- 1TCM Regulating Metabolic Diseases Key Laboratory of Sichuan Province, Hospital of Chengdu University of Traditional Chinese Medicine, Chengdu, Sichuan, China
- 2Second Department of Oncology, Hospital of Chengdu University of Traditional Chinese Medicine, Chengdu, Sichuan, China
Introduction: The detection of Volatile Organic Compounds (VOCs) could provide a potential diagnostic modality for the early detection and surveillance of colorectal cancers. However, the overall diagnostic accuracy of the proposed tests remains uncertain.
Objective: This systematic review is to ascertain the diagnostic accuracy of using VOC analysis techniques and electronic noses (e-noses) as noninvasive diagnostic methods for colorectal cancer within the realm of clinical practice.
Methods: A systematic search was undertaken on PubMed, EMBASE, Web of Science, and the Cochrane Library to scrutinize pertinent studies published from their inception to September 1, 2023. Only studies conducted on human subjects were included. Meta-analysis was performed using a bivariate model to obtain summary estimates of sensitivity, specificity, and positive and negative likelihood ratios. The Quality Assessment of Diagnostic Accuracy Studies 2 tool was deployed for quality assessment. The protocol for this systematic review was registered in PROSPERO, and PRISMA guidelines were used for the identification, screening, eligibility, and selection process.
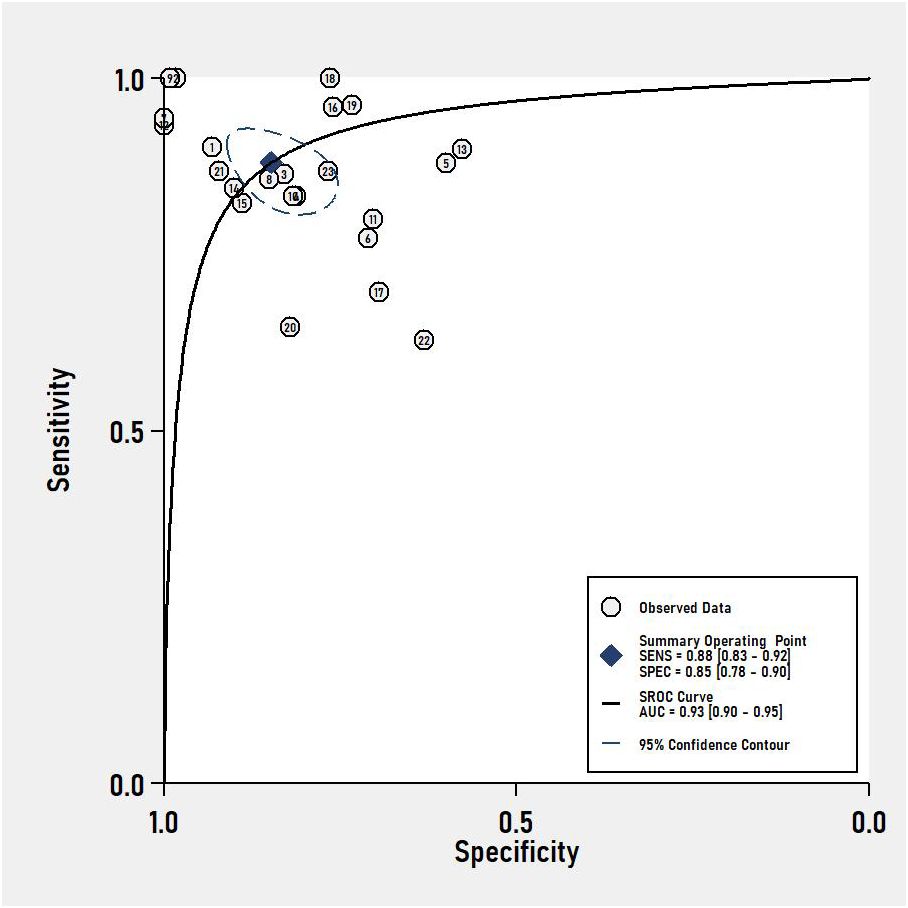
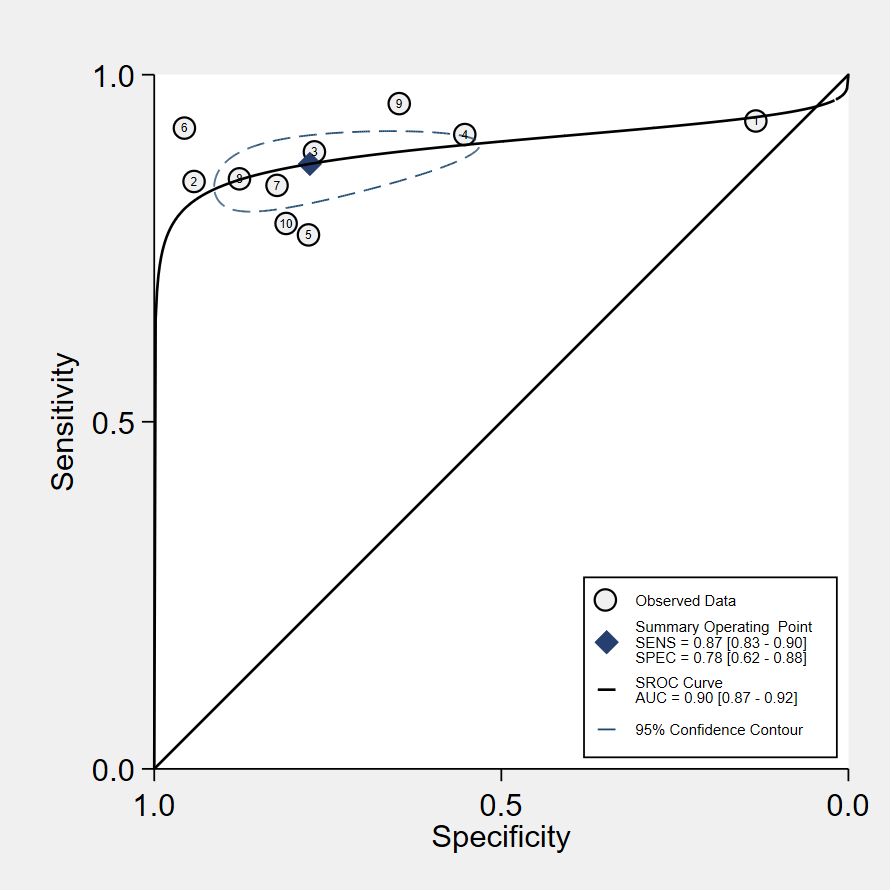
Results: This review encompassed 32 studies, 22 studies for VOC analysis and 9 studies for e-nose, one for both, with a total of 4688 subjects in the analysis. The pooled sensitivity and specificity of VOC analysis for CRC detection were 0.88 (95% CI, 0.83-0.92) and 0.85 (95% CI, 0.78-0.90), respectively. In the case of e-nose, the pooled sensitivity was 0.87 (95% CI, 0.83-0.90), and the pooled specificity was 0.78 (95% CI, 0.62-0.88). The area under the receiver operating characteristic analysis (ROC) curve for VOC analysis and e-noses were 0.93 (95% CI, 0.90-0.95) and 0.90 (95% CI, 0.87-0.92), respectively.
Conclusion: The outcomes of this review substantiate the commendable accuracy of VOC analysis and e-nose technology in detecting CRC. VOC analysis has a higher specificity than e-nose for the diagnosis of CRC and a sensitivity comparable to that of e-nose. However, numerous limitations, including a modest sample size, absence of standardized collection methods, lack of external validation, and a notable risk of bias, were identified. Consequently, there exists an imperative need for expansive, multi-center clinical studies to elucidate the applicability and reproducibility of VOC analysis or e-nose in the noninvasive diagnosis of colorectal cancer.
Systematic review registration: https://www.crd.york.ac.uk/prospero/#recordDetails, identifier CRD42023398465.
1 Introduction
Colorectal carcinoma (CRC) stands as a substantial global public health concern, with an estimated 1.93 million new cases and 0.93 million deaths in 2020 (1). CRC is known to develop from precursor lesions, in most cases adenomas, through the adenoma-carcinoma sequence (2) which can be diagnosed earlier through screening even in its early stages. Through standardized early diagnosis and treatment, the 5-year survival rate for early-stage CRC could exceed 90% (1). Fecal immunochemical test (FIT) and colonoscopy screening for colorectal cancer are pivotal tools for early diagnosis of colorectal cancer (3). However, the detection performance of FIT falls short, with a miss detection rate of 9-29% for CRC and 60-75% for advanced CRC (4). FIT-positive patients are recommended to undergo colonoscopy, but colonoscopy is painful, expensive, and invasive, with the risk of complications such as perforation and bleeding. So not all FIT-positive individuals undergo regular colonoscopy follow-up (5, 6). Therefore, there is an urgent need for convenient, non-invasive, reliable, simple, and cost-effective diagnostic methods to enhance early diagnosis and screening of colorectal cancer.
The analysis of Volatile organic compounds (VOCs) has been applied as a novel and promising diagnostic technique for exploration of non-invasive colorectal neoplasia biomarker. VOCs constitute the by-products of biochemical processes within the human body and typically mirror metabolic states (7, 8). Pathological conditions precipitate aberrant metabolic processes, resulting in a marked increase in VOC production (9). Investigations into cancer-related VOCs have explored various matrices, including breath, blood, urine, saliva, and feces (10–13). Many studies have demonstrated that the applicability of VOC analysis could be used in cancer diagnosis (14–20).
