- Head and Neck Oncology Ward, West China Hospital of Sichuan University, Chengdu, China
Background: Histiocytic sarcoma originates in various tissues, including the skin, lymph nodes, gastrointestinal tract, lungs, bone marrow, and central nervous system. Primary central nervous system histiocytic sarcoma (PCNSHS) is exceptionally rare, known for its aggressive behavior and poor prognosis. This report describes a case of PCNSHS in the cerebellum treated with surgery and radiotherapy.
Case presentation: A 30-year-old woman presented with progressive dizziness and headache. Magnetic resonance imaging scans showed right cerebellar neoplastic lesions approximately 3.6 cm*3.0 cm with cerebral edema and fourth ventricle and brainstem compression. The patient underwent surgical debulking, and the pathological diagnosis was PCNSHS. Two months after the surgery, the patient underwent adjuvant radiotherapy at a dose of 60 Gy. No tumor progression has been observed during the one-year follow-up period.
Conclusions: This case report provides an example of effective central nervous system control using resection and radiation therapy. A review of the literature confirms that surgery alone or combined concurrent or sequential treatment of radiotherapy and chemotherapy is often used; however, the best treatment plan remains unclear. Moreover, the prognosis is poor, with a median survival of six months. Thus, ongoing research aims to better understand the biology of histiocytic sarcomas and find more effective strategies.
1 Introduction
Histiocytic sarcoma (HS) is a rare and aggressive lymphohematopoietic tumor derived from non-Langerhans histiocytic cells of the mononuclear macrophage system (1, 2), which most often involves the lymph nodes and/or gastrointestinal tract (3). Primary central nervous system HS (PCNSHS) is extremely rare, accounting for less than 1% of all lymphohematopoietic neoplasms (4). Although the pathogenesis of HS remains unclear, histologically, PCNSHS is characterized by the infiltration of inflammatory cells in the central nervous system and plays a crucial role in confirming the diagnosis; CD68, CD163, and lysozyme are recognized as typical markers that distinguish PCNSHS from other hematopoietic neoplasms, such as B-cell or T-cell non-Hodgkin’s lymphoma (1, 5–7). Unfortunately, there are no standard or effective treatment strategies for PCNSHS.
Here, we describe a patient with primary cerebellar HS and review the available literature on this aggressive disease.
2 Case description
2.1 Clinical presentation
The treatment timeline is shown in Figure 1. A 30-year-old woman presented with dizziness and headache that had persisted for three months. On February 23, 2023, magnetic resonance imaging (MRI) revealed lamellar abnormal signals at the right cerebellar hemispheres, approximately 3.6 cm*3.0 cm in size, with clear boundaries, slightly low signal on T1WI, equal or slightly high signal on T2WI, low signal envelope on the edge, and unrestricted diffusion. The lesions showed obvious uniform enhancement on the enhanced scan. Abnormal signals with slightly longer T1 and T2 images were observed around the lesions. The adjacent fourth ventricle and brainstem were compressed and narrowed, respectively. The ventricular system was slightly enlarged, and there were a few symmetrical distributions of slightly longer abnormal T1 and T2 signals around the bilateral ventricles (Figure 2A). Preliminary diagnoses were medulloblastoma, pilocytic astrocytoma, or lymphoma.
