- 1Drug Development Department, Gustave Roussy Cancer Campus, Villejuif, France
- 2Medical Biology and Pathology Department, Gustave Roussy Cancer Campus, Villejuif, France
- 3Medical Oncology Department, Gustave Roussy Cancer Campus, Villejuif, France
- 4Biology and Genetics Department, Centre Eugène Marquis, Rennes, France
- 5Department of Genetics, Hôpital Pitié-Salpêtrière, Assistance Publique – Hôpitaux de Paris (AP-HP), Sorbonne Université, Paris, France
- 6Gastroenterology and Hepatology Department, Institut Mutualiste Montsouris, Paris, France
Introduction: Microsatellite instability (MSI) is a genetic marker that is useful in the detection and treatment of Lynch syndrome (Sd). Although conventional techniques such as immunohistochemistry (IHC) and polymerase chain reaction (PCR) are the standards for MSI detection, the advent of next-generation sequencing (NGS) has offered new possibilities, especially with circulating DNA.
Case report: We present the case of a 26-year-old patient with Lynch Sd and a BRAF-mutated metastatic colon cancer. The discordant MSI results between the conventional methods and NGS posed challenges in making treatment decisions. Subsequent NGS analysis revealed a high MSI status, leading to participation in an immunotherapy trial, with remarkable clinical response.
Conclusion: This case emphasizes the importance of comprehensive molecular profiling and strong interdisciplinary collaborations, especially in cases with ambiguous MSI results.
Introduction
Microsatellite instability (MSI) is the genetic fingerprint of a defective DNA mismatch repair (dMMR) system (1), in which the accumulation of mutations occur throughout the genome and is particularly grouped in repetitive regions of microsatellites (2). It is routinely assessed in solid tumors for the initial detection of Lynch syndrome, for treatment orientation, and for cancer prognosis (3).
Most MSI-high (MSI-H) tumors arise sporadically (4), often associated with hypermethylation of the MLH1 promoter or a mutation in BRAF V600E (specifically in colorectal cancer, CRC) (5), while others result from hereditary cancer predisposition syndromes such as Lynch syndrome [originated from a monoallelic germline mutation in one of the four major mismatch repair (MMR) genes: MLH1, MSH2, MSH6, PMS2, or the EPCAM gene] (6, 7). The tumor phenotype of MSI-H CRC is characterized by a right-sided colon presentation, poorly differentiated mucinous adenocarcinomas, an early-disease onset, and a high response to immune checkpoint blockers (8).
Currently, the gold standard for dMMR detection is immunohistochemistry (IHC) and polymerase chain reaction (PCR) using tumor tissue samples. However, the evolution and the development of next-generation sequencing (NGS) techniques have offered the opportunity to extend MSI-H determination particularly using circulating cell-free DNA (cfDNA) (3). The latter has gained significant interest as it is a minimally invasive and easily repeatable tool that overcomes the problem of spatial and temporal heterogeneity and allows longitudinal monitoring of the disease through iterative sampling (9). Thus, there has been special interest in demonstrating the concordance between the use of conventional techniques and NGS tools in MSI-H detection (for validation as a detection method) with promising results (10, 11). However, discordant results could arise and therefore pose challenges in making treatment decisions. In this paper, we present a case report as an example of this issue.