The electronic nose (e-nose) emerges as an instrument equipped with a suite of sensors endowed with specificity and an adept pattern recognition system capable of discerning both simple and complex odors (21). As a relatively recent development, the e-nose has become widely accepted for detecting diseases, owing to its portability, expeditious, cost-effective, and user-friendly diagnostic capabilities, rendering it particularly suited for routine clinical applications. Multiple researchers (22–24) have substantiated the commendable diagnostic accuracy of available e-nose technologies across diverse indications. Notably, van Keulen et al. (25) analyzed exhaled breath from patients with CRC and advanced adenomas (AAs), proving that the Aeonose electronic nose can distinguish CRC and AAs from controls. Additionally, de Meij et al. (26) reported an e-nose sensitivity of 0.85 and a specificity of 0.87 in CRC detection.
Our article aims to systematically review published studies on VOC analysis and e-nose technology concerning colorectal cancer (CRC) detection. Furthermore, we aim to compare their diagnostic performance, with the aspiration of offering a valuable reference for the application of diagnostic techniques in CRC diagnosis.
2 Methods
2.1 Registration
This systematic review has been registered with PROSPERO, under registration number CRD42023398465. The Preferred Reporting Items for Systematic Review and Meta-Analysis (PRISMA) guidelines were adhered to in both the identification and reporting phases of this review (27).
2.2 Search strategy
A comprehensive literature search encompassing PubMed, Embase, Cochrane Library, and Web of Science was conducted from inception up to September 1, 2023. This search, void of language or data publication restrictions, utilized keywords such as “Volatile Organic Compounds,” “VOCs,” “electronic nose,” “e-nose,” “Colorectal neoplasms,” and “diagnosis” or “diagnostic” as search strategy terms. A detailed search strategy is provided in the Supplement.
2.3 Study selection
A total of 192 articles were retrieved. The eligibility of each article was assessed through a meticulous examination of titles and abstracts by two independent reviewers (Y.F. and S.Y.T.). Inclusion criteria were as follows: (1) studies conducted on adult subjects; (2) studies involving colorectal patients; and (3) studies that identified evaluating the diagnostic accuracy of using VOC analysis or e-nose technology. Exclusion criteria encompassed: (1) studies lacking information on the number of cases, controls, sensitivity, and specificity; and (2) studies published as review articles or case reports. Discrepancies between reviewers were resolved through consensus or, if necessary, with the involvement of a third investigator (Q.L.W.). A total of 32 articles met the inclusion criteria and were subsequently included in this systematic review.
2.4 Data collection process
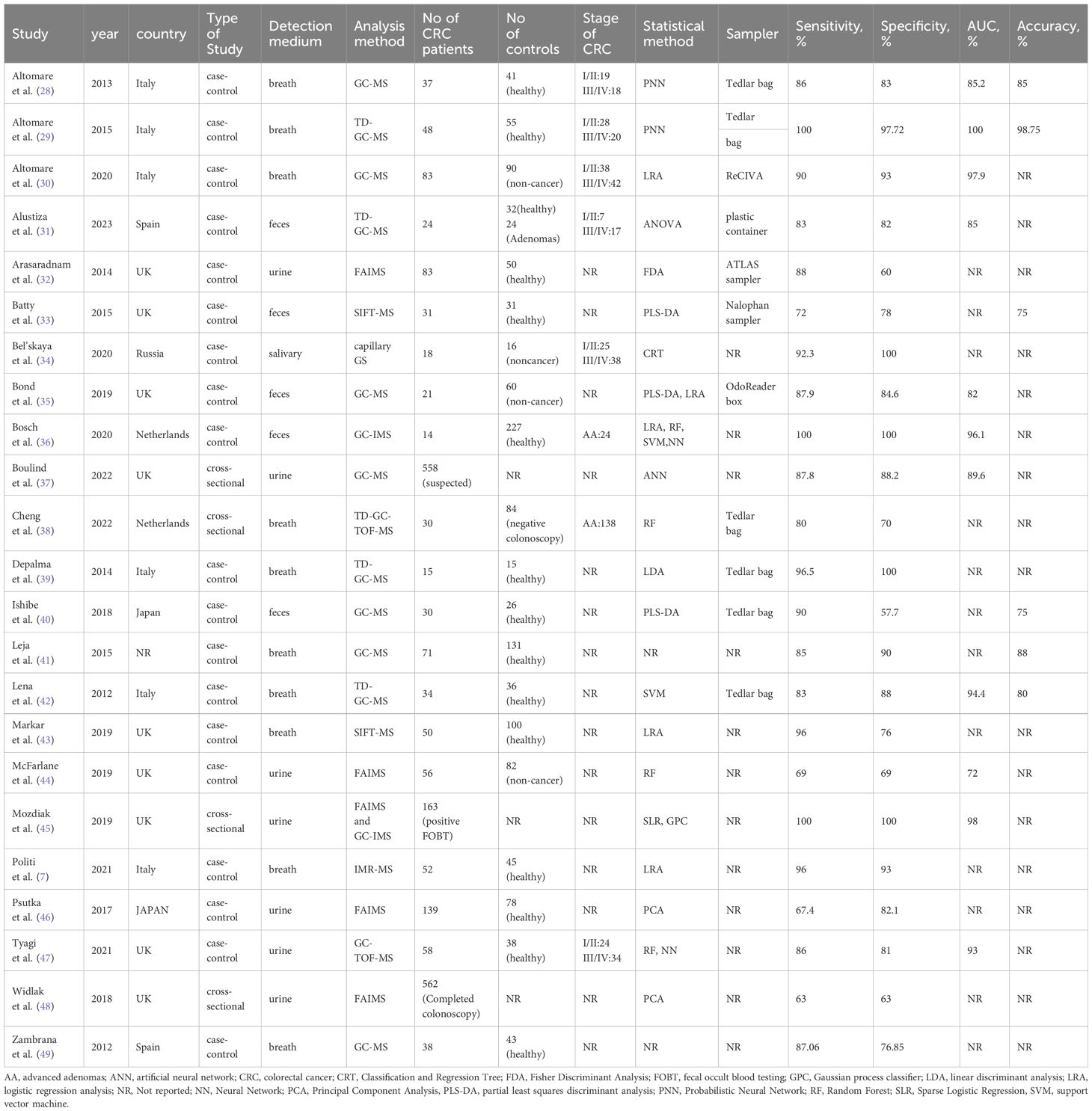
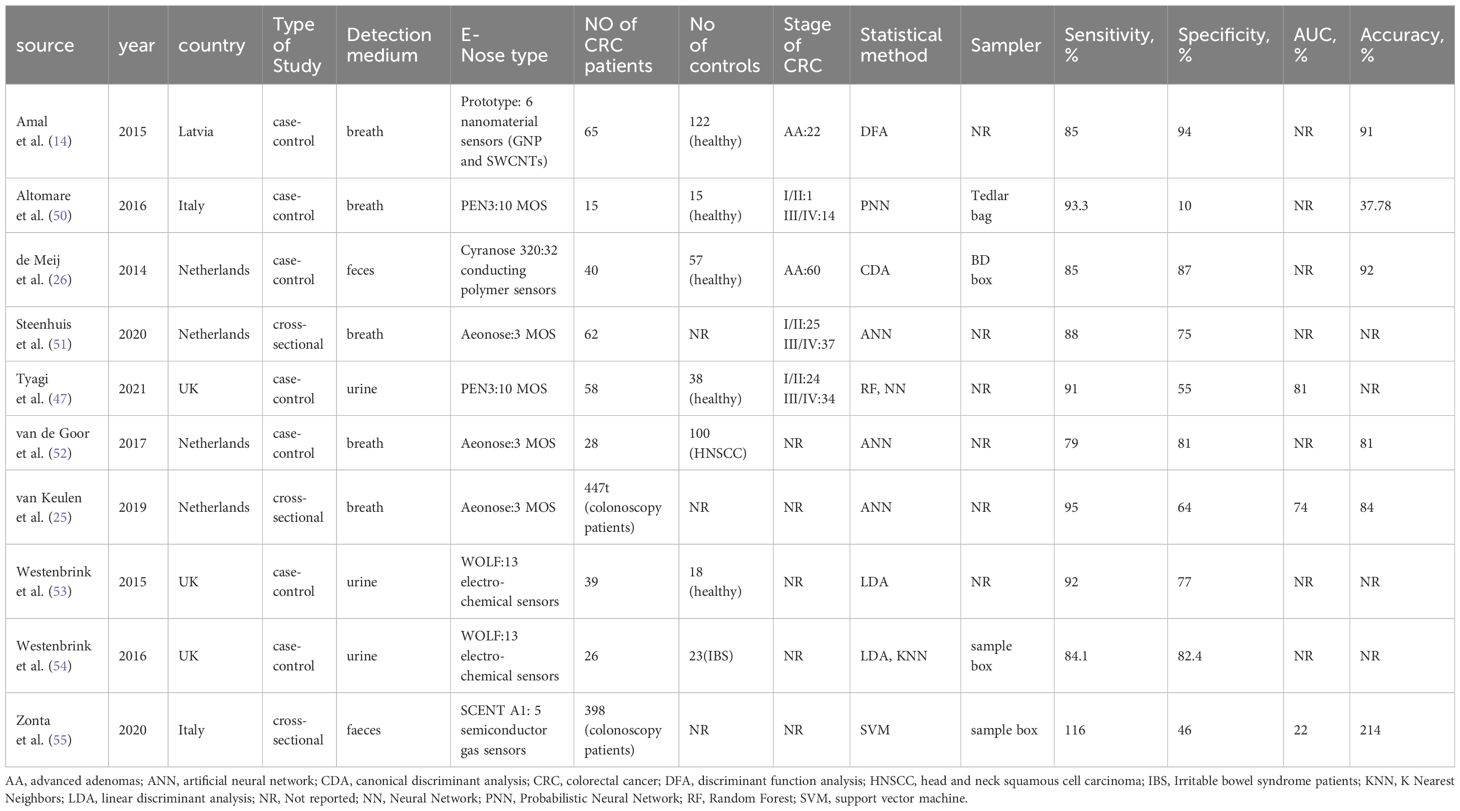
The data extraction and tabulation process from the selected studies was undertaken by two reviewers (S.Y.T. and R.Y.Z.). Tables 1, 2 summarized basic study characteristics, including authorship, country and year of publication, study type, detection medium, analysis method, sample size, CRC stage, statistical analysis methodology, sampler, sensitivity, specificity, and the area under the curve (AUC), as well as accuracy.