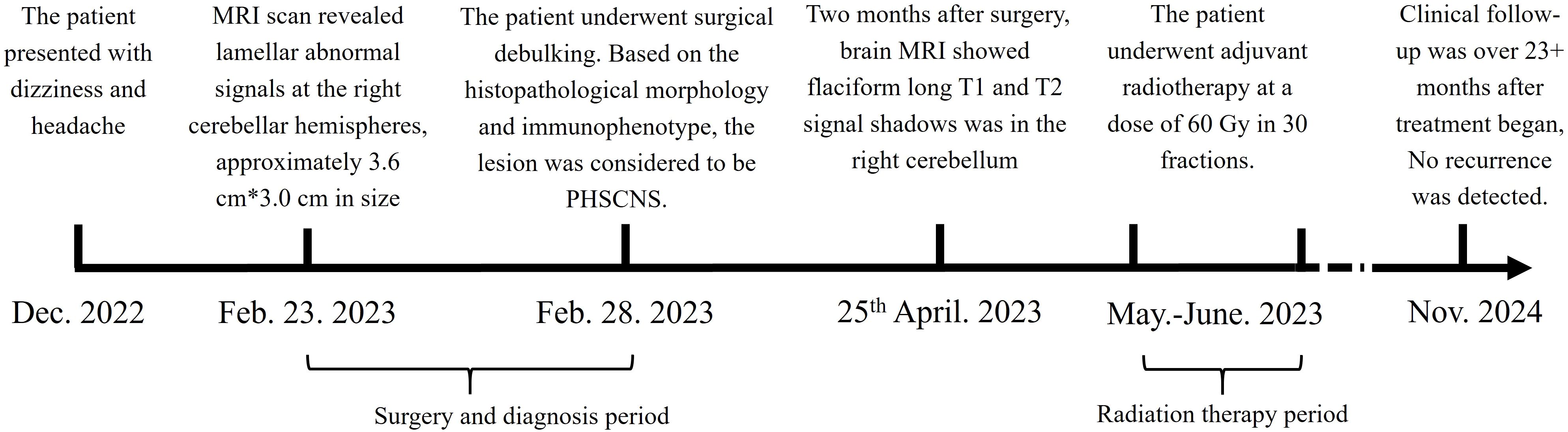
Figure 1. Treatment timeline. The patient suffered from PCNSHS since December. 2022, and diagnosed in February. 2023. After diagnosed, the patient underwent surgical debulking, and received radiation therapy. CR respond reached.
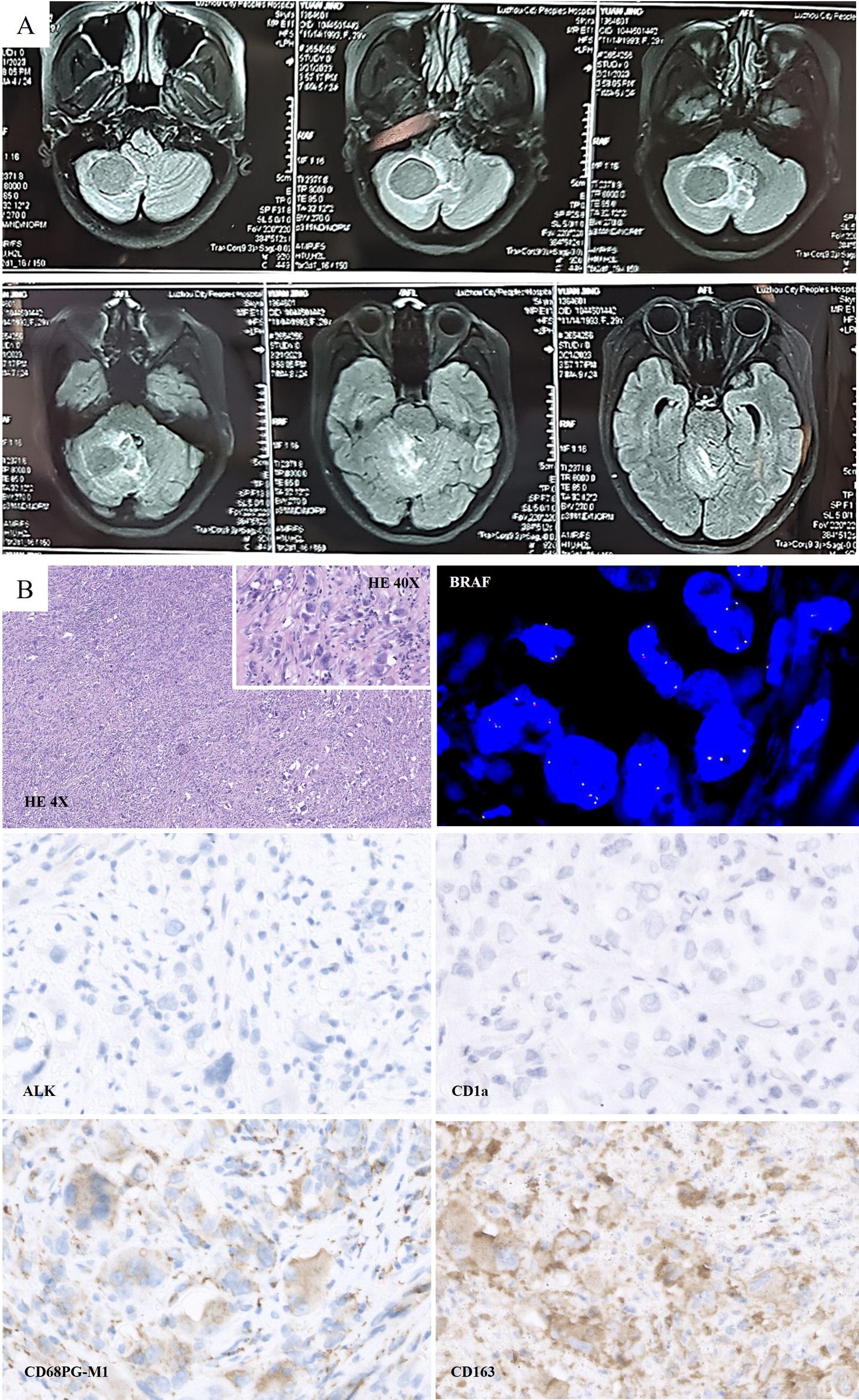
Figure 2. (A) Preoperative Magnetic resonance imaging (MRI) scan on February 2023: Lamellar abnormal signals at the right cerebellar hemispheres were detected, which was approximately 3.6 cm*3.0 cm in size. (B) Histologically, large cells with abundant cytoplasm were presented. Morphologically, CD163 (+), CD68 (PGM1) (+), CD1a (-), and ALK (-) were detected. FISH test: No BRAF gene translocation was detected.
2.1 Treatment and pathology
On February 28, 2023, the patient underwent surgical debulking. Histologically, large cells with abundant cytoplasm were mostly present in cerebellar biopsies. Morphologically, these cells had characteristics of histiocytes, and immunohistochemistry showed GFAP (-), Oligo2 (-), P53 (+), ATRX (+, expressed), HMB45 (-), CK (-), H3K27ME3 (+, expressed), H3K27M (-), CD68 (PGM1) (+), CD163 (+), CD1a (-), langerin (-), and ALK (-). FISH test: No BRAF translocations were detected. Mutations in TERT (P250/P228), BRAF (V600E), or H3F3A/HIS1H3B (K27/G34/K36) were not detected. The examination revealed no clear glial differentiation (Figure 2B). Based on the histopathological morphology and immunophenotype, the lesion was considered to be HS.
On April 25, 2023, two months after surgery, brain MRI showed falciform long T1 and T2 signal shadows was in the right cerebellum, FLAIR represented circular high signal, and annular enhancement was observed on the contrast scan. For staging evaluation, chest and abdominal Computed Tomography (CT), positron emission tomography (PET), and bone marrow examinations were performed (Figure 3A). Only the brain lesions were found. To decrease the risk of recurrence, the patient underwent adjuvant radiotherapy at a dose of 60 Gy in 30 fractions. CT with a fixed mask was performed with the patient in the supine position. The RayStation treatment planning system (RaySearch Laboratories, USA) was used for radiation therapy, contouring, and planning. The daily dose of 2.0 Gy was delivered to the patient with an Elekta Linear Accelerator (Edge™) using intensity-modulated radiation therapy plus volumetric modulated arc therapy (Figure 3B). The patients were evaluated weekly for radiation toxicity. After adjuvant RT was completed, head MRI was obtained every three months, and no evidence of residual neoplasm or tumor recurrence was found after RT. The patient remains in complete remission (Figure 3C).
