
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol., 24 June 2024
Sec. Thoracic Oncology
Volume 14 - 2024 | https://doi.org/10.3389/fonc.2024.1396843
Objective: The inflammatory response and the nutritional status are associated with overall survival (OS) in patients with non-small cell lung cancer (NSCLC), but it is unclear which biomarkers are better suited to predict prognosis. This study sought to determine which of the commonly existing inflammatory and nutritional indicators best predicted the OS.
Methods: This study included 15 compound indicators based on inflammation or nutrition, with cutoff points obtained through the receiver operating characteristic (ROC) curve. Univariate and multivariate Cox proportional risk models were used to evaluate the relationship between these predictors and OS. Kaplan–Meier curves were used for survival analysis, and log-rank tests were used to compare differences between groups. The C-index was calculated to evaluate the predictive ability of the different indicators.
Results: The study included 899 patients with NSCLC. In the univariate analysis, all 15 measures were significantly associated with the OS of patients (all p < 0.05). The results of the C-index analysis showed that the fibrinogen-to-albumin ratio (FAR), the systemic immune-inflammation index (SII), and the albumin-to-alkaline phosphatase ratio (AAPR) were the three indices with the best predictive performance. Among them, FAR (C-index = 0.639) had the best predictive power for OS in patients with NSCLC. In the different subgroups, FAR had the highest C-index in male, non-smoking, adenocarcinoma, and stage II patients. The C-index of the platelet-to-lymphocyte ratio (PLR) in female patients was the highest. SII was the highest in smokers, in those aged <65 and ≥65 years, and in stage III patients. The C-index of AAPR was the highest in non-adenocarcinomas. The C-index of the pan-immune-inflammation value (PIV) was the highest in stage I patients. In the multivariate Cox regression analysis, among FAR, SII, and AAPR, only FAR was an independent predictor of OS in patients with NSCLC. A high FAR was associated with a higher risk of death in patients with NSCLC (HR = 1.601, 95% CI = 1.028–2.495). In order to further evaluate the potential prognostic value of FAR, SII, and AAPR in patients with different stages, Cox regression analysis was performed for those with stage I–II and stage III NSCLC. The results showed that FAR was an independent prognostic factor for OS in patients with stage I–II NSCLC.
Conclusion: For all patients with NSCLC, the prognostic power of FAR was superior to that of other inflammatory and nutritional indicators.
Lung cancer represents 11.4% of all malignancies and causes 18% of all cancer-related deaths, making it the primary cause of cancer mortality according to the Global Cancer Statistics 2020 report (1). Among these, non-small cell lung cancer (NSCLC) accounts for approximately 80%–85% of all lung cancer cases and is associated with a poor 5-year survival rate (2). Patients with early-stage NSCLC are mainly treated with surgery, and postoperative adjuvant therapy is usually recommended thereafter to prevent cancer recurrence and to effectively improve patient survival (3). With significant advances in clinical diagnosis and treatment techniques and in antitumor treatment options (such as immunotherapy), 5-year survival rates have improved (4). However, predicting the prognosis of patients with lung cancer is still challenging. Therefore, there is an urgent need for effective biomarkers to predict the survival prognosis of patients in order to help identify patients and conduct timely and effective treatment. The American Joint Committee on Cancer (AJCC) tumor node metastasis staging system is an important factor in the assessment of the prognosis of patients with NSCLC (5). In addition, the patient’s age, sex, smoking status, weight status, and other clinical indicators are also factors that affect the survival outcomes of individual cancer patients (6–8).
Recent research has indicated that many inflammation and nutritional markers can serve as reliable prognostic indicators for lung cancer. The indicators of systemic inflammatory response, including the neutrophil-to-lymphocyte ratio (NLR) (9), the platelet-to-lymphocyte ratio (PLR) (10), the lymphocyte-to-monocyte ratio (LMR) (11), the systemic immune-inflammation index (SII) (12), and the pan-immune-inflammation value (PIV) (13), are significant in determining the prognosis of patients with lung cancer. The fibrinogen-to-albumin ratio (FAR) (14), the fibrinogen-to-prealbumin ratio (FPR) (15), the albumin-to-globulin ratio (AGR) (16), the advanced lung cancer inflammation index (ALI) (17), the prognostic nutritional index (PNI) (18), the albumin-to-alkaline phosphatase ratio (AAPR) (19), and the C-reactive protein-to-albumin ratio (CAR) (20), as well as other inflammatory and nutritional complex indicators, are also biomarkers for predicting the prognosis of lung cancer. Furthermore, red cell distribution width-to-albumin ratio (RAR) (21), alkaline phosphatase-to-prealbumin ratio (APR) (22), free fatty acid (FFA) content (23), and other markers are significant predictors for cancer patients. However, despite the predictive value of RAR, APR, and FFA in cancer, their prognostic value for lung cancer remains unknown.
Although studies have confirmed the value of some of the aforementioned nutrition- and inflammation-related indicators in predicting the survival outcomes of patients with lung cancer, it is necessary to identify which indicators are the best prognostic factors in patients with NSCLC. In this study, we evaluated and compared the predictive value of 15 nutritional and inflammatory biomarkers for overall survival (OS) for patients with NSCLC. We also assessed which indicator had a higher predictive value in different groups.
