
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Oncol. , 23 April 2024
Sec. Gastrointestinal Cancers: Gastric and Esophageal Cancers
Volume 14 - 2024 | https://doi.org/10.3389/fonc.2024.1383076
This article is part of the Research Topic Immune Checkpoint Inhibitors in Advanced Gastric and Esophageal Cancers View all 12 articles
This case describes the benefits of perioperative chemo-immunotherapy for advanced gastric cancer and incomplete pyloric obstruction, supplemented with nutritional support. Early parenteral nutrition to stabilize nutritional status and mitigate nutrition impact symptoms, and in addition, throughout the chemo-immunotherapy perioperative period also maintained oral nutrition support and a tailored dietary plan. Above nutritional support maintained the patient’s physical condition during immunotherapy. Eventually, this combination therapy plan leads to a partial response. On the other hand, a combination of therapies that focus more on immune checkpoint inhibitor may be able to mitigate the side effects of chemotherapy. Such findings may yield novel prospects for patients with advanced gastric cancer and incomplete pyloric obstruction, enabling them to achieve better outcomes.
Gastric cancer (GC) ranks among the top five malignant tumors worldwide in terms of incidence and mortality, with most patients were diagnosed with advanced cancer (1). In patients with advanced gastric cancer, obstruction is a common accompanying symptom, and surgery remains a critical treatment for patients with obstruction. However, high recurrence rate and elevated perioperative complication risk were observed in patients of advanced gastric cancer with obstruction accepting radical operation sequential adjuvant therapy due to poorly controlled tumor burden and inferior physical condition (2, 3). For such patients, perioperative therapy may significantly extend the survival of patients with gastric cancer, but nutrient intake disorders often limit the use of perioperative therapy. Therefore, nutritional therapy is necessary for some patients receiving perioperative therapy.
Over the past decade, immune checkpoint inhibitors (ICIs) have emerged as a promising alternative to conventional systemic chemotherapy for selective advanced GC cohorts (4). Various ICIs have demonstrated considerable advantages for individuals with GC, approved by FDA bureau (4, 5). In addition, the overlooked nutritional status is critical to the success of immune-based combination therapy (6). In our case, both tumor progression and the toxic side effects of chemotherapy deteriorate nutrient intake, while immunotherapy offers the possibility of reducing this deterioration. Meanwhile, the addition of early nutritional intervention contributes to the success of perioperative chemo-immunotherapy.
In this case, a patient with advanced GC accompanying incomplete pyloric obstruction received short-term parenteral nutrition and oral nutrition support and a tailored dietary plan throughout the perioperative chemo-immunotherapy. The patient achieved a partial response (PR) and underwent radical surgery. This case suggests the potential of combination of perioperative chemo-immunotherapy and nutritional intervention for patients with advanced GC and incomplete pyloric obstruction.
A 62-year-old female patient was diagnosed with gastric adenocarcinoma at a local hospital and came to our hospital for better treatment outcomes. The patient experienced recurrent abdominal pain and vomiting over the past month, which progressively worsened over time. She did not have any other disease or a family history of cancer. Physical examination revealed tenderness in the upper abdomen but no palpable abdomen mass was detected. Upon admission, serum tumor marker test revealed a significant increase in alpha-fetoprotein (AFP) levels, carcinoembryonic antigen (CEA), carbohydrate antigen 125 (CA-125). PG-SGA score can provide a comprehensive assessment of her physical, anthropometric and functional condition, recent oral intake capacity and the subjective recall of nutrition impact symptoms which is assessed by our dieticians (7, 8). The nutritional assessment results indicated that she suffered from severe malnutrition (Figure 1).
