
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
BRIEF RESEARCH REPORT article
Front. Oncol., 08 May 2024
Sec. Hematologic Malignancies
Volume 14 - 2024 | https://doi.org/10.3389/fonc.2024.1393191
This article is part of the Research TopicCurrent Challenges in Hematology: The Biological and Therapeutic Advances in Chronic Myeloid LeukemiaView all 6 articles
 Bruna Murbach1
Bruna Murbach1 Gislaine Duarte1
Gislaine Duarte1 Leonardo Carvalho Palma2
Leonardo Carvalho Palma2 Eliana Miranda1
Eliana Miranda1 Guilherme Duffles1
Guilherme Duffles1 Graziele Pavan Furlin1
Graziele Pavan Furlin1 Isabella Toni1
Isabella Toni1 Carmino De Souza1
Carmino De Souza1 Larissa Binelli2
Larissa Binelli2 Vitor Leonardo Bassan3
Vitor Leonardo Bassan3 Fabiola Attie de Castro3
Fabiola Attie de Castro3 Lorena Lobo de Figueiredo-Pontes2
Lorena Lobo de Figueiredo-Pontes2 Katia Borgia Barbosa Pagnano1*
Katia Borgia Barbosa Pagnano1*Tyrosine kinase inhibitors (TKI) have revolutionized the treatment of patients with chronic myeloid leukemia. Patients who achieve sustained deep molecular response are eligible for treatment discontinuation. DES-CML is an ongoing, phase 2 multicentric discontinuation trial. Adult patients with CML in chronic phase with typical BCR::ABL1 transcripts, stable deep molecular response (MR4.5 IS) for two years, and no previous resistance were eligible. Patients underwent a phase of TKI dose de-escalation for six months before discontinuation. TKI was reintroduced at the previous dose if the patient lost major molecular response (MMR) at any time. This study aimed to assess the impact of BCR-ABL transcript kinetics during TKI de-escalation and discontinuation phases on treatment-free survival. So far, the study recruited 41 patients, and 38 patients discontinued therapy (4 were in the second discontinuation attempt). Eleven patients lost MMR, one during the de-escalation phase and ten after discontinuation. 24-month treatment-free survival was 66% (95% CI: 48-84%) in a median follow-up of 7 (1–30) months. No patient lost hematological response or had disease progression. A higher rate of molecular relapses occurred in patients with fluctuating BCR::ABL1 levels after the discontinuation phase (with loss of MR4.5, but no loss of MMR) (P=0.04, HR-4.86 (1.03-22.9) but not confirmed in the multivariate analysis. The longer duration of TKI treatment (P=0.03, HR-1.02, 95%CI - 1.00-1.04) and MMR (P=0.004, HR-0.95, 95%CI - 0.92-098) were independent factors of a lower relapse rate.
Chronic myeloid leukemia (CML) is a myeloproliferative neoplasm characterized by the reciprocal chromosomal translocation t(9;22), which results in the formation of the Philadelphia (Ph) chromosome containing the BCR::ABL1 gene. This gene encodes a protein with tyrosine kinase activity, a therapeutic target of tyrosine kinase inhibitors (TKI) (1, 2). CML is a disease with an incidence of 1–2 cases per 100,000 adults, accounting for approximately 15% of diagnosed cases of leukemia (3).
The development of TKI changed the natural history of the disease, drastically reducing the rates of transformation to the blastic phase, increasing patient survival from a historical rate of 10-20% to above 80%, resulting in a life expectancy close to that of the normal population, as long as they receive appropriate TKI treatment and maintain treatment adherence (4–7). Several studies confirm that discontinuation of TKI is feasible and safe in patients who achieve a sustained deep molecular response (8, 9). Discontinuation of TKI therapy can be successful in approximately 20% of the total number of CML patients who start therapy (10).
The duration of previous treatment, the depth of molecular response, previous resistance, type of BCR::ABL1 transcripts, halving time, and lymphocyte subtypes are known predictive factors of molecular recurrence after tyrosine kinase inhibitors (TKI) discontinuation (11–20). Few studies investigated the kinetics of BCR::ABL1 transcripts during a dose de-escalation phase before discontinuation in predicting molecular relapse (21, 22).
In the present study, we aimed to evaluate the kinetics of BCR::ABL1 transcripts during the de-escalation and discontinuation phases of the DES-CML TKI discontinuation trial and the impact on treatment-free survival (TFS).