Patients with NSCLC who received surgical treatment in our hospital from January 2017 to June 2021 were selected as the study subjects. All of the patients included in this study met the following criteria: 1) at least 18 years of age; 2) undergoing thoracoscopic surgery for pathologically proven NSCLC (classified as stage I–III lung cancer according to TNM edition 8) (24); 3) no previous history of malignant tumors or the presence of a second primary cancer; and 4) with complete preoperative clinical data available. The exclusion criteria were as follows: 1) patients with NSCLC that is unresectable or who cannot tolerate surgical treatment; 2) patients with blood system and immune system diseases or blood abnormalities of unknown cause; 3) those with severe underlying disease in the past (such as grade IV heart function, liver and kidney failure, and stroke with severe sequelae, among others), resulting in unclear outcome indicators; and 4) those with incomplete clinical data or incomplete follow-up records. The flowchart for patient screening is shown in Figure 1.
In this study, the baseline information of the patients, including age, sex, smoking status, and body mass index (BMI), was collected through the hospital’s electronic medical record filing system, quality control registration management system, and laboratory examination reporting system. The Eastern Cooperative Oncology Group Performance Status (ECOG PS) score, respiratory diseases, tumor diameter, pathological type and TNM stage, the presence of vascular invasion, endovascular thrombus, lymphatic vessel invasion, and perineural invasion, and the survival status of the patients, among others, were assessed.
After fasting for at least 9 h, routine blood tests were performed within 24 h of hospitalization to measure the inflammatory indicators (including neutrophils, lymphocytes, monocytes, C-reactive protein, platelets, serum FFAs, globulins, alkaline phosphatase, and fibrinogen) and the nutritional status indicators (including albumin, prealbumin, and red blood cell distribution width). The 15 nutrition/inflammation-based measures used in this study included FPR, CAR, AAPR, FAR, APR, RAR, AGR, NLR, LMR, FFA/Alb, PNI, ALI, PLR, PIV, and SII. The methodology for the calculation of each nutrition/inflammation indicator and the specificity and sensitivity of the receiver operating characteristic (ROC) curve can be found in Supplementary Tables S1, S2, respectively.
The patients were followed up according to the hospital’s outpatient and inpatient medical record system and by telephone, with the follow-up ending in December 2022. The date when the patients were pathologically diagnosed with NSCLC was first defined as the starting point, death due to lung cancer was defined as the outcome event, and the Overall Survival (OS) was the time from the starting point to death or the end of follow-up.
The SPSS 27.0 software, R4.2.1 software, and GraphPad Prism 9.4.1 software were used for data processing and analysis. The measurement data were expressed as the mean plus or minus the standard deviation or median (quartile range), while the counting data were expressed as the number of cases (percentage). Differences between groups were assessed using the Mann–Whitney U test (for continuous variables with non-normal distributions) and the chi-squared test (for categorical variables). The best cutoff values for each inflammatory or nutritional index were obtained using the ROC curve and were divided into two categorical variables. Survival analysis was presented by the Kaplan–Meier curve, and differences between groups were compared using the log-rank test. Cox’s proportional hazards regression models were used to examine the relationship between nutritional and inflammatory markers and OS in patients with NSCLC. The predictive accuracy of each indicator was assessed using the C-index. To determine whether the same indicator applied to the entire subgroup and to gain insight into the most valuable biomarkers in the different subgroups, subgroup analyses were performed for patients’ age, sex, pathology type, and smoking status. A two-tailed p-value of <0.05 was considered statistically significant.
A total of 899 patients were included in the study. There were 413 men and 486 women. The mean age of the patients was 61.36 ± 9.64 years. Of all the patients in the study, 283 (31.5%) had a history of smoking, 220 (24.5%) had a history of lung disease, and 170 (18.9%) had an ECOG PS score of 2 or higher. In all patients, the mean tumor diameter was 1.96 ± 1.37 cm, 793 (88.2%) were pathologically diagnosed as adenocarcinomas, and 106 (11.8%) were non-adenocarcinomas. There were 707 (78.6%), 87 (9.7%), and 105 (11.7%) patients with TNM stages I, II, and III, respectively. Vascular invasion, endovascular thrombus, lymphatic vessel invasion, and perineural invasion were observed in 62.8%, 11.0%, 61.4%, and 2.7% of patients, respectively. In addition, the average BMI of the patients included in the study was 24.67 ± 3.38 kg/m2. The patient characteristics are shown in Table 1.
By December 2022, a total of 97 patients with lung cancer had died. The median OS was 34.23 months. The median OS for the adenocarcinoma and non-adenocarcinoma patients was 33.97 and 35.40 months, respectively. The cutoff points of the 15 inflammation- and malnutrition-based indicators were 0.013 (FPR), 0.017 (CAR), 0.045 (AAPR), 0.079 (FAR), 0.34 (APR), 1.13 (RAR), 1.49 (AGR), 1.64 (NLR), 4.17 (LMR), 9.81 (FFA/Alb), 45.51 (PNI), 70.06 (ALI), 143.73 (PLR), 156.18 (PIV), and 487.10 (SII). The univariate and multivariate analyses showed that FPR (p < 0.001), CAR (p = 0.001), AAPR (p < 0.001), FAR (p < 0.001), APR (p = 0.002), RAR (p < 0.001), AGR (p = 0.005), NLR (p < 0.001), LMR (p = 0.007), FFA/Alb (p = 0.005), PNI (p < 0.001), ALI (p < 0.001), PLR (p < 0.001), PIV (p < 0.001), and SII (p < 0.001) were independent risk factors affecting the survival of patients with NSCLC (Table 2). The Kaplan–Meier curves showed that lung cancer patients with malnutrition and inflammation have a more unfavorable OS compared with NSCLC patients without malnutrition or inflammation (Figure 2; Supplementary Figure S1).