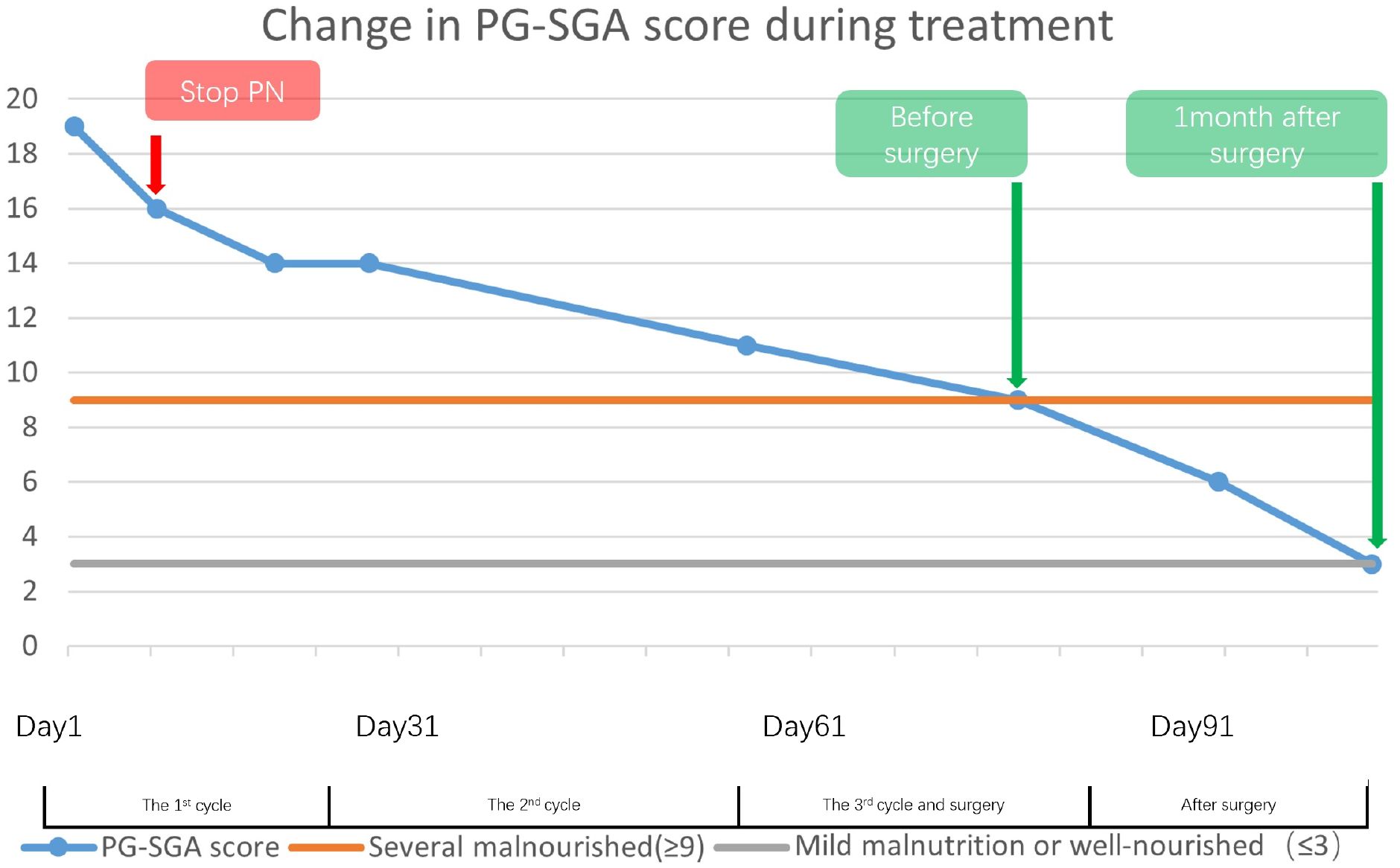
Figure 1 This illustrates the trajectory of patients’ PG-SGA scores from the first cycle of the treatment through to one-month post-surgery, indicating a progressive shift in nutritional status from severe to mild malnutrition. Despite the patient being severely malnourished before surgery, the marked reduction in scores throughout the course of treatment lends credence to the efficacy of the nutrition therapy.
During the first multidisciplinary team (MDT) discussion, we diagnosed the patient had advanced gastric cancer with incomplete pyloric obstruction based on the imaging, endoscopic, and pathological evidence, and determined a clinical stage of cT4bN2M0 (Figure 2). At the beginning of our communication with this patient and her family, they chose surgery first. But, after we analyzed the current state of the disease and communicated with them about the risk of duodenal stump fistula (DSF) and high tumor mutation burden (TMB), they finally agreed to receive perioperative chemoimmunotherapy every 3 weeks first, consisting of intravenous oxaliplatin 200mg on day 1, oral tegafur–gimeracil–oteracil potassium (S-1) 60mg twice daily on days 1–14, oral Apatinib Mesylate Tablets 250mg on days 1-21 and intravenous Camrelizumab 200mg on day1 first.