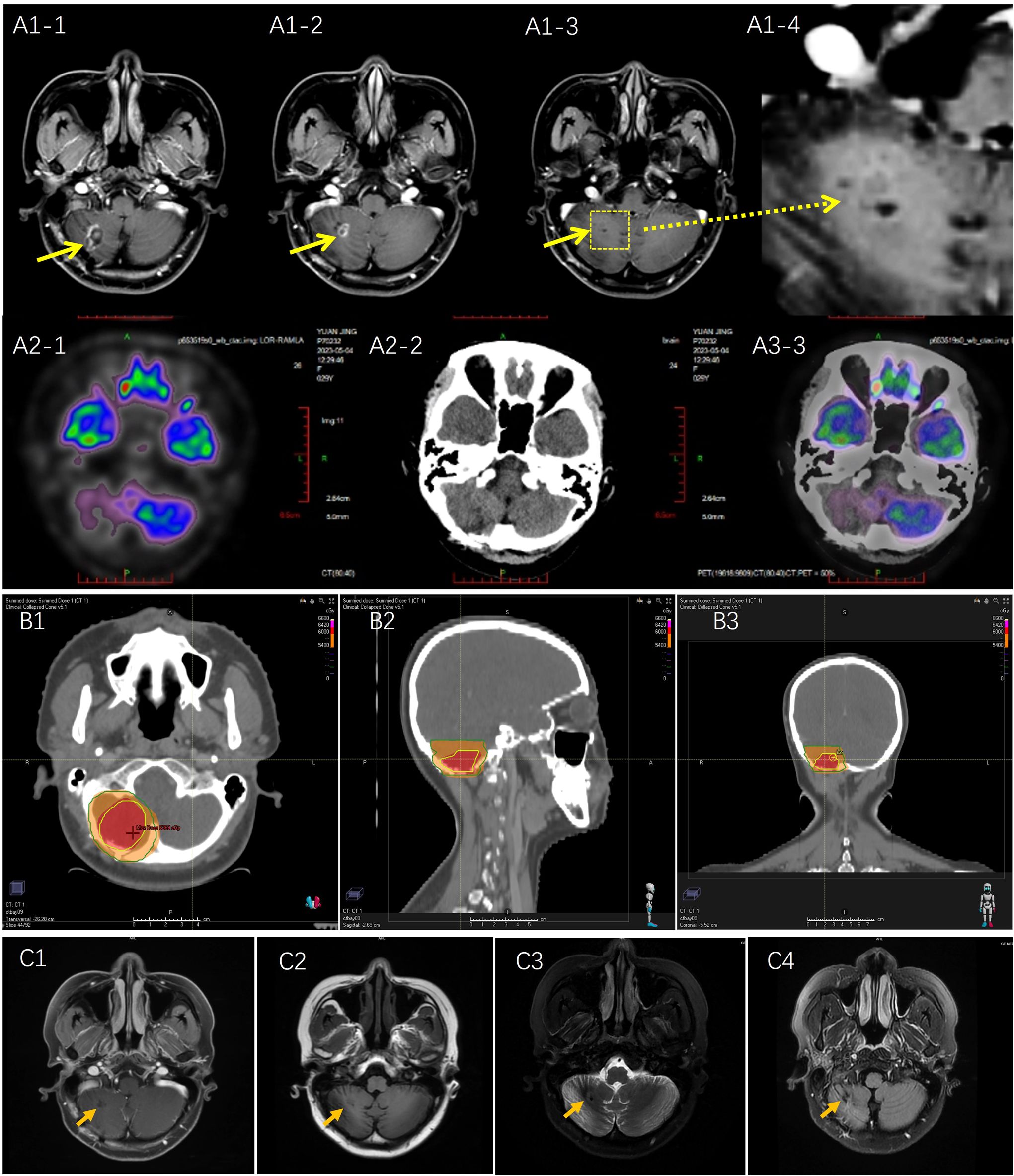
Figure 3. (A) Postoperative Magnetic resonance imaging (MRI) and Positron Emission Tomography (PET) scan on April 2023: (A1) MRI scan: long T1 and T2signal shadows were observed with annular enhancement; (A2) PET scan no metastasis and recurrent tumor were detected. (B) Radiotherapy planning: According to RTOG radiotherapy standard, tumor bed as the gross target volume (GTV) was delineated based on MRI T1 enhanced image and T2 Flair image, including postoperative area and edema area, and expanding the GTV range by 2.0 cm became clinical target volume (CTV). Red area: GTV was at a dose of 60 Gy; Orange area: CTV area was at a dose of 54 Gy. (C) Brain MRI scan was performed on October 11, 2023.No new recurrent lesion was detected. (C1) T1 enhancement; (C2) T1 FLAIR; (C3) T2 propeller; (C4) T2 FLAIR.