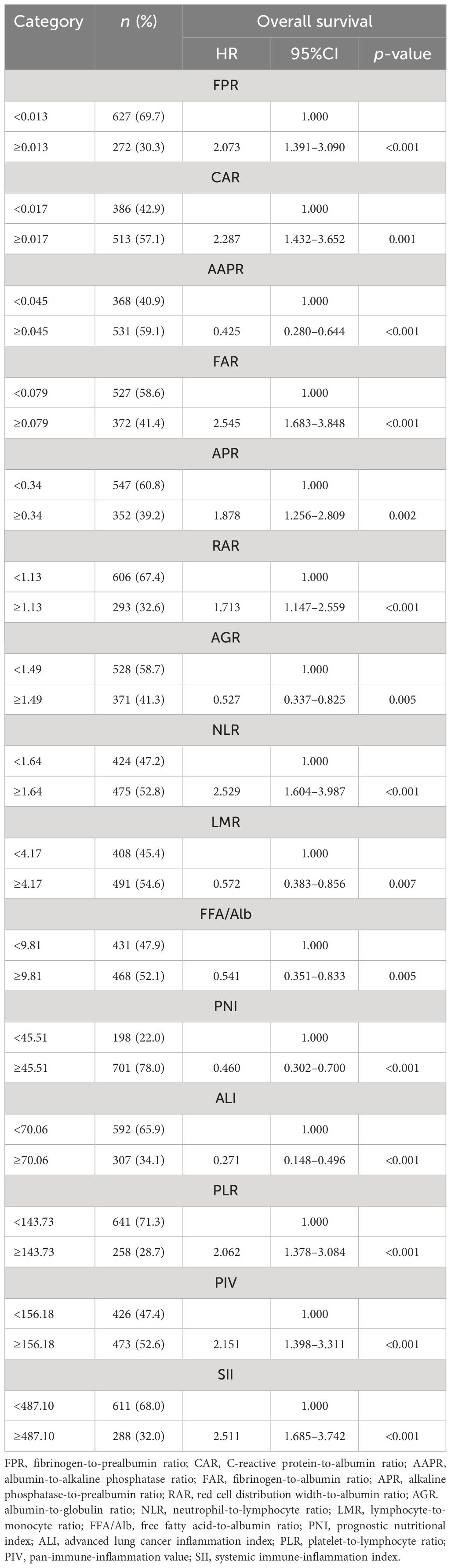
Table 2 Univariate analysis of the 15 inflammatory/nutritional markers and overall survival (OS) in patients with non-small cell lung cancer.

Figure 2 Kaplan–Meier curves of the fibrinogen-to-albumin ratio (FAR) (A), systemic immune-inflammation index (SII) (B), and albumin-to-alkaline phosphatase ratio (AAPR) (C) in patients with non-small cell lung cancer (NSCLC).
The patients were categorized into two groups based on their survival outcomes at the conclusion of the follow-up period: a survival group (n = 802) and a non-survival group (n = 97). The purpose was to compare the survival rates of each inflammatory and nutritional composite index between these two groups. The results of the statistical analysis revealed significant differences (p < 0.05) among the 15 inflammatory and nutritional complex indices, as presented in Supplementary Table S3.
A C-index analysis was performed on the 15 inflammatory and nutritional indicators to compare their prognostic ability. Compared with other inflammation- and nutrition-based measures, FAR showed the highest C-index of OS in patients with NSCLC at 1, 2, and 3 years: 0.701 (95%CI = 0.681–0.721), 0.667 (95%CI = 0.647–0.687), and 0.639 (95%CI = 0.619–0.659), respectively (Table 3). According to the 3-year C-index, FAR, SII, and AAPR were the top 3 inflammation-based indicators. Supplementary Table S4 shows that FAR, SII, and AAPR contributed significantly to the prognostic value of the TNM classification system.
