Abstract
Introduction:
Early oral feeding (EOF) has been shown to improve postoperative recovery for many surgeries. However, surgeons are still skeptical about EOF after gastric cancer surgery due to possible side effects. This updated systematic review and meta-analysis aimed to investigate the efficacy and safety of EOF in patients after gastric cancer surgery.
Methods:
Randomized controlled trials (RCTs) investigating EOF in patients after gastric cancer surgery were searched in the databases of PubMed, Embase, Clinicaltrials.gov, and Cochrane from 2005 to 2023, and an updated meta-analysis was performed using RevMan 5.4 software.
Results:
The results of 11 RCTs involving 1,352 patients were included and scrutinized in this analysis. Hospital days [weighted mean difference (WMD), −1.72; 95% confidence interval (CI), −2.14 to −1.30; p<0.00001), the time to first flatus (WMD, −0.72; 95% CI, −0.99 to −0.46; p<0.00001), and hospital costs (WMD, −3.78; 95% CI, −4.50 to −3.05; p<0.00001) were significantly decreased in the EOF group. Oral feeding tolerance [risk ratio (RR), 1.00; 95% CI, 0.95–1.04; p=0.85), readmission rates (RR, 1.28; 95% CI, 0.50–3.28; p=0.61), postoperative complications (RR, 1.02; 95% CI, 0.81–1.29; p=0.84), anastomotic leakage (RR, 0.83; 95% CI, 0.25–2.78; p=0.76), and pulmonary infection (RR, 0.65; 95% CI, 0.31–1.39; p=0.27) were not significantly statistical between two groups.
Conclusion:
This meta-analysis reveals that EOF could reduce hospital days, the time to first flatus, and hospital costs, but it was not associated with oral feeding tolerance, readmission rates, or postoperative complications especially anastomotic leakage and pulmonary infection, regardless of whether laparoscopic or open surgery, partial or total gastrectomy, or the timing of EOF initiation.
Introduction
Gastric cancer (GC) constitutes a significant global health challenge, characterized by a poor prognosis (1). It ranks fifth in incidence among malignancies and fourth in cancer-related mortality worldwide (1). Surgical intervention remains the primary therapeutic approach for gastric cancer (2). Subtotal and total gastrectomy represent the most frequently employed surgical techniques in the management of gastric cancer patients (3). Nevertheless, malnutrition frequently afflicts individuals diagnosed with gastric cancer. Malnutrition in gastric cancer patients significantly elevates the risk of postoperative complications and adversely affects overall survival rates following gastrectomy (4). Consequently, ensuring adequate nutritional support is paramount for enhancing postoperative outcomes in gastric cancer patients.
In order to accelerate the postoperative recovery of patients after gastrointestinal surgery, the concept of fast track surgery (FTS) or enhanced recovery after surgery (ERAS) has been proposed and applied in a variety of operations (5, 6), such as colorectal cancer (7), lung cancer (8), liver cancer (9), and gynecological surgery (10). Early oral feeding (EOF) is widely recognized as a critical component of FTS and ERAS protocols and has demonstrated favorable outcomes following gynecological tumor surgery and colorectal procedures (11, 12). Despite evidence from several studies indicating the safety and efficacy of EOF post-gastrectomy (13–15), clinical surgeons remain cautious about its implementation in gastric cancer surgery, citing potential adverse effects such as anastomotic leakage, oral feeding intolerance, and aspiration pneumonia.
To further elucidate the efficacy and safety of EOF, we performed a comprehensive and updated systematic review and meta-analysis of randomized controlled trials (RCTs), aimed at offering evidence-based guidance for clinicians. Additionally, we examined the impact of various surgical approaches (laparoscopy versus open surgery, total gastrectomy versus subtotal gastrectomy) and the timing of early feeding on the outcomes of EOF in postoperative gastric cancer patients.
Methods
Search strategy
The relevant RCTs in which language was restricted to English from 2005 to 2023 were searched from PubMed, Embase, Clinicaltrials.gov, and Cochrane. Search terms include “early oral feeding or early oral intake,” “gastric cancer,” “gastrectomy”, “fast track surgery,” and “enhanced recovery after surgery.” Only RCTs were included in the meta-analysis after all studies were browsed by two independent reviewers.
Criteria for selection
The inclusion criteria were as follows: (1) RCTs, (2) adult patients (≥18 years old), (3) gastric cancer patients receiving oral feeding after gastrectomy, and (4) data on the outcomes related to hospital days, the time to first flatus, hospital costs, oral feeding tolerance, readmission rates, postoperative complications, anastomotic leakage, and pulmonary infection.
The exclusion criteria were as follows: animal trials, non-RCTs, comments, case reports, letters, ongoing trials, protocols, and studies lacking of applicable data.
Data extraction
Two researchers extracted the following data independently: first author, year, country, trial design, surgical approach, sample size, time of oral feeding, age, and gender (m/f). The outcomes analyzed in the meta-analysis included (1) hospital days, (2) the time to first flatus, (3) hospital costs, (4) oral feeding tolerance, (5) postoperative complications, (6) readmission rates, (7) anastomotic leakage, and (8) pulmonary infection. Two sets of data were collected when there were two operation methods within the same RCTs.
Quality assessment
The quality of the retrieved RCTs was assessed through the Cochrane risk of bias tool (16). The quality items were random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective outcome reporting, and other bias. Any uncertainties about the quality assessment were resolved through the discussion among all reviewers.
Data analysis
Review Manager version 5.4 was used to analyze the data included in the meta-analysis. The continuous data of the included RCTs were elucidated using weighted mean difference (WMD) along with the corresponding 95% confidence interval (CI). The dichotomous data of the included RCTs were performed by using the risk ratio (RR). Estimation of the sample mean and standard deviation from the sample median and range was accomplished according previously published methods (17, 18). The result of meta-analysis was statistical significant when p-value < 0.05. The heterogeneity in data analysis was analyzed by using I2 statistic. A fixed effect model would be used for results where the I2 value is <50% and has insignificant heterogeneity. Otherwise, the random effect model was applied.
Subgroup analysis, sensitivity analysis, and publication bias
Subgroup analyses were performed according to the pre-specified factors: operation methods (laparoscopy vs. open surgery, total gastrectomy vs. subtotal gastrectomy) and early feeding time on EOF of patients with gastric cancer after operation. A sensitivity analysis was performed by excluding trials recruiting participants with particular conditions or trials with characteristics that were different from those in other trials. Publication bias was assessed with funnel plots and Egger’s and Begg’s tests. A p-value < 0.05 was considered statistically significant.
Results
Characteristics of the individual studies
A total of 686 articles were identified according to the searching terms from each database. Following a preliminary screening of titles and abstracts, 331 trials were excluded. A total of 47 articles were exclude due to lack of useful data, non-RCTs, study protocol, etc. Finally, 11 RCTs (19–29) encompassing 1,352 participants (EOF group: 671 participants vs. traditional oral feeding (TOF) group: 681 participants) were included in our meta-analysis (Figure 1). The characteristics of the 11 selected RCTs are revealed in Table 1.
