- 1Department of Surgery, Ewha Womans University College of Medicine, Ewha Womans University Mokdong Hospital, Seoul, Republic of Korea
- 2Department of Surgery, University of Ulsan College of Medicine, Asan Medical Center, Seoul, Republic of Korea
Introduction: Male breast cancer (MBC) is a rare condition, and recent research has underscored notable distinctions between MBC and breast cancer in women. This study aimed to assess and contrast the long-term survival outcomes and disease patterns of MBC patients with those of their female counterparts.
Methods: We analyzed data from 113,845 patients diagnosed with breast cancer who had undergone curative surgery from the Korean Breast Cancer Registry (KBCR) between January 1990 and August 2014 in Seoul, Korea. The five-year overall survival was analyzed according to clinicopathological characteristics.
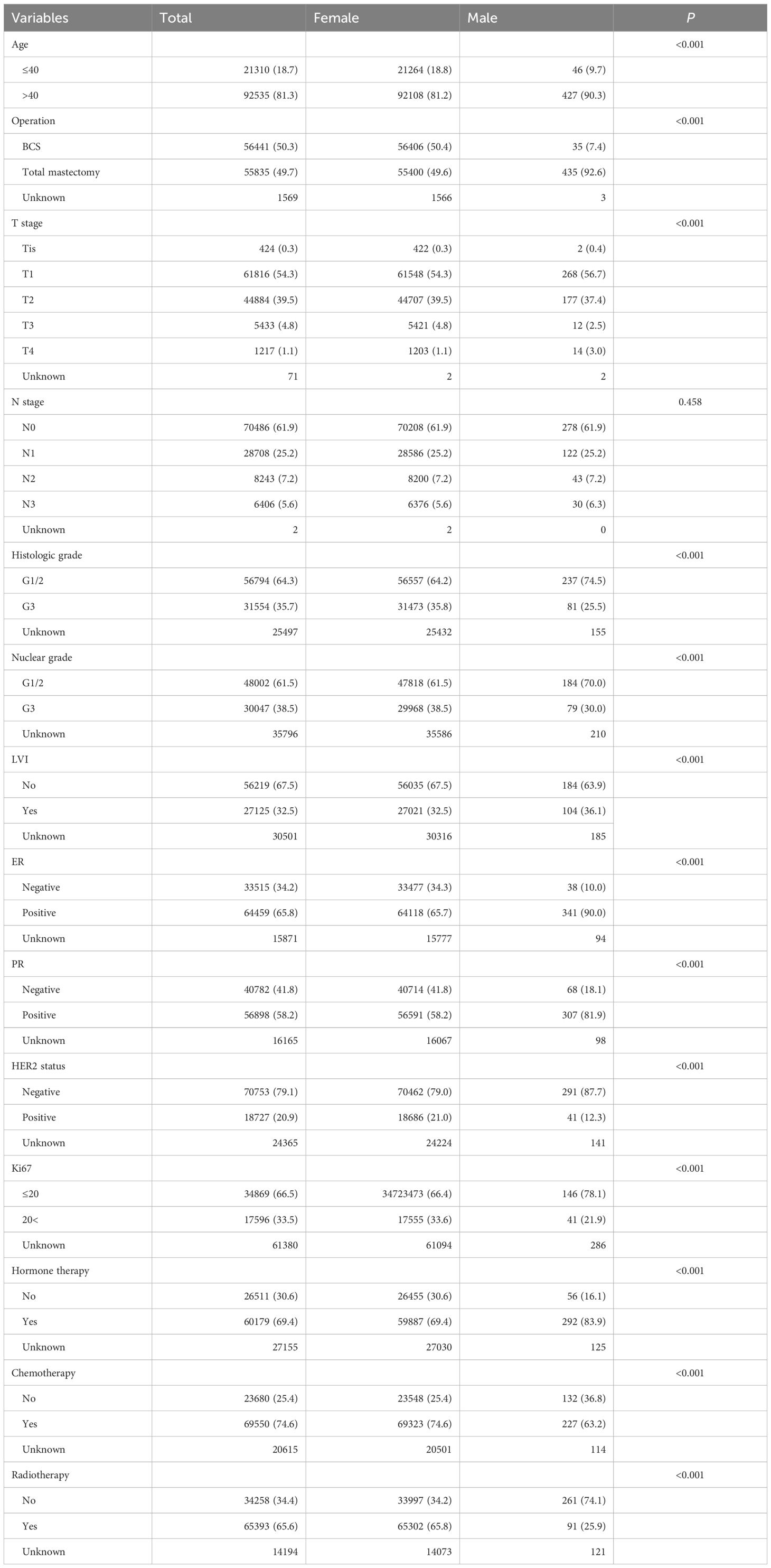
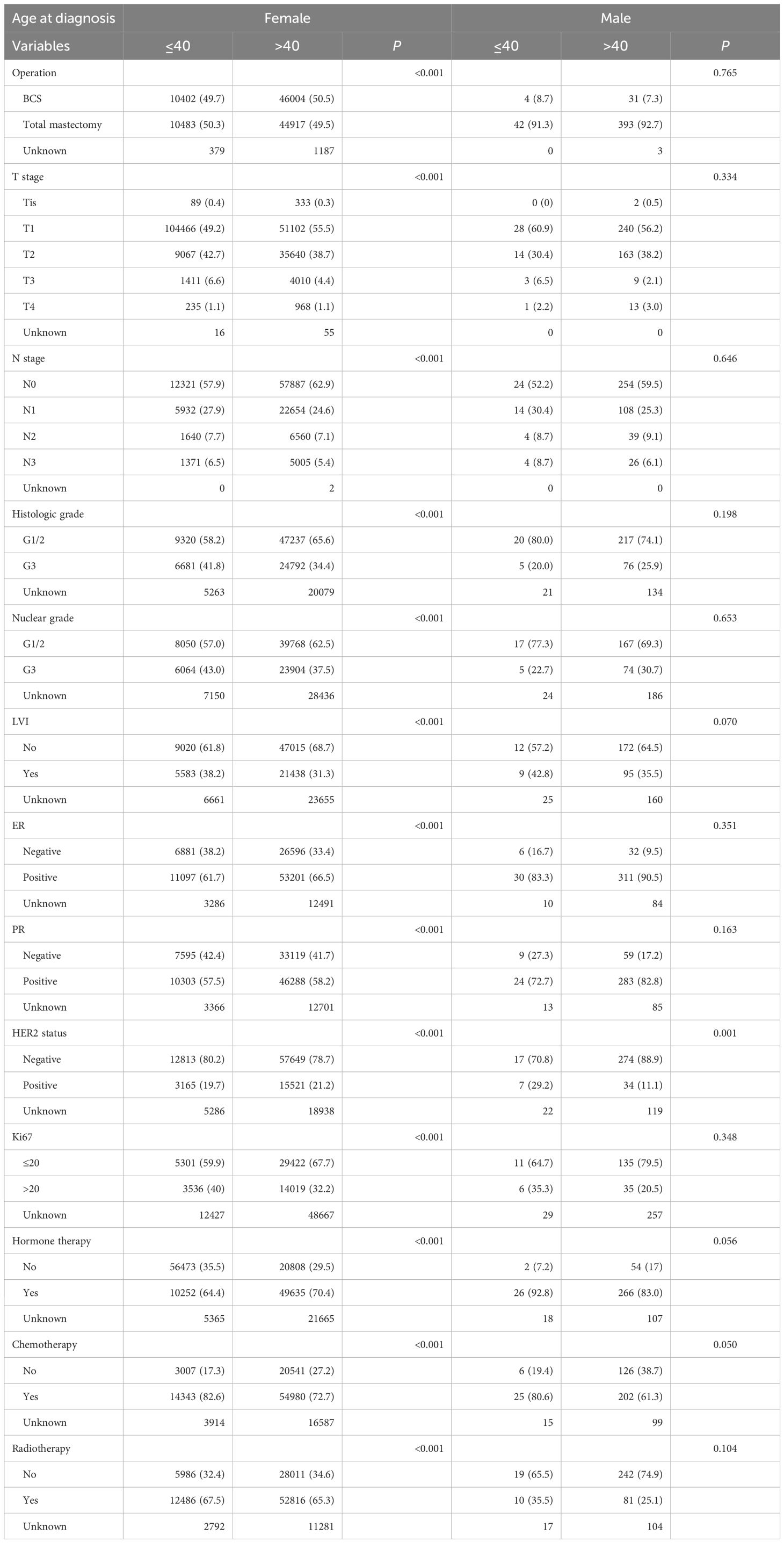
Results: Among 113,845 patients with breast cancer, 473 MBC cases were included. The median duration of follow-up was 72 months. The median age at diagnosis was 60 and 48 years for MBC and female breast cancer, respectively. Most male patients (92.6%) underwent total mastectomy, while 50.4% of female patients underwent breast-conserving surgery. Among MBC, 63.2% received chemotherapy, and 83.9% of hormone receptor-positive male patients received endocrine therapy. In survival analysis, MBC demonstrated distinct 5-year overall survival patterns compared with female breast cancer, according to age at diagnosis. In women with breast cancer, the younger age group (≤40 years) demonstrated worse 5-year overall survival than did the older age group (>40 years) (91.3% vs 92.7%, p <0.05). While in MBC, the younger age group (≤40 years) demonstrated better 5-year overall survival than did the older age group (>40 years) (97.4% vs 86.4%, p <0.05).
Discussion: In conclusion within this extensive cohort, we have revealed unique survival patterns in MBC that diverge from those observed in women with breast cancer. This study enhances our comprehension of MBC prognosis and can potentially shed light on unresolved questions, paving the way for future research in the realm of MBC.
1 Introduction
Male breast cancer (MBC) is rare, comprising roughly 1% of all cancers in men and approximately 1% of the total breast cancer cases worldwide (1–6). Less than 0.2% of cancer-related deaths in men can be attributed to MBC (7, 8). Owing to the exceptionally low incidence of MBC, studies, clinical trials, and the development of new treatment approaches have primarily centered around BC in women. While insights from studies of BC in women undoubtedly offer valuable guidance in the MBC diagnosis and treatment, it is crucial to emphasize the significant molecular and clinicopathologic differences between the two. A notable difference is the age at which BC is typically diagnosed in men, with men generally developing the condition at an older age compared with women (9).
Until recently, MBC was considered similar to its post-menopausal female counterpart, primarily characterized by estrogen receptor (ER) positivity. However, advancements in research and clinical trials have highlighted significant disparities between the two. MBC typically presents at an older age as well as exhibits more frequent lymph node metastases and a higher prevalence of hormone-receptor positive tumors compared with female BC (10, 11). Furthermore, the risk factors for MBC differ slightly; unlike BC in women, MBC is more likely to occur in individuals with a BRCA2 instead of a BRCA1 mutation (12). In addition, a low androgen state is a recognized risk factor for MBC (13). This study aimed to compare the clinicopathologic characteristics, survival outcomes, and disease patterns of MBC patients with those of their female counterparts.
2 Methods
2.1 Korean breast cancer registry
The Korean Breast Cancer Registry (KBCR) is a prospectively maintained, multi-institutional registry of the Korean Breast Cancer Society. Breast surgeons in 102 teaching hospitals nationwide participate in this program. As of 2004, this registry was estimated to include 50% of all newly diagnosed patients with BC in Korea. Essential data include the patient’s identification number, sex, age, surgical method, and cancer stage based on the American Joint Committee on Cancer classification. Patients’ age at diagnosis, family history, menopausal status, and tumor characteristics such as subtype and histological grades are recorded. For follow-up, patients were divided into four categories: no evidence of disease (NED), with recurrence, alive with disease, and dead. The type of first recurrence (locoregional or distant metastasis) and causes of death have been further categorized.
2.2 Patients and study design
This study was supported by the grant “Elimination of Cancer Project Fund” from the Asan Cancer Institute of Asan Medical Center, Seoul (Institutional review board approval no. 2017-1341), and by the Korean Breast Cancer Society. In this population-based study, we used data from KBCR. The key data elements comprised the patient’s identification number, sex, age, surgical approach, and cancer stage, categorized according to the American Joint Committee on Cancer classification. Additionally, information regarding the patient’s age at the time of diagnosis, family medical history, menopausal status, and tumor characteristics, including subtype and histological grades, were documented. Patients with an unknown cancer stage, a prior cancer diagnosis, or lacking follow-up data were excluded from the study.
The initial diagnostic and follow-up assessments comprised a range of procedures, including mammography, breast ultrasound imaging, magnetic resonance imaging, chest radiography, blood sampling, and clinical examinations. The expression levels of estrogen (ER) and progesterone receptors (PR) were evaluated using the Allred score (14). The HER2 status was considered negative if the immunohistochemistry score was either 1+ or 2+, and HER2 amplification was confirmed as negative based on the results of fluorescence or silver in situ hybridization (15). The clinical and histopathologic staging adhered to the guidelines outlined in the seventh edition of the Cancer Staging Manual by the American Joint Committee on Cancer (16).
2.3 Statistical analysis
The characteristics of BC in both the female and male groups were compared using the chi-squared (χ²) test. The overall survival was analyzed using the Kaplan–Meier method, and the log-rank test was performed to compare various subgroups. Multivariate Cox proportional hazard analysis was performed to calculate the hazard ratios (HRs) with corresponding 95% confidence intervals (CIs) for survival. Variables with p-value ≤ 0.2 in the univariate analysis were included in multivariate analysis.
All statistical analyses were performed using IBM SPSS Statistics version 26.0 for Windows (IBM Co. in Armonk, NY, USA). P-values < 0.05 were considered statistically significant.
3 Results
3.1 Baseline characteristics
The data of 113,845 patients diagnosed with BC between January 1990 and August 2014 in Seoul, Korea, were analyzed. Among these cases, a total of 473 were MBC cases. Table 1 presents an overview of the baseline characteristics of the patients. In the MBC group, the median age at diagnosis was 60 years, while it was 48 years for females with BC. Approximately 93.6% of MBC patients exhibited hormone receptor-positive tumors. Among the 473 MBC patients with ER, PR, and HER2 status data, 16 (3.4%) had triple-negative BR. Most (41.2%) MBC patients were diagnosed at stage II, followed by diagnosis at stage I (40.2%). Moreover, 92.6% underwent total mastectomy, while 7.4% opted for breast-conserving surgery (BCS). In contrast, 50.3% of female patients selected BCS.
Regarding treatment, a total of 91 (25.9%) MBC patients received adjuvant radiation. Among those who underwent BCS, 42.9% received adjuvant radiation, while among those who underwent mastectomy, 14.3% received post-mastectomy radiation (data not shown). In the MBC group, 62.9% received chemotherapy, and 83.7% of hormone receptor-positive male patients were treated with endocrine therapy.
Table 2 shows the result of a comparative analysis between male and female patients with BC classified according to an age threshold of 40 years. In the group aged 40 years and younger, the proportion of female patients with BC who were ER-negative was higher than that in the group over the age of 40 years (38.2% vs. 33.4%, p <0.05); however, in MBC patients, no significant difference was observed, regardless of age (16.7% vs 9.5%, p = 0.351). Additionally, the histological (41.8% vs 34.4%) and nuclear grade (43.0% vs 37.5%) in female patients with BC aged 40 years and younger was higher; meanwhile, there was no difference in MBC patients in terms of histologic and nuclear grade.
3.2 Survival analysis
The median follow-up duration was 72 months. The 5-year overall survival rate for the entire group was 92.4%. Depending on the age at diagnosis, the MBC patients exhibited distinct patterns in 5-year overall survival compared with that in female patients with BC (Figure 1). In female patients with BC, the younger age group (≤40 years) showed a lower 5-year overall survival rate than that in the older age group (>40 years) (91.3% vs 92.7%, p <0.05). Conversely, in MBC patients, the younger age group (≤40 years) exhibited a better 5-year overall survival rate compared with the older age group (>40 years) (97.4% vs 86.4%, p <0.05).
