
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol., 24 May 2024
Sec. Gastrointestinal Cancers: Hepato Pancreatic Biliary Cancers
Volume 14 - 2024 | https://doi.org/10.3389/fonc.2024.1395740
This article is part of the Research TopicLiver Cancer Awareness Month 2023: Current Progress and Future Prospects on Advances in Primary Liver Cancer Investigation and TreatmentView all 21 articles
Background: Hepatocellular carcinoma (HCC) is one of the common causes of tumor death in elderly patients. However, there is a lack of individualized prognostic predictors for elderly patients with HCC after surgery.
Method: We retrospectively analyzed HCC patients over 65 years old who underwent hepatectomy from 2015 to 2018, and randomly divided them into training cohort and validation cohort in a ratio of 3:1. Univariate Cox regression was used to screen the risk factors related to prognosis. Prognostic variables were further selected by least absolute shrinkage and selection operator regression model (LASSO) and multivariate Cox regression to identify the predictors of overall survival (OS) and disease-free survival (DFS). These indicators were then used to construct a predictive nomogram. The receiver operating characteristic curve (ROC curve), calibration curve, consistency index (C-index) and decision analysis curve (DCA) were used to test the predictive value of these independent prognostic indicators.
Result: A total of 188 elderly HCC patients who underwent hepatectomy were enrolled in this study. The independent prognostic indicators of OS included albumin (ALB), cancer embolus, blood loss, viral hepatitis B, total bilirubin (TB), microvascular invasion, overweight, and major resection. The independent prognostic indicators of DFS included major resection, ALB, microvascular invasion, laparoscopic surgery, blood loss, TB, and pleural effusion. In the training cohort, the ROC curve showed that the predictive values of these indicators for OS and DFS were 0.827 and 0.739, respectively, while in the validation cohort, they were 0.798 and 0.694. The calibration curve nomogram exhibited good prediction for 1-year, 2-year, and 3-year OS and DFS. Moreover, the nomogram models exhibited superior performance compared to the T-staging suggested by C-index and DCA.
Conclusion: The nomogram established in this study demonstrate commendable predictive efficacy for OS and DFS in elderly patients with HCC after hepatectomy.
Core Tip: The purpose of this retrospective study is to screen the risk factors of survival and recurrence in elderly patients with HCC after hepatectomy. The nomogram included cancer embolus, viral hepatitis B, overweight, major resection, ALB, microvascular invasion, laparoscopic surgery, blood loss, TB, and pleural effusion as predictors. The calibration curve of this nomogram was good, indicating credible predictive value and clinical feasibility.
Primary hepatic cancer is a common malignant tumor of the digestive system, more than 90% of which is hepatocellular carcinoma (HCC). It is the sixth-highest incidence of malignant tumors in the world, accounting for 8.3% of cancer-related deaths worldwide, and is the third most frequent cause of cancer-related death (1). As one of the most populous countries with the largest number of hepatitis B patients in the world, the new cases of HCC in China account for 45% of the new cases in the world every year, and this proportion is also unceasingly growing (2). Therefore, HCC is still a major global health problem to be solved.
In recent years, the aging of the population has become a global problem, which has caused people’s concern that it will increase the morbidity of cancer. Clinical research has already confirmed that aging is an established risk factor for HCC (3). Research related to aging shows that cell and tissue aging caused by DNA damage, epigenetic changes, oxidative stress, and mitochondrial dysfunction will also increase the risk of malignant tumors (4). According to statistics, about 80% of HCC cases are elderly patients (5). Given the increase in life expectancy and the aging of the population around the world, it is expected that the number of elderly HCC patients may continue to rise.
At present, hepatectomy, liver transplantation, or radiofrequency ablation has become a recognized surgical treatment for HCC. As far as hepatectomy is concerned, the methods of surgery have developed from wedge-shaped resection or conventional hepatectomy to minimally invasive and precise hepatectomy (6). The concept of Enhanced Recovery After Surgery (ERAS) put forward in recent years emphasizes the integration of preoperative individualized disease assessment, surgical plan formulation, and perioperative rehabilitation treatment to minimize surgical trauma, protect remaining liver function, and strive for the best rehabilitation effect (7).
Although liver resection, the most commonly used surgical modality, has shown good results in elderly patients (8, 9), it is still a complex procedure (10). At the same time, due to the decline of physical function and underlying health conditions, elderly patients have a higher probability of severe postoperative complications, which leads to poor prognosis (11). Therefore, for such elderly patients, there may be large differences in prognosis due to individual conditions. However, there is no individualized prognostic prediction model for elderly HCC patients after liver resection.
This study aims to retrospectively analyze the relationship between perioperative indicators and tumor prognosis in elderly patients. Consequently, the objective is to propose an individualized prognosis prediction scheme for elderly HCC patients after surgery.
We collected clinical and follow-up data of elderly patients > 65 years of age who underwent radical hepatectomy for HCC at West China Hospital of Sichuan University (Chengdu, Sichuan Province, China) from January 2015 to September 2018. The preoperative diagnosis of HCC was performed according to the criteria of the American Association for the Study of Liver Diseases (AASLD) (12). The absolute contraindications for liver resection are American Society of Anesthesiologists (ASA) grade >= III, ascites, extrahepatic metastasis, unresectable large vessel tumor invasion, or future residual liver <40%-50% (13). The inclusion criteria were as follows:(1) Patients aged >= 65 years (male or female); (2) Primary hepatocellular carcinoma was confirmed by postoperative pathological examination; (3) The years of diagnosis were 2015–2018; (4) Without the absolute contraindications for liver resection; (5) no preoperative anticancer treatments. The exclusion criteria were as follows: (1) Patients aged < 65 years; (2) Intrahepatic cholangiocarcinoma or mixed-type HCC was confirmed by postoperative pathological examination; (3) Patients have absolute contraindications for liver resection; (4) Severe dysfunction of vital organs; (5) History of any other malignancy.
For the included patients, we collected the basic information of the patients at the time of admission in the medical records, including age, sex, overweight (BMI>24), viral hepatitis B, liver cirrhosis, abdominal surgery history, hypertension, diabetes, chronic obstructive pulmonary disease (COPD), tumor size (cm), tumor numbers (single or multiply), tumor location (VII/VIII/IVa or not), alpha-fetoprotein (AFP, ng/L), indocyanine green retention rate at 15 minutes (ICGR15, %), TB (mmol/L), ALB (g/L), aspartate aminotransferase (AST, IU/L), alanine aminotransferase (ALT, IU/L), platelet (PLT, IU/L), white blood count (WBC, 109/L).
In addition, we also collected the data of patients undergoing surgical treatment, postoperative pathological data, and postoperative complications in the medical records. The information includes ASA(I~IV), surgical type(laparoscopic or open), major resection, blood loss(ml), intraoperative transfusion, total pringle time(min), margin distance(mm), cancer embolus, microvascular invasion, capsular invasion, microsatellite nodules, poor differentiation, necrosis (0~4), fibrosis (0~4), overall complications, major complications, liver-specific complications, liver failure, hemorrhage, ascites, biliary leakage, general complications, respiratory complications, respiratory infection, wound infection, pleural effusion, atelectasis respiratory insufficiency, Clavien-Dindo Grade(I~IV) and hospital stay.
All patients were followed up every 1 month for the first 3 months after discharge and every 3 months thereafter. The median follow-up time was 34.5 months. The primary endpoints of the study were overall survival (OS) and disease-free survival (DFS), and the secondary endpoint was complication rate. DFS was defined from the end of surgery to death or recurrence; OS was defined from the end of surgery to death.
All patients were randomly assigned into training cohort and validation cohort in a ratio of 3:1. For descriptive statistics of patient clinical characteristics data, the statistical description median [interquartile range (IQR)] was used for continuous variables, and frequency (%) was used for categorical variables. Univariate Cox regression was used to screen the risk factors related to prognosis. Prognostic variables were further selected by least absolute shrinkage and selection operator regression model (LASSO) and multivariate Cox regression to identify the predictors of overall survival (OS) and disease-free survival (DFS). Proportional hazards assumption was used to assessed the Cox regression models. These indicators were then used to construct a predictive nomogram.