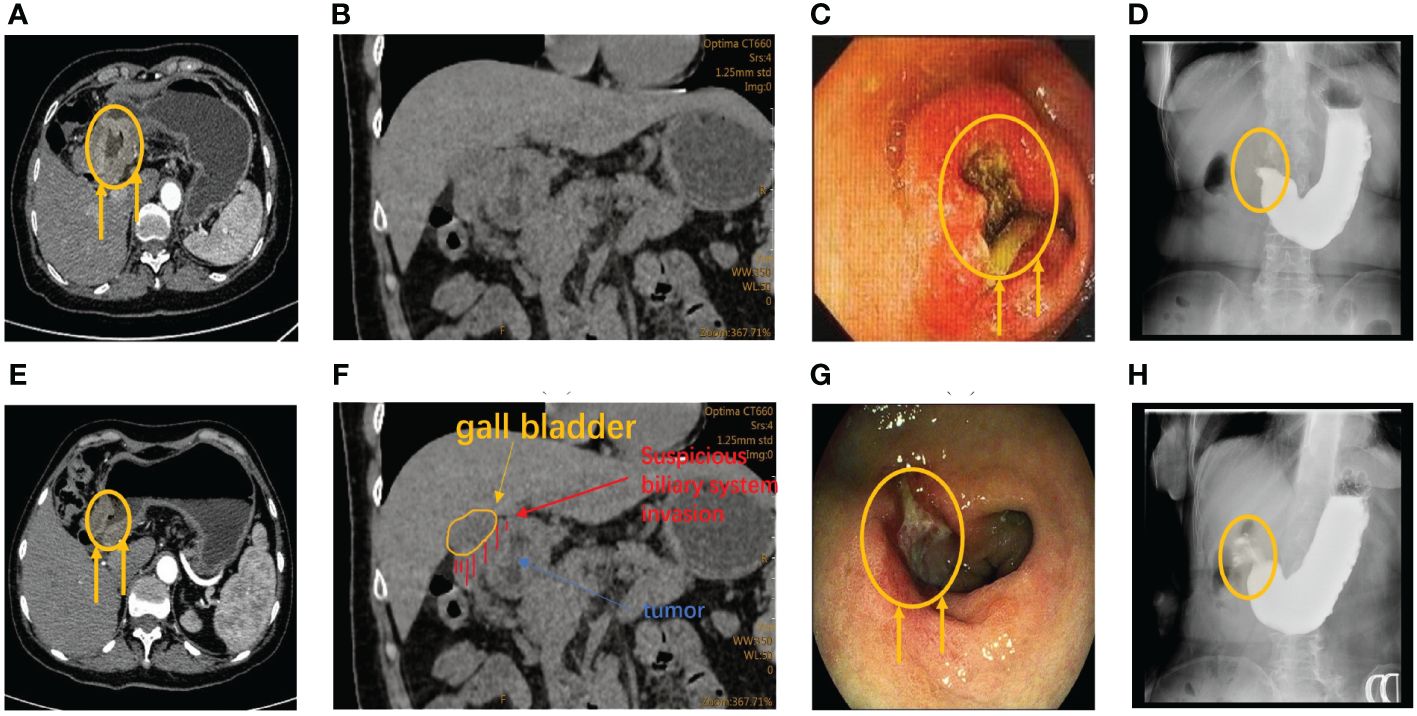
Figure 2 (A) CT reveals abnormal thickening of the antrum wall and incomplete obstruction prior to therapy. (E) Following three treatment cycles, CT scans showed significant improvement with disease regression, tumor retraction, and alleviated obstruction. (B, F) CT examination after admission showed suspicious biliary invasion. (C) Before therapy, gastroscopy revealed nearly complete stenosis of the pyloric ring cavity, accompanied by obstructive and edematous surrounding tissues. (G) Preoperative gastroscopy revealed obvious tumor retraction, along with significantly reduced obstruction and tissue edema compared to pretherapy gastroscopy. (D, H) Barium meal shows incomplete pyloric obstruction before the second cycle of treatment.
Initially, the patient suffered significant side effects, including nausea and vomiting. Given the nutrition intake disorder stemming from incomplete obstruction and post-chemotherapy side effects of this patient, we adopted a combination regimen including parenteral nutrition (PN), oral nutrition support and a tailored dietary plan. Additionally, we continued to monitor various indicators of nutrition status, such as BMI, total protein, albumin, red blood cell, CRP levels, and hemoglobin (Figure 3).