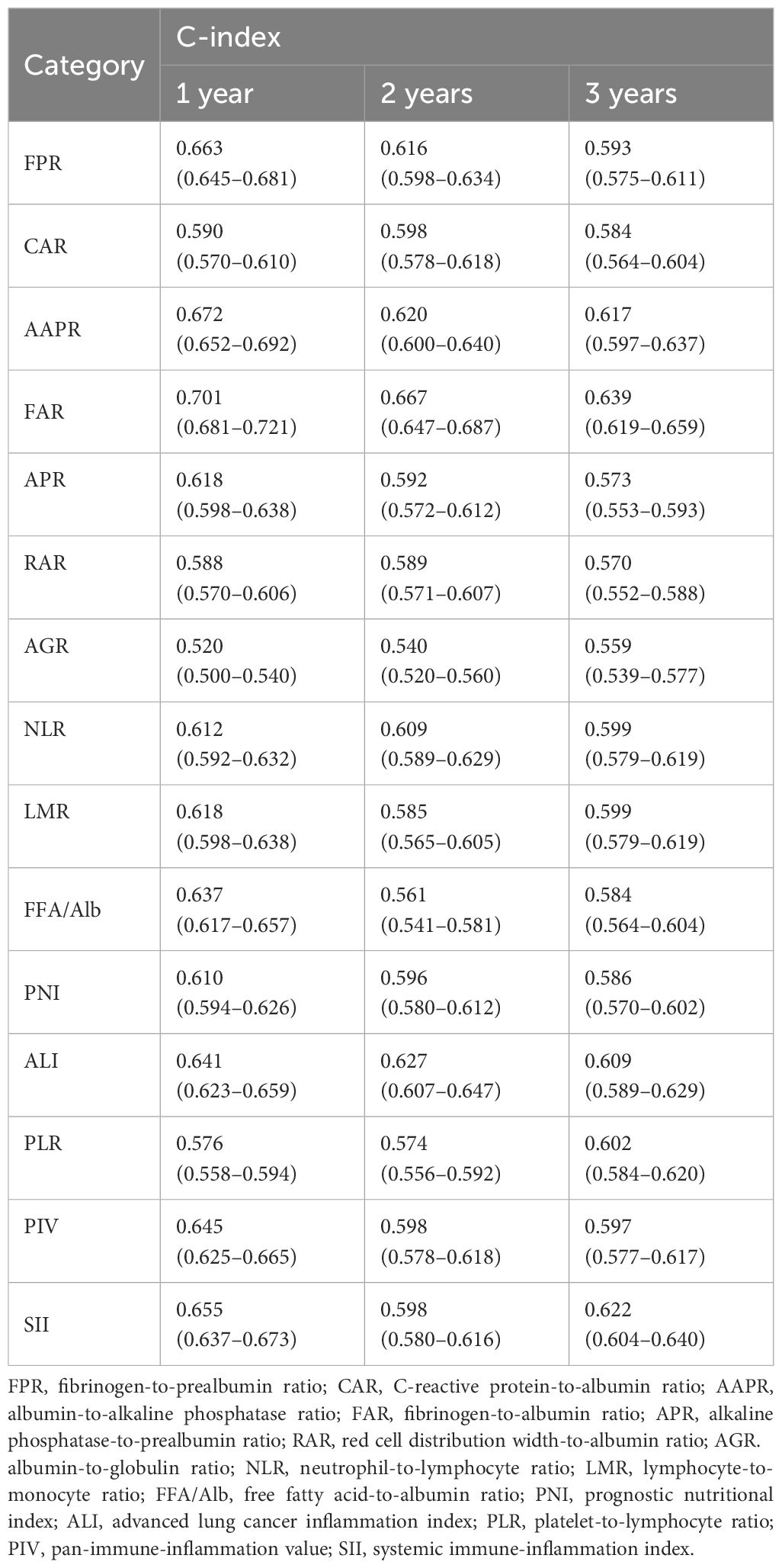
Table 3 C-index of the 15 indicators of overall survival (OS) in non-small cell lung cancer (NSCLC) patients.
In the different subgroups, FAR had the highest C-index compared with the other indicators in men, non-smokers, adenocarcinomas, and stage II patients (Supplementary Tables S5–S9). The C-index of PLR was the highest in female patients (Supplementary Table S5). SII was the highest in smokers (Supplementary Table S6), in those aged <65 and ≥65 years (Supplementary Table S7), and in stage III patients (Supplementary Table S9). The C-index of AAPR was the highest in non-adenocarcinomas (Supplementary Table S8). The C-index of PIV was the highest in stage I patients (Supplementary Table S9).
Overall, FAR, SII, and AAPR were the top 3 inflammatory and nutritional markers that predicted prognosis in patients with NSCLC. Supplementary Tables S10–S12 displays the baseline characteristics of patients with NSCLC stratified by high/low FAR, SII, and AAPR. The Kaplan–Meier curves of FAR, SII, and AAPR for the subgroups of NSCLC patients were constructed and stratified according to sex, smoking status, and pathological type. The results showed that even within subgroups, patients with high FAR, high SII, and low AAPR still had poor OS performance (Supplementary Figure S2).
The univariate analysis showed that sex (p = 0.012), smoking status (p = 0.002), pathologic type (p < 0.001), TNM stage (p < 0.001), tumor diameter (p < 0.001), endovascular thrombus (p < 0.001), perineural invasion (p = 0.001), FAR (p < 0.001), SII (p < 0.001), and AAPR (p < 0.001) were predictive of OS. In the multivariate Cox regression analysis, only the TNM stage (p < 0.001), perineural invasion (p = 0.035), and FAR (p = 0.037) were independent prognostic factors for OS in patients with NSCLC (Table 4).