3 Discussion
According to the literature review, PCNSHS is a rare subtype of HS, and its occurrence in the central nervous system distinguishes it from HSs that may occur in other organs. PCNSHS often presents with non-specific symptoms, including headache, seizures, focal neurological deficits, and changes in mental status.
Neurological HS images showed mainly brain parenchymal involvement with single lesions (60%). Commonly, CT is the first choice for HS lesions, which mostly represent high-density lesions due to the high nuclear-cytoplasmic ratio of tumor cells (8); however, MRI is better than CT. According to the MRI scan, the lesions are mostly round or oval, with clear boundaries, and are often located at the junction of the gray and white matter, which may be accompanied by cystic components, necrotic areas (20%), or hemorrhages (4%). This characteristic is helpful for identifying other diseases; however, it still lacks typical characteristics. The lesions mostly appear as T1 equal or slightly high signal, T2 low or slightly higher signal. PET examination revealed hypermetabolic changes (9).
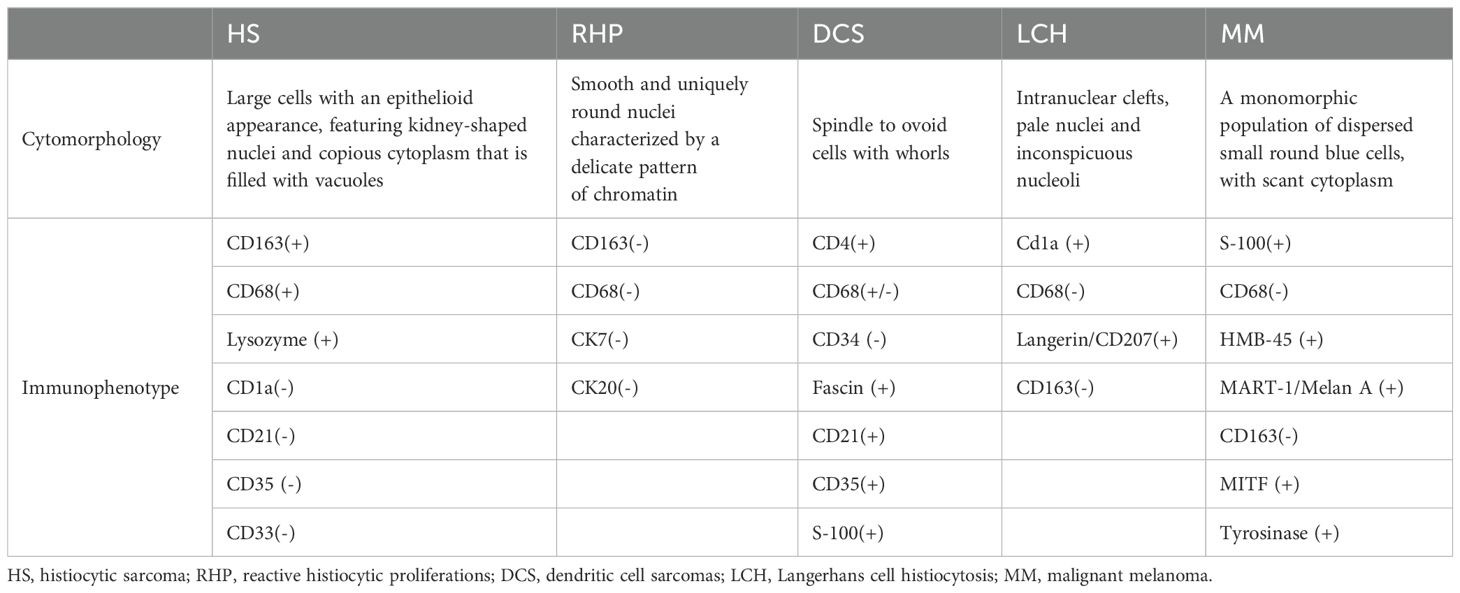
Histologically, PCNSHS is characterized by the infiltration of large pleomorphic histiocytes into the central nervous system (1). Immunohistochemistry plays a crucial role in confirming the diagnosis, with markers, such as CD68, CD163, and lysozyme, being commonly positive, and not expressing specific T or B cell markers, myeloid markers, follicular dendritic cell markers, or epithelial cell markers (1, 8, 10) (Table 1).
