- 1Department of Radiation Oncology, University of California Irvine, Orange, CA, United States
- 2School of Medicine, University of California Irvine, Irvine, CA, United States
- 3Department of Hematology/Oncology, University of California Irvine, Orange, CA, United States
- 4Department of Orthopedic Surgery, University of California Irvine, Orange, CA, United States
Introduction: This study investigates the impact of pre- and post-treatment hematologic markers, specifically neutrophil-to-lymphocyte ratio (NLR) and platelet-to-lymphocyte ratio (PLR), on treatment outcomes in soft tissue sarcoma (STS) patients undergoing radiation therapy (RT).
Methods: Data from 64 patients who underwent RT for curative management of STS were reviewed. Pre-RT and post-RT hematologic measures were evaluated for associations with survival outcomes. A normal tissue complication probability (NTCP) curve for predicting ΔPLR ≥ 75 was modeled using a probit function.
Results: Elevated baseline NLR was associated with worse overall survival (OS) and disease-free survival (DFS), while elevated PLR was associated with worse DFS. Post-RT, elevated PLR was linked to worse OS and DFS. Increasing PLR change post-RT was associated with worse OS and DFS. Receiver operating characteristics analysis determined ΔPLR ≥ 75 to be a robust cutoff associated with worse DFS. Bone V10Gy ≥362 cc corresponded to a 50% risk of developing ΔPLR ≥ 75.
Discussion: These results suggest that hematologic markers could serve as prognostic biomarkers in both pre- and post-treatment settings for STS patients undergoing RT. Future studies can consider using bone V10Gy < 362 cc as a potential cutoff to reduce the risk of increased PLR after RT.
Introduction
Soft-tissue sarcomas (STS) constitute a heterogenous disease characterized by varied anatomical presentations and over 50 histological subtypes with disease courses spanning a wide spectrum (1, 2). Consequently, there is a necessity for improved understanding of the prognosis of STS beyond established staging criteria such as anatomical presentation, tumor size, and grading (3).
Recent studies have demonstrated that inflammatory markers obtained from routine blood tests can serve as valuable prognostic markers in different types of cancers, including STS (4–6). The presence of tumor-associated neutrophils is believed to have a critical role in promoting tumor growth and metastasis, and elevated neutrophil-to-lymphocyte ratio (NLR) has been linked to a worse prognosis in various cancer types (7–9). Similarly, platelets, which serve as acute phase reactants, have shown utility in determining cancer prognosis, with elevated platelet-to-lymphocyte ratio being associated with worse outcomes (10–12).
The management of non-metastatic STS may involve a combination of surgery and radiation (RT). Previous research has shown that pre-operative elevations in NLR and PLR are associated with worse overall survival (OS) in STS (13–16). While neutrophil, platelet, and lymphocyte progenitor cells are well-known to be sensitive to radiation, the relative impact of RT on NLR or PLR is not well understood (13–15).
Sarcomas are generally thought to have low immunogenicity and low tumor mutational burden (17, 18). However, recent clinical trials have shown promise with the use of immune checkpoint inhibitors (ICIs) in certain immunogenic subtypes such as undifferentiated pleomorphic sarcoma and de-differentiated liposarcoma (19, 20). Understanding the effects of RT on the immune response in sarcoma is crucial, especially given recent efforts to combine RT with ICIs to enhance the immune response and improve the efficacy of these therapies (21).
This study had two main objectives. First, to investigate the association between the relatively unexplored hematologic markers of immune response (neutrophil-to-lymphocyte ratio (NLR), and platelet-to-lymphocyte ratio (PLR)) with treatment and survival outcomes in patients undergoing RT. Second, to assess the impact of RT and dosimetric parameters on these hematologic markers before and after treatment.
Materials and methods
Patient selection
A retrospective study was conducted on patients who underwent management of non-metastatic STS at a single academic institution between September 2009 and January 2023. A total of 64 patients were included in the analysis. Treatment consisted of neoadjuvant or adjuvant radiation RT with surgery, or definitive RT alone. Chemotherapy was sometimes employed for high-risk patients or with rhabdomyosarcoma histology. Staging was performed based on criteria outlined in the 8th edition of the American Joint Committee on Cancer staging.
Hematologic assessments
Routine follow-up, at provider discretion, consisted of a physical exam, CBC with differential, and radiologic assessments. ANC, ALC, and PLT were recorded prior to the initiation of any treatment (pre-RT) and between 0 to 4 months post-RT (post-RT). To minimize the capture of transient changes resulting from infection or medication adverse effects, CBC measures with significant leukopenia (WBC < 4 x 103 cells/μL) or leukocytosis (WBC > 12 x 103 cells/μL) were excluded from the analysis. NLR and PLR were calculated by dividing ANC by ALC and PLT by ALC, respectively. Delta (Δ) values were calculated by subtracting the post-RT measures from the pre-RT measures.
Dosimetric analysis
Evaluation of RT treatment plans was performed on MIM (MIM Software Inc., Cleveland, OH). Organ volumes were individually delineated for the body and bones to determine dose-volume histograms (DVH). Total dose was converted to equivalent dose in 2 Gy fractions (EQD2) to account for different dose regimens. Mean doses to body and bone as well as volumetric doses, defined as volume of body or bone receiving 10 Gy, 20 Gy, or 30 Gy (V10Gy, V20 Gy, V30 Gy), were recorded.
Statistical analysis
Kaplan-Meier curves were used to estimate OS and DFS and log-rank testing was used to compare groups. OS was calculated as the duration in months from the initiation of RT to death from any cause. Disease-free survival DFS was calculated as the duration in months from the initiation of RT to disease recurrence, distant or local progression, or death. Baseline and follow-up ALC, ANC, PLT, NLR, and PLR were compared using paired T-test and effect size was estimated with Cohen’s d. Univariate and multivariate Cox proportional hazards regression models were used to analyze associations between clinical factors and hematologic markers with survival outcomes. Spearman’s rank correlation coefficient was used to evaluate for correlations between ΔPLR and dosimetric parameters. Normal Tissue Complication Probability (NTCP) for developing ΔPLR ≥ 75 was modeled with a probit regression function (22, 23). Data analysis was performed using SPSS Version 24.0 (IBM Corp., Armonk, NY) and R Version 4 (R Foundation for Statistical Computing, Vienna, Austria).
Results
Clinical characteristics
64 patients were included in this study (Table 1). Median age was 59 years old (range: 22 - 89 years old). Primary disease sites were extremities in 37 patients (58%), trunk in 12 patients (19%), and head and neck in 15 patients (23%). T1 disease was present in 9 patients (14%), T2 in 27 patients (42%), T3 in 15 patients (23%), and T4 in 13 patients (20%). N1 disease was present in 3 patients (4.7%). Grade 1 disease was found in 8 patients (14%), grade 2 disease in 6 patients (9.4%), and grade 3 disease in 50 patients (78%). Goal of radiation was definitive in 7 patients (11%) and either neoadjuvant or adjuvant in 57 patients (89%). 3D conformal technique was utilized in 17 patients (27%) and IMRT in 47 patients (73%). Median RT dose was 51 Gy (range: 39 - 78 Gy). 30 patients (47%) received chemotherapy and 54 patients (84%) underwent surgical resection as a part of their definitive management.
Hematologic effects
The median pre-RT values for ANC, ALC, PLT, NLR, and PLR were 4900 cells/μL (IQR: 3600 - 6600 cells/μL), 1600 cells/μL (IQR: 1200 - 2200 cells/μL), 256 x 103 cells/μL (IQR: 203 x 103 - 321 x 103 cells/μL), 3.0 (IQR: 2.0 - 4.4), and 160 (120 – 230), respectively (Figure 1). At the 3 month post-RT time point, the median values for ANC, ALC, PLT, NLR, and PLR were 4300 cells/μL (IQR: 3200 - 5600 cells/μL), 950 cells/μL (IQR: 600 - 1400 cells/μL), 225 x 103 cells/μL (IQR: 204 x 103 - 284 x 103 cells/μL), 4.2 (IQR: 2.5 - 7.8), and 250 (150 – 420), respectively. Significant decreases in ANC and ALC, as well as significant increases in NLR and PLR, were noted between the 3 month post-RT and pre-RT timepoints. The decrease in ALC and in the increase in PLR showed stronger effect sizes, with Cohen’s d > 0.8.
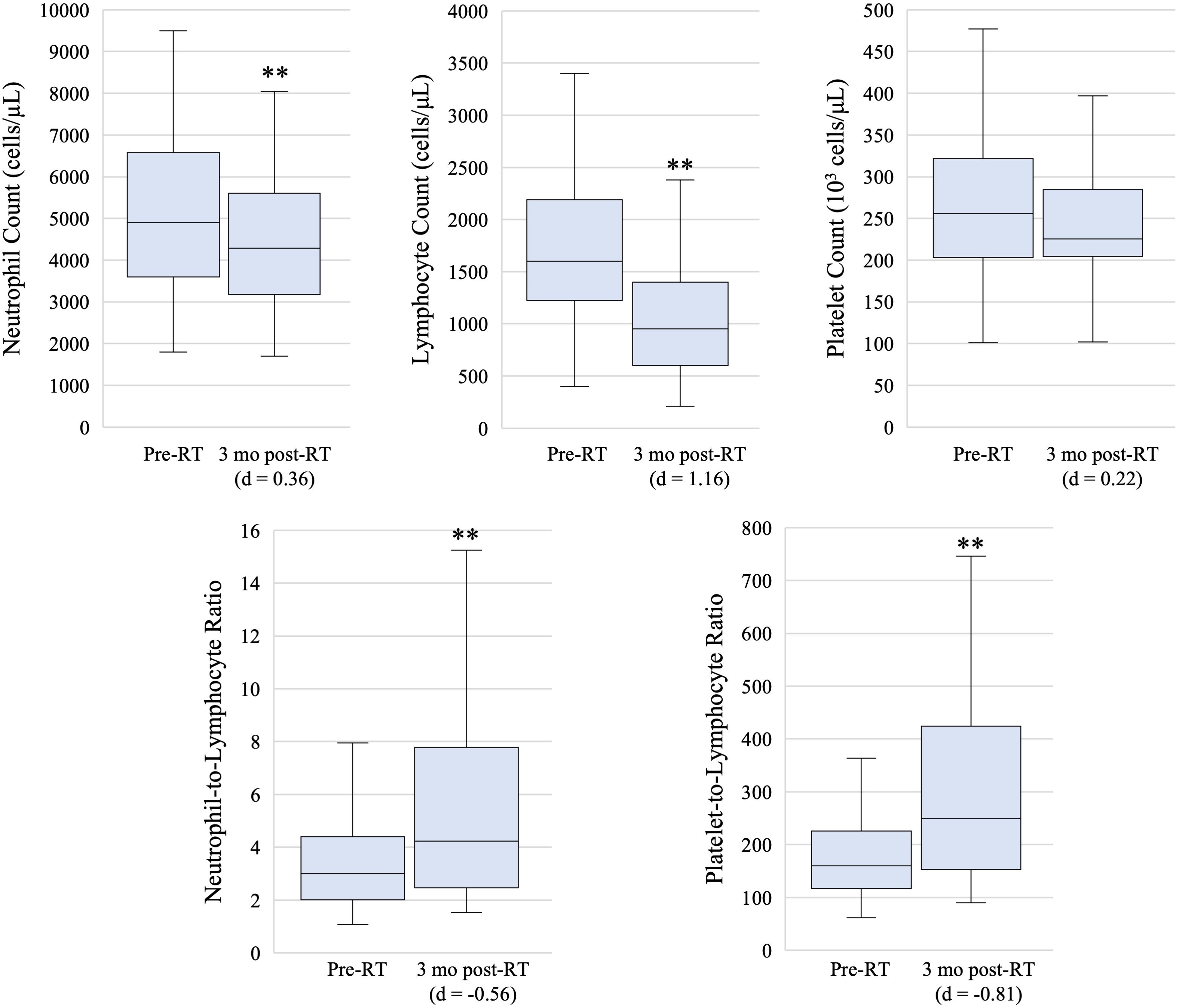
Figure 1. Box and whisker plots showing pre-radiation therapy (pre-RT) and 3 month post-radiation therapy (3 mo post-RT) hematologic marker measures. Box, line, and whiskers represent interquartile range, median, and 95% confidence interval, respectively. ** indicates p-value < 0.05 on paired T-test and effect size is estimated with Cohen’s d.
Survival outcomes
The median follow-up duration was 23 months. The median OS was 84 months (95% CI: 57 - 112 months), and the median DFS was 20 months (95% CI: 1 - 72 months). Several baseline clinical factors were associated with worse OS on univariate Cox proportional hazards regression, including advanced T-stage disease, head and neck primary disease, receipt of chemotherapy, lack of surgical resection, receipt of definitive RT alone, and use of intensity-modulated radiation therapy (IMRT) technique (Supplementary Table S1). Male sex, advanced T-stage disease, receipt of chemotherapy, lack of surgical resection, and use of IMRT technique were associated with worse DFS.
At the pre-RT timepoint, elevated ANC, elevated NLR, and elevated PLR were associated with worse OS (Supplementary Table S1). However, on multivariate regression analysis considering surgical resection and chemotherapy, only NLR remained associated with OS. Similarly, elevated ANC, elevated NLR, and elevated PLR were associated with worse DFS. All three markers remained associated with DFS on multivariate regression analysis. Kaplan-Meier curves for DFS shown in Figures 2A, B. DFS data by histological subtype and stratified by ΔPLR and pre-RT/post-RT NLR cut-offs is shown in Supplementary Table S2.
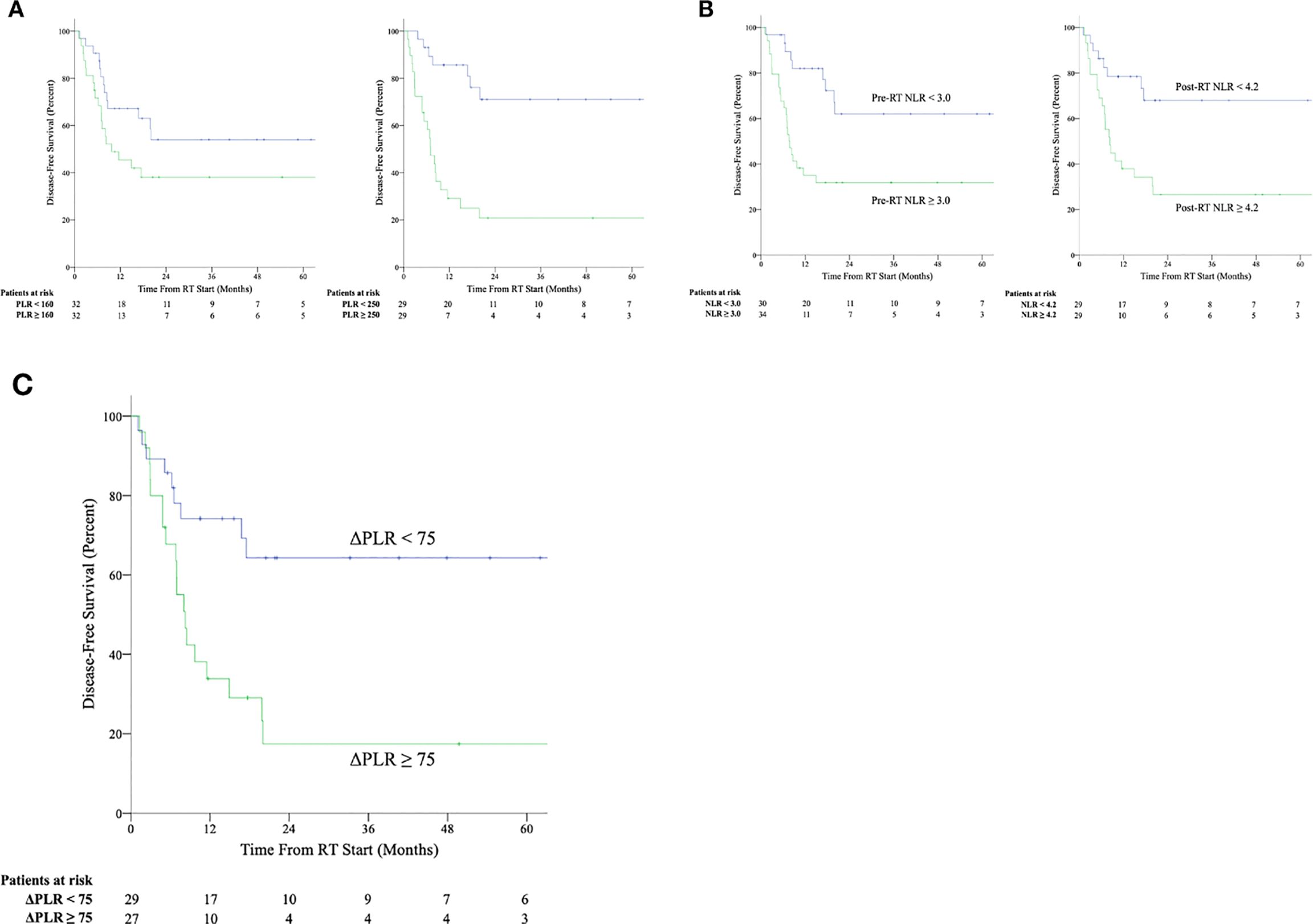
Figure 2. Kaplan-Meier survival curves for disease-free survival stratified by above median and below median values of platelet-to-lymphocyte ratio (NLR) (A) and neutrophil-to-lymphocyte ratio (PLR) (B) at the pre-radiation therapy (pre-RT) and post-radiation therapy (post-RT) timepoints as well as by values of change in platelet-to-lymphocyte ratio between post-radiation therapy and pre-radiation therapy timepoints (ΔPLR) ≥ 75 or < 75 (C).
At the 3-month post-RT timepoint, lower ALC, elevated PLT, and elevated PLR were associated with worse OS. PLT and PLR remained associated with OS on multivariate regression. Lower ALC, elevated PLT, elevated NLR, and elevated PLR were associated with worse DFS. ALC, PLT, and PLR continued to show associations with DFS on multivariate regression.
ΔANC, ΔALC, ΔPLT, and ΔNLR were not associated with worse outcomes, whereas an increased ΔPLR was associated with both OS and DFS on univariate and multivariate regression (Figure 2C). ROC analysis was used to determine optimum cutoff values for predicting DFS (AUC 0.745, p = 0.002) and determined to be ΔPLR ≥ 75 (Sn = 66%, Sp = 75%) using the concordance probability method (24). A ΔPLR ≥ 75 was associated with a 5-year OS of 40% and 5-year DFS of 18%, compared to 74% OS (p = 0.046) and 64% DFS (p = 0.005) for those with a ΔPLR < 75.
Dosimetric analysis
Dosimetric parameters that significantly correlated with ΔPLR were mean body dose (rs = 0.29), body V10 Gy (rs = 0.34), body V20 Gy (rs = 0.31), body V30 Gy (rs = 0.28), mean bone dose (rs = 0.37), bone V10 Gy (rs = 0.48), bone V20 Gy (rs = 0.46), and bone V30 Gy (rs = 0.38).
With a ΔPLR “toxicity” of ≥ 75, dosimetric parameters and their associations with this cutoff were evaluated. Among the dosimetric parameters, bone V10Gy demonstrated the highest capability for predicting ΔPLR ≥ 75 (AUC > 0.8, p < 0.001) (Figure 3). The dosimetric parameter values corresponding to a 50% risk (TD50) of developing ΔPLR ≥ 75 were body mean, body V10 Gy, body V20 Gy, bone mean, bone V10 Gy, and bone V20 Gy of 8.4 Gy, 4952 cc, 3,246 cc, 8.8 Gy, 362 cc, and 254 cc, respectively (Figure 4).
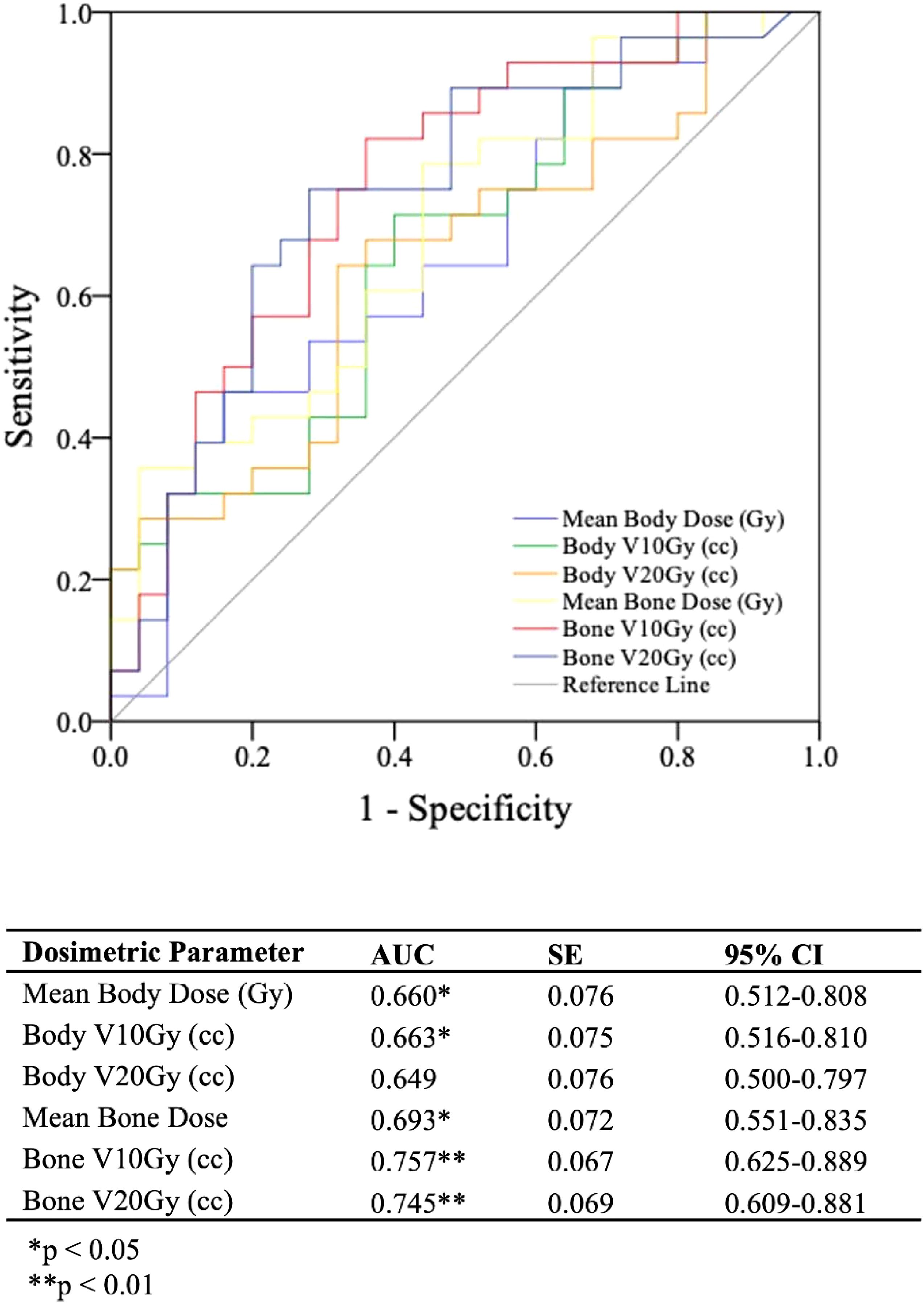
Figure 3. Receiver operating characteristics (ROC) curves and analysis for evaluating bone and body dosimetric parameters ability to predict a change in platelet-to-lymphocyte ratio between post-radiation therapy and pre-radiation therapy timepoints (ΔPLR) ≥ 75.
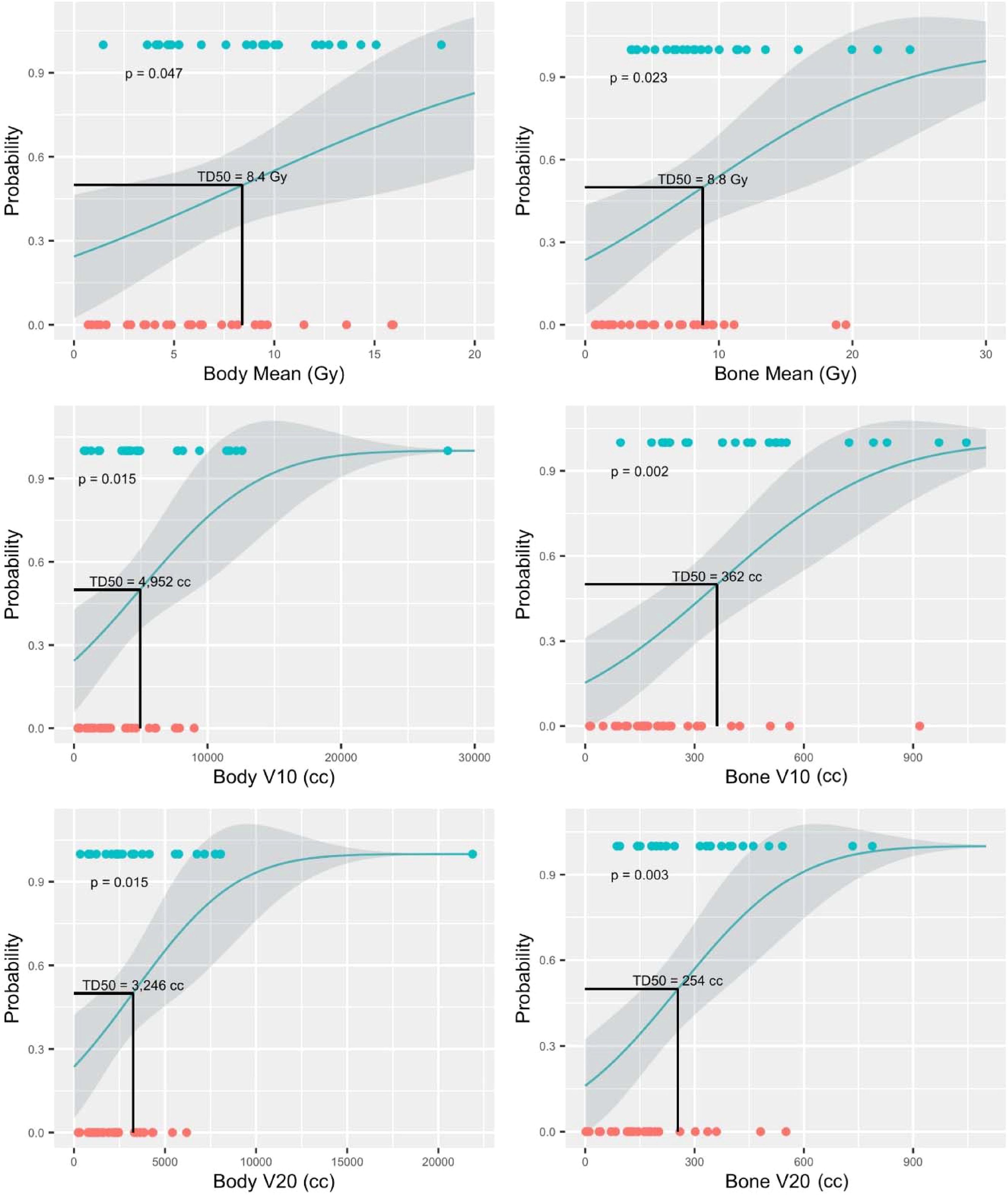
Figure 4. Normal tissue complication probability (NTCP) models for predicting change in platelet-to-lymphocyte ratio (ΔPLR) ≥ 75. Blue circles represent patients with ΔPLR ≥ 75 and orange circles represent patients with ΔPLR < 75.
Discussion
Our findings were that elevated baseline NLR and PLR were associated with worse survival outcomes in patients undergoing RT for STS. Additionally, we observed a greater increase in PLR between pre-RT and post-RT measurements was associated with worse survival outcomes. We then identified a suggested threshold for bone V10Gy that could help mitigate the risk of increased ΔPLR.
There have been a number of retrospective studies that have shown the usefulness of pre-treatment NLR and PLR in determining prognosis in STS (13, 14, 16, 25–27). However, the existing evidence is conflicting, as a recent multicenter sarcoma database study found both NLR and PLR to be poor predictors of mortality and recurrence-free survival (28). Notably, the patients in that study consisted exclusively of STS of the retroperitoneum and trunk, while our study included a majority of patients with extremity tumors. Moreover, our study is unique as it focused on a subset of patients receiving RT for STS, and the results regarding prognostic utility of NLR and PLR are consistent with previous work on radiation treatment outcomes in other malignancies, including lung, esophageal, cervical, and pancreatic cancers (29–34).