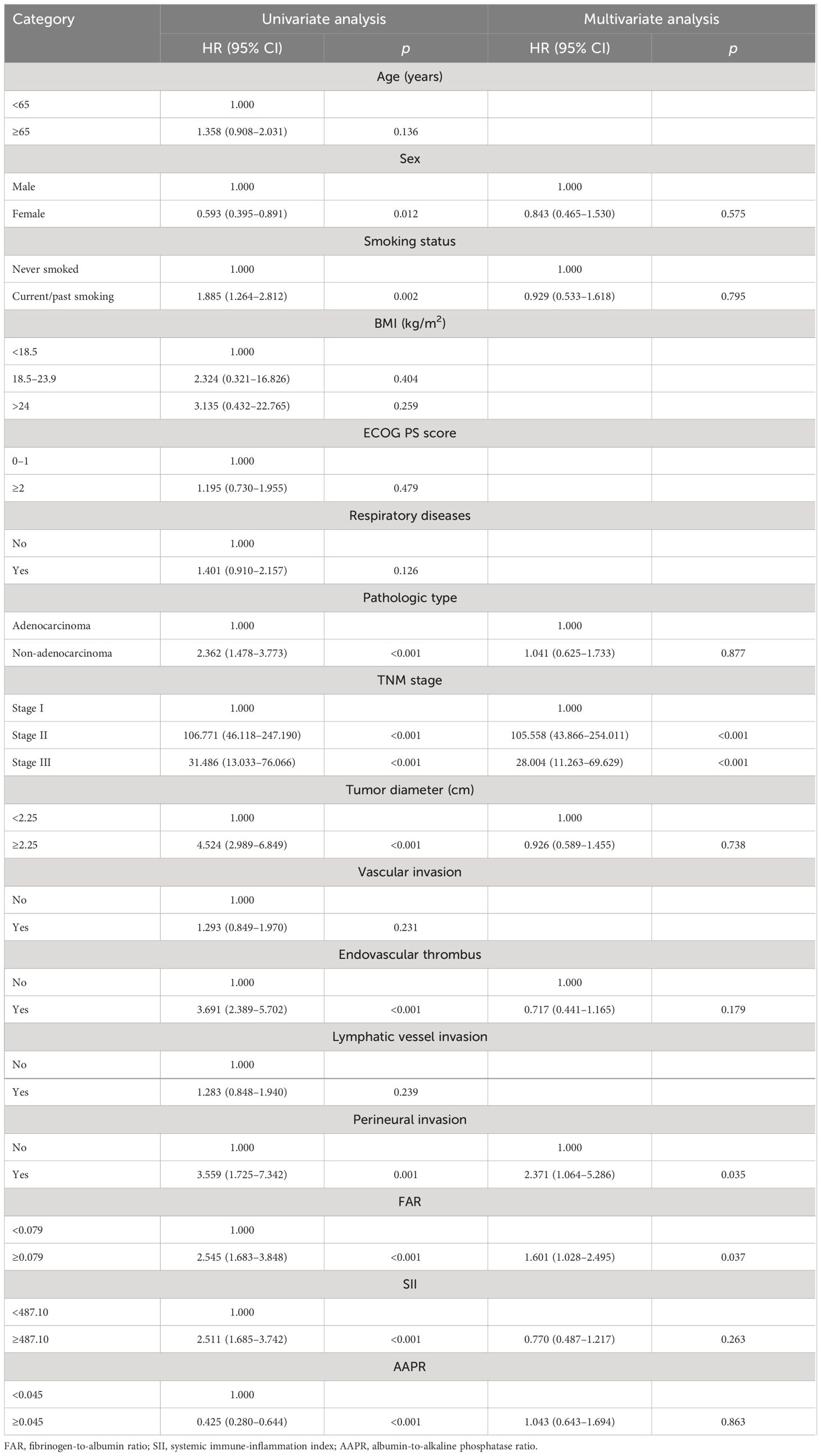
Table 4 Cox regression analysis of overall survival (OS) in all non-small cell lung cancer (NSCLC) patients.
Considering that there may be significant differences in the nutritional status and the inflammatory status between patients with early-stage and middle-advanced lung cancer, Cox regression analysis was performed for patients with stage I–II and stage III NSCLC in order to further evaluate the potential prognostic value of FAR, SII, and AAPR in patients with different stages of the disease. The results of the univariate Cox regression analysis are shown in Supplementary Tables S13, S14. The multivariate Cox regression analysis showed that tumor diameter (p = 0.047) and FAR (p = 0.049) were independent prognostic factors for OS in patients with stage I–II NSCLC. In those with stage III NSCLC, variables with p < 0.1 (SII and perineural invasion) were added to the multifactorial model after correction. However, only perineural invasion (p = 0.032) was a strong predictor of the outcome.
Increasing research suggests that inflammation- and nutrition-based indicators are dependable predictors of OS in patients with cancer. Chronic inflammation produces several cytokines that promote the initiation and progression of malignancies through pathophysiological mechanisms (25). Furthermore, malnutrition in patients with cancer may result in a weakened immune function and an increased inflammatory response (26). Cancer patients with lower nutritional markers or higher inflammatory markers tend to have worse outcomes (27). However, the optimal indicator for patients with NSCLC is unclear. Our study evaluated and compared 15 inflammation- and nutrition-based measures and found that FAR had stable and good predictive performance in predicting the prognosis of patients with NSCLC and their subgroups in risk stratification.
Consistent with previous studies (9–20), our study found that NLR, PLR, LMR, SII, PIV, FAR, FPR, AGR, ALI, PNI, AAPR, CAR, RAR, APR, and FFA/Alb were all associated with OS in the univariate analysis. Even RAR, APR, and FFA/Alb, which were not evaluated in previous lung cancer association studies, were found to be independent predictors of prognosis in patients with NSCLC. Some studies have claimed that FAR, SII, and AAPR are associated with OS in patients with NSCLC (12, 19, 28). However, no previous studies have compared these three indicators in these patients. A recent study found that ALI was more effective in predicting the prognosis of patients with lung cancer compared with other inflammatory or nutritional markers (29). Among the 15 indicators related to inflammation and nutrition investigated in this study, FAR had the most accurate predictive ability in assessing the prognosis of patients with NSCLC, surpassing ALI in predictive effectiveness. FAR is an objective, easy-to-use, and simplified approach that helps facilitate the timely, individualized treatment of patients with NSCLC in clinical practice. However, the results need to be confirmed in more prospective studies.
