- 1Department of Urology, The First Affiliated Hospital of Soochow University, Suzhou, China
- 2Department of Urology, Taixing People’s Hospital, Taizhou, China
Objective: To compare the differential therapeutic effects of Bacillus Calmette-Guérin (BCG) instillation and radical cystectomy (RC) for high-risk non-muscle–invasive urothelial cancer (NMIBC) classified as high-grade T1 in initial and repeat transurethral resection of bladder tumors (TURBT) and to construct a prediction model.
Methods: We retrospectively analyzed the clinical data of patients with malignant bladder tumors treated at the First Affiliated Hospital of Soochow University from January 2016 to December 2017 and compared the differences in 1-year, 2-year, 3-year, 5-year, and comprehensive overall survival (OS) and progression-free survival (PFS) between BCG instillation treatment and RC treatment. Survival curves were drawn to show differences in OS and PFS between the two groups. Concurrently, univariate and multivariate COX analyses were performed to identify risk factors affecting OS and PFS, and a nomogram was created.
Results: In total, 146 patients were included in the study, of whom 97 and 49 were in the BCG and RC groups, respectively. No statistical differences were observed in the 1- and 2-year OS and PFS between the two groups, whereas significant statistical differences were found in the 3-year, 5-year, and comprehensive OS and PFS. Survival curves also confirmed the statistical differences in OS and PFS between the BCG and RC groups. Multivariate COX analysis revealed that the treatment method, concomitant satellite lesions, and albumin-to-alkaline phosphatase ratio (AAPR) were independent risk factors affecting OS and PFS. The nomogram that was further plotted showed good predictive ability for OS and PFS.
Conclusion: For patients who exhibit high-level T1 pathology after both initial and repeat TURBT, especially those with low AAPR, and concomitant satellite lesions, choosing RC as a treatment method offers a better prognosis.
Introduction
Bladder cancer, the ninth most prevalent cancer worldwide, has an alarming estimated 430,000 new cases annually (1). For patients initially treated for bladder tumors, if the pathology results from the first transurethral resection of the bladder tumor (TURBT) indicate a high-grade T1 stage tumor, then conducting repeat TURBT (re-TURBT) can significantly increase progression-free survival (PFS) and disease-free survival rates (2, 3). However, for patients whose pathology results from re-TURBT are still in the high-grade T1 stage, the recommended course of action is either Bacillus Calmette-Guérin (BCG) instillation therapy or radical cystectomy (RC) (4). However, the superiority of either of these treatment options remains controversial (5–7).
Nutritional indicators, including the prognostic nutritional index (PNI) and the controlling nutritional status (CONUT) score, have recently been found to be associated with the prognosis of bladder tumors (8, 9). At the same time, inflammatory factors and their related derivative indicators, including albumin-to-alkaline phosphatase ratio (AAPR), neutrophil-lymphocyte ratio (NLR), platelet-lymphocyte ratio (PLR), lymphocyte-monocyte ratio (LMR), and systemic immune-inflammation index (SII) have also recently been found to be associated with the prognosis of bladder tumors (10–12). Our study further validated the relationship between these indicators and the PFS and overall survival (OS) after bladder cancer surgery.
Our study mainly aimed to follow up patients with high-risk non-muscle-invasive urothelial cancer (NMIBC) who were diagnosed with high-level T1 stage via two TURBT pathology results. After receiving either BCG instillation or RC therapy, we compared the differences in OS and PFS between the two groups of patients. This study aimed to provide a useful reference for future clinical decision-making for this type of patients.
Materials and methods
Clinical data
This was a single-center, retrospective study. We collected and analyzed data from patients who underwent TURBT at the First Affiliated Hospital of Soochow University between January 2016 and December 2018. The inclusion criteria were as follows: (1) having undergone both initial TURBT and re-TURBT, with both pathologies being high-grade T1 stage tumors; (2) not accompanied by carcinoma in situ; and (3) not including all three clinically relevant risk factors; age >70 years, multiple papillary tumors, and diameter >3 cm. The exclusion criteria were as follows: (1) immunodeficiency or active tuberculosis; (2) allergy to BCG; and (3) refusal to participate in the study. In total, 146 patients were included in this study. Among them, 97 received BCG instillation, and 49 underwent RC treatment. We followed up with all enrolled patients for a period ranging from 10 to 84 months, with an average follow-up duration of 54.21 ± 20.29 months.