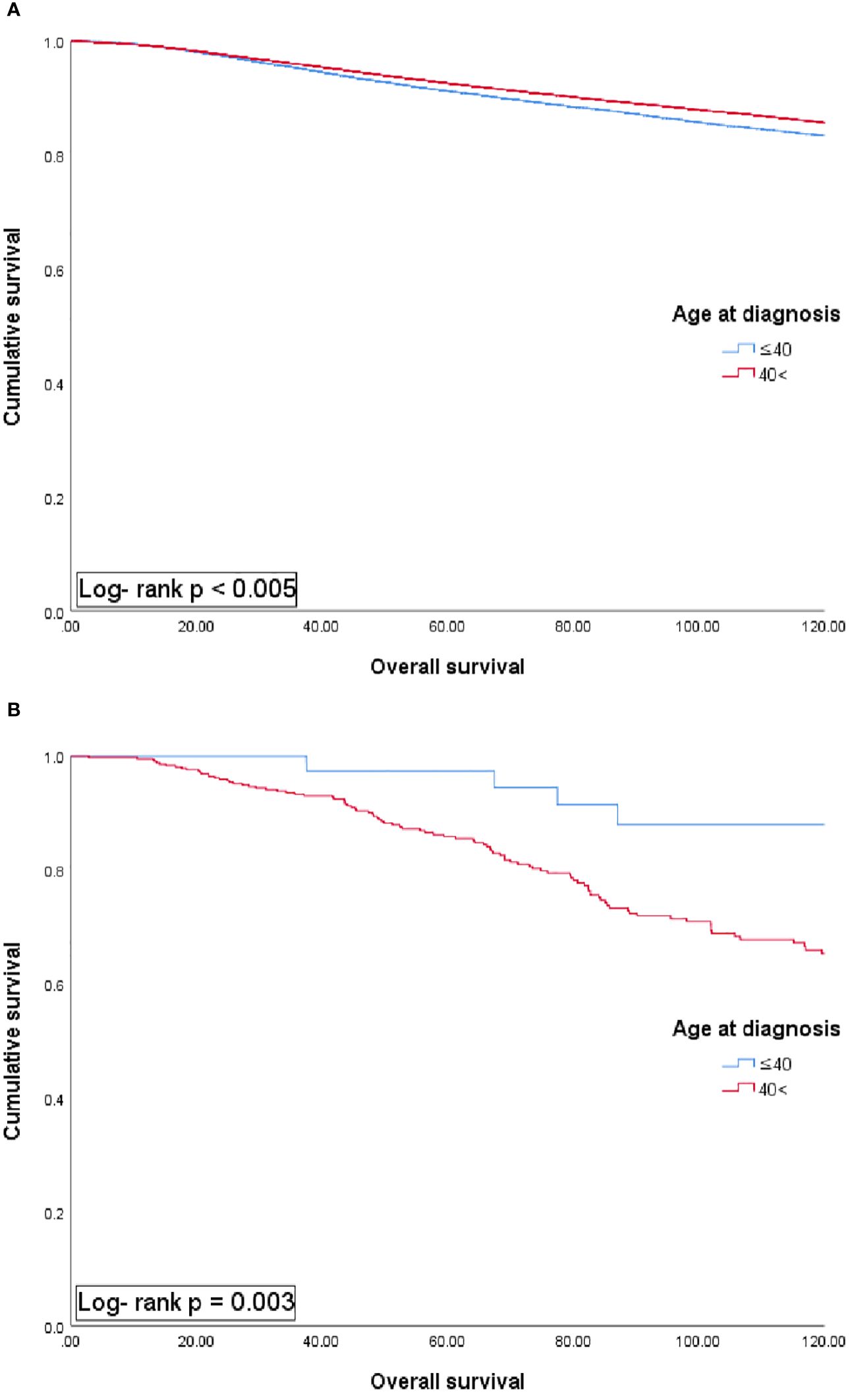
Figure 1 Univariate analysis of overall survival in female (A) and male patients with breast cancer (B) according to each age group.
The results of the comparative analysis between male and female patients with BC classified based on an age threshold of 40 years showed no significant survival difference between female and male patients in the group under 40 years. Meanwhile, the survival rate of MBC patients was worse in the group aged 40 years and older (Figure 2, p <0.05).
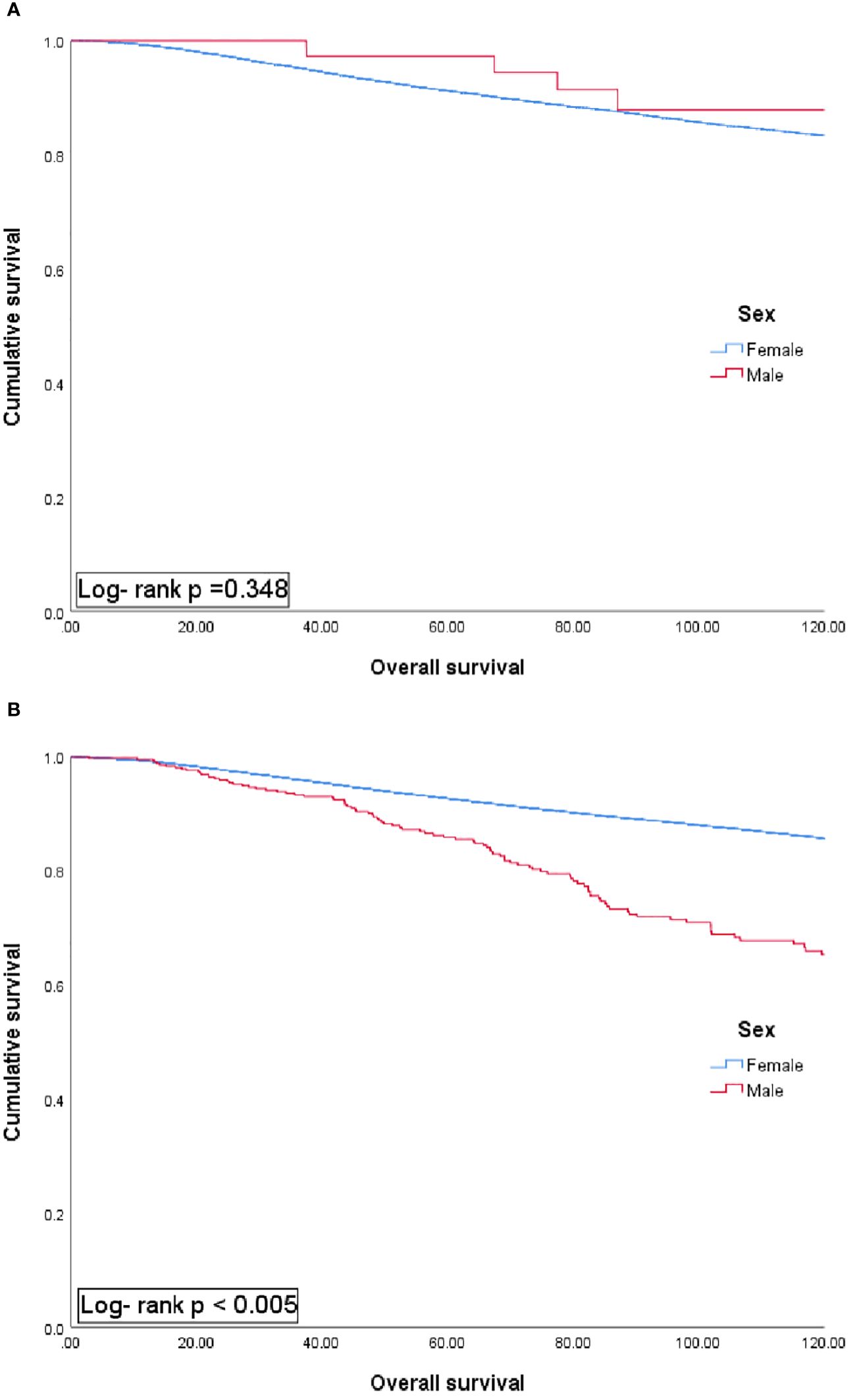
Figure 2 Univariate analysis of overall survival in patients aged 40 years or younger (A) and over 40 years (B) according to sex group.
