- 1Department of Interventional Radiology, Ruijin Hospital Luwan Branch, Shanghai JiaoTong University School of Medicine, Shanghai, China
- 2Department of Interventional Therapy, The Second Affiliated Hospital of Soochow University, Jiangsu, China
- 3Department of Intervention Vascular, The Third Affiliated Hospital of Shihezi University, Shihezi, China
- 4Ruijin Hospital, School of Medicine, Shanghai Jiao Tong University, Shanghai, China
Background: Irreversible electroporation has been proved as a feasible and safe method against tumor in liver. However, few studies focused on tumors adjacent to perihepatic important structure like vessels, biliary system and gall bladder. These structures limit the effectiveness of conventional treatments. The aim of this article is to analyze the clinical outcomes of patients with hepatic tumors at the special sites who received IRE treatment and provide reliable evidence for broadening the scope of IRE’s clinical application.
Methods: The clinical information of patients who underwent IRE ablation for tumors adjacent to perihepatic important structure between February 2017 and December 2021 was collected and retrospectively analyzed. All patients underwent contrast-enhanced CT or MRI for further evaluation at the 1-month follow-up and every 3 months thereafter. Post-ablation complications, recurrence, progression-free survival and overall survival were evaluated to analyze the prognosis of IRE ablation adjacent to perihepatic important structure. Categorical variables are presented as numbers followed by percentages. Continuous data are presented as the mean ± deviation. The tumor size and IRE ablation size were evaluated by the maximum diameters.
Results: Thirty-two patients who underwent IRE ablation for tumor adjacent to perihepatic important structure were studied in this research. There were 39 lesions in 32 patients treated with IRE ablation. Fourteen of them (35.9%) were located adjacent to the porta hepatis, and 8 of them (20.5%) were located adjacent to the hepatocaval confluence. Subcapsular lesions accounted for 15.4% (6 of 39 lesions). The other 11 lesions were in the para gallbladder (5 of 39 lesions, 12.8%), the caudate lobe (5 of 39 lesions, 12.8%) and the colonic hepatic flexure (1 of 39 lesions, 2.6%). According to the Clavien−Dindo classification system for complications, all relative patients with cancer experienced complications below class III except one patient who developed postoperative hemorrhagic shock and improved after timely treatment. Recurrence in situ was observed in 5 of 32 (15.6%) patients. The median PFS of the patients who received IRE ablation was 384 days, and the median OS was 571 days.
Conclusion: IRE ablation is a feasible and safe treatment strategy for tumors adjacent to perihepatic important structure. With improved equipment, optimized therapeutic parameters and long-term clinical trials, IRE will play an increasingly important role in the treatment of tumors in liver.
Introduction
Liver cancer including primary hepatic tumor and liver metastasis is the fourth most deadly type of cancer worldwide that has an increasing rate of death and a consistently low survival rate (1). At present, in clinical practice, surgical resection is still considered the main treatment strategy for primary hepatic carcinoma. However, it was reported that only 10-15% of patients with liver metastases will experience long-term benefits of resection, and about 30% of the liver cancer patients are suitable for resection (2, 3).
Nowadays, minimally invasive techniques are rapidly developing, and interventional therapy, especially ablative treatment combined with chemotherapy, targeted therapy, or immunotherapy, is feasible and effective for patients with unresectable liver metastases or multiple liver tumors (4–6). Remarkably, ablative treatment for small liver tumors (maximum diameter is less than 3 cm) is expected to achieve comparable effects as surgical resection (7). However, ablative therapy is ineffective in treating some liver tumors at special sites. For example, ablative therapy for residual lesions adjacent to the porta is most often ineffective due to the heat sink effect and the safe distance between the needle electrode and visceral vessels, and tumors near the biliary system and hepatic flexure have an increased risk of iatrogenic perforations after ablation and tumors adjacent to the diaphragm, especially those protruding beyond the outline of the liver, are prone to diaphragmatic injury and diaphragmatic hernia because of ablation (8–10).
Irreversible electroporation (IRE), a relatively new modality to treat cancer, uses high-voltage electrical pulses to induce cancer cell apoptosis through nanoscale irreversible cell membrane pores, which results in an imbalance of ion exchange and destruction of the homeostasis of the intracellular environment (10, 11). The main advantage of IRE is that there is no significant temperature change during the process and no severely irreversible damage to adjacent normal tissue (12). And IRE is not affected by the heat sink effect. This kind of highly selective ablation modality is especially suitable for hepatic carcinoma adjacent to perihepatic important structure. In several studies, researchers have reported the feasibility and safety of IRE in the liver, however few studies show a focus on tumors adjacent to perihepatic important structure like porta hepatis, hepatocaval confluence, subcapsular, gall bladder, caudate lobe and hepatic flexure of colon (13, 14). Therefore, the treatment strategies for these types of tumors are supposed to be discussed in clinical practice. The aim of this research is to analyze the clinical outcomes of patients with hepatic tumors adjacent to perihepatic important structure who receive IRE treatment and provide reliable evidence for broadening the scope of clinical application of IRE.
Materials and methods
This retrospective study was approved by the institutional review board. All patients with liver tumors were assessed preoperatively using the following criteria in Figure 1 to determine suitability for IRE treatment. However, only patients who underwent IRE for tumors adjacent to special sites, including the diaphragm, capsule, inferior vena cava, hepatic vein, gall bladder or secondary branch of the portal vein, were included in this study. All patients signed informed consent forms.