2.5 Quality assessment
The Quality Assessment of Diagnostic Studies 2 tool (QUADAS-2) (56) was conducted to assess the quality of the included studies. This evaluation encompassed four domains: patient selection, index test, reference standard, and patient flow and timing. Ratings were assigned as “low risk,” “unclear,” or “high risk”. The assessment was conducted independently by two investigators (Y.F.J. and Z.H.L.), and any disparities were resolved through the involvement of a third investigator (X.P.H). The complete QUADAS-2 version can be found in Supplement.
2.6 Statistical analysis
This meta-analysis was performed by a bivariate model to obtain summary estimates of sensitivity, specificity, and positive and negative likelihood ratios. The Deeks funnel plot asymmetry test was employed to discern publication bias (57). A two-sided P<0.10 was deemed statistically significant. Statistical heterogeneity was evaluated among pooled studies using I2 index. STATA software (version 16 SE; Stata Corporation, College Station, TX, USA) was used to aggregate analysis and the statistical package MIDAS was used for bivariate meta-analysis and summary receiving operate characteristic (SROC) curve calculation with 95% confidence region. Subgroup analyses were performed by Open Meta-Analyst software to explore sources of heterogeneity based on the characteristics of the included articles.
3 Results
3.1 Study selection
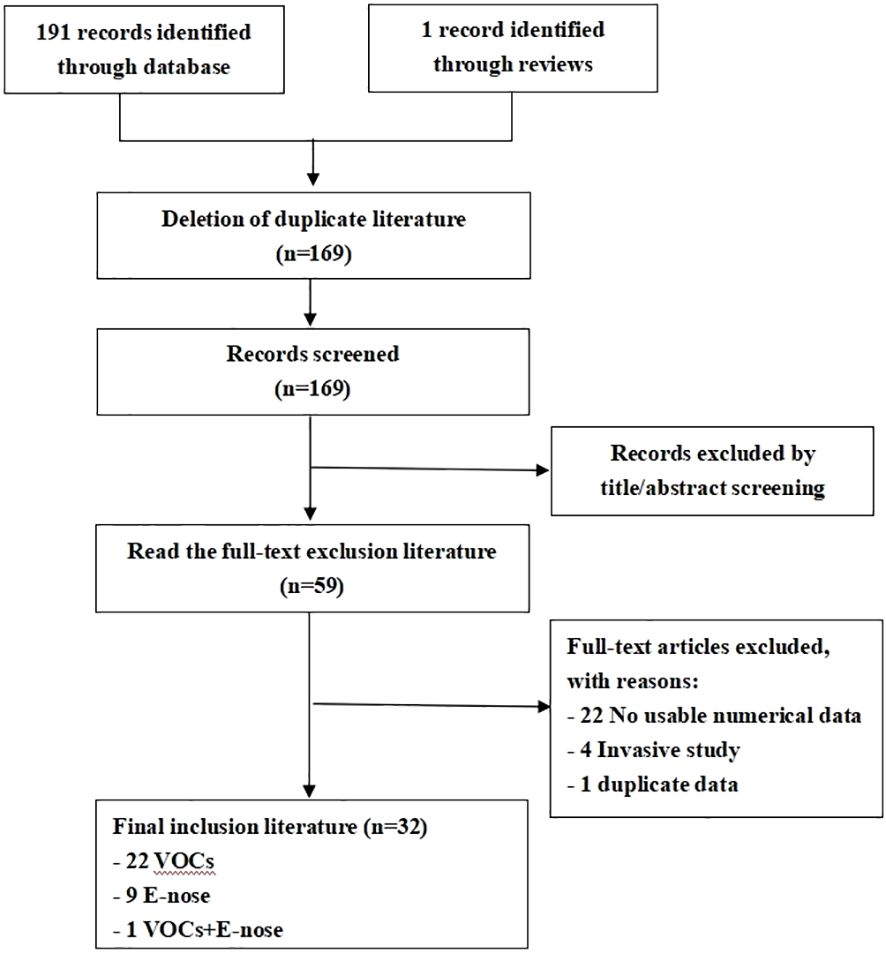
The literature search strategy yielded an initial pool of 192 articles. Following review, 110 articles were excluded based on title and abstract screening. Subsequently, 59 full-text articles, with a total of 4688 subjects underwent scrutiny against the inclusion criteria. Ultimately, 32 studies fulfilled the inclusion criteria for this review. The selection process of the studies is shown in the PRISMA diagram-Figure 1.
3.2 Study characteristics
All thirty-two studies included in this review were published in English (7, 25, 26, 28–55, 58). Among them, 22 studies employed VOC analysis for the diagnosis of colorectal cancer (7, 28–46, 48, 49), 9 studies utilized e-nose technology (25, 26, 50–55, 58), and one study used both VOC analysis and e-nose (47). In the VOC studies, 10 studies used breath samples (7, 28–30, 38, 39, 41–43, 49), 6 studies used urine samples (32, 37, 44–46, 48), 5 studies used fecal samples (31, 33, 35, 36, 40), and one study used salivary sample (34). Most studies used MS-based techniques, principally GC-MS (n=7), TD-GC-MS (n=4), FAIM (n=4), and SIFT-MS (n=2). In E-nose studies, 5 studies used breath samples (25, 50–52, 58), two studies used urine samples (53, 54), and two studies used fecal samples (26, 55). One study used both VOC analysis and e-nose technology in testing urine samples (47). The most commonly used e-noses were Aeonose (n=3), PEN3 (n=2), and WOLF (n=2). All studies were prospective, 25 were case-control studies, and 7 employed cross-sectional studies. Logistic regression analysis (LRA) and partial least squares discriminant analysis (PLS-DA) emerged as the most frequently reported analytical methods. Other reported analytical methods encompassed artificial neural network (ANN), support vector machine (SVM), linear discriminant analysis (LDA), random forest (RF), probabilistic neural network (PNN), discriminant function analysis (DFA), and neural network (NN). The majority of studies were conducted in hospital settings, with 29 studies in Europe, two in Asia, and one with an undisclosed location. Tables 1, 2 provides an overview of the fundamental characteristics of the studies.
3.3 Risk of bias
The quality appraisal of all incorporated literature was conducted according to the QUADAS-2 scale through Review Manager 5.4 software. The results of the risk of bias assessment are visually presented in Figures 2A, B.
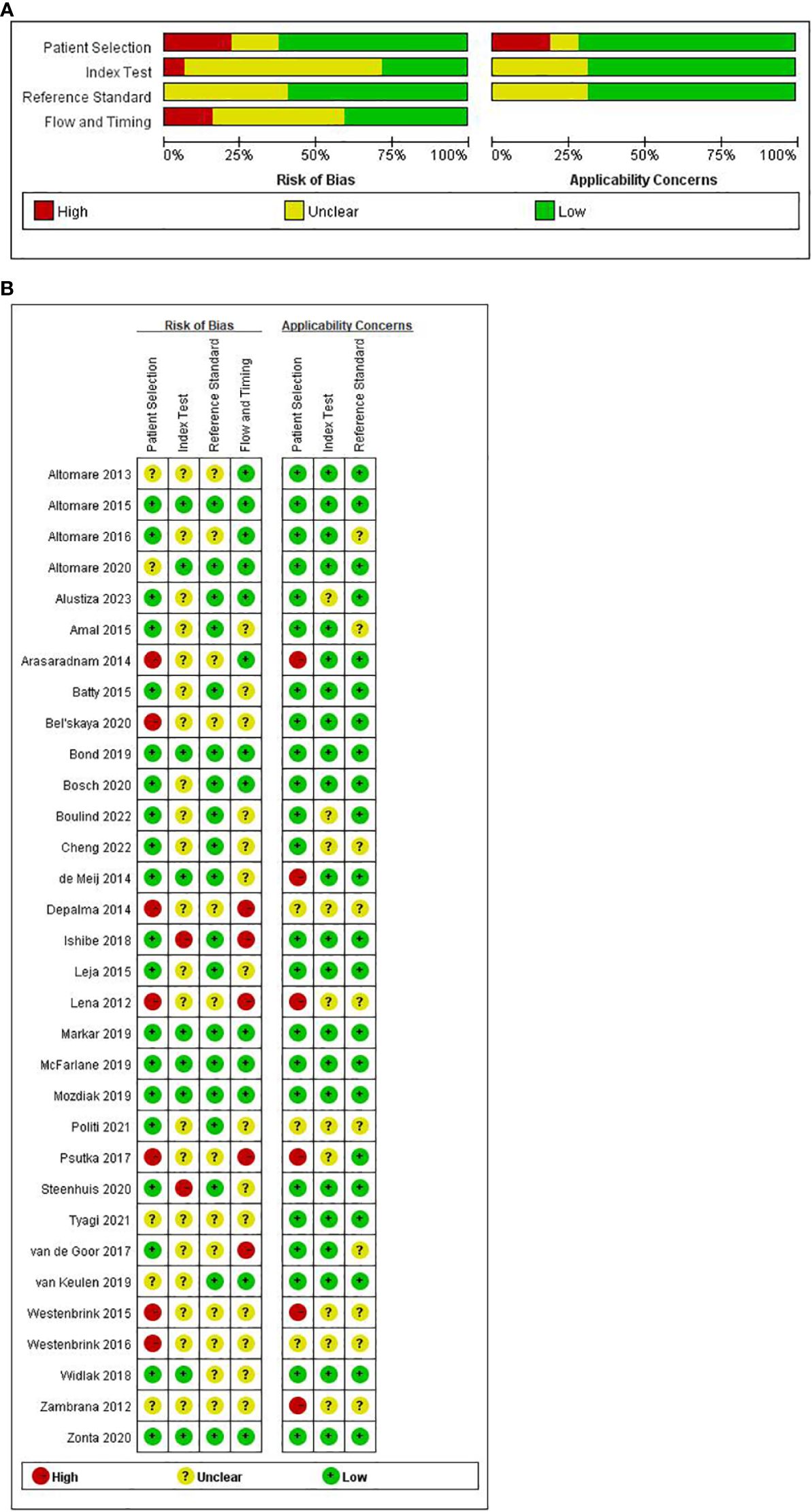
Figure 2 (A) Summary and separate outcome of risk of bias and concerns. (B) Summary and separate outcome of risk of bias and concerns regarding applicability for included studies using QUADAS-2 tool.
