
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Oncol. , 02 July 2024
Sec. Head and Neck Cancer
Volume 14 - 2024 | https://doi.org/10.3389/fonc.2024.1392245
Objective: This study aims to assess the current research status, focus areas, and developmental trends in nasopharyngeal carcinoma (NPC) through a bibliometric analysis.
Methods: Articles focusing on NPC published from 2000 to 2023 were retrieved from the Web of Science database. VOSviewer and CiteSpace were used for bibliometric and visual analysis.
Results: A total of 14516 related publications were retrieved. There has been a steady increase in the number of NPC-related publications from 2000 to 2023. China was the dominant country in this field with 8948 papers (61.64%), followed by the USA (2234, 15.39%). Sun Yat-sen University was the most influential institution, while Ma J was the most prolific author. Furthermore, Head And Neck-journal For The Sciences And Specialties Of The Head And Neck was the most prolific journal. International Journal of Radiation Oncology Biology Physics had the highest total citation counts. "Introduction chemotherapy", "Concurrent chemotherapy", "Epithelial-mesenchymal transition", "Cancer stem cells", "MicroRNAs", "LncRNA", "Exosomes", and "Biomarker" were the most common keywords. The reference "Chen YP, 2019, Lancet" had the highest citations and strong outbreak value.
Conclusion: The past two decades have witnessed a significant increase in research on NPC. The optimization of treatment mode is the most widely studied aspect at present. The mechanism of occurrence and development and the most favorable diagnostic and therapeutic targets are the research hotspots in the future.
Nasopharyngeal carcinoma (NPC) is a malignant tumor originating from the mucosal epithelium of the nasopharynx that exhibits uniqueness in epidemiology, histology, and virology. NPC has an obvious geographical distribution, and more than 77% of the incidence rate of NPC occurs in East Asia and Southeast Asia, especially in southern China (1). Epstein-Barr virus (EBV) is generally considered a significant cause of NPC, and lifestyle habits such as eating preserved food, smoking, and drinking alcohol are associated with an increasing risk of NPC (2). Nonetheless, the exact pathogenesis of NPC still needs to be elucidated. NPC is mostly a poorly differentiated squamous cell carcinoma that occurs in the fossa of Rosenmüller (3). Compared to the patients with late-stage NPC, the overall 5-year survival rate of patients with early-stage exceeds 90% (4). However, due to the hidden location of NPC, most patients have already reached extremely poor staging when diagnosed. Even if patients receive active treatment, lesions in the local nasopharyngeal and cervical lymph nodes of cancer are still more likely to remain or recur, which greatly affects the overall survival (OS) rate of patients (5). Therefore, NPC causes serious health burden.
NPC is a topic of continuous concern. Many studies related to etiology, histopathology, epidemiology, and treatment are published annually. However, the number of studies has grown rapidly, and cannot keep up with the latest findings on all issues. Bibliometric analysis is an emerging tool that quickly explores the structure and trends of a field through specific computational methods and visual analysis. In recent years, this method has been widely applied in the analysis of massive scientific research data (6). Bibliometric analysis of medical paper data is necessary and valuable, as it can objectively reflect the current development status, research hotspots, and future trends (7). Although there have been bibliometric studies on NPC before, we cannot identify research hotspots due to temporal updates and methodological variations (8–10). Therefore, this study evaluates the global publications in the field of NPC from 2000 to 2023 in order to provide new insights into the research trends of NPC. Specifically, this study mainly used VOSviewer and CiteSpace software to capture the growth trend of NPC publications, measure the contributions of countries, institutions, journals and authors, and determine the main keywords and important literatures, so as to reveal the research hotspots and development trends in this field.
Web of Science is the primary research platform for hard science, social sciences, arts, and humanities information, as well as the independent global citation database of the world’s most trusted publishers (11). To improve data representativeness and accessibility, we retrieved the Web of Science core collection (WoSCC).
The following search terms were employed to retrieve literature from WoSCC: TS = (“nasopharyngeal carcinoma”). The publication date was from 2000-01-01 to 2023-12-31. The language type is selected as English, and the article type is selected as article and review. The search results were exported with a “Plain Text file” and the record content was chosen “Full Record and Cited References” and stored in “download_*.txt” format. Two researchers independently completed literature screening, data extraction, and analysis to ensure the reliability of the results. The retrieval framework is shown in Figure 1.
Bibliometric analysis was conducted using four tools, namely VOSviewer (version 1.6.19), CiteSpace (version 6.2. R6), R (version 4.2.2), and GraphPad Prism (version 9.5.1).
VOSviewer is a distance-based bibliometric tool that has advantages in visualizing bibliometric networks (12). VOSviewer was used to analyze the countries, institutions, and authors with the most production/collaboration, as well as the journals with the most citations and the most frequently appearing keywords, and generate a visual knowledge graph. Link strength (LS) represents the strength of cooperation between nodes, while total link strength (TLS) reflects the overall level of cooperation.
CiteSpace is a Java application developed by Professor Chen Chaomei for bibliometric analysis, which focuses on the dynamic visualization of bibliometrics and reflects the evolution of bibliometric networks over time (13, 14). In this study, CiteSpace was used to identify the highly cited keywords and references with the strongest citation burst over a certain period.
Bibliometrix is an R package that includes the functionality of scientific quantitative research (15). This study used it to summarize the publication and citation count of bibliometric analysis and visualize a Three-Fields Plot.
GraphPad Prism was used to generate a line graph of the number of publications, cumulative number of publications, citation count, and H-index.
A total of 14516 publications related to NPC were obtained from the WoSCC. The distribution of annual publication volume from 2000 to 2023 is shown in Figure 2A. The number of annual publications showed an overall increasing trend, indicating that attention to the field of NPC increased. The number of published articles peaked in 2020 (1034, 7.12%). The cumulative number of publications increased steadily from 2000 to 2023 (Figure 2B). From 2009 to 2019, citations were relatively high (Figure 2C). The annual H-index from 2000 to 2018 was above 55, while the H-index from 2019 to 2023 declined (Figure 2D).
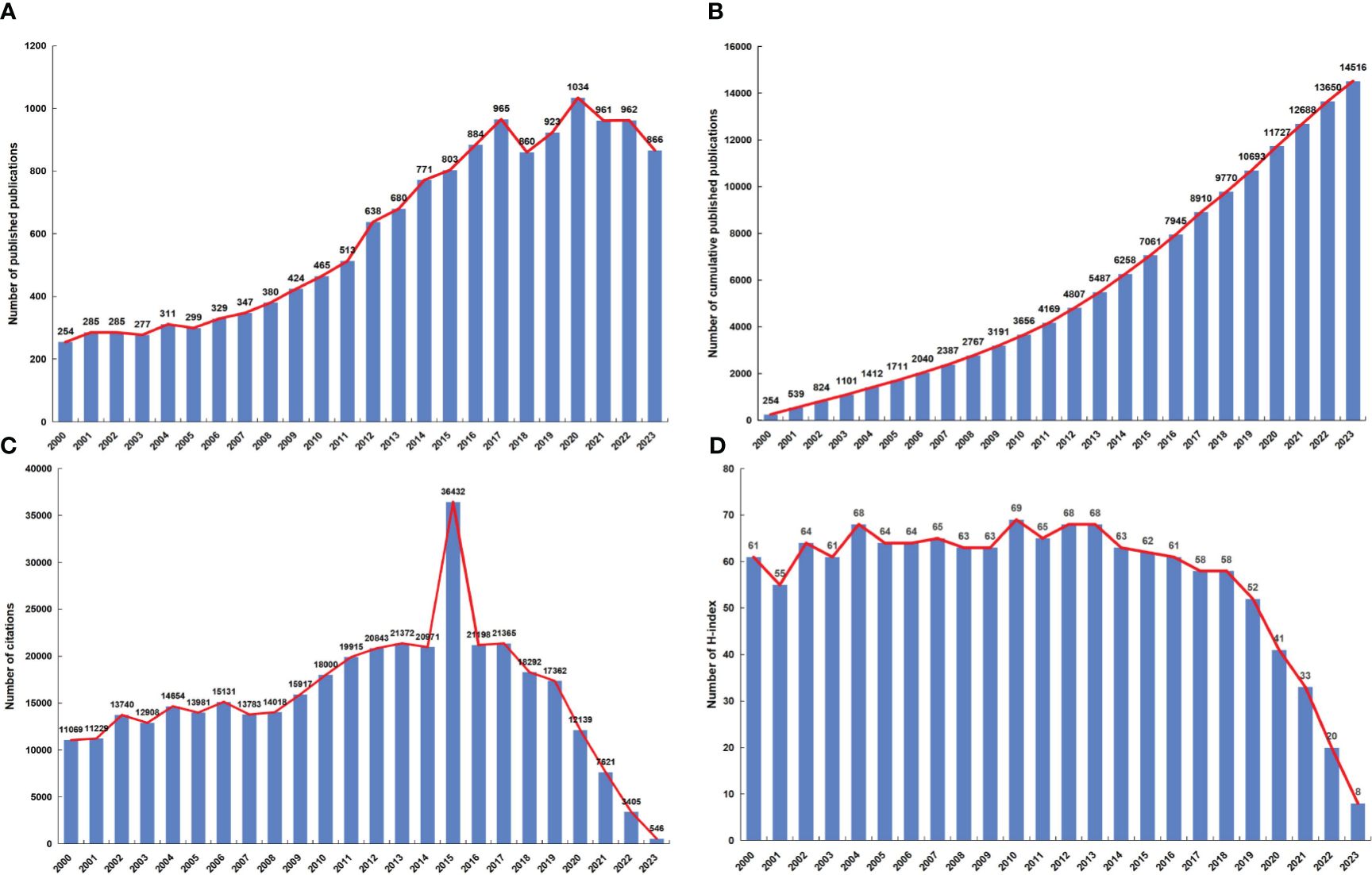
Figure 2 (A) The global annual number of publications related to NPC research. (B) The global annual number of cumulative publications related to NPC research. (C) The global annual number of citations of the publications related to NPC research. (D) The global annual H-index values of the publications related to NPC research.
113 Counties/regions have contributed to research related to NPC. Figure 3A shows 44 countries/regions with more than 15 submissions and 588 collaboration instances. China had the strongest international cooperation network (TLS=1890) and the closest cooperation with the United States (LS=808).