Figure 1
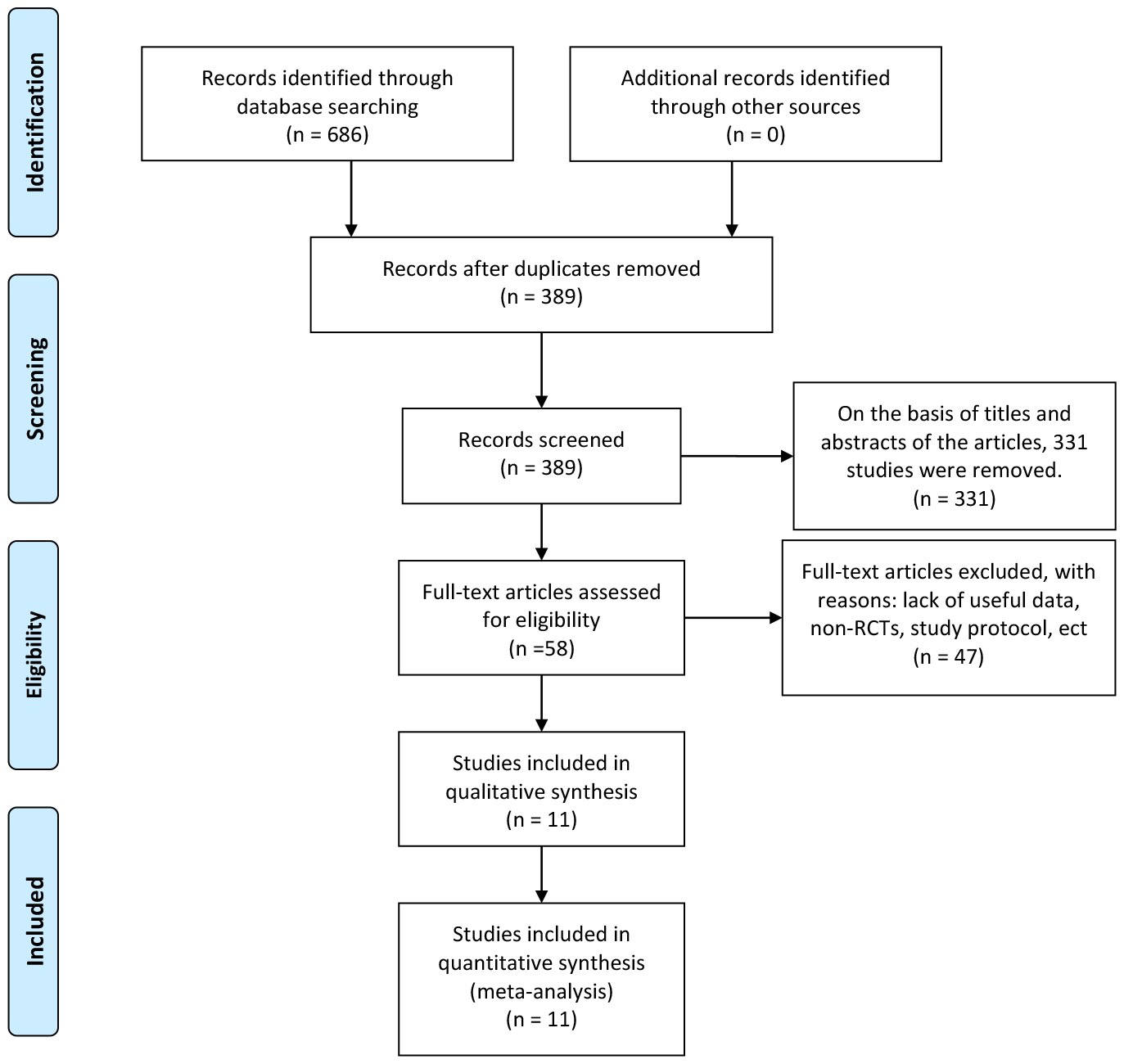
Flowchart of study inclusion.
Table 1
| Study, year, country | Trial design | Surgical approach | Sample size | Time of oral feeding | Age | Gender (m/f) | ||
|---|---|---|---|---|---|---|---|---|
| EOF | DOF | EOF | DOF | |||||
| Liu et al., 2010 (19), China | RCT | Open gastrectomy, with lymph node dissection | 33 | 30 | Day of surgery: Oral intake clear fluid ≈50 mL POD1: Semiliquid diet 50–100 mL POD2: Semiliquid diet 100–200 mL POD3: Semiliquid diet 200–400 mL POD4: Semiliquid diet POD5: Solid diet |
Diet reintroduced in same manner as in optimized group on PODs 1, 2, and 3 after bowel venting. POD5: Semiliquid diet POD5+: Solid diet |
28–81 | 33/29 |
| Wang et al., 2010 (20), China | RCT | Open distal or proximal subtotal resection or total gastrectomy, standard D2 total gastrectomy | 45 | 47 | Day of surgery: a little clear water POD1: 0.5 L liquid (follow a stepwise plan from water to other liquids to semi-fluids to normal food; adhere to the premise of eating little and often) POD2: 1 L liquid+others as above POD3: Continue as above |
Oral intake is initiated if normal bowel sounds are heard (follow a stepwise plan from water to other liquids to semi-fluids to normal food; adhere to the premise of eating little and often) | <80 | 61/31 |
| Hur et al., 2011 (21), Korea | RCT | Open subtotal gastrectomy or total gastrectomy | 28 | 26 | POD1: sips of water POD2: liquid diet POD3: a soft diet |
POD3: sips of water POD4–5: liquid diet POD6: a soft diet |
<65 or >=65 | 33/21 |
| Chen Hu et al., 2012 (22), China | RCT | laparoscopy-assisted radical distal gastrectomy or open distal gastrectomy | 40 | 42 | 6–8h after surgery, following a stepwise program from warm clear water to carbohydrate drink to TPF, then to semi-fluids to normal food. | After the first flatus, the patient progressed from fluids to semi-fluids and then to normal food. |
25–75 | 41/41 |
| Kim et al., 2012 (23), Korea | RCT | laparoscopic distal gastrectomy with intracorporeal anastomosis (Billroth I, Billroth II, or Roux-en-Y type) | 22 | 22 | SOW were started 48 h after surgery POD2: Clear liquid diet at dinner POD3: Clear liquid diet at breakfast, full liquid diet at lunch and dinner POD4: Soft diet at breakfast and lunch |
SOW after flatus, diet build-up; three steps (clear liquid–full liquid–soft diet), one step a day, from the day after start day of SOW | Unclear, mean 55.05 | |
| Feng et al., 2013 (24), China | RCT | Standard laparotomy approach, standard D2 total gastrectomy | 59 | 60 | Day of surgery: 500–1,000 mL glucose saline. POD1: 2,000–3,000 mL liquid food containing 1,000–1,200 kcal per day |
Oral intake initiated after flatus (following a stepwise plan from water to other liquids to semi-fluids to normal food) | 28–75 | 85/34 |
| Hong et al., 2014 (25), China | RCT | laparoscopic distal gastrectomy | 40 | 44 | POD1–2: a clear liquid diet POD3: a soft diet |
POD 3–4: clear liquid diet; POD 5: soft diet. | 18–75 | 59/25 |
| Li et al., 2015 (26), China | RCT | Open radical gastrectomy | 150 | 150 | POD1: a small amount of drinking water. POD2: 500 mL of fractionated oral enteral nutrition POD3: 1,000 mL of oral Jevity was given multiple times in addition to a small amount of liquid diet |
After anal exhaust, the patient began to drink water orally. If no discomfort, the intake of water and liquid and semi-liquid diets were gradually increased. | Unclear, mean 59.8 | 154/146 |
| Shimizu et al., 2018 (27), Japan | RCT | distal or total gastrectomy either laparoscopically or via laparotomy | 102 | 114 | POD1–3: a diet of iEAT® POD 4: ordinary hospital diets |
conventional nutritional management | 20–80 | 137/79 |
| Wang et al., 2019 (28), China | RCT | total laparoscopic radical gastrectomy, standard D2 lymphadenectomy, and the main digestive tract anastomoses | 51 | 49 | POD1: liquid food on the morning POD2–6: liquid foods and semi-liquids were adopted according to the different tolerances of different individuals |
POD4–6: liquid food POD7: the same oral intake as the EOF group |
18–75 | 71/29 |
| Gao et al., 2019 (29), China | RCT | laparoscopic distal gastrectomy or laparoscopic radical gastrectomy | 101 | 97 | POD2: the oral fluid diet POD3: Semi-liquid food and soft food Insufficient intake of oral nutrition was supplemented by intravenous fluids. |
Oral intake initiated after flatus (following a stepwise plan from water to other liquids to semi-fluids to normal food) | 44–80 | 123/75 |
General characteristics of the studies included.
EOF, early oral feeding; TOF, traditional oral feeding; POD, postoperative day; SOW, sips of water; iEAT®, a commercially available food (EN Otsuka Pharmaceutical Co., Ltd., Hanamaki, Japan).
Quality of the RCTs
All of the 11 articles included in the meta-analysis were RCTs. The assessment of risk of bias of studies is shown in Figure 2. It was difficult to ensure blinding because the oral feeding was easy to distinguish.