A series of validation methods were used to validate the accuracy and discrimination of the nomogram, including AUC, calibration curve and consistency index (C-index). In order to explore clinical application value of the model, decision analysis curve (DCA) was used to calculate net benefit under different thresholds. At the same time, we divided all patients into low-risk and high-risk groups based on each patient’s nomogram score. Log-rank test and Kaplan-Meier (K-M) curve were used to compare survival differences among patients in different groups.
All data analyses were performed using R 4.1.1. and SPSS 25.0. All tests were two-sided, and a P<0.05 was considered statistically significant.
A total of 188 elderly HCC patients who underwent hepatectomy were included in this study, as shown in Figure 1. Of these patients, males accounted for 77.66% and the median age was 69 (66,72) years. Among them, 40.43% of the patients were overweight (BMI>24). 39.36% and 41.49% of the patients had a history of hepatitis B and liver cirrhosis respectively. 29.26% of the patients had abdominal surgery history. In terms of common chronic diseases in the elderly, hypertension accounted for 34.57%, diabetes accounted for 17.55%, and COPD accounted for only 3.19% (Table 1).
The median tumor size of patients was 5.65cm (4.00,7.00). The tumors of 72.87% of the patients were single lesions, and the tumors of 51.06% of the patients were located in the posterosuperior segments (VII/VIII/IVa) (Table 1). Part of the blood routine, blood biochemical, and serum tumor marker data of the patients are shown in Table 1. All data were collected at the time of admission. Table 2 shows the surgical, pathological, and postoperative information of 188 patients.
In the training cohort, 46 patients died, while in the validation cohort, 15 patients died. The 1-year and 3-year OS of the training cohort was 83.7% and 72.3% of that of the validation cohort, respectively, and the OS of the validation cohort was 85.1% and 74.5%, respectively.
In order to initially determine the factors related to the OS and DFS of the elderly patients, univariate Cox regression analysis was performed. The results showed that ALB, viral hepatitis B, TB, PLT, cancer embolus, capsular invasion, major resection, microvascular invasion, blood loss, and overweight (BMI>24) were significantly related to the OS of the patients (Table 3). ALB, major resection, capsular invasion, TB, pleural effusion, microvascular invasion, and PLT were significantly related to the DFS of the patients (Table 3).
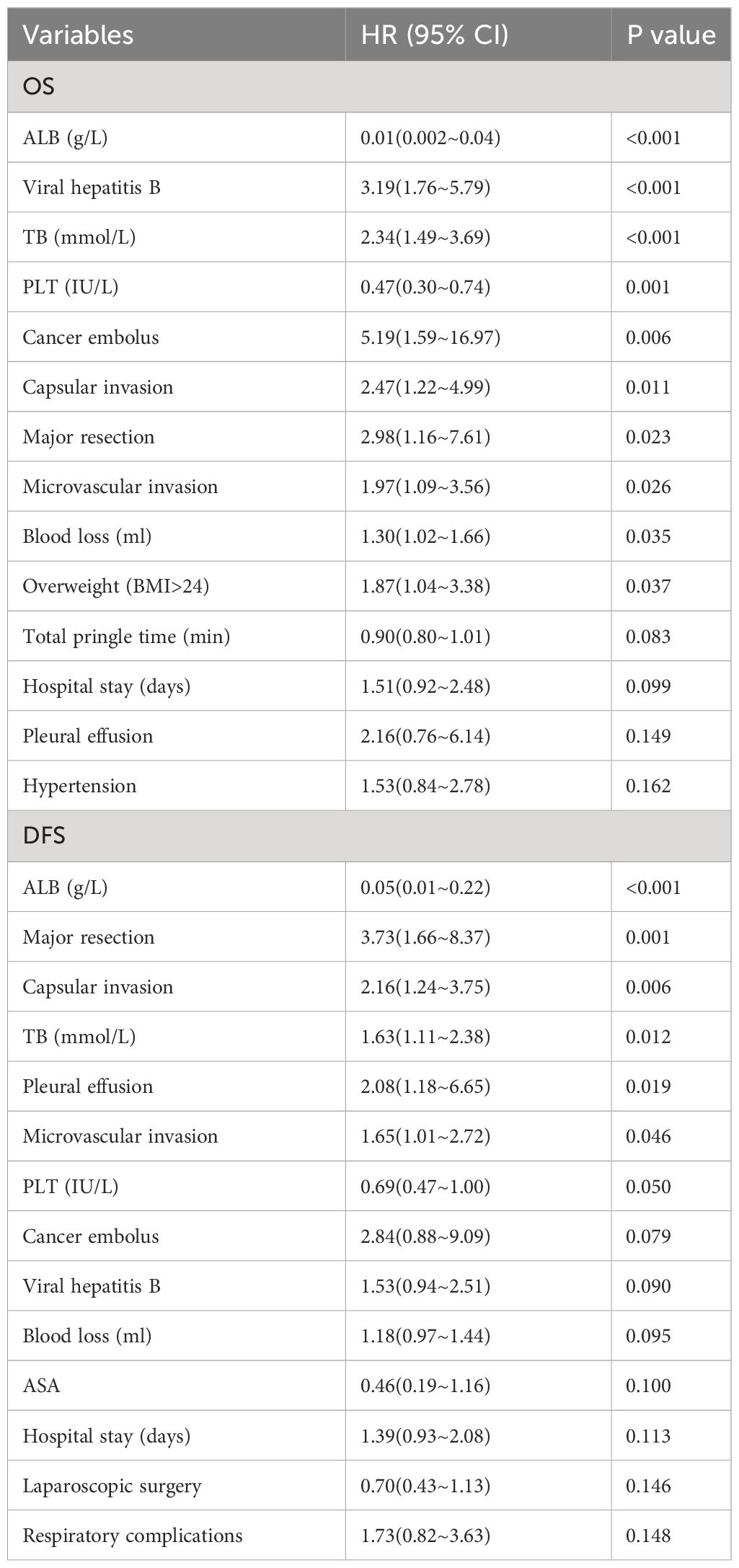
Table 3 Univariate Cox regression analysis of OS and DFS in 141 elderly patients with hepatocellular carcinoma after operation.
The LASSO regression analysis was used to reduce high-dimensional data (14). The features with a P value <0.2 in the univariate Cox regression analysis of OS and DFS were included in the LASSO. The cross-validation was used to determine the most appropriate λ value as optimal parameters (Figure 2). The results for OS yield a λ value of 12, and the results for DFS yield a λ value of 13. Indicating that 12 features associated with OS and 13 features associated with DFS included in LASSO were important.