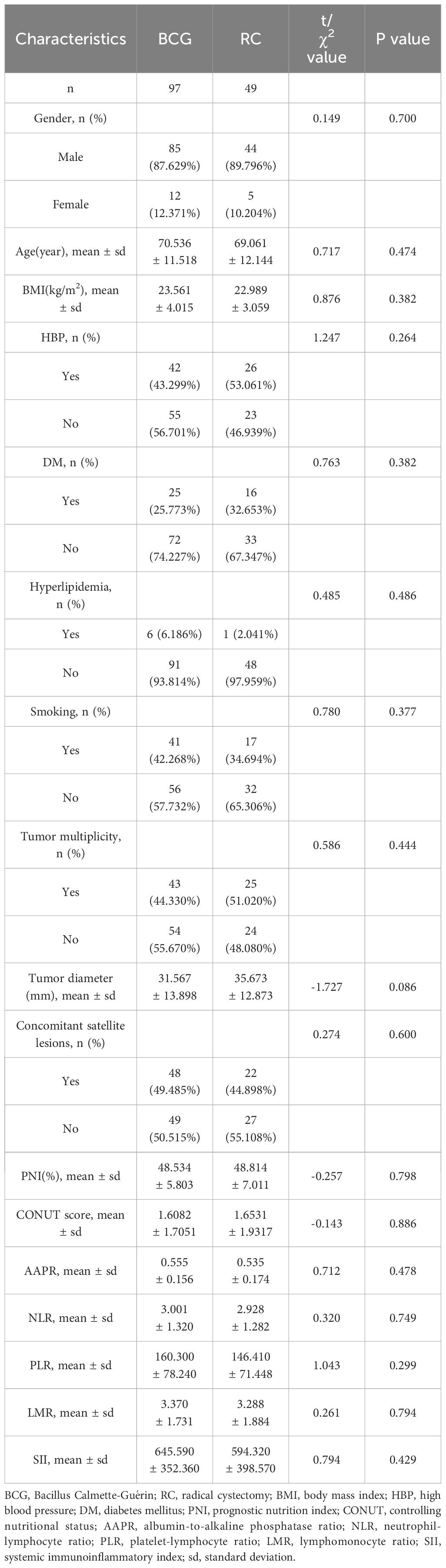
The clinical data collected includes sex, age, body mass index (BMI), high blood pressure (HBP), diabetes mellitus (DM), hyperlipidemia, smoking history, tumor multiplicity, tumor diameter, concurrent satellite lesions, PNI, CONUT score, AAPR, NLR, PLR, LMR, and SII. The calculation formula for PNI is PNI= Serum Albumin (g/L) + 5 x Total Peripheral Blood Lymphocyte Count (x109/L). The CONUT score is calculated based on serum albumin levels, total cholesterol levels, and total lymphocyte count, with a total score ranging from 0–12 points. The calculation formula for AAPR is the pre-operative ratio of albumin to alkaline phosphatase. The calculation formula for SII is SII = Platelet Count x Neutrophil Count/Lymphocyte Count in the blood.
This study adhered to the ethical standards stipulated in the Declaration of Helsinki, which was revised in 2013. Ethical approval was obtained from the Ethics Committee of the First Affiliated Hospital of Soochow University (Approval ID: No. 113[2024]). Before the study, all participating patients signed informed consent forms.
Treatment protocols
RC Group: After the initial TURBT and re-TURBT, each patient underwent preoperative chest, abdominal, and pelvic computed tomography (CT) scans to ensure the absence of distant metastases. Patients were deemed eligible if their Eastern Cooperative Oncology Group (ECOG) performance status was 0–2 points. Patients and their families provided informed consent for surgery, after which RC and urinary diversion were performed under general anesthesia. Details of this procedure have been described previously (13).
BCG Group: After undergoing two rounds of TURBT, all patients underwent chest, abdominal, and pelvic CT scans to rule out distant metastases, with an ECOG score ranging from 0 to 2. After the patients and their families were fully informed and signed consent forms were obtained, the BCG instillation therapy was initiated. The instillation began two weeks after re-TURBT and was conducted in the following sequence: (1) induction instillation, once a week, for a total of six times with 120 mg each instillation; (2) intensification instillation, once every two weeks for three sessions; maintenance instillation was performed once a month for a total of 10 sessions; and (3) continuous instillations were performed once a month for three continuous years.
Follow-up
All patients underwent examinations every three months for the first two years, semi-annually from the second to the fifth year, and annually thereafter. These examinations included chest, abdominal, and pelvic CT and urinary cytology tests. In addition, patients who received BCG instillation therapy underwent supplementary cystoscopy. None of the participants withdrew midway through the study period. For the RC group, the occurrence of tumor recurrence or progression during the follow-up period is considered as the endpoint event for PFS. For the BCG group, the occurrence of high-grade recurrence or tumor progression during the follow-up period is considered as the endpoint event for PFS. And death was regarded as the endpoint event for OS for both two groups.
Statistical analysis
R software (version 4.2.1) with related accessory packages including “ggplot2[3.3.6], survival[3.3.1], rms[6.3–0], stats[4.2.1], ResourceSelection[0.3–5]” was utilized in this study. Qualitative data were compared between the two groups using a t-test. Cumulative data between the two groups were examined the χ2 test. Kaplan–Meier and log-rank tests were used for survival analysis. Univariate and multivariate COX regression analyses were used to identify independent risk factors. A nomogram model was established to predict the OS and PFS. A calibration curve was constructed to evaluate the model. Statistical significance was set at p < 0.05. The sample size was calculated using R software, which required significant results from 103 participants in our study (14). Two senior statisticians independently verified the data.
Results
Baseline levels comparison between the BCG and RC groups
There were no statistically significant differences between the two groups in terms of sex, age, BMI, HBP, DM, hyperlipidemia, smoking history, tumor multiplicity, tumor diameter, concomitant satellite lesions, PNI, CONUT score, AAPR, NLR, PLR, LMR, or SII (Table 1). Meanwhile, the postoperative pathology of RC group patients includes 39 cases classified as T1N0M0, 6 cases as T2aN0M0, 1 case as T2aN2M0, 1 case as T2bN1M0, 1 case as T2bN2M0, and 1 case as T3aN2M0.