In this study, we also observed that TNM staging combined with FAR, SII, and AAPR had better predictive value than the TNM staging system alone. Subsequently, Kaplan–Meier survival analysis was performed to examine these three indicators. The results showed that higher levels of FAR and SII and lower levels of AAPR were linked to worse OS. However, after excluding potential confounders based on multifactor regression analysis, only FAR was found to be an independent prognostic factor for patients with NSCLC. This suggests that timely intervention should be carried out during the treatment of patients to improve their nutritional status, inhibit inflammatory responses, and improve coagulation function.
The C-index can be used to assess the differentiation ability of various models; that is, all of the research content in the research data are randomly paired. In this cohort, if a patient with a longer survival time is predicted to live longer than another patient with a relatively shorter survival time, the prediction is said to match the actual outcome. In other words, the patient who was predicted to have a high survival rate actually achieved a higher survival rate than the other patient, a phenomenon known as consensus prediction. In other words, the area under the curve (AUC) mainly reflects the predictive power of the model, but the C-index can evaluate the accuracy of the prediction results of various models, which can be simply understood as the C-index is an extension of the AUC, while the AUC is a special case of the C-index. Although the survival analysis and the C-index results all suggested that FAR, SII, and AAPR had a certain predictive value for OS in NSCLC patients, the multivariate Cox regression analysis suggested that only FAR was an independent prognostic factor for OS in NSCLC patients, while SII and AAPR were not. At the same time, in this study, we have observed that the C-index of FAR was better than that of SII and AAPR. These results suggest that FAR is better than SII and AAPR in terms of prognostic value for patients with NSCLC.
As a composite indicator based on fibrinogen and albumin, FAR has been reported to be a potential predictor of adverse outcomes in various malignancies, such as esophageal squamous cell carcinoma (30), hepatocellular carcinoma (31), and NSCLC (28). The mechanism of action of FAR in cancer prognosis can be explained by studying the functions of its components. Research has indicated that fibrinogen levels will increase to different degrees when the body is in a pathophysiological condition, such as a tumor, surgery, infection, inflammation, or trauma (32). Research has also indicated that fibrinogen serves as a cytoskeleton within the tumor extracellular matrix, shielding tumor cells from immune cell attack, facilitating interactions between platelets and circulating tumor cells (CTCs), enhancing platelet adhesion to CTCs, and boosting the metastatic potential of tumor cells (33, 34). Furthermore, fibrinogen can bind directly to the intercellular adhesion molecule-1 (ICAM-1) on endothelial cells, enhancing tumor cell adhesion, proliferation, and migration (35).
Previous studies have shown that nutritional status plays an important prognostic role in disease progression and long-term survival in patients with cancer (36). Albumin is the most abundant circulating protein in plasma and not only reflects the nutritional status of the human body but also participates in the systemic inflammatory response (37). Low albumin levels or poor nutritional status could lead to impaired immune function in tumor patients and promote tumor proliferation, invasion, and migration (38). For the aforementioned reasons, the composite index composed of fibrinogen and albumin is more beneficial for evaluating a patient’s overall condition, and its predictive value is higher than that of single inflammation indicators, such as NLR and PLR.
The study has several limitations. First, this is a retrospective analysis with a single-center design and a limited sample size, which inevitably leads to selection bias in the study subjects and the clinical data collection. Second, there is no clear consensus on the optimal cutoff values for FAR, SII, and AAPR, and the impact of dynamic changes in FAR, SII, and AAPR on long-term prognosis remains to be evaluated. Third, this study is a retrospective analysis with a long follow-up duration, and the recommended NSCLC treatment regimen has been constantly updated. Therefore, prospective, large-sample, multicenter studies are needed in the future to further validate the findings of this study, and subgroup analysis should be conducted using the treatment approach as a stratification factor to explore other variables that are meaningful for prognostic assessment in order to further improve the predictive efficacy of this model.
In conclusion, among the other indicators, FAR was the best predictor of prognosis in patients with NSCLC. Assessment of FAR can identify patients at potential risk of poor prognosis and is expected to be a useful prognostic marker in clinical practice.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
This study was reviewed and approved by Institutional Review Board of Qingdao Municipal Hospital, which waived the informed consent requirement due to the retrospective design of the study. This study complied with the Declaration of Helsinki. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements.