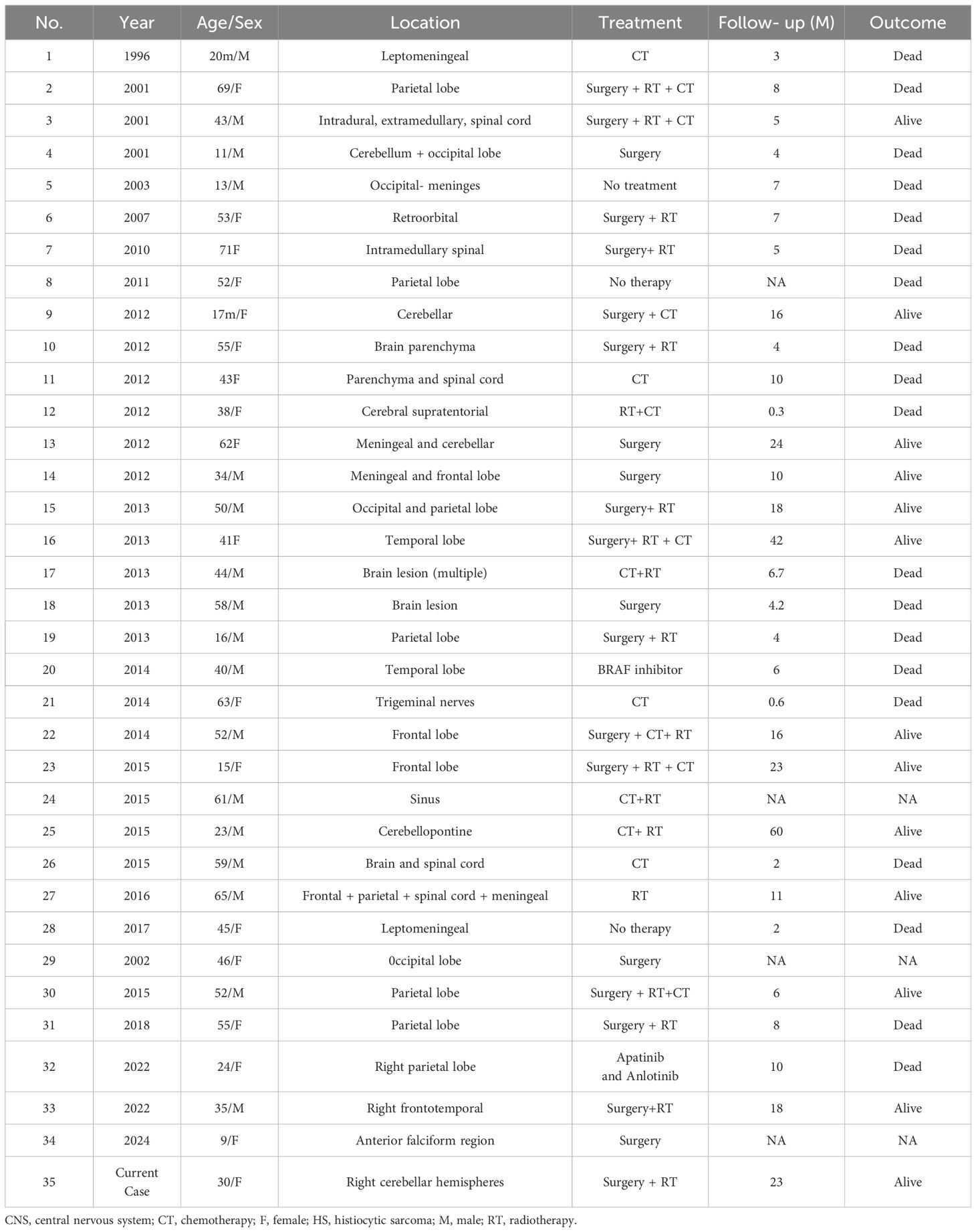
Unfortunately, there is no standard therapy for HS or PHSCNS. Patients were treated using multimodal strategies, such as surgery, chemotherapy, and/or radiation therapy. In contrast, surgical resection combined with adjuvant radiotherapy is recommended for patients with single lesions (7). Patients with multiple lesions have a more aggressive clinical course, and combined chemotherapy is recommended; however, the optimal chemotherapy regimen is unclear, and lymphoma treatment regimens are commonly used, such as the CHOP regimen (cyclophosphamide, doxorubicin, vincristine, and prednisone) alone or combined with Etoposide (10). Additionally, genomic sequencing may play an important role in the identification of molecularly targeted agents and immune checkpoint inhibitors. IDBAIH et al. reported a case of neurological HS with BRAF V600E mutation, and the BRAF inhibitor Vemurafenib was used to achieve a good therapeutic effect in the short term (11), and May et al. reported the use of Dasatinib in a case of PHSCNS with platelet-derived growth factor receptor mutation (4). However, even if PHSCNS received treatment, the median survival time is 7.0 ± 0.98 months (95% confidence interval: 5.08–8.92) and an average survival time is 24.07 ± 5.1 months (95% confidence interval: 14.08–34.06) (Table 2) (2, 12–15).
In this case, the disease was located in the cerebellum, and no metastasis was observed on PET. Thus, only radiation at a dose of 60 Gy was administered after surgery. To date, no recurrence has been reported, and the survival time was 23 months. Meanwhile, such integrated therapies with surgery and radiotherapy might be useful in all cases of sarcoma (16, 17).
4 Conclusion
HS is an extremely rare disease with a poor prognosis, and intensive radiation-based chemotherapy is a treatment option. This case demonstrates that patients can benefit from radiotherapy for localized lesions. Furthermore, owing to its rarity, ongoing research aims to better understand the biology of HS and develop more effective treatment strategies.
Data availability statement
The datasets presented in this article are not readily available because of ethical and privacy restrictions. Requests to access the datasets should be directed to the corresponding author/s.
Ethics statement
The studies involving humans were approved by the West China Hospital of Sichuan University. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