The DES-CML study is a multicenter, prospective, open-label, single-arm, phase 2, non-randomized, ongoing trial that enrolled patients with chronic myeloid leukemia treated in the Unified Health System from two Brazilian centers (Study of treatment discontinuation of chronic myeloid leukemia in the Unified Health System - DES-CML Study). This study is registered at the Brazilian Registry of Clinical Trials (ReBEC) platform (RR-6f5xbq; UTN code: U1111-1252-7312), and the ethics committees of the participating centers (Universidade Estadual de Campinas and USP-Ribeirão Preto) approved the protocol. All patients signed informed consent. Inclusion criteria used were adult CML chronic phase patients (18 years or older) receiving first or second-line treatment (if toxicity with the first-line therapy) with TKI for a minimum of 3 years that achieved sustained deep molecular response (DMR), defined by a minimum duration of MR4.5 for two years, confirmed by four real-time quantitative reverse-transcription polymerase chain reaction (qPCR) tests within six months interval. Exclusion criteria were patients with atypical BCR::ABL1 transcripts, previous or current advanced phase CML, previous resistance to TKI or ABL mutations, and patients with prior allogeneic bone marrow transplantation. The TKI dose was reduced by 50% for six months before discontinuation (de-escalation phase). Molecular monitoring was performed by qPCR, using ABL as the control gene, and the results were reported using the International Scale (23–26). BCR::ABL1 transcript levels were evaluated monthly during the de-escalation phase. After discontinuation, assessments were performed monthly for six months, every two months at months 7-24, and then every three months. Molecular recurrence definition was the MMR loss (qPCR>0.1%) (11) and was a trigger for treatment reintroduction. TKI was reintroduced at the same dose before dose de-escalation.
The statistical analysis was performed using the IBM-SPSS software. Study data were collected and managed using REDCap (Research Electronic Data Capture) tools hosted at University of Campinas (27). Treatment-free survival (TFS) was calculated from the date of discontinuation until the loss of MMR or TKI reintroduction. The rates of TFS were determined using the Kaplan-Meier method and Log-Rank test for comparison. Gray’s test was also applied to calculate Cumulative Incidence. Death in MMR was considered a competitive event. in significant molecular remission was the only occurring event. The Backward method of Cox regression was used to identify the prognostic factors. In the univariate analysis, a P-value of 0.10 was used to choose the variables for multivariate analysis. P values <.05 were considered statistically significant. The cut-off date for this analysis was November 29, 2023.