OS and PFS comparison between the BCG and RC groups
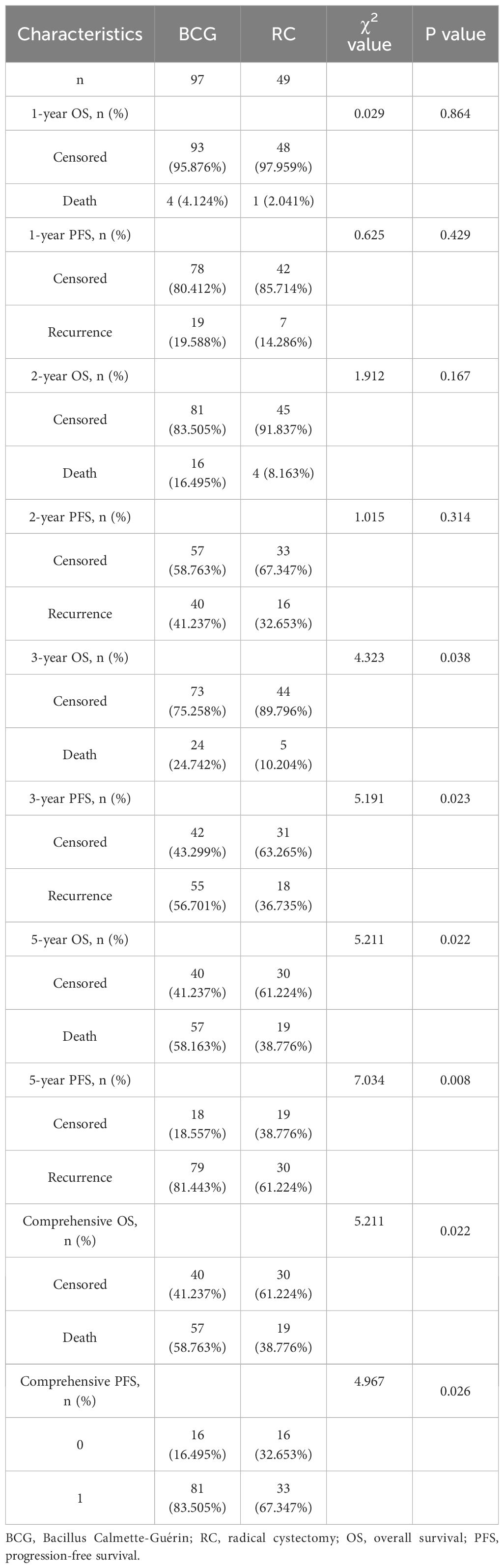
The follow-up period for the BCG group ranged from 10 to 84 months, averaging 51.43 ± 20.50 months, whereas for the RC group, it ranged from 11 to 84 months, averaging 59.71 ± 18.89 months. There was a statistically significant difference in the follow-up time between the two groups (t=2.365, p=0.019). There were no statistically significant differences in the 1-year and 2-year OS (95.876% vs. 97.959% and 83.505% vs. 91.837%, respectively) and PFS (80.412% vs. 85.714% and 58.763% vs. 67.347%, respectively) between the BCG and RC groups. However, statistical differences were found in 3-year, 5-year, and comprehensive OS (75.258% vs. 89.796%, 41.237% vs. 61.224%, and 41.237% vs. 61.224%, respectively) and PFS (43.299% vs. 63.265%, 18.557% vs. 38.776%, and 16.495% vs. 32.653%, respectively) (Table 2). The survival curves indicated that the RC group significantly outperformed the BCG group in terms of both OS [Hazard Ratio (HR) = 0.60, p = 0.013] and PFS (HR = 0.55, p = 0.025) (Figure 1).
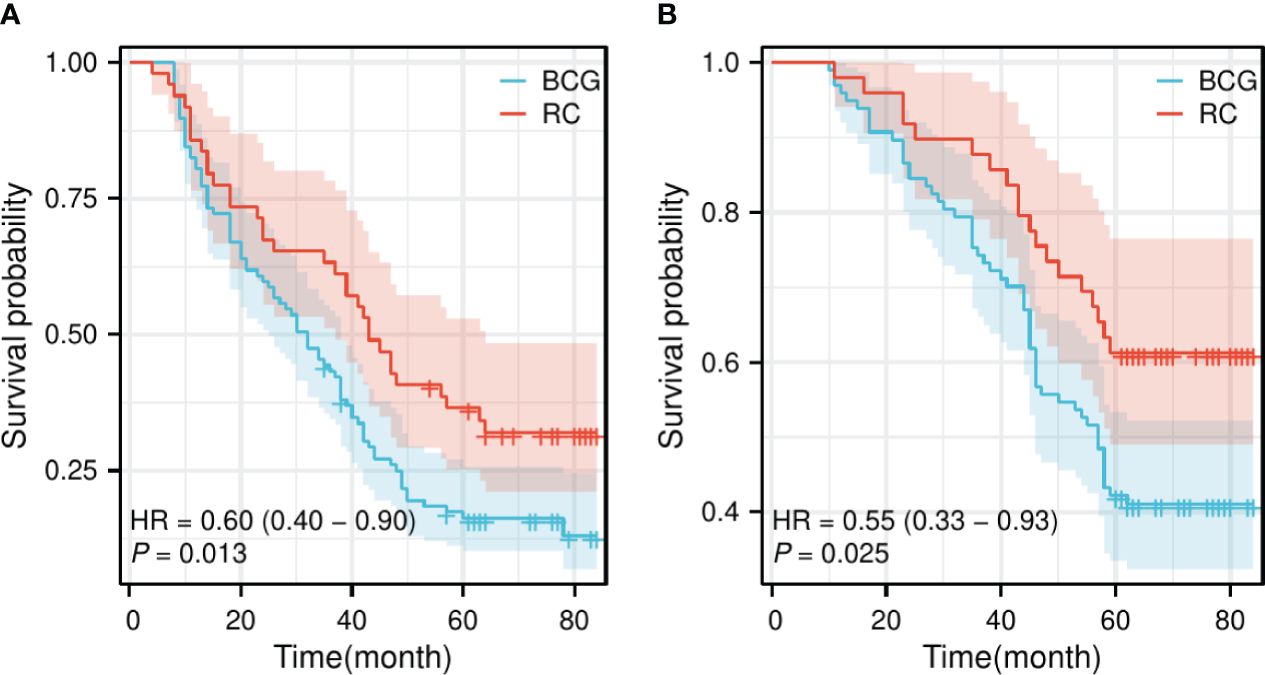
Figure 1 Survival curve of OS and PFS for the BCG and RC groups. (A) The survival curve of OS shows a significant difference between the BCG and RC groups. (B) The survival curve of PFS also demonstrates a significant difference between the BCG and RC groups. OS, overall survival; PFS, progress-free survival; BCG, Bacillus Calmette-Guérin; RC, radical cystectomy; HR, hazard ratio.