Figure 2
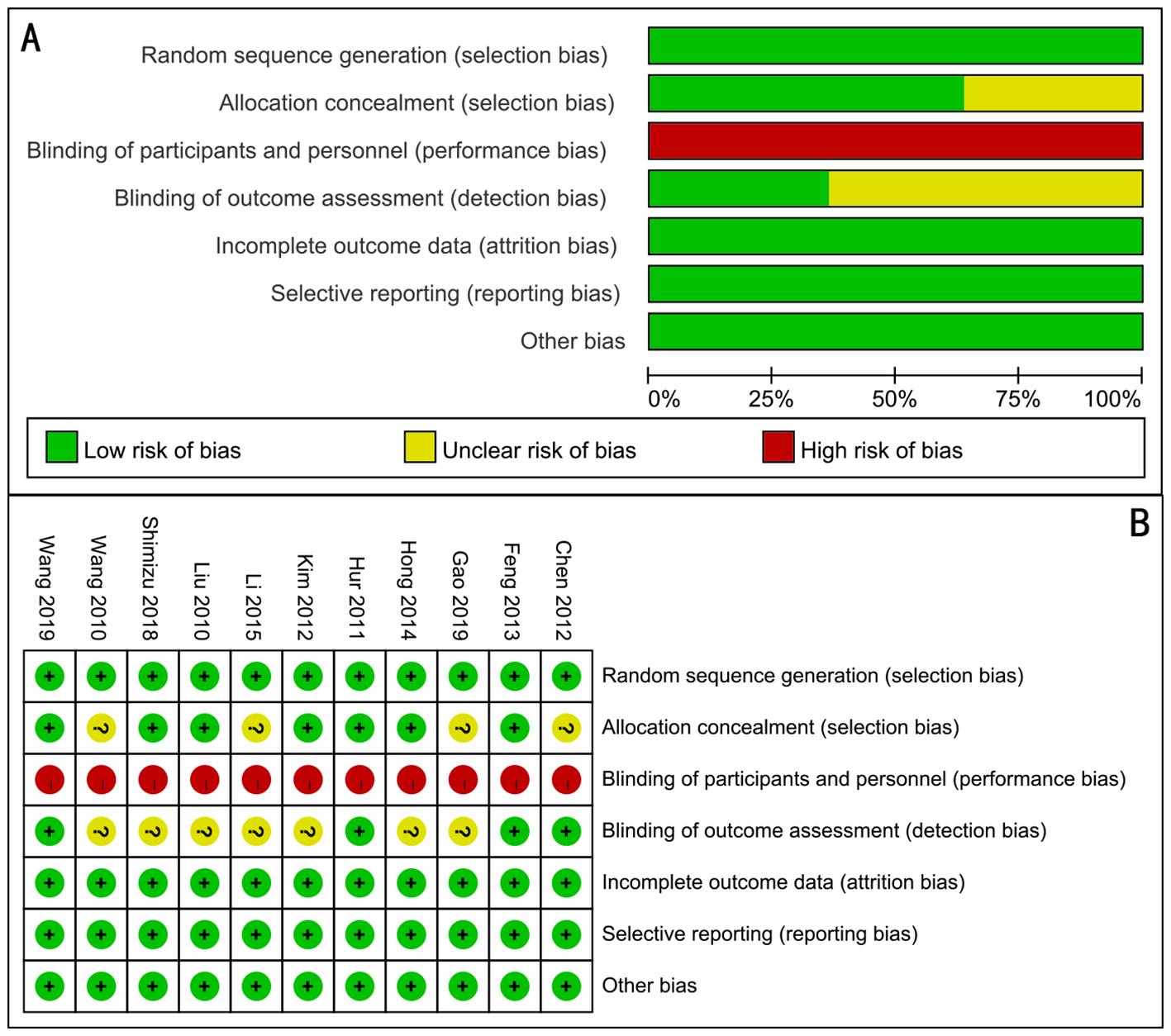
Assessment of randomized study quality. (A) Risk of bias graph, (B) risk of bias summary.
Result of meta-analysis
Hospital days
A total of 10 RCTs encompassing a total of 1,154 patients were analyzed to assess the length of hospital stay. A random-effects model was employed to evaluate the difference in hospital day duration between the two groups. The forest plot illustrated a significant reduction in hospital stay duration for the EOF group compared to the TOF group (WMD, −1.72; 95% CI, −2.14 to −1.30; p<0.00001) (Figure 3A).
Figure 3
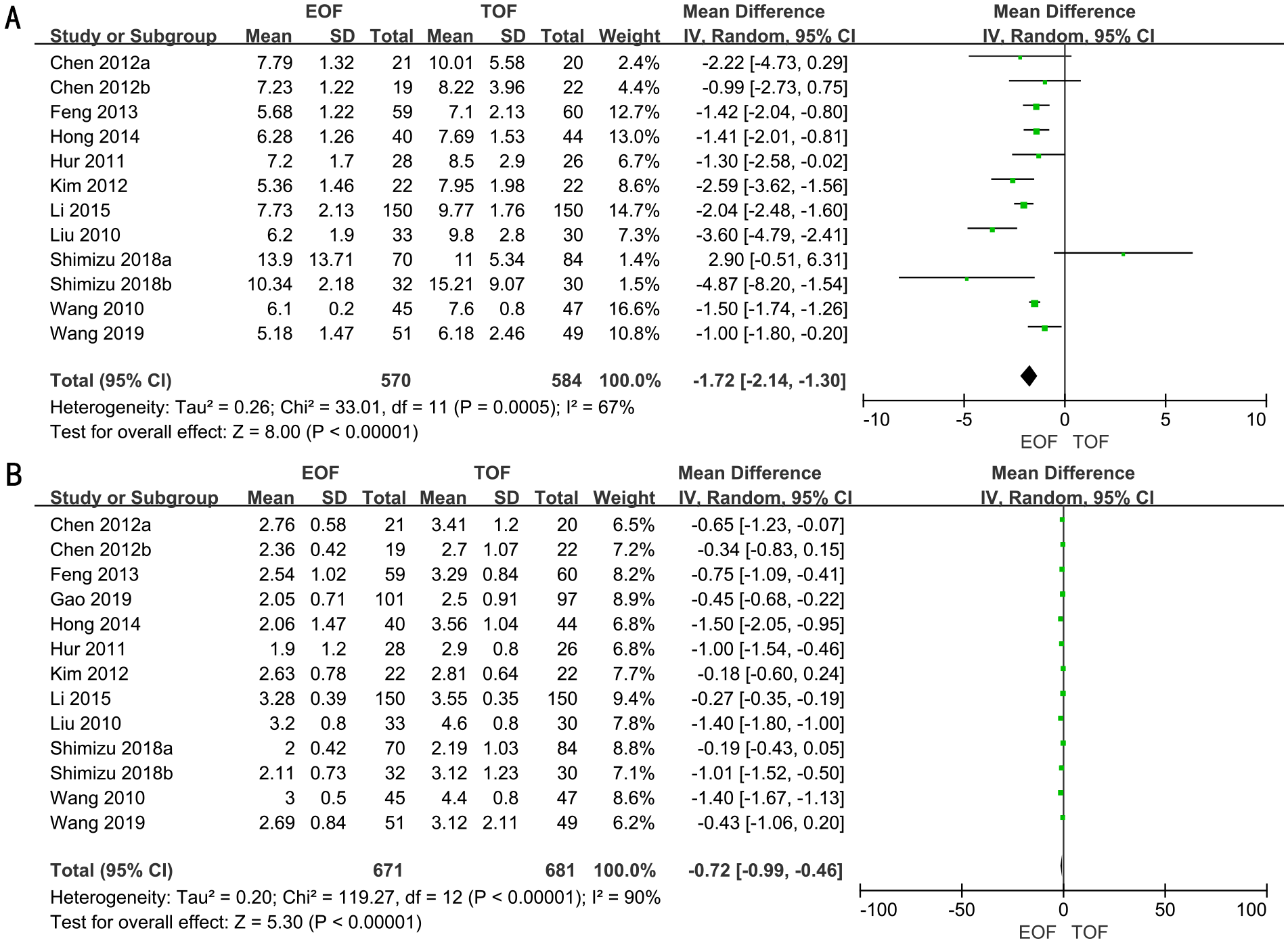
Forest plots of hospital day (A) and the time to first flatus (B).
Subgroup analyses revealed that patients in the EOF group experienced significantly shorter hospital stays compared to the TOF group, irrespective of surgical approach (laparoscopic vs. open surgery), extent of gastrectomy (partial vs. total), or the timing of EOF initiation (Supplementary Figure 1).
The time to first flatus
A total of 11 RCTs, involving 1,352 patients, were adopted to analyze the time to first flatus. Employing a random-effects model, we assessed the difference in time to first flatus between two groups. The result revealed that the time to first flatus was significantly shorter in the EOF groups (WMD, −0.72; 95% CI, −0.99 to −0.46; p<0.00001) (Figure 3B).
Subgroup analyses indicated that patients in the EOF group experienced a significantly shorter time to first flatus compared to the TOF group, regardless of the type of surgery (laparoscopic vs. open), the extent of gastrectomy (partial vs. total), or the timing of EOF initiation (Supplementary Figure 2).
Hospital costs
Seven RCTs full of 791 patients were applied to assess the hospital costs. We adopted a fixed-effects model to evaluate the hospital costs between two groups (WMD, −3.78; 95% CI, −4.50 to −3.05; p<0.00001). The forest plot revealed a significant decrease in hospital costs within the EOF group compared to the TOF group (Figure 4A).