Between September 2020 and November 2023, 41 CML patients were recruited for the study (Figure 1). Four patients were in the second discontinuation attempt, but all were treated with imatinib for at least four years before the second attempt. The patients’ median age at study entry was 62 years (24-79); the majority were male (61%); 35% Sokal low risk, 49% intermediate, 16% high; 79% ELTS low risk, 18% intermediate, 3% high; 82% had b3a2 BCR::ABL1 transcripts; 86% were using imatinib, 7% nilotinib, 5% dasatinib and 2% bosutinib. The median duration of TKI treatment until the date of discontinuation was 12 years (4-20). CML patient’s characteristics are described in Table 1. The median follow-up was 20 months (3-39 months) from the dose de-escalation and seven months (1-30) from the date of discontinuation. Two patients were in the discontinuation phase at the cut-off date (November 2023).
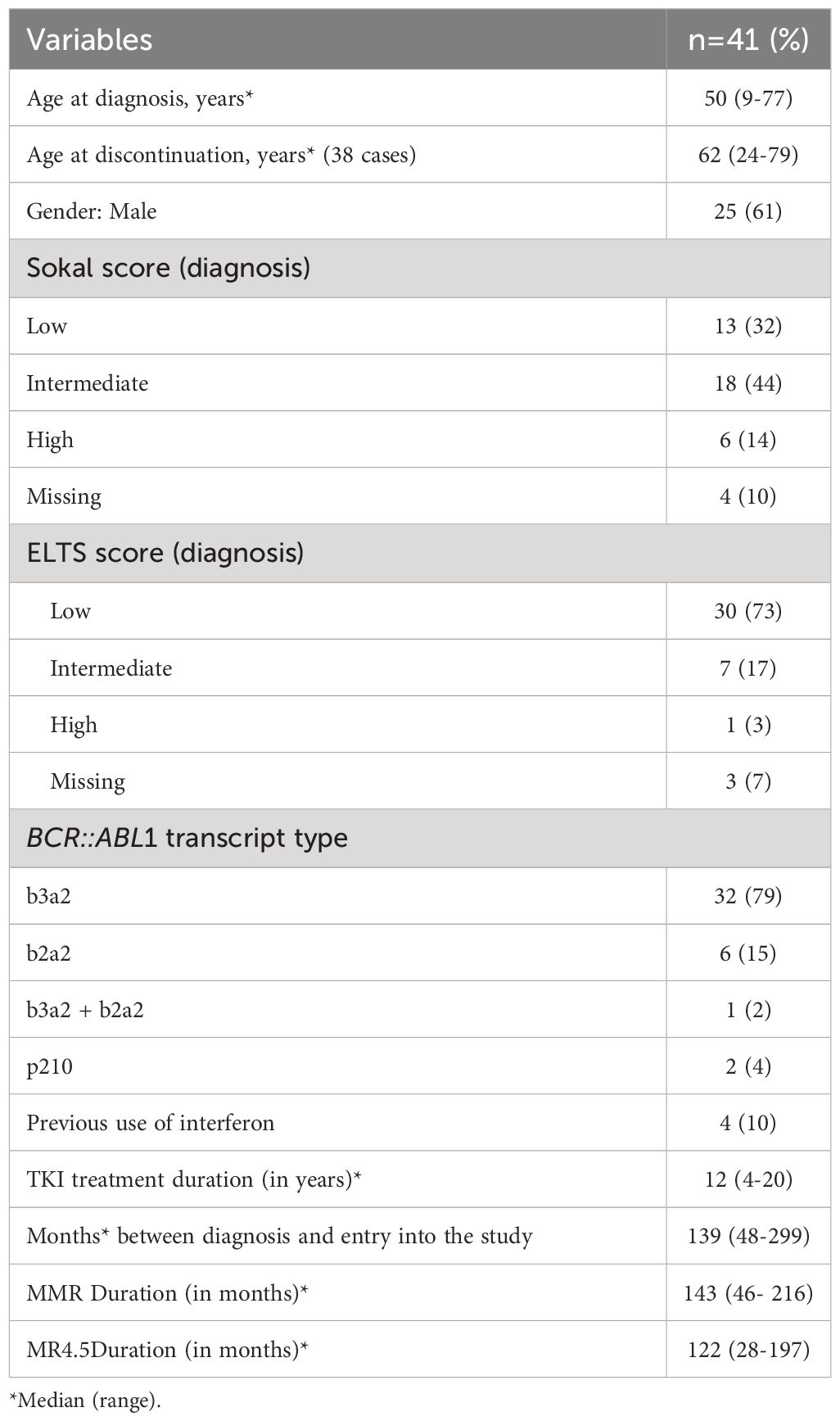
Table 1 Clinical and laboratory characteristics of CML patients with chronic myeloid leukemia at diagnosis and study entry (n=41).
Forty-one patients de-escalated therapy to half of the previous dose (Supplementary Table 1). Two patients did not complete the six months of de-escalation due to lack of medication and went earlier to the discontinuation phase. One patient lost MMR in the de-escalation phase and returned to the previous dose. Two patients were still in the de-escalation phase at the moment of the present analysis. Among the 38 patients that interrupted TKI, 13 (34%) had lost MR4.5 in the de-escalation phase, and 5/13 (38.4%) lost MMR after discontinuation. Among the 25 patients who did not lose MR4.5 during the de-escalation phase, there were five relapses after discontinuation (20.0%).
Ten out of 38 patients lost MMR (26%) after discontinuation. The median time between discontinuation and MMR loss was four months (1-11). The median time for recovering MMR was two months (1-5). All patients recovered their response, and only one did not recover MR4.5.
The molecular response status before discontinuation was 30 (79%) RM4.5, 7(18%) RM4.0, and 1 (3%) MMR. There were no relapses after 12 months of treatment interruption. BCR::ABL1 levels from the first day of TKI dose de-escalation and TKI discontinuation until 12 months are demonstrated in Figure 2.
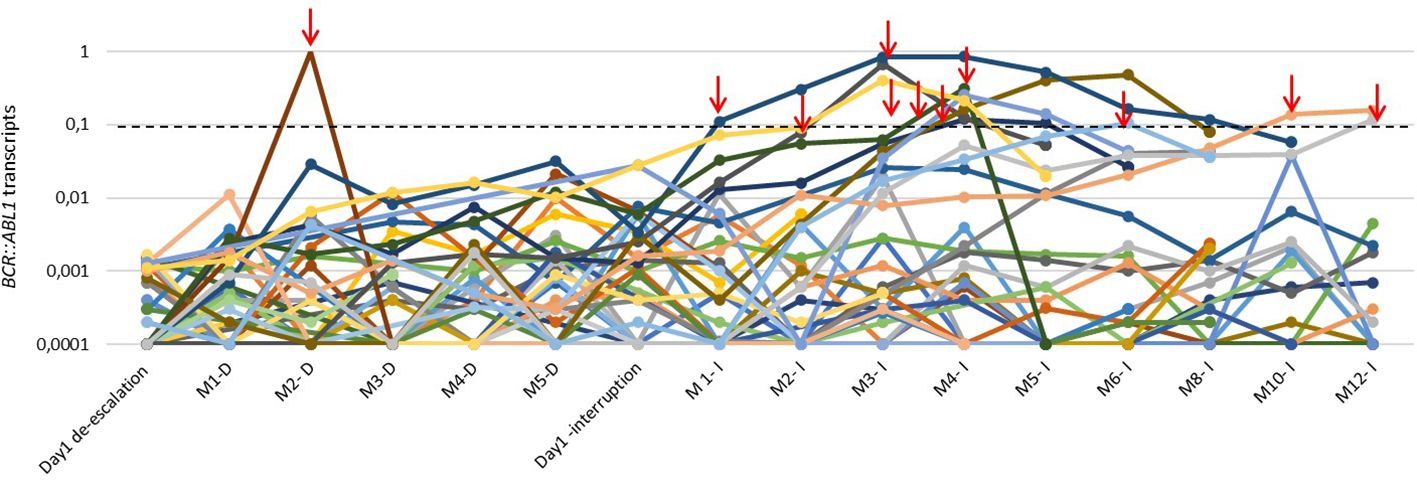
Figure 2 BCR::ABL1 transcript levels of 41 patients with CML during the de-escalation phase and along the first 12 months of TKI interruption. The red arrows highlight the loss of MMR and treatment reintroduction. M: month; D:de-escalation; I:interruption.