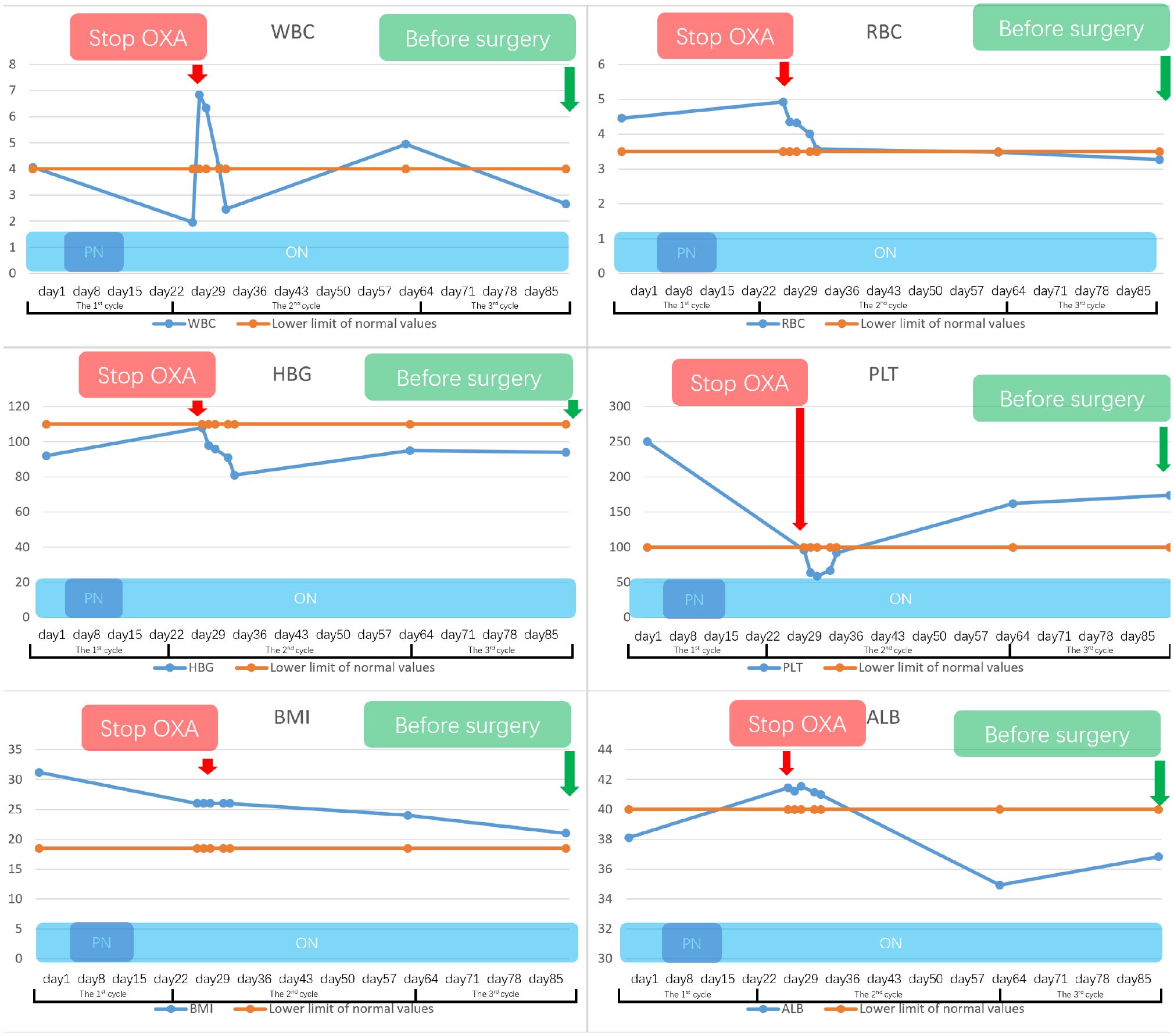
Figure 3 This study presents the variations in the patient’s nutritional examination indicators from admission to the preoperative period. With oral nutritional support, supplemented by parenteral nutrition and a tailored dietary plan, this patient’s nutritional indicators were generally maintained within the normal range. However, there was a notable occurrence of severe bone marrow suppression following the initial cycle of Oxaliplatin treatment, which subsided after discontinuing the medication. Notably, the patient did not exhibit any evident surgical contraindications based on the preoperative indicators.