Case report
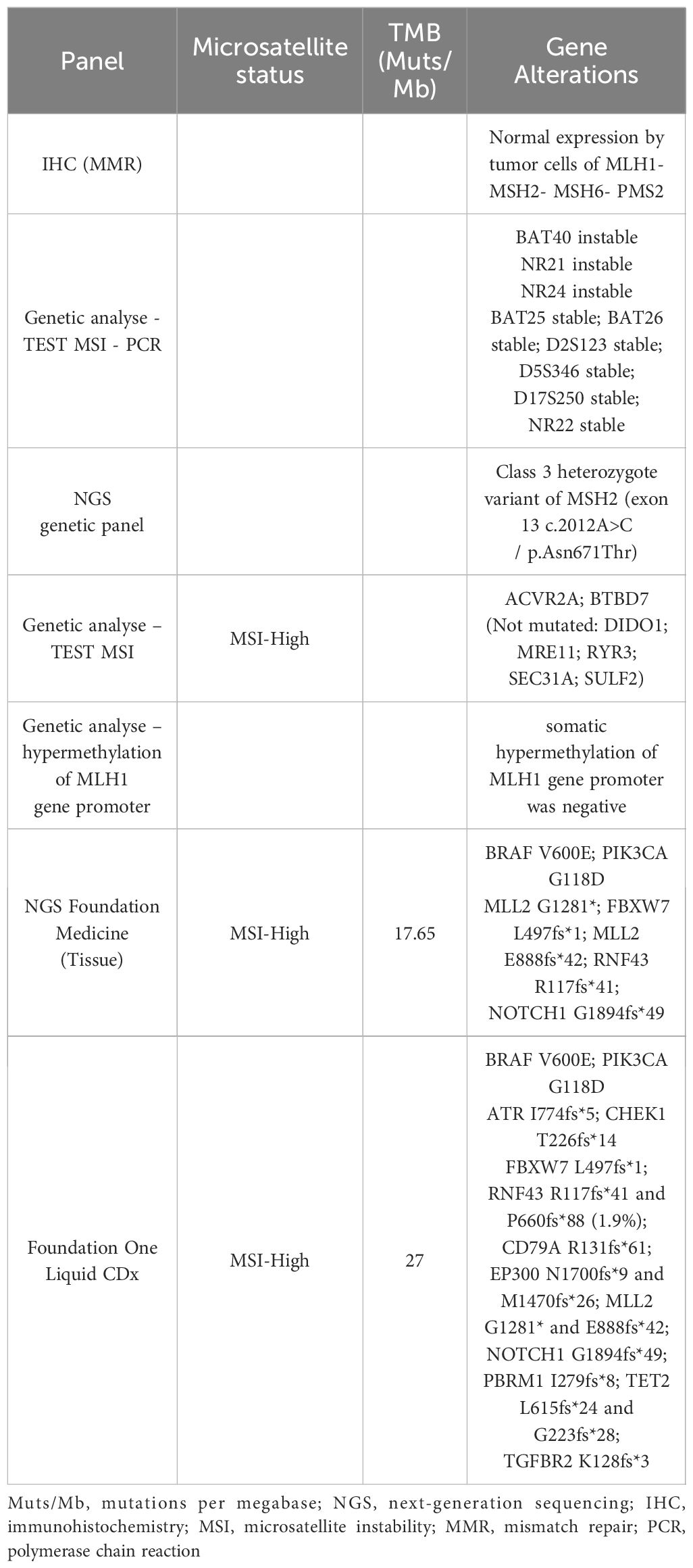
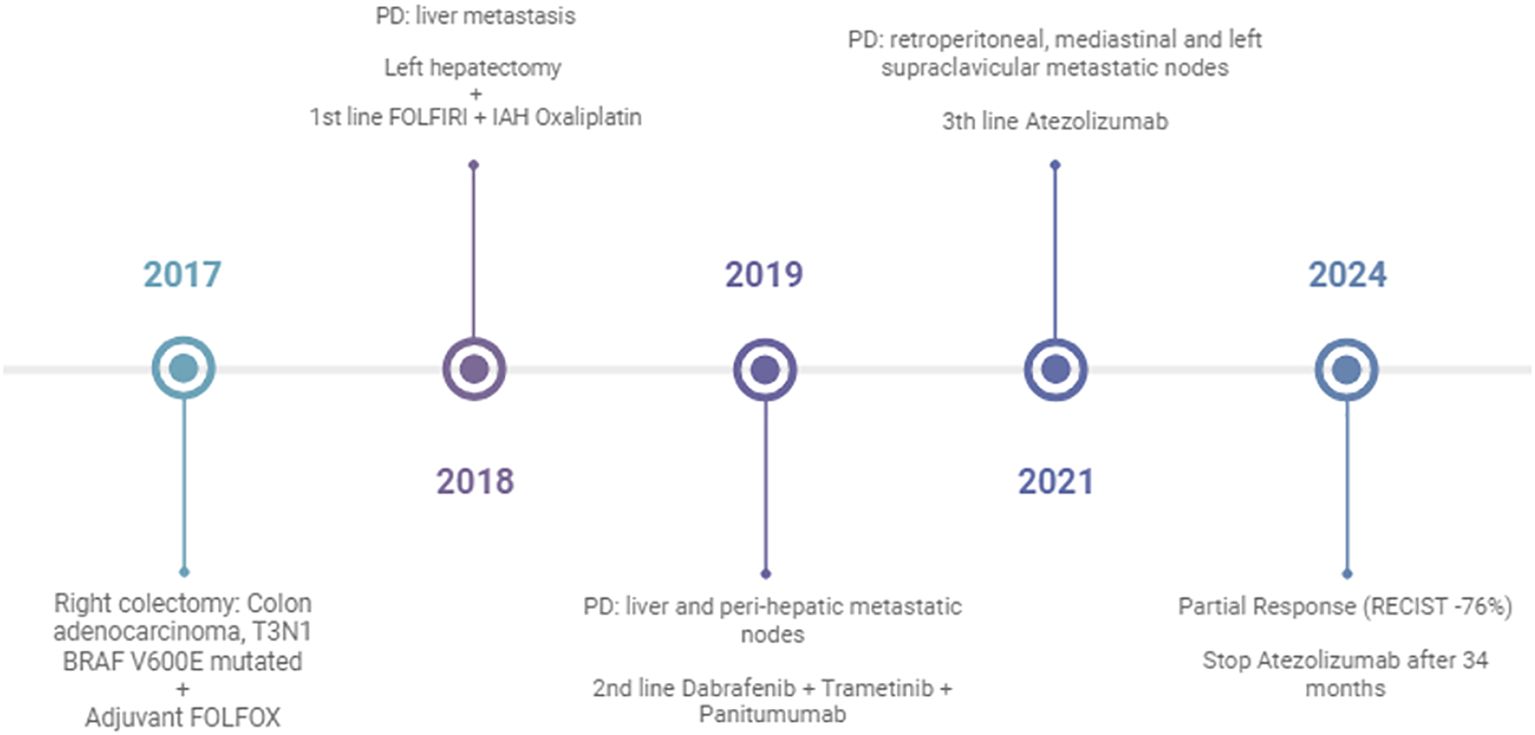
A 26-year-old man with no personal or familial history of cancer was hospitalized after 1 week of fever and abdominal pain. The computed tomography (CT) scan showed a primary right colonic mass associated with local inflammation and free pelvic effusion, without distant metastasis. In this context, he underwent emergency surgery. Pathological specimen revealed R0 resection, a 7-cm infiltrative mucinous adenocarcinoma of the right colon, and two positive lymph nodes out of 47 (T3N1 stage IIIA). Molecular testing on the primary tumor found KRAS wild type, BRAF V600E mutation, and normal expression of MLH1–MSH2–MSH6–PMS2 as assessed by IHC. The result of the PCR analysis for MSI detection was considered uninterpretable due to insufficient tumor cells (<20%).