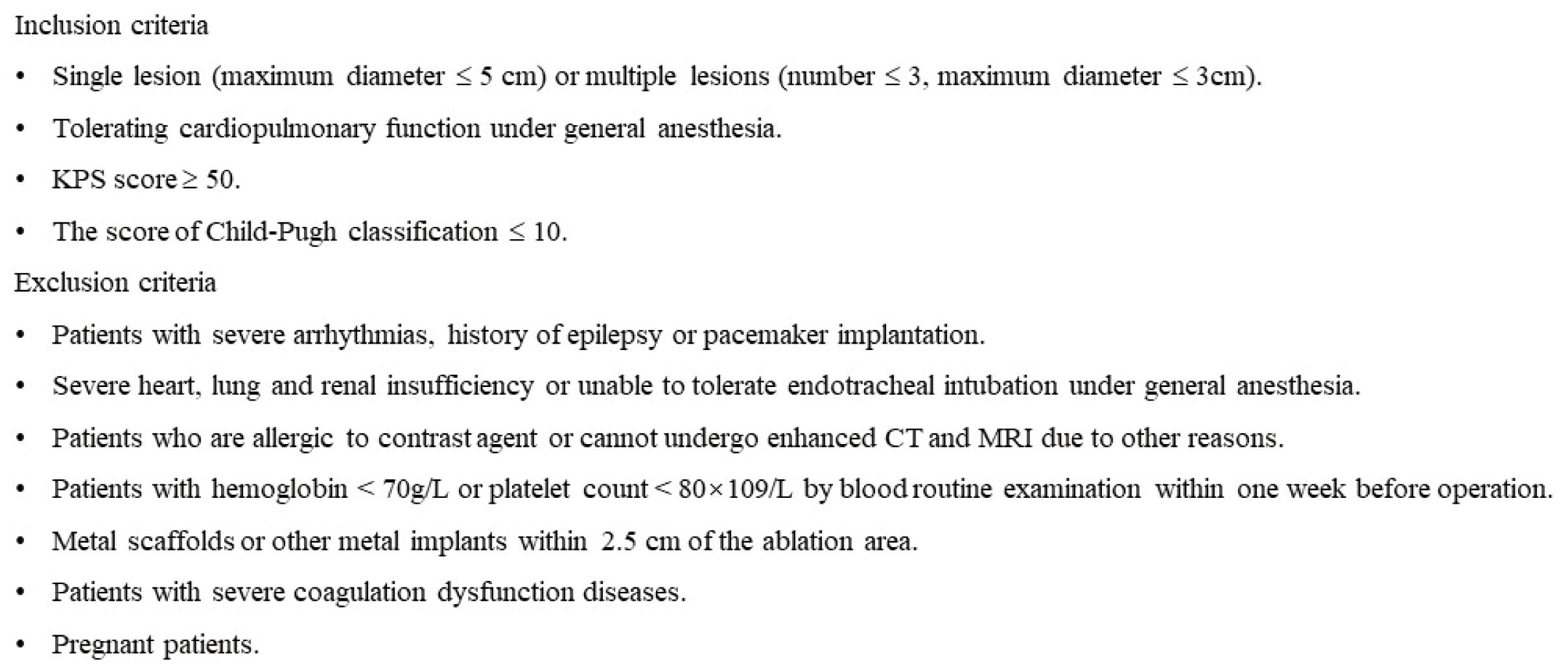
Figure 1. The inclusion and exclusion criteria of IRE ablation for tumors in special sites of liver.
Equipment
A NanoKnife (Angiodynamic, USA) was used for IRE treatment. The main needle electrode was a 19G monopolar needle electrode, which was combined with other electrodes to adapt to the size and shape of tumors. A total of 2 to 5 electrodes were used in this research according to the parameter of tumors and IRE settings. There is an insulation layer around the needle electrode. The exposed section of the electrode can be adjusted by adjusting the length of the insulation layer subcutaneously. The common electrode exposure length is 1-2cm.
Procedure
Preoperative preparation
An anesthetist, an itinerant nurse, and an experienced equipment technician comprised the IRE treatment team to assist the interventional radiologists. General anesthesia combined with neuromuscular blockade was required to reduce the effects of muscle contractions.
The image-guiding mode of IRE
Multiplanar reconstruction using CT showed the relationship between the electrode and lesions or the perifocal structures satisfactorily and the electrode distribution could be adjusted in real time.
The setting of electrode route (pre-operation plan)
Contrast-enhanced CT and multiplanar reconstruction were performed to obtain images to determine the location and size of the lesions and to show the relationship between the lesions and the adjacent structures. Then, the access and amount of needle electrodes were chosen, and interventional radiologists placed the electrodes in the planned positions. The electrodes were supposed to be parallel to each other, and the distance between every two electrodes was 1 to 2.2 cm. The ideal electrode route form an ablation area that covers the whole tumor in all cases.
The strategy for IRE treatment
The parameters of the NanoKnife system were adjusted after confirming suitable electrode positions. The IRE planning system was used to adjust the appropriate ablation parameters to achieve the ablation area covering all tumors. Initially, test pulses (20 pulses of 1500 V/cm for 70 μs) were delivered to evaluate the impedance and current of the tissue. Then the IRE ablation parameters were set as follows: voltage 1500V/cm, 100 pulses/2 cycles, pulse width 70-90μs. electrode distribution was adjusted for an ideal ablation zone, including the margins of the target lesion and the surrounding normal tissue. Meanwhile, the variation trend of current (2550-3000mA) should be closely monitored during the operation. For some cases, electrodes were repositioned between applications of pulses according to the monitored images.
Assessment of the IRE procedure and follow-up
Contrast-enhanced CT was performed immediately to assess the completeness of IRE ablation and to detect intraoperative complications. IRE ablation was considered to be successful if there was no obvious enhancement in the arterial phase or washout in the venous phase or the delayed phase. All patients receiving IRE underwent contrast-enhanced CT or MRI for further evaluation at the 1-month follow-up and every 3 months thereafter (Figures 2, 3). Recurrence was defined as the appearance of new lesions on contrast-enhanced CT or MRI at the follow-up. Lesions adjacent to the primary IRE site were defined as recurrence in situ, while those away from the IRE site were termed recurrence ex situ. Postablation complications were classified into five grades according to the Clavien−Dindo classification (15). The follow-up treatment received by the patients should be recorded during follow-up. Progression-free survival (PFS) was defined as the time interval from IRE ablation to tumor progression or death. Overall survival (OS) was defined as the time interval from IRE ablation to death from any cause (for patients who were lost to follow-up before death, the time of death was counted as the last follow-up).