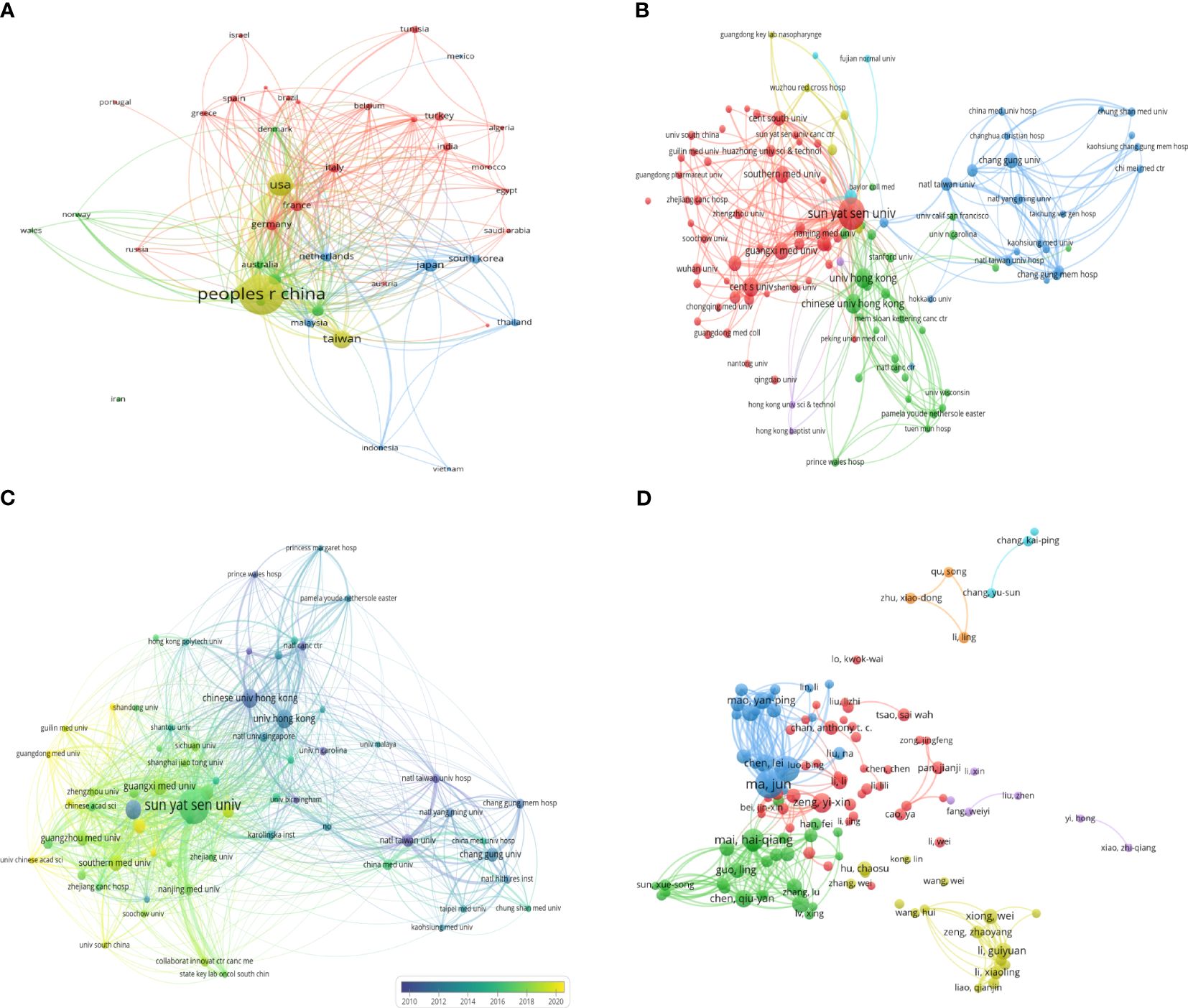
Figure 3 The coauthorship network map of countries/regions (A), and institutions (B). (C) The coauthorship overlay map of institutions. (D) The coauthorship network map of authors.
Next, we analyzed the number of publications and total citations for the 10 most productive countries/regions. As shown in Table 1, China had the most publications (8948, 61.64%), followed by the USA (2234, 15.39%) and the Taiwan(1191, 8.20%). Each of them published fewer than 1000 articles. In addition, China had the highest number of citations.
A total of 7478 institutions contributed to NPC research. Figure 3B shows 109 institutions with more than 50 documents and 1928 instances. Sun Yat-sen University had the largest cooperative network (TLS=2036). The top 10 most productive institutions are shown in Table 2. Sun Yat-sen University was the most productive institution (2116, 14.58%), followed by Central South University (883, 6.08%), and the University of Hong Kong (600, 4.13%). Sun Yat-sen University also had the highest number of total citations (58162).
In the overlay network analyzed by the co-authors, the size of the circle represents the number of publications, and the color represents the average start year of each institution’s publications in a particular field of study. As shown in Figure 3C, the study identified 66 institutions with at least 75 publications. The Prince of Wales Hospital, the University of Hong Kong, and the Chinese University of Hong Kong started earlier. In contrast, Sun Yat-sen University, Guangzhou Medical University, and Southern Medical University recently conducted research in this area.
A total of 53787 authors contributed to publications related to NPC. Table 3 shows the top 10 authors ranked by the number of published articles according to co-author analysis. Professor Ma J had the highest efficiency (305, 2.10%), followed by Sun Y (259, 1.78%) and Mai HQ (191, 1.32%), all from Sun Yat-sen University. In addition, Ma J was the author with the highest total citations and H-index. Figure 3D shows a collaborative map among 118 researchers, with a minimum of 40 papers. Ma J had the highest number of collaborative relationships with other authors (TLS=1869). Figure 4A visually illustrates the inflow and outflow of ten authors, affiliated institutions, and countries who have contributed to NPC research from 2000 to 2023.
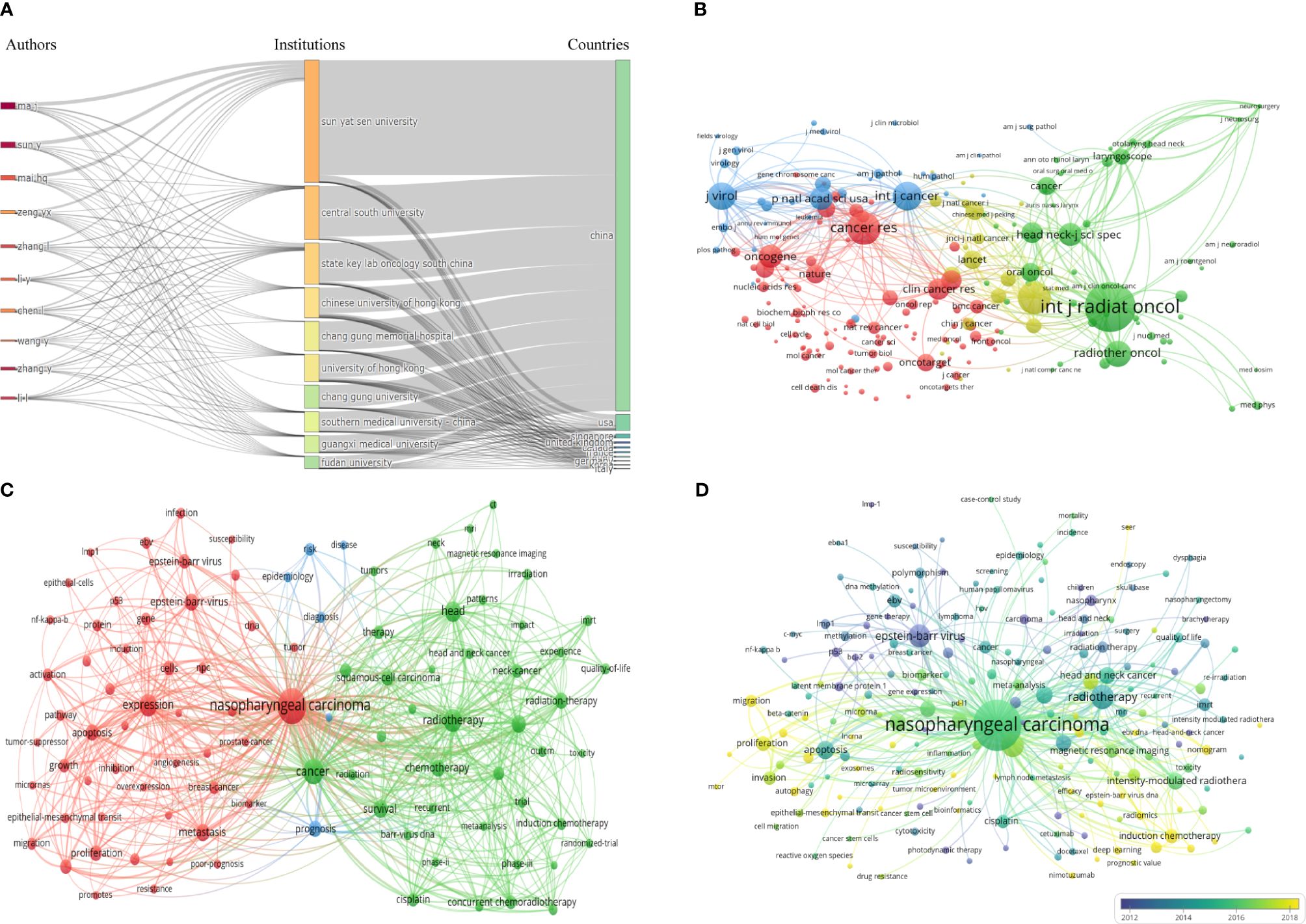
Figure 4 (A) Three Fields Plot. (B) The co-citation network map of journals. (C) Keyword co-occurrence network diagram of NPC. (D) Keyword co-occurrence average years network diagram of NPC.
Publications on NPC research were published in 1709 journals. Table 4 shows the top 10 most widely published journals and their latest issues of JournaI Citation Reports (JCR), Impact Factor (IF), and H-index. Head And Neck-journal For The Sciences And Specialties Of The Head And Neck had the highest number of articles (publication counts=373), followed by International Journal of Radiation Oncology Biology Physics (publication counts=340) and PLoS One (publication counts=292). International Journal of Radiation Oncology Biology Physics had the highest total citation counts (23825) and average citation counts (70.07). In terms of JCR, most journals were classified as Q1 and Q2 (80%). In terms of journal direction, these 10 journals cover head and neck diseases, oncology, tumor radiotherapy, etc. IF is an important parameter for evaluating the value of journals. International Journal of Radiation Oncology Biology Physics had the highest IF (7), followed by International Journal of Cancer (6.4) and Radiotherapy and Oncology (5.7). In addition, PLoS One had the highest H-index (268), followed by International Journal of Radiation Oncology Biology Physics (228) and International Journal of Cancer (212). The journal co-citation network map is shown in Figure 4B. The top 3 co-cited journals were International Journal Of Radiation Oncology Biology Physics (26354 citations), Journal Of Clinical Oncology (13104 citations), and Cancer Research (12992 citations).
Citation analysis is a valuable way to evaluate the most cited articles, and the number of citations can reflect the impact of an article in a particular field of research. Figure 5 and Supplementary Table 1 list the most cited publications on NPC each year from 2000 to 2023. Among the 24 articles, 8 were published in Journal of Clinical Oncology, 4 in the New England Journal of Medicine, 4 in International Journal of Radiation Oncology Biology Physics, 2 in Radiation and Oncology, and 2 in Lancet Oncology. Others were published in JAMA Oncology, Cancer Research, Jnci-journal of the National Cancer Institute, and Seminars in Cancer Biology. Furthermore, 24 articles included 11 studies on chemotherapy, 5 on EBV, 4 on intensity-modulated radiotherapy (IMRT), 2 on immunotherapy, 1 on epidemiology, and 1 on retrospective analysis.