In the aggregate, a few studies exhibited a high risk of bias. Concerning ‘patient selection’ seven studies (32, 34, 39, 42, 46, 53, 54) (21.9%) incurred a high risk of bias. The primary contributor to this high risk pertained to the absence of a detailed description of the sampling process and the implementation of a case-control study design. Regarding the ‘index test’ while most studies employed reference diagnostic tests to delineate the definition of a positive test, only nine studies ensured adequate blinding (26, 29, 30, 35, 43–45, 48, 55), leaving 23 studies with an unspecified risk of bias concerning the ‘index test’. Concerning ‘reference standard’, none of the 13 studies (28, 32, 34, 39, 42, 46–50, 52–54) reported the reference standard test. Concerning ‘flow and timing’, five studies (39, 40, 42, 46, 52) faced a high risk of bias. The primary reason for this was that these studies do not account for the time interval between the index test and the reference test.
In evaluating clinical applicability, significant concerns in patient selection arose from the absence of matched patient groups, inadequate patient selection criteria, and applicability of the study design to the research question. Six studies exhibited a high applicability concern for patient selection criteria (26, 32, 42, 46, 49, 53). No high-risk concerns were identified regarding the applicability of the index and reference tests to the research questions.
3.4 Diagnostic accuracy
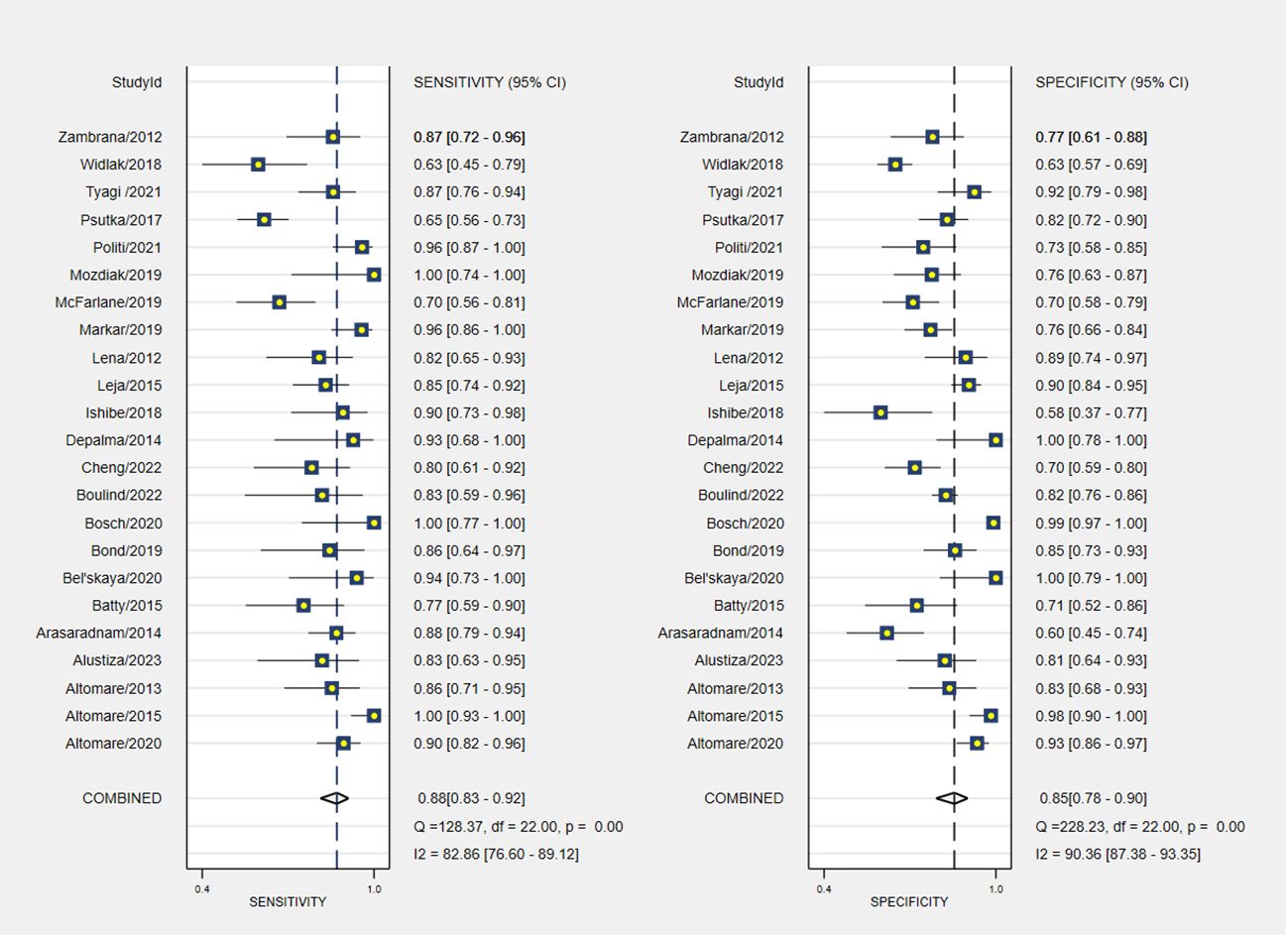
The pooled sensitivity and specificity of VOC analysis for detecting CRC were 0.88 (95% CI, 0.83-0.92) and 0.85 (95% CI, 0.78-0.90), respectively (Figure 3). Similarly, the pooled sensitivity of the e-nose was 0.87 (95% CI, 0.83-0.90), with a specificity of 0.78 (95% CI, 0.62-0.88) (Figure 4). Notably, in VOC studies, the I2 index was 82.86% for sensitivity and 90.36% for specificity, while for e-nose studies, it was 23.31% for sensitivity and 89.46% for specificity. Pooled receiver operating characteristic analysis of VOC studies resulted in an area under the curve (AUC) of 0.93 (95% CI, 0.90-0.95) (Figure 5). For e-nose studies, the AUC was 0.90 (95% CI, 0.87-0.92) (Figure 6). The Positive Likelihood Ratio (PLR), Negative Likelihood Ratio (NLR), and Diagnostic Odds Ratio (DOR) of VOC studies were 5.8 (95% CI, 3.9-8.7), 0.14 (95% CI, 0.09-0.21), and 41 (95% CI, 19-87), respectively. For e-nose studies, the PLR, NLR, and DOR were 3.9 (95% CI, 2.2-6.7), 0.17 (95% CI, 0.13-0.21), and 23 (95% CI, 13-44), respectively.
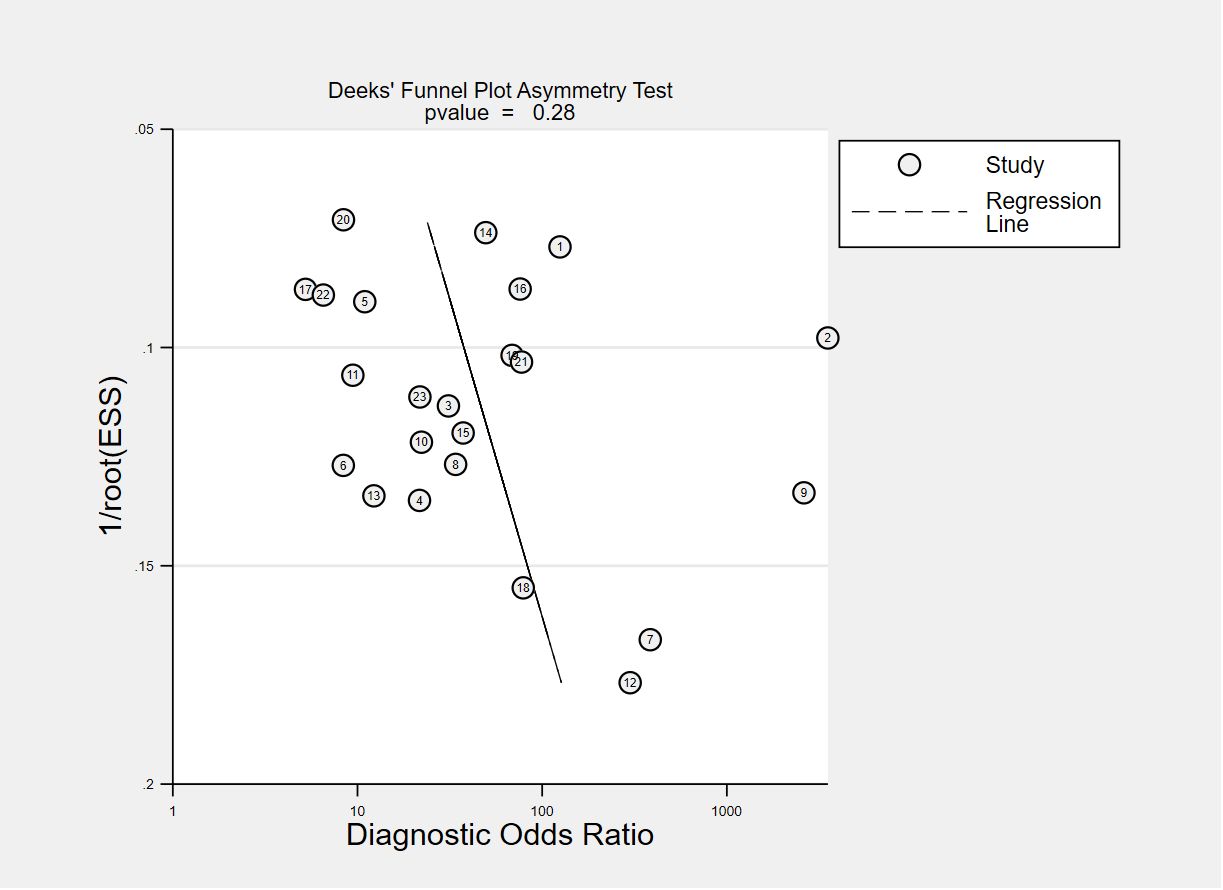
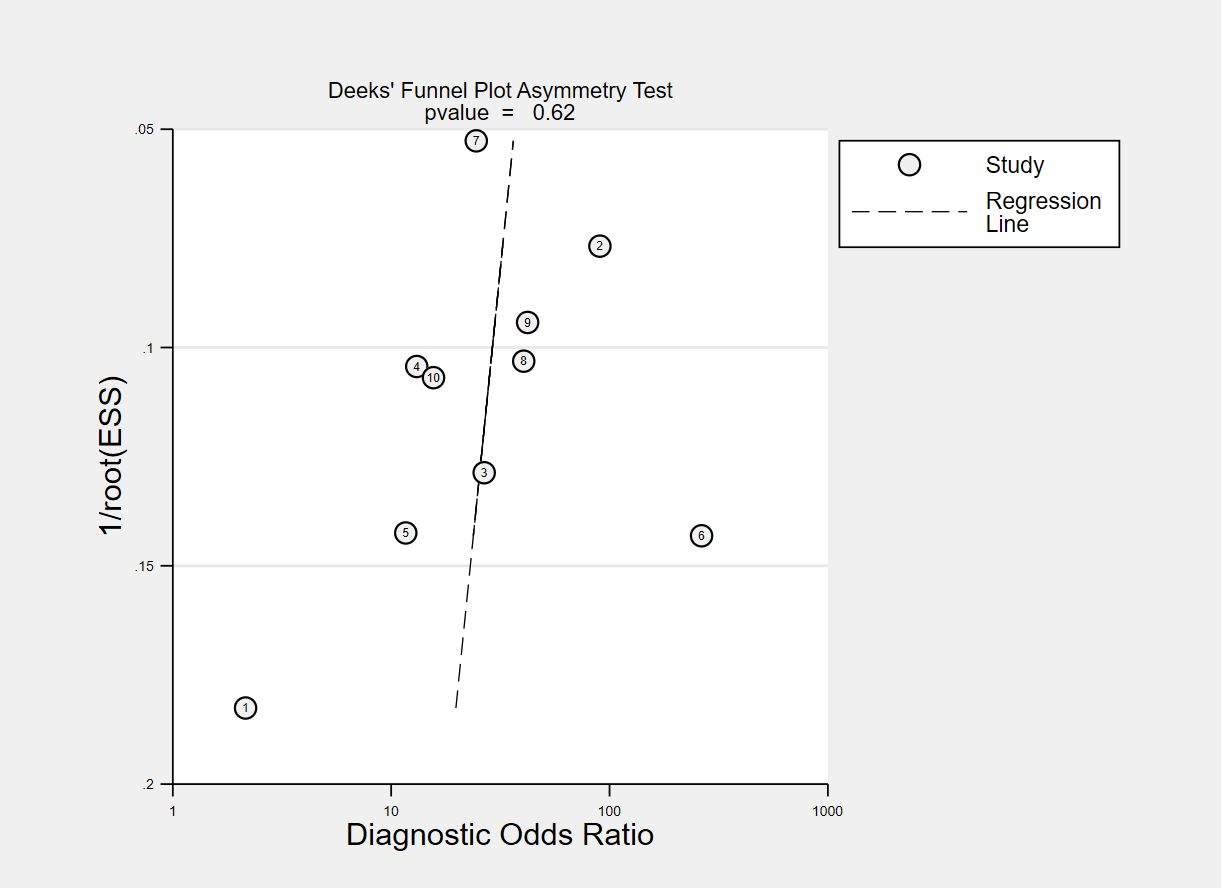
The funnel plots for publication bias are displayed in Figures 7, 8. The Deeks’ regression test for funnel plot asymmetry demonstrated an absence of publication bias among the studies included, with slope coefficients P values of 0.28 and 0.62 for using VOC analysis and e-nose.
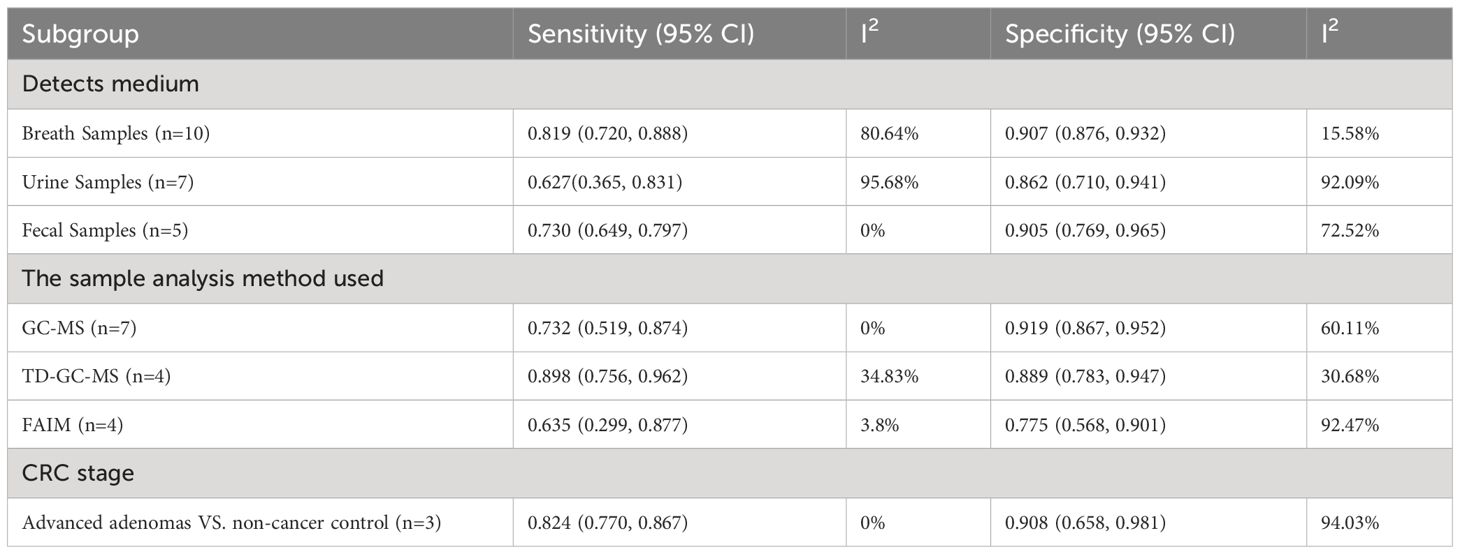
3.5 Subgroup analysis
We compared the accuracy of different samples of included studies. A separate pooled analysis of breath VOCs studies exhibited good efficacy, with a sensitivity of 0.819 (95% CI, 0.720-0.888) and a specificity of 0.907 (95% CI, 0.876-0.932) (Table 3). A Separate pooled analysis of GC-MS, TD-GC-MS, and FAIMS methods, showed a sensitivity of 0.732 (95%CI, 0.519-0.874) and a specificity of 0.919 (95%CI, 0.867-0.952) for GC-MS, and a sensitivity of 0.898 (95% CI, 0.756-0.962) and a specificity of 0.889 (95% CI, 0.783-0.947) for TD-GC-MS, and a sensitivity of 0.635 (95% CI, 0.299-0.877) and a specificity of 0.775 (95% CI, 0.568-0.901) for FAIMS (Table 3).
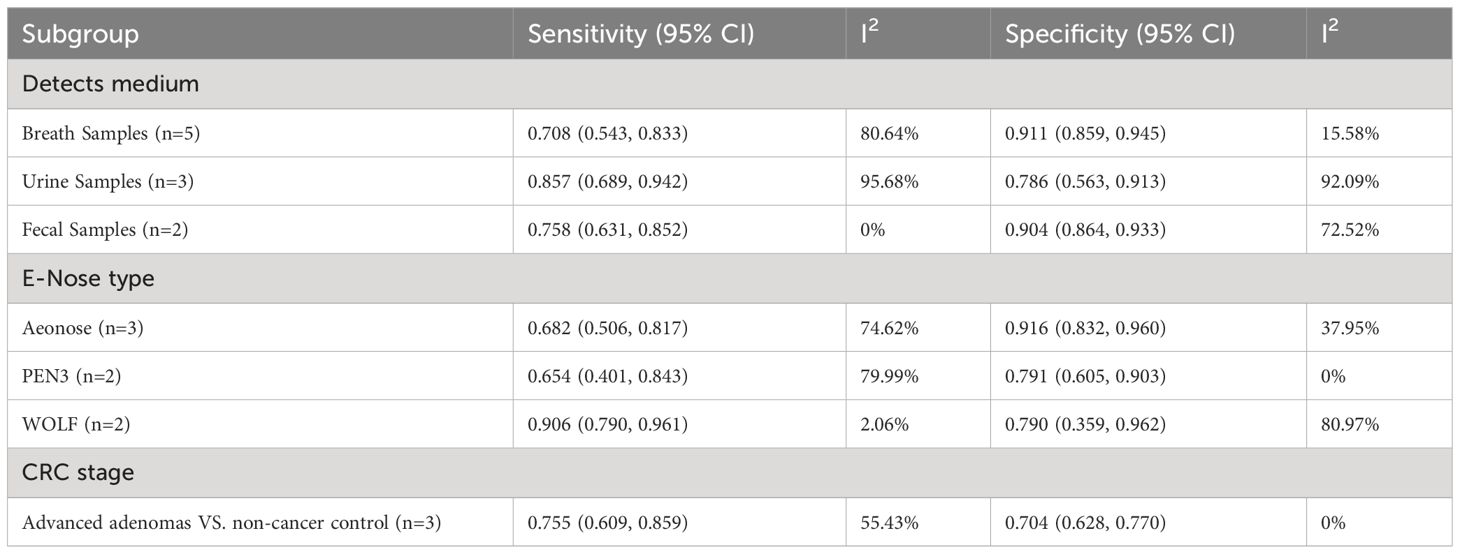
For e-nose studies, exhaled breath samples demonstrated a better specificity of 0.911 (95% CI, 0.859-0.945) but a lower sensitivity of 0.708 (95% CI, 0.543-0.833) (Table 4). A separate pooled analysis for different types of e-Nose demonstrated that Aeonose could detect colorectal with a sensitivity of 0.682 (95% CI, 0.506-0.817) and a specificity of 0.916 (95% CI, 0.832-0.960). Separate pooled analysis for PEN3 showed a sensitivity of 0.654 (95% CI, 0.401-0.843) and a specificity of 0.791 (95% CI, 0.605-0.903). For WOLF the sensitivity was 0.906 (95%CI, 0.790-0.961) and the specificity was 0.790 (95%CI, 0.359-0.962) (Table 4).