Figure 4
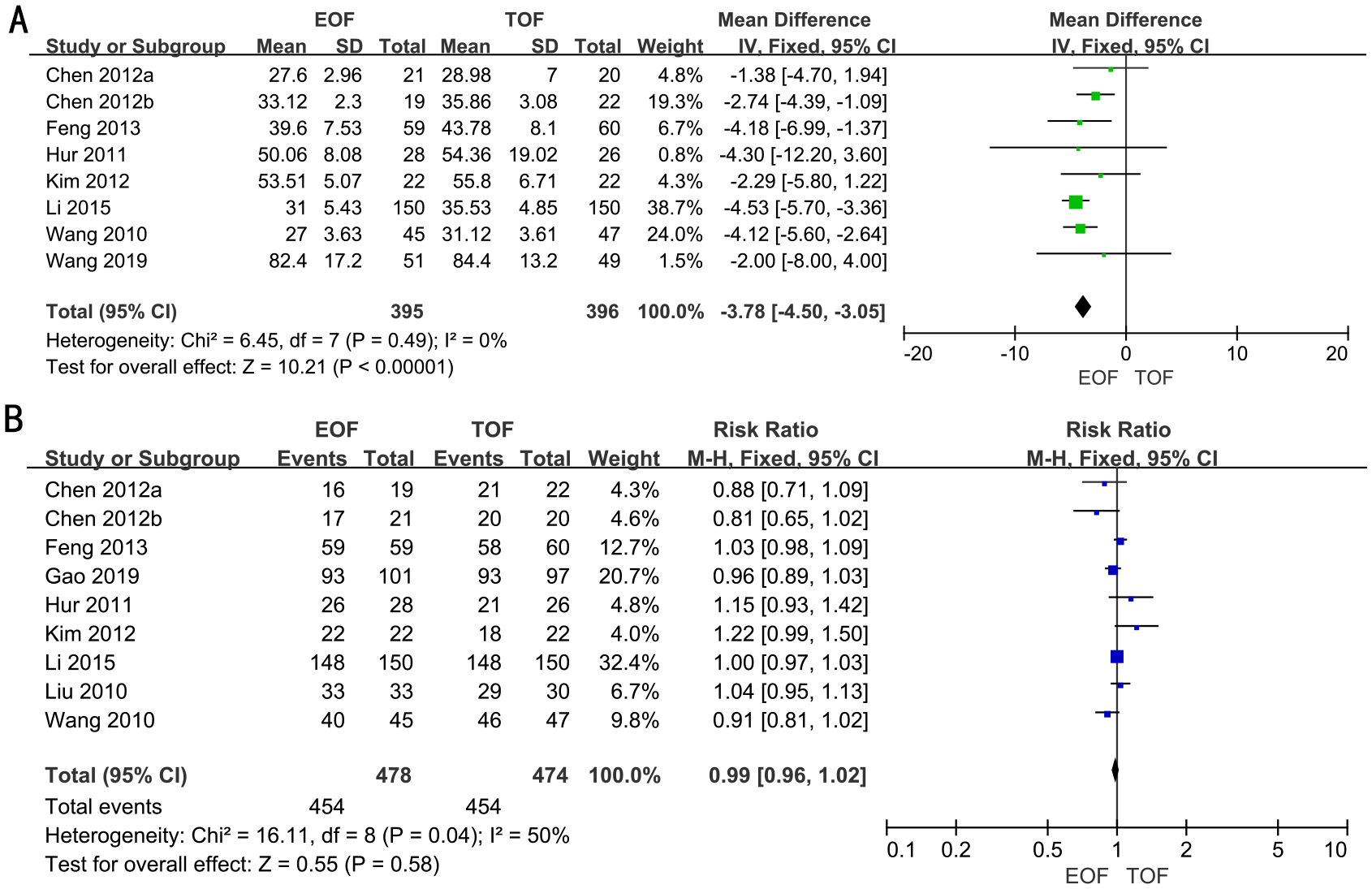
Forest plots of hospital costs (A) and oral feeding tolerance (B).
Subgroup analyses demonstrated that patients in the EOF group incurred lower hospital costs compared to those in the TOF group, irrespective of surgical method (laparoscopic vs. open), extent of gastrectomy (partial vs. total), or the timing of EOF initiation (Supplementary Figure 3).
Oral feeding tolerance
Eight RCTs full of 952 patients were adopted to evaluate oral feeding tolerance. A random-effects model was used to assess oral feeding tolerance between two groups [risk ratio (RR), 1.00; 95% CI, 0.95–1.04; p=0.85). The results revealed that there was no significant difference in oral feeding tolerance between the two groups (Figure 4B).
Subgroup analyses revealed that patients in both groups exhibited similar oral feeding tolerance, irrespective of the surgical approach (laparoscopic vs. open), the extent of gastrectomy (partial vs. total), or the timing of EOF initiation (Supplementary Figure 4).
Postoperative complications
In 11 RCTs, enrolling 1,352 patients, the rate of postoperative complications was analyzed. We adopted a fixed-effects model. The forest plot revealed that the improvements in the rate of postoperative complications were similar between the two groups (RR, 1.02; 95% CI, 0.81–1.29; p=0.84) (Figure 5A).
Figure 5
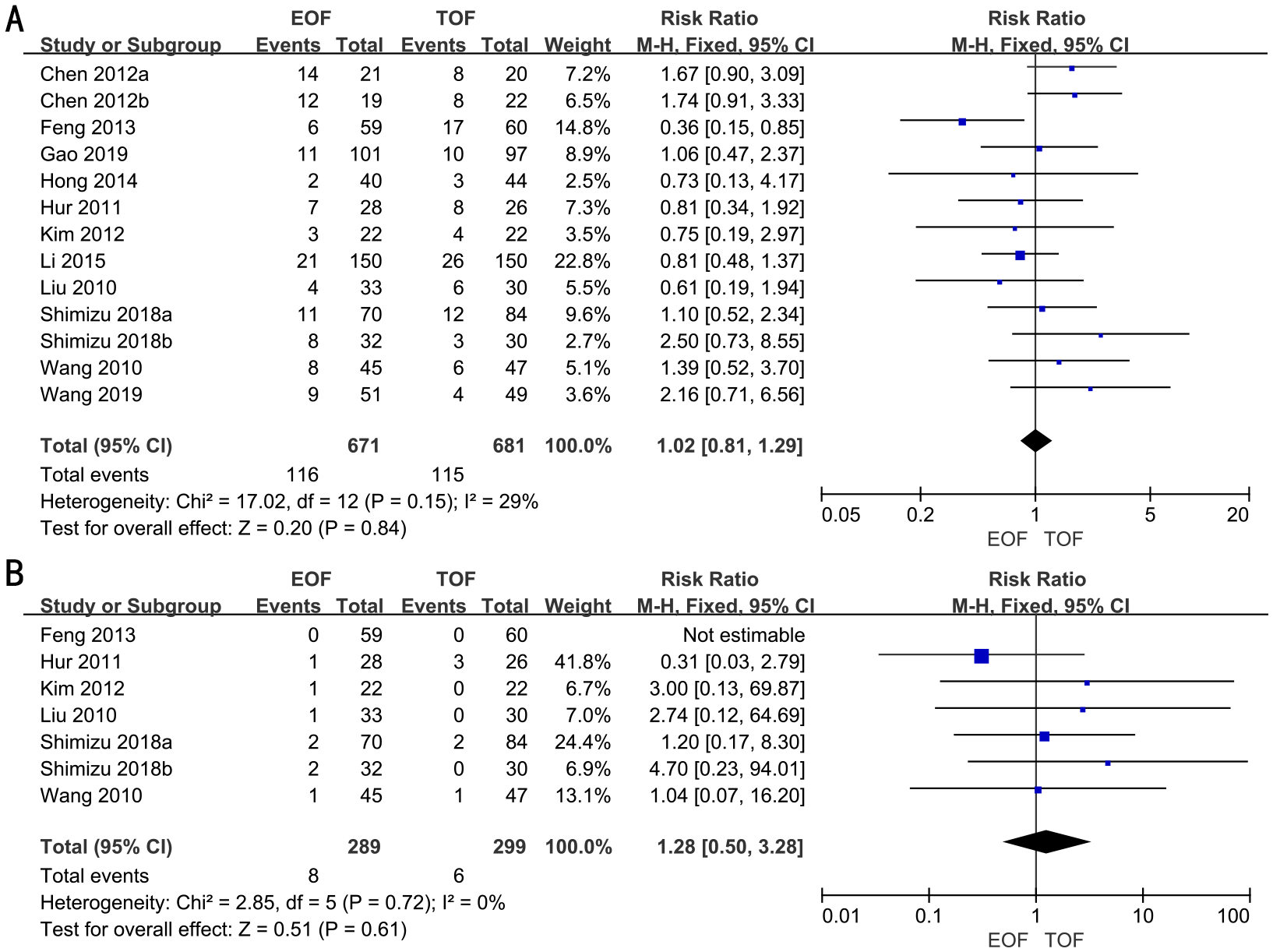
Forest plots of postoperative complications (A) and readmission rates (B).
Subgroup analyses indicated that there was no significant difference in the rate of postoperative complications between the groups, irrespective of surgical approach (laparoscopic vs. open), extent of gastrectomy (partial vs. total), or timing of EOF initiation (Supplementary Figure 5).
