xiao and Geng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qing Geng, Z2VuZ3Fpbmd3aHVAd2h1LmVkdS5jbg==; Yongguang Xiao, eW9uZ2d1YW5nLnhpYW9Ad2h1LmVkdS5jbg==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.