Influencing factors of OS and PFS
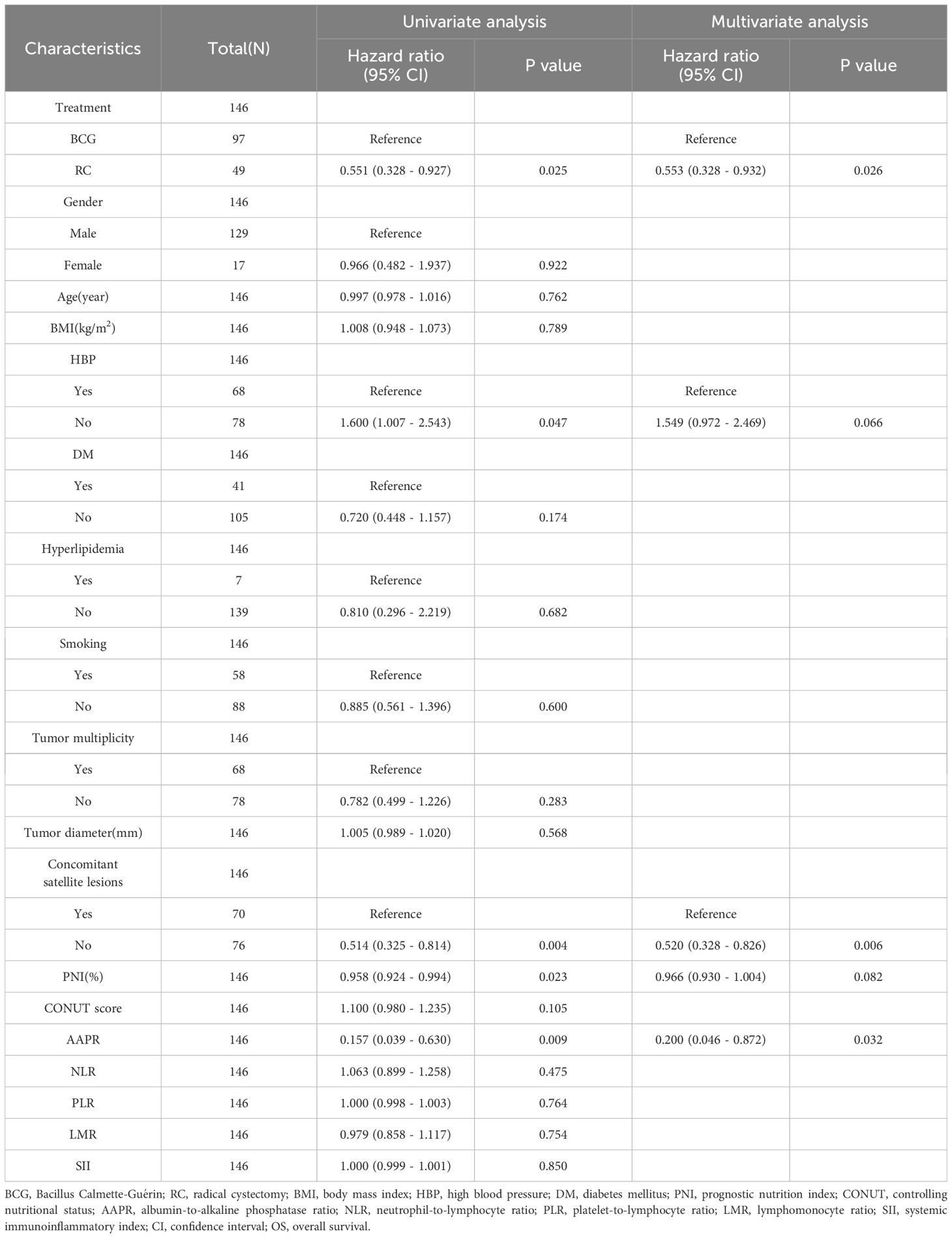
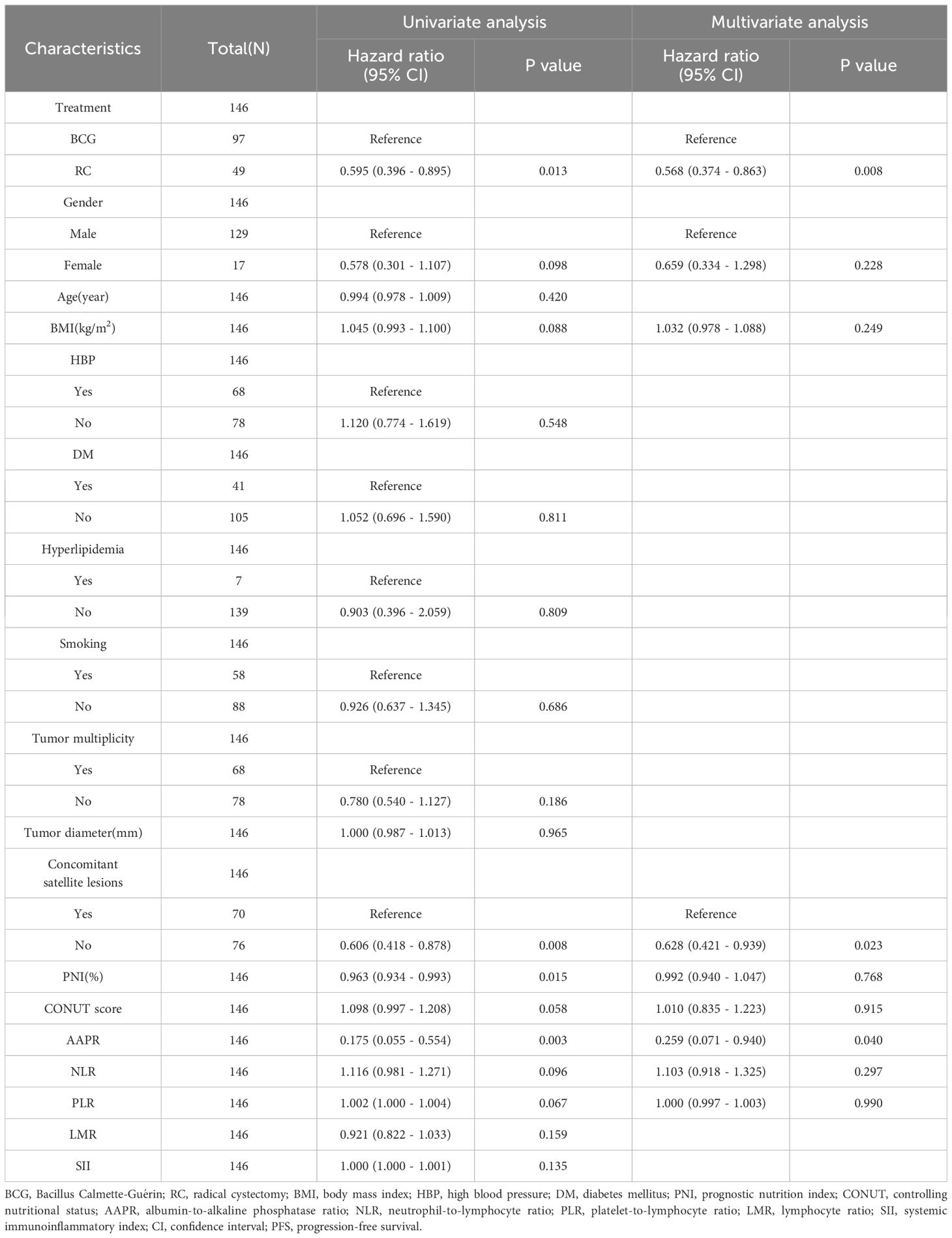
Univariate COX analysis showed that treatment method, HBP, concomitant satellite lesions, PNI, and AAPR were associated with OS, whereas treatment method, concomitant satellite lesions, PNI, and AAPR were associated with PFS. However, multivariate COX analysis demonstrated that the treatment method, concomitant satellite lesions, and AAPR were independent risk factors for OS and PFS (Tables 3, 4).
Establishment of predictive nomograms for OS and PFS
According to the results of multivariate logistic analysis, nomograms predicting OS [C-index: 0.665, 95% confidence interval (CI): 0.629–0.700] and PFS (C-index: 0.593, 95% CI: 0.564–0.622) were obtained (Figures 2A, C). Calibration curves indicated that the nomograms had a good degree of fit (Figures 2B, D).
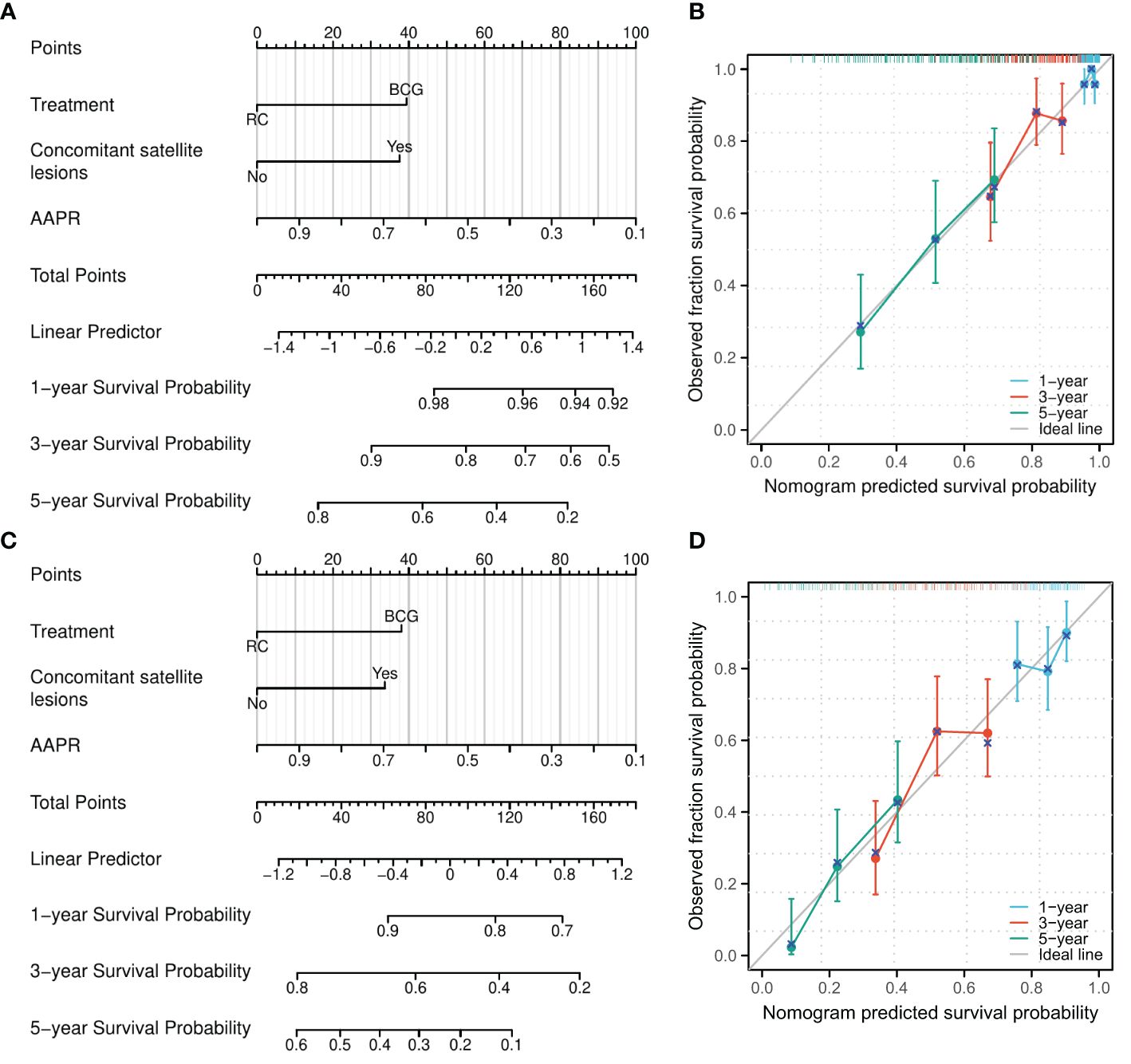
Figure 2 A nomogram model was established to predict the risk of OS and PFS for patients pathologically confirmed as high-grade T1 in initial and repeat transurethral resection of bladder tumor. (A) A nomogram for predicting 1-, 3-, and 5-year OS for patients pathologically confirmed as high-grade T1 in initial and repeat transurethral resection of bladder tumor. (B) Calibration of the nomogram model for OS. (C) A nomogram for predicting 1-, 3-, and 5-year PFS for patients pathologically confirmed as high-grade T1 in initial and repeat transurethral resection of bladder tumor. (D) Calibration of the nomogram model for PFS. AAPR, albumin-to-alkaline phosphatase ratio.
Discussion