Postoperative imaging was clear, as well as the tumor markers [i.e., carcinoembryonic antigen (CEA) and carbohydrate antigen 19–9 (CA19–9)]. As recommended by the guidelines, between January and July 2018, the patient received 12 cycles of adjuvant chemotherapy with an intravenous (iv) FOLFOX regimen [oxaliplatin, 85 mg/m2 iv; 5-fluoruracil (5-FU), 2,400 mg/m2 iv over 46 h; 5-FU, 400 mg/m2 bolus; leucovorin (LV), 400 mg/m2]. Genetic counseling was considered due to the patient’s young age, and a standard constitutional NGS panel was performed, which included the genes MLH1, MSH2, MSH6, PMS2, EPCAM, APC, MUTYH POLD1, and POLE, without detection of any deleterious mutations (Table 1). A class 3 heterozygous VUS (variant of uncertain significance) of the MSH2 gene located in exon 13 c.2012A>C/p.Asn671Thr was found, and due to the patient’s young age, the panel recommended upper and lower endoscopic surveillance as a Lynch-like case (every 2 years in France).
In December 2018, the patient had resectable liver relapse, and a left hepatectomy was performed. As the relapse was intrahepatic and occurred 6 months after the end of adjuvant chemotherapy, the multidisciplinary team (MDT) decided on an adjuvant systemic treatment with an iv FOLFIRI regimen (irinotecan, 180 mg/m2; 5-FU, 2,400 mg/m2 iv over 46 h; 5-FU, 400 mg/m2 bolus; LV, 400 mg/m2) plus intra-arterial hepatic oxaliplatin. The patient received 3 months of chemotherapy from February to May 2019, with good tolerance. He was disease free at the end of the treatment.
After 3 months, in September 2019, a CT scan revealed progressive disease and the appearance of retroperitoneal lymph nodes. As the patient harbored BRAF V600E mutation, the MDT decided on targeted therapy with dabrafenib, trametinib, and panitumumab [at the time, we did not have the results of the BEACON trial (12)]. The patient had partial response and a progression-free period of 16 months (between September 2019 and February 2021), but with limited tolerance due to grade 2–3 cutaneous toxicity related to panitumumab.
In January 2021, the disease became progressive again at the retroperitoneal, mediastinal, and left supraclavicular lymph nodes. Despite the fact of a known BRAF V600E mutation and the proficient mismatch repair (pMMR) status, we decided to perform a new molecular NGS analysis with the FoundationOne CDx panel, which used both liquid biopsy and the archival specimen (from the hepatic surgery).
The results revealed, on tissue, MSI-H, tumor mutational burden (TMB) of 17.65 mutations per megabase (Muts/Mb), and a BRAF V600E mutation, while the liquid biopsy confirmed the MSI-H, TMB-H (27 Muts/Mb), and the BRAF V600E mutation, among others (Table 1).