Before the second cycle of treatment, the imaging showed that the patient still suffered the incomplete pyloric obstruction (Figure 2C, F). And the blood tests showed a significant drop in her platelets and white blood cells (Figure 3). This patient and her family are concerned about whether subsequent treatment can be carried out and whether early surgery will have better results.
For the gastrointestinal adverse reactions and myelosuppression in the first cycle, our MDT team initially excluded the possibility of tumor progression according to the Barium meal results and attributed these side effects to the intravenous of oxaliplatin (Figures 2, 3). Meanwhile considering the non-regressive state of the disease, we communicated with the patient and her family about the necessary of subsequent combination therapy for diminishing the TMB and the risk of DSF. Finally, we stop the use of oxaliplatin to alleviate adverse effects and gave the patient platelets transfusion and white blood cells raising drugs to treat the abnormalities of hemogram.
On the other hand, hemoglobin and albumin were significantly increased, and the PG-SGA scores decreased, indicating that nutritional therapy was effective (Figures 1, 3). Given that the PG-SGA score is a comprehensive nutritional score that combines the patient´s physical, anthropometric and functional condition, recent oral intake capacity and the subjective recall of nutrition impact symptoms and is evaluated by professional physicians, we judge that the patient’s nutritional and physical condition was better than before treatment (7, 8). Subsequently, we discontinued PN and maintained the oral nutrition support and a tailored dietary plan.
All treatment options are used in consultation with the patient and her family.
Before the third cycle of treatment, the patient could start eat semi-liquid diet which indicated the sign of tumor regression. Taking into account the improvement of the patient’s dietary status, we further reduced the proportion of oral nutrition support and instead increased the proportion of the tailored dietary plan. Moreover, the recovery from myelosuppression confirmed the correctness of stopping oxaliplatin (Figure 3). The patient then completed the third cycle of perioperative chemo-immunotherapy with an increasing food intake.
After three cycles of treatment, the disease was reevaluated in the second MDT discussion. Imaging and endoscopic examinations showed that the tumor had shrunk significantly and the obstruction had been relieved (Figure 2D, E). The patient achieved PR during the treatment. Meanwhile, considering that the physical condition of the patient can tolerate the surgical injury, radical surgery was performed on the patient (Figure 3).
The radical surgery was ensured, and the pathological examination revealed a significant regression of the tumor (Figure 4). The postoperative pathological stage of this patient was ypT2N3aM0. Pathology showed that showed it was microsatellite stable (MSS) gastric cancer. After the operation, the patient was re-examined regularly in our hospital follow the guidelines for follow-up of patients, and there was no obvious abnormality in imaging and tumor marker examination (9). She continues to receive postoperative adjuvant therapy after treatment and there was no obvious recurrence so far.
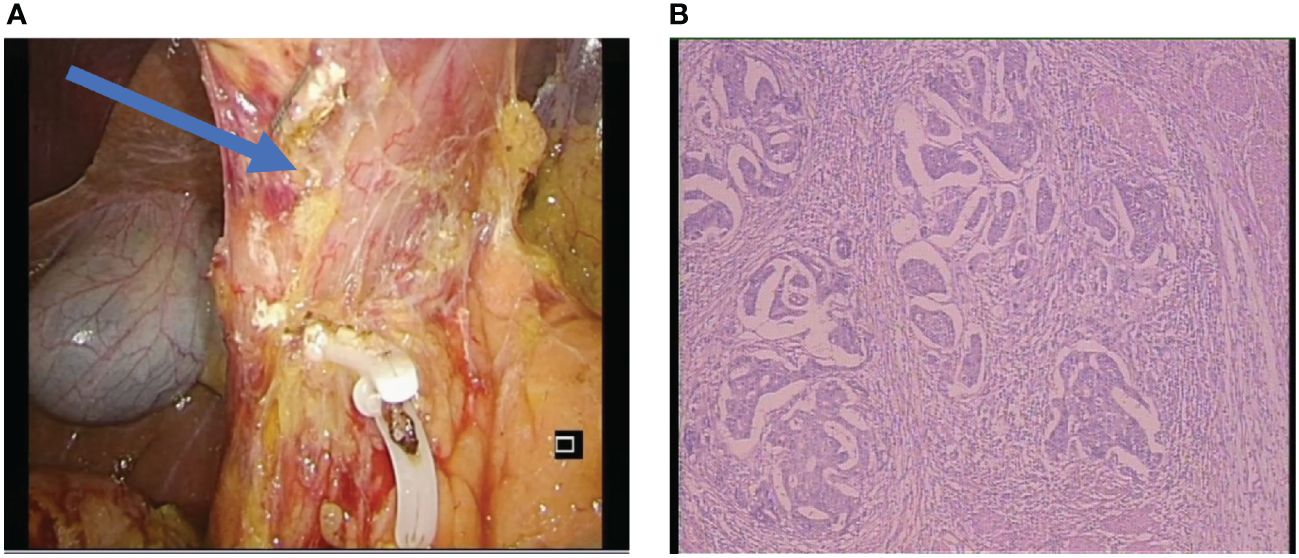
Figure 4 (A) The surgical photograph displayed the post-dissection of the sixth group of lymph nodes at the pylorus and revealed the absence of tissue edema. (B) Postoperative pathological results, the Becker tumor regression grading showed grade 1b (living tumor cells accounted for less than 10% of the original focus area). The postoperative pathological stage of this patient was ypT2N3aM0.