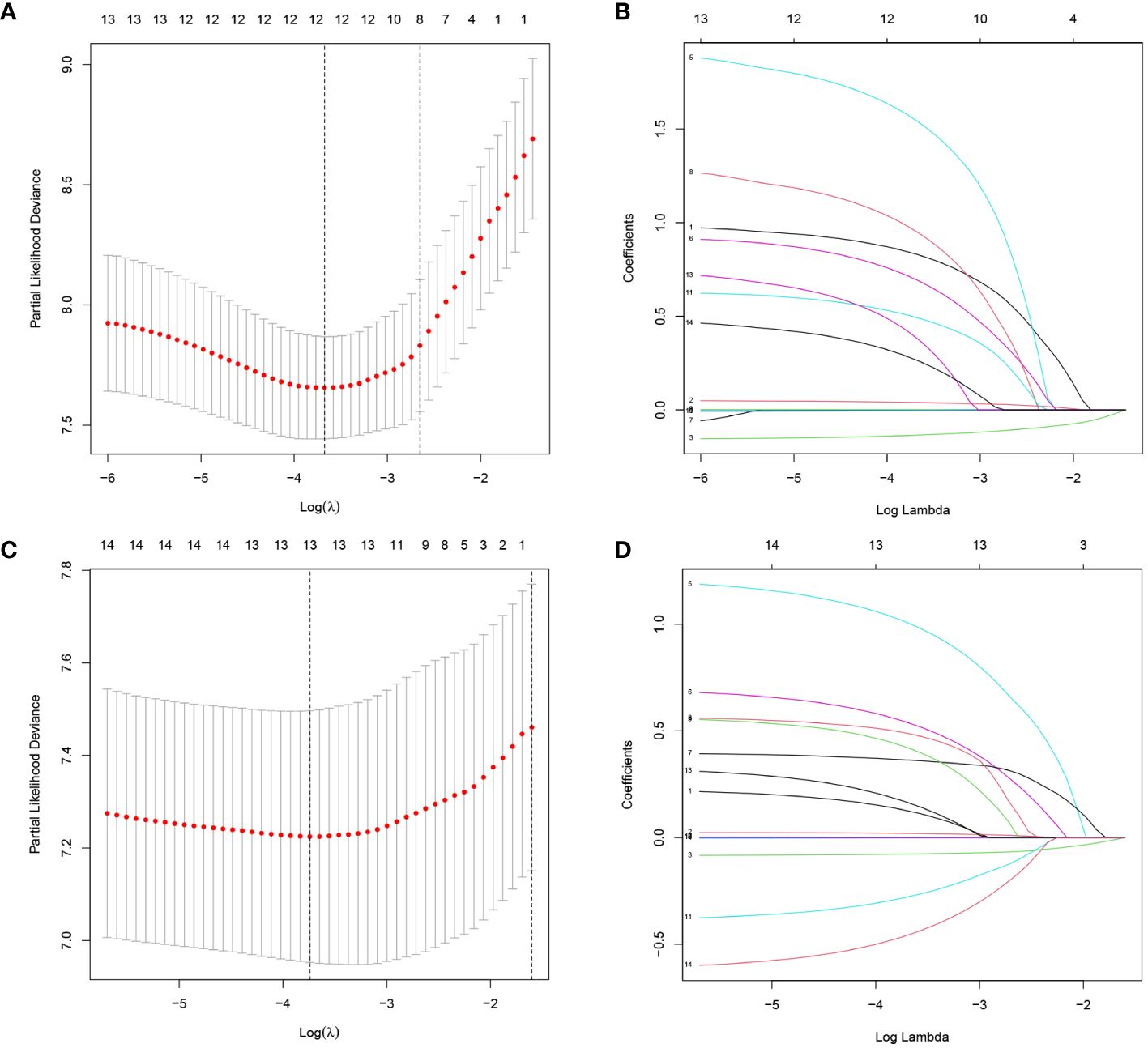
Figure 2 Screening of variables based on Lasso regression. (A) OS, (C) DFS: The selection process of the optimum value of the parameter λ in the Lasso regression model by cross-validation method. (B) OS, (D) DFS: The variation characteristics of the coefficient of variables.
Significant variables output by LASSO regression analysis were included in multivariate Cox regression analysis. The results of multivariate Cox regression analysis showed that ALB, cancer embolus, blood loss, viral hepatitis B, TB, microvascular invasion, overweight (BMI>24), and major resection were independent risk factors for OS in elderly patients (Table 4). Major resection, ALB, microvascular invasion, laparoscopic surgery, blood loss, TB, and pleural effusion were independent risk factors for DFS in elderly patients (Table 4). The proportional hazards assumption was evaluated and found reasonable for each variable (Table S1).
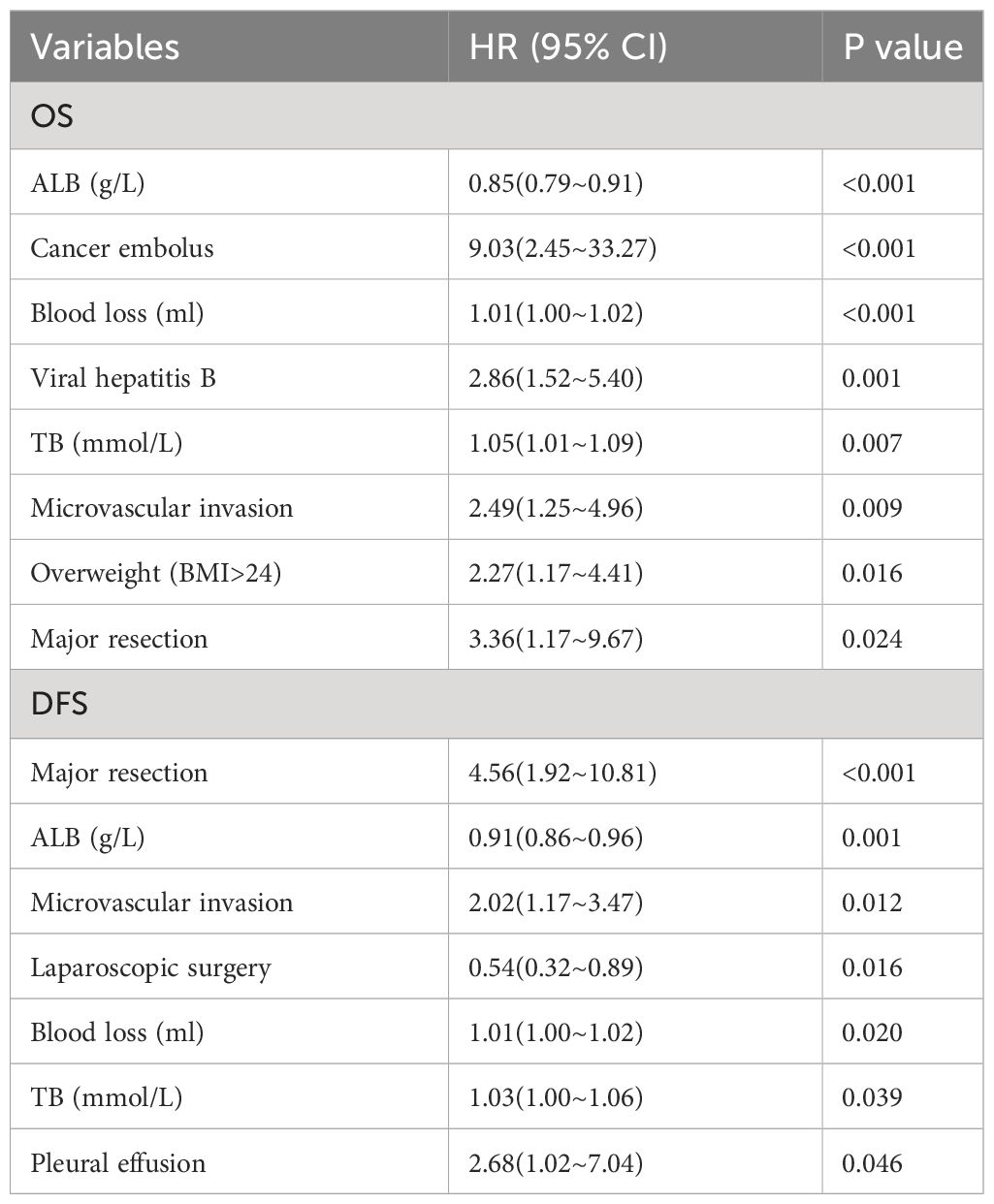
Table 4 Multivariate Cox regression analysis of OS and DFS in elderly patients with hepatocellular carcinoma after operation.
The following factors were used to construct the nomogram (Figures 3, 4). 1-, 2-, and 3-year OS and DFS in elderly patients were predicted by the nomogram. We used ROC curves to verify the predictive value of these independent prognostic factors. As shown in Figures 3 and 4, the area under the curve (AUC value) of the eight independent prognostic factors for the prediction of OS was 0.827. The AUC value of 6 independent prognostic factors for the prediction of DFS was 0.739. In the validation cohort, the AUC values were 0.798 and 0.694, respectively. This suggests that these factors have good predictive value for OS and DFS. Subsequently, calibration curves were depicted, and the results showed that the predicted values of the prediction models were generally consistent with the actual observed values (Figures 3, 4).