Based on the MSI-H result, the patient was enrolled in a basket clinical trial, in which he underwent treatment for 34 months with second-line atezolizumab with partial response (−76%) and complete metabolic response with excellent treatment tolerance (Figure 1). The diagnosis and the treatment process of the patient are displayed in Figure 2.
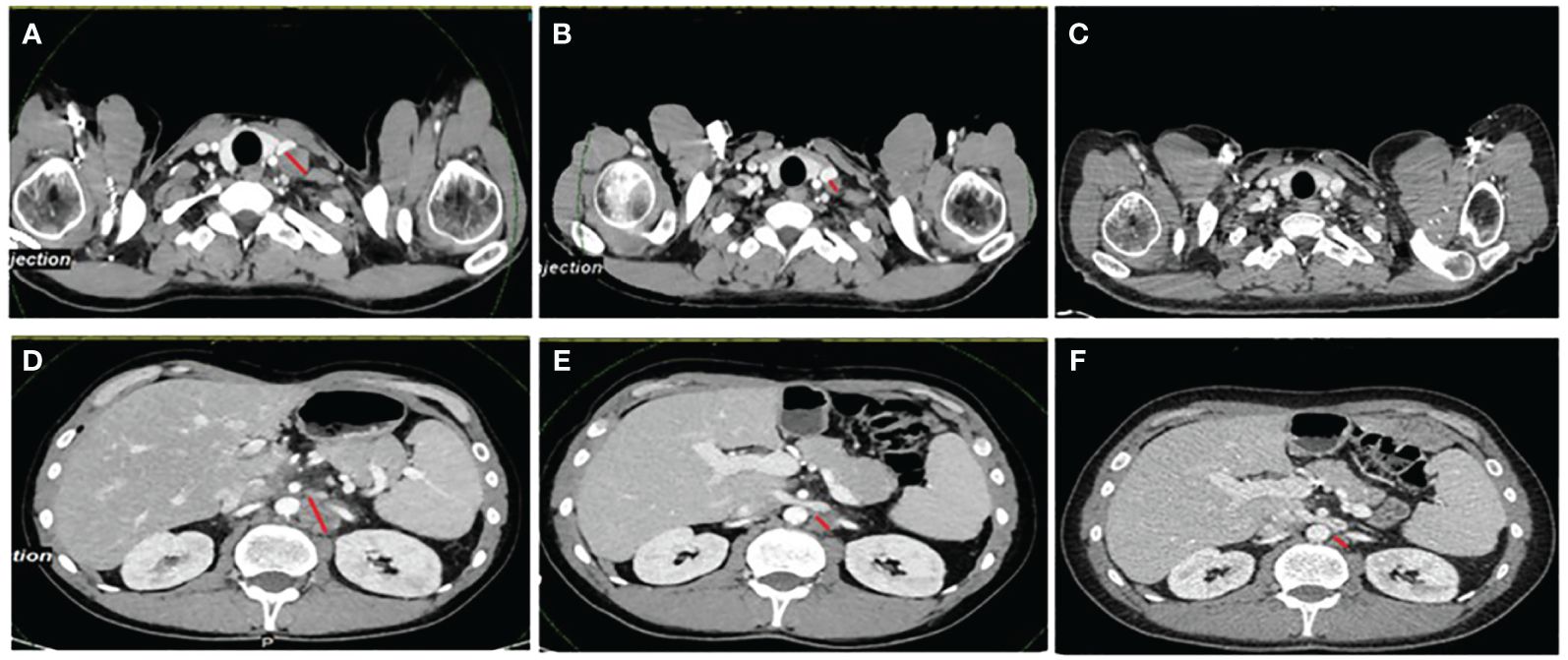
Figure 1 The patient’s CT scans at baseline, at best response, and at the last evaluation. (A–C) Evolution of a left supraventricular node. (D–F) Retroperitoneal node. The images in (A) and (D) were taken before starting immunotherapy treatment, while the images in (B) and (E) are those at the best response (this patient experienced a partial response with a −76% reduction in the target lesions). The images in (C) and (F) are the last scans of the patient in January 2024, when the decision to stop the treatment was made after 34 months. The red lines denote measurable disease.
In the face of these discordant results, we performed an MSI analysis using the specimen from the liver metastasis. The analysis revealed an MSI-H phenotype in NGS, but still a microsatellite stable (MSS) in IHC. A second NGS using the Idylla panel confirmed the presence of MSI-H in the liver specimen. The case was discussed with a pathologist and a biologist, and a somatic hypermethylation of MLH1 was performed, which came back negative. The patient was considered to harbor an MSI-H tumor.
After the MSI-H results were confirmed, we went back on the germline analysis and decided to perform a methylation tolerance-based functional assay (13) for the MSH2 exon 13 c.2012A>C/p.Asn671Thr. The results confirmed the pathogenicity of the variant, and the case was discussed in genetic MDT. The variant was classified as likely pathogenic (in the national FrOG (14) database), and it was considered that the patient harbored Lynch syndrome. The evaluation performed by the end of January 2024 showed a complete metabolic response, and it was decided to stop immunotherapy. His healthy relatives had not performed any genetic testing at this stage.
Discussion
We present the case of a 26-year-old male patient with a metastatic colon cancer that presented discordant MSI results between IHC/PCR and NGS. The NGS panel done at progression (liquid biopsy and the most recent tissue) yielded results of MSI-H and TMB-H, offering the possibility to be exposed to immunotherapy with impressive results.
Before DNA sequencing became available, MSI detection mainly relied on IHC for the MMR proteins and PCR evaluation of the five highly conserved loci of the “Bethesda panel” (15). However, given that, on certain occasions, biopsies may not contain a sufficient percentage of tumor cells for correct analysis of the MMR status (as well as other genes of special interest), a significant number of patients may find themselves limited in their therapeutic options (16).
According to the recommendations of the European Society of Medical Oncology (ESMO) and the American Society of Clinical Oncology (ASCO), the MMR status should be assessed in all patients at the time of CRC diagnosis whenever Lynch syndrome is suspected, but also as an initial molecular workup in metastatic disease for its predictive value for the use of immunotherapy [Level of evidence (I,A)] (17, 18). Testing should be carried out using conventional techniques such as MMR-IHC and/or MSI by PCR, which are the primary recommendations. Furthermore, if MMR detection is conducted with an NGS panel, this must show equivalency to the aforementioned techniques (18).
In the context of this case, the outcome of the IHC, indicating a typical expression of the MSH2 protein despite a missense variant, raises intriguing questions about its functional impact. While Lynch syndrome screening typically relies on IHC and/or MSI tests, with genetic testing reserved for specific cases, further investigations were warranted due to the patient’s young age and the potentially inconclusive MSI test, leading to the accurate identification of the syndrome.
Our patient presented an uninterpretable MSI status from the use of conventional techniques, assessed at the initial diagnosis, probably due to specimen cellularity (<20%) or the mucinous histology. He was treated with standard therapies with short periods of disease control.
Several causes could lead to a false-negative MSI result, as follows: 1) technical issues (MSI testing on tissue fragments <5.5 mm could produce a false-negative MSI result) (19); 2) mucinous histology (20); and 3) some Lynch syndrome (a number of individuals with Lynch syndrome could have tumors with an MSI-L or an MSS phenotype (20–22), leading to false-negative results). Thanks to the high availability of NGS platforms in our center, we were able to discover that the patient had an MSI-H tumor. He was then treated with atezolizumab in the basket trial, which was the only possibility at the time.
In 2020, the Keynote-177 trial presented the results of pembrolizumab treatment in patients with MSI-H, which showed an improvement in progression-free survival and, notably, a response rate of 43.8% compared with standard chemotherapy, then becoming the first-line treatment for this population, even for patients harboring an BRAF V600E mutation (23). Approval for pembrolizumab was followed by nivolumab (with or without its combination with ipilimumab) for the same setting, thanks to the results of the phase II trial Checkmate 142 (2022) (24). We would also like to mention that our patient had a BRAF V600E mutation, which was considered only until recently to be more a marker for the sporadic MSI cancer. However, of late, the presence of a BRAF V600E mutation has also been found in patients with Lynch syndrome; therefore, its presence should not exclude germline testing if clinically indicated (25).
In conclusion, our focus on the MSI status, particularly in younger patients, underscores the critical role it plays in making treatment decisions. When confronted with unclear results, consulting biologists and geneticists become imperative, given the life-changing potential of an accurate diagnosis. If direct determination from the tumor tissue is not feasible, cfDNA is an option in such cases.
Data availability statement
The data that support the finding of this case are available from the corresponding author upon reasonable request.
Ethics statement
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
JR: Conceptualization, Investigation, Methodology, Visualization, Writing – original draft, Writing – review & editing. DV: Investigation, Writing – review & editing. MB: Formal analysis, Writing – review & editing. OdC: Formal analysis, Writing – review & editing. SC: Formal analysis, Writing – review & editing. MM: Formal analysis, Writing – review & editing. VG: Formal analysis, Writing – review & editing. DM: Formal analysis, Writing – review & editing. TP: Validation, Writing – review & editing. OlC: Formal analysis, Writing – review & editing. CS: Investigation, Supervision, Validation, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Definition of microsatellite instability. National Cancer Institute: NCI Dictionary of Cancer Terms - NCI (2011). Available at: https://www.cancer.gov/publications/dictionaries/cancer-terms/def/microsatellite-instability.