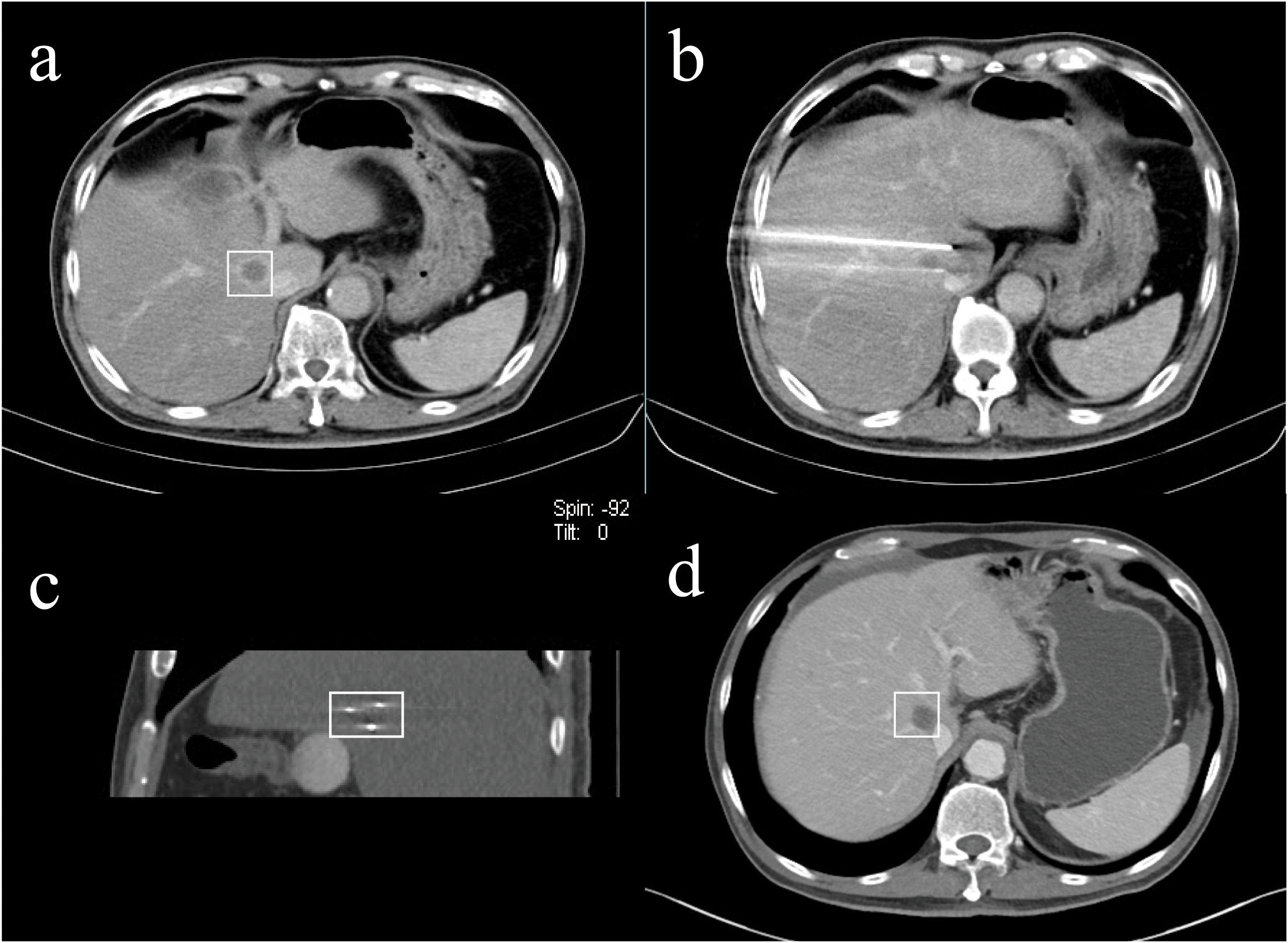
Figure 2. A 66-year-old male with liver metastasis of pancreatic cancer adjacent to the superior vena cava underwent IRE ablation. [(A) venous phase of preoperative enhanced CT (rectangular); (B, C) the approximate equilateral triangle distribution of electrodes; (D) venous phase of postoperative enhanced CT at the 13th-month follow-up indicated satisfactory ablation effect (rectangular)].

Figure 3. A 54-year-old female patient with liver metastasis of colon cancer underwent IRE ablation for the lesion adjacent to middle hepatic vein. [(A, B) preoperative enhanced CT and MRI; (C) the distribution of electrodes; (D) postoperative CT at the 3-month follow-up; (E) postoperative CT at the 19-month follow-up].
Statistical analysis
Categorical variables are presented as numbers followed by percentages. Continuous data are presented as the mean ± deviation. The tumor size and IRE ablation size were evaluated by the maximum diameters. PFS and OS were calculated by using GraphPad Prism 8 (GraphPad Software).
Results
Patient characteristics
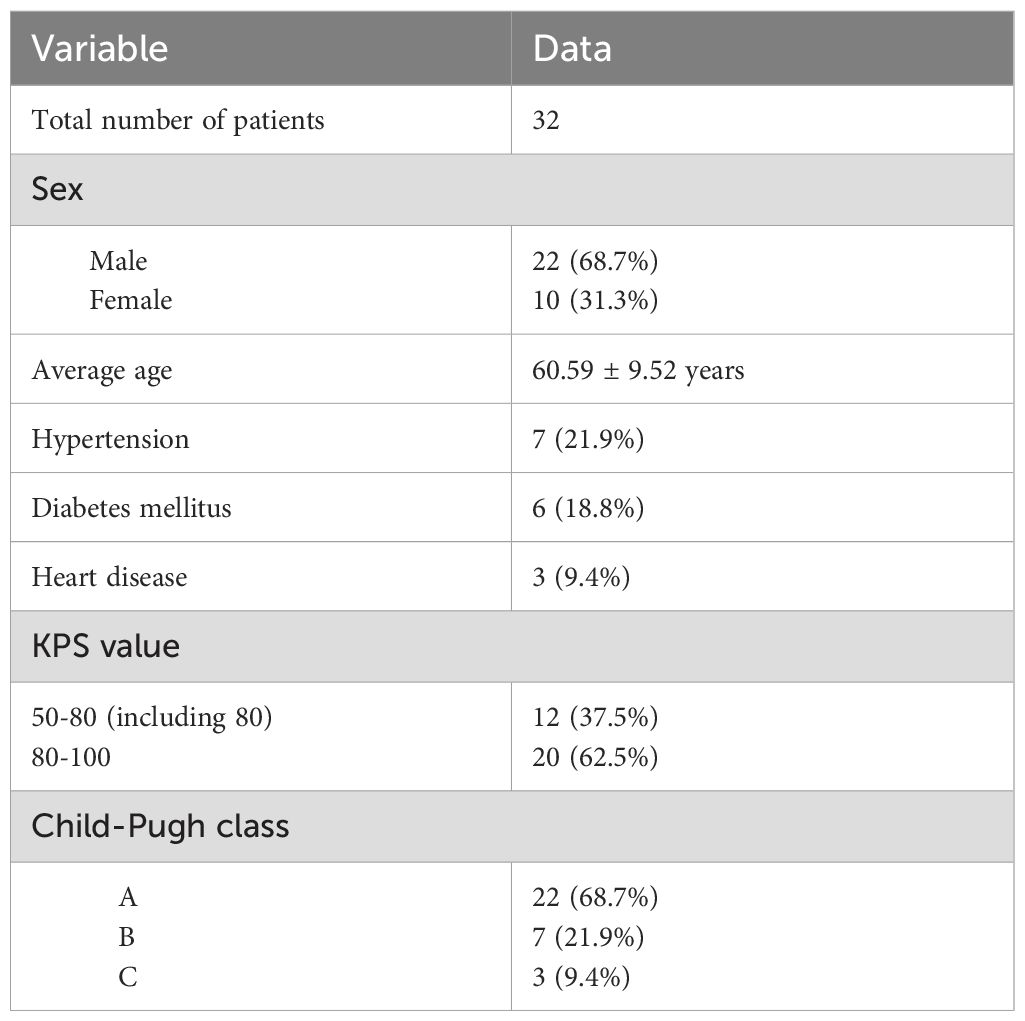
Thirty-two patients (22 male, 10 female) who underwent IRE ablation between February 2017 and December 2021 were included in this study (Table 1). Regarding underlying health problems, hypertension was diagnosed in 7 (21.9%) patients, diabetes mellitus in 6 (18.8%) patients, and heart disease in 3 (9.4%) patients. Furthermore, 6 of 32 patients (18.8%) had a confirmed history of hepatitis or cirrhosis.