The increase in NLR and PLR shortly after completing RT aligns with the sensitivity of lymphocytes to radiation and the relative lower sensitivity of neutrophil and platelet progenitor cells. However, as expected, drops were observed in all cell lines (35). Recent research has focused on radiation-induced lymphopenia and its impact on treatment outcomes across cancer subtypes (36). The capability of neutrophils to hinder lymphocyte infiltration within the tumor microenvironment plays a critical role in the tumor immune response. This is achieved in part, by inhibiting antitumor responses and releasing anti-inflammatory cytokines (37, 38). Therefore, NLR may be viewed as a balance between host pro-inflammatory and anti-inflammatory mediators. While evidence exists linking platelets to cancer progression through mechanisms like increased angiogenesis and subsequent increased metastatic risk, the precise mechanism by which elevated PLR leads to worse outcomes is not as firmly established (39–41). Another theory suggests that PLR might reflect a more intense tumor-induced host systemic inflammatory response, potentially contributing to worse outcomes (42). Notably, in our study, only an increase in ΔPLR was associated with worse overall survival. These findings underscore the need for further investigation into how radiation treatment may affect the tumor immune response, the tumor microenvironment, and certain hematologic markers such as PLR.
Several studies have examined dosimetric parameters and their influence on NLR and PLR (30, 32, 33, 43, 44). For example, Wolf et al. found NLR to be associated with worse outcomes and that increased spleen dose may contribute to a greater change in NLR after RT for locally advanced pancreatic cancer (32). However, we did not find an association between ΔNLR, ΔANC, or ΔALC and any survival outcomes, suggesting possible differences in hematologic adverse effects between treating STS and intraabdominal cancers. Another study focusing on lung cancer and chemoradiation discovered that volumetric heart doses and mean body dose were related to post-RT NLR and PLR. Interestingly, contrary to our findings, they observed that a higher mean body dose was associated with lower PLR (43). Our work supports the idea of reducing dosimetric parameters, particularly bone V10Gy, as a strategy to improve treatment outcomes in STS.
This research has several limitations. The small sample size and retrospective design may introduce selection bias and limit the generalizability of the findings. A major limitation is the lack of a validation cohort, which is necessary to confirm the robustness and generalizability of ΔPLR as prognostic marker and bone V10 Gy < 362 cc as a dosimetric parameter. Additionally, dosimetric studies of sarcomas present inherently challenges due to the diverse patient population, variations in sarcoma presentation, and heterogeneity in treatment options. Although we implemented strict inclusion criteria and utilized multivariate analyses to address these issues, the small sample size and the number of analyses conducted require caution in interpreting the results. Further research with larger cohorts is needed to validate these findings and assess their applicability to a broader sarcoma patients.
Current findings provide additional support for the significance of routinely collected hematologic markers, such as NLR and PLR, as important prognostic indicators in patients receiving therapy for STS. Furthermore, our results suggest that minimizing the rise in PLR following RT by keeping the bone V10Gy under 362 cc could potentially enhance beneficial outcomes in STS.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by UC Irvine School of Medicine IRB. The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants’ legal guardians/next of kin because without a waiver of the consent the research could not be practically carried out since this will be a chart review of any patients who have a sarcoma treated at UCI. Patients will be identified from those seen in the Department of Radiation Oncology, Medical Oncology, Surgical Oncology, or Orthopedic Oncology, or the sarcoma multidisciplinary tumor board. We will not be contacting subjects for this study and so consent would not be practical. We will also not be contacting subjects who may be identified in the future as eligible for this study and so consent would not be practical.
Author contributions
EK: Conceptualization, Methodology, Formal analysis, Investigation, Writing – original draft, Writing – review & editing, Visualization. GH: Formal analysis, Methodology, Writing – review & editing. GL: Investigation, Writing – review & editing. AM: Investigation, Writing – review & editing. NP: Investigation, Writing – review & editing. JP: Investigation, Writing – review & editing. WC: Conceptualization, Writing – review & editing. RS: Conceptualization, Writing – review & editing. CL: Conceptualization, Writing – review & editing. JH: Conceptualization, Formal analysis, Funding acquisition, Investigation, Methodology, Supervision, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. Funded by the National Cancer Institute of the National Institutes of Health award number P30CA062203 and the UC Irvine Comprehensive Cancer Center using UCI Anti-Cancer Challenge funds.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2024.1392705/full#supplementary-material
Supplementary Table 2 | Disease-free survival data by sarcoma histological subtype, stratified by ΔPLR and pre-RT/post-RT NLR cut-offs.
References
1. Gómez J, Tsagozis P. Multidisciplinary treatment of soft tissue sarcomas: An update. World J Clin Oncol. (2020) 11:180–9. doi: 10.5306/wjco.v11.i4.180
2. Pisters PW, Leung DH, Woodruff J, Shi W, Brennan MF. Analysis of prognostic factors in 1,041 patients with localized soft tissue sarcomas of the extremities. JCO. (1996) 14:1679–89. doi: 10.1200/jco.1996.14.5.1679
3. von Mehren, Kane. The AJCC 8th Edition Staging System for Soft Tissue Sarcoma of the Extremities or Trunk: A Cohort Study of the SEER Database in. J Natl Compr Cancer Network. (2018) 16:66. https://jnccn.org/view/journals/jnccn/16/2/article-p144.xml.