This study also analyzes the most frequently cited references. Supplementary Table 2 lists the 10 most cited articles, all of which were cited more than 400 times. Specifically, the article titled “Nasopharyngeal Carcinoma” published in Lancet in 2019 had the highest citation count (1092). Other articles included 2 reviews on NPC, 2 reviews on NPC epidemiology, 3 phase III randomized intergroup studies on chemotherapy in different treatment stages of patients with NPC, 1 clinical study on IMRT, and 1 global cancer data analysis.
This study counted 29447 keywords, and Figure 4C shows the network visualization of some keywords (with a minimum occurrence of 50, including a total of 86 keywords). The top 10 most frequently used keywords are “nasopharyngeal cancer”, “cancer”, “radiation”, “expression”, “head”, “survival”, “intensive modulated radiation”, “chemotherapy”, “metastasis”, and “Epstein-Barr virus”.
Figure 4D shows the overlay visualization of author keywords. The term marked in purple indicates an average publication year of 2012 or earlier, while the term marked in bright yellow appears after 2018. “Epstein-Barr virus”, “p53”, and “late member protein 1” were the main topics in the early stages. The keywords “introduction chemotherapy”, “concurrent chemotherapy”, “epithelial-mesenchymal transition”, “cancer stem cells”, “lncRNA”, “microRNAs”, “exosomes”, and “biomarker” appeared relatively late during the research period.
To obtain the research hotspots in the field of NPC in recent years, this study conducted cluster analysis on keywords within the past 5 years (2019-2023) and finally formed 5 representative clusters (Figure 6A). The clustering map shows that the modularity value is 0.475 (> 0.3), indicating a significant clustering structure; the silhouette value is 0.8049 (> 0.5), indicating a reasonable clustering. Among the 5 cluster labels, the largest cluster size is #0 (Promotion, Size=100), followed by #1 (Introduction chemotherapy, Size=78), #2 (Epstein-Barr virus, Size=43), #3 (Head and neck cancer, Size=40), and #4 (Magnetic resonance imaging, Size=30). Based on clustering vocabulary analysis, the research focus of NPC mainly includes molecular mechanisms, treatment strategies, EBV, and diagnostic methods.
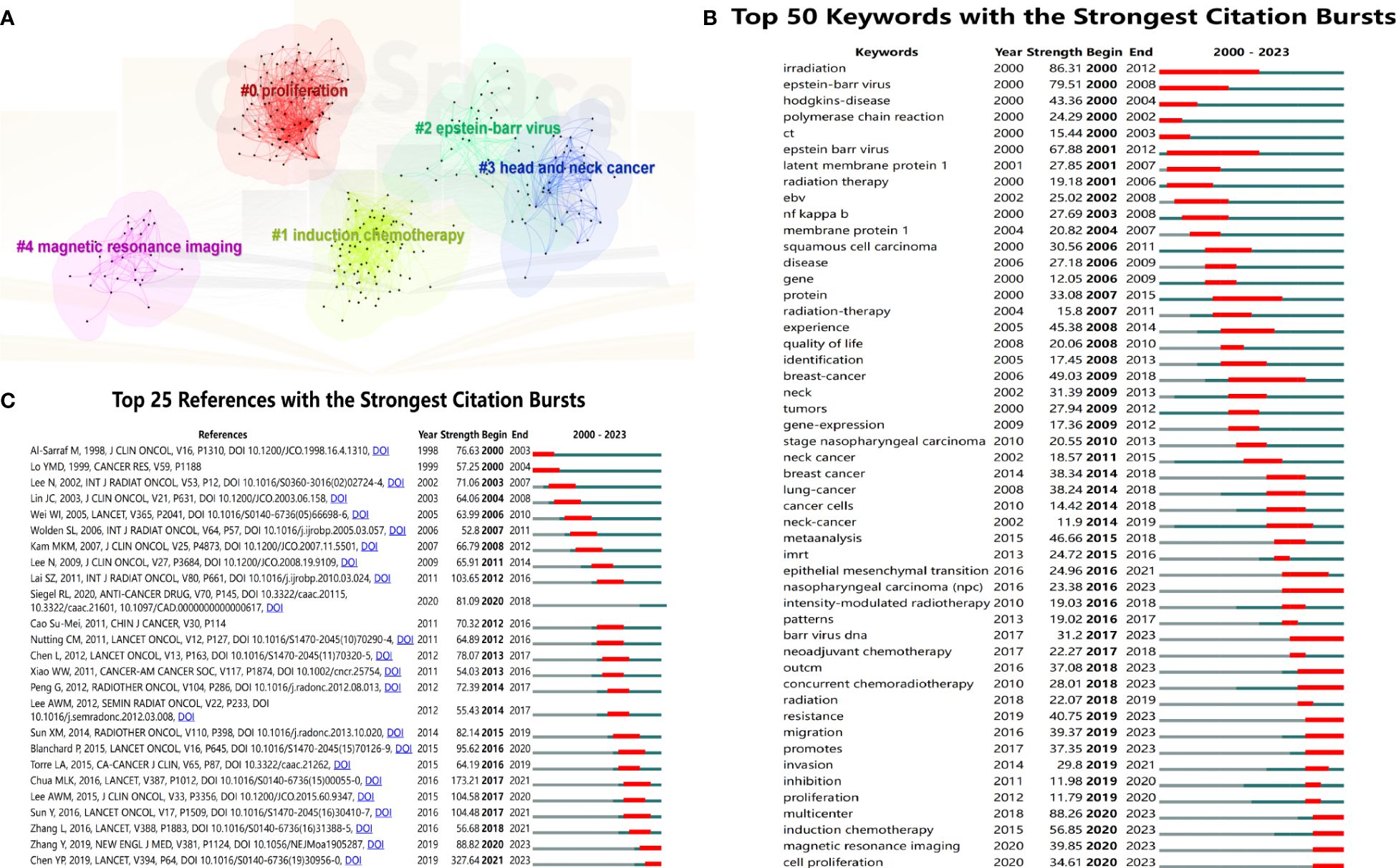
Figure 6 (A) Five representative clusters of keyword clustering from 2019 to 2023. The top 50 keywords (B) and 25 references (C) with the strongest citation bursts.
Figure 6B shows the top 50 keywords with the strongest citation bursts, and the red line represents the duration of the burst. The keywords “radiation” (2000-2012) and “Epstein-Barr virus” (2001-2012) received considerable attention in the past period. The vocabulary of “concurrent chemotherapy” (2018-2023), “resistance” (2019-2023), “migration” (2019-2023), “promotes” (2019-2023), “multicenter” (2020-2023), “introduction chemotherapy” (2020-2023), “magnetic response imaging” (2020-2023), and “cell promotion” (2020-2023) received high attention in recent years. They may still be research hotspots in the future.
Figure 6C shows the top 25 references with the highest citation burst. The article “Nasopharyngeal Carcinoma” published by Chen YP et al. in Lancet had a strong outbreak value and is still ongoing. Meanwhile, the citation explosion of “Gemcitabine and Cisplatin Induction Chemotherapy in Nasopharyngeal Carcinoma” published by Zhang Y et al. in the New England Journal of Medicine is also ongoing. This article and Sun Ying et al.’s article are both prospective randomized controlled studies targeting locally advanced NPC. The results showed that the use of a GP regimen (gemcitabine plus cisplatin) and an improved TPF regimen (docetaxel, cisplatin, 5-FU) for induction chemotherapy significantly improved OS of patients.
To our knowledge, this is the first study to conduct a comprehensive bibliometric analysis of publications related to NPC from 2000 to 2023. Our research findings indicate that the number of annual publications in this field is on the rise. The highest citation count was in 2015, and the highest H-index was in 2010. The decrease in citation frequency and H-index after 2018 may be attributed to the approaching data collection time.
In this bibliometric analysis, we analyzed the most influential countries, institutions, authors, and journals in the field of NPC. China was the major contributor to this research field, with the highest number of publications, citations, and international cooperation. The top 10 institutions and 10 authors with the highest productivity are all from China. Sun Yat-sen University was the most influential institution, while Ma J was the most prolific author. NPC is common in some ethnic groups, especially in southern China, Hong Kong, and Southeast Asia (16). Therefore, as a high-risk area for NPC, China ranks first in the world in terms of scholars and institutions conducting research in this field. The above results are consistent with the results of two previous studies (8, 10). It is worth noting that in another study with a large statistical time span (1970-2018), the most influential institutions and authors were the Prince of Wales Hospital and Chan ATC (9). But our research results show that the Prince of Wales Hospital and Chan ATC are the early founders of NPC (Figure 3C; Supplementary Figure 1). In addition, our results show that Head And Neck Journal For The Sciences And Specialties Of The Head And Neck, International Journal of Radiation Oncology Biology Physics, and PLoS One were considered the most influential journals in this field. Head And Neck Journal For The Sciences And Specialties Of The Head And Neck had the highest number of publications; International Journal of Radiation Oncology Biology Physics had the highest citation counts and journal IF; PLoS One had the highest H-index. Although these results are different from previous research results, in general, International Journal of Radiation Oncology Biology Physics has always been an influential journal in the NPC field (8–10). Because NPC is sensitive to radiotherapy, relevant professional journal continues to attract researchers’ attention.
Citation, co-citation, and keyword analysis offer insights into the hotspots and developmental trends in NPC-related research. Compared with the previous NPC bibliometric research findings, this study proposes some new conclusions (8–10). Firstly, the citation and co-citation results show that the development of effective treatment schemes (radiotherapy, concurrent chemoradiotherapy, induction chemotherapy, and adjuvant chemotherapy) has always been a research hotspot, which is similar to the previous research results. But currently, researchers are working to reduce the area of radiotherapy and identify the most suitable candidates for chemotherapy, aiming to make treatment plans more targeted. At the same time, targeted therapy and immunotherapy are increasingly gaining attention from researchers. Secondly, the keyword analysis results show that in recent years, researchers have focused on “epithelial-mesenchymal transition”, “cancer stem cells”, “lncRNA”, “microRNAs”, and “exosomes”, aiming to identify key diagnostic and therapeutic biomarkers for NPC. But in previous studies, researchers have paid more attention to EBV, gene expression, RNA guided endonuclease. Thirdly, keyword burst analysis and citation burst analysis mentioned “EBV” and “MRI”, indicating that they are of great significance for the diagnosis and therapeutic evaluation of NPC. Fourthly, combined with the latest literature, we find that the global concept of NPC has changed, but previous studies have not mentioned this problem.