Readmission rates
Six RCTs full of 588 patients were applied to assess the readmission rates. A fixed-effects model was adopted. The result revealed that there was no significant difference in the readmission rates in both groups (RR, 1.28; 95% CI, 0.50–3.28; p=0.61) (Figure 5B).
Subgroup analyses revealed that readmission rates were similar between the two groups, regardless of the type of surgery (laparoscopic vs. open), the extent of gastrectomy (partial vs. total), or the timing of EOF initiation (Supplementary Figure 6).
Anastomotic leakage
In 11 RCTs, enrolling 1,352 patients, the rate of anastomotic leakage was analyzed. We adopted a fixed-effects model. The forest plot revealed that the improvements in the rates of anastomotic leakage were similar between the two groups (RR, 0.83; 95% CI, 0.25–2.78; p=0.76) (Figure 6A).
Figure 6
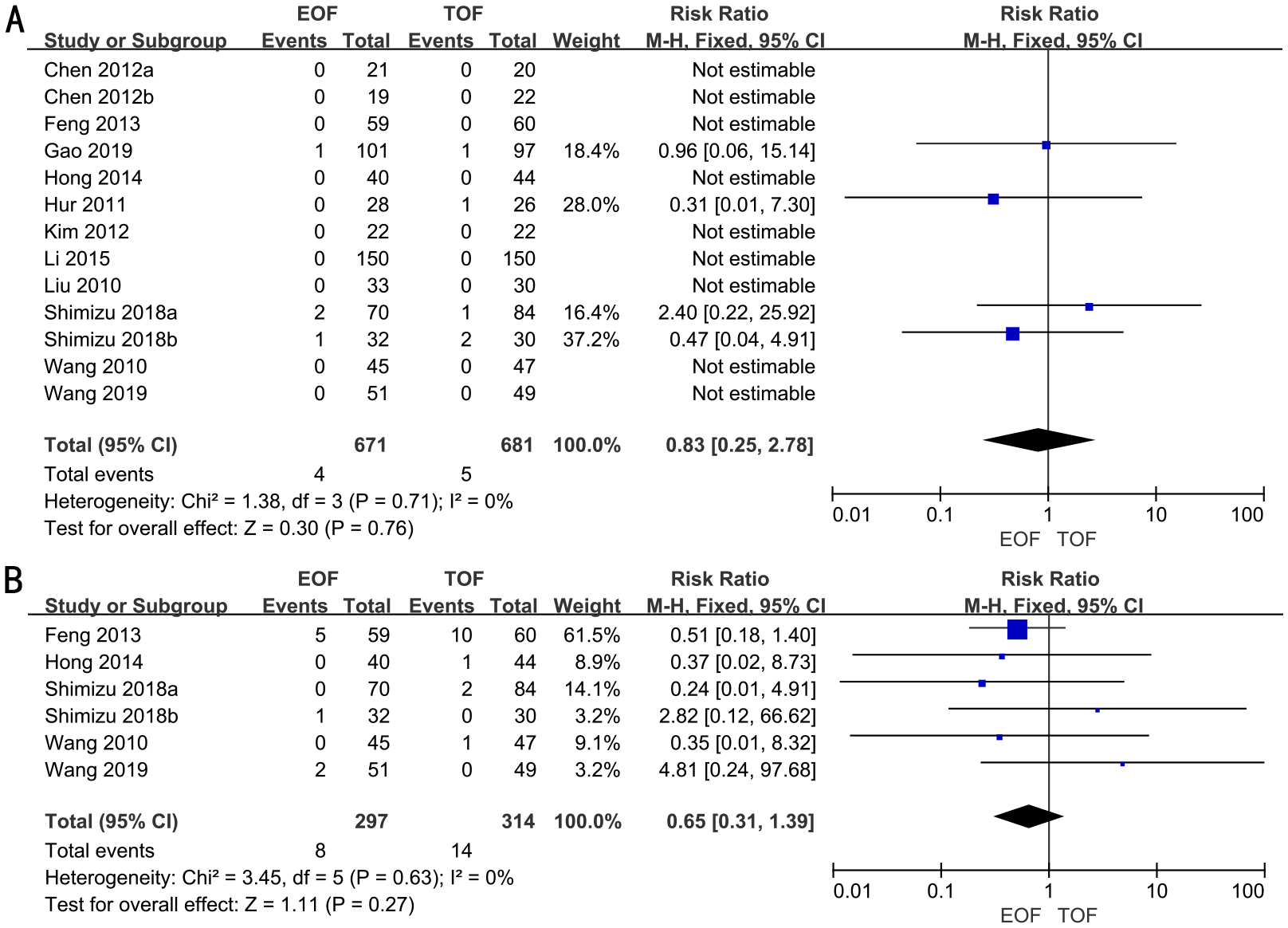
Forest plots of anastomotic leakage (A) and pulmonary infection (B).
Subgroup analyses indicated that there was no significant difference in the rates of anastomotic leakage between the groups, irrespective of surgical approach (laparoscopic vs. open), extent of gastrectomy (partial vs. total), or timing of EOF initiation (Supplementary Figure 7).
Pulmonary infection
Five RCTs full of 611 patients were applied to assess the rate of pulmonary infection. A fixed-effects model was adopted. The result revealed that there was no significant difference in the rates of pulmonary infection in both groups (RR, 0.65; 95% CI, 0.31–1.39; p=0.27) (Figure 6B).
Subgroup analyses indicated that the rates of pulmonary infection were similar between the two groups, irrespective of the surgical approach (laparoscopic vs. open), extent of gastrectomy (partial vs. total), or timing of EOF initiation (Supplementary Figure 8).
Sensitivity analysis
We performed a sensitivity analysis by including only studies with total sample size of at least 50 participants to evaluate the robustness of the outcomes (Table 2). The results indicated that the sensitivity analysis did not substantially alter the outcomes. The sensitivity analysis results affirmed the reliability of the evidence presented in this meta-analysis.
Table 2
| Outcomes | Number of studies | Patients | WMD/RR | 95% CI | p-value | I2 | |
|---|---|---|---|---|---|---|---|
| EOF | DOF | ||||||
| Hospital days | 9 | 508 | 520 | −1.66 | −2.12, −1.19 | <0.00001 | 72 |
| The time to first flatus | 9 | 609 | 617 | −0.82 | −1.14, −0.50 | <0.00001 | 92 |
| Hospital costs | 5 | 333 | 332 | −4.31 | −5.16, −3.45 | <0.00001 | 0 |
| Oral feeding tolerance | 6 | 416 | 410 | 1.00 | 0.97, 1.02 | 0.78 | 36 |
| Postoperative complications | 9 | 609 | 617 | 0.92 | 0.71, 1.21 | 0.56 | 20 |
| Readmission rates | 6 | 267 | 277 | 1.15 | 0.43, 3.13 | 0.78 | 0 |
| Anastomotic leakage | 9 | 609 | 617 | 0.83 | 0.25, 2.78 | 0.76 | 0 |
Sensitivity analysis results.
EOF, early oral feeding; TOF, traditional oral feeding; WMD, weighted mean difference; RR, risk ratio; CI, confidence interval.
Publication bias
A funnel plot was generated to evaluate publication bias in the meta-analysis of postoperative complications. The plot indicated no evidence of publication bias (Figure 7). Furthermore, publication bias was not detected in the analyses of “Hospital days,” “Time to first flatus,” “Hospital costs,” “Oral feeding tolerance,” “Readmission rates,” “Anastomotic leakage,” and “Pulmonary infection” (Supplementary Figure 9).
Figure 7
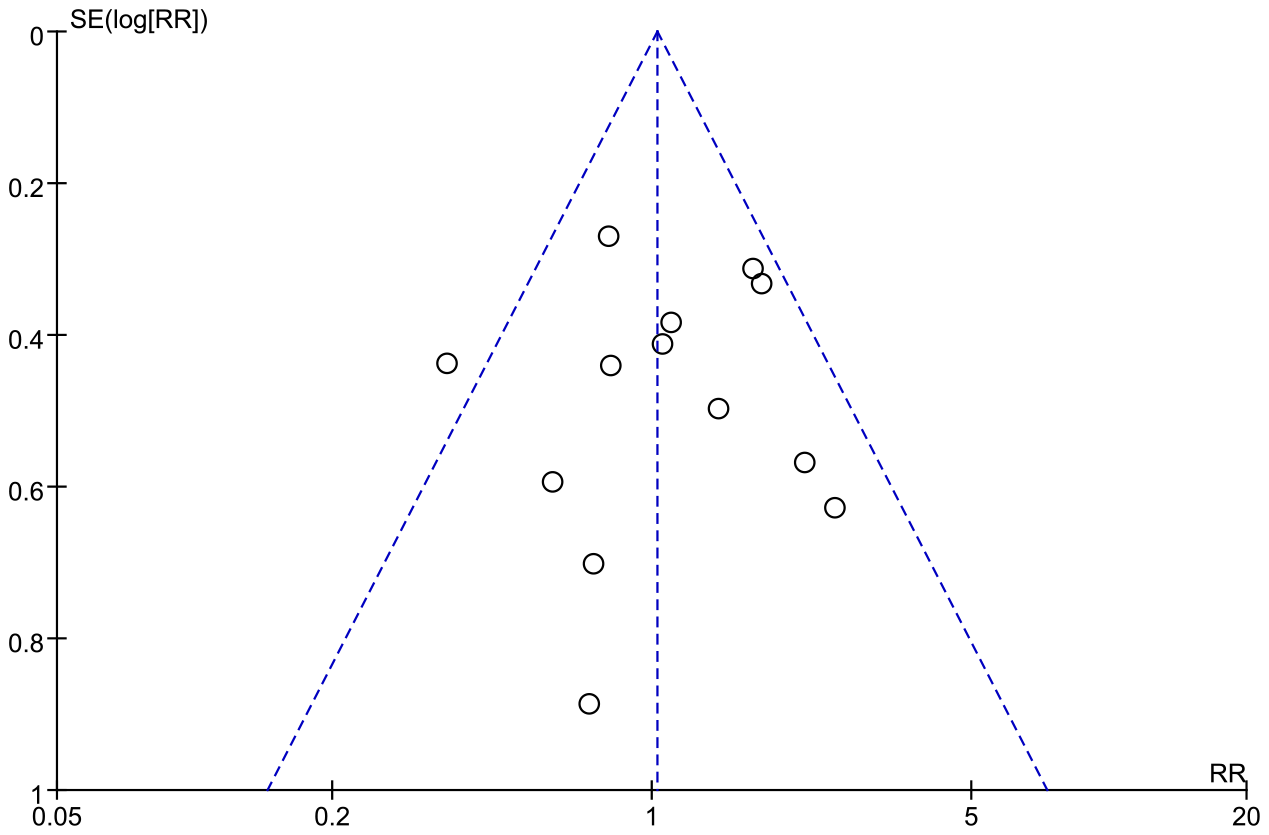
Funnel plot of the studies represented in the meta-analysis. RR, risk ratio; SE, standard error.
Discussion
The updated systematic review and meta-analysis included 11 RCTs that provided the most recent data on the safety and efficacy of EOF after gastrectomy for gastric cancer. The results indicated that EOF significantly reduced hospital stay duration, expedited time to first flatus, and lowered hospitalization costs. Additionally, EOF did not increase the incidence of oral feeding intolerance, readmission rates, or postoperative complications, including anastomotic leakage and pulmonary infection. Subgroup analyses based on surgical approach (laparoscopic vs. open), extent of gastrectomy (partial vs. total), and timing of EOF initiation, along with sensitivity analyses, corroborated these findings.
Gastrectomy is the definitive treatment for patients with gastric cancer (30), and their nutritional status is also very important. However, malnutrition is prevalent among gastric cancer patients due to tumor-related factors, nausea, vomiting, bleeding, obstruction, surgical trauma, and metabolic disorders, and it is associated with increased postoperative complication rates (31–33). Therefore, nutritional support is particularly important for patients with gastric cancer after surgery. EOF, an essential part of FTS and ERAS, has been suggested to benefit patients with gastric cancer after surgery (34). However, the clinical application of EOF after gastric cancer surgery remains controversial. The main reason is the worry about the incidence of postoperative complications, such as anastomotic leakage, oral feeding intolerance, and aspiration pneumonia.
The present meta-analysis revealed that EOF after gastrectomy for gastric cancer would not increase the incidence of oral feeding intolerance, readmission rates, or postoperative complications, especially anastomotic leakage and pulmonary infection. In the traditional surgical treatment of gastric cancer, patients underwent routine fasting and gastrointestinal decompression for 5–7 days after surgery until exhausted based on the concern that EOF would increase vomiting, flatulence, anastomotic fistula, etc. (29, 35, 36). Nelson reported that gastrointestinal decompression did not reduce the incidence of postoperative complications (37). Even gastrointestinal decompression was found to increase the rate of pulmonary complications and prolong the time to first flatus (38–40). Lu showed that there was no significant difference in the incidence of EOF intolerance, such as abdominal distension and nausea (14). A meta-analysis also reported that feeding intolerance was comparable between EOF and TOF groups (41). For readmission rates, multiple studies and meta-analyses showed that readmission rates were similar between two groups (41–43). Concerning postoperative complications, especially anastomotic leakage and pulmonary infection, in 2004, Suehiro first showed that there was no evidence that EOF would increase postoperative complications and mortality, including anastomotic leakage (44). Jang’s study demonstrated EOF could facilitate early bowel recovery without increasing the incidence of complications, including anastomotic leakage and aspiration pneumonia (45). They also showed a lower incidence of anastomotic leakage in the EOF group; this may be related to improvement in operation techniques, devices, and perioperative care with time (45). A propensity score matching analysis reported that no significant differences were found in the postoperative complications (42). Tadano revealed that EOF was beneficial in the recovery of peristalsis and promoted anastomotic healing in the rat model (46). Another animal study also reported that EOF can promote anastomotic healing and strengthen anastomotic strength of intestinal and somatic tissues after upper digestive tract surgery (47). However, Shimizu demonstrated that EOF increased the incidence of postoperative complications in the distal gastrectomy group, especially delayed gastric emptying (27). The possible reason is that EOF may increase food consumption on postoperative day (POD) 4 and thereafter, which led to delayed gastric emptying. A meta-analysis showed that EOF could reduce the incidence of postoperative complications in the total gastrectomy group but increase it in the distal gastrectomy group, possibly due to the small number of RCTs in each subgroup (43). The subgroup analyses in the present meta-analysis showed that EOF was not associated with oral feeding tolerance, readmission rates, or postoperative complications especially anastomotic leakage and pulmonary infection, regardless of whether laparoscopic and open surgery or partial and total gastrectomy or the timing of EOF initiation. Therefore, EOF is safe and feasible for gastric cancer patients after gastrectomy.
The findings of this meta-analysis showed that EOF could significantly shorten the hospital days, accelerate exhaustion, and reduce hospitalization costs. Minig reported that EOF was not only easily absorbed but also could accelerate intestinal peristalsis recovery, protect intestinal mucosal barrier function, and enhance immune response (48). Moreover, intestinal nutrients play an important role in regulating gastrointestinal function (49). Pilichiewicz also demonstrated that dietary macronutrients regulate gastrointestinal motor function and hormone secretion in a load-dependent manner (50). Both Lu and Gao reported that the levels of gastrointestinal hormones were significantly higher on POD 5 in the EOF group, which could speed up the recovery of gastrointestinal function (14, 29). Other studies showed that EOF could be beneficial in leading to faster gastrointestinal function recovery and nutritional improvement of patients with gastric cancer after surgery (51, 52). All these findings provide a theoretical basis for reducing hospital stays and accelerating exhaustion. Shoar revealed that EOF after surgery can promote gastrointestinal function recovery and reduce postoperative hospitalization for patients with upper gastrointestinal malignant tumors (53). Two retrospective studies also indicated that EOF could shorten the length of hospital stay and improve the recovery of bowel functions after the surgery of gastric cancer (14, 45). Two meta-analyses showed that EOF significantly decreased hospital costs for patients with gastric cancer after surgery (41, 43). Sindler also reported that EOF had no risk of several possible postoperative morbidities after upper GI surgeries but has several advantageous effects on a patient’s recovery (54). However, Wang and Hur reported that there were no differences in hospital costs between the two groups, which may be related to the charging standards of different hospitals, and oral on-site nutritional formulations (21, 28). Conversely, the reduction in hospitalization costs by EOF may be related to the shortened length of hospitalization. Subgroup analyses also showed consistent results regardless of whether laparoscopic and open surgery or partial and total gastrectomy or the timing of EOF initiation.
This meta-analysis encompasses a greater number of RCTs and patients compared to prior meta-analyses. Notably, we also analyzed the effect of EOF on postoperative pneumonia, an aspect frequently overlooked in prior meta-analyses. The subgroup analyses further confirm the robustness and reliability of our findings. However, there are still some limitations in our study. First, the inclusion criteria of these RCTs were different; some studies excluded patients with advanced gastric cancer, which contributed to potential heterogeneity in the results. Second, most RCTs had small sample sizes and were single-center studies. Third, the methodological quality was poor due to easy differentiation of feeding methods. Fourth, all RCTs were conducted in Asian countries; thus, the population may not be representative. Fifth, oral feeding regimens were inconsistent, with some patients receiving water or liquid diets and others receiving enteral nutrition preparations. Additionally, the timing of oral feeding varied. Finally, some surgeons and nurses were hesitant to implement early oral feeding in gastric cancer surgery patients, potentially introducing selection bias in the clinical trials and our study. Thus, given these limitations, we conducted subgroup and sensitivity analyses, which yielded consistent results.