The Karnofky Performance Status (KPS) of the patients was evaluated before IRE ablation. All patients received a score of more than 50. Among them, 20 patients (62.5%) were in the independent state, and the other 12 patients (37.5%) were in the semi-independent state. For the Child−Pugh class of liver function, 22 of 32 patients (68.7%) were divided into Class A, 7 of 32 patients (21.9%) were divided into Class B, and the other 3 patients (9.4%) were divided into Class C, but all of them received a score of 10, which indicated that they were in a relatively tolerating state.
Tumor characteristics
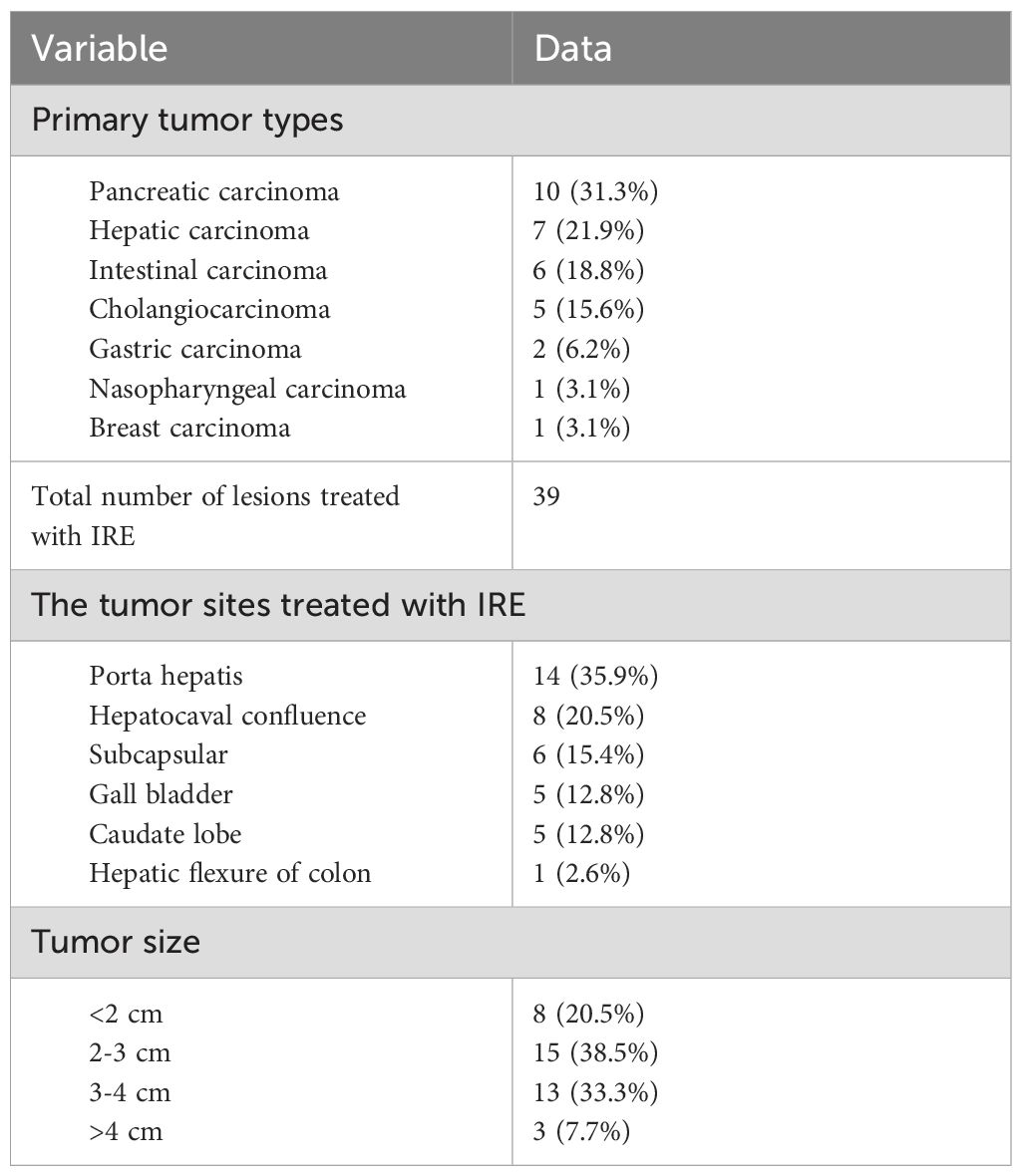
All the primary tumors of 32 patients were confirmed pathologically by comprehensive analysis of tissue biopsy, clinical findings and imaging characteristics (Table 2). Ten of 32 (31.3%) patients were suffering from pancreatic carcinoma as the primary tumor, and 7 of 32 (21.9%) patients were diagnosed with hepatic carcinoma. Other primary tumor types in this study are shown in Table 3. There were 39 lesions in 32 patients treated with IRE ablation. Fourteen of them (35.9%) were located adjacent to the porta hepatis, and 8 of them (20.5%) were located adjacent to the hepatocaval confluence. Subcapsular lesions accounted for 15.4% (6 of 39 lesions). The other 11 lesions were in the para gallbladder (5 of 39 lesions, 12.8%), the caudate lobe (5 of 39 lesions, 12.8%) and the colonic hepatic flexure (1 of 39 lesions, 2.6%). The maximum diameter of 15 (38.5%) lesions ranged between 2 cm and 3 cm and that of 13 (33.3%) lesions ranged between 3 cm and 4 cm. There were also 8 (20.5%) lesions with a maximum diameter of less than 2 cm and 3 (7.7%) lesions with a maximum diameter of more than 4 cm.