Due to NPC cells being sensitive to radiotherapy, 2-dimensional radiotherapy (2DRT) was used earlier to treat NPC. However, the anticancer efficiency of 2DRT is limited and the side effects are obvious, resulting in many patients refusing treatment. IMRT is an advanced external radiotherapy technology, which can precisely adjust the radiation dose distribution so that the tumor receives a higher dose and the normal tissue receives a lower dose. Lee et al. showed that IMRT provides excellent tumor target coverage and allows for high-dose delivery to the target while significantly preserving salivary glands and other key normal tissues nearby (17). Kam, M. K et al. showed that IMRT significantly reduces the incidence of severe late-onset dry mouth (18). Lai SZ et al. conducted a retrospective analysis of data from 1276 patients with non-metastatic NPC confirmed by biopsy, and the results showed that IMRT has better local tumor control rates than traditional 2DRT, especially in patients with early T-stage (19). Peng, G et al. found that IMRT improves local recurrence-free survival, especially in patients with advanced NPC (20). Research by Sun, X et al. showed that the survival outcomes of IMRT treatment for NPC are excellent, but distant metastasis is a concern (21). However, the potential harm of IMRT to oral muscles, thyroid, and cervical tissues cannot be overlooked. Recently, Huang, C. L et al. reported that Upper-Neck Irradiation (UNI), a novel radiotherapy approach tailored for patients with stage N0-1 NPC, significantly protects cervical organs and tissues while maintaining therapeutic efficacy, in contrast to Whole-Neck Irradiation (WNI) (22). Mao, Y. P et al. found that for newly diagnosed adult patients (18-65 years old) with non-keratinizing, non-distant metastatic, non-medial retropharyngeal lymph node (MRLN) metastasis, preserving the MRLN reduces the incidence of dysphagia without altering outcomes when compared to standard radiotherapy (23). Consequently, the precision of radiotherapy for NPC is continuously improving.
Chemotherapy is also an important method for treating cancer, and the 0099 study laid the foundation for the combination of radiotherapy and chemotherapy in the treatment of NPC. Al-Sarraf M et al.’s research indicated that patients in the combination group of radiotherapy and chemotherapy have a higher OS rate and better safety compared to those in the simple radiotherapy group (24). Another study found that although IMRT has good therapeutic effects on NPC with or without chemotherapy, patients with poor TNM staging still have a higher rate of distant metastasis (25). Lin JC et al. showed that concurrent chemoradiotherapy (CCRT) is superior to radiotherapy alone for patients with advanced NPC (26). Two clinical studies showed that synchronous chemotherapy combined with adjuvant chemotherapy significantly improves the survival of patients with locally advanced NPC (27, 28). Two meta-analyses showed that adding chemotherapy to radiotherapy significantly improves the survival rate of patients with locally advanced NPC (29, 30). Sun Y et al. showed that for patients with locally advanced NPC, adding induction chemotherapy based on chemoradiotherapy leads to higher tumor control rates and survival rates (31). Zhang, Y et al. found that the combination of gemcitabine and cisplatin as an induction chemotherapy regimen can significantly improve OS in patients with locally advanced NPC (32, 33). Therefore, Chen, Y. P et al. developed evidence-based recommendations for chemotherapy combined with radiotherapy for the treatment of stage II-IVa NPC (34). In addition, results from a retrospective study of patients with stage III-IVa NPC showed that although there was no difference in OS, progression-free survival (PFS), and local progression-free survival (LRFS) between neoadjuvant chemotherapy (NCT) + CCRT and NCT + IMRT in the low-risk group of EBV DNA cutoff values, patients receiving NCT + CCRT treatment have a better distant metastasis-free survival rate (DMFS) than those receiving NCT + IMRT treatment (35). In pediatric patients with advanced NPC, the use of NCT + CCRT regimen tends to improve long-term DMFS and has acceptable toxicity (36). A recent meta-analysis showed that the addition of induction or adjuvant chemotherapy to CCRT enhances overall survival in NPC compared to chemotherapy alone (37). However, the inclusion of chemotherapy has led to an increase in acute toxicities (38). Results from a recent randomized clinical trial indicated that patients with locally advanced NPC treated with induction chemotherapy followed by radiotherapy alone achieved a 3-year progression-free survival rate that was non-inferior to that of CCRT (39). Another study found that patients with NPC at stage II and T3N0 who exhibit adverse characteristics (defined as: lymph node size ≥ 3cm, radiological evidence of extranodal extension, or Epstein-Barr virus DNA titer ≥ 4000 copies/mL), IMRT combined with chemotherapy did not improve survival rates but led to increased acute toxicities (40). Consequently, the treatment paradigm for NPC has evolved from exclusive radiotherapy to CCRT, followed by the addition of induction and adjuvant chemotherapy. Currently, researchers are focusing on the adverse effects of chemotherapy, aiming to assess through rigorous clinical trials which patients with NPC may benefit from chemotherapy.
Although radiation therapy and chemotherapy have achieved strong therapeutic effects, there are still patients who progress to treatment failure, including recurrence and metastasis. Recent clinical trials showed that nimotuzumab (a humanized monoclonal antibody targeting EGFR) has certain advantages in treating patients with locally advanced NPC, or in combination with chemotherapy, radiotherapy, and CCRT for patients with recurrent or metastatic NPC (41–43). In addition, immunotherapy showed good efficacy in treating patients with recurrent/metastatic NPC (44–46). Thus, targeted therapy and immunotherapy provide another strategy for patients with NPC.
The prognosis of NPC is closely related to its staging. The 5-year OS of patients with early-stage (I-II) NPC is as high as 94%, while the 5-year survival rate of patients with advanced-stage (III-IV) has sharply decreased, below 80% (47). Lee AW et al. believed that improving baseline assessment level is crucial for distant metastasis of NPC, and magnetic response imaging (MRI) achieves significantly better results than those staged by computed tomography (48). Another prospective clinical study showed that the staging of NPC after neoadjuvant therapy based on MRI determines the OS (49). Recent research indicates that PET/CT offers more precise imaging for patients with locoregional recurrent NPC (RIII-IVA) (50). Additionally, the recursive partitioning analysis (RPA) staging model, which incorporates cell-free Epstein-Barr virus (cfEBV) DNA, lactate dehydrogenase (LDH), and C-reactive protein-to-albumin ratio (CAR) along with TNM staging, surpasses the current TNM system in prognostic prediction and clinical decision-making (51). In a separate proposal, researchers have proposed a more refined classification of T, N, and M: reclassifying T3 NPC cases with early skull base invasion as T2; elevating N1-N2 cases with level 3 extranodal extension (ENE) to N3; combining T2N0 and T1N0 into a single level IA; and subclassifying newly diagnosed metastatic NPC (M1) into M1a (1-3 non-liver-involved metastatic lesions) and M1b (>3 metastatic lesions or liver involvement) (52). Therefore, developing a more suitable staging approach for NPC greatly benefits patient treatment.
Unfortunately, despite significant progress in the treatment of NPC, due to the lack of specificity in the early symptoms (headache, nasal congestion, nosebleeds, etc.), over 60% of patients are diagnosed with advanced stage at the initial treatment, resulting in extremely poor prognosis (53). Therefore, identifying biomarkers with early diagnostic significance is currently a research hotspot. EBV is a key factor causing NPC, so it has always been the subject of research. Lo, Y. M et al. observed a rapid decrease in plasma EBV DNA concentration in patients with NPC after radiation therapy (54). Pre-treatment EBV DNA level is an important risk factor for distant metastasis (55). In addition, studies showed that plasma EBV DNA levels can help screen for early asymptomatic NPC and predict treatment outcomes (56, 57). At present, plasma EBV-DNA has been used for population screening, prognosis, and prediction of treatment response to adapt to treatment and disease monitoring (3). A meta-analysis compared the diagnostic efficacy of 14 diagnostic biomarkers for NPC. The results showed that VCA-IgG had the highest combination sensitivity, EA-IgG had the highest combination specificity, and ZTA-IgG had the highest combination AUC value (58). However, there is heterogeneity in the results of this study, and further exploration is needed in the future.
Epithelial-mesenchymal transition (EMT) refers to the biological process in which epithelial cells lose polarity and intercellular adhesion through specific procedures, transforming into cells with mesenchymal phenotype. It is one of the key mechanisms for NPC cells to resist anti-tumor measures, leading to metastasis and invasion (59). Multiple studies have confirmed that TGF-β (60), NF-κB (61), Wnt (62), Akt (63), Notch (64), and other signaling pathways are involved in EMT in NPC, while EBV infection (65), abnormal gene expression (66), hypoxia (67), and abnormal expression of non-coding RNA (68) are involved in regulating these signaling pathways. Stanniocalcin-2 promotes cell EMT by activating the ITGB2/FAK/SOX6 signaling pathway in NPC (69). MiR-296-5p inhibits EMT-related NPC metastasis by targeting TGF-β (70). Circular RNA CRIM1 promotes EMT and chemotherapy resistance in NPC by upregulating FOXQ1 (71). In addition, metabolic reprogramming promotes NPC metastasis by promoting TGFβ1-induced EMT (72). Moreover, EMT is reversible: tumor cells undergo EMT before metastasis, and tumor cells colonize in distant sites and then undergo a mesenchymal-epithelial transition (MET), which then exhibits more malignant behaviors (73). Recent research results showed that EBV-encoded LMP1 and LMP2A coordinate to produce different EMT states in NPC cells, leading to tumor initiation, angiogenesis, and metastasis (74). Therefore, this precise regulation of EMT is crucial for the occurrence and development of NPC.
Cancer stem cells (CSCs) are stem-like cells located at the top of the NPC niche. Although the number of CSCs is rare, their characteristics of tumor initiation, self-renewal, differentiation potential, plasticity, formation of microenvironment, promotion of metastasis, treatment resistance, and immune escape endow them with strong carcinogenic potential (75). Based on these characteristics, CSCs can maintain tumor heterogeneity and protect them from the killing effects of current therapeutic methods (76). Therefore, although patients with NPC actively undergo anti-tumor therapy, most of them still suffer from the painful experience of recurrence and metastasis caused by nasopharyngeal carcinoma stem cells (NPCSCs) (77). Early studies have found that CD44+ and aldehyde dehydrogenase 1 (ALDH1) are associated with the recurrence and metastasis of NPC, and they have been identified as specific markers of NPCSCs (78, 79). The embryonic stem cell markers SOX2, OCT4, and Nanog have also been shown to be associated with the EMT in NPC (80). Recent studies have found that the protein C receptor (PROCR), which is associated with poor prognosis in patients with NPC, has the potential to maintain the stemness of tumor cells by regulating lipid metabolism and mitochondrial fission (81). CD166 induces the formation of CSCs by activating the EGFR/ERK1/2 signaling pathway in NPC cells (82). It is noteworthy that EMT and CSCs are interconnected: cancer cells acquire CSCs stemness through EMT processes, while CSCs can also regulate EMT (83, 84). For example, EBV latent membrane protein promotes EMT in NPC cells by activating mTORC1 and mTORC2 pathways, which endows NPC cells with stemness characteristics and promotes cancer cells migration and invasion (85, 86). SLC27A6 promotes EMT and maintains NPCSCs stemness by upregulating NPC cell lipid uptake (87). However, there is evidence that complete EMT is not the best state for maintaining the stemness of CSCs (88). Therefore, in-depth exploration of EMT and CSCs may bring hope to researchers in overcoming the problems of proliferation, metastasis, and drug resistance in NPC.