Despite the severe malnutrition and the side effects of chemotherapy drugs, the treatment and surgery proceed smoothly. Nutritional therapy played a key role in maintaining the nutritional, physical and functional status of the patient. The change of PG-SGA scores and the nutritional markers also indicated its efficiency (Figures 1, 3). Considering the potential complications of PN, we administered short-term PN united with oral nutrition support and a tailored dietary plan and no nutrition-related complications were found throughout the treatment.
This is a case of a patient with advanced GC and incomplete pyloric obstruction who benefit from perioperative chemo-immunotherapy (10). It also emphasizes the crucial role of nutritional therapy as an adjunctive treatment. To our knowledge, this is the first report of such a case.
Nutritional support therapy is often imperative during the treatment of advanced GC, particularly as disease progression leads to obstruction. The importance of supportive care including nutritional support, especially for cancer patients who are at high risk of malnutrition have been highlighted by some research (11, 12). Besides, keeping an adequate body-mass index (BMI) and nutritional status can also benefit for advanced patients managed with perioperative therapy, even in second-line regimens as paclitaxel plus ramucirumab (13). In this case, given the risk of DSF after surgery and the high TMB, prioritization of perioperative therapy was deemed necessary (3, 4). According to an international consensus, cachexia should be judged by a combination of BMI, loss of energy stores, and weight loss (14). Although the patient’s BMI is normal, she experienced rapid weight loss and an imbalance in energy intake and expenditure before the therapy. To avoid the deterioration of physical condition, early parenteral nutrition united with oral nutrition support and a tailored dietary plan was consistently implemented throughout the whole treatment. This regimen led to a notable enhancement in patient physical condition and nutritional status, even following the initial cytotoxic phase involving oxaliplatin.
Previous studies have demonstrated that the early implementation of PN can improve physical condition in patients with GC during perioperative chemo-immunotherapy and prolonged the effectiveness of drug responses (15, 16). But we also need to consider the risks of long-term PN (17). Meanwhile, oral nutrition support and a tailored dietary plan, another important component of nutrition support therapy, plays an important supporting role in the treatment of patients with advanced gastric cancer. Thus, based on the pros and cons of above nutritional therapy, our MDT team applied short-term PN combined with oral nutrition support and a tailored dietary plan when the patient had severe gastrointestinal side effects, and stopped PN when the symptoms improved. The absence of nutrition-related complications and the smooth progress of the overall treatment confirmed the efficacy of our protocol.
This case demonstrates a feasible nutritional regimen to improve the physical condition of patients with advanced GC and incomplete pyloric obstruction during perioperative chemo-immunotherapy. However, the specific role of nutritional support therapy in the treatment of gastric cancer is still being explored. At the same time, the oral intake and tolerance with enteral nutritional may vary with the progression of disease (18). Therefore, more research and evidence are needed to confirm if this nutritional regimen can be applied to more patients.
In comparison to traditional chemotherapy regimens, ICIs combined with chemotherapy have shown superior efficacy and fewer side effects as a first-line treatment for selective advanced GC patients (19, 20). Although this regimen has fewer complications and better treatment effects, the limitations and high cost of its application are not suitable for every patient. In recent years, some experiments have confirmed the potential effect of this regimen compared to previous treatments (10, 21). After comparing and summarizing several previous studies, immunