
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol. , 12 June 2024
Sec. Head and Neck Cancer
Volume 14 - 2024 | https://doi.org/10.3389/fonc.2024.1401165
 Maximilian Gottsauner*
Maximilian Gottsauner* Johannes Meier
Johannes Meier Jonas Eichberger
Jonas Eichberger Stephanie Eckmüller
Stephanie Eckmüller Johannes Schuderer
Johannes Schuderer Mathias Fiedler
Mathias Fiedler Michael Maurer
Michael Maurer Torsten E. Reichert
Torsten E. Reichert Tobias Ettl
Tobias EttlBackground: The aim of this study was to investigate the effect of antiresorptive agents on the ossification of reconstructed mandibles by free bone grafts for the first time.
Methods: A total of 38 reconstructions of the jaw were retrospectively evaluated for ossification between bone segments by two raters based on postoperative panoramic radiographs. The study group (n = 13) had segmental resection of the mandible and free bone flap reconstruction due to medication-related osteonecrosis of the jaw (MRONJ). The control group (noMRONJ, n = 25) comprised segmental mandibular resections and free bone flap reconstructions due to tumors, chronic osteomyelitis, or trauma without any radiation. Ossification time and influencing factors were evaluated.
Results: Both duration of surgery (346 ± 90 min. vs. 498 ± 124 min.; p < 0.001) and hospitalization (8.7 ± 2.8 days vs. 13.4 ± 5.3 days, p = 0.006) were shorter in the MRONJ group compared to the noMRONJ group. Ossification after mandibular reconstruction was significantly faster in the MRONJ study group [224 days, interquartile range (IQR) 175–287] compared to the control group (288 days, IQR 194–445; p < 0.001). Moreover, good initial contact between the segments resulted in faster ossification (p < 0.001) in the MRONJ group. Ossification rate between original and grafted bone or between grafted bone segments only did not differ in both the study and control groups (MRONJ, p = 0.705 vs. control, p = 0.292). The type of antiresorptive agent did not show any significance for ossification. The rate of wound healing disturbances did also not differ between the study and control groups (p = 0.69).
Conclusion: Advanced MRONJ (stage 3) can be resected and reconstructed safely with free microvascular bone flaps. Antiresorptive agents enhance the ossification of the bone segments. Optimal initial contact of the bone segments accelerates bone healing. Surgery and hospitalization are markedly shortened in this vulnerable group of MRONJ patients compared to oncologic patients.
First used against hypercalcemia, bisphosphonates became an important adjuvant drug for patients suffering from bone metastases (1). Nowadays, the group of antiresorptive drug agents includes the group of bisphosphonates and denosumab (2).
Bisphosphonates interact with the osteoclasts, leading to inhibition and suppression of bone resorption. After incorporation orally or intravenously, the agent binds to hydroxyapatite and can be detected after more than one decade (3). Denosumab, however, is a monoclonal antibody, which prevents the binding of the receptor activator of nuclear factor-κB (RANK) and RANK ligand (RANKL). By docking RANKL on RANK, osteoclast precursor cells become stimulated and lead, among others, to the differentiation of the osteoclasts. This control mechanism plays a major role in bone remodeling, and by being specifically blocked, bone resorption is reduced (4).
With worldwide age-standardized incidence rates (ASIR) in 2020 of 47.8 per 100,000 for breast cancer (female) and 30.7 per 100,000 for prostate cancer (male), the two most common malignant tumors separated by gender enable a large group of patients to be treated with these medications (5, 6).
In addition to the treatment of bone metastases, antiresorptive therapy is well-established for multiple myeloma. With more than 150,000 cases a year worldwide in 2020, these patients set an additional part of the oncological prescriptions of antiresorptive drugs (5–7).
Moreover, the prevention of osteoporotic fractures has become a major socioeconomic factor in a worldwide aging population (8). With reduced doses and enlarged time intervals compared to the malignant indications, all antiresorptive agents are approved for osteoporotic treatment (9).
With the spread of bisphosphonates, a severe complication, the bisphosphonate-related osteonecrosis of the jaw (BRONJ), appeared and accumulated (10, 11). Later with anti-RANKL therapy, similar complications were observed and renamed to medication-related osteonecrosis of the jaw (MRONJ) (12). In general, patients with oncological indications have therefore a higher risk for MRONJ than those with osteoporosis (13).
Over the years, resection of the necrotic bone and stable, stressless soft tissue wound closure have turned out to be the key steps for curing MRONJ (14, 15).
In a very advanced stage of MRONJ (usually stage 3), segmental resection of the mandible may become necessary. Immediate reconstruction with free vascularized bone grafts has evolved as a feasible and reliable option (16, 17).
In addition to vascular flap complications or surgical site infections, non-union of the grafted bone segments presents a further important complication of free bone grafting. Proper osseous healing of the grafted bone is decisive for successful mandibular rehabilitation and the precondition for the removal of osteosynthesis material. Modulating bone metabolism antiresorptive agents are supposed to influence the ossification of reconstructed mandibles with free bone grafts.
Small case studies reported the feasibility and successful ossification of microvascular bone reconstructions in cases with stage 2 and 3 BRONJ (18, 19). However, no single study to date has presented more detailed data about the time and quality of bone ossification after free bone graft transfer.
Therefore, the aim of this retrospective study was to investigate the timeline and influencing factors for ossification after mandibular reconstruction with free vascularized bone grafts in MRONJ in comparison to a control group without antiresorptive agents or radiotherapy.
The study design was approved by the ethics committee of the University of Regensburg (ref. 23–3559-104) in accordance with the Declaration of Helsinki and its later amendments or comparable ethical standards.
Between 2009 and 2022, 38 operations with free microvascular bony reconstructions of the mandible were identified in the Department of Oral and Maxillofacial Surgery at the University Hospital in Regensburg and were included in this study. Exclusion criteria were pre- or postoperative radiation therapy and no follow-up postoperative panoramic radiograph in the first 12 months. Four reconstructed patients dropped out because of missing follow-ups. Moreover, regular follow-ups with panoramic radiographs starting from 2 months until 2 years after reconstruction were available. Three different types of microvascular bony reconstruction were performed. The predominant type of reconstruction was the free fibula flap (n = 33), followed by microvascular iliac crest (n = 3) and scapular flaps (n = 2). No patient received two separate independent free flaps.
The following data were collected (Tables 1A–C): age, gender, diagnosis, antiresorptive agent, duration of dose delivery, preoperative computer-aided design/computer-aided manufacturing (CAD/CAM) planning, defect classification after Jewer and Boyd (20), type of bony reconstruction, amount and length of the bone segments, duration on intensive care unit (ICU) and duration of hospitalization, previous operations in the head and neck region, wound healing disorders, and revisions regarding the free graft.
The cohort was divided into two groups regarding the primary reason for the reconstruction of the mandible: one study group with MRONJ and one control group (noMRONJ).
All available postoperative panoramic radiographs were examined (Figure 1). Location and quality of contact (no contact, moderate contact, and good contact) between the bone graft and original bone as well as between graft segments were documented and associated with healing time after surgery (Figure 2).

Figure 1 Example of ossification under antiresorptive agent: (A) patient with severe MRONJ of the mandible, resection, and reconstruction with a three-segmented fibula CAD/CAM-planned. (B) After 5 months, complete ossification in all four contact points. (C) Two months later, after removal of reconstruction plate and augmentation with avascular iliac crest. (D) Thirteen months after microvascular reconstruction and insertion of dental implants. MRONJ, medication-related osteonecrosis of the jaw; CAD/CAM, computer-aided design/computer-aided manufacturing.
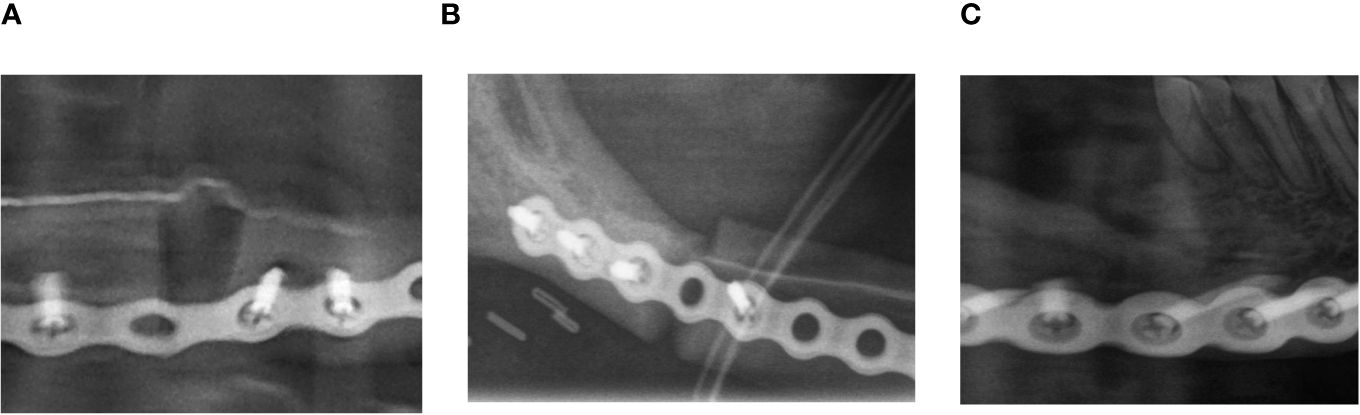
Figure 2 Example of evaluation of the initial contact point. (A) Example of no initial contact (radiographic gap >2 mm). (B) Example of moderate initial contact (radiographic gap ≤2 mm). (C) Example of good initial contact (no radiographic gap).
The segment number and segment length of the bone graft were evaluated, too.
Panoramic radiographs around the seventh postoperative day and the 6th, 11th, and 21st postoperative months were examined. After full ossification at each contact point, no further examinations were made.
Two raters (T.E. and M.G.) examined all radiographs independently and valued every point of contact with a three-part score. The raters faced a choice between no ossification (no sign of ossification vertically between the segments), partial ossification (less than 50% ossification between the segments), and complete ossification (more than 50% ossification between the segments). In the case of diverse evaluation between the raters, a review was performed, and a final statement was expressed.
Continuous data are presented as mean ± SD or as median (first quartile, third quartile) depending on the underlying distribution and were compared between groups using a t-test for independent samples or the non-parametric Kruskal–Wallis test. Revisions and wound healing disturbance were analyzed by chi-square tests. Time to ossification was analyzed using Kaplan–Meier plots and log-rank tests as well as a multivariable Cox proportional hazards model, including all significant variables of the univariable analyses. Hazard ratios (HRs) and corresponding 95% confidence intervals (95% CI) are reported as effect estimates. A p-value <0.05 was considered statistically significant for all tests. All statistical analyses and plots were performed using IBM SPSS Statistics, version 29 (IBM Corp., Armonk, NY, USA).