Additional sensitivity analysis for advanced adenomas demonstrated good accuracy in VOC analysis, with a sensitivity of 0.824 (95% CI, 0.770-0.867) and specificity of 0.908 (95% CI, 0.658-0.981) (Table 3). For e-nose studies, the sensitivity and specificity for the detection of advanced adenomas were 0.755 (95% CI, 0.609-0.859) and 0.704 (95% CI, 0.628-0.770), respectively (Table 4).
4 Discussion
We conducted a systematic review and meta-analysis to evaluate VOC analysis and electronic nose in detecting colorectal cancer, aiming to compare the diagnostic accuracy and clinical application value of these two methods. Pooled analysis of VOC and electronic-nose studies demonstrated high diagnostic accuracy for CRC detection, with a pooled sensitivity of 0.88 and specificity of 0.85 for VOC analysis and a sensitivity of 0.87 and specificity of 0.78 for e-nose studies. The visually assessed SROC curves indicated clinical accuracy, with VOC analysis and e-nose having SROC curves of approximately 0.93 and 0.90, respectively, both close to 1, signifying superior accuracy and diagnostic efficacy in CRC diagnosis. These findings align with prior reviews (22, 59, 60), but the notable heterogeneity between studies and the identified high risk of bias warrant cautious interpretation. The heterogeneity was largely due to the sample media and the analytical methods used.
Subgroup analyses revealed that breath samples in VOC analysis and urine and breath samples in e-nose studies exhibited higher sensitivity or specificity. Breath sampling is easily performed and well-received by patients, and urine samples, boasting high sensitivity and specificity, emerge as valuable alternatives. Recent meta-analysis evaluated the performance of the combined FIT and urinary. The findings revealed that the combined FIT-VOC approach could detect 33% more cases of colorectal cancers (60). Chandrapalan S et al. (61) showed that the combination of FIT and VOC can be a better triage tool, for CRC in patients with lower gastrointestinal symptoms than FIT alone.
Due to the lack of standardization in sample collection, handling, and storage, technical barriers exist in measuring and analyzing various VOC characteristics during sampling, whether it involves alveolar air, urine, or feces. In several studies, exhaled breath was collected into a bag and subsequently analyzed (28, 29, 33, 38–40, 42, 50). The use of bag collection aligns more closely with real-world medical applications. However, this approach may be influenced by several factors, including interference from ambient VOCs, the material used for collection, and the impact of temperature, humidity, and storage time on specimens (62). For breath samples, it is essential to examine them within 6 hours of the collection’s conclusion to ensure test accuracy (63). Therefore, developing methods for the collection, transmission, and handling of breath samples is crucial for the success of this approach. Some studies have indicated that the diagnostic accuracy of fecal and urine VOCs is not significantly affected by storage time (20 months for fecal and 12 months for urine VOCs) (64, 65).
Urine samples are ideal detection medium because they have limited confounding factors compared to breath samples which is influenced by smoking or fecal samples influenced by diet. Further research should standardize the method of collection of such samples and investigate the effects of potential confounding factors.
Among all studies, only six reported on CRC stages, indicating limited generalizability and clinical applicability. Multi-center validation studies of the diagnostic performance of VOCs on early stages of CRC and its precursor lesions (adenomas or not) is warranted, which could reduce the incidence of CRC.
It has been demonstrated that various factors, such as age, gender, smoking, alcohol consumption, coffee intake, and the consumption of stimulating foods like leeks and garlic, as well as comorbidities and medication, may influence the composition of VOCs in exhaled breath (66). However, only a few studies considered confounding or modifying effects, limiting the validity and reliability of the results. Therefore, future studies should account for the impact of such factors on breath prints during the design phase.
Gas chromatography-mass spectrometry (GC-MS), a traditional method for VOC analysis, is a highly standardized technique providing qualitative and quantitative information on exhaled VOCs (67, 68). In this study, TD-GC-MS demonstrated high sensitivity and specificity in detecting colorectal cancer, while GC-MS exhibited improved specificity but suboptimal sensitivity. The use of GC-MS and newer mass spectrometry technology devices remains the gold standard for identifying specific VOCs for analysis. However, GC-MS technology is costly and complex, with long analysis times, and it demands a high level of expertise from operators.
Based on sensors, electronic nose technology serves as a novel analytical method for disease diagnosis, offering the advantages of being cost-effective, user-friendly, portable, sensitive, and responsive. Nevertheless, there are existing shortcomings that require refinement in the application of e-nose in clinical practice. Unlike GC-MS and other techniques, e-nose lacks the precision to measure specific types and composition ratios of components in VOCs (24). It also cannot identify specific pathophysiological pathways or therapeutic targets. Furthermore, as the e-nose relies on arrays of gas sensors to distinguish and identify response spectra of mixtures composed of multiple VOCs, the diverse sensor types with distinct signal responses prevent the integration of results from one e-nose with different devices or sensor types (69). Van der Sar IG (70) recommends the establishment of a comprehensive worldwide shared database encompassing patient characteristics and other pretest probabilities.
Various algorithms and methods were employed to analyze VOCs in this study, with PLA-DA and logistic regression analysis emerging as the most commonly used approaches. However, the majority of studies fail to elucidate the rationale behind selecting a specific machine learning model for analysis, only reporting the highest accuracy value, thereby impacting the reliability of the results. Additionally, studies with small sample sizes may compromise the reported accuracy. Few studies have conducted external validation to affirm the validity and reliability of these findings. Consequently, large, multi-center external validation studies should be conducted in the future to explore the applicability and reproducibility of the results in different study settings and among diverse target populations.
4.1 Limitation
This study has certain limitations. Heterogeneity was observed among studies, potentially attributed to variations in sample media and analytical methods. Some studies exhibited a high risk of bias, with seven showing concern regarding patient selection and ten having applicability concerns in one or two domains. Furthermore, the study included fewer investigations employing both VOC analysis and e-nose technology, thus impeding an accurate evaluation of the complementary effects of the two methods. In addition, VOC combined with FIT approach could increase the detection of colorectal cancer. However, there are no prospective studies evaluating the positive effect on VOC-FIT for screening prior to the onset of CRC.