Treatment-free survival (TFS) was 66% (95% CI: 48-84%, Figure 3A). TFS was 71% in the first-attempt discontinuation group. Three of four cases of second discontinuation attempts relapsed (P<0.0001, Figure 3B). There was no difference in TFS, according to the type of BCR::ABL1 transcripts (Supplementary Figure 1), TKI (Supplementary Figure 2), and Sokal (Figure 3C). There was a trend for a higher TFS in patients with MR4.5 before TKI interruption than in patients with MR4.0 and MMR, but it was not statistically significant (68% versus 40%, P=0.17) (Figure 3D). No patient lost hematological response or had disease progression during the study. One patient died from sepsis after a urinary infection (unrelated to CML) during the discontinuation phase.
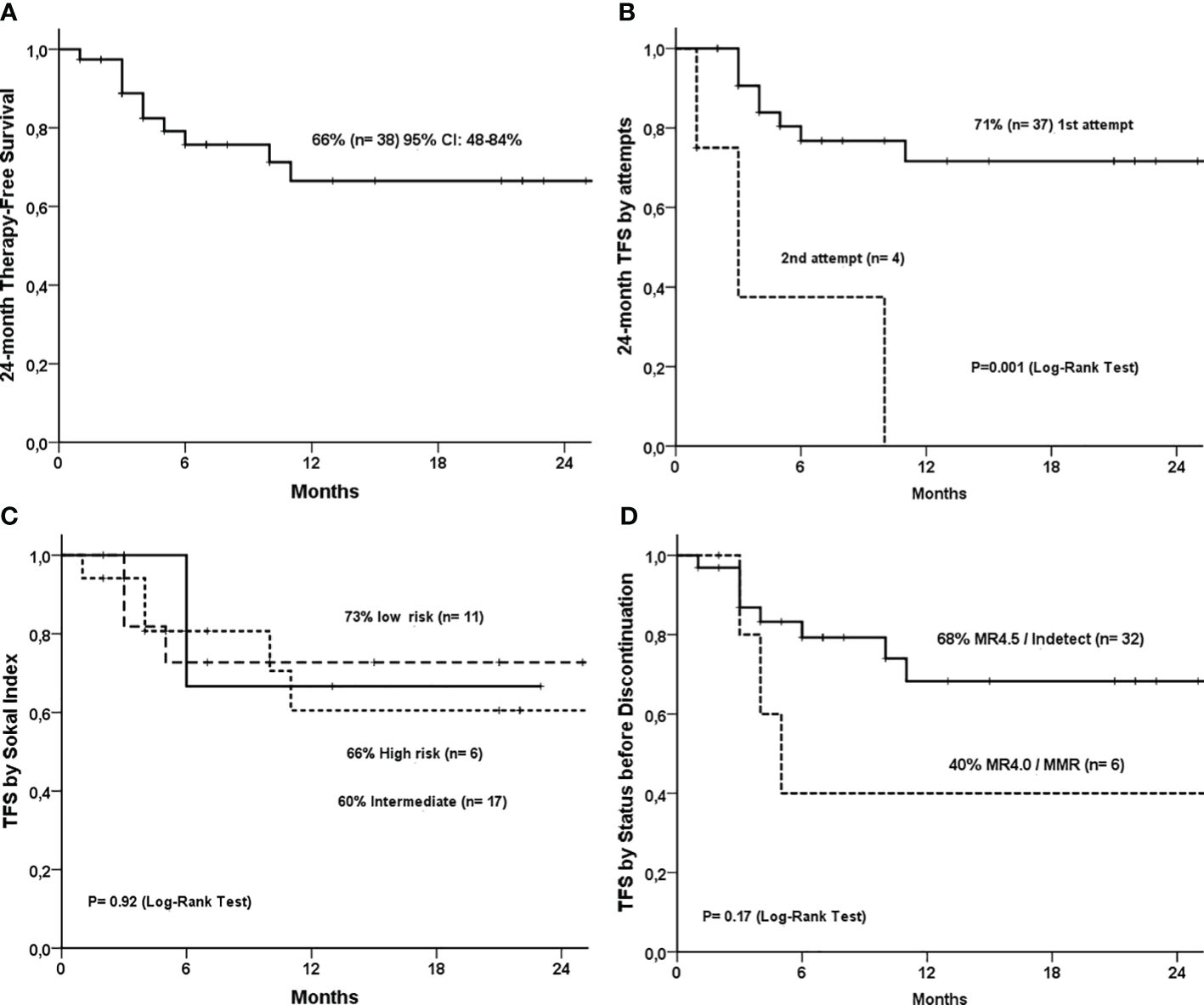
Figure 3 Treatment-free survival curves of 38 CML patients that discontinued TKI. (A) 24-month treatment-free survival (TFS); (B) 24-month TFS by attempts (first vs second attempt); (C) TFS by Sokal Index; (D) TFS by molecular response status before discontinuation (MR4.5 vs MR4.0).