The first panoramic radiograph was performed around the seventh postoperative day (7.4 ± 5.9) as a starting point and was used for evaluation of the primary contacts between the segments. Mean surveillance periods were at three different postoperative check-up dates. The first was around the sixth month (170 ± 64 days), the second around the 11th month (320 ± 92) days, and the last control X-ray around the 21st month (629 ± 233 days) after surgery. Overall, 114 points of contact between the segments were documented in panoramic radiographs. The duration of the operation was 498 ± 124 minutes significantly longer in the noMRONJ subgroup than the MRONJ subgroup, p < 0.001 (346 ± 90 minutes). The average postoperative stay in ICU showed no significant difference (noMRONJ subgroup 4.0 ± 3.4 days vs. MRONJ group 2.5 ± 1.6 days, p = 0.14). The control group had a significant (p = 0.006) longer overall hospitalization with 13.4 ± 5.3 days compared to the MRONJ group with 8.7 ± 2.8 days. The defect size showed no significant difference (p = 0.51) between the groups. The control group had a smaller average defect with 102 ± 37 mm in relation to the MRONJ group with 110 ± 36 mm. Revisions (p = 0.117) and wound healing disturbance (p = 0.69) showed no significant difference between the subgroups (noMRONJ vs. MRONJ).
The overall median for complete ossification at the point of contact was 273 days (interquartile range (IQR) 184–373). The fastest ossification was observed in patients with antiresorptive agents MRONJ in a median of 224 days (IQR 175–287). Patients without these agents showed 288 days (IQR 194–445). The difference between both groups was significant with p < 0.001 (Figure 3).
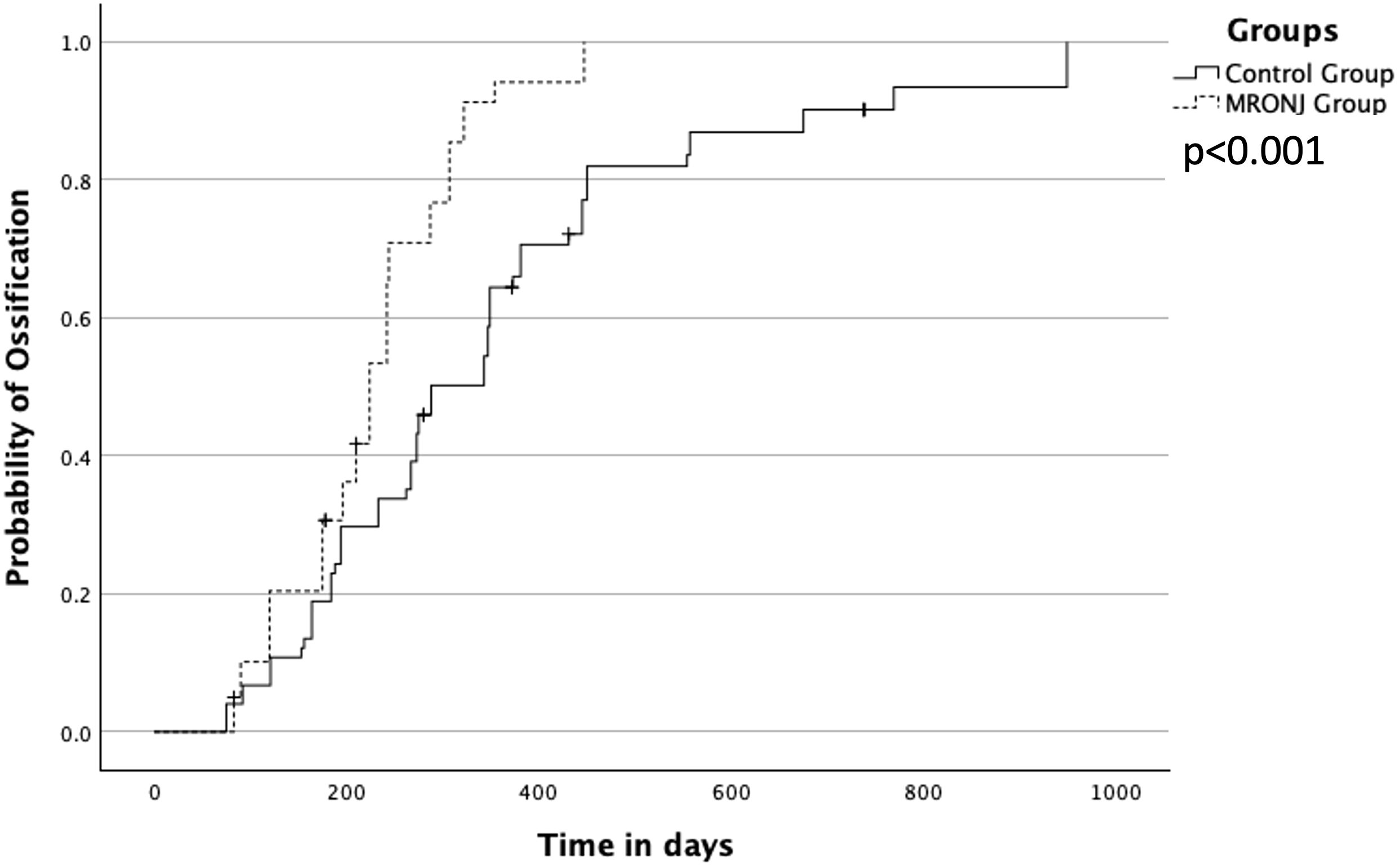
Figure 3 Overall ossification between MRONJ group and the control group (noMRONJ). MRONJ, medication-related osteonecrosis of the jaw.
In the control group noMRONJ, the median ossification of contact points between two segments of the microvascular transplant showed was 273 (IQR 188–381) days, a slightly faster ossification than the ossification between one segment of the transplant and the original recipient bone of 343 (IQR 194–450) days.
A similar distribution was observed in the MRONJ group with 210 (IQR 175–244) days for median ossification between transplanted segments and 224 (IQR 175–307) days for contact points between original bone and transplant. In both groups, the difference was not significant (noMRONJ, p = 0.292; MRONJ, p = 0.705) (Figure 4).
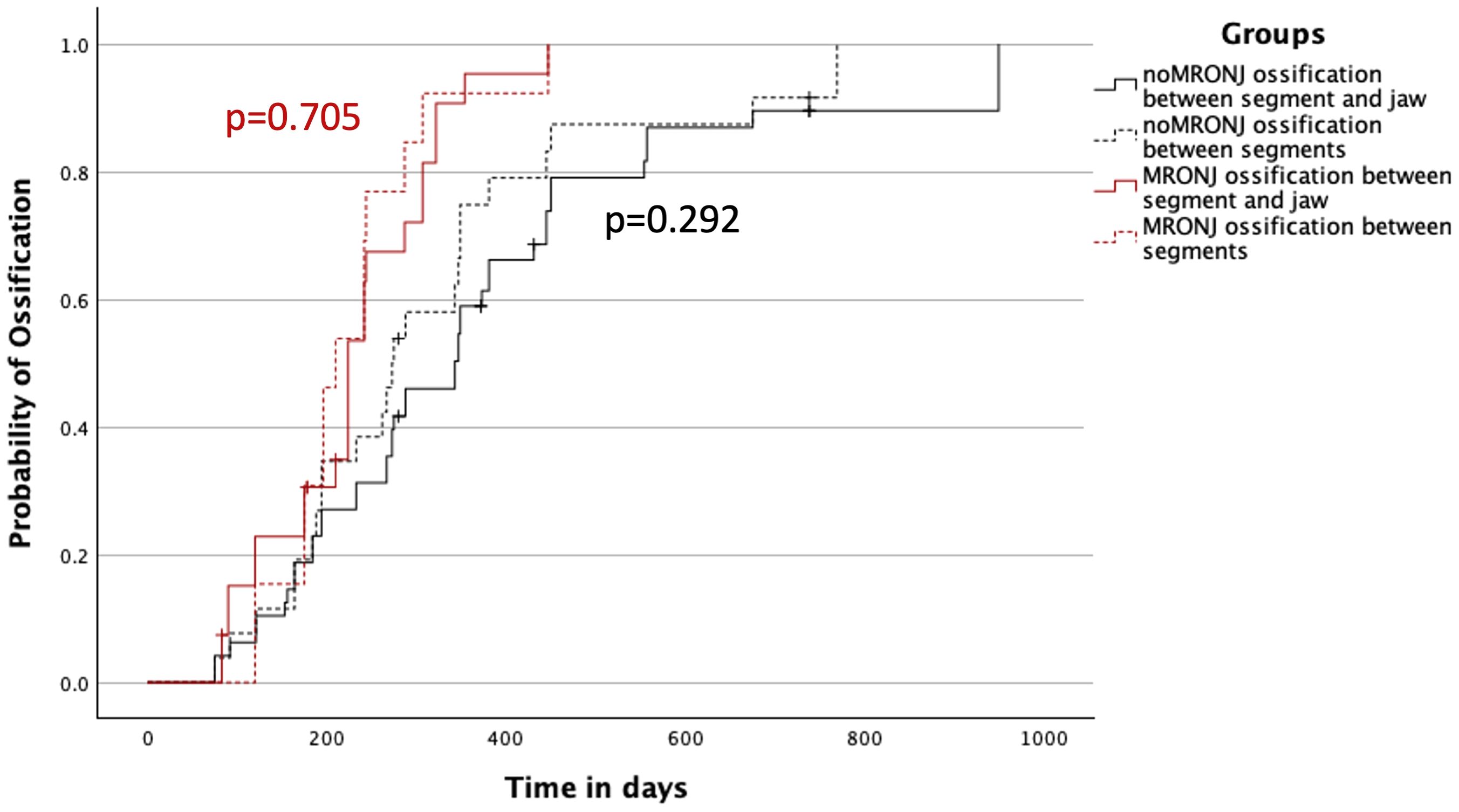
Figure 4 Ossification concerning the contact between two segments of the transplant and between one segment of the transplant and the original bone of the jaw divided into both MRONJ and noMRONJ groups. MRONJ, medication-related osteonecrosis of the jaw.
The quality of the initial contact showed an impact on ossification between noMRONJ and MRONJ. While a moderate initial contact showed, p = 0.533, no faster ossification between noMRONJ (median 349 days, IQR 275–431) and MRONJ (median 322 days, IQR 242–354) (Figure 5A), a good initial contact could demonstrate significant, p < 0.001, faster ossification for the MRONJ group with a median of 210 days (IQR 120–242) compared to noMRONJ with 288 days (IQR 184–450) (Figure 5B).