IRE characteristics
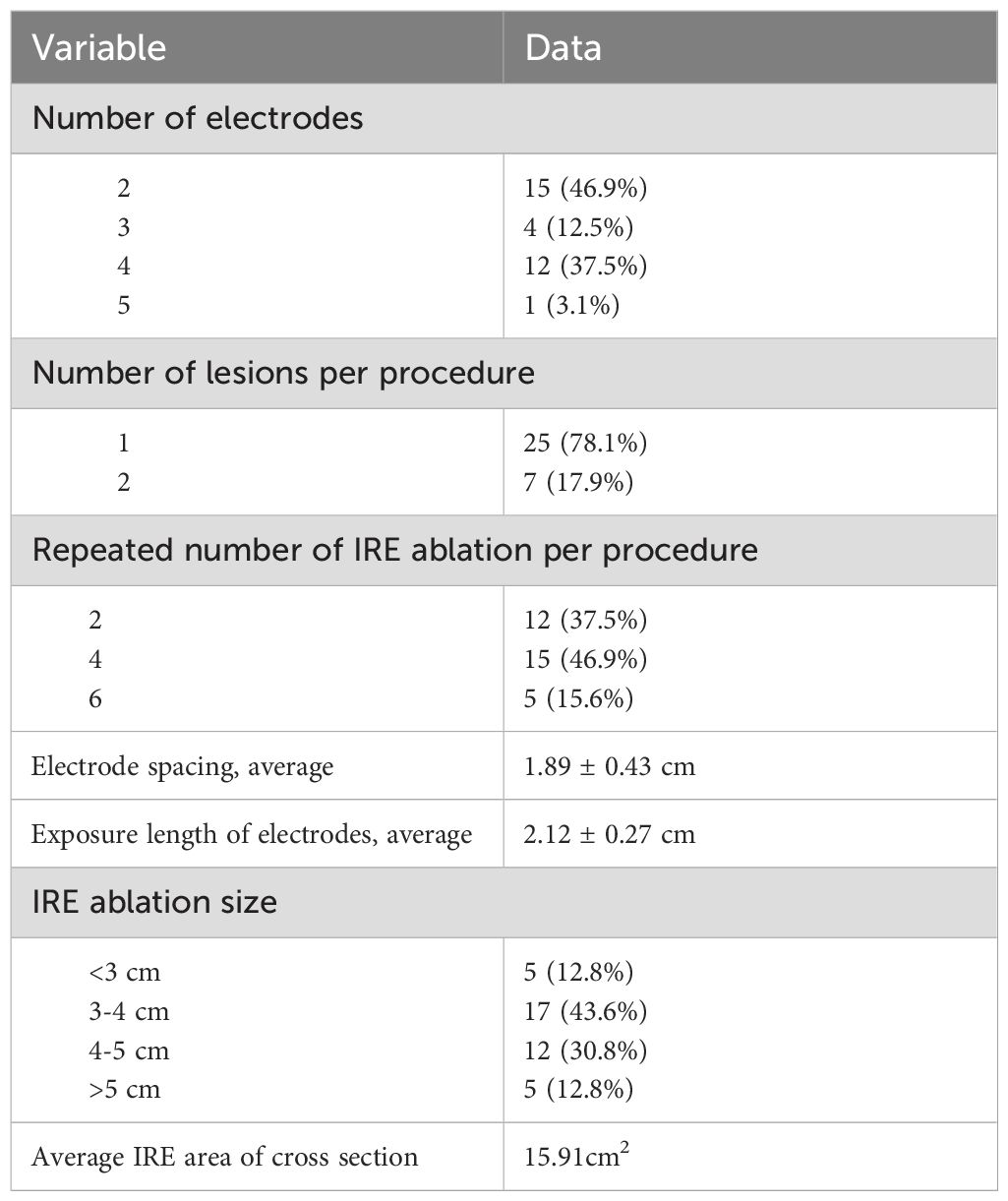
The number of electrodes, lesions per procedure and repeated IRE ablations per procedure were determined by the IRE team according to the intraoperative positioning and real-time adjustment. Related data are described in Table 3. The average effective ablation distance between the electrode needles was 1.8 ±0.43 cm, and the electrode needles were parallel to each other. The electrode needles were arranged to ensure that the entire ablation area covered all the lesions and extended beyond the edge by 1 cm. The average exposure length of the electrodes was 2.1 ± 0.27 cm, which was adjusted according to the location of the lesions. The zone of IRE ablation was evaluated immediately by abdominal contrast-enhanced CT or MRI in the postablation imaging assessment. Most of the imaging showed a reduced density of the liver tissue in the ablation area, and the degree of enhancement was lower than that of the surrounding liver tissue. There were also bubble shadows of different sizes in the ablation area. The maximum diameter of the IRE ablation zone was 3-4 cm in 17 of 39 lesions (43.6%), 4-5 cm in 12 of 39 lesions (30.8%), and less than 3 cm or more than 5 cm in 10 of 39 lesions (25.6%). The average IRE area of cross section was 15.91cm2.
Outcomes
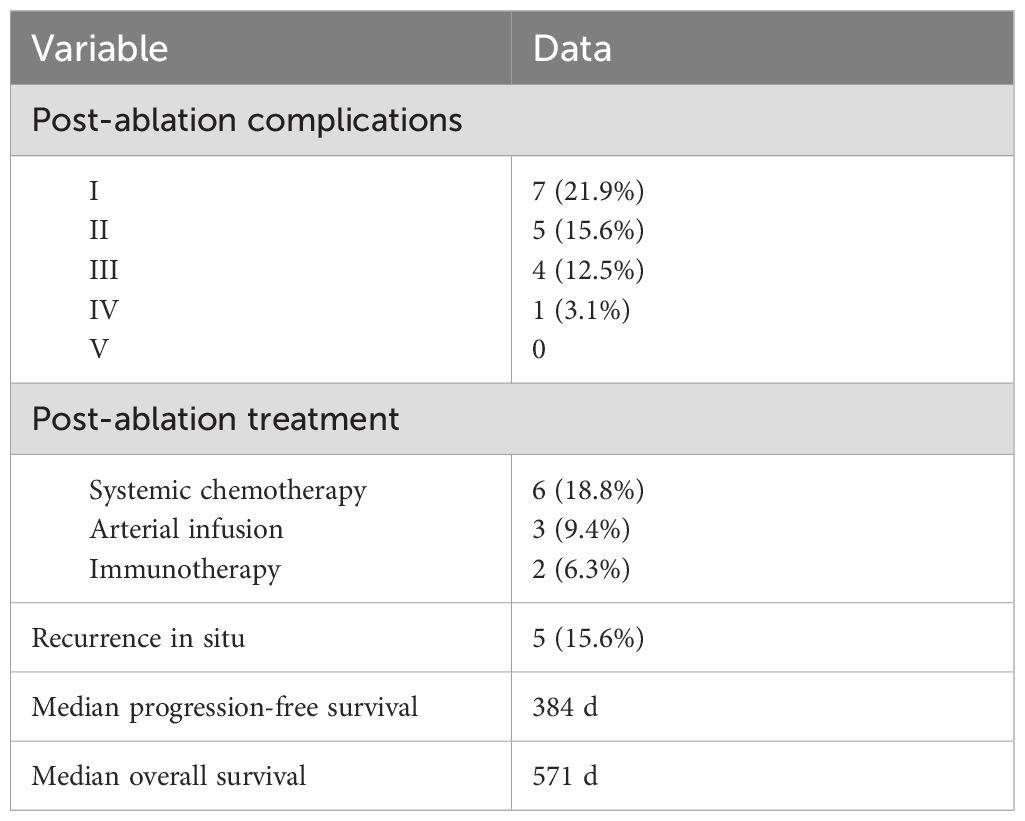
According to the Clavien−Dindo classification for complications, 7 of 32 (21.9%) patients developed class I complications. Five of 32 (15.6%) patients developed class II complications, and 4 of 32 (12.5%) patients suffered from class III complications (drainage for pleural or peritoneal effusions). One patient had a class IV complication (hemorrhagic shock). The most common complication was pleural or peritoneal effusions (8 of 32 patients, 25.0%). One patient was transfused with 2 units of platelets because of the critical value after IRE ablation. Another patient developed postoperative hemorrhagic shock and improved after timely treatment. Regarding the subsequent treatment after IRE ablation, 6 of 32 (18.8%) patients received systemic chemotherapy, 3 of 32 (9.4%) patients received transcatheter arterial infusion, and 2 of 32 (6.3%) patients received immunotherapy. Recurrence in situ was observed in 5 of 32 (15.6%) patients. The median overall PFS of the patients who received IRE ablation was 384 (95%CI: 361-403) days, and the median OS was 571 (95%CI: 545-595) days (Figure 4 and Table 4).
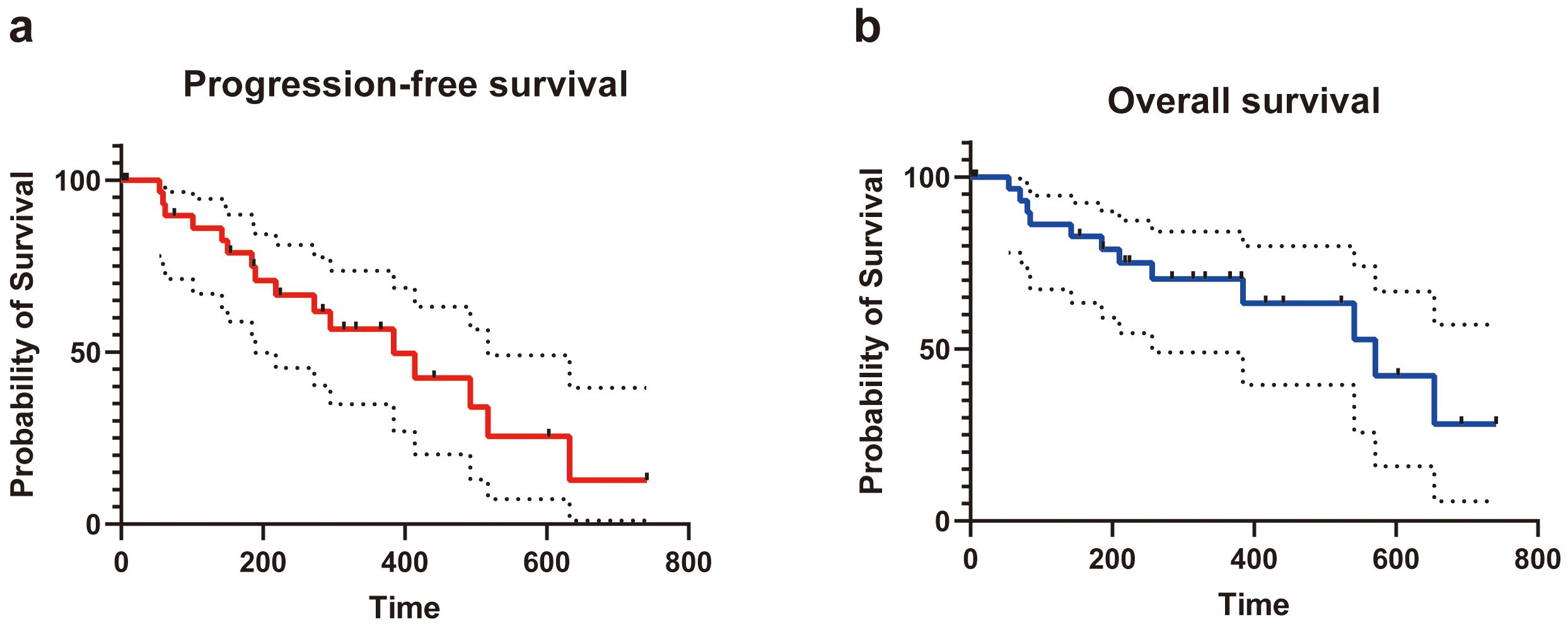
Figure 4. The progression-free survival (A) and overall survival (B) of patients underwent IRE for tumors in special sites of liver.
Discussion
Thermal ablation techniques (including radiofrequency ablation and microwave ablation) have been widely used in the radical treatment of tumors in liver and have achieved curative effects similar to those of surgical treatment for small liver cancer. It is advantageous because it is a simple operation, causes minimal trauma, has a quick recovery and a short hospitalization period. However, thermal ablation still has certain limitations. On the one hand, when the lesion is adjacent to a large blood vessel, the temperature of the targeted region is likely to be lower than 60°C due to the “heat sink effect” of blood flow, which has a major influence on the effect of thermal ablation. On the other hand, high temperature may damage the large blood vessels, bile duct, gallbladder and gastrointestinal tract, which could cause complications such as bleeding, liver abscess, gallbladder perforation and gastrointestinal perforation (16, 17). However, some evidence of thermal ablation with hydrodissection technique has confirmed safe for perivascular, peripheral and subdiaphragmatic tumors (18, 19). Some techniques using hypothermia induction chemotherapy have also been reported (20).
According to its special mechanism, irreversible electroporation therapy, in theory, only kills cells with lipid bilayers and has little effect on the fibrous structure and collagen tissue in vessels and organ lumen (21). Cells in blood vessels, bile ducts, gallbladder and gastrointestinal tract adjacent to the liver tissue were induced to undergo apoptosis during irreversible electroporation. However, normal cells can regenerate and repair in a relatively short time because the frame structure of the organ still exists (22). Therefore, the important structure will not be affected. Moreover, because apoptotic vascular endothelial cells shed within 7 days after IRE ablation, an embolus may form in small blood vessels and further reduce the risk of bleeding (23, 24).