Bladder cancer not only has a high prevalence rate but also imposes a higher lifetime treatment cost than all other forms of cancer (1, 4, 15). Therefore, determining the most effective treatment measures for patients with different clinicopathological stages and grades to improve PFS and OS has significant clinical implications. There exists the possibility of inadequate tumor grading and staging, as well as residual tumors after the initial TURBT surgery, which are significant factors leading to a poor prognosis of NMIBC. Although the benefits of re-TURBT for patients remain controversial, it proves beneficial particularly for those patients where the detrusor muscle was not obtained during the initial TURBT surgery (16). For these reasons, authorities such as the EAU and AUA recommend that patients with NMIBC who meet the criteria for secondary TURBT should undergo re-TURBT 2 to 6 weeks after the initial TURBT (4, 17). Nevertheless, the choice of treatment for patients at high risk with NMIBC with a high-grade T1 pathological result, whether BCG instillation or RC treatment, remains a point of contention in contemporary clinical practices (5–7). Our study conducted a long-term follow-up and compared the differences in OS and PFS among patients who underwent BCG instillation or RC treatment. We observed a significant difference in OS and PFS beginning from three years post-treatment, suggesting a long-term superiority of RC over BCG instillation. Hence, our study contributes valuable insights for the strategic selection of treatments for this patient cohort.
It should be noted that the prognosis varies among different histologies of bladder tumors (18). For patients with urothelial carcinomas, clear-cell, plasmacytoid, small-cell, and sarcomatoid variant histologies (VH) have poorer disease-specific survival, while those with lymphoepithelioma-like VH tend to have better outcomes. Suh et al. (19) conducted a study on 45 patients with high-risk NMIBC with squamous or glandular histological variants, of whom 30 underwent BCG instillation and 15 underwent RC. The 5-year OS (p=0.893) and PFS (p=0.811) rates were similar. The findings from the study by Lonati et al. (6) on 188 patients with high-risk NMIBC echoed these results. A large-scale multi-center study from Sweden followed up 3862 patients with high-risk NMIBC who received BCG instillation treatment and 687 who received RC treatment and found that the 5-year PFS in the BCG group was superior to that in the RC group (87% vs. 71%) (5). Hautmann et al. (20), in their study of 1,100 patients with malignant bladder tumors, posited that patients with high-risk NMIBC could benefit from immediate RC surgery, with a 5-year OS exceeding 80%. Our research data indicated that for patients with high-risk NMIBC with high-grade T1 staging, there were no statistically significant differences in OS and PFS within the first 2 years, regardless of whether they received BCG or RC treatment. However, starting in the third year, patients receiving RC demonstrated a significant survival advantage in terms of both OS and PFS. Although BCG instillation therapy demonstrated similar clinical benefits to RC treatment in the initial two years, its effectiveness diminished over time. Therefore, for this group of patients, assuming conditions permit, opting for RC treatment over BCG instillation therapy would yield better clinical outcomes.