LY: Writing – original draft, Writing – review & editing. ZL: Writing – original draft, Writing – review & editing. ZQ: Writing – review & editing. DJ: Data curation, Writing – review & editing. WF: Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
We would like to thank Professor Zhou Qiao of the West China Hospital for his assistance in confirming the pathological diagnosis.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Takahashi E, Nakamura S. Histiocytic sarcoma: an updated literature review based on the 2008 WHO classification. J Clin Exp Hematopathol. (2013) 53:1–8. doi: 10.3960/jslrt.53.1
2. Shahrokh S, Rakhsha A, Shahin M, Javadzadegan A, Ahadi M, Azghandi S, et al. Successful treatment of central nervous system histiocytic sarcoma with craniectomy and adjuvant radiotherapy. Cureus. (2022) 14:e24690. doi: 10.7759/cureus.24690
3. Toshkezi G, Waddle MR, Miller DH, Stross WC, Kaleem TA, May BC, et al. Primary intramedullary histiocytic sarcoma. World Neurosurg. (2010) 74:523–7. doi: 10.1016/j.wneu.2010.07.002
4. May JM, Waddle MR, Miller DH, Stross WC, Kaleem TA, May BC, et al. Primary histiocytic sarcoma of the central nervous system: a case report with platelet derived growth factor receptor mutation and PD-L1/PD-L2 expression and literature review. Radiat Oncol. (2018) 13:167–174. doi: 10.1186/s13014-018-1115-x
5. So H, Kim SA, Yoon DH, Khang SK, Hwang J, Suh CH, et al. Primary histiocytic sarcoma of the central nervous system. Cancer Res Treat. (2014) 47:322–8. doi: 10.4143/crt.2013.163
6. Campo E, Swerdlow SH, Harris NL, Pileri S, Stein H, Jaffe ES. The 2008 WHO classification of lymphoid neoplasms and beyond: evolving concepts and practical applications. Blood. (2011) 117:5019–32. doi: 10.1182/blood-2011-01-293050
7. Hornick JL, Jaffe ES, Fletcher CDM. Extranodal histiocytic sarcoma: clinicopathologic analysis of 14 cases of a rare epithelioid Malignancy. Am J Surg Pathol. (2004) 28:1133–44. doi: 10.1097/01.pas.0000131541.95394.23
8. Wu W, Sayit AT, Vinters HV, Pope W, Mirsadraei L, Said J. Primary central nervous system histiocytic sarcoma presenting as a postradiation sarcoma: case report and literature review. Hum Pathol. (2013) 44:1177–83. doi: 10.1016/j.humpath.2012.11.002
9. Takahashi E, Sakakibara A, Tsuzuki T, Nakamura S. Case of primary central nervous system histiocytic sarcoma with prominent proliferation of histiocytic cells between the trabeculae of reactive glial cells. Neuropathology. (2018) 38:609–18. doi: 10.1111/neup.2018.38.issue-6