Conclusion
EOF could reduce hospital stays, the time to first flatus, and hospital costs, but it was not associated with oral feeding tolerance, readmission rates, or postoperative complications especially anastomotic leakage and pulmonary infection, regardless of whether laparoscopic or open surgery, partial or total gastrectomy, or the timing of EOF initiation. Therefore, EOF might be safe and effective for gastric cancer patients after gastrectomy. However, further multi-center, multi-regional, and more standardized RCTs are required to address the limitations of the current studies.
Statements
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author contributions
DX: Software, Writing – original draft. JPL: Data curation, Writing – original draft. JCL: Data curation, Writing – original draft. PW: Data curation, Writing – original draft. JD: Project administration, Resources, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2024.1390065/full#supplementary-material
Supplementary Figure 1Subgroup analysis of Hospital day based on operative approach (A), the range of gastrectomy (B), the time to start EOF (C).
Supplementary Figure 2Subgroup analysis of The time to first flatus based on operative approach (A), the range of gastrectomy (B), the time to start EOF (C).
Supplementary Figure 3Subgroup analysis of Hospital costs based on operative approach (A), the range of gastrectomy (B), the time to start EOF (C).
Supplementary Figure 4Subgroup analysis of Oral feeding tolerance based on operative approach (A), the range of gastrectomy (B), the time to start EOF (C).
Supplementary Figure 5Subgroup analysis of Postoperative complications based on operative approach (A), the range of gastrectomy (B), the time to start EOF (C).
Supplementary Figure 6Subgroup analysis of Readmission rates based on operative approach (A), the range of gastrectomy (B), the time to start EOF (C).
Supplementary Figure 7Subgroup analysis of Anastomotic leakage based on operative approach (A), the range of gastrectomy (B), the time to start EOF (C).
Supplementary Figure 8Subgroup analysis of Pulmonary infection based on operative approach (A), the range of gastrectomy (B), the time to start EOF (C).
Supplementary Figure 9Funnel plot of Hospital days (A), The time to first flatus (B), Hospital costs (C), Oral feeding tolerance (D), Readmission rates (E), Anastomotic leakage (F), Pulmonary infection (G).
References
1
Sung H Ferlay J Siegel RL Laversanne M Soerjomataram I Jemal A et al . Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2021) 71:209–49. doi: 10.3322/caac.21660
2
Zhao L Ling R Chen J Shi A Chai C Ma F et al . Clinical outcomes of proximal gastrectomy versus total gastrectomy for proximal gastric cancer: a systematic review and meta-analysis. Dig Surg. (2021) 38:1–13. doi: 10.1159/000506104
3
Hyung WJ Yang HK Han SU Lee YJ Park JM Kim JJ et al . A feasibility study of laparoscopic total gastrectomy for clinical stage I gastric cancer: a prospective multi-center phase II clinical trial, KLASS 03. Gastric Cancer. (2019) 22:214–22. doi: 10.1007/s10120-018-0864-4
4
Kanda M Mizuno A Tanaka C Kobayashi D Fujiwara M Iwata N et al . Nutritional predictors for postoperative shortterm and long-term outcomes of patients with gastric cancer. Medicine. (2016) 95:e3781. doi: 10.1097/MD.0000000000003781
5
Mortensen K Nilsson M Slim K Schäfer M Mariette C Braga M et al . Consensus guideline for enhanced recovery after gastrectomy: Enhanced Recovery After Surgery (ERAS®) Society recommendations. Br J Surg. (2014) 101:1209–29. doi: 10.1002/bjs.9582
6
Jeong O Ryu SY Jung MR Choi WW Park YK . The safety and feasibility of early postoperative oral nutrition on the first postoperative day after gastrectomy for gastric carcinoma. Gastric Cancer. (2014) 17:324–31. doi: 10.1007/s10120-013-0275-5
7
Gustafsson UO Scott MJ Hubner M Nygren J Demartines N Francis N et al . Guidelines for perioperative care in elective colorectal surgery: enhanced recovery after surgery (ERAS®) society recommendations: 2018. World J Surg. (2019) 43:659–95. doi: 10.1007/s00268-018-4844-y
8
Che G . Establishment and optimization of enhanced recovery after surgery system for lung cancer. Zhongguo Fei Ai Za Zhi. (2017) 20:795–9. doi: 10.3779/j.issn.1009-3419.2017.12.01
9
Melloul E Hübner M Scott M Snowden C Prentis J Dejong CH et al . Guidelines for perioperative care for liver surgery: enhanced recovery after surgery (ERAS) society recommendations. World J Surg. (2016) 40:2425–40. doi: 10.1007/s00268-016-3700-1
10
Nelson G Bakkum-Gamez J Kalogera E Glaser G Altman A Meyer LA et al . Guidelines for perioperative care in gynecologic/oncology: enhanced recovery after surgery (ERAS) society recommendations-2019 update. Int J Gynecol Cancer. (2019) 29:651–68. doi: 10.1136/ijgc-2019-000356
11
Altman AD Helpman L McGee J Samouëlian V Auclair MH Brar H et al . Enhanced recovery after surgery: implementing a new standard of surgical care. Can Med Assoc J. (2019) 191:E469–75. doi: 10.1503/cmaj.180635
12
Reece L Hogan S Allman-Farinelli M Carey S . Oral nutrition interventions in patients undergoing gastrointestinal surgery for cancer: a systematic literature review. Support Care Cancer. (2020) 28:5673–91. doi: 10.1007/s00520-020-05673-w
13
Ye M Jin K Xu G Lin F Zhou Q Tao K et al . Short- and long-term outcomes after conversion of laparoscopic total gastrectomy for gastric cancer: A single-center study. JBUON. (2017) 22:126–33.
14
Lu YX Wang YJ Xie TY Li S Wu D Li XG et al . Effects of early oral feeding after radical total gastrectomy in gastric cancer patients. World J Gastroenterol. (2020) 26:5508–19. doi: 10.3748/wjg.v26.i36.5508
15
Shinohara T Maeda Y Koyama R Minagawa N Hamaguchi J Hamada T . Feasibility and safety of early oral feeding in patients with gastric cancer after radical gastrectomy. Indian J Surg Oncol. (2020) 11:47–55. doi: 10.1007/s13193-019-00999-2
16
Vader JP . Randomised controlled trials: A User’s guide. BMJ. (1998) 317:1258. doi: 10.1136/bmj.317.7167.1258
17
Wan X Wang W Liu J Tong T . Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. (2014) 14:135. doi: 10.1186/1471-2288-14-135
18
Luo D Wan X Liu J Tong T . Optimally estimating the sample mean from the sample size, median, mid-range, and/or mid-quartile range. Stat Methods Med Res. (2018) 27:1785–805. doi: 10.1177/0962280216669183
19
Liu XX Jiang ZW Wang ZM Li JS . Multimodal optimization of surgical care shows beneficial outcome in gastrectomy surgery. JPEN J Parenter Enteral Nutr. (2010) 34:313–21. doi: 10.1177/0148607110362583
20
Wang D Kong Y Zhong B Zhou X Zhou Y . Fast-track surgery improves postoperative recovery in patients with gastric cancer: a randomized comparison with conventional postoperative care. J Gastrointest Surg. (2010) 14:620–627. doi: 10.1007/s11605-009-1139-5
21
Hur H Kim SG Shim JH Song KY Kim W Park CH et al . Effect of early oral feeding after gastric cancer surgery: a result of randomized clinical trial. Surgery. (2011) 149:561–8. doi: 10.1016/j.surg.2010.10.003
22
Chen Hu J Xin Jiang L Cai L Tao Zheng H Yuan Hu S Bing Chen H et al . Preliminary experience of fast-track surgery combined with laparoscopy-assisted radical distal gastrectomy for gastric cancer. J Gastrointest Surg. (2012) 16:1830–9. doi: 10.1007/s11605-012-1969-4
23
Kim JW Kim WS Cheong JH Hyung WJ Choi SH Noh SH . Safety and efficacy of fast-track surgery in laparoscopic distal gastrectomy for gastric cancer: a randomized clinical trial. World J Surg. (2012) 36:2879–87. doi: 10.1007/s00268-012-1741-7
24
Feng F Ji G Li JP Li XH Shi H Zhao ZW et al . Fast-track surgery could improve postoperative recovery in radical total gastrectomy patients. World J Gastroenterol. (2013) 19:3642–8. doi: 10.3748/wjg.v19.i23.3642
25
Hong L Han Y Zhang H Zhao Q Liu J Yang J et al . Effect of early oral feeding on short-term outcome of patients receiving laparoscopic distal gastrectomy: a retrospective cohort study. Int J Surg. (2014) 12:637–9. doi: 10.1016/j.ijsu.2014.05.062
26
Li B Liu HY Guo SH Sun P Gong FM Jia BQ . Impact of early postoperative enteral nutrition on clinical outcomes in patients with gastric cancer. Genet Mol Res. (2015) 14:7136–41. doi: 10.4238/2015.June.29.7
27
Shimizu N Oki E Tanizawa Y Suzuki Y Aikou S Kunisaki C et al . Effect of early oral feeding on length of hospital stay following gastrectomy for gastric cancer: a Japanese multicenter, randomized controlled trial. Surg Today. (2018) 48:865–874. doi: 10.1007/s00595-018-1665-4
28
Wang Q Yang KL Guo BY Shang LF Yan ZD Yu J et al . Safety of early oral feeding after total laparoscopic radical gastrectomy for gastric cancer (SOFTLY-1): a single-center randomized controlled trial. Cancer Manag Res. (2019) 11:4839–46. doi: 10.2147/CMAR.S199552
29
Gao L Zhao Z Zhang L Shao G . Effect of early oral feeding on gastrointestinal function recovery in postoperative gastric cancer patients: a prospective study. J BUON. (2019) 24:194–200.