4. Howard R, Kanetsky PA, Egan KM. Exploring the prognostic value of the neutrophil-to-lymphocyte ratio in cancer. Sci Rep. (2019) 9:19673. doi: 10.1038/s41598-019-56218-z
5. Templeton AJ, McNamara MG, Šeruga B, Vera-Badillo FE, Aneja P, Ocaña A, et al. Prognostic role of neutrophil-to-lymphocyte ratio in solid tumors: a systematic review and meta-analysis. J Natl Cancer Inst. (2014) 106:dju124. doi: 10.1093/jnci/dju124
6. Chan JY, Zhang Z, Chew W, Tan GF, Lim CL, Zhou L, et al. Biological significance and prognostic relevance of peripheral blood neutrophil-to-lymphocyte ratio in soft tissue sarcoma. Sci Rep. (2018) 8:11959. doi: 10.1038/s41598-018-30442-5
7. Cupp MA, Cariolou M, Tzoulaki I, Aune D, Evangelou E, Berlanga-Taylor AJ. Neutrophil to lymphocyte ratio and cancer prognosis: an umbrella review of systematic reviews and meta-analyses of observational studies. BMC Med. (2020) 18:360. doi: 10.1186/s12916-020-01817-1
8. Yan M, Zheng M, Niu R, Yang X, Tian S, Fan L, et al. Roles of tumor-associated neutrophils in tumor metastasis and its clinical applications. Front Cell Dev Biol. (2022) 10:938289. doi: 10.3389/fcell.2022.938289
9. Nemoto T, Endo S, Isohata N, Takayanagi D, Nemoto D, Aizawa M, et al. Change in the neutrophil−to−lymphocyte ratio during chemotherapy may predict prognosis in patients with advanced or metastatic colorectal cancer. Mol Clin Oncol. (2021) 14:1–7. doi: 10.3892/mco.2021.2269
10. Zhou X, Du Y, Huang Z, Xu J, Qiu T, Wang J, et al. Prognostic value of PLR in various cancers: A meta-analysis. PloS One. (2014) 9:e101119. doi: 10.1371/journal.pone.0101119
11. Wang W, Tong Y, Sun S, Tan Y, Shan Z, Sun F, et al. Predictive value of NLR and PLR in response to preoperative chemotherapy and prognosis in locally advanced gastric cancer. Front Oncol. (2022) 12:936206. doi: 10.3389/fonc.2022.936206
12. Ding N, Pang Z, Shen H, Ni Y, Du J, Liu Q. The prognostic value of PLR in lung cancer, a meta-analysis based on results from a large consecutive cohort. Sci Rep. (2016) 6:34823. doi: 10.1038/srep34823
13. Idowu OK, Ding Q, Taktak AFG, Chandrasekar CR, Yin Q. Clinical implication of pretreatment neutrophil to lymphocyte ratio in soft tissue sarcoma. Biomarkers. (2012) 17:539–44. doi: 10.3109/1354750x.2012.699554
14. Liang Y, Wang W, Li J, Guan Y, Que Y, Xiao W, et al. Combined use of the neutrophil-lymphocyte and platelet-lymphocyte ratios as a prognostic predictor in patients with operable soft tissue sarcoma. J Cancer. (2018) 9:2132–9. doi: 10.7150/jca.24871
15. Kaur P, Asea A. Radiation-induced effects and the immune system in cancer. Front Oncol. (2012) 2:191. doi: 10.3389/fonc.2012.00191
16. Lee IH, Na J, Lee SJ. Preoperative and postoperative platelet-lymphocyte ratio is a prognostic marker for patients with soft tissue sarcoma treated with curative resection. In Vivo. (2024) 38:2049–57. doi: 10.21873/invivo.13663
17. Chalmers ZR, Connelly CF, Fabrizio D, Gay L, Ali SM, Ennis R, et al. Analysis of 100,000 human cancer genomes reveals the landscape of tumor mutational burden. Genome Med. (2017) 9:34. doi: 10.1186/s13073-017-0424-2
18. Abeshouse A, Adebamowo C, Adebamowo SN, Akbani R, Akeredolu T, Ally A, et al. Comprehensive and integrated genomic characterization of adult soft tissue sarcomas. Cell. (2017) 171:950–965.e28.doi: 10.1016/j.cell.2017.10.014
19. Tawbi HA, Burgess M, Bolejack V, Tine BAV, Schuetze SM, Hu J, et al. Pembrolizumab in advanced soft-tissue sarcoma and bone sarcoma (SARC028): a multicentre, two-cohort, single-arm, open-label, phase 2 trial. Lancet Oncol. (2017) 18:1493–501. doi: 10.1016/s1470-2045(17)30624-1
20. Wood GE, Meyer C, Petitprez F, D’Angelo SP. Immunotherapy in sarcoma: current data and promising strategies. Am Soc Clin Oncol Educ Book. (2024) 44:e432234. doi: 10.1200/edbk_432234
21. Lussier DM, Alspach E, Ward JP, Miceli AP, Runci D, White JM, et al. Radiation-induced neoantigens broaden the immunotherapeutic window of cancers with low mutational loads. Proc Natl Acad Sci U S A. (2021) 118:e2102611118. doi: 10.1073/pnas.2102611118
22. Marks LB, Yorke ED, Jackson A, Ten Haken RK, Constine LS, Eisbruch A, et al. The use of normal tissue complication probability (NTCP) models in the clinic. Int J Radiat Oncol Biol Phys. (2010) 76:S10–9. doi: 10.1016/j.ijrobp.2009.07.1754
23. R: Normal tissue complication probability (NTCP) . Available online at: https://search.r-project.org/CRAN/refmans/DVHmetrics/html/getNTCP.html (Accessed September 01, 2023).