The cumulative incidence of MMR loss was 34% (95% CI: 16-52%) at 30 months (Figure 4). In the multivariate analysis, the independent factors for a longer TFR were the duration of the TKI treatment and the longer duration of MMR before discontinuation (Table 2). Among the patients who discontinued therapy, 12 (32%) presented symptoms of withdrawal syndrome in the first four months after discontinuation. Most of the events were of mild intensity (CTCAE Grades 1 and 2), and 8% were grade 3, but they did not require hospitalization or reintroduction of the medication.
The preliminary results of this discontinuation trial, which includes a de-escalation phase, confirmed that a more prolonged TKI treatment and a longer duration of MMR were independent factors for a longer TFR. Fluctuations of MR4.5 during the discontinuation phase were predictive of MMR loss in the univariate analysis, but not confirmed in the multivariate analysis, probably due to the small sample size and short follow-up. The present study is still recruiting patients, which will be accessed in the future.
Treatment-free remission is currently one of the central goals of CML treatment. Several discontinuation trials show it is successful in 50-60% of patients. These studies demonstrated that discontinuation is safe and effective for patients with a deep molecular response, improving patients’ quality of life and reducing treatment costs (11, 12, 16, 28, 29).
Few discontinuation studies included a de-escalation phase before TKI interruption. In most trials, TKI is interrupted abruptly. In our study, TKI was reduced by half of the current dose for six months before discontinuation. Only one case (2.4%) lost MMR during this phase. However, 5/13 patients who lost MR4.5 during de-escalation lost MMR after interruption.
Moreover, the fluctuation of qPCR with loss of MR4.5 in the discontinuation phase was related to a trend of higher rate of molecular relapse in the univariate analysis. In the DESTINY trial, 174 CML patients in stable MMR for the last 12 months went to a phase of 12 months of dose de-escalation to half of the standard TKI dose for 12 months before discontinuation. Seventy-two percent (72%) of the patients who in stable MR4 after the de-escalation phase did not relapse two years after stopping treatment (21). A recent trial randomized 125 CML patients with MR4.5 for over five years in two groups: one for discontinuation and the other for dose de-escalation before TKI discontinuation for 12 months. The molecular recurrence-free survival was lower in the discontinued group compared to the dose de-escalation group (59.9 versus 88.3) p= 0.0002. In the 12 months of dose de-escalation, 8% of patients lost MMR (22).
Regarding other prognostic factors, the present study did not find higher relapse rates in patients with intermediate and high Sokal scores. This finding is in contrast to other studies, such as STIM1, in which the Sokal score was a prognostic factor, leading to a higher relapse rate after discontinuation (13).
Therapy-free survival observed in our study was 66%, similar to other TKI discontinuation trials. TFS was higher in the first attempt group, while most of the patients of the second attempt failed (3 patients). Those patients were retreated with imatinib after the first TFR attempt. The French group was the first to demonstrate the feasibility of a second TKI discontinuation attempt, with a TFR rate of 36% at 24 months (30). In the TRAD study, 59 patients who failed in the first TFR attempt were treated with dasatinib, and 35 achieved MR4.5 or more profound molecular responses and were able to try a second attempt, but 74% lost MMR (31). In the DAstop2 trial, for patients eligible for a second discontinuation attempt, the TFR rate after stopping dasatinib was 46% at 24 months (32).
Eight out of the ten patients lost MMR after discontinuation (80%) within the first six months, and none occurred after 12 months. As observed in most discontinuation studies, relapse tends to occur within the first six months. Twelve (30%) patients have reached the second year of discontinuation and are maintaining MMR. MMR loss is rare after the first year of TFR. In the long-term follow-up of the STIM trial, there were late relapses after two years in 14% of the patients. There was a correlation between fluctuating levels of deep molecular response and late relapse (33). However, due to the short follow-up period in our study, we were unable to analyze this potential correlation.
In the univariate analysis, gender, Sokal score, type of BCR::ABL1 transcript, and withdrawal syndrome did not impact the TFR rate. The treatment’s longer duration and MMR were independent factors of a lower molecular relapse rate, similar to the findings of the EURO-SKI discontinuation trial (11).
Withdrawal syndrome, which is characterized by musculoskeletal pain after TKI interruption, is expected and occurs in around 30% of patients (34). In our study, we had a similar percentage of patients with withdrawal syndrome. There was no relationship between this adverse event and MMR loss, and it was not necessary to reintroduce TKI in these cases.
In summary, the preliminary results of this study reinforce the importance of the duration of TKI treatment and the longer duration of MMR as prognostic factors for TFR. The fluctuations of molecular responses during the de-escalation and discontinuation phases may require further investigation, particularly with a longer follow-up period, to assess their impact on treatment-free survival more comprehensively.
The raw data supporting the findings of this study will be made available by the authors, upon request, and subject to review.
The studies involving humans were approved by Comitê de Ética em Pesquisa da Universidade Estadual de Campinas, Campinas-SP, Brazil and Comitê de Ética em Pesquisa em Seres Humanos do Hospital das Clínicas de Ribeirão Preto e da Faculdade de Medicina de Ribeirão Preto (CEP HCRP e FMRP), Ribeirão Preto-SP, Brazil. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
BM: Data curation, Formal analysis, Investigation, Writing – original draft, Writing – review & editing. GiD: Investigation, Writing – review & editing. LP: Investigation, Writing – review & editing. EM: Investigation, Writing – review & editing, Data curation, Formal analysis. GuD: Investigation, Writing – review & editing. GF: Investigation, Writing – review & editing. IT: Writing – review & editing, Investigation. CD: Writing – review & editing, Investigation. LB: Writing – review & editing, Investigation. VB: Writing – review & editing, Investigation. FC: Writing – review & editing, Investigation. LF: Investigation, Writing – review & editing, Funding acquisition, Resources, Supervision. KP: Investigation, Writing – review & editing, Conceptualization, Data curation, Formal analysis, Funding acquisition, Methodology, Project administration, Resources, Supervision, Writing – original draft.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. The Brazilian National Council for Scientific and Technological Development (CNPq) supported this project (grant 406361/2021-5). VLB is a recipient of São Paulo Research Foundation (FAPESP) grant number 2022/13366-2.
The authors would like to express their gratitude to all patients and staff from Universidade Estadual de Campinas, USP Ribeirão Preto, CML Brazilian Working Group (GB-LMC), especially to Nicete Romano for regulatory support, and EMS for support regarding sample exchange.
The authors declare the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2024.1393191/full#supplementary-material
Supplementary Figure 1 | Treatment-free survival according to TKI (imatinib vs others) (n=38).
Supplementary Figure 2 | Treatment-free survival according to type of BCR::ABL1 transcript (b3a2 vs b2a2) (n=35).
1. Jabbour E, Kantarjian H. Chronic myeloid leukemia: 2018 update on diagnosis, therapy and monitoring. Am J Hematol. (2018). 93(3):442–59.
2. Chereda B, Melo JV. Natural course and biology of CML. In: Annals of hematology, vol. 94. New York, United States: Springer Verlag (2015). p. 107–21.
3. Hehlmann R, Hochhaus A, Baccarani M. Chronic myeloid leukaemia. Lancet. (2007) 370 (9584):342–50. doi: 10.1016/S0140-6736(07)61165-9
4. Deininger MWN. Basic science going clinical: Molecularly targeted therapy of chronic myelogenous leukemia. J Cancer Res Clin Oncol. (2004) 130:59–72. doi: 10.1007/s00432-003-0502-2
5. Kantarjian H, O’Brien S. The chronic leukemias. Chapter 190. In: Goldman L, Schafer A, Arend W, Armitage J, Clemmons D, Drazen J, Griggs R, Landry D, Levinson W, Rustgi A, Scheld W, editors. Cecil medicine, 24th Edition. vol. 2012. Elsevier Saunders, Philadelphia, United States. (2011) p. 1209–18.
6. Gambacorti-Passerini C, Antolini L, Mahon FX, Guilhot F, Deininger M, Fava C, et al. Multicenter independent assessment of outcomes in chronic myeloid leukemia patients treated with imatinib. J Natl Cancer Inst. (2011) 103:553–61. doi: 10.1093/jnci/djr060
7. SEER (Surveillance, Epidemiology, and End Results Program). Cancer stat facts: leukemia - chronic myeloid leukemia (CML). National Cancer Institute. Available at: https://seer.cancer.gov/statfacts/html/cmyl.html.