The presence of satellite lesions can increase the probability of residual tumors after surgery, thereby increasing the patient’s chance of tumor recurrence (4, 17). This can ultimately delay treatment and negatively affect patient survival. Our study confirmed that the presence of satellite lesions was an independent risk factor for OS and PFS. AAPR, the ratio of albumin to alkaline phosphatase, was first introduced by Anthony W H Chan et al. (21) in 2015 as an independent risk factor for PFS after liver cancer surgery. Researches conducted by Shijie Li et al. (10) and Zhao et al. (22) found that AAPR is an independent risk factor for prognosis after surgery for malignant bladder tumors. This conclusion aligns with our study showing that AAPR is an independent risk factor for OS and PFS in patients with high-risk NMIBC classified as a high-grade T1 stage. A retrospective study involving 516 patients diagnosed with bladder cancer revealed that PNI served as an independent predictor for OS and PFS (8). This discovery highlights the potential for PNI to be a reliable, novel biomarker for bladder cancer. Concurrently, another retrospective study focused on delineating the relationship between the CONUT score and bladder cancer (9). The study found that a higher CONUT score predicted worse recurrence-free survival, OS, and cancer-specific survival. Although our study identified a correlation between PNI and the prognosis of high-risk, high-grade T1 NMIBC patients, PNI was not found to be an independent risk factor for postoperative OS or PFS in this patient population. Neutrophils are important inflammatory cells in humans. Previous studies have found that under the influence of the tumor microenvironment, they promote tumor cell proliferation and angiogenesis, ultimately accelerating tumor development (11). Lymphocytes are considered the primary executors of immune functions. However, owing to the high metabolic nutritional consumption of tumor tissues within the body, patients may experience a relative reduction in lymphocytes owing to progressively declining immune function. The primary role of platelets is to promote coagulation, but they also contain a large number of angiogenesis regulatory proteins that can be utilized by tumor cells and tumor matrix cells to promote the formation of new blood vessels, thereby accelerating tumor cell proliferation and invasion (11). The inflammatory indices derived from these indicators were related to the prognosis of bladder tumors (11, 23). However, we did not find these indices to be independent risk factors for the prognosis of high-risk high-grade T1 NMIBC.
Our study has certain limitations. Initially, this was a single-center study with a relatively small sample size; hence, the conclusions drawn require further validation through subsequent multi-center studies involving larger sample sizes. Second, our study exclusively targeted patients with high-risk NMIBC classified as a high-grade T1 stage. We aimed to extend our research to explore the OS and PFS outcomes in all types of patients with high-risk NMIBC. Thirdly, our analysis fell short as it omitted T1 substaging, an evolving and pivotal prognostic classification. This oversight could potentially impact the scope of our study and depth of insight into the disease prognosis. Going forward, we are intent on exploring the prognostic disparities between T1 substaging patients administered BCG instillation therapy versus those subjected to RC (24). Finally, the duration of follow-up in our study may be considered too short; however, we plan to continue these follow-ups in the future.
In summary, our study found that for patients with high-risk NMIBC classified as high-grade T1 stage, RC could confer survival benefits compared with BCG instillation if satellite lesions, and low AAPR are concurrently present.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: All original raw data of this study can be accessed from https://figshare.com/s/9c42afea3f51c6f1159e.
Ethics statement
The studies involving humans were approved by the ethics committee of the First Affiliated Hospital of Soochow University. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
SZ: Writing – original draft, Visualization, Software, Resources, Investigation, Formal analysis, Data curation. CH: Writing – original draft, Software, Resources, Formal analysis, Data curation. YY: Writing – original draft, Visualization, Validation, Investigation, Formal Analysis, Data curation. LY: Writing – review & editing, Supervision, Project administration, Methodology, Funding acquisition. OJ: Writing – review & editing, Supervision, Project administration, Methodology. ZZ: Writing – review & editing, Supervision, Software, Project administration, Methodology, Conceptualization.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the Science and Technology Program of Suzhou (grant number SLJ201906).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Shkolyar E, Jia X, Chang TC, Trivedi D, Mach KE, Meng MQ, et al. Augmented bladder tumor detection using deep learning. Eur Urol. (2019) 76(6):714–8. doi: 10.1016/j.eururo.2019.08.032
2. Divrik RT, Sahin AF, Yildirim U, Altok M, Zorlu F. Impact of routine second transurethral resection on the long-term outcome of patients with newly diagnosed pT1 urothelial carcinoma with respect to recurrence, progression rate, and disease-specific survival: a prospective randomised clinical trial. Eur Urol. (2010) 58(2):185–90. doi: 10.1016/j.eururo.2010.03.007
3. Hashine K, Ide T, Nakashima T, Hosokawa T, Ninomiya I, Teramoto N. Results of second transurethral resection for high-grade T1 bladder cancer. Urol Ann. (2016) 8(1):10–5. doi: 10.4103/0974–7796.163798
4. Babjuk M, Burger M, Capoun O, Cohen D, Compérat EM, Dominguez Escrig JL, et al. European association of urology guidelines on non-muscle-invasive bladder cancer (Ta, T1, and carcinoma in situ). Eur Urol. (2022) 81(1):75–94. doi: 10.1016/j.eururo.2021.08.010