30
Association JGC . Japanese gastric cancer treatment guidelines 2014 (ver. 4). Gastric Cancer. (2017) 20:1–19. doi: 10.1007/s10120-016-0622-4
31
Fukuda Y Yamamoto K Hirao M Nishikawa K Maeda S Haraguchi N et al . Prevalence of malnutrition among gastric cancer patients undergoing gastrectomy and optimal preoperative nutritional support for preventing surgical site infections. Ann Surg Oncol. (2015) 22 Suppl 3:S778–85. doi: 10.1245/s10434-015-4820-9
32
Rinninella E Cintoni M Raoul P Pozzo C Strippoli A Bria E et al . Effects of nutritional interventions on nutritional status in patients with gastric cancer: a systematic review and meta-analysis of randomized controlled trials. Clin Nutr ESPEN. (2020) 38:28–42. doi: 10.1016/j.clnesp.2020.05.007
33
Xishan Z Ye Z Feiyan M Liang X Shikai W . The role of prognostic nutritional index for clinical outcomes of gastric cancer after total gastrectomy. Sci Rep. (2020) 10:17373. doi: 10.1038/s41598-020-74525-8
34
Gianotti L Sandini M Romagnoli S Carli F Ljungqvist O . Enhanced recovery programs in gastrointestinal surgery: actions to promote optimal perioperative nutritional and metabolic care. Clin Nutr. (2020) 39:2014–24. doi: 10.1016/j.clnu.2019.10.023
35
Lewis SJ Andersen HK Thomas S . Early enteral nutrition within 24 h of intestinal surgery versus later commencement of feeding: a systematic review and meta-analysis. J Gastrointest Surg. (2009) 13:569–75. doi: 10.1007/s11605-008-0592-x
36
Petrelli NJ Cheng C Driscoll D Rodriguez-Bigas MA . Early postoperative oral feeding after colectomy: an analysis of factors that may predict failure. Ann Surg Oncol. (2001) 8:796–800. doi: 10.1007/s10434-001-0796-8
37
Nelson R Tse B Edwards S . Systematic review of prophylactic nasogastric decompression after abdominal operations. Br J Surg. (2005) 92:673–80. doi: 10.1002/bjs.5090
38
Carrère N Seulin P Julio CH Bloom E Gouzi JL Pradère B . Is nasogastric or nasojejunal decompression necessary after gastrectomy? A prospective randomized trial. World J Surg. (2007) 31:122–7. doi: 10.1007/s00268-006-0430-9
39
Yoo CH Son BH Han WK Pae WK . Nasogastric decompression is not necessary in operations for gastric cancer: prospective randomised trial. Eur J Surg. (2002) 168:379–83. doi: 10.1080/110241502320789041
40
Yang Z Zheng Q Wang Z . Meta-analysis of the need for nasogastric or nasojejunal decompression after gastrectomy for gastric cancer. Br J Surg. (2008) 95:809–16. doi: 10.1002/bjs.6198
41
He H Ma Y Zheng Z Deng X Zhu J Wang Y . Early versus delayed oral feeding after gastrectomy for gastric cancer: A systematic review and meta-analysis. Int J Nurs Stud. (2022) 126:104120. doi: 10.1016/j.ijnurstu.2021.104120
42
Wang J Yang M Wang Q Ji G . Comparison of early oral feeding with traditional oral feeding after total gastrectomy for gastric cancer: A propensity score matching analysis. Front Oncol. (2019) 9:1194. doi: 10.3389/fonc.2019.01194
43
Liu X Wang D Zheng L Mou T Liu H Li G . Is early oral feeding after gastric cancer surgery feasible? A systematic review and meta-analysis of randomized controlled trials. PloS One. (2014) 9:e112062. doi: 10.1371/journal.pone.0112062
44
Suehiro T Matsumata T Shikada Y Sugimachi K . Accelerated rehabilitation with early postoperative oral feeding following gastrectomy. Hepatogastroenterology. (2004) 51:1852–5.
45
Jang A Jeong O . Early postoperative oral feeding after total gastrectomy in gastric carcinoma patients: A retrospective before-after study using propensity score matching. JPEN J Parenter Enteral Nutr. (2019) 43:649–57. doi: 10.1002/jpen.1438
46
Tadano S Terashima H Fukuzawa J Matsuo R Ikeda O Ohkohchi N . Early postoperative oral intake accelerates upper gastrointestinal anastomotic healing in the rat model. J Surg Res. (2011) 169:202–8. doi: 10.1016/j.jss.2010.01.004
47
Fukuzawa J Terashima H Ohkohchi N . Early postoperative oral feeding accelerates upper gastrointestinal anastomotic healing in the rat model. World J Surg. (2007) 31:1234–9. doi: 10.1007/s00268-007-9003-9
48
Minig L Biffi R Zanagnolo V Attanasio A Beltrami C Bocciolone L et al . Early oral versus ‘‘traditional’’ postoperative feeding in gynecologic oncology patients undergoing intestinal resection: a randomized controlled trial. Ann Surg Oncol. (2009) 16:1660–8. doi: 10.1245/s10434-009-0444-2
49
Ryan AT Luscombe-Marsh ND Saies AA Little TJ Standfield S Horowitz M et al . Effects of intraduodenal lipid and protein on gut motility and hormone release, glycemia, appetite, and energy intake in lean men. Am J Clin Nutr. (2013) 98:300–11. doi: 10.3945/ajcn.113.061333
50
Pilichiewicz AN Chaikomin R Brennan IM Wishart JM Rayner CK Jones KL et al . Load-dependent effects of duodenal glucose on glycemia, gastrointestinal hormones, antropyloroduodenal motility, and energy intake in healthy men. Am J Physiol Endocrinol Metab. (2007) 293:E743–53. doi: 10.1152/ajpendo.00159.2007
51
Laffitte AM Polakowski CB Kato M . Early oral re-feeding on oncology patients submitted to gastrectomy for gastric cancer. Arq Bras Cir Dig. (2015) 28:200–3. doi: 10.1590/S0102-67202015000300014
52
Sierzega M Choruz R Pietruszka S Kulig P Kolodziejczyk P Kulig J . Feasibility and outcomes of early oral feeding after total gastrectomy for cancer. J Gastrointest Surg. (2015) 19:473–9. doi: 10.1007/s11605-014-2720-0
53
Shoar S Naderan M Mahmoodzadeh H Hosseini-Araghi N Mahboobi N Sirati F et al . Early oral feeding after surgery for upper gastrointestinal Malignancies: A prospective cohort study. Oman Med J. (2016) 31:182–7. doi: 10.5001/omj.2016.36
54
Sindler DL Mátrai P Szakó L Berki D Berke G Csontos A et al . Faster recovery and bowel movement after early oral feeding compared to late oral feeding after upper GI tumor resections: a meta-analysis. Front Surg. (2023) 10:1092303. doi: 10.3389/fsurg.2023.1092303
Summary
Keywords
early oral feeding, traditional oral feeding, gastric cancer, gastrectomy, meta-analysis
Citation
Xu D, Li J, Liu J, Wang P and Dou J (2024) An updated systematic review and meta-analysis of the efficacy and safety of early oral feeding vs. traditional oral feeding after gastric cancer surgery. Front. Oncol. 14:1390065. doi: 10.3389/fonc.2024.1390065
Received
22 February 2024
Accepted
29 July 2024
Published
04 September 2024
Volume
14 - 2024
Edited by
Francesco Giovinazzo, Agostino Gemelli University Polyclinic (IRCCS), Italy
Reviewed by
Qian Xu, Shandong Provincial Qianfoshan Hospital, China
Andras Papp, University of Pecs, Hungary
Updates
Copyright
© 2024 Xu, Li, Liu, Wang and Dou.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jianjian Dou, lanjiniao123@163.com
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.