MicroRNA (miRNA) is a non-coding single-stranded RNA molecule with a length of approximately 22 nucleotides, playing a crucial role in various biological processes (89). MiRNA is a potential biomarker for predicting prognosis and diagnosing radiotherapy resistance in NPC (90, 91). Transactivated MIR106A-5p exerts the effect of macroautophagy/autophagy inhibitors by targeting BTG3 (BTG anti-proliferative factor 3) and activating autophagy to regulate MAPK signaling, thereby promoting the malignant phenotype of NPC (92). EBV-miRNA-BART2-5p directly targets RNase III endonuclease DICER1, inhibiting its ability to cleave double-stranded stem ring RNA into short double-stranded RNA, leading to changes in the expression of a series of key EMT molecules (93). EBV-miR-BART11 and EBV-miR-BART17-3p promote tumor immune escape by inhibiting FOXP1 and PBRM1 and enhancing PD-L1 transcription (61). The expression of let-7i-5p is significantly increased in NPC tissues, and it promotes malignant phenotype by targeting ATG10 and ATG16L1 to inhibit autophagy (62). Lin C et al. found that EBV-encoded miRNA BART8-3p is upregulated in NPC and its expression level in plasma is associated with patient diagnosis and prognosis. Therefore, miRNA is a promising biomarker (94).
Long non-coding RNA (lncRNA) is typically defined as a non-protein coding RNA molecule with a length exceeding 200 nucleotides. LncRNA promotes the occurrence and development of cancer by activating proliferation, migration, and invasion signals, causing cellular energy metabolism disorders, acquiring cancer stem cells characteristics, and regulating microenvironments (95). LncRNA FAM225A promotes NPC tumor occurrence and metastasis by acting as ceRNA on sponge miR-590-3p/miR-1275 and upregulating ITGB3 (96). LINC00173 promotes NPC cell proliferation, migration, and invasion by directly binding and interacting with RAB1B, promoting the secretion of PA2G4 and SDF4 through the exocytosis pathway (97). Wilms tuber 1-associated protein (WTAP) maintains the stability of lncRNA DIAPH1-AS1 in an m6A-dependent manner, promotes the formation of MTDH-LASP1 complex, protects LASP1 from ubiquitin degradation, and promotes the growth and metastasis of NPC (98). It is worth noting that the positive diagnostic rate of tumor-associated platelets (TEP) lncRNA-ROR for NPC is similar to EBV DNA (58.3%), and the positive rate of TEP lncRNA-ROR combined with EBV DNA for NPC can reach 74% (99). Therefore, LncRNA may provide direction for the diagnosis and treatment of NPC.
Exosomes are small (30-100nm) extracellular vesicles originating in the plasma membrane. Studies showed that exosomes mediate tumor immune microenvironment (100), enhance angiogenesis (101), promote metastasis (102), and promote chemoradiotherapy resistance to NPC (103). Wu A et al. showed that exosome LBH inhibits EMT and angiogenesis of NPC by regulating VEGFA expression (104). Jiang J et al. showed that exosomal miR-197-3p (EXO-miR-197-3p) reduces the proliferation, migration, and radiation resistance of NPC cells by regulating AKT/mTOR phosphorylation activation and HSPA5 mediated autophagy (105). Exosomes derived from γδ- T cells (γδ- T Exos) combined with radiotherapy can overcome the radiotherapy resistance of NPC stem cells (106). It is worth considering that extracellular vesicles can carry miRNA, DNA, metabolites, and small molecule drugs, and can accurately transport the carried “goods” from parent cells to recipient cells (107). This provides ideas for the precise treatment of NPC. For example, miRNAs with anti-cancer and improved drug resistance can be loaded into exosomes, which are then taken up and expressed by NPC cells, ultimately enhancing the therapeutic effect (108).
Although a large number of articles related to NPC have been published, due to the complexity of this tumor, researchers still can not get a glimpse of its full picture. We have paid attention to some recently published literatures and found that researchers are increasingly concerned about the integrity of cancer: cancer is not only a local disease, but a systemic disease that interacts with various physiological systems and the external environment of the body (109). It is worth noting that the ecological theory of NPC is a new theory with historical significance. From a macro perspective, this theory defines NPC as a multi-dimensional spatio-temporal “ecological and evolutionary unity” disease: an evolutionary adaptive pathological ecosystem composed of four interdependent parts: primary ecosystem, circular ecosystem, transfer ecosystem, and multi-directional ecosystem (110). The core connotation of this system is evolution and adaptability. Specifically, NPC cells produce heterogeneity, metastasis, drug resistance, etc. through intraspecific competition, species evolution, and interspecific interaction, etc. For example, in terms of metabolism, primary tumor cells and circulating tumor cells show “Warburg effect” phenotype and “anti-Warburg effect” phenotype respectively, to adapt to different peripheral environments and achieve the purpose of proliferation and metastasis (111). In terms of immunity, macrophages and neutrophils can be induced into M2 and N2 phenotypes to help tumor cells achieve immune escape (112, 113). In terms of drug resistance, tumor repopulating cells (TRCs) with stem cell-like cancer cell characteristics resist chemotherapy and radiotherapy by reducing the sensitivity to iron death (114). Based on such an ecological theory, researchers can consider using ecological therapies to combat NPC (by altering the surrounding environment to eliminate specific targets), such as destroying tumor habitats through hyperthermia (68) and reducing drug resistance in NPC cells through adaptive therapies (115). Therefore, it is helpful for researchers to develop more effective anti-cancer strategies to examine NPC in a new system.
This study provides insights into possible future trends and potential impacts of NPC. In the future, The clinical treatment of NPC will change from the overall strategy of the whole population to the specific strategy of the specific population. Developing a more accurate NPC evaluation system will help clinicians complete more accurate staging and make better clinical decisions. With the progress of medicine, the molecular mechanism of NPC has gradually become the focus of research. The study of EMT and CSCs is very important for further understanding the mechanism of recurrence, metastasis, and drug resistance of NPC. Identification of special biomarkers is of great significance for the diagnosis and treatment of NPC. In addition, the global concept of NPC has changed dramatically. Integrating the micro molecular mechanisms of NPC and understanding NPC from a macro perspective will help researchers develop more advanced treatment options.
The significant advantage of our research lies in the extensive analysis of global publications on NPC from the perspective of scientific literature. This study also has some limitations. The first is the selection of a database. Although we used the WoSCC database, one of the most extensive and comprehensive global databases, as the literature source database, it may still lead to omissions in literature retrieval. The second is the retrieval strategy. This study only selected the research published in English and ignored the literature in other languages. This can also lead to selection bias. The third is the retrieval time range. Although we have retrieved some high-quality articles published recently as supplements beyond the statistical time, there are still omissions.
In summary, we conducted a bibliometric analysis using Citespace, VOSviewer, and R version 4.2.2 to outline the current research situation and development trend of nasopharyngeal carcinoma. The article demonstrates the characteristics of the publication, identifies the most influential countries, institutions, authors, journals, articles, and references, and displays keywords and references with the strongest citation bursts. In addition, we also discussed the research hotspots and trends of NPC. At present, optimizing clinical treatment strategies, exploring the molecular mechanisms, and improving diagnostic and staging methods are current research hotspots. The future research trends will be to find advantageous populations for precise treatment, develop more accurate NPC evaluation systems, clarify the progression mechanism of NPC, search for more favorable diagnostic and therapeutic biomarkers, and interpret NPC from a holistic perspective.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
GA: Writing – original draft. JL: Writing – original draft. TL: Writing – original draft. LH: Writing – review & editing. YH: Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was supported by the National Natural Science Foundation of China (No. 82305329), the Natural Science Foundation of Hunan Province (No. 2023JJ30449; No. 2023JJ40500), the National Natural Science Foundation of Changsha City (No. kq2208206), the Project of Hunan Provincial Department of Education (No. 23A0298), the Project of Hunan Provincial Health Commission (No. D202307018448), the Hunan Provincial Health High-Level Talent Scientific Research Project (No. R2023111), the Chinese Academy of Engineering Academician Liang Liu’s Workstation of Hunan University of Chinese Medicine (No. 22YS001), the First-class Discipline Construction Project of Hunan University of Chinese Medicine (No. 22JBZ011), and the University level project of Hunan University of Chinese Medicine (No. 2022YYZK010).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2024.1392245/full#supplementary-material
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2021) 71:209–49. doi: 10.3322/caac.21660
2. Chua MLK, Wee JTS, Hui EP, Chan ATC. Nasopharyngeal carcinoma. Lancet. (2016) 387:1012–24. doi: 10.1016/S0140-6736(15)00055-0
3. Chen YP, Chan ATC, Le QT, Blanchard P, Sun Y, Ma J. Nasopharyngeal carcinoma. Lancet. (2019) 394:64–80. doi: 10.1016/S0140-6736(19)30956-0
4. Yang K, Hu Y, Feng Y, Li K, Zhu Z, Liu S, et al. IGF-1R mediates crosstalk between nasopharyngeal carcinoma cells and osteoclasts and promotes tumor bone metastasis. J Exp Clin Cancer Res. (2024) 43:46. doi: 10.1186/s13046-024-02970-8
5. Lee AW, Ma BB, Ng WT, Chan AT. Management of nasopharyngeal carcinoma: current practice and future perspective. J Clin Oncol. (2015) 33:3356–64. doi: 10.1200/JCO.2015.60.9347
6. Geng JW, Li Q, Quan WX, Lin XY, Miao YD. Bibliometric analysis of worldwide research trends on tumor burden and immunotherapy: A correspondence. Int J Surg. (2024) 110:3088–3090. doi: 10.1097/JS9.0000000000001157
7. Wang J, Wang S, Zhang Y, Zhang W. Bibliometric analysis of evolutionary trajectory and prospective directions of LAG-3 in cancer. Front Immunol. (2024) 15:1329775. doi: 10.3389/fimmu.2024.1329775
8. Hung CC, Tu MY, Chien TW, Lin CY, Chow JC, Chou W. The model of descriptive, diagnostic, predictive, and prescriptive analytics on 100 top-cited articles of nasopharyngeal carcinoma from 2013 to 2022: Bibliometric analysis. Med (Baltimore). (2023) 102:e32824. doi: 10.1097/MD.0000000000032824
9. Wu Q, Yuan T, Zhang Z, Yang Q, Chen M, Wang Q, et al. The top 100 most impactful articles and recent trends in nasopharyngeal carcinoma from 1970 to 2018: a bibliometric analysis. J Int Med Res. (2020) 48:300060519896149. doi: 10.1177/0300060519896149
10. Xing CY, Lin MQ, Luo WT, Chen LF, Wu SG, Cai YJ. The 100 most cited papers in nasopharyngeal carcinoma between 2000 and 2019: a bibliometric study. Transl Cancer Res. (2023) 12:848–58. doi: 10.21037/tcr-22-2621