5. Wang EY, Larsson U, Gårdmark T, Malmström PU. Radical cystectomy compared to intravesical BCG immunotherapy for high-risk non-muscle invasive bladder cancer - is there a long-term survival difference? A Swedish nationwide analysis. Scand J Urol. (2021) 55(1):46–52. doi: 10.1080/21681805.2020.1851763
6. Lonati C, Afferi L, Mari A, Minervini A, Krajewski W, Borghesi M, et al. Immediate radical cystectomy versus BCG immunotherapy for T1 high-grade non-muscle-invasive squamous bladder cancer: an international multi-centre collaboration. World J Urol. (2022) 40(5):1167–74. doi: 10.1007/s00345–022-03958–9
7. Catto JWF, Gordon K, Collinson M, Poad H, Twiddy M, Johnson M, et al. Radical cystectomy against intravesical BCG for high-risk high-grade nonmuscle invasive bladder cancer: results from the randomized controlled BRAVO-feasibility study. J Clin Oncol. (2021) 39(3):202–14. doi: 10.1200/JCO.20.01665
8. Peng D, Gong YQ, Hao H, He ZS, Li XS, Zhang CJ, et al. Preoperative Prognostic Nutritional Index is a Significant Predictor of Survival with Bladder Cancer after Radical Cystectomy: a retrospective study. BMC Cancer. (2017) 17(1):391. doi: 10.1186/s12885-017-3372-8
9. Claps F, Mir MC, van Rhijn BWG, Mazzon G, Soria F, D'Andrea D, et al. Impact of the controlling nutritional status (CONUT) score on perioperative morbidity and oncological outcomes in patients with bladder cancer treated with radical cystectomy. Urol Oncol. (2023) 41(1):49.e13–22. doi: 10.1016/j.urolonc.2022.09.023
10. Li S, Lu S, Liu X, Chen X. Association between the pretreatment albumin-to-alkaline phosphatase ratio and clinical outcomes in patients with bladder cancer treated with radical cystectomy: A retrospective cohort study. Front Oncol. (2021) 11:664392. doi: 10.3389/fonc.2021.664392
11. Chen H, Wu X, Wen Z, Zhu Y, Liao L, Yang J. The clinicopathological and prognostic value of NLR, PLR and MLR in non-muscular invasive bladder cancer. Arch Esp Urol. (2022) 75(5):467–71. doi: 10.56434/j.arch.esp.urol.20227505.68
12. Li J, Cao D, Huang Y, Xiong Q, Tan D, Liu L, et al. The prognostic and clinicopathological significance of systemic immune-inflammation index in bladder cancer. Front Immunol. (2022) 13:865643. doi: 10.3389/fimmu.2022.865643
13. Zhiyu Z, Qi Z, Zhen S, Jun O, Jianglei Z. The effect of tri-modality therapy with bladder preservation for selective muscle-invasive bladder cancer. Technol Cancer Res Treat. (2021) 20:15330338211062323. doi: 10.1177/15330338211062323
14. Schmidt SAJ, Lo S, Hollestein LM. Research techniques made simple: sample size estimation and power calculation. J Invest Dermatol. (2018) 138:1678–82. doi: 10.1016/j.jid.2018.06.165
15. Hurst CD, Alder O, Platt FM, Droop A, Stead LF, Burns JE, et al. Genomic subtypes of non-invasive bladder cancer with distinct metabolic profile and female gender bias in KDM6A mutation frequency. Cancer Cell. (2017) 32(5):701–715.e7. doi: 10.1016/j.ccell.2017.08.005
16. Gontero P, Sylvester R, Pisano F, Joniau S, Oderda M, Serretta V, et al. The impact of re-transurethral resection on clinical outcomes in a large multicentre cohort of patients with T1 high-grade/Grade 3 bladder cancer treated with bacille Calmette-Guérin. BJU Int. (2016) 118(1):44–52. doi: 10.1111/bju.13354
17. Jubber I, Ong S, Bukavina L, Black PC, Compérat E, Kamat AM, et al. Epidemiology of bladder cancer in 2023: A systematic review of risk factors. Eur Urol. (2023) 84(2):176–90. doi: 10.1016/j.eururo.2023.03.029
18. Claps F, van de Kamp MW, Mayr R, Bostrom PJ, Shariat SF, Hippe K, et al. Prognostic impact of variant histologies in urothelial bladder cancer treated with radical cystectomy. BJU Int. (2023) 132(2):170–80. doi: 10.1111/bju.15984
19. Suh J, Moon KC, Jung JH, Lee J, Song WH, Kang YJ, et al. BCG instillation versus radical cystectomy for high-risk NMIBC with squamous/glandular histologic variants. Sci Rep. (2019) 9(1):15268. doi: 10.1038/s41598–019-51889–0
20. Hautmann RE, de Petriconi RC, Pfeiffer C, Volkmer BG. Radical cystectomy for urothelial carcinoma of the bladder without neoadjuvant or adjuvant therapy: long-term results in 1100 patients. Eur Urol. (2012) 61(5):1039–47. doi: 10.1016/j.eururo.2012.02.028
21. Chan AW, Chan SL, Mo FK, Wong GL, Wong VW, Cheung YS, et al. Albumin-to-alkaline phosphatase ratio: a novel prognostic index for hepatocellular carcinoma. Dis Markers. (2015) 2015:564057. doi: 10.1155/2015/564057
22. Zhao M, Zhang M, Wang Y, Yang X, Teng X, Chu G, et al. Prognostic value of preoperative albumin-to-alkaline phosphatase ratio in patients with muscle-invasive bladder cancer after radical cystectomy. Onco Targets Ther. (2020) 13:13265–74. doi: 10.2147/OTT.S285098
23. Li DX, Wang XM, Feng DC, Han P. Neutrophil-to-lymphocyte ratio (NLR) during induction is a better predictor than preoperative NLR in non-muscle-invasive bladder cancer receiving Bacillus Calmette-GuÉRin. Asian J Surg. (2023) 46(3):1348–51. doi: 10.1016/j.asjsur.2022.08.108
Keywords: high-risk non-muscle-invasive urothelial cancer, Bacillus Calmette-Guérin instillation, radical cystectomy, overall survival, progression-free survival
Citation: Zhen S, Hao C, Yanhang Y, Yuxin L, Jun O and Zhiyu Z (2024) Comparative efficacy of Bacillus Calmette–Guérin instillation and radical cystectomy treatments for high-risk non-muscle-invasive urothelial cancer classified as high-grade T1 in initial and repeat transurethral resection of bladder tumor. Front. Oncol. 14:1394451. doi: 10.3389/fonc.2024.1394451
Received: 01 March 2024; Accepted: 31 May 2024;
Published: 18 June 2024.
Edited by:
Alexandre Zlotta, University of Toronto, CanadaReviewed by:
Francesco Claps, The Netherlands Cancer Institute (NKI), NetherlandsRodolfo Hurle, Humanitas Research Hospital, Italy
Copyright © 2024 Zhen, Hao, Yanhang, Yuxin, Jun and Zhiyu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhang Zhiyu, YWJuZXJfNjY2QDEyNi5jb20=
†These authors have contributed equally to this work
 Song Zhen1,2†
Song Zhen1,2†