In this study, 39 lesions in 32 patients underwent IRE ablation. Although these lesions were in special locations, the technical success rate was still 100% without severe intraoperative complications. Safety is particularly important for the clinical application of IRE ablation. It’s worth noting that circumferential enhancement bands related to hyperemia may be seen in the surrounding liver tissue during the venous phase but should not be considered active lesions. The common complications that have been reported include liver abscess, hemorrhage, pneumothorax, atrial fibrillation, and pleural effusion (25–27). In this research, postoperative complications were divided into five grades according to the Clavien−Dindo classification for complications. The postoperative complication with the highest incidence in this study was pleural or peritoneal effusion. In this study, the reason for the high risk of pleural or peritoneal effusion is thought to be the special locations of the lesions. These special locations are often close to the capsule, and inflammatory stimulation occurs during IRE ablation. However, some peritoneal effusions are more likely bloody or bilious. It should be noticed for the possibility of hemorrhage or biliary fistula. In this study, one patient developed hemorrhagic shock, which improved after timely treatment. For all types of ablations, hemorrhage is often a common complication, and damage to large vessels during ablation can sometimes be life-threatening. Similar to IRE, common complications after RFA include pleural effusion, liver function impairment, pneumothorax, hemoperitoneum. Skin burn and secondary biliary dilatation have also been reported (28, 29). Since IRE ablation does not have a thermal effect, the method of slow withdrawal is recommended, which uses self-blood clots to reduce the risk of bleeding. Moreover, gelatin sponges can be used to treat definite hemorrhage.
In our research, the average PFS of the patients who received IRE ablation was 315 days, and the median OS was 571 days. Residual tumor recurrence is a significant concern after ablation and surgery. Five of 32 (15.6%) patients experienced recurrence in situ, and 3 of 32 (9.4%) patients experienced recurrence ex situ.
There are some limitations in our research. First, the sample size was small, and there was a large difference in the number of cases between different locations. Second, there was a short follow-up period for some patients. Third, this study was retrospective, which may have caused selection bias.
Conclusion
In conclusion, this study showed that IRE ablation is feasible and safe for lesions in special locations of the liver. With improved equipment, optimized therapeutic parameters and long-term clinical trials, IRE will play an increasingly important role in the treatment of tumors in liver.
The importance and relevance of this study to the field lie in several areas. Firstly, the study provides important insights into the safety and efficacy of IRE ablation for treating liver lesions adjacent to perihepatic important structure, which can inform clinical practice and help doctors make more informed treatment decisions. Secondly, the study highlights the need for further research and improvement in the treatment of liver tumors adjacent to perihepatic important structure, particularly in terms of reducing residual tumor recurrence rates.
In addition, the study’s findings underscore the importance of continued technological advancements and refinement of therapeutic parameters in the field of IRE ablation focusing on the tumors adjacent to perihepatic important structure. By addressing the limitations of this study, such as small sample size and short follow-up period, future research could further establish the role of IRE ablation in tumor treatment and potentially improve these patient’s outcomes. Overall, this study contributes valuable knowledge to the field and underscores the importance of continued research in this area.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by Ruijin Hospital Luwan Branch Ethics Committee, Shanghai Jiao Tong University, Shanghai Jiao Tong University (protocol code 2016 EC new technology (1) and date of approval is 2016/10/1). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
JG: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Validation, Writing – original draft. ShW: Conceptualization, Data curation, Investigation, Methodology, Writing – original draft. StW: Conceptualization, Data curation, Investigation, Methodology, Writing – original draft. CL: Data curation, Investigation, Methodology, Writing – original draft. WL: Investigation, Methodology, Writing – original draft. YC: Conceptualization, Methodology, Validation, Writing – original draft. NX: Investigation, Methodology, Writing – original draft. CW: Data curation, Methodology, Validation, Writing – review & editing. ZW: Funding acquisition, Project administration, Supervision, Validation, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This research was funded by National Natural Science Foundation of China (contract grant number: 82272089) and Famous Medical Studio Project of Huangpu District of Shanghai (2023MY02).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Villanueva A. Hepatocellular carcinoma. N Engl J Med. (2019) 380:1450–62. doi: 10.1056/NEJMra1713263
2. Kornprat P, Jarnagin WR, Gonen M, DeMatteo RP, Fong Y, Blumgart LH, et al. Outcome after hepatectomy for multiple (four or more) colorectal metastases in the era of effective chemotherapy. Ann Surg Oncol. (2007) 14:1151–60. doi: 10.1245/s10434-006-9068-y
3. Choti MA, Sitzmann JV, Tiburi MF, Sumetchotimetha W, Rangsin R, Schulick RD, et al. Trends in long-term survival following liver resection for hepatic colorectal metastases. Ann Surg. (2002) 235:759–66. doi: 10.1097/00000658-200206000-00002
4. Buisman FE, Filipe WF, Kemeny NE, Narayan RR, Srouji RM, Balachandran VP, et al. Recurrence after liver resection of colorectal liver metastases: repeat resection or ablation followed by hepatic arterial infusion pump chemotherapy. Ann Surg Oncol. (2021) 28:808–16. doi: 10.1245/s10434-020-08776-0
5. Xing R, Gao J, Cui Q, Wang Q. Strategies to improve the antitumor effect of immunotherapy for hepatocellular carcinoma. Front Immunol. (2021) 12:783236. doi: 10.3389/fimmu.2021.783236