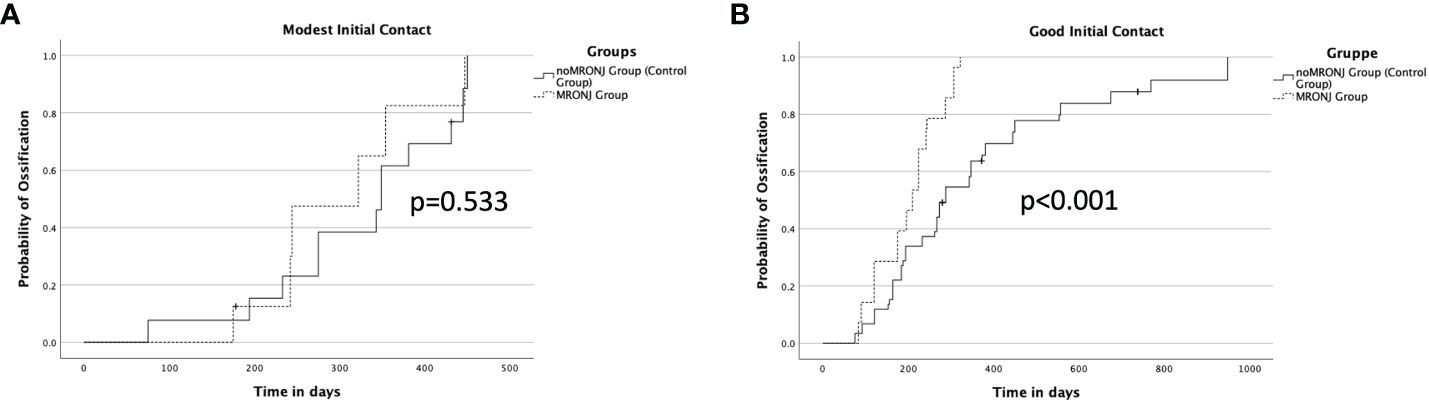
Figure 5 (A) Ossification concerning a modest initial contact between both groups. (B) Ossification concerning good initial contact between both groups.
By analyzing the subgroup MRONJ, the effects of different antiresorptive agents were examined. The fastest ossification was observed by patients having combined therapy of bisphosphonate and denosumab within 175 days (IQR 90–175), followed by patients with only bisphosphonates within 224 days (IQR 196–244) and patients with only denosumab therapy within 242 days (IQR 242–287) without significance, p = 0.291 (Figure 6).
Moreover, all cofactors were examined in a multivariable Cox regression model on ossification. Only the main variable of antiresorptive agent showed a significant impact on ossification [HR (95% CI), 4.71 (2.36; 9.39), p < 0.001] (Table 2).
The influences of antiresorptive agents on the metabolism of the bone are well known, but the effects on the ossification of bony reconstructions of the mandible have not been investigated in detail so far. The aim of this study was to evaluate the bony healing after mandibular reconstruction with free microvascular bone grafts in patients with advanced MRONJ. During follow-up, panoramic radiographs revealed a reliable ossification in most contact points of the reconstructed mandible. Moreover, ossification was significantly faster compared to the control group (noMRONJ) consisting of free bone graft reconstructions due to tumor or osteomyelitis without a history of antiresorptive medication or radiation therapy.
The effects on indirect fracture healing have already been examined in animal models. Rats treated with alendronate showed faster radiographic healing. In biomechanical testing, the callus even showed higher stability than in the control group. Nevertheless, remodeling processes of the callus were delayed in comparison to those in animals of the control group (21).
Similar results could be achieved in a rabbit model for the examination of bone healing after mandibular fractures under zoledronate, leading to accelerated bone healing with higher stability compared to the control group (22). These results support the outcome of the current study with faster ossification for patients with antiresorptive agents in their history. In this context, human studies on patients with fractures and bisphosphonate intake showed no delay in bony healing under antiresorptive medication (23).
The safe reconstruction of the mandible via free microvascular bone graft in patients with MRONJ has been proven in the past (16, 24). Described fistulas or postoperative infections could be treated with antibiotics or minor surgical revisions in most cases; very rarely is removal of osteosynthesis necessary. Stable intra- and extraoral soft tissue closure remains a key prerequisite according to general MRONJ treatment recommendations (15).
Both groups of drug agents, bisphosphonates and denosumab, affect the bone remodeling of the whole skeleton (25). Therefore, this study showed no significant difference in bone healing between grafted segments and between grafted and mandibular segments. This differs from irradiated patients with significantly faster bone healing between the non-irradiated grafted segments (26). The slightly faster ossification of intersegmental graft contact points—as found in the current study—may be attributed to a better matching contact surface compared to an incongruent graft/mandibular angle junction.
For both subgroups (noMRONJ and MRONJ), the initial contact defines the progress of ossification, and a good initial bone contact is warranted for predictable bony union and stable mandibular reconstruction (27). The differences between both subgroups appear significantly only in cases with good initial contact and is therefore an important goal for the surgical procedure. Higher accuracy of CAD/CAM-planned microvascular reconstructions of the mandible may lead to better initial contact and therefore may additionally improve ossification (28). However, patient-specific manufactured reconstruction plates may even delay ossification due to higher rigidity (29). The pooled analysis could not find a statistically significant difference in non-union between CAD/CAM and conventional planned cases (30).
The variable pharmacokinetics of bisphosphonates and denosumab are well documented. While integrated into the bone, the half-life of bisphosphonates is up to 10–12 years; meanwhile, the half-life of denosumab with binding RANKL is only 24–26 days (25). The switch from bisphosphonates to denosumab is still under investigation. Due to the overlap of the pharmacokinetics, synergistic effects of both medications on the metabolism of the bone seem likely (31). These tendencies may be seen in the different types of antiresorptive agents.
This study is based on a limited number of clinical cases, and imaging examinations were performed using panoramic X-rays instead of 3D radiographs like CT or cone-beam computer tomography (CBCT). Important associations may miss statistical significance due to missing power. With regard to imaging, post-surgical panoramic X-rays are performed in clinical routine after mandibular reconstructions, whereas 3D diagnostics are preserved for special indications such as mandibular reconstructions including temporomandibular joint (TMJ). Of course, 3D diagnostics are supposed to enable a more exact determination of ossification. Additionally, our grading of ossification relies on X-ray imaging. The gold standard would be a clinical validation. However, the removal of the reconstruction plate was performed rarely in our department (Figure 7).
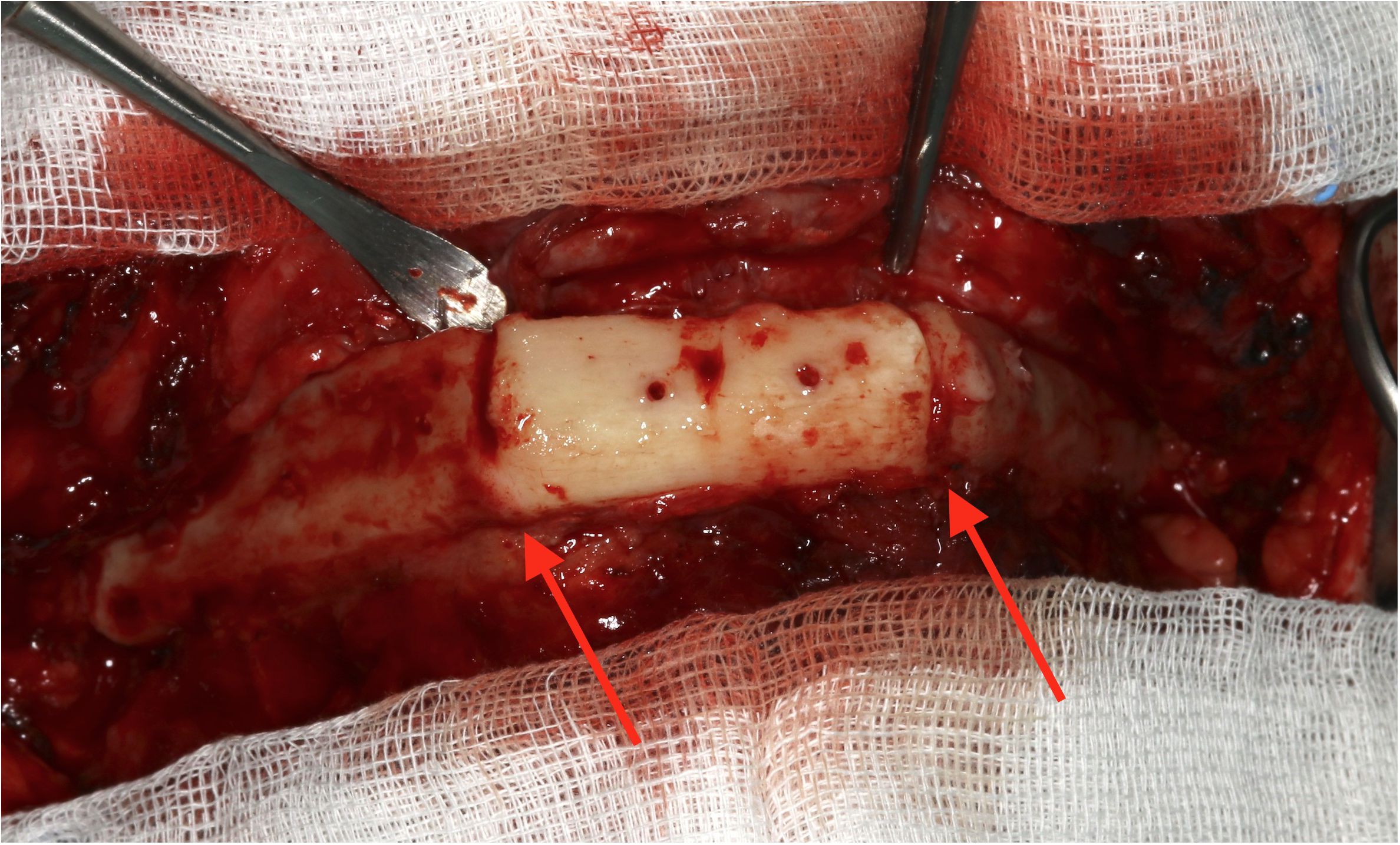
Figure 7 Intraoperative example of clinical complete ossification during removal of a reconstruction plate and before augmentation 7 months after microvascular reconstruction.
At this point, it must be mentioned that although showing faster ossification, the MRONJ group came up with more revisions and wound healing disorders compared to the control group but without significant differences. Bisphosphonates not only affect bone metabolism but also have been shown to reduce cell viability, reduce proliferation, and increase apoptosis in oral keratinocytes and fibroblasts. Moreover, bisphosphonates have been demonstrated to reduce epithelial thickness and prevent epithelial formation in three-dimensional tissue-engineered models of the oral mucosa (32).
A further important aspect of this group of compromised patients is to keep reconstructive surgery short. This study clearly shows that fibula reconstruction is safely possible with successful bony healing within 5 to 6 hours of surgery. Surgery in MRONJ patients can be performed faster because typical oncological steps such as extended tumor resection and neck dissection are not necessary. In comparison with infected osteoradionecrosis (IORN), mandibular reconstruction in MRONJ patients is easier, as typical radiation-induced vessel fibrosis is absent, facilitating vascular anastomosis (33). Apart from technical ease, healing can be challenging in cases with IORN as well as MRONJ.
This study shows for the first time an enhanced ossification of microvascular mandibular reconstructions with free bone grafts in patients with advanced medication-related osteonecrosis of the jaw. Close initial segmental bone contact additionally accelerates ossification. A foregoing therapy with antiresorptive agents is no contraindication for major reconstructive surgery. Surgery in this group of compromised patients with jaw resection and free flap reconstruction can be safely performed with short recovery times.
The datasets presented in this article are not readily available because patients ID are part of the dataset. Requests to access the datasets should be directed to bWF4aW1pbGlhbi5nb3R0c2F1bmVyQHVrci5kZQ==.
The studies involving humans were approved by ethics committee of the University of Regensburg (ref. 23-3559-104). The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements.
MG: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Project administration, Resources, Visualization, Writing – original draft, Writing – review & editing. JM: Conceptualization, Investigation, Validation, Writing – review & editing. JE: Conceptualization, Validation, Writing – review & editing. SE: Conceptualization, Validation, Writing – review & editing. JS: Conceptualization, Validation, Writing – review & editing. MF: Conceptualization, Validation, Writing – review & editing. MM: Conceptualization, Validation, Writing – review & editing. TR: Validation, Writing – review & editing. TE: Conceptualization, Supervision, Validation, Writing – original draft, Writing – review & editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Diel IJ, Solomayer E-F, Costa SD, Gollan C, Goerner R, Wallwiener D, et al. Reduction in new metastases in breast cancer with adjuvant clodronate treatment. New Engl J Med. (1998) 339:357–63. doi: 10.1056/NEJM199808063390601
2. O'Carrigan B, Wong MH, Willson ML, Stockler MR, Pavlakis N, Goodwin A. Bisphosphonates and other bone agents for breast cancer. Cochrane Database Syst Rev. (2017) 10:Cd003474. doi: 10.1002/14651858.CD003474.pub4
3. Nogueira D, Caldas IM, Dinis-Oliveira RJ. Bisphosphonates and osteonecrosis of the jaws: Clinical and forensic aspects. Arch Oral Biol. (2023) 155:105792. doi: 10.1016/j.archoralbio.2023.105792
4. McClung M. Role of RANKL inhibition in osteoporosis. Arthritis Res Ther. (2007) 9:S3. doi: 10.1186/ar2167
5. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2021) 71:209–49. doi: 10.3322/caac.21660
6. Agarwal AR, Librizzi CL, Wessel L, Thakkar SC, Levin AS. The low and disproportionate utilization of antiresorptive therapy in patients with osseous metastasis. J Bone Oncol. (2023) 43:100507. doi: 10.1016/j.jbo.2023.100507
7. Terpos E, Ntanasis-Stathopoulos I. Controversies in the use of new bone-modifying therapies in multiple myeloma. Br J Haematol. (2021) 193:1034–43. doi: 10.1111/bjh.17256
8. Wong RMY, Wong PY, Liu C, Wong HW, Chung YL, Chow SKH, et al. The imminent risk of a fracture-existing worldwide data: a systematic review and meta-analysis. Osteoporos Int. (2022) 33:2453–66. doi: 10.1007/s00198-022-06473-0
9. Mcgreevy C, Williams D. Safety of drugs used in the treatment of osteoporosis. Ther Adv Drug Saf. (2011) 2:159–72. doi: 10.1177/2042098611411012
10. Marx RE. Pamidronate (Aredia) and zoledronate (Zometa) induced avascular necrosis of the jaws: a growing epidemic. J Oral Maxillofac Surg. (2003) 61:1115–7. doi: 10.1016/S0278-2391(03)00720-1
11. Ruggiero SL, Mehrotra B. Bisphosphonate-related osteonecrosis of the jaw: diagnosis, prevention, and management. Annu Rev Med. (2009) 60:85–96. doi: 10.1146/annurev.med.60.063007.134350
12. Taylor KH, Middlefell LS, Mizen KD. Osteonecrosis of the jaws induced by anti-RANK ligand therapy. Br J Oral Maxillofac Surg. (2010) 48:221–3. doi: 10.1016/j.bjoms.2009.08.030
13. Khan AA, Morrison A, Kendler DL, Rizzoli R, Hanley DA, Felsenberg D, et al. Case-based review of osteonecrosis of the jaw (ONJ) and application of the international recommendations for management from the international task force on ONJ. J Clin Densitom. (2017) 20:8–24. doi: 10.1016/j.jocd.2016.09.005
14. Carlson ER. Management of antiresorptive osteonecrosis of the jaws with primary surgical resection. J Oral Maxillofac Surg. (2014) 72:655–7. doi: 10.1016/j.joms.2013.12.007
15. Ristow O, Rückschlos T, Bodem J, Berger M, Bodem E, Kargus S, et al. Double-layer closure techniques after bone surgery of medication-related osteonecrosis of the jaw - A single center cohort study. J Craniomaxillofac Surg. (2018) 46:815–24. doi: 10.1016/j.jcms.2018.03.005
16. Caldroney S, Ghazali N, Dyalram D, Lubek JE. Surgical resection and vascularized bone reconstruction in advanced stage medication-related osteonecrosis of the jaw. Int J Oral Maxillofac Surg. (2017) 46:871–6. doi: 10.1016/j.ijom.2017.01.023