10. Ansari J, Naqash AR, Munker R, El-Osta H, Master S, Cotelingam JD, et al. Histiocytic sarcoma as a secondary Malignancy: pathobiology, diagnosis, and treatment. Eur J Haematol. (2016) 97:9–16. doi: 10.1111/ejh.2016.97.issue-1
11. Idbaih A, Mokhtari K, Emile J-F, Galanaud D, Belaid H, de Bernard S, et al. Dramatic response of a BRAF V600E-mutated primary CNS histiocytic sarcoma to vemurafenib. Neurology. (2014) 83:1478–80. doi: 10.1212/WNL.0000000000000880
12. Wang J, Li T, Chen H, Liu Q. A case of primary central nervous system histiocytic sarcoma. Clin Neurol Neurosurg. (2012) 114:1074–6. doi: 10.1016/j.clineuro.2012.02.009
13. Ma S, Schild M, Tran D, Zhang X, Zhang W-L, Shen S, et al. Primary central nervous system histiocytic sarcoma: A case report and review of literature. Med (Baltimore). (2018) 97:e11271. doi: 10.1097/MD.0000000000011271
14. Wang W, Zhang Y, Dong Y, Li S, Qin H. Primary central nervous system histiocytic sarcoma with somatic NF2 mutation: Case report and review of literature. Clin Neuropathol. (2022) 41:253–62. doi: 10.5414/NP301473
15. Zhang L, Zhang G, Zheng H, Jiang B, Ju Y, Duan Q, et al. A rare case of primary central nervous system histiocytic sarcoma harboring a novel ARHGAP45::BRAF fusion: a case report and literature review. Brain Tumor Pathol. (2024) 4:18–29. doi: 10.1007/s10014-023-00471-8
16. Spatola C, Migliore M, Liardo RLE, Bevilacqua R, Luigi R, Vincenzo S, et al. Follicular dendritic cell sarcoma of mediastinum: a key role of radiotherapy in a multidisciplinary approach. Future Oncol. (2015) 11:57–61. doi: 10.2217/fon.15.315
Keywords: histiocytic sarcoma, central nervous system, cerebellum, radiation, surgery
Citation: Yanchu L, Li Z, Qiongwen Z, Jiayu D and Feng W (2024) Case report: Treatment of a rare primary cerebellum histiocytic sarcoma with surgery and radiotherapy. Front. Oncol. 14:1398350. doi: 10.3389/fonc.2024.1398350
Received: 14 March 2024; Accepted: 20 November 2024;
Published: 20 December 2024.
Edited by:
Liam Chen, University of Minnesota, United StatesReviewed by:
Corrado Spatola, University of Catania, ItalyFarzad Taghizadeh-Hesary, Iran University of Medical Sciences, Iran
Copyright © 2024 Yanchu, Li, Qiongwen, Jiayu and Feng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Li Yanchu, bHljMzI4QHllYWgubmV0; Wang Feng, d2FuZ2Zlbmc1MDI0QDEyNi5jb20=
†These authors share first authorship
 Li Yanchu
Li Yanchu Zhang Li†
Zhang Li† Zhang Qiongwen
Zhang Qiongwen Wang Feng
Wang Feng