Factors associated with overall survival in the univariate analysis are presented in Table 3. In the male group, the only factor associated with worse overall survival was the age over 40. In the female group, higher TNM stage, ER and PR negativity, and undergoing total mastectomy were associated with worse overall survival, however, the group over the age of 40 years showed better survival (HR 0.94; 95%CI 0.91-0.98; Table 3, p <0.05).
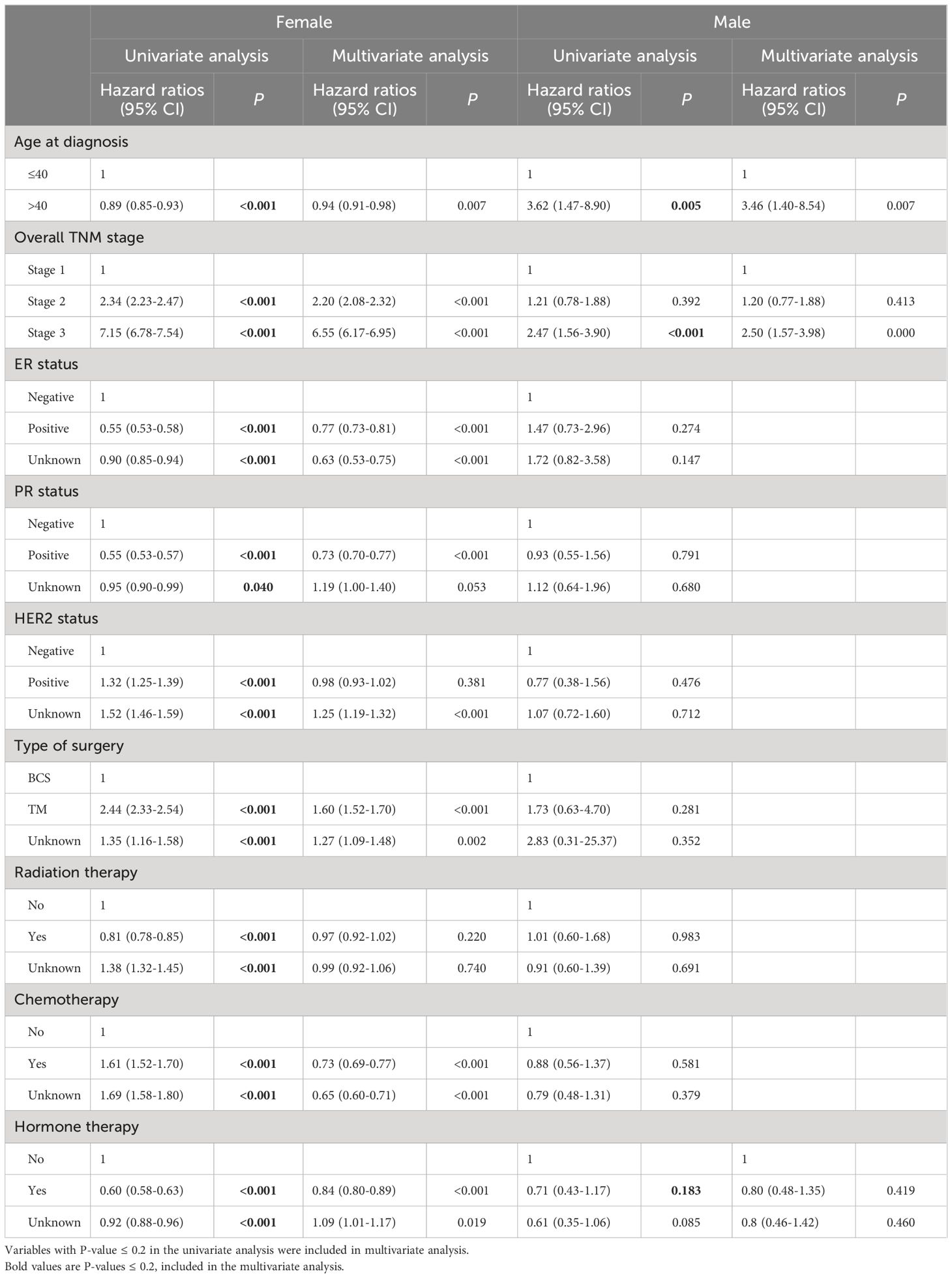
Table 3 Univariate and multivariate cox proportional hazard regression analysis for overall survival.
4 Discussion
In this study, we demonstrated that MBC patients exhibited a distinct pattern in 5-year-overall survival compared with female BC patients, classified according to age at diagnosis. In female BC patients, the younger age group (≤40 years) demonstrated worse 5-year overall survival compared with the older age group (>40 years) (91.3% vs 92.7%). Meanwhile, in MBC, the younger age group (≤40 years) demonstrated better 5-year overall survival compared with the older age group (>40 years) (97.4% vs 86.4%). These differences could be attributed to several factors. Men are often initially diagnosed with BC at a more advanced stage compared with women. Approximately 10% of MBCs are in situ carcinoma, with the remaining 90% being infiltrating ductal carcinoma (2, 5, 6, 8, 10). MBC tends to exhibit more advanced disease characteristics, including larger tumor size, lymph node involvement, and the presence of distant metastases at the time of diagnosis (5, 6, 8, 17–21). Moreover, MBCs typically express the ER and PR and are most commonly found as unilateral tumors (3, 10, 17, 18, 22–24). A common physical examination finding in MBC is nipple retraction or retroareolar mass detection, which may be the first clinical sign of the disease (6).
In general, women diagnosed with BC at a younger age harbor aggressive clinicopathologic features and have been recognized as a unique biologic entity. Colleonia et al. reported a higher percentage of ER- and PR-negative, vascular or lymphatic invasion, and pathologic grade 3 tumors in young patients compared with older women (25). Additionally, young age is an independent predictor of adverse outcomes (26–30). A retrospective study of more than 1,200 women diagnosed with early-stage BC evaluated the relationship between age, typical prognostic factors, treatment, and patient outcome. In multivariate analyses, younger age is a potent independent prognostic factor, including all potential patient, treatment, and pathology variables (28).
In contrast, MBC exhibits a comparatively mild nature, characterized by low-grade features and hormone receptor-positive expression. The age-specific incidence rate curve for MBC consistently increases with advancing age. The age-specific rates for BC in men demonstrate a parallel increase over time, aligning with a pattern indicative of hormone-independent epithelial carcinogenesis (31). Furthermore, certain high-risk conditions, such as Klinefelter’s syndrome, gynecomastia, obesity, and testicular or liver dysfunction (32–35), have been implicated in some MBCs due to excessive hormonal exposures. However, these conditions may only contribute to a small fraction of MBC cases. The mean ages at diagnosis for Klinefelter’s syndrome and gynecomastia are younger than the mean and/or median ages at diagnosis for MBC (32, 35).
More than two-thirds (90%) of MBC patients opted for a mastectomy, which aligns with findings from the previous studies on MBC (10, 36). Conversely, approximately two-thirds of female patients with BC opted for BCS, and one-third underwent mastectomy (37, 38). The difference in treatment options between men and women can be attributed to concerns in men that complete removal of all at-risk breast tissue with sufficient margins may be challenging because of smaller breast size. Additionally, sex-specific differences in cosmetic preferences might play a role. Moreover, MBCs are frequently located centrally and involve the nipple, which often necessitates the removal of the nipple-areolar complex, limiting the potential aesthetic benefits of BCS (39). Nevertheless, the approach to MBC treatment may have followed a pattern similar to BC in women, with more patients opting for mastectomy and potentially avoiding radiation despite the availability of BCS (37, 40).
The importance of using adjuvant endocrine therapy for hormone receptor-positive MBC is underscored by its association with improved overall survival, aligning with prior studies on men (41, 42). While there was an overall increase in the utilization of adjuvant endocrine therapy over the study period, nearly a third of men with ER+ breast cancer did not receive any endocrine therapy. This study could not assess long-term compliance or the duration of endocrine therapy use, both of which may pose an issue in men (43, 44). Further research is warranted to investigate the factors influencing the utilization of adjuvant endocrine therapy, which is the most effective form of systemic therapy for hormone receptor-positive breast cancer.
This study demonstrated that a positive ER status is a worse prognostic factor, which could be attributed to the limited number of ER-negative patients in this cohort. Additionally, the ER-negative cohort may have included patients with low ER expression due to changes in classification over time (45). The previously observed patterns in population-based incidence suggest a significant causal connection between early-onset hormonal events and ER-negative tumors in pre-menopausal women. Conversely, the importance of accumulated lifetime exposures appears to be more pronounced in the context of ER-positive tumors, post-menopausal women with breast cancer, and overall MBC (11).
Our study had certain limitations owing to its retrospective database reliance. When conducting this research based on the KBCS dataset, several limitations become evident, including the non-population-based nature of the dataset, leading to geographical and sociodemographic disparities in case coverage and lack of recurrence data. Similar datasets have reported issues related to the under-ascertainment of treatment-related variables. We did not consider the impact of changes in treatment practices over time when assessing factors associated with overall survival. Furthermore, the relatively small number of patients in certain sub-groups, such as those with hormone receptor-negative status, restricts the generalizability of our findings to the entire MBC population.
5 Conclusion
Although MBC is infrequent and frequently overlooked, there is an increasing recognition of the biological distinctions of BC between men and women. These disparities suggest that MBC should be regarded as a unique condition, separate from female breast cancer. Within this cohort, we have demonstrated distinct survival trends in MBC based on age groups, diverging from patterns observed in female BC. Our study advances our understanding of MBC prognosis and can potentially uncover unresolved issues that could guide future research on MBC.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by University of Ulsan College of Medicine, Asan Medical Center, Seoul, South Korea. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
SG: Data curation, Formal analysis, Investigation, Methodology, Software, Validation, Visualization, Writing – original draft, Writing – review & editing. JK: Resources, Writing – review & editing. IYC: Conceptualization, Resources, Supervision, Writing – review & editing. HJK: Conceptualization, Resources, Supervision, Writing – review & editing. BSK: Conceptualization, Resources, Supervision, Writing – review & editing. JWL: Conceptualization, Resources, Supervision, Writing – review & editing. BHS: Conceptualization, Resources, Supervision, Writing – review & editing. SHA: Conceptualization, Resources, Supervision, Writing – review & editing. SBL: Conceptualization, Data curation, Funding acquisition, Methodology, Project administration, Resources, Supervision, Validation, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was supported by the grant “Elimination of Cancer Project Fund” from Asan Cancer Institute of Asan Medical Center, Seoul (2017-1341).