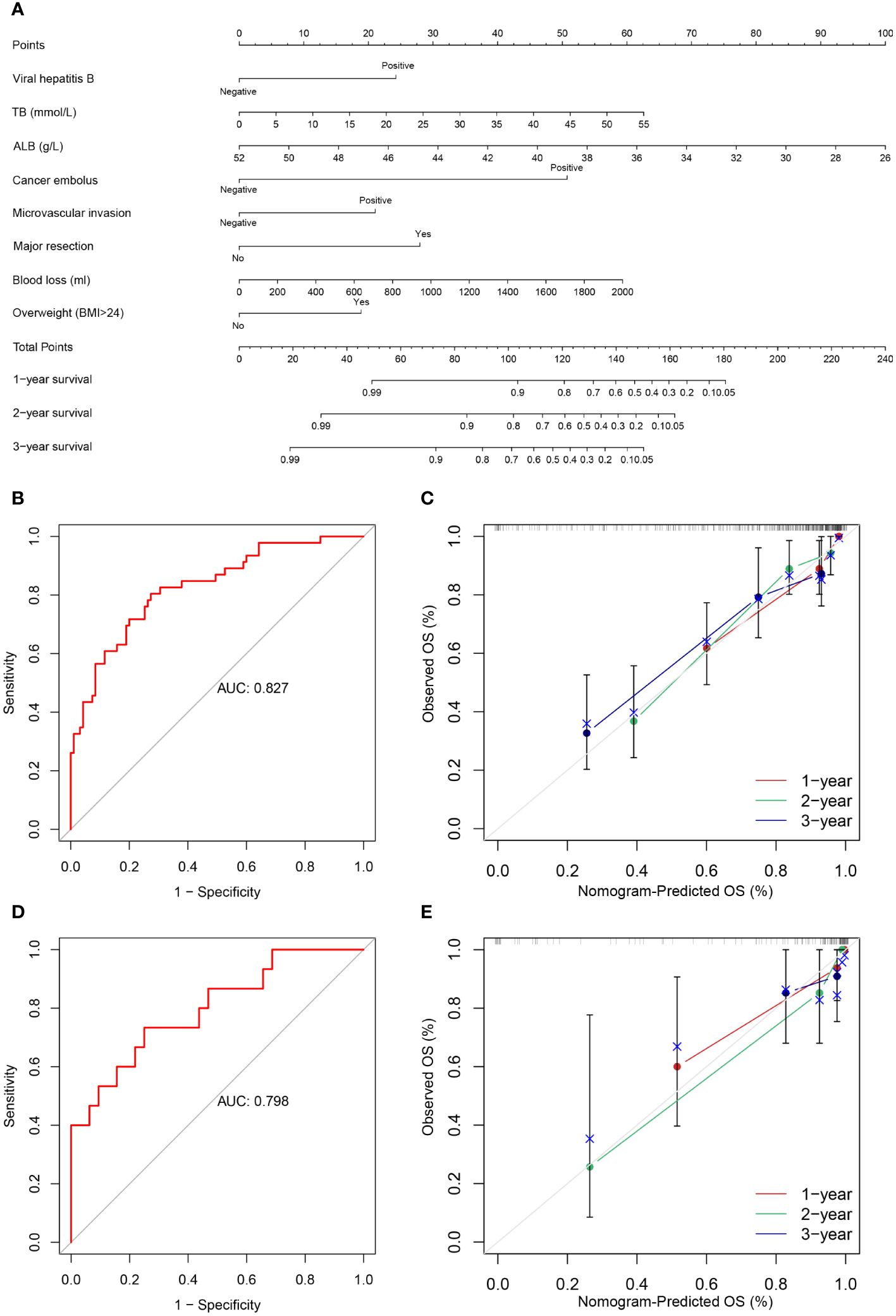
Figure 3 (A) Nomogram prediction model and prediction OS curve; (B) AUC for predicting OS in training cohort; (C) Calibration curves of 1-, 2-, and 3-year OS in training cohort; (D) AUC for predicting OS in validation cohort; (E) Calibration curves of 1-, 2-, and 3-year OS in the validation cohort.
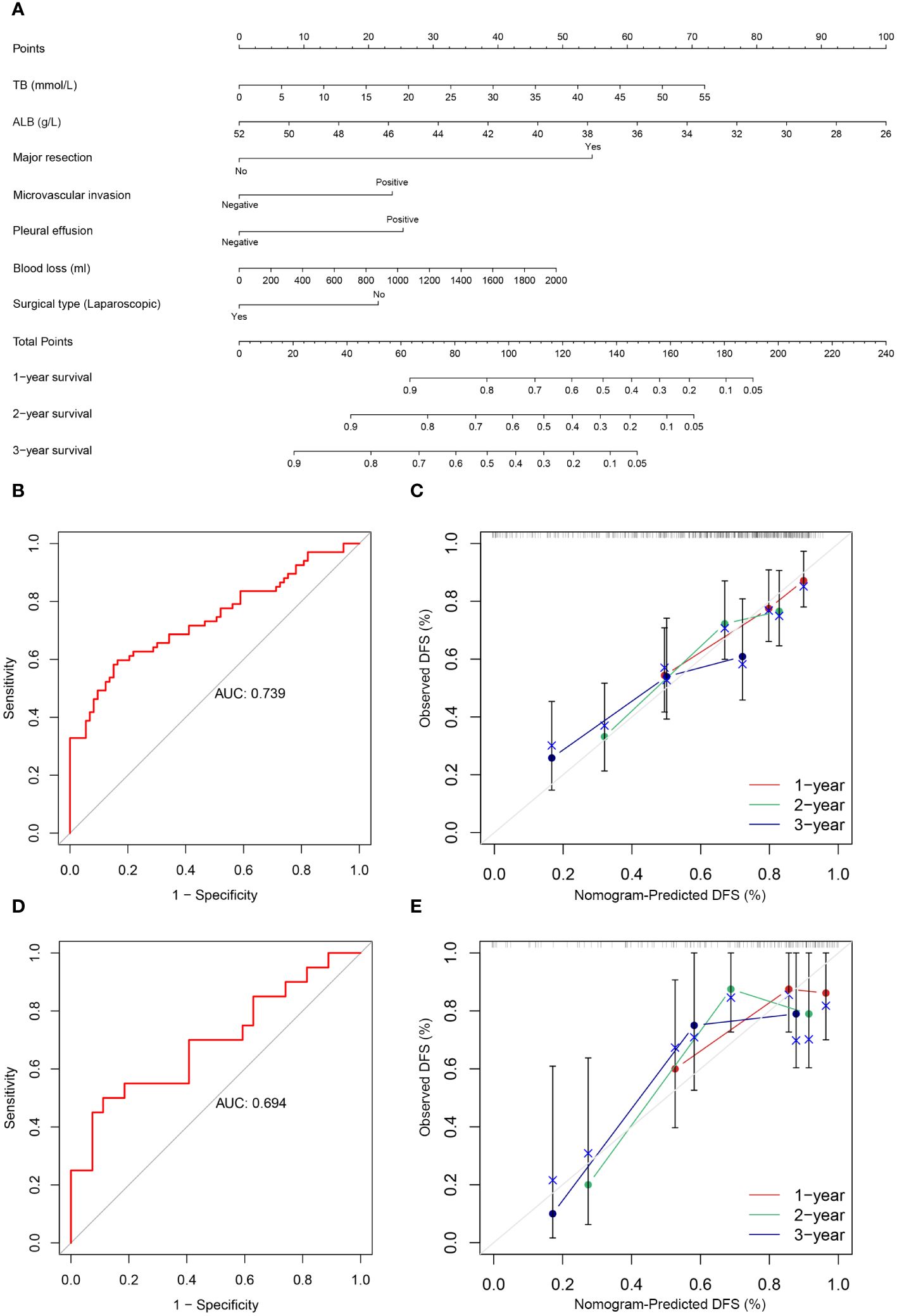
Figure 4 (A) Nomogram prediction model and prediction DFS curve; (B) AUC for predicting DFS in training cohort; (C) Calibration curves of 1-, 2-, and 3-year DFS in training cohort; (D) AUC for predicting DFS in validation cohort; (E) Calibration curves of 1-, 2-, and 3-year DFS in the validation cohort.
The predictive value of the constructed nomogram was compared with the 8th edition American Joint Committee on Cancer (AJCC) T-staging system in terms of clinical practicability. The results are shown in Figure 5. In the training cohort, the C-index of the nomogram for OS and DFS was 0.825 and 0.699, respectively, which was significantly higher than that of the T-staging (OS: 0.590; DFS: 0.537). Similarly, in the validation cohort, the C-index of the nomogram for OS (0.914) and DFS (0.761) was also significantly higher than that of the T-staging (OS: 0.613; DFS: 0.621). Additionally, DCA suggested that the nomogram had better predictive power than the T-staging (Figure 6). Overall, the nomogram models exhibited superior performance compared to the T-staging.