24. Unal I. Defining an optimal cut-point value in ROC analysis: an alternative approach. Comput Math Methods Med. (2017) 2017:3762651. doi: 10.1155/2017/3762651
25. Maio ED, Touati N, Litière S, Sleijfer S, van der Graaf WTA, Cesne AL, et al. Evolution in neutrophil-to-lymphocyte ratio (NLR) among advanced soft tissue sarcoma (STS) patients treated with pazopanib within EORTC 62043/62072 trials. Ann Oncol. (2017) 28::v531. doi: 10.1093/annonc/mdx387.028
26. Choi ES, Kim HS, Han I. Elevated preoperative systemic inflammatory markers predict poor outcome in localized soft tissue sarcoma. Ann Surg Oncol. (2014) 21:778–85. doi: 10.1245/s10434-013-3418-3
27. Szkandera J, Absenger G, Liegl-Atzwanger B, Pichler M, Stotz M, Samonigg H, et al. Elevated preoperative neutrophil/lymphocyte ratio is associated with poor prognosis in soft-tissue sarcoma patients. Br J Cancer. (2013) 108:1677–83. doi: 10.1038/bjc.2013.276
28. Schwartz PB, Poultsides G, Roggin K, Howard JH, Fields RC, Clarke CN, et al. PLR and NLR are poor predictors of survival outcomes in sarcomas: A new perspective from the USSC. J Surg Res. (2020) 251:228–38. doi: 10.1016/j.jss.2020.01.008
29. Cannon NA, Meyer J, Iyengar P, Ahn C, Westover KD, Choy H, et al. Neutrophil–lymphocyte and platelet–lymphocyte ratios as prognostic factors after stereotactic radiation therapy for early-stage non–small-cell lung cancer. J Thorac Oncol. (2015) 10:280–5. doi: 10.1097/jto.0000000000000399
30. Yu Y, Zheng H, Liu L, Li H, Zheng Q, Wang Z, et al. Predicting severe radiation esophagitis in patients with locally advanced esophageal squamous cell carcinoma receiving definitive chemoradiotherapy: construction and validation of a model based in the clinical and dosimetric parameters as well as inflammatory indexes. Front Oncol. (2021) 11:687035. doi: 10.3389/fonc.2021.687035
31. Sebastian N, Miller ED, Williams TM, Pardo DAD. Transarterial radioembolization (TARE) vs stereotactic body radiation therapy (SBRT) in the treatment of unresectable intrahepatic cholangiocarcinoma. Int J Radiat Oncology Biology Physics. (2018) 102:e61. doi: 10.1016/j.ijrobp.2018.07.491
32. Wolfe AR, Siedow M, Nalin A, DiCostanzo D, Miller ED, Diaz DA, et al. Increasing neutrophil-to-lymphocyte ratio following radiation is a poor prognostic factor and directly correlates with splenic radiation dose in pancreatic cancer. Radiother Oncol. (2021) 158:207–14. doi: 10.1016/j.radonc.2021.02.035
33. Chen M, Wang D, Bao Z, Yi Z, Mei Z, Sun S, et al. The impact of bone marrow irradiation dose on acute haematologic toxicity in cervical cancer patients treated with concurrent chemoradiotherapy. Radiat Oncol. (2023) 18:66. doi: 10.1186/s13014-023-02248-x
34. Liang S, Li C, Gao Z, Li J, Zhao H, Yu J, et al. A nomogram to predict short-term outcome of radiotherapy or chemoradiotherapy based on pre/post-treatment inflammatory biomarkers and their dynamic changes in esophageal squamous cell carcinoma. Int Immunopharmacol. (2021) 90:107178. doi: 10.1016/j.intimp.2020.107178
35. Heylmann D, Ponath V, Kindler T, Kaina B. Comparison of DNA repair and radiosensitivity of different blood cell populations. Sci Rep. (2021) 11:2478. doi: 10.1038/s41598-021-81058-1
36. Damen PJJ, Kroese TE, van Hillegersberg R, Schuit E, Peters M, Verhoeff JJC, et al. The influence of severe radiation-induced lymphopenia on overall survival in solid tumors: A systematic review and meta-analysis. Int J Radiat Oncol Biol Phys. (2021) 111:936–48. doi: 10.1016/j.ijrobp.2021.07.1695
37. Coffelt SB, Wellenstein MD, de Visser KE. Neutrophils in cancer: neutral no more. Nat Rev Cancer. (2016) 16:431–46. doi: 10.1038/nrc.2016.52
38. Gooden MJM, de Bock GH, Leffers N, Daemen T, Nijman HW. The prognostic influence of tumour-infiltrating lymphocytes in cancer: a systematic review with meta-analysis. Br J Cancer. (2011) 105:93–103. doi: 10.1038/bjc.2011.189
39. Battinelli EM, Markens BA, Italiano JE. Release of angiogenesis regulatory proteins from platelet alpha granules: modulation of physiologic and pathologic angiogenesis. Blood. (2011) 118:1359–69. doi: 10.1182/blood-2011-02-334524
40. Gay LJ, Felding-Habermann B. Contribution of platelets to tumour metastasis. Nat Rev Cancer. (2011) 11:123–34. doi: 10.1038/nrc3004
41. van der Laan P, van der Graaf WTA, van den Broek D, van Boven H, Heeres BC, Schrage Y, et al. Interleukin-6 in relation to early recurrence in primary, localized soft tissue sarcoma: An addition for existing risk classification systems? Eur J Surg Oncol. (2024) 50. https://www.ejso.com/article/S0748-7983(24)00582-1/abstract.
42. Kwon HC, Kim SH, Oh SY, Lee S, Lee JH, Choi HJ, et al. Clinical significance of preoperative neutrophil-lymphocyte versus platelet-lymphocyte ratio in patients with operable colorectal cancer. Biomarkers. (2012) 17:216–22. doi: 10.3109/1354750x.2012.656705
43. Xia WY, Zhu XR, Feng W, Liu J, Wang JM, Lv CX, et al. Neutrophil-lymphocyte ratio and platelet-lymphocyte ratio associations with heart and body dose and their effects on patient outcomes in locally advanced non-small cell lung cancer treated with definitive radiotherapy. Transl Lung Cancer Res. (2020) 9:1996–2007. doi: 10.21037/tlcr-20-831
Keywords: sarcoma, radiation, NLR, PLR, dosimetry
Citation: Ku E, Harada G, Lee G, Munjal A, Peterson N, Park J, Chow W, Stitzlein R, Limoli C and Harris J (2024) A study of pre- and post-treatment hematologic markers of immune response in patients undergoing radiotherapy for soft tissue sarcoma. Front. Oncol. 14:1392705. doi: 10.3389/fonc.2024.1392705
Received: 27 February 2024; Accepted: 09 September 2024;
Published: 03 October 2024.
Edited by:
Eric Chi-ching Ko, University of Massachusetts Medical School, United StatesReviewed by:
Nuradh Joseph, Government of Sri Lanka, Sri LankaMarla Weetall, PTC Therapeutics, United States
Copyright © 2024 Ku, Harada, Lee, Munjal, Peterson, Park, Chow, Stitzlein, Limoli and Harris. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jeremy Harris, anBoYXJyaXMxQGhzLnVjaS5lZHU=
 Eric Ku
Eric Ku Garrett Harada1
Garrett Harada1 Warren Chow
Warren Chow Charles Limoli
Charles Limoli