8. Mahon FX, Pfirrmann M, Dulucq S, Hochhaus A, Panayiotidis P, Almeida A, et al. European stop tyrosine kinase inhibitor trial (EURO-SKI) in chronic myeloid leukemia: final analysis and novel prognostic factors for treatment-free remission. J Clin Oncol. (2024), JCO2301647. doi: 10.1200/JCO.23.01647
9. Mahon FX, Boquimpani C, Kim DW, Benyamini N, Clementino NCD, Shuvaev V, et al. Treatment-free remission after second-line nilotinib treatment in patients with chronic myeloid leukemia in chronic phase. Ann Intern Med. (2018) 168:461. doi: 10.7326/M17-1094
10. Hochhaus A, Baccarani M, Silver RT, Schiffer C, Apperley JF, Cervantes F, et al. European LeukemiaNet 2020 recommendations for treating chronic myeloid leukemia. Leukemia. (2020) 34(4):966-84. doi: 10.1038/s41375-020-0776-2
11. Saussele S, Richter J, Guilhot J, Gruber FX, Hjorth-Hansen H, Almeida A, et al. Discontinuation of tyrosine kinase inhibitor therapy in chronic myeloid leukaemia (EURO-SKI): a prespecified interim analysis of a prospective, multicentre, non-randomised, trial. Lancet Oncol. (2018) 19:747–57. doi: 10.1016/S1470-2045(18)30192-X
12. Ross DM, Branford S, Seymour JF, Schwarer AP, Arthur C, Yeung DT, et al. Safety and efficacy of imatinib cessation for CML patients with stable undetectable minimal residual disease: results from the TWISTER study. Blood. (2013) 122:515–22. doi: 10.1182/blood-2013-02-483750
13. Etienne G, Guilhot J, Rea D, Rigal-Huguet F, Nicolini F, Charbonnier A, et al. Long-term follow-up of the french stop imatinib (STIM1) study in patients with chronic myeloid leukemia. J Clin Oncol. (2017) 35:298–305. doi: 10.1200/JCO.2016.68.2914
14. Takahashi N, Kyo T, Maeda Y, Sugihara T, Usuki K, Kawaguchi T, et al. Discontinuation of imatinib in Japanese patients with chronic myeloid leukemia. Haematologica. (2012) 97:903–6. doi: 10.3324/haematol.2011.056853
15. Mahon FX, Nicolini FE, Noël MP, Escoffre M, Charbonnier A, Rea D, et al. Preliminary report of the STIM2 study: A multicenter stop imatinib trial for chronic phase chronic myeloid leukemia de novo patients on imatinib. Blood. (2013) 122:654–4. doi: 10.1182/blood.V122.21.654.654
16. Pagnano KBB, Lopes ABP, Miranda EC, Delamain MT, Duarte GO, Rodrigues BRV, et al. Efficacy and safety of pioglitazone in a phase 1/2 imatinib discontinuation trial (EDI-PIO) in chronic myeloid leukemia with deep molecular response. Am J Hematol. (2020) 95(12):E321-3. doi: 10.1002/ajh.25986
17. Shanmuganathan N, Pagani IS, Ross DM, Park S, Yong ASM, Braley JA, et al. Early BCR- ABL1 kinetics are predictive of subsequent achievement of treatment-free remission in chronic myeloid leukemia. Blood. (2021) 137:1196–207. doi: 10.1182/blood.2020005514
18. Rea D, Henry G, Khaznadar Z, Etienne G, Guilhot F, Nicolini F, et al. Natural killer-cell counts are associated with molecular relapse-free survival after imatinib discontinuation in chronic myeloid leukemia: the IMMUNOSTIM study. Haematologica. (2017) 102:1368–77. doi: 10.3324/haematol.2017.165001
19. Braga AGO, Barbosa Pagnano KB, Campioni MDP, Lopes ABP, Duarte GO, Metze K, et al. Peripheral lymphocyte subsets as predicting factors for molecular recurrence after imatinib discontinuation in a phase 2 imatinib discontinuation trial in patients with chronic myeloid leukemia. Hematol Transfus Cell Ther. (2023) 5:S2531–1379(23)00110-4. doi: 10.1016/j.htct.2023.06.001
20. Seguro FS, Maciel FVR, Santos FM, Abdo ANR, Pereira TDM, Nardinelli L, et al. MR 4log and low levels of NK cells are associated with higher molecular relapse after imatinib discontinuation: Results of a prospective trial. Leuk Res. (2021) 101:106516. doi: 10.1016/j.leukres.2021.106516
21. Clark RE, Polydoros F, Apperley JF, Milojkovic D, Rothwell K, Pocock C, et al. De-escalation of tyrosine kinase inhibitor therapy before complete treatment discontinuation in patients with chronic myeloid leukaemia (DESTINY): a non-randomised, phase 2 trial. Lancet Haematol. (2019) 6:e375–83. doi: 10.1016/S2352-3026(19)30094-8
22. Luo J, Du X, Lou J, Wu J, Ma L, Huang J, et al. De-escalation or discontinuation of tyrosine kinase inhibitor in patients with chronic myeloid leukemia: A multicentral, open-label, prospective trial in China. EJHaem. (2022) 3:1220–30. doi: 10.1002/jha2.550