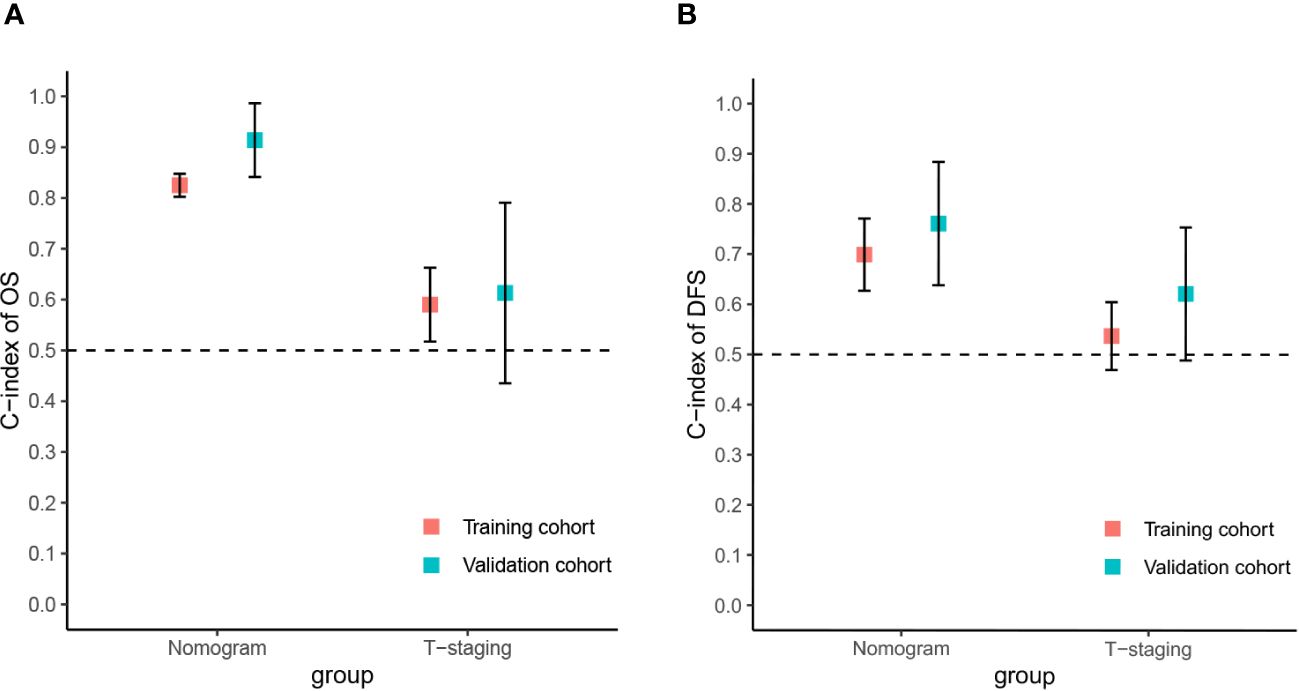
Figure 5 The C-index of the nomograms and T-staging. (A) The C-index of the OS nomogram and T-staging; (B) The C-index of the DFS nomogram and T-staging.
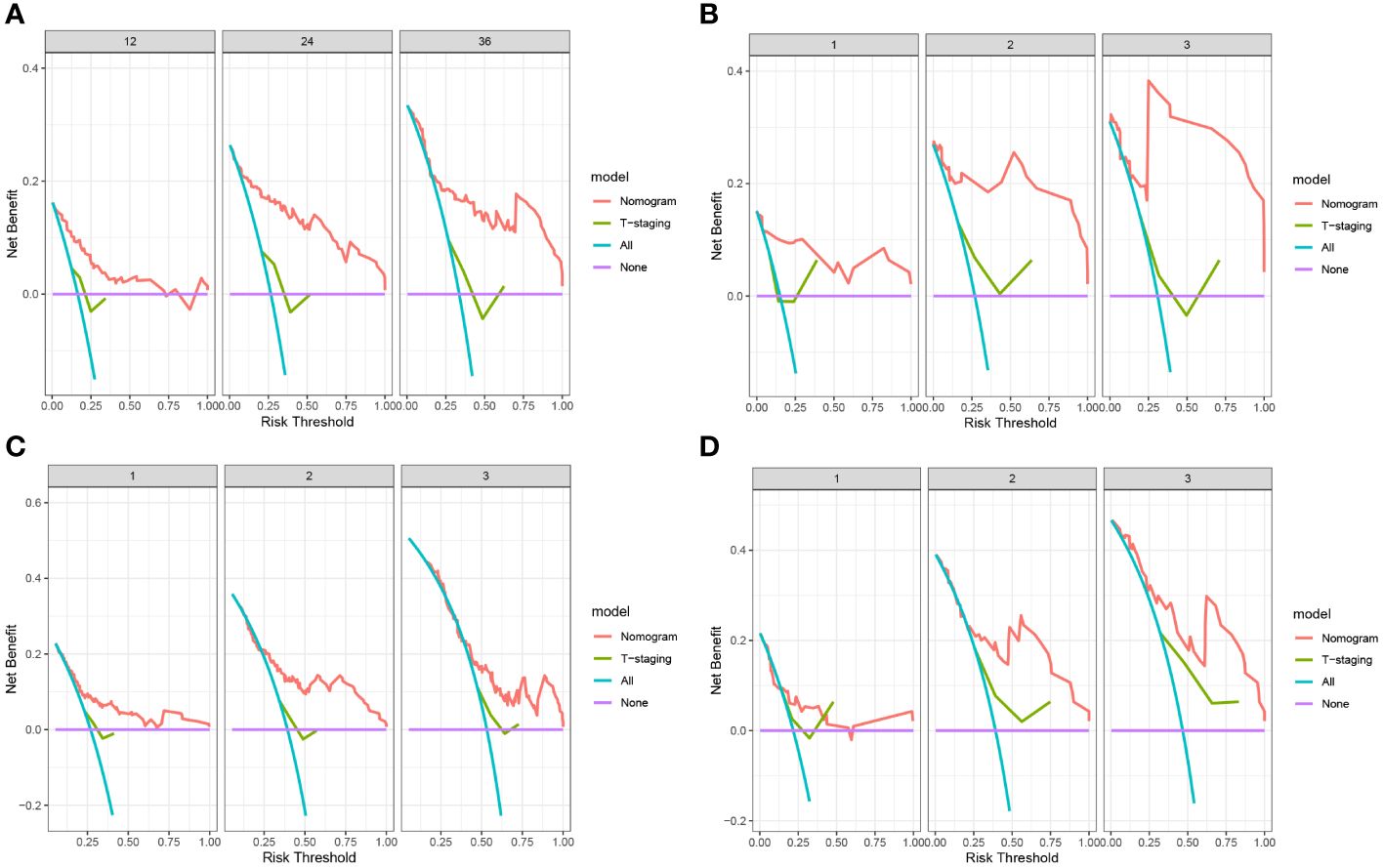
Figure 6 (A) DCA of OS in training cohort; (B) DCA of OS in validation cohort; (C) DCA of DFS in training cohort; (D) DCA of DFS in validation cohort.
All patients were assigned to the high-risk group or the low-risk group based on their nomogram scores. In both the training and validation cohorts, patients in the low-risk group exhibited significantly higher survival rates and lower recurrence rates than those in the high-risk group (Figure 7).