17. Sacco R, Sacco N, Hamid U, Ali SH, Singh M, Blythe JSJ. Microsurgical reconstruction of the jaws using vascularised free flap technique in patients with medication-related osteonecrosis: A systematic review. BioMed Res Int. (2018) 2018:9858921. doi: 10.1155/2018/9858921
18. Hanasono MM, Militsakh ON, Richmon JD, Rosenthal EL, Wax MK. Mandibulectomy and free flap reconstruction for bisphosphonate-related osteonecrosis of the jaws. JAMA Otolaryngol Head Neck Surg. (2013) 139:1135–42. doi: 10.1001/jamaoto.2013.4474
19. Neto T, Horta R, Balhau R, Coelho L, Silva P, Correia-Sá I, et al. Resection and microvascular reconstruction of bisphosphonate-related osteonecrosis of the jaw: The role of microvascular reconstruction. Head Neck. (2016) 38:1278–85. doi: 10.1002/hed.24395
20. Jewer DD, Boyd JB, Manktelow RT, Zuker RM, Rosen IB, Gullane PJ, et al. Orofacial and mandibular reconstruction with the iliac crest free flap: a review of 60 cases and a new method of classification. Plast Reconstr Surg. (1989) 84:391–403; discussion 404–5. doi: 10.1097/00006534-198909000-00001
21. Fu LJ, Tang TT, Hao YQ, Dai KR. Long-term effects of alendronate on fracture healing and bone remodeling of femoral shaft in ovariectomized rats. Acta Pharmacol Sin. (2013) 34:387–92. doi: 10.1038/aps.2012.170
22. Tatli U, Ustün Y, Kürkçü M, Erdoğan O, Gürbüz CC, Ozgür H, et al. Effects of zoledronic acid on healing of mandibular fractures: an experimental study in rabbits. J Oral Maxillofac Surg. (2011) 69:1726–35. doi: 10.1016/j.joms.2010.07.063
23. Kates SL, Ackert-Bicknell CL. How do bisphosphonates affect fracture healing? Injury. (2016) 47:S65–8. doi: 10.1016/S0020-1383(16)30015-8
24. Oh H, Kwon D, Ahn J, Paeng JY. Reconstruction of mandibular defects in osteoradionecrosis and medication-related osteonecrosis of the jaw using fibula free flap and management of postoperative wound infections. Maxillofac Plast Reconstr Surg. (2022) 44:37. doi: 10.1186/s40902-022-00366-2
25. Baron R, Ferrari S, Russell RG. Denosumab and bisphosphonates: different mechanisms of action and effects. Bone. (2011) 48:677–92. doi: 10.1016/j.bone.2010.11.020
26. Gottsauner M, Fehrer C, Spoerl S, Schuderer J, Zeman F, Fiedler M, et al. Influence of radiotherapy on ossification of vascularized osseous reconstruction of the jaw: A radiological retrospective cohort study based on panoramic radiographs. J Clin Med. (2022) 11. doi: 10.3390/jcm11175041
27. Swendseid B, Kumar A, Sweeny L, Zhan T, Goldman RA, Krein H, et al. Natural history and consequences of nonunion in mandibular and maxillary free flaps. Otolaryngology–Head Neck Surg. (2020) 163:956–62. doi: 10.1177/0194599820931069
28. van Baar GJC, Forouzanfar T, Liberton NPTJ, Winters HAH, Leusink FKJ. Accuracy of computer-assisted surgery in mandibular reconstruction: A systematic review. Oral Oncol. (2018) 84:52–60. doi: 10.1016/j.oraloncology.2018.07.004
29. Knitschke M, Yonan M, Roller FC, Pons-Kühnemann J, Attia S, Howaldt HP, et al. Osseous Union after Jaw Reconstruction with Fibula-Free Flap: Conventional vs. CAD/CAM Patient-Specific Implants. Cancers (Basel). (2022) 14. doi: 10.3390/cancers14235774
30. Padilla PL, Mericli AF, Largo RD, Garvey PB. Computer-aided design and manufacturing versus conventional surgical planning for head and neck reconstruction: A systematic review and meta-analysis. Plast Reconstr Surg. (2021) 148:183–92. doi: 10.1097/PRS.0000000000008085
31. Stopeck AT, Fizazi K, Body JJ, Brown JE, Carducci M, Diel I, et al. Safety of long-term denosumab therapy: results from the open label extension phase of two phase 3 studies in patients with metastatic breast and prostate cancer. Support Care Cancer. (2016) 24:447–55. doi: 10.1007/s00520-015-2904-5
32. Bullock G, Miller CA, Mckechnie A, Hearnden V. A review into the effects of pamidronic acid and zoledronic acid on the oral mucosa in medication-related osteonecrosis of the jaw. Front Oral Health. (2021) 2:822411. doi: 10.3389/froh.2021.822411
Keywords: ossification, MRONJ, antiresorptive agent, microvascular reconstruction, jaw, mandible, fibula
Citation: Gottsauner M, Meier J, Eichberger J, Eckmüller S, Schuderer J, Fiedler M, Maurer M, Reichert TE and Ettl T (2024) Antiresorptive agents enhance ossification of free flap reconstructions of the mandible: a radiological retrospective cohort study. Front. Oncol. 14:1401165. doi: 10.3389/fonc.2024.1401165
Received: 14 March 2024; Accepted: 23 May 2024;
Published: 12 June 2024.
Edited by:
Steffen Koerdt, Charité University Medicine Berlin, GermanyReviewed by:
Jeremie Oliver Piña, National Institutes of Health (NIH), United StatesCopyright © 2024 Gottsauner, Meier, Eichberger, Eckmüller, Schuderer, Fiedler, Maurer, Reichert and Ettl. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Maximilian Gottsauner, bWF4aW1pbGlhbi5nb3R0c2F1bmVyQHVrci5kZQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.