5 Conclusion
Based on our meta-analysis, VOC analysis and e-nose technology show promise in the detection of CRC. However, several milestones must be achieved in colorectal cancer detection with these two non-invasive methods before clinical implementation. Firstly, for patients presenting with common non-specific symptoms, which may be an early indication of CRC, an exhaled breath test or a urine test or FIT+VOC could serve as screening tool. Secondly, electronic nose could be utilized in primary care units and community healthcare centers for mass screening of various intestinal diseases due to their portability, ease of use, cost-effectiveness, speed, and independence from specialized technicians. Thirdly, the identification of colorectal cancer-specific VOC biomarkers and combinations of biomarkers for colorectal cancer diagnosis is still necessary. This requires comprehensive metabolomics studies to elucidate the production of endogenous VOCs and the metabolic transformation of exogenous VOCs in colorectal cancer, aiding in the identification of VOC markers for cancer. Finally, large, multi-center external validation trials should be conducted to verify the generalizability and reproducibility of the results in different research settings and at different stages of CRC.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Author contributions
QW: Formal analysis, Funding acquisition, Supervision, Writing – original draft. YF: Investigation, Project administration, Writing – original draft. ST: Methodology, Writing – review & editing. ZL: Data curation, Project administration, Writing – review & editing. RZ: Data curation, Investigation, Writing – review & editing. YR: Methodology, Project administration, Writing – original draft. YJ: Project administration, Software, Writing – original draft. XH: Supervision, Validation, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was supported by the Natural Science Foundation of Sichuan Province [No. 2022NSFSC0670] and [No. 24NSFSC5858]. The fund sponsor had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2024.1397259/full#supplementary-material
References
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: Cancer J Clin. (2021) 71:209–49. doi: 10.3322/caac.21660
2. Dekker E, Tanis PJ, Vleugels JLA, Kasi PM, Wallace MB. Colorectal cancer. Lancet. (2019) 394:1467–80. doi: 10.1016/S0140-6736(19)32319-0
3. Shaukat A, Levin TR. Current and future colorectal cancer screening strategies. Nat Rev Gastroenterol Hepatol. (2022) 19:521–31. doi: 10.1038/s41575-022-00612-y
4. Imperiale TF, Gruber RN, Stump TE, Emmett TW, Monahan PO. Performance characteristics of fecal immunochemical tests for colorectal cancer and advanced adenomatous polyps: A systematic review and meta-analysis. Ann Internal Med. (2019) 170:319–29. doi: 10.7326/M18-2390
5. Bertels L, Lucassen P, van Asselt K, Dekker E, van Weert H, Knottnerus B. Motives for non-adherence to colonoscopy advice after a positive colorectal cancer screening test result: a qualitative study. Scandinavian J primary Health Care. (2020) 38:487–98. doi: 10.1080/02813432.2020.1844391
6. Chen H, Li N, Ren J, Feng X, Lyu Z, Wei L, et al. Participation and yield of a population-based colorectal cancer screening programme in China. Gut. (2019) 68:1450–7. doi: 10.1136/gutjnl-2018-317124
7. Politi L, Monasta L, Rigressi MN, Princivalle A, Gonfiotti A, Camiciottoli G, et al. Discriminant profiles of volatile compounds in the alveolar air of patients with squamous cell lung cancer, lung adenocarcinoma or colon cancer. Molecules. (2021) 26:550. doi: 10.3390/molecules26030550
8. Shirasu M, Touhara K. The scent of disease: volatile organic compounds of the human body related to disease and disorder. J Biochem. (2011) 150:257–66. doi: 10.1093/jb/mvr090
9. Hakim M, Broza YY, Barash O, Peled N, Phillips M, Amann A, et al. Volatile organic compounds of lung cancer and possible biochemical pathways. Chem Rev. (2012) 112:5949–66. doi: 10.1021/cr300174a
10. Wang C, Li P, Lian A, Sun B, Wang X, Guo L, et al. Blood volatile compounds as biomarkers for colorectal cancer. Cancer Biol Ther. (2014) 15:200–6. doi: 10.4161/cbt.26723
11. Sethi S, Nanda R, Chakraborty T. Clinical application of volatile organic compound analysis for detecting infectious diseases. Clin Microbiol Rev. (2013) 26:462–75. doi: 10.1128/CMR.00020-13
12. Monedeiro F, Dos Reis RB, Peria FM, Sares CTG, De Martinis BS. Investigation of sweat VOC profiles in assessment of cancer biomarkers using HS-GC-MS. J Breath Res. (2020) 14:026009. doi: 10.1088/1752-7163/ab5b3c
13. de Boer NK, de Meij TG, Oort FA, Ben Larbi I, Mulder CJ, van Bodegraven AA, et al. The scent of colorectal cancer: detection by volatile organic compound analysis. Clin Gastroenterol Hepatol. (2014) 12:1085–9. doi: 10.1016/j.cgh.2014.05.005
14. Amal H, Shi DY, Ionescu R, Zhang W, Hua QL, Pan YY, et al. Assessment of ovarian cancer conditions from exhaled breath. Int J Cancer. (2015) 136:E614–22. doi: 10.1002/ijc.29166
15. Peng G, Hakim M, Broza YY, Billan S, Abdah-Bortnyak R, Kuten A, et al. Detection of lung, breast, colorectal, and prostate cancers from exhaled breath using a single array of nanosensors. Br J Cancer. (2010) 103:542–51. doi: 10.1038/sj.bjc.6605810
16. Barash O, Zhang W, Halpern JM, Hua QL, Pan YY, Kayal H, et al. Differentiation between genetic mutations of breast cancer by breath volatolomics. Oncotarget. (2015) 6:44864–76. doi: 10.18632/oncotarget.v6i42
17. Corradi M, Poli D, Banda I, Bonini S, Mozzoni P, Pinelli S, et al. Exhaled breath analysis in suspected cases of non-small-cell lung cancer: a cross-sectional study. J Breath Res. (2015) 9:027101. doi: 10.1088/1752-7155/9/2/027101
18. Bouza M, Gonzalez-Soto J, Pereiro R, de Vicente JC, Sanz-Medel A. Exhaled breath and oral cavity VOCs as potential biomarkers in oral cancer patients. J Breath Res. (2017) 11:016015. doi: 10.1088/1752-7163/aa5e76
19. Guo L, Wang C, Chi C, Wang X, Liu S, Zhao W, et al. Exhaled breath volatile biomarker analysis for thyroid cancer. Trans Res. (2015) 166:188–95. doi: 10.1016/j.trsl.2015.01.005
20. Qin T, Liu H, Song Q, Song G, Wang HZ, Pan YY, et al. The screening of volatile markers for hepatocellular carcinoma. Cancer epidemiology Biomarkers Prev. (2010) 19:2247–53. doi: 10.1158/1055-9965.EPI-10-0302
21. Gardner JW, Bartlett PN. A brief history of electronic noses. Sensors Actuators B: Chem. (1994) 18:210–1. doi: 10.1016/0925-4005(94)87085-3
22. Scheepers M, Al-Difaie Z, Brandts L, Peeters A, van Grinsven B, Bouvy ND. Diagnostic performance of electronic noses in cancer diagnoses using exhaled breath: A systematic review and meta-analysis. JAMA network Open. (2022) 5:e2219372. doi: 10.1001/jamanetworkopen.2022.19372
23. Licht JC, Grasemann H. Potential of the electronic nose for the detection of respiratory diseases with and without infection. Int J Mol Sci. (2020) 21:9416. doi: 10.3390/ijms21249416
24. van Keulen KE, Jansen ME, Schrauwen RWM, Kolkman JJ, Siersema PD. The smell of lung disease: a review of the current status of electronic nose technology. Respir Res. (2021) 22:246. doi: 10.1186/s12931-021-01835-4
25. van Keulen KE, Jansen ME, Schrauwen RWM, Kolkman JJ, Siersema PD. Volatile organic compounds in breath can serve as a non-invasive diagnostic biomarker for the detection of advanced adenomas and colorectal cancer. Alimentary Pharmacol Ther. (2019) 51:334–46. doi: 10.1111/apt.15622
26. de Meij TG, Larbi IB, van der Schee MP, Lentferink YE, Paff T, Terhaar Sive Droste JS, et al. Electronic nose can discriminate colorectal carcinoma and advanced adenomas by fecal volatile biomarker analysis: proof of principle study. Int J Cancer. (2014) 134:1132–8. doi: 10.1002/ijc.28446
27. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ (Clinical Res ed). (2009) 339:b2700. doi: 10.1136/bmj.b2700
28. Altomare DF, Di Lena M, Porcelli F, Travaglio E, Longobardi F, Tutino M, et al. Exhaled volatile organic compounds identify patients with colorectal cancer. Br J Surg. (2013) 100:144–50. doi: 10.1002/bjs.8942
29. Altomare DF, Picciariello A, Rotelli MT, De Fazio M, Aresta A, Zambonin CG, et al. Effects of curative colorectal cancer surgery on exhaled volatile organic compounds and potential implications in clinical follow-up. Ann Surg. (2015) 262:862–7. doi: 10.1097/SLA.0000000000001471
30. Altomare DF, Porcelli F, Picciariello A, Pinto M, Di Lena M, Caputi Iambrenghi O, et al. Chemical signature of colorectal cancer: case–control study for profiling the breath print. BJS Open. (2020) 4:1189–99. doi: 10.1002/bjs5.50354
31. Alustiza M, Ripoll L, Canals A, Murcia O, Martínez-Roca A, García-Heredia A, et al. A novel non-invasive colorectal cancer diagnostic method: Volatile organic compounds as biomarkers. Clinica Chimica Acta. (2023) 542:1189–99. doi: 10.1016/j.cca.2023.117273
32. Arasaradnam RP, McFarlane MJ, Ryan-Fisher C, Westenbrink E, Hodges P, Thomas MG, et al. Detection of colorectal cancer (CRC) by urinary volatile organic compound analysis. PloS One. (2014) 9. doi: 10.1371/journal.pone.0108750
33. Batty CA, Cauchi M, Lourenço C, Hunter JO, Turner C. Use of the analysis of the volatile faecal metabolome in screening for colorectal cancer. PLoS One. (2015) 10. doi: 10.1371/journal.pone.0130301
34. Bel'skaya LV, Sarf EA, Shalygin SP, Postnova TV, Kosenok VK. Identification of salivary volatile organic compounds as potential markers of stomach and colorectal cancer: A pilot study. J Oral Biosci. (2020) 62:212–21. doi: 10.1016/j.job.2020.05.002