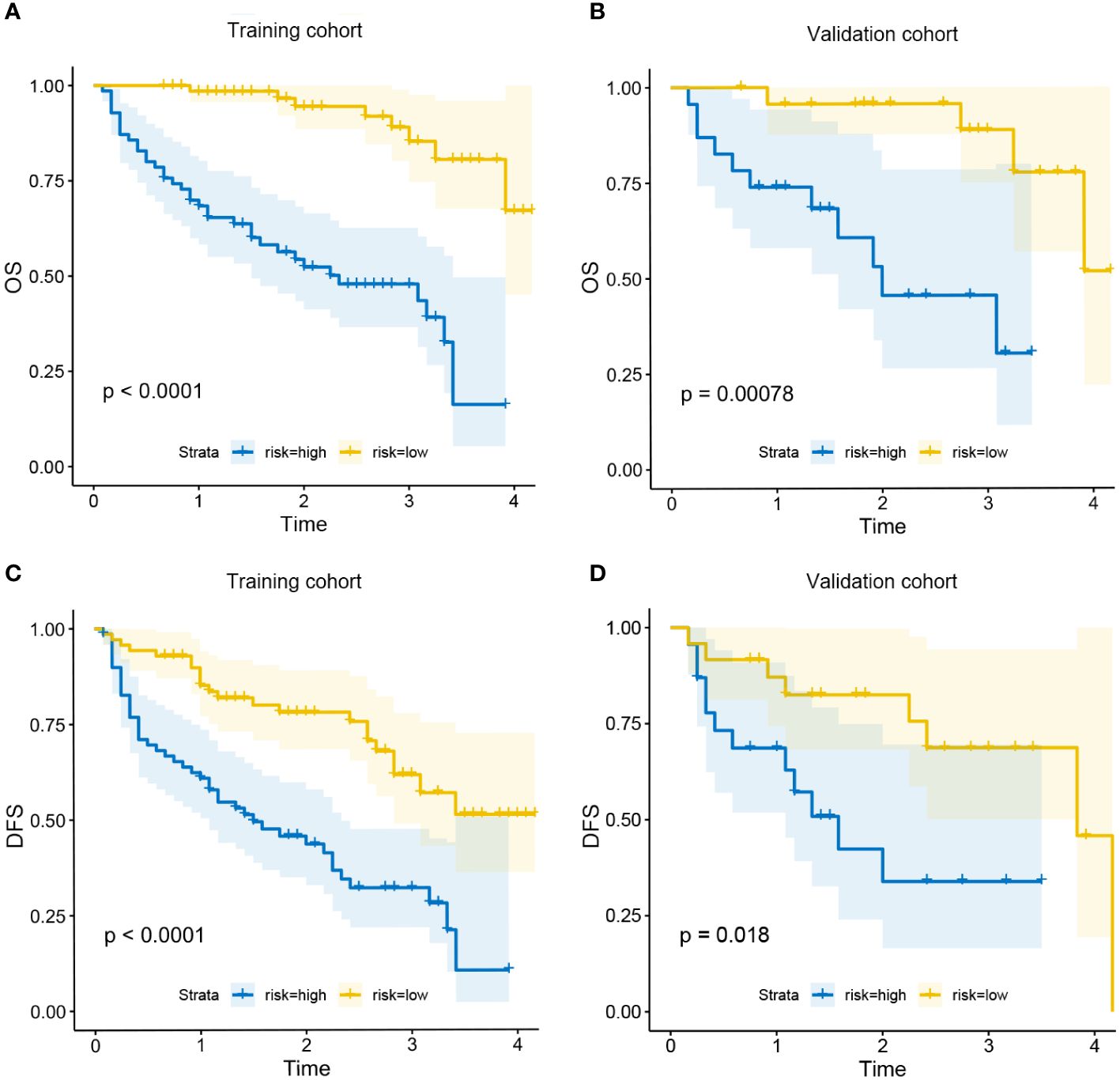
Figure 7 Kaplan-Meier curves for OS in the low-risk and high-risk groups in training cohort (A) and validation cohort (B); Kaplan-Meier curves for DFS in the low-risk and high-risk groups in training cohort (C) and validation cohort (D).
At present, the prognosis prediction model of malignant tumor patients has been widely used. In recent years, the nomogram related to HCC has been gradually developed, which has played a very active role in the diagnosis, treatment, and prognosis prediction of HCC. For example, Li et al. constructed a nomogram to predict the risk of liver nodule malignant transformation into HCC, which is helpful for the early diagnosis of HCC (15). Lin et al. established a simplified model to help guide decisions about prophylactic transarterial chemoembolization after hepatectomy for patients with HCC (16). Wang et al. developed a nomogram to predict recurrence in patients with early-stage HCC (17). However, the prognosis of HCC patients is closely related to age. In addition, the incidence of HCC is highest in the elderly population, whose postoperative prognostic factors are different from other age groups. For example, the underlying health conditions of elderly patients and the higher incidence of postoperative complications will affect the prognosis of patients. Currently, there is still a lack of predictive models for recurrence and survival after liver resection in elderly patients, so we constructed a nomogram to predict the prognosis of such elderly patients.
Unlike earlier prognostic indicators such as BCLC and Child–Pugh grade (18), the nomogram we developed calculates the survival rate and recurrence rate for each patient rather than simply categorizing patients into different risk groups. This approach reduces the impact of heterogeneity, thereby aiding clinicians in making individualized treatment decisions for elderly HCC patients and establishing a foundation for managing high-risk patients in clinical practice.
Our study collected the clinical information of elderly HCC patients over 65 years old who underwent liver resection in our hospital. After univariate analysis, LASSO regression analysis, and multivariate Cox analysis, it was found that ALB, cancer embolus, blood loss, viral hepatitis B, TB, microvascular invasion, overweight (BMI>24), and major resection were closely related to the OS of this group of people. Major resection, ALB, microvascular invasion, laparoscopic surgery, blood loss, TB, and pleural effusion are closely related to the DFS of this group. Among them, ALB, blood loss, TB, microvascular invasion, and major resection are related to both DFS and OS. Previous studies have shown that these factors are associated with the prognosis of HCC.
Many previous studies have shown that a serum ALB level of <35g/L is a risk factor for prognosis of HCC. As for the mechanism, ALB has been shown to inhibit HCC growth, migration and invasion (19, 20). In a recent investigation by Zeng et al. (21), focusing on young patients with HCC, it was elucidated that a lower ALB level correlated with increased recurrence after liver resection. ALB and TB are important parameters to access liver functional estimation in the Child-Pugh score. But in recent years, more and more studies have used the Albumin-Bilirubin (ALBI) grade to evaluate the liver reserve function and prognosis prediction ability of HCC patients (22–24). Our study discovered that ALB and TB levels affect the prognosis of elderly HCC patients as independent predictive factors, which is consistent with previous studies.
For elderly patients, there is still insufficient evidence of whether overweight increases the risk of poor prognosis after liver resection. Currently, there is only limited evidence that obesity itself does not affect the prognosis of postoperative patients with HCV-related HCC (25). Another study shows that overweight and obese patients with cirrhosis have an increased morbidity rate after hepatectomy (26). However, we found that being overweight may be a risk factor for OS in elderly HCC patients undergoing hepatectomy.
HBV has long been considered an independent risk factor associated with poor prognosis in HCC. High HBV replication rate and related non-resolving inflammation are major risk factors of postoperative recurrence, and antiviral treatment can effectively prolong postoperative survival (27). But our study only found that HBV was associated with OS in elderly patients, not with DFS.
The effect of whether to perform major hepatectomy on prognosis is still controversial. On the one hand, extended liver resection can benefit those patients with large and locally advanced HCC (28), but at the same time, it will increase the burden on the residual liver and increase the risk of liver failure (29). However, we found that major resection is a risk factor in older patients.
Suh et al. (30) revealed that intraoperative blood loss ≥ 700 mL were risk factors for tumor recurrence after surgical resection for HCC, consistent with the findings of our study. Large-volume blood loss may impede the immune reaction against tumor cells and induce hypoxic ischemia, thereby increasing the likelihood of tumor recurrence (31).
As for the choice of surgical method, many studies recommend laparoscopic surgery for elderly patients (32). Our study further confirmed that laparoscopic surgery is sufficiently safe, has no significant impact on OS, and can reduce the risk of recurrence. On the one hand, laparoscopic resection is significantly associated with less blood loss, wider resection margins, shorter hospital stays, and lower morbidity (33). On the other hand, smaller tumors are more inclined to be eligible for laparoscopic resection. Challenges persist in laparoscopic approaches, particularly with lesions located in the posterosuperior segments, large and recurrent tumors, and in cases of advanced cirrhosis (34).