2. Capper D, Voigt A, Bozukova G, Ahadova A, Kickingereder P, von Deimling A, et al. BRAF V600E-specific immunohistochemistry for the exclusion of Lynch syndrome in MSI-H colorectal cancer. Int J Cancer. (2013) 133:1624–30. doi: 10.1002/ijc.28183
3. Gilson P, Merlin J-L, Harlé A. Detection of microsatellite instability: state of the art and future applications in circulating tumour DNA (ctDNA). Cancers. (2021) 13:1491. doi: 10.3390/cancers13071491
4. Colle R, Cohen R. [Epidemiology of microsatellite instability across solid neoplasms]. Bull Cancer. (2019) 106:114–8. doi: 10.1016/j.bulcan.2018.07.019
5. Yamamoto H, Imai K. Microsatellite instability: an update. Arch Toxicol. (2015) 89:899–921. doi: 10.1007/s00204-015-1474-0
6. Pellat A, Netter J, Perkins G, Cohen R, Coulet F, Parc Y, et al. [Lynch syndrome: What is new]? Bull Cancer. (2019) 106:647–55. doi: 10.1016/j.bulcan.2018.10.009
7. Buza N, Ziai J, Hui P. Mismatch repair deficiency testing in clinical practice. Expert Rev Mol Diagn. (2016) 16:591–604. doi: 10.1586/14737159.2016.1156533
8. Mei W-J, Mi M, Qian J, Xiao N, Yuan Y, Ding PR. Clinicopathological characteristics of high microsatellite instability/mismatch repair-deficient colorectal cancer: A narrative review. Front Immunol. (2022) 13. doi: 10.3389/fimmu.2022.1019582
9. Deininger P. Alu elements: know the SINEs. Genome Biol. (2011) 12:236. doi: 10.1186/gb-2011-12-12-236
10. Shimozaki K, Hayashi H, Tanishima S, Horie S, Chida A, Tsugaru K, et al. Concordance analysis of microsatellite instability status between polymerase chain reaction based testing and next generation sequencing for solid tumors. Sci Rep. (2021) 11. doi: 10.1038/s41598-021-99364-z
11. Willis J, et al. Validation of microsatellite instability detection using a comprehensive plasma-based genotyping panel. Clin Cancer Res. (2019) 25:7035–45. doi: 10.1158/1078-0432.CCR-19-1324
12. Kopetz S, Grothey A, Yaeger R, Van Cutsem E, Desai J, Yoshino T. Encorafenib, binimetinib, and cetuximab in BRAF V600E-mutated colorectal cancer. N Engl J Med. (2019) 381(17):1632–43. doi: 10.1056/NEJMoa190807
13. Bouvet D, Bodo S, Munier A, Guillerm E, Bertrand R, Colas C, et al. Methylation tolerance-based functional assay to assess variants of unknown significance in the MLH1 and MSH2 genes and identify patients with lynch syndrome. Gastroenterology. (2019) 157:421–31. doi: 10.1053/j.gastro.2019.03.071
14. FrOG. Available online at: https://frog-db.fr/.
15. Boland CR, Thibodeau SN, Hamilton SR, Sidransky D, Eshleman JR, Burt RW, et al. A National Cancer Institute Workshop on Microsatellite Instability for cancer detection and familial predisposition: development of international criteria for the determination of microsatellite instability in colorectal cancer. Cancer Res. (1998) 58:5248–57.