23. Cross NCP, White HE, Colomer D, Ehrencrona H, Foroni L, Gottardi E, et al. Laboratory recommendations for scoring deep molecular responses following treatment for chronic myeloid leukemia. Leukemia. (2015) 29:999–1003. doi: 10.1038/leu.2015.29
24. White HE, Salmon M, Albano F, Andersen CSA, Balabanov S, Balatzenko G, et al. Standardization of molecular monitoring of CML: results and recommendations from the European treatment and outcome study. Leukemia. (2022) 36:1834–42. doi: 10.1038/s41375-022-01607-z
25. Branford S, Cross NCP, Hochhaus A, Radich J, Saglio G, Kaeda J, et al. Rationale for the recommendations for harmonizing current methodology for detecting BCR-ABL transcripts in patients with chronic myeloid leukaemia. Leukemia. (2006) 20:1925–30. doi: 10.1038/sj.leu.2404388
26. Ribeiro BF, Vergílio BR, Miranda ECM, Almeida MH, Delamain MT, da Silveira RA, et al. BCR-ABL1 Transcript Levels at 3 and 6 Months Are Better for Identifying Chronic Myeloid Leukemia Patients with Poor Outcome in Response to Second-Line Second-Generation Tyrosine Kinase Inhibitors after Imatinib Failure: A Report from a Single Institution. Acta Haematol. (2015) 134:248–54. doi: 10.1159/000430835
27. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG, et al. Research electronic data capture (REDCap) – A metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. (2009) 42(2):377-81. doi: 10.1016/j.jbi.2008.08.010
28. Mahon FX, Réa D, Guilhot J, Guilhot F, Huguet F, Nicolini F, et al. Discontinuation of imatinib in patients with chronic myeloid leukaemia who have maintained complete molecular remission for at least 2 years: the prospective, multicentre Stop Imatinib (STIM) trial. Lancet Oncol. (2010) 11:1029–35. doi: 10.1016/S1470-2045(10)70233-3
29. Hochhaus A, Masszi T, Giles FJ, Radich JP, Ross DM, Gómez Casares MT, et al. Treatment-free remission following frontline nilotinib in patients with chronic myeloid leukemia in chronic phase: results from the ENESTfreedom study. Leukemia. (2017) 31:1525–31. doi: 10.1038/leu.2017.63
30. Legros L, Nicolini FE, Etienne G, Rousselot P, Rea D, Giraudier S, et al. Second tyrosine kinase inhibitor discontinuation attempt in patients with chronic myeloid leukemia. Cancer. (2017) 123:4403–10. doi: 10.1002/cncr.30885
31. Perusini MA, Novitzky-Basso I, Atenafu EG, Forrest D, Bence-Bruckler I, Savoie L, et al. Final report of TKI discontinuation trial with dasatinib for the second attempt of treatment-free remission after failing the first attempt with imatinib: Treatment-free Remission Accomplished by Dasatinib (TRAD) study. Br J Haematol. (2023) 203:781–91. doi: 10.1111/bjh.19058
32. Flygt H, Söderlund S, Richter J, Saussele S, Koskenvesa P, Stenke L, et al. Treatment-free remission after a second TKI discontinuation attempt in patients with Chronic Myeloid Leukemia re-treated with dasatinib – interim results from the DAstop2 trial. Leukemia. (2024) 38(4):781–7. doi: 10.1038/s41375-024-02145-6
33. Rousselot P, Loiseau C, Delord M, Cayuela JM, Spentchian M. Late molecular recurrences in patients with chronic myeloid leukemia experiencing treatment-free remission. Blood Adv. (2020) 4:3034–40. doi: 10.1182/bloodadvances.2020001772
Keywords: Chronic myeloid leukemia, tyrosine kinase inhibitors, discontinuation, dose de-escalation, BCR::ABL1 transcripts, molecular relapse
Citation: Murbach B, Duarte G, Palma LC, Miranda E, Duffles G, Furlin GP, Toni I, De Souza C, Binelli L, Bassan VL, de Castro FA, Figueiredo-Pontes LLd and Pagnano KBB (2024) Kinetics of BCR::ABL1 transcript levels and molecular relapse after tyrosine kinase inhibitors discontinuation in chronic myeloid leukemia patients: preliminary results from the DES-CML study. Front. Oncol. 14:1393191. doi: 10.3389/fonc.2024.1393191
Received: 28 February 2024; Accepted: 16 April 2024;
Published: 08 May 2024.
Edited by:
Mohamed A. Yassin, Qatar University, QatarReviewed by:
Philippe Rousselot, Université de Versailles Saint-Quentin-en-Yvelines, FranceCopyright © 2024 Murbach, Duarte, Palma, Miranda, Duffles, Furlin, Toni, De Souza, Binelli, Bassan, de Castro, Figueiredo-Pontes and Pagnano. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Katia Borgia Barbosa Pagnano, a2JvcmdpYUB1bmljYW1wLmJy
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.