As is known to us, tumor embolism is seen most commonly in metastatic renal cell carcinoma; hepatocellular carcinoma; and carcinomas of the breast, stomach, and prostate (35). For tumor embolism caused by HCC, studies have shown that it is associated with poor prognosis of HCC (36, 37). This is also consistent with our conclusion. For microvascular invasion, it is also an established risk factor for HCC (38), which not only can affect OS but also DFS for elderly HCC patients, due to its association with microscopic residual metastatic disease after resection (39). Microvascular invasion and tumor embolism are associated with circulating tumor cells (CTCs). CTCs are independent significant risk factors for HCC recurrence and can be identified as biomarkers for diagnosis, prognostication, and therapeutic monitoring.
Previous studies have shown that complications after hepatectomy in HCC patients will affect their prognosis (40). For the elderly, their reserve capacity is low, and many comorbidities, such as cardiovascular disease, diabetes, and respiratory system disease, etc., make postoperative rehabilitation and treatment difficult (32, 41). Therefore, the incidence of postoperative complications is higher than that of patients in other age groups. Our data showed that postoperative pleural effusion was an independent risk factor for DFS. Due to the limited number of patients with various complications in our included patients, we did not find that the occurrence of other postoperative complications was related to the prognosis of the patients.
Overall, the clinical features we used to construct the nomogram after statistical screening were reported in previous studies. It is suggested that these factors may be related to the OS and DFS of patients. However, in terms of these clinical features, no studies have systematically analyzed them in elderly HCC patients undergoing hepatectomy and determined their impact on prognosis. We initially confirmed the validity of the nomogram for OS and DFS prediction in elderly HCC patients who underwent hepatectomy, and found that it exhibited superior predictive power compared to T-staging, as suggested by DCA. This nomogram can effectively identify patients at high risk of death and recurrence. Therefore, the model we developed is an intuitive clinical tool with good predictive performance, assisting physicians in making rational treatment decisions for elderly HCC patients.
However, this study still has some limitations. First of all, we constructed the nomogram based on the clinical data of West China Hospital of Sichuan University. This does not necessarily represent other countries and regions. Secondly, as a retrospective analysis, this study inevitably has information bias and selection bias, which may affect the conclusion to a certain extent. Third, the nomogram we constructed has only been internally verified, and more clinical data and multi-center studies are still needed for external verification to further prove the effectiveness of the nomogram.
We have built and internally validated a nomogram to identify the risk factors of overall survival and recurrence in elderly HCC patients who underwent hepatectomy. Although the nomograms have exhibited better prediction for OS and DFS, further multi-center studies and external verification are still needed.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
The studies involving humans were approved by Biomedical Ethics Review Committee of West China Hospital of Sichuan University. The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants’ legal guardians/next of kin because 1.The medical records used in this study were obtained from previous clinical records. 2.In this study, the risk to patients is no greater than the minimum risk. 3.Exemption from informed consent will not have adverse effects on the rights and health of the subjects. 4.The privacy and personal identity information of the subjects are protected. 5.This study does not utilize medical records that patients have explicitly refused to use in the past. 6.This study does not involve personal privacy and commercial interests.
YT: Data curation, Methodology, Validation, Writing – original draft. YW: Formal analysis, Investigation, Resources, Visualization, Writing – original draft. NW: Investigation, Methodology, Software, Visualization, Writing – original draft. YL: Data curation, Resources, Supervision, Writing – review & editing. GL: Project administration, Resources, Software, Writing – review & editing. BL: Conceptualization, Funding acquisition, Resources, Supervision, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by National Natural Science Foundation of China (Grant No.82002578); Sichuan Science and Technology Program (Grant No.2022YSF0060, Grant No.2022YSF0114, Grant No.2022NSFSC0680, Grant No. 2023YFS0094); 1·3·5 project for disciplines of excellence–Clinical Research Incubation Project, West China Hospital, Sichuan University (20HXFH021); 1·3·5 project for disciplines of excellence, West China Hospital, Sichuan University (ZYJC21049).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2024.1395740/full#supplementary-material
AFP, Alpha Fetoprotein; ALB, Albumin; ALT, Alanine Aminotransferase; ASA, American Society of Anesthesiologists; AST, Aspartate Aminotransferase; AUC, Area Under the Curve; BMI, Body mass index; COPD, Chronic Obstructive Pulmonary Disease; DFS, Disease-Free Survival; DCA, decision curve analysis; HBV, Hepatitis B Virus; HCC, Hepatocellular carcinoma; HCV, Hepatitis C Virus; ICGR15, Indocyanine green retention rate at 15 minutes; LASSO, Least Absolute Shrinkage and Selection Operator regression model; OS, Overall Survival; PLT, Platelet; ROC, Receiver Operating Characteristic Curve; TB, Total Bilirubin; WBC, White Blood Count.
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2021) 71:209–49. doi: 10.3322/caac.21660
2. Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, et al. Cancer statistics in China, 2015. CA Cancer J Clin. (2016) 66:115–32. doi: 10.3322/caac.21338
3. Bosch FX, Ribes J, Cléries R, Díaz M. Epidemiology of hepatocellular carcinoma. Clin Liver Dis. (2005) 9:191–211. doi: 10.1016/j.cld.2004.12.009
4. Campisi J. Aging, cellular senescence, and cancer. Annu Rev Physiol. (2013) 75:685–705. doi: 10.1146/annurev-physiol-030212-183653
5. Macias RIR, Monte MJ, Serrano MA, González-Santiago JM, Martín-Arribas I, Simão AL, et al. Impact of aging on primary liver cancer: epidemiology, pathogenesis and therapeutics. Aging (Albany NY). (2021) 13:23416–34. doi: 10.18632/aging.v13i19
6. Lv A, Li Y, Qian HG, Qiu H, Hao CY. Precise navigation of the surgical plane with intraoperative real-time virtual sonography and 3D simulation in liver resection. J Gastrointest Surg. (2018) 22:1814–8. doi: 10.1007/s11605-018-3872-0
7. Niu X, Liu J, Feng Z, Zhang T, Su T, Han W. Short-term efficacy of precise hepatectomy and traditional hepatectomy for primary liver cancer: a systematic review and meta-analysis. J Gastrointest Oncol. (2021) 12:3022–32. doi: 10.21037/jgo
8. Kishida N, Hibi T, Itano O, Okabayashi K, Shinoda M, Kitago M, et al. Validation of hepatectomy for elderly patients with hepatocellular carcinoma. Ann Surg Oncol. (2015) 22:3094–101. doi: 10.1245/s10434-014-4350-x
9. Oishi K, Itamoto T, Kohashi T, Matsugu Y, Nakahara H, Kitamoto M. Safety of hepatectomy for elderly patients with hepatocellular carcinoma. World J Gastroenterol. (2014) 20:15028–36. doi: 10.3748/wjg.v20.i41.15028
10. Wu FH, Shen CH, Luo SC, Hwang JI, Chao WS, Yeh HZ, et al. Liver resection for hepatocellular carcinoma in oldest old patients. World J Surg Oncol. (2019) 17:1. doi: 10.1186/s12957-018-1541-0
11. Inoue Y, Tanaka R, Fujii K, Kawaguchi N, Ishii M, Masubuchi S, et al. Surgical outcome and hepatic regeneration after hepatic resection for hepatocellular carcinoma in elderly patients. Dig Surg. (2019) 36:289–301. doi: 10.1159/000488327
12. Marrero JA, Kulik LM, Sirlin CB, Zhu AX, Finn RS, Abecassis MM, et al. Diagnosis, staging, and management of hepatocellular carcinoma: 2018 practice guidance by the American association for the study of liver diseases. Hepatology. (2018) 68:723e50. doi: 10.1002/hep.29913
13. Altekruse SF, McGlynn KA, Dickie LA, Kleiner DE. Hepatocellular carcinoma confirmation, treatment, and survival in surveillance, epidemiology, and end results registries, 1992–2008. Hepatology. (2012) 55:476e82. doi: 10.1002/hep.24710
14. Tibshirani R. Regression shrinkage and selection via the Lasso. J R Stat Soc Ser B Methodol. (1996) 58:267–88. doi: 10.1111/j.2517-6161.1996.tb02080.x
15. Li J, Xu Y, Liu H, Guo B, Guo X, Li Y, et al. Construction and validation of NSMC-ASIL, a predictive model for risk assessment of Malignant hepatic nodules. Am J Cancer Res. (2022) 12:5315–24.