35. Bond A, Greenwood R, Lewis S, Corfe B, Sarkar S, O'Toole P, et al. Volatile organic compounds emitted from faeces as a biomarker for colorectal cancer. Alimentary Pharmacol Ther. (2019) 49:1005–12. doi: 10.1111/apt.15140
36. Bosch S, Bot R, Wicaksono A, Savelkoul E, van der Hulst R, Kuijvenhoven J, et al. Early detection and follow-up of colorectal neoplasia based on faecal volatile organic compounds. Colorectal Dis. (2020) 22:1119–29. doi: 10.1111/codi.15009
37. Boulind CE, Gould O, de Lacy Costello B, Allison J, White P, Ewings P, et al. Urinary volatile organic compound testing in fast-track patients with suspected colorectal cancer. Cancers. (2022) 14:2127. doi: 10.3390/cancers14092127
38. Cheng HR, van Vorstenbosch RWR, Pachen DM, Meulen LWT, Straathof JWA, Dallinga JW, et al. Detecting colorectal adenomas and cancer using volatile organic compounds in exhaled breath: A proof-of-principle study to improve screening. Clin Trans Gastroenterol. (2022) 13. doi: 10.14309/ctg.0000000000000518
39. Depalma N, Di Lena M, Porcelli F, Travaglio E, Longobardi F, Demarinis Loiotile A, et al. Detection of colorectal polyps by exhaled VOCs. Preliminary data. Techniques Coloproctology. (2014) 18:92–3. doi: 10.1007/s10151-013-1096-6
40. Ishibe A, Ota M, Takeshita A, Tsuboi H, Kizuka S, Oka H, et al. Detection of gas components as a novel diagnostic method for colorectal cancer. Ann Gastroenterological Surg. (2018) 2:147–53. doi: 10.1002/ags3.12056
41. Leja M, Amal H, Funka K, Vanags A, Kikuste I, Sivins A, et al. Nanoarray sensor technology-based volatile marker tests to detect colorectal cancer and colonic adenomas. Gastroenterology. (2015) 148. doi: 10.1016/S0016-5085(15)30059-7
42. Lena MD, Porcelli F, Trizio L, Giuratrabocchetta S, Travaglio E, Tutino M, et al. Colorectal cancer screening by breath analysis: A specific pattern of volatile organic compounts (VOCs) can discriminate between patients and healthy controls. Gastroenterology. (2012) 142:S528. doi: 10.1016/S0016-5085(12)62029-0
43. Markar SR, Chin S-T, Romano A, Wiggins T, Antonowicz S, Paraskeva P, et al. Breath volatile organic compound profiling of colorectal cancer using selected ion flow-tube mass spectrometry. Ann Surg. (2019) 269:903–10. doi: 10.1097/SLA.0000000000002539
44. McFarlane M, Millard A, Hall H, Savage R, Constantinidou C, Arasaradnam R, et al. Urinary volatile organic compounds and faecal microbiome profiles in colorectal cancer. Colorectal Dis. (2019) 21:1259–69. doi: 10.1111/codi.14739
45. Mozdiak E, Wicaksono AN, Covington JA, Arasaradnam RP. Colorectal cancer and adenoma screening using urinary volatile organic compound (VOC) detection: early results from a single-centre bowel screening population (UK BCSP). Techniques Coloproctology. (2019) 23:343–51. doi: 10.1007/s10151-019-01963-6
46. Psutka C, Yamada M, Matsuda A, Yamahatsu K, Matsumoto S, Kitayama T, et al. Abstract 5303: FAIMS technology in urinary volatile organic compound analysis to detect colorectal cancer. Cancer Res. (2017) 77:5303–. doi: 10.1158/1538-7445.AM2017-5303
47. Tyagi H, Daulton E, Bannaga AS, Arasaradnam RP, Covington JA. Non-invasive detection and staging of colorectal cancer using a portable electronic nose. Sensors. (2021) 21:5440. doi: 10.3390/s21165440
48. Widlak MM, Neal M, Daulton E, Thomas CL, Tomkins C, Singh B, et al. Risk stratification of symptomatic patients suspected of colorectal cancer using faecal and urinary markers. Colorectal Dis. (2018) 20:O335–O42. doi: 10.1111/codi.14431
49. Zambrana Tevar F, Herrero A, Vidal-de-Miguel G, Bailador G, Criado E, Marquina I, et al. On-line breath analysis of volatile organic compounds as a method for colorectal cancer detection. J Clin Oncol. (2012) 30:1570–. doi: 10.1200/jco.2012.30.15_suppl.1570
50. Altomare DF, Porcelli F, Picciariello A, Pinto M, Di Lena M, Caputi Iambrenghi O, et al. The use of the PEN3 e-nose in the screening of colorectal cancer and polyps. Techniques Coloproctology. (2016) 20:405–9yang. doi: 10.1007/s10151-016-1457-z
51. Steenhuis EGM, Schoenaker IJH, de Groot JWB, Fiebrich HB, de Graaf JC, Brohet RM, et al. Feasibility of volatile organic compound in breath analysis in the follow-up of colorectal cancer: A pilot study. Eur J Surg Oncol. (2020) 46:2068–73. doi: 10.1016/j.ejso.2020.07.028
52. van de Goor RMGE, Leunis N, van Hooren MRA, Francisca E, Masclee A, Kremer B, et al. Feasibility of electronic nose technology for discriminating between head and neck, bladder, and colon carcinomas. Eur Arch Oto-Rhino-Laryngology. (2017) 274:1053–60. doi: 10.1007/s00405-016-4320-y
53. Westenbrink E, Arasaradnam RP, O'Connell N, Bailey C, Nwokolo C, Bardhan KD, et al. Development and application of a new electronic nose instrument for the detection of colorectal cancer. Biosensors Bioelectronics. (2015) 67:733–8. doi: 10.1016/j.bios.2014.10.044
54. Westenbrink E, O’Connell N, Bailey C, Nwokolo C, Bardhan K, Arasaradnam R, et al. Detection of colorectal cancer from urinary volatile organic compounds using a new chromatograph/electronic-nose instrument – wolf system. Gut. (2016) 65:A195.2–A6. doi: 10.1136/gutjnl-2016-312388.361
55. Zonta G, Malagù C, Gherardi S, Giberti A, Pezzoli A, De Togni A, et al. Clinical validation results of an innovative non-invasive device for colorectal cancer preventive screening through fecal exhalation analysis. Cancers. (2020) 12:1471. doi: 10.3390/cancers12061471
56. Whiting PF, Rutjes AW, Westwood ME, Mallett S, Deeks JJ, Reitsma JB, et al. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Internal Med. (2011) 155:529–36. doi: 10.7326/0003-4819-155-8-201110180-00009
57. Deeks JJ, Macaskill P, Irwig L. The performance of tests of publication bias and other sample size effects in systematic reviews of diagnostic test accuracy was assessed. J Clin Epidemiol. (2005) 58:882–93. doi: 10.1016/j.jclinepi.2005.01.016
58. Amal H, Leja M, Funka K, Lasina I, Skapars R, Sivins A, et al. Breath testing as potential colorectal cancer screening tool. Int J Cancer. (2016) 138:229–36. doi: 10.1002/ijc.29701
59. Hanna GB, Boshier PR, Markar SR, Romano A. Accuracy and methodologic challenges of volatile organic compound-based exhaled breath tests for cancer diagnosis: A systematic review and meta-analysis. JAMA Oncol. (2019) 5:e182815. doi: 10.1001/jamaoncol.2018.2815
60. van Liere E, van Dijk LJ, Bosch S, Vermeulen L, Heymans MW, Burchell GL, et al. Urinary volatile organic compounds for colorectal cancer screening: A systematic review and meta-analysis. Eur J Cancer (Oxford England: 1990). (2023) 186:69–82. doi: 10.1016/j.ejca.2023.03.002
61. Chandrapalan S, Bosch S, Cubiella J, Guardiola J, Kimani P, Mulder C, et al. Systematic review with meta-analysis: volatile organic compound analysis to improve faecal immunochemical testing in the detection of colorectal cancer. Alimentary Pharmacol Ther. (2021) 54:14–23. doi: 10.1111/apt.16405
62. Ghimenti S, LoMonaco T, Bellagambi FG, Tabucchi S, Onor M, Trivella MG, et al. Comparison of sampling bags for the analysis of volatile organic compounds in breath. J Breath Res. (2015) 9:047110. doi: 10.1088/1752-7155/9/4/047110
63. Mochalski P, King J, Unterkofler K, Amann A. Stability of selected volatile breath constituents in Tedlar, Kynar and Flexfilm sampling bags. Analyst. (2013) 138:1405–18. doi: 10.1039/c2an36193k
64. Esfahani S, Sagar NM, Kyrou I, Mozdiak E, O'Connell N, Nwokolo C, et al. Variation in gas and volatile compound emissions from human urine as it ages, measured by an electronic nose. Biosensors. (2016) 6:4. doi: 10.3390/bios6010004
65. Chan DK, Leggett CL, Wang KK. Diagnosing gastrointestinal illnesses using fecal headspace volatile organic compounds. World J Gastroenterol. (2016) 22:1639–49. doi: 10.3748/wjg.v22.i4.1639
66. Krilaviciute A, Leja M, Kopp-Schneider A, Barash O, Khatib S, Amal H, et al. Associations of diet and lifestyle factors with common volatile organic compounds in exhaled breath of average-risk individuals. J Breath Res. (2019) 13:026006. doi: 10.1088/1752-7163/aaf3dc
67. Boots AW, Bos LD, van der Schee MP, van Schooten FJ, Sterk PJ. Exhaled molecular fingerprinting in diagnosis and monitoring: validating volatile promises. Trends Mol Med. (2015) 21:633–44. doi: 10.1016/j.molmed.2015.08.001
68. Fiehn O. Metabolomics by gas chromatography–mass spectrometry: combined targeted and untargeted profiling. Curr Protoc Mol Biol. (2016) 114:30.4.1–30.4.32. doi: 10.1002/0471142727.mb3004s114
69. Baldini C, Billeci L, Sansone F, Conte R, Domenici C, Tonacci A. Electronic nose as a novel method for diagnosing cancer: A systematic review. Biosensors. (2020) 10:84. doi: 10.3390/bios10080084
Keywords: volatile organic compounds, VOCs, electronic nose, E-nose, colorectal cancer, diagnosis
Citation: Wang Q, Fang Y, Tan S, Li Z, Zheng R, Ren Y, Jiang Y and Huang X (2024) Diagnostic performance of volatile organic compounds analysis and electronic noses for detecting colorectal cancer: a systematic review and meta-analysis. Front. Oncol. 14:1397259. doi: 10.3389/fonc.2024.1397259
Received: 12 March 2024; Accepted: 24 April 2024;
Published: 13 May 2024.
Edited by:
Francesk Mulita, General University Hospital of Patras, GreeceReviewed by:
Dimitrios Kehagias, General University Hospital of Patras, GreeceChristos Pitros, General University Hospital of Patras, Greece
Copyright © 2024 Wang, Fang, Tan, Li, Zheng, Ren, Jiang and Huang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qiaoling Wang, qiaoling86@126.com; Xiaopeng Huang, huangxiaopeng@cdutcm.edu.cn
†These authors share first authorship
 Qiaoling Wang
Qiaoling Wang