Acknowledgments
This article was supported by the Korean Breast Cancer Society.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Weiss JR, Moysich KB, Swede H. Epidemiology of male breast cancer. Cancer Epidemiol Biomarkers Prev. (2005) 14:20–6. doi: 10.1158/1055-9965.20.14.1
2. Fentiman IS, Fourquet A, Hortobagyi GN. Male breast cancer. Lancet. (2006) 367:595–604. doi: 10.1016/S0140-6736(06)68226-3
3. Yoney A, Kucuk A, Unsal M. Male breast cancer: a retrospective analysis. Cancer/Radiothérapie. (2009) 13:103–7. doi: 10.1016/j.canrad.2008.11.011
4. Gennari R, Curigliano G, Jereczek-Fossa BA, Zurrida S, Renne G, Intra M, et al. Male breast cancer: a special therapeutic problem. Anything new? Int J Oncol. (2004) 24:663–70. doi: 10.3892/ijo
5. Korde LA, Zujewski JA, Kamin L, Giordano S, Domchek S, Anderson WF, et al. Multidisciplinary meeting on male breast cancer: summary and research recommendations. J Clin Oncol. (2010) 28:2114. doi: 10.1200/JCO.2009.25.5729
6. Cutuli B. Strategies in treating male breast cancer. Expert Opin Pharmacother. (2007) 8:193–202. doi: 10.1517/14656566.8.2.193
7. Burga AM, Fadare O, Lininger RA, Tavassoli FA. Invasive carcinomas of the male breast: a morphologic study of the distribution of histologic subtypes and metastatic patterns in 778 cases. Virchows Archiv. (2006) 449:507–12. doi: 10.1007/s00428-006-0305-3
8. Darkeh MHSE, Azavedo E. Male breast cancer clinical features, risk factors, and current diagnostic and therapeutic approaches. Int J Clin Med. (2014) 5:1068–86. doi: 10.4236/ijcm.2014.517138
9. Howlader N, Noone A, Krapcho M, Miller D, Bishop K, Kosary C. Cancer Statistics Review, 1975-2014-SEER Statistics, National Cancer Institute. SEER Cancer Statistics Review, 1975-2014. (2016).
10. Cardoso F, Bartlett J, Slaets L, Van Deurzen C, van Leeuwen-Stok E, Porter P, et al. Characterization of male breast cancer: results of the EORTC 10085/TBCRC/BIG/NABCG International Male Breast Cancer Program. Ann Oncol. (2018) 29:405–17. doi: 10.1093/annonc/mdx651
11. Anderson WF, Althuis MD, Brinton LA, Devesa SS. Is male breast cancer similar or different than female breast cancer? Breast Cancer Res Treat. (2004) 83:77–86. doi: 10.1023/B:BREA.0000010701.08825.2d
12. Pritzlaff M, Summerour P, McFarland R, Li S, Reineke P, Dolinsky JS, et al. Male breast cancer in a multi-gene panel testing cohort: insights and unexpected results. Breast Cancer Res Treat. (2017) 161:575–86. doi: 10.1007/s10549-016-4085-4
13. Evans DB, Crichlow RW. Carcinoma of the male breast and Klinefelter’s syndrome: is there an association? CA: Cancer J Clin. (1987) 37:246–51. doi: 10.3322/canjclin.37.4.246
14. Allred DC, Harvey JM, Berardo M, Clark GM. Prognostic and predictive factors in breast cancer by immunohistochemical analysis. Mod Pathol. (1998) 11:155–68.
15. Wolff AC, Hammond MEH, Hicks DG, Dowsett M, McShane LM, Allison KH, et al. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: american society of clinical oncology/college of american pathologists clinical practice guideline update. Arch Pathol Lab Med. (2014) 138:241–56. doi: 10.5858/arpa.2013-0953-SA
16. Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. (2010) 17:1471–4. doi: 10.1245/s10434-010-0985-4
17. Anderson WF, Jatoi I, Tse J, Rosenberg PS. Male breast cancer: a population-based comparison with female breast cancer. J Clin Oncol. (2010) 28:232. doi: 10.1200/JCO.2009.23.8162
18. Arslan ÜY, Öksüzoğlu B, Özdemir N, Aksoy S, Alkış N, Gök A, et al. Outcome of non-metastatic male breast cancer: 118 patients. Med Oncol. (2012) 29:554–60. doi: 10.1007/s12032-011-9978-9
19. Donegan WL, Redlich PN, Lang PJ, Gall MT. Carcinoma of the breast in males: a multiinstitutional survey. Cancer: Interdiscip Int J Am Cancer Soc. (1998) 83:498–509. doi: 10.1002/(ISSN)1097-0142
20. Giotta F, Acito L, Candeloro G, Del Medico P, Gadaleta-Caldarola G, Giordano G, et al. Eribulin in male patients with breast cancer: the first report of clinical outcomes. Oncologist. (2016) 21:1298–305. doi: 10.1634/theoncologist.2016-0022
21. Joshi MG, Lee AK, Loda M, Camus MG, Pedersen C, Heatley GJ, et al. Male breast carcinoma: an evaluation of prognostic factors contributing to a poorer outcome. Cancer: Interdiscip Int J Am Cancer Soc. (1996) 77:490–8. doi: 10.1002/(SICI)1097-0142(19960201)77:3<490::AID-CNCR10>3.0.CO;2-#
22. Giordano SH, Cohen DS, Buzdar AU, Perkins G, Hortobagyi GN. Breast carcinoma in men: a population-based study. Cancer: Interdiscip Int J Am Cancer Soc. (2004) 101:51–7. doi: 10.1002/cncr.20312
23. Ruddy KJ, Winer E. Male breast cancer: risk factors, biology, diagnosis, treatment, and survivorship. Ann Oncol. (2013) 24:1434–43. doi: 10.1093/annonc/mdt025
24. Everson RB, Lippman ME, Thompson EB, McGuire WL, Wittliff JL, De Sombre ER, et al. Clinical correlations of steroid receptors and male breast cancer. Cancer Res. (1980) 40:991–7.
25. Colleoni M, Rotmensz N, Robertson C, Orlando L, Viale G, Renne G, et al. Very young women (< 35 years) with operable breast cancer: features of disease at presentation. Ann Oncol. (2002) 13:273–9. doi: 10.1093/annonc/mdf039
26. De La Rochefordiere A, Campana F, Fenton J, Vilcoq J, Fourquet A, Asselain B, et al. Age as prognostic factor in premenopausal breast carcinoma. Lancet. (1993) 341:1039–43. doi: 10.1016/0140-6736(93)92407-K
27. Albain KS, Allred DC, Clark GM. Breast cancer outcome and predictors of outcome: are there age differentials? J Natl Cancer Inst Monogr. (1994) 16:35–42.