SM: Formal analysis, Investigation, Methodology, Software, Validation, Visualization, Writing – original draft, Writing – review & editing. LW: Data curation, Methodology, Project administration, Supervision, Visualization, Writing – review & editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2024.1396843/full#supplementary-material
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2021) 71:209–49. doi: 10.3322/caac.21660
2. Molinier O, Goupil F, Debieuvre D, Auliac JB, Jeandeau S, Lacroix S, et al. Five-year survival and prognostic factors according to histology in 6101 non-small-cell lung cancer patients. Respir Med Res. (2020) 77:46–54. doi: 10.1016/j.resmer.2019.10.001
3. Pirker R, Filipits M. Adjuvant therapy in patients with completely resected non-small-cell lung cancer: current status and perspectives. Clin Lung Cancer. (2019) 20:1–6. doi: 10.1016/j.cllc.2018.09.016
4. Debieuvre D, Locher C, Asselain B, Dayen C, Molinier O, Falchero L, et al. Evidence of slight improvement in five-year survival in non-small-cell lung cancer over the last 10 years: Results of the French KBP-CPHG real-world studies. Bull Cancer. (2019) 106:283–92. doi: 10.1016/j.bulcan.2019.01.010
5. Hwang JK, Page BJ, Flynn D, Passmore L, Mccaul E, Brady J, et al. Validation of the eighth edition TNM lung cancer staging system. J Thorac Oncol. (2020) 15:649–54. doi: 10.1016/j.jtho.2019.11.030
6. Mytelka DS, Li L, Benoit K. Post-diagnosis weight loss as a prognostic factor in non-small cell lung cancer. J Cachexia Sarcopenia Muscle. (2018) 9:86–92. doi: 10.1002/jcsm.12253
7. Zhan C, Jiang T, Yang X, Guo W, Tan L. [Clinical characteristics and prognostic factors of lung adenosquamous carcinoma in SEER database between 2010 and 2015]. Zhongguo Fei Ai Za Zhi. (2018) 21:600–9. doi: 10.3779/j.issn.1009-3419.2018.08.14
8. Liang J, Sui Q, Zheng Y, Bi G, Chen Z, Li M, et al. A nomogram to predict prognosis of patients with lung adenosquamous carcinoma: a population-based study. J Thorac Dis. (2020) 12:2288–303. doi: 10.21037/jtd
9. Zhang N, Jiang J, Tang S, Sun G. Predictive value of neutrophil-lymphocyte ratio and platelet-lymphocyte ratio in non-small cell lung cancer patients treated with immune checkpoint inhibitors: A meta-analysis. Int Immunopharmacol. (2020) 85:106677. doi: 10.1016/j.intimp.2020.106677
10. Zhang H, Gao L, Zhang B, Zhang L, Wang C. Prognostic value of platelet to lymphocyte ratio in non-small cell lung cancer: a systematic review and meta-analysis. Sci Rep. (2016) 6:22618. doi: 10.1038/srep22618
11. Zhai B, Chen J, Wu J, Yang L, Guo X, Shao J, et al. Predictive value of the hemoglobin, albumin, lymphocyte, and platelet (HALP) score and lymphocyte-to-monocyte ratio (LMR) in patients with non-small cell lung cancer after radical lung cancer surgery. Ann Transl Med. (2021) 9:976. doi: 10.21037/atm
12. Yan X, Li G. Preoperative systemic immune-inflammation index predicts prognosis and guides clinical treatment in patients with non-small cell lung cancer. Biosci Rep. (2020) 40(3):BSR20200352. doi: 10.1042/BSR20200352
13. Guven DC, Sahin TK, Erul E, Kilickap S, Gambichler T, Aksoy S. The association between the pan-immune-inflammation value and cancer prognosis: A systematic review and meta-analysis. Cancers (Basel). (2022) 14(11). doi: 10.3390/cancers14112675
14. Li B, Deng H, Lei B, Chen L, Zhang X, Sha D. The prognostic value of fibrinogen to albumin ratio in Malignant tumor patients: A meta-analysis. Front Oncol. (2022) 12:985377. doi: 10.3389/fonc.2022.985377
15. Ying HQ, Liao YC, Luo YR, Xiong G, Huang Y, Nie RW, et al. Cancer-elicited inflammation attenuates response and outcome in tyrosine kinase inhibitor naive patients with advanced NSCLC. Pharmacol Res. (2021) 170:105734. doi: 10.1016/j.phrs.2021.105734
16. Zhang H, Zhang B, Zhu K, Wu C, Gao L, Sun X, et al. Preoperative albumin-to-globulin ratio predicts survival in patients with non-small-cell lung cancer after surgery. J Cell Physiol. (2019) 234:2471–9. doi: 10.1002/jcp.26766
17. Han Z, Hu Z, Zhao Q, Xue W, Duan G. The advanced lung cancer inflammation index predicts outcomes of patients with non-small cell lung cancer following video-assisted thoracic surgery. J Int Med Res. (2021) 49:3000605211062442. doi: 10.1177/03000605211062442
18. Wang Z, Wang Y, Zhang X, Zhang T. Pretreatment prognostic nutritional index as a prognostic factor in lung cancer: Review and meta-analysis. Clin Chim Acta. (2018) 486:303–10. doi: 10.1016/j.cca.2018.08.030
19. Li SJ, Lv WY, Du H, Li YJ, Zhang WB, Che GW, et al. Albumin-to-alkaline phosphatase ratio as a novel prognostic indicator for patients undergoing minimally invasive lung cancer surgery: Propensity score matching analysis using a prospective database. Int J Surg. (2019) 69:32–42. doi: 10.1016/j.ijsu.2019.07.008