16. Stadler ZK, Battaglin F, Middha S, Hechtman JF, Tran C, Cercek A, et al. Reliable detection of mismatch repair deficiency in colorectal cancers using mutational load in next-generation sequencing panels. J Clin Oncol. (2016) 34:2141–7. doi: 10.1200/JCO.2015.65.1067
17. Cervantes A, Adam R, Roselló S, Arnold D, Normanno N, Taïeb J, et al. Metastatic colorectal cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann Oncol. (2023) 34:10–32. doi: 10.1016/j.annonc.2022.10.003
18. Vikas P, Messersmith H, Compton C, Sholl L, Broaddus RR, Davis A, et al. Mismatch repair and microsatellite instability testing for immune checkpoint inhibitor therapy: ASCO endorsement of college of American pathologists guideline. JCO. (2023) 41:1943–8. doi: 10.1200/JCO.22.02462
19. Trusky CL, Sepulveda AR, Hunt JL. Assessment of microsatellite instability in very small microdissected samples and in tumor samples that are contaminated with normal DNA. Diagn Mol Pathol. (2006) 15:63. doi: 10.1097/00019606-200606000-00001
20. Rudzki Z, Zazula M, Okoń K, Stachura J. Low-level microsatellite instability colorectal carcinomas: do they really belong to a ‘gray zone’ between high-level microsatellite instability and microsatellite-stable cancers? Int J Colorectal Dis. (2003) 18:216–21. doi: 10.1007/s00384-002-0460-1
21. Kim Y-H, Min BH, Choi HK, Kim SJ, Kim KM, Kim JY, et al. Sporadic colorectal carcinomas with low-level microsatellite instability in Korea: do they form a distinct subgroup with distinguished clinicopathological features? J Surg Oncol. (2009) 99:351–5. doi: 10.1002/jso.21239
22. Pawlik TM, Raut CP, Rodriguez-Bigas MA. Colorectal carcinogenesis: MSI-H versus MSI-L. Dis Markers. (2004) 20:199–206. doi: 10.1155/2004/368680
23. André T, Shiu KK, Kim TW, Jensen BV, Jensen LH, Punt C, et al. Pembrolizumab in microsatellite-instability–high advanced colorectal cancer. N Engl J Med. (2020) 383:2207–18. doi: 10.1056/NEJMoa2017699
24. Lenz H-J, Cutsem EV, Limon ML, Wong KYM, Aglietta M, García-Alfonso P, et al. First-line nivolumab plus low-dose ipilimumab for microsatellite instability-high/mismatch repair-deficient metastatic colorectal cancer: the phase II checkMate 142 study. JCO. (2022) 40:161–70. doi: 10.1200/JCO.21.01015
Keywords: discordant MSI, colorectal cancer, next-generation sequencing, circulating DNA, case report
Citation: Rodriguez JE, Vasseur D, Bani MA, Cabaret O, Cotteret S, Muleris M, Golbarg V, Malka D, Pudlarz T, Caron O and Smolenschi C (2024) Case report: Microsatellite instability determination is not always black and white in Lynch syndrome diagnosis. Front. Oncol. 14:1396869. doi: 10.3389/fonc.2024.1396869
Received: 06 March 2024; Accepted: 24 May 2024;
Published: 18 June 2024.
Edited by:
Sakti Chakrabarti, University Hospitals Seidman Cancer Center, United StatesReviewed by:
Gianluca Tedaldi, Scientific Institute of Romagna for the Study and Treatment of Tumors (IRCCS), ItalySona Vodenkova, Institute of Experimental Medicine (ASCR), Czechia
Copyright © 2024 Rodriguez, Vasseur, Bani, Cabaret, Cotteret, Muleris, Golbarg, Malka, Pudlarz, Caron and Smolenschi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Cristina Smolenschi, Y3Jpc3RpbmEuc21vbGVuc2NoaUBndXN0YXZlcm91c3N5LmZy
†ORCID: Julieta E. Rodriguez, orcid.org/0000-0002-4060-6993
Mohamed Amine Bani, orcid.org/0000-0003-4834-4905
 Julieta E. Rodriguez
Julieta E. Rodriguez Damien Vasseur
Damien Vasseur Mohamed Amine Bani
Mohamed Amine Bani Odile Cabaret2
Odile Cabaret2 Cristina Smolenschi
Cristina Smolenschi