16. Lin N, Wang L, Huang Q, Zhou W, Liu X, Liu J. A simplified model for prophylactic transarterial chemoembolization after resection for patients with hepatocellular carcinoma. PloS One. (2022) 17:e0276627. doi: 10.1371/journal.pone.0276627
17. Wang Q, Qiao W, Zhang H, Liu B, Li J, Zang C, et al. Nomogram established on account of Lasso-Cox regression for predicting recurrence in patients with early-stage hepatocellular carcinoma. Front Immunol. (2022) 13:1019638. doi: 10.3389/fimmu.2022.1019638
18. Reig M, Forner A, Rimola J, Ferrer-Fàbrega J, Burrel M, Garcia-Criado Á, et al. BCLC strategy for prognosis prediction and treatment recommendation: The 2022 update. J Hepatology. (2022) 76:681–93. doi: 10.1016/j.jhep.2021.11.018
19. Bağırsakçı E, Şahin E, Atabey N, Erdal E, Guerra V, Carr BI. Role of albumin in growth inhibition in hepatocellular carcinoma. Oncology. (2017) 93:136–42. doi: 10.1159/000471807
20. Fu X, Yang Y, Zhang D. Molecular mechanism of albumin in suppressing invasion and metastasis of hepatocellular carcinoma. Liver Int. (2022) 42:696–709. doi: 10.1111/liv.15115
21. Zeng J, Lin K, Liu H, Huang Y, Guo P, Zeng Y, et al. Prognosis factors of young patients undergoing curative resection for hepatitis B virus-related hepatocellular carcinoma: A multicenter study. Cancer Manag Res. (2020) 12:6597–606. doi: 10.2147/CMAR.S261368
22. Zhao S, Wang M, Yang Z, Tan K, Zheng D, Du X, et al. Comparison between Child-Pugh score and Albumin-Bilirubin grade in the prognosis of patients with HCC after liver resection using time-dependent ROC. Ann Transl Med. (2020) 8:539. doi: 10.21037/atm
23. Liao J, Liu J, Li C, Fan J, Wang Z. Albumin-bilirubin grade as a prognostic factor of hepatocellular carcinoma after treatment with orthotopic liver transplantation. Transl Cancer Res. (2019) 8:1457–65. doi: 10.21037/tcr
24. Wang Z, Fan Q, Wang M, Wang E, Li H, Liu L. Comparison between Child-Pugh Score and albumin-bilirubin grade in patients treated with the combination therapy of transarterial chemoembolization and sorafenib for hepatocellular carcinoma. Ann Transl Med. (2020) 8:537. doi: 10.21037/atm
25. Nishikawa H, Arimoto A, Wakasa T, Kita R, Kimura T, Osaki Y. The relation between obesity and survival after surgical resection of hepatitis C virus-related hepatocellular carcinoma. Gastroenterol Res Pract. (2013) 2013:430438. doi: 10.1155/2013/430438
26. Tanaka S, Iimuro Y, Hirano T, Hai S, Suzumura K, Nakamura I, et al. Safety of hepatic resection for hepatocellular carcinoma in obese patients with cirrhosis. Surg Today. (2013) 43:1290–7. doi: 10.1007/s00595-013-0706-2
27. Du Y, Han X, Ding YB, Yin JH, Cao GW. Prediction and prophylaxis of hepatocellular carcinoma occurrence and postoperative recurrence in chronic hepatitis B virus-infected subjects. World J Gastroenterol. (2016) 22:6565–72. doi: 10.3748/wjg.v22.i29.6565
28. Pamecha V, Sasturkar SV, Sinha PK, Mahansaria SS, Bharathy KGS, Kumar S, et al. Major liver resection for large and locally advanced hepatocellular carcinoma. Indian J Surg. (2017) 79:326–31. doi: 10.1007/s12262-016-1545-3
29. Wang J, Zhang Z, Shang D, Li J, Liu C, Yu P, et al. Noninvasively assessed portal hypertension grade predicts post-hepatectomy liver failure in patients with hepatocellCarcinoma: A multicenter study. Front Oncol. (2022) 12:934870. doi: 10.3389/fonc.2022.934870
30. Suh SW, Lee SE, Choi YS. Influence of intraoperative blood loss on tumor recurrence after surgical resection in hepatocellular carcinoma. J Pers Med. (2023) 13:1115. doi: 10.3390/jpm13071115
31. Nakanishi K, Kanda M, Kodera Y. Long-lasting discussion: Adverse effects of intraoperative blood loss and allogeneic transfusion on prognosis of patients with gastric cancer. World J Gastroenterol. (2019) 25:2743–51. doi: 10.3748/wjg.v25.i22.2743
32. Wang S, Ye G, Wang J, Xu S, Ye Q, Ye H. Laparoscopic versus open liver resection for hepatocellular carcinoma in elderly patients: A systematic review and meta-analysis of propensity score-matched studies. Front Oncol. (2022) 12:939877. doi: 10.3389/fonc.2022.939877
33. Sotiropoulos GC, Prodromidou A, Kostakis ID, Machairas N. Meta-analysis of laparoscopic vs open liver resection for hepatocellular carcinoma. Updates Surg. (2017) 69:291–311. doi: 10.1007/s13304-017-0421-4
34. Berardi G, Muttillo EM, Colasanti M, Mariano G, Meniconi RL, Ferretti S, et al. Challenging scenarios and debated indications for laparoscopic liver resections for hepatocellular carcinoma. Cancers (Basel). (2023) 15:1493. doi: 10.3390/cancers15051493
35. Silva CIS, Müller NL. Online case 45. In: Silva CIS, Müller NL, editors. Teaching files in radiology, the teaching files: chest. W.B. Saunders (2010). p. 90–1. doi: 10.1016/B978-1-4160-6110-6.10045-9
36. Zhou Z, Qi L, Mo Q, Liu Y, Zhou X, Zhou Z, et al. Effect of surgical margin on postoperative prognosis in patients with solitary hepatocellular carcinoma: A propensity score matching analysis. J Cancer. (2021) 12:4455–62. doi: 10.7150/jca.57896
37. Ou H, Huang Y, Xiang L, Chen Z, Fang Y, Lin Y, et al. Circulating tumor cell phenotype indicates poor survival and recurrence after surgery for hepatocellular carcinoma. Dig Dis Sci. (2018) 63:2373–80. doi: 10.1007/s10620-018-5124-2
38. Xu L, Li L, Wang P, Zhang M, Zhang Y, Hao X, et al. Novel prognostic nomograms for hepatocellular carcinoma patients with microvascular invasion: experience from a single center. Gut Liver. (2019) 13:669–82. doi: 10.5009/gnl18489
39. Erstad DJ, Tanabe KK. Prognostic and therapeutic implications of microvascular invasion in hepatocellular carcinoma. Ann Surg Oncol. (2019) 26:1474–93. doi: 10.1245/s10434-019-07227-9
40. Harimoto N, Shirabe K, Ikegami T, Yoshizumi T, Maeda T, Kajiyama K, et al. Postoperative complications are predictive of poor prognosis in hepatocellular carcinoma. J Surg Res. (2015) 199:470–7. doi: 10.1016/j.jss.2015.06.012
Keywords: prognosis, recurrence, nomogram, hepatocellular carcinoma, elderly
Citation: Tian Y, Wang Y, Wen N, Lin Y, Liu G and Li B (2024) Development and validation of nomogram to predict overall survival and disease-free survival after surgical resection in elderly patients with hepatocellular carcinoma. Front. Oncol. 14:1395740. doi: 10.3389/fonc.2024.1395740
Received: 04 March 2024; Accepted: 06 May 2024;
Published: 24 May 2024.
Edited by:
Francisco Tustumi, University of São Paulo, BrazilReviewed by:
Wei Wang, First Affiliated Hospital of Wannan Medical College, ChinaCopyright © 2024 Tian, Wang, Wen, Lin, Liu and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yixin Lin, bGlueWl4aW5fcGhkQDEyNi5jb20=; Geng Liu, bGl1Mmdlbmc2QDE2My5jb20=; Bei Li, bGliZWlAc2N1LmVkdS5jbg==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.