11. Liu X, Zhao S, Tan L, Tan Y, Wang Y, Ye Z, et al. Frontier and hot topics in electrochemiluminescence sensing technology based on CiteSpace bibliometric analysis. Biosens Bioelectron. (2022) 201:113932. doi: 10.1016/j.bios.2021.113932
12. Liu Z, Yu Q, Liu H. Mesenchymal stem cells in heterotopic ossification in ankylosing spondylitis: A bibliometric study based on citeSpace and VOSViewer. J Inflammation Res. (2023) 16:4389–98. doi: 10.2147/JIR.S421962
13. Jian C, Jing Z, Yinhang W, Jinlong D, Yuefen P, Quan Q, et al. Colorectal cancer and gut viruses: a visualized analysis based on CiteSpace knowledge graph. Front Microbiol. (2023) 14:1239818. doi: 10.3389/fmicb.2023.1239818
14. Chen C. A glimpse of the first eight months of the COVID-19 literature on microsoft academic graph: themes, citation contexts, and uncertainties. Front Res Metr Anal. (2020) 5:607286. doi: 10.3389/frma.2020.607286
15. Li C, Zhao C, Zhao J, Wang M, Luo F, Zhou J. Global research trends of acupuncture therapy on cancer pain: A bibliometric and visualized study. Front Oncol. (2023) 13:1077961. doi: 10.3389/fonc.2023.1077961
16. Zhou F, Wang W, Xu R, Liu L, Lin T, He L, et al. Unraveling the mechanism of Yiqi Jiedu formula against nasopharyngeal carcinoma: An investigation integrating network pharmacology, serum pharmacochemistry, and metabolomics. J Ethnopharmacol. (2024) 319:117343. doi: 10.1016/j.jep.2023.117343
17. Lee N, Xia P, Quivey JM, Sultanem K, Poon I, Akazawa C, et al. Intensity-modulated radiotherapy in the treatment of nasopharyngeal carcinoma: an update of the UCSF experience. Int J Radiat Oncol Biol Phys. (2002) 53:12–22. doi: 10.1016/S0360-3016(02)02724-4
18. Kam MK, Leung SF, Zee B, Chau RM, Suen JJ, Mo F, et al. Prospective randomized study of intensity-modulated radiotherapy on salivary gland function in early-stage nasopharyngeal carcinoma patients. J Clin Oncol. (2007) 25:4873–9. doi: 10.1200/JCO.2007.11.5501
19. Lai SZ, Li WF, Chen L, Luo W, Chen YY, Liu LZ, et al. How does intensity-modulated radiotherapy versus conventional two-dimensional radiotherapy influence the treatment results in nasopharyngeal carcinoma patients? Int J Radiat Oncol Biol Phys. (2011) 80:661–8. doi: 10.1016/j.ijrobp.2010.03.024
20. Peng G, Wang T, Yang KY, Zhang S, Zhang T, Li Q, et al. A prospective, randomized study comparing outcomes and toxicities of intensity-modulated radiotherapy vs. conventional two-dimensional radiotherapy for the treatment of nasopharyngeal carcinoma. Radiother Oncol. (2012) 104:286–93. doi: 10.1016/j.radonc.2012.08.013
21. Sun X, Su S, Chen C, Han F, Zhao C, Xiao W, et al. Long-term outcomes of intensity-modulated radiotherapy for 868 patients with nasopharyngeal carcinoma: an analysis of survival and treatment toxicities. Radiother Oncol. (2014) 110:398–403. doi: 10.1016/j.radonc.2013.10.020
22. Huang CL, Zhang N, Jiang W, Xie FY, Pei XQ, Huang SH, et al. Reduced-volume irradiation of uninvolved neck in patients with nasopharyngeal cancer: updated results from an open-label, noninferiority, multicenter, randomized phase III trial. J Clin Oncol. (2024) 42:2021–2025. doi: 10.1200/JCO.23.02086
23. Mao YP, Wang SX, Gao TS, Zhang N, Liang XY, Xie FY, et al. Medial retropharyngeal nodal region sparing radiotherapy versus standard radiotherapy in patients with nasopharyngeal carcinoma: open label, non-inferiority, multicentre, randomised, phase 3 trial. BMJ. (2023) 380:e072133. doi: 10.1136/bmj-2022-072133
24. Al-Sarraf M, LeBlanc M, Giri PG, Fu KK, Cooper J, Vuong T, et al. Chemoradiotherapy versus radiotherapy in patients with advanced nasopharyngeal cancer: phase III randomized Intergroup study 0099. J Clin Oncol. (1998) 16:1310–7. doi: 10.1200/JCO.1998.16.4.1310
25. Lee N, Harris J, Garden AS, Straube W, Glisson B, Xia P, et al. Intensity-modulated radiation therapy with or without chemotherapy for nasopharyngeal carcinoma: radiation therapy oncology group phase II trial 0225. J Clin Oncol. (2009) 27:3684–90. doi: 10.1200/JCO.2008.19.9109
26. Lin JC, Jan JS, Hsu CY, Liang WM, Jiang RS, Wang WY. Phase III study of concurrent chemoradiotherapy versus radiotherapy alone for advanced nasopharyngeal carcinoma: positive effect on overall and progression-free survival. J Clin Oncol. (2003) 21:631–7. doi: 10.1200/JCO.2003.06.158
27. Chen Y, Liu MZ, Liang SB, Zong JF, Mao YP, Tang LL, et al. Preliminary results of a prospective randomized trial comparing concurrent chemoradiotherapy plus adjuvant chemotherapy with radiotherapy alone in patients with locoregionally advanced nasopharyngeal carcinoma in endemic regions of China. Int J Radiat Oncol Biol Phys. (2008) 71:1356–64. doi: 10.1016/j.ijrobp.2007.12.028
28. Lee AW, Tung SY, Chua DT, Ngan RK, Chappell R, Tung R, et al. Randomized trial of radiotherapy plus concurrent-adjuvant chemotherapy vs radiotherapy alone for regionally advanced nasopharyngeal carcinoma. J Natl Cancer Inst. (2010) 102:1188–98. doi: 10.1093/jnci/djq258
29. Baujat B, Audry H, Bourhis J, Chan AT, Onat H, Chua DT, et al. Chemotherapy in locally advanced nasopharyngeal carcinoma: an individual patient data meta-analysis of eight randomized trials and 1753 patients. Int J Radiat Oncol Biol Phys. (2006) 64:47–56. doi: 10.1016/j.ijrobp.2005.06.037
30. Blanchard P, Lee A, Marguet S, Leclercq J, Ng WT, Ma J, et al. Chemotherapy and radiotherapy in nasopharyngeal carcinoma: an update of the MAC-NPC meta-analysis. Lancet Oncol. (2015) 16:645–55. doi: 10.1016/S1470-2045(15)70126-9
31. Sun Y, Li WF, Chen NY, Zhang N, Hu GQ, Xie FY, et al. Induction chemotherapy plus concurrent chemoradiotherapy versus concurrent chemoradiotherapy alone in locoregionally advanced nasopharyngeal carcinoma: a phase 3, multicentre, randomised controlled trial. Lancet Oncol. (2016) 17:1509–20. doi: 10.1016/S1470-2045(16)30410-7
32. Zhang Y, Chen L, Hu GQ, Zhang N, Zhu XD, Yang KY, et al. Gemcitabine and cisplatin induction chemotherapy in nasopharyngeal carcinoma. N Engl J Med. (2019) 381:1124–35. doi: 10.1056/NEJMoa1905287
33. Zhang Y, Chen L, Hu GQ, Zhang N, Zhu XD, Yang KY, et al. Final overall survival analysis of gemcitabine and cisplatin induction chemotherapy in nasopharyngeal carcinoma: A multicenter, randomized phase III trial. J Clin Oncol. (2022) 40:2420–5. doi: 10.1200/JCO.22.00327
34. Chen YP, Ismaila N, Chua MLK, Colevas AD, Haddad R, Huang SH, et al. Chemotherapy in combination with radiotherapy for definitive-intent treatment of stage II-IVA nasopharyngeal carcinoma: CSCO and ASCO guideline. J Clin Oncol. (2021) 39:840–59. doi: 10.1200/JCO.20.03237
35. Ji P, Lu Q, Chen X, Chen Y, Peng X, Chen Z, et al. Individualized concurrent chemotherapy for patients with stage III-IVa nasopharyngeal carcinoma receiving neoadjuvant chemotherapy combined with definitive intensity-modulated radiotherapy. Cancer Res Treat. (2023) 55:1113–22. doi: 10.4143/crt.2022.1651
36. Jin YN, Ruan ZH, Cao WW, Yang L, Yao W, Pei XF, et al. Concurrent chemoradiotherapy with or without neoadjuvant chemotherapy in pediatric patients with stage III-IVa nasopharyngeal carcinoma: a real-world propensity score-matched cohort study. J Cancer Res Clin Oncol. (2023) 149:11929–40. doi: 10.1007/s00432-023-05041-1
37. Petit C, Lee A, Ma J, Lacas B, Ng WT, Chan ATC, et al. Role of chemotherapy in patients with nasopharynx carcinoma treated with radiotherapy (MAC-NPC): an updated individual patient data network meta-analysis. Lancet Oncol. (2023) 24:611–23. doi: 10.1016/S1470-2045(23)00163-8
38. Wang F, Zhou L, Zhang LJ, Xie CB, Liao ZW, Lin XD, et al. Concurrent chemoradiotherapy versus radiotherapy alone in older patients with stage II nasopharyngeal carcinoma after intensity-modulated radiotherapy: A propensity score-matched cohort study. Radiother Oncol. (2024) 191:110081. doi: 10.1016/j.radonc.2024.110081
39. Dai J, Zhang B, Su Y, Pan Y, Ye Z, Cai R, et al. Induction chemotherapy followed by radiotherapy vs chemoradiotherapy in nasopharyngeal carcinoma: A randomized clinical trial. JAMA Oncol. (2024) 10:456–63. doi: 10.1001/jamaoncol.2023.6552
40. Zhang WW, Lin JY, Wang GY, Huang CL, Tang LL, Mao YP, et al. Radiotherapy alone versus concurrent chemoradiotherapy in patients with stage II and T3N0 nasopharyngeal carcinoma with adverse features: A propensity score-matched cohort study. Radiother Oncol. (2024) 194:110189. doi: 10.1016/j.radonc.2024.110189
41. Liang R, Yang L, Zhu X. Nimotuzumab, an anti-EGFR monoclonal antibody, in the treatment of nasopharyngeal carcinoma. Cancer Control. (2021) 28:1073274821989301. doi: 10.1177/1073274821989301
42. You R, Hua YJ, Liu YP, Yang Q, Zhang YN, Li JB, et al. Concurrent chemoradiotherapy with or without anti-EGFR-targeted treatment for stage II-IVb nasopharyngeal carcinoma: retrospective analysis with a large cohort and long follow-up. Theranostics. (2017) 7:2314–24. doi: 10.7150/thno.19710
43. Patil VM, Noronha V, Joshi A, Agarwal J, Ghosh-Laskar S, Budrukkar A, et al. A randomized phase 3 trial comparing nimotuzumab plus cisplatin chemoradiotherapy versus cisplatin chemoradiotherapy alone in locally advanced head and neck cancer. Cancer. (2019) 125:3184–97. doi: 10.1002/cncr.32179
44. Ma BBY, Lim WT, Goh BC, Hui EP, Lo KW, Pettinger A, et al. Antitumor activity of nivolumab in recurrent and metastatic nasopharyngeal carcinoma: an international, multicenter study of the mayo clinic phase 2 consortium (NCI-9742). J Clin Oncol. (2018) 36:1412–8. doi: 10.1200/JCO.2017.77.0388
45. Ding X, Zhang WJ, You R, Zou X, Wang ZQ, Ouyang YF, et al. Camrelizumab plus apatinib in patients with recurrent or metastatic nasopharyngeal carcinoma: an open-label, single-arm, phase II study. J Clin Oncol. (2023) 41:2571–82. doi: 10.1200/JCO.22.01450
46. Mai HQ, Chen QY, Chen D, Hu C, Yang K, Wen J, et al. Toripalimab plus chemotherapy for recurrent or metastatic nasopharyngeal carcinoma: the JUPITER-02 randomized clinical trial. JAMA. (2023) 330:1961–70. doi: 10.1001/jama.2023.20181
47. Su ZY, Siak PY, Leong CO, Cheah SC. Nasopharyngeal carcinoma and its microenvironment: past, current, and future perspectives. Front Oncol. (2022) 12:840467. doi: 10.3389/fonc.2022.840467
48. Lee AW, Sze WM, Au JS, Leung SF, Leung TW, Chua DT, et al. Treatment results for nasopharyngeal carcinoma in the modern era: the Hong Kong experience. Int J Radiat Oncol Biol Phys. (2005) 61:1107–16. doi: 10.1016/j.ijrobp.2004.07.702
49. Liu LT, Chen QY, Tang LQ, Zhang L, Guo SS, Xie CM, et al. Advanced-stage nasopharyngeal carcinoma: restaging system after neoadjuvant chemotherapy on the basis of MR imaging determines survival. Radiology. (2017) 282:171–81. doi: 10.1148/radiol.2016152540
50. Dong Z, Wang GY, Dai DY, Qin GJ, Tang LL, Xu C, et al. Prognostic value of pre-treatment [(18)F] FDG PET/CT in recurrent nasopharyngeal carcinoma without distant metastasis. BMC Cancer. (2024) 24:466. doi: 10.1186/s12885-024-12189-7
51. Ding C, Dai DY, Luo ZK, Wang GY, Dong Z, Qin GJ, et al. Evaluation of a novel model incorporating serological indicators into the conventional TNM staging system for nasopharyngeal carcinoma. Oral Oncol. (2024) 151:106725. doi: 10.1016/j.oraloncology.2024.106725
52. Du XJ, Wang GY, Zhu XD, Han YQ, Lei F, Shen LF, et al. Refining the 8th edition TNM classification for EBV related nasopharyngeal carcinoma. Cancer Cell. (2024) 42:464–73 e3. doi: 10.1016/j.ccell.2023.12.020
53. Siti-Azrin AH, Norsa’adah B, Naing NN. Prognostic factors of nasopharyngeal carcinoma patients in a tertiary referral hospital: a retrospective cohort study. BMC Res Notes. (2017) 10:705. doi: 10.1186/s13104-017-2990-1
54. Lo YM, Leung SF, Chan LY, Chan AT, Lo KW, Johnson PJ, et al. Kinetics of plasma Epstein-Barr virus DNA during radiation therapy for nasopharyngeal carcinoma. Cancer Res. (2000) 60:2351–5.