28. Nixon AJ, Neuberg D, Hayes DF, Gelman R, Connolly JL, Schnitt S, et al. Relationship of patient age to pathologic features of the tumor and prognosis for patients with stage I or II breast cancer. J Clin Oncol. (1994) 12:888–94. doi: 10.1200/JCO.1994.12.5.888
29. Anders CK, Hsu DS, Broadwater G, Acharya CR, Foekens JA, Zhang Y, et al. Young age at diagnosis correlates with worse prognosis and defines a subset of breast cancers with shared patterns of gene expression. J Clin Oncol. (2008) 26:3324–30. doi: 10.1200/JCO.2007.14.2471
30. Anders CK, Johnson R, Litton J, Phillips M, Bleyer A. editors. Breast cancer before age 40 years. Semin Oncol. (2009) 36(3):237–249. doi: 10.1053/j.seminoncol.2009.03.001
31. Pike MC, Krailo M, Henderson B, Casagrande J, Hoel D. ‘Hormonal’risk factors,’breast tissue age’and the age-incidence of breast cancer. Nature. (1983) 303:767–70. doi: 10.1038/303767a0
32. Sasco AJ, Lowenfels AB, Jong PPD. epidemiology of male breast cancer. A meta-analysis of published case-control studies and discussion of selected aetiological factors. Int J Cancer. (1993) 53:538–49. doi: 10.1002/ijc.2910530403
33. Keller AZ. Demographic, clinical and survivorship characteristics of males with primary cancer of the breast. Am J Epidemiol. (1967) 85:183–99. doi: 10.1093/oxfordjournals.aje.a120682
34. Hsing AW, McLaughlin JK, Cocco P, Co Chien HT, Fraumeni JF. Risk factors for male breast cancer (United States). Cancer Causes Control. (1998) 9:269–75. doi: 10.1023/A:1008869003012
36. Fields EC, DeWitt P, Fisher CM, Rabinovitch R. Management of male breast cancer in the United States: a surveillance, epidemiology and end results analysis. Int J Radiat Oncol Biol Phys. (2013) 87:747–52. doi: 10.1016/j.ijrobp.2013.07.016
37. Mahmood U, Hanlon AL, Koshy M, Buras R, Chumsri S, Tkaczuk KH, et al. Increasing national mastectomy rates for the treatment of early stage breast cancer. Ann Surg Oncol. (2013) 20:1436–43. doi: 10.1245/s10434-012-2732-5
38. McGuire KP, Santillan AA, Kaur P, Meade T, Parbhoo J, Mathias M, et al. Are mastectomies on the rise? A 13-year trend analysis of the selection of mastectomy versus breast conservation therapy in 5865 patients. Ann Surg Oncol. (2009) 16:2682–90. doi: 10.1245/s10434-009-0635-x
39. Leon-Ferre RA, Giridhar KV, Hieken TJ, Mutter RW, Couch FJ, Jimenez RE, et al. A contemporary review of male breast cancer: current evidence and unanswered questions. Cancer Metastasis Rev. (2018) 37:599–614. doi: 10.1007/s10555-018-9761-x
40. Kummerow KL, Du L, Penson DF, Shyr Y, Hooks MA. Nationwide trends in mastectomy for early-stage breast cancer. JAMA Surg. (2015) 150:9–16. doi: 10.1001/jamasurg.2014.2895
41. Giordano SH, Perkins GH, Broglio K, Garcia SG, Middleton LP, Buzdar AU, et al. Adjuvant systemic therapy for male breast carcinoma. Cancer: Interdiscip Int J Am Cancer Soc. (2005) 104:2359–64. doi: 10.1002/cncr.21526
42. Ribeiro G, Swindell R. Adjuvant tamoxifen for male breast cancer (MBC). Br J Cancer. (1992) 65:252–4. doi: 10.1038/bjc.1992.50
43. Oke O, Niu J, Chavez-MacGregor M, Zhao H, Giordano SH. Adjuvant tamoxifen adherence in men with early stage breast cancer. Am Soc Clin Oncol. (2018) 128(1):59–64. doi: 10.1200/JCO.2018.36.15_suppl.550
44. Xu S, Yang Y, Tao W, Song Y, Chen Y, Ren Y, et al. Tamoxifen adherence and its relationship to mortality in 116 men with breast cancer. Breast Cancer Res Treat. (2012) 136:495–502. doi: 10.1007/s10549-012-2286-z
45. Hammond MEH, Hayes DF, Dowsett M, Allred DC, Hagerty KL, Badve S, et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer (unabridged version). Arch Pathol Lab Med. (2010) 134:e48–72. doi: 10.5858/134.7.e48
Keywords: breast cancer, male breast cancer, prognosis, overall survival, survival pattern
Citation: Gwark S, Kim J, Chung IY, Kim HJ, Ko BS, Lee JW, Son BH, Ahn SH and Lee SB (2024) Survival pattern in male breast cancer: distinct from female breast cancer. Front. Oncol. 14:1392592. doi: 10.3389/fonc.2024.1392592
Received: 27 February 2024; Accepted: 11 June 2024;
Published: 28 June 2024.
Edited by:
Valentina Silvestri, Sapienza University of Rome, ItalyReviewed by:
Hasan Cagri Yildirim, Niğde Ömer Halisdemir University Training and Research Hospital, TürkiyeSinziana Ionescu, Carol Davila University of Medicine and Pharmacy, Romania
Copyright © 2024 Gwark, Kim, Chung, Kim, Ko, Lee, Son, Ahn and Lee. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sae Byul Lee, bmV3c3RhcjE1M0BoYW5tYWlsLm5ldA==
 Sungchan Gwark
Sungchan Gwark Jisun Kim
Jisun Kim Il Yong Chung
Il Yong Chung Hee Jeong Kim
Hee Jeong Kim Beom Seok Ko2
Beom Seok Ko2 Jong Won Lee
Jong Won Lee Sei Hyun Ahn
Sei Hyun Ahn