20. Miyazaki T, Yamasaki N, Tsuchiya T, Matsumoto K, Kunizaki M, Kamohara R, et al. Ratio of C-reactive protein to albumin is a prognostic factor for operable non-small-cell lung cancer in elderly patients. Surg Today. (2017) 47:836–43. doi: 10.1007/s00595-016-1448-8
21. Lu C, Long J, Liu H, Xie X, Xu D, Fang X, et al. Red blood cell distribution width-to-albumin ratio is associated with all-cause mortality in cancer patients. J Clin Lab Anal. (2022) 36:e24423. doi: 10.1002/jcla.24423
22. Li Y, Wang JS, Guo Y, Zhang T, Li LP. Use of the alkaline phosphatase to prealbumin ratio as an independent predictive factor for the prognosis of gastric cancer. World J Gastroenterol. (2020) 26:6963–78. doi: 10.3748/wjg.v26.i44.6963
23. Zhang L, Han L, He J, Lv J, Pan R, Lv T. A high serum-free fatty acid level is associated with cancer. J Cancer Res Clin Oncol. (2020) 146:705–10. doi: 10.1007/s00432-019-03095-8
24. Goldstraw P, Chansky K, Crowley J, Rami-Porta R, Asamura H, Eberhardt WE, et al. The IASLC lung cancer staging project: proposals for revision of the TNM stage groupings in the forthcoming (Eighth) edition of the TNM classification for lung cancer. J Thorac Oncol. (2016) 11:39–51. doi: 10.1016/j.jtho.2015.09.009
25. Wei X, Xie F, Zhou X, Wu Y, Yan H, Liu T, et al. Role of pyroptosis in inflammation and cancer. Cell Mol Immunol. (2022) 19:971–92. doi: 10.1038/s41423-022-00905-x
26. Medina-Jiménez AK, Monroy-Torres R. Repurposing individualized nutritional intervention as a therapeutic component to prevent the adverse effects of radiotherapy in patients with cervical cancer. Front Oncol. (2020) 10:595351. doi: 10.3389/fonc.2020.595351
27. Mcmillan DC. Systemic inflammation, nutritional status and survival in patients with cancer. Curr Opin Clin Nutr Metab Care. (2009) 12:223–6. doi: 10.1097/MCO.0b013e32832a7902
28. Yuan C, Huang M, Wang H, Jiang W, Su C, Zhou S. Pretreatment fibrinogen-albumin ratio (FAR) associated with treatment response and survival in advanced non-small cell lung cancer patients treated with first-line anti-PD-1 therapy plus platinum-based combination chemotherapy. Cancer Manag Res. (2022) 14:377–86. doi: 10.2147/CMAR.S347547
29. Song M, Zhang Q, Song C, Liu T, Zhang X, Ruan G, et al. The advanced lung cancer inflammation index is the optimal inflammatory biomarker of overall survival in patients with lung cancer. J Cachexia Sarcopenia Muscle. (2022) 13:2504–14. doi: 10.1002/jcsm.13032
30. Tan Z, Zhang M, Han Q, Wen J, Luo K, Lin P, et al. A novel blood tool of cancer prognosis in esophageal squamous cell carcinoma: the Fibrinogen/Albumin Ratio. J Cancer. (2017) 8:1025–9. doi: 10.7150/jca.16491
31. Xu Q, Yan Y, Gu S, Mao K, Zhang J, Huang P, et al. A novel inflammation-based prognostic score: the fibrinogen/albumin ratio predicts prognoses of patients after curative resection for hepatocellular carcinoma. J Immunol Res. (2018) 2018:4925498. doi: 10.1155/2018/4925498
32. Pieters M, Wolberg AS. Fibrinogen and fibrin: An illustrated review. Res Pract Thromb Haemost. (2019) 3:161–72. doi: 10.1002/rth2.12191
33. Zheng S, Shen J, Jiao Y, Liu Y, Zhang C, Wei M, et al. Platelets and fibrinogen facilitate each other in protecting tumor cells from natural killer cytotoxicity. Cancer Sci. (2009) 100:859–65. doi: 10.1111/j.1349-7006.2009.01115.x
34. Desgrosellier JS, Cheresh DA. Integrins in cancer: biological implications and therapeutic opportunities. Nat Rev Cancer. (2010) 10:9–22. doi: 10.1038/nrc2748
35. Pluskota E, D'souza SE. Fibrinogen interactions with ICAM-1 (CD54) regulate endothelial cell survival. Eur J Biochem. (2000) 267:4693–704. doi: 10.1046/j.1432-1327.2000.01520.x
36. Mantzorou M, Koutelidakis A, Theocharis S, Giaginis C. Clinical Value of Nutritional Status in Cancer: What is its Impact and how it Affects Disease Progression and Prognosis? Nutr Cancer. (2017) 69:1151–76. doi: 10.1080/01635581.2017.1367947
37. Artigas A, Wernerman J, Arroyo V, Vincent JL, Levy M. Role of albumin in diseases associated with severe systemic inflammation: Pathophysiologic and clinical evidence in sepsis and in decompensated cirrhosis. J Crit Care. (2016) 33:62–70. doi: 10.1016/j.jcrc.2015.12.019
Keywords: non-small cell lung cancer, inflammatory index, nutritional index, prognosis, overall survival
Citation: Ma S and Wang L (2024) Fibrinogen-to-albumin ratio (FAR) is the best biomarker for the overall survival of patients with non-small-cell lung cancer. Front. Oncol. 14:1396843. doi: 10.3389/fonc.2024.1396843
Received: 06 March 2024; Accepted: 29 May 2024;
Published: 24 June 2024.
Edited by:
Jelena Stojsic, University of Belgrade, SerbiaReviewed by:
Wenkai Xia, Ludwig Maximilian University of Munich, GermanyCopyright © 2024 Ma and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lunqing Wang, d2FuZ2x1bnFpbmcxOTczQDE2My5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.