55. Tang LQ, Chen QY, Fan W, Liu H, Zhang L, Guo L, et al. Prospective study of tailoring whole-body dual-modality [18F]fluorodeoxyglucose positron emission tomography/computed tomography with plasma Epstein-Barr virus DNA for detecting distant metastasis in endemic nasopharyngeal carcinoma at initial staging. J Clin Oncol. (2013) 31:2861–9. doi: 10.1200/JCO.2012.46.0816
56. Lin JC, Wang WY, Chen KY, Wei YH, Liang WM, Jan JS, et al. Quantification of plasma Epstein-Barr virus DNA in patients with advanced nasopharyngeal carcinoma. N Engl J Med. (2004) 350:2461–70. doi: 10.1056/NEJMoa032260
57. Chan KCA, Woo JKS, King A, Zee BCY, Lam WKJ, Chan SL, et al. Analysis of plasma epstein-barr virus DNA to screen for nasopharyngeal cancer. N Engl J Med. (2017) 377:513–22. doi: 10.1056/NEJMoa1701717
58. Feng Y, Xia W, He G, Ke R, Liu L, Xie M, et al. Accuracy evaluation and comparison of 14 diagnostic markers for nasopharyngeal carcinoma: A meta-analysis. Front Oncol. (2020) 10:1779. doi: 10.3389/fonc.2020.01779
59. Nieto MA, Huang RY, Jackson RA, Thiery JP. Emt: 2016. Cell. (2016) 166:21–45. doi: 10.1016/j.cell.2016.06.028
60. Oshimori N, Oristian D, Fuchs E. TGF-beta promotes heterogeneity and drug resistance in squamous cell carcinoma. Cell. (2015) 160:963–76. doi: 10.1016/j.cell.2015.01.043
61. Wang J, Ge J, Wang Y, Xiong F, Guo J, Jiang X, et al. EBV miRNAs BART11 and BART17-3p promote immune escape through the enhancer-mediated transcription of PD-L1. Nat Commun. (2022) 13:866. doi: 10.1038/s41467-022-28479-2
62. You B, Zhang P, Gu M, Yin H, Fan Y, Yao H, et al. Let-7i-5p promotes a Malignant phenotype in nasopharyngeal carcinoma via inhibiting tumor-suppressive autophagy. Cancer Lett. (2022) 531:14–26. doi: 10.1016/j.canlet.2022.01.019
63. Pan S, Liang S, Wang X. ADORA1 promotes nasopharyngeal carcinoma cell progression through regulation of PI3K/AKT/GSK-3beta/beta-catenin signaling. Life Sci. (2021) 278:119581. doi: 10.1016/j.lfs.2021.119581
64. Guo H, Wang F, Diao Y, Zhang Z, Chen Q, Qian CN, et al. Knockdown of Notch1 inhibits nasopharyngeal carcinoma cell growth and metastasis via downregulation of CCL2, CXCL16, and uPA. Mol Carcinog. (2019) 58:1886–96. doi: 10.1002/mc.23082
65. Zhang T, Chen Z, Deng J, Xu K, Che D, Lin J, et al. Epstein-Barr virus-encoded microRNA BART22 serves as novel biomarkers and drives Malignant transformation of nasopharyngeal carcinoma. Cell Death Dis. (2022) 13:664. doi: 10.1038/s41419-022-05107-x
66. Zou Y, Yang R, Huang ML, Kong YG, Sheng JF, Tao ZZ, et al. NOTCH2 negatively regulates metastasis and epithelial-Mesenchymal transition via TRAF6/AKT in nasopharyngeal carcinoma. J Exp Clin Cancer Res. (2019) 38:456. doi: 10.1186/s13046-019-1463-x
67. Shan Y, You B, Shi S, Shi W, Zhang Z, Zhang Q, et al. Hypoxia-induced matrix metalloproteinase-13 expression in exosomes from nasopharyngeal carcinoma enhances metastases. Cell Death Dis. (2018) 9:382. doi: 10.1038/s41419-018-0425-0
68. Lin TY, Jia JS, Luo WR, Lin XL, Xiao SJ, Yang J, et al. ThermomiR-377-3p-induced suppression of Cirbp expression is required for effective elimination of cancer cells and cancer stem-like cells by hyperthermia. J Exp Clin Cancer Res. (2024) 43:62. doi: 10.1186/s13046-024-02983-3
69. Li J, Zhang Z, Feng X, Shen Z, Sun J, Zhang X, et al. Stanniocalcin-2 promotes cell EMT and glycolysis via activating ITGB2/FAK/SOX6 signaling pathway in nasopharyngeal carcinoma. Cell Biol Toxicol. (2022) 38:259–72. doi: 10.1007/s10565-021-09600-5
70. Chen M, Chen C, Luo H, Ren J, Dai Q, Hu W, et al. MicroRNA-296-5p inhibits cell metastasis and invasion in nasopharyngeal carcinoma by reversing transforming growth factor-beta-induced epithelial-mesenchymal transition. Cell Mol Biol Lett. (2020) 25:49. doi: 10.1186/s11658-020-00240-x
71. Hong X, Liu N, Liang Y, He Q, Yang X, Lei Y, et al. Circular RNA CRIM1 functions as a ceRNA to promote nasopharyngeal carcinoma metastasis and docetaxel chemoresistance through upregulating FOXQ1. Mol Cancer. (2020) 19:33. doi: 10.1186/s12943-020-01149-x
72. Quan J, Li N, Tan Y, Liu H, Liao W, Cao Y, et al. PGC1alpha-mediated fatty acid oxidation promotes TGFbeta1-induced epithelial-mesenchymal transition and metastasis of nasopharyngeal carcinoma. Life Sci. (2022) 300:120558. doi: 10.1016/j.lfs.2022.120558
73. Tsai JH, Donaher JL, Murphy DA, Chau S, Yang J. Spatiotemporal regulation of epithelial-mesenchymal transition is essential for squamous cell carcinoma metastasis. Cancer Cell. (2012) 22:725–36. doi: 10.1016/j.ccr.2012.09.022
74. Zhu N, Xu X, Wang Y, Zeng MS, Yuan Y. EBV latent membrane proteins promote hybrid epithelial-mesenchymal and extreme mesenchymal states of nasopharyngeal carcinoma cells for tumorigenicity. PloS Pathog. (2021) 17:e1009873. doi: 10.1371/journal.ppat.1009873
75. Loh JJ, Ma S. Hallmarks of cancer stemness. Cell Stem Cell. (2024) 31:617–39. doi: 10.1016/j.stem.2024.04.004
76. Huang T, Song X, Xu D, Tiek D, Goenka A, Wu B, et al. Stem cell programs in cancer initiation, progression, and therapy resistance. Theranostics. (2020) 10:8721–43. doi: 10.7150/thno.41648
77. Tang KD, Holzapfel BM, Liu J, Lee TK, Ma S, Jovanovic L, et al. Tie-2 regulates the stemness and metastatic properties of prostate cancer cells. Oncotarget. (2016) 7:2572–84. doi: 10.18632/oncotarget.v7i3
78. Lun SW, Cheung ST, Cheung PF, To KF, Woo JK, Choy KW, et al. CD44+ cancer stem-like cells in EBV-associated nasopharyngeal carcinoma. PloS One. (2012) 7:e52426. doi: 10.1371/journal.pone.0052426
79. Wu A, Luo W, Zhang Q, Yang Z, Zhang G, Li S, et al. Aldehyde dehydrogenase 1, a functional marker for identifying cancer stem cells in human nasopharyngeal carcinoma. Cancer Lett. (2013) 330:181–9. doi: 10.1016/j.canlet.2012.11.046
80. Luo W, Li S, Peng B, Ye Y, Deng X, Yao K. Embryonic stem cells markers SOX2, OCT4 and Nanog expression and their correlations with epithelial-mesenchymal transition in nasopharyngeal carcinoma. PloS One. (2013) 8:e56324. doi: 10.1371/journal.pone.0056324
81. Zhang P, He Q, Wang Y, Zhou G, Chen Y, Tang L, et al. Protein C receptor maintains cancer stem cell properties via activating lipid synthesis in nasopharyngeal carcinoma. Signal Transduct Target Ther. (2022) 7:46. doi: 10.1038/s41392-021-00866-z
82. Chen X, Liang R, Lin H, Chen K, Chen L, Tian G, et al. CD166 promotes cancer stem cell-like phenotype via the EGFR/ERK1/2 pathway in the nasopharyngeal carcinoma cell line CNE-2R. Life Sci. (2021) 267:118983. doi: 10.1016/j.lfs.2020.118983
83. Wang L, Yang G, Zhao D, Wang J, Bai Y, Peng Q, et al. CD103-positive CSC exosome promotes EMT of clear cell renal cell carcinoma: role of remote MiR-19b-3p. Mol Cancer. (2019) 18:86. doi: 10.1186/s12943-019-0997-z
84. Wilson MM, Weinberg RA, Lees JA, Guen VJ. Emerging mechanisms by which EMT programs control stemness. Trends Cancer. (2020) 6:775–80. doi: 10.1016/j.trecan.2020.03.011
85. Kong QL, Hu LJ, Cao JY, Huang YJ, Xu LH, Liang Y, et al. Epstein-Barr virus-encoded LMP2A induces an epithelial-mesenchymal transition and increases the number of side population stem-like cancer cells in nasopharyngeal carcinoma. PloS Pathog. (2010) 6:e1000940. doi: 10.1371/journal.ppat.1000940
86. Zhu N, Wang Q, Wu Z, Wang Y, Zeng MS, Yuan Y. Epstein-Barr Virus LMP1-Activated mTORC1 and mTORC2 Coordinately Promote Nasopharyngeal Cancer Stem Cell Properties. J Virol. (2022) 96:e0194121. doi: 10.1128/jvi.01941-21
87. Zhong X, Yang Y, Li B, Liang P, Huang Y, Zheng Q, et al. Downregulation of SLC27A6 by DNA hypermethylation promotes proliferation but suppresses metastasis of nasopharyngeal carcinoma through modulating lipid metabolism. Front Oncol. (2021) 11:780410. doi: 10.3389/fonc.2021.780410
88. Schmidt JM, Panzilius E, Bartsch HS, Irmler M, Beckers J, Kari V, et al. Stem-cell-like properties and epithelial plasticity arise as stable traits after transient Twist1 activation. Cell Rep. (2015) 10:131–9. doi: 10.1016/j.celrep.2014.12.032
89. Chen Y, Lin T, Tang L, He L, He Y. MiRNA signatures in nasopharyngeal carcinoma: molecular mechanisms and therapeutic perspectives. Am J Cancer Res. (2023) 13:5805–24.
90. Vimalraj S, Sekaran S. Exploring the potential of MiRNAs as predictive biomarkers for radioresistance in nasopharyngeal carcinoma. Oral Oncol. (2023) 145:106521. doi: 10.1016/j.oraloncology.2023.106521
91. Liu N, Chen NY, Cui RX, Li WF, Li Y, Wei RR, et al. Prognostic value of a microRNA signature in nasopharyngeal carcinoma: a microRNA expression analysis. Lancet Oncol. (2012) 13:633–41. doi: 10.1016/S1470-2045(12)70102-X
92. Zhu Q, Zhang Q, Gu M, Zhang K, Xia T, Zhang S, et al. MIR106A-5p upregulation suppresses autophagy and accelerates Malignant phenotype in nasopharyngeal carcinoma. Autophagy. (2021) 17:1667–83. doi: 10.1080/15548627.2020.1781368
93. Wu Y, Zhang X, Liu C, Li Z, Wen Y, Zheng R, et al. Epstein-Barr virus microRNA miR-BART2-5p accelerates nasopharyngeal carcinoma metastasis by suppressing RNase III endonuclease DICER1. J Biol Chem. (2023) 299:105082. doi: 10.1016/j.jbc.2023.105082
94. Lin C, Lin K, Zhang B, Su Y, Guo Q, Lu T, et al. Plasma epstein-barr virus microRNA BART8-3p as a diagnostic and prognostic biomarker in nasopharyngeal carcinoma. Oncologist. (2022) 27:e340–e9. doi: 10.1093/oncolo/oyac024
95. Wang H, Wang W, Fan S. Emerging roles of lncRNA in Nasopharyngeal Carcinoma and therapeutic opportunities. Int J Biol Sci. (2022) 18:2714–28. doi: 10.7150/ijbs.70292
96. Zheng ZQ, Li ZX, Zhou GQ, Lin L, Zhang LL, Lv JW, et al. Long Noncoding RNA FAM225A Promotes Nasopharyngeal Carcinoma Tumorigenesis and Metastasis by Acting as ceRNA to Sponge miR-590-3p/miR-1275 and Upregulate ITGB3. Cancer Res. (2019) 79:4612–26. doi: 10.1158/0008-5472.CAN-19-0799
97. Miao J, Chen B, Xiao Y, Huang R, Xiao X, Lu S, et al. Long noncoding RNA LINC00173 induces radioresistance in nasopharyngeal carcinoma via inhibiting CHK2/P53 pathway. Cancer Gene Ther. (2023) 30:1249–59. doi: 10.1038/s41417-023-00634-x
98. Li ZX, Zheng ZQ, Yang PY, Lin L, Zhou GQ, Lv JW, et al. WTAP-mediated m(6)A modification of lncRNA DIAPH1-AS1 enhances its stability to facilitate nasopharyngeal carcinoma growth and metastasis. Cell Death Differ. (2022) 29:1137–51. doi: 10.1038/s41418-021-00905-w
99. Wei J, Meng X, Wei X, Zhu K, Du L, Wang H. Down-regulated lncRNA ROR in tumor-educated platelets as a liquid-biopsy biomarker for nasopharyngeal carcinoma. J Cancer Res Clin Oncol. (2023) 149:4403–9. doi: 10.1007/s00432-022-04350-1
100. Yu C, Xue B, Li J, Zhang Q. Tumor cell-derived exosome RNF126 affects the immune microenvironment and promotes nasopharyngeal carcinoma progression by regulating PTEN ubiquitination. Apoptosis. (2022) 27:590–605. doi: 10.1007/s10495-022-01738-9
101. Duan B, Shi S, Yue H, You B, Shan Y, Zhu Z, et al. Exosomal miR-17-5p promotes angiogenesis in nasopharyngeal carcinoma via targeting BAMBI. J Cancer. (2019) 10:6681–92. doi: 10.7150/jca.30757
102. Cheng Q, Li Q, Xu L, Jiang H. Exosomal microRNA-301a-3p promotes the proliferation and invasion of nasopharyngeal carcinoma cells by targeting BTG1 mRNA. Mol Med Rep. (2021) 23:328. doi: 10.3892/mmr.2021.11967
103. Yuan F, Zhou ZF. Exosomes derived from Taxol-resistant nasopharyngeal carcinoma (NPC) cells transferred DDX53 to NPC cells and promoted cancer resistance to Taxol. Eur Rev Med Pharmacol Sci. (2021) 25:127–38. doi: 10.26355/eurrev_202101_24375
104. Wu A, Luo N, Xu Y, Du N, Li L, Liu Q. Exosomal LBH inhibits epithelial-mesenchymal transition and angiogenesis in nasopharyngeal carcinoma via downregulating VEGFA signaling. Int J Biol Sci. (2022) 18:242–60. doi: 10.7150/ijbs.66506
105. Jiang J, Tang Q, Gong J, Jiang W, Chen Y, Zhou Q, et al. Radiosensitizer EXO-miR-197-3p inhibits nasopharyngeal carcinoma progression and radioresistance by regulating the AKT/mTOR axis and HSPA5-mediated autophagy. Int J Biol Sci. (2022) 18:1878–95. doi: 10.7150/ijbs.69934
106. Wang X, Zhang Y, Mu X, Tu CR, Chung Y, Tsao SW, et al. Exosomes derived from gammadelta-T cells synergize with radiotherapy and preserve antitumor activities against nasopharyngeal carcinoma in immunosuppressive microenvironment. J Immunother Cancer. (2022) 10:e003832. doi: 10.1136/jitc-2021-003832
107. Li HL, Deng NH, He XS, Li YH. Small biomarkers with massive impacts: PI3K/AKT/mTOR signalling and microRNA crosstalk regulate nasopharyngeal carcinoma. biomark Res. (2022) 10:52. doi: 10.1186/s40364-022-00397-x
108. Arjmand B, Alavi-Moghadam S, Rezaei-Tavirani M, Kokabi-Hamidpour S, Arjmand R, Gilany K, et al. GMP-compliant mesenchymal stem cell-derived exosomes for cell-free therapy in cancer. Methods Mol Biol. (2024) 2736:163–76. doi: 10.1007/7651_2022_467
109. Swanton C, Bernard E, Abbosh C, Andre F, Auwerx J, Balmain A, et al. Embracing cancer complexity: Hallmarks of systemic disease. Cell. (2024) 187:1589–616. doi: 10.1016/j.cell.2024.02.009
110. Luo W. Nasopharyngeal carcinoma ecology theory: cancer as multidimensional spatiotemporal “unity of ecology and evolution” pathological ecosystem. Theranostics. (2023) 13:1607–31. doi: 10.7150/thno.82690
111. Jiang Z, He J, Zhang B, Wang L, Long C, Zhao B, et al. A potential “Anti-warburg effect” in circulating tumor cell-mediated metastatic progression? Aging Dis. (2024). doi: 10.14336/AD.2023.1227
112. Fridlender ZG, Sun J, Kim S, Kapoor V, Cheng G, Ling L, et al. Polarization of tumor-associated neutrophil phenotype by TGF-beta: “N1” versus “N2” TAN. Cancer Cell. (2009) 16:183–94. doi: 10.1016/j.ccr.2009.06.017
113. Liu Q, Yang T, Zhang Y, Hu ZD, Liu YM, Luo YL, et al. ZIC2 induces pro-tumor macrophage polarization in nasopharyngeal carcinoma by activating the JUNB/MCSF axis. Cell Death Dis. (2023) 14:455. doi: 10.1038/s41419-023-05983-x
114. Li Z, Xu ZM, Chen WP, Du XJ, Ou CX, Luo ZK, et al. Tumor-repopulating cells evade ferroptosis via PCK2-dependent phospholipid remodeling. Nat Chem Biol. (2024). doi: 10.1038/s41589-024-01612-6
Keywords: nasopharyngeal carcinoma, bibliometric analysis, research trends, VOSviewer, CiteSpace
Citation: An G, Liu J, Lin T, He L and He Y (2024) Global trends in research of nasopharyngeal carcinoma: a bibliometric and visualization analysis. Front. Oncol. 14:1392245. doi: 10.3389/fonc.2024.1392245
Received: 27 February 2024; Accepted: 17 June 2024;
Published: 02 July 2024.
Edited by:
Omar Kujan, University of Western Australia, AustraliaReviewed by:
Weiren Luo, The Second Affiliated hospital of Southern University of Science and Technology, ChinaCopyright © 2024 An, Liu, Lin, He and He. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yingchun He, aGV5aW5nY2h1bkBobnVjbS5lZHUuY24=; Lan He, MzgyNzExMDA5QHFxLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.