6. Meng M, Li W, Yang X, Huang G, Wei Z, Ni Y, et al. Transarterial chemoembolization, ablation, tyrosine kinase inhibitors, and immunotherapy (TATI): A novel treatment for patients with advanced hepatocellular carcinoma. J Cancer Res Ther. (2020) 16:327–34. doi: 10.4103/jcrt.JCRT_101_20
7. Kuang M, Xie XY, Huang C, Wang Y, Lin MX, Xu ZF, et al. Long-term outcome of percutaneous ablation in very early-stage hepatocellular carcinoma. J Gastrointest Surg. (2011) 15:2165–71. doi: 10.1007/s11605-011-1716-2
8. Chen R, Lu F, Wu F, Jiang T, Xie L, Kong D. An analytical solution for temperature distributions in hepatic radiofrequency ablation incorporating the heat-sink effect of large vessels. Phys Med Biol. (2018) 63:235026. doi: 10.1088/1361-6560/aaeef9
9. Yamane T, Imai K, Umezaki N, Yamao T, Kaida T, Nakagawa S, et al. Perforation of the esophagus due to thermal injury after laparoscopic radiofrequency ablation for hepatocellular carcinoma: a case for caution. Surg Case Rep. (2018) 4:127. doi: 10.1186/s40792-018-0534-0
10. Head HW, Dodd GD 3rd, Dalrymple NC, Prasad SR, El-Merhi FM, Freckleton MW, et al. Percutaneous radiofrequency ablation of hepatic tumors against the diaphragm: frequency of diaphragmatic injury. Radiology. (2007) 243:877–84. doi: 10.1148/radiol.2433060157
11. Wah TM, Lenton J, Smith J, Bassett P, Jagdev S, Ralph C, et al. Irreversible electroporation (IRE) in renal cell carcinoma (RCC): a mid-term clinical experience. Eur Radiol. (2021) 31:7491–9. doi: 10.1007/s00330-021-07846-5
12. Llovet JM, De Baere T, Kulik L, Haber PK, Greten TF, Meyer T, et al. Locoregional therapies in the era of molecular and immune treatments for hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol. (2021) 18:293–313. doi: 10.1038/s41575-020-00395-0
13. Meijerink MR, Ruarus AH, Vroomen LGPH, Puijk RS, Geboers B, Nieuwenhuizen S, et al. Irreversible electroporation to treat unresectable colorectal liver metastases (COLDFIRE-2): A phase II, two-center, single-arm clinical trial. Radiology. (2021) 299:470–80. doi: 10.1148/radiol.2021203089
14. Gupta P, Maralakunte M, Sagar S, Kumar-M P, Bhujade H, Chaluvashetty SB, et al. Efficacy and safety of irreversible electroporation for Malignant liver tumors: a systematic review and meta-analysis. Eur Radiol. (2021) 31:6511–21. doi: 10.1007/s00330-021-07742-y
15. Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. (2004) 240:205–13. doi: 10.1097/01.sla.0000133083.54934.ae
16. Shi QM, Xue C, He YT, Hu XB, Yu ZJ. Massive abdominal hemorrhage after radiofrequency ablation of recurrent hepatocellular carcinoma with successful hemostasis achieved through transarterial embolization: a case report. J Int Med Res. (2020) 48:300060519898012. doi: 10.1177/0300060519898012
17. Schembri V, Piron L, Le Roy J, Hermida M, Lonjon J, Escal L, et al. Percutaneous ablation of obscure hypovascular liver tumours in challenging locations using arterial CT-portography guidance. Diagn Interv Imaging. (2020) 101:707–13. doi: 10.1016/j.diii.2020.09.005
18. Makovich Z, Logemann J, Chen L, Mhaskar R, Choi J, Parikh N, et al. Liver tumor ablation in difficult locations: Microwave ablation of perivascular and subdiaphragmatic hepatocellular carcinoma. Clin imaging. (2021) 71:170–7. doi: 10.1016/j.clinimag.2020.11.010
19. Smolock AR, Lubner MG, Ziemlewicz TJ, Hinshaw JL, Kitchin DR, Brace CL, et al. Microwave ablation of hepatic tumors abutting the diaphragm is safe and effective. AJR Am J Roentgenol. (2015) 204:197–203. doi: 10.2214/AJR.14.12879
20. Stefano M, Prosperi E, Fugazzola P, Benini B, Bisulli M, Coccolini F, et al. Case report: cytoreductive surgery and HIPEC associated with liver electrochemotherapy in a cholangiocarcinoma patient with peritoneal carcinomatosis and liver metastasis case report. Front Surg. (2021) 8:624817. doi: 10.3389/fsurg.2021.624817
21. Rubinsky B, Onik G, Mikus P. Irreversible electroporation: a new ablation modality–clinical implications. Technol Cancer Res Treat. (2007) 6:37–48. doi: 10.1177/153303460700600106
22. Rai ZL, Feakins R, Pallett LJ, Manas D, Davidson BR. Irreversible electroporation (IRE) in locally advanced pancreatic cancer: A review of current clinical outcomes, mechanism of action and opportunities for synergistic therapy. J Clin Med. (2021) 10:1609. doi: 10.3390/jcm10081609
23. Salati U, Barry A, Chou FY, Ma R, Liu DM. State of the ablation nation: a review of ablative therapies for cure in the treatment of hepatocellular carcinoma. Future Oncol. (2017) 13:1437–48. doi: 10.2217/fon-2017-0061
24. Cohen EI, Field D, Lynskey GE, Kim AY. Technology of irreversible electroporation and review of its clinical data on liver cancers. Expert Rev Med Devices. (2018) 15:99–106. doi: 10.1080/17434440.2018.1425612
25. Li T, Huang W, Wu Z, Wang Y, Wang Q, Wang Z, et al. Percutaneous ablation of hepatic tumors at the hepatocaval confluence using irreversible electroporation: A preliminary study. Curr Oncol. (2022) 29:3950–61. doi: 10.3390/curroncol29060316
26. Wichtowski M, Nowaczyk P, Kocur J, Murawa D. Irreversible electroporation in the treatment of locally advanced pancreas and liver metastases of colorectal carcinoma. Contemp Oncol (Pozn). (2016) 20:39–44. doi: 10.5114/wo.2016.57815
27. Kalra N, Gupta P, Gorsi U, Bhujade H, Chaluvashetty SB, Duseja A, et al. Irreversible electroporation for unresectable hepatocellular carcinoma: initial experience. Cardiovasc Intervent Radiol. (2019) 42:584–90. doi: 10.1007/s00270-019-02164-2
28. Fan H, Wang X, Qu J, Lu W, Xu S, Wu X, et al. Comparison of percutaneous radiofrequency ablation for subcapsular and non-subcapsular colorectal cancer liver metastases. Front Oncol. (2021) 11:678490. doi: 10.3389/fonc.2021.678490
Keywords: irreversible electroporation, ablation, interventional radiology, tumor, liver metastasis
Citation: Gong J, Wang S, Wang S, Li C, Li W, Chen Y, Xia N, Wang C and Wang Z (2024) A retrospective study of irreversible electroporation for tumors adjacent to perihepatic important structure. Front. Oncol. 14:1387952. doi: 10.3389/fonc.2024.1387952
Received: 19 February 2024; Accepted: 09 August 2024;
Published: 12 September 2024.
Edited by:
Alberto Brolese, Department of General Surgery and HPB Unit - APSS, ItalyReviewed by:
Enrico Prosperi, University of Bologna, ItalyJi Hoon Shin, University of Ulsan, Republic of Korea
Copyright © 2024 Gong, Wang, Wang, Li, Li, Chen, Xia, Wang and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhongmin Wang, d3ptMTE4OTZAcmpoLmNvbS5jbg==; Chen Wang, Y2hlbm5pYW84MjJAMTYzLmNvbQ==
†These authors have contributed equally to this work and share first authorship
 Ju Gong1†
Ju Gong1† Zhongmin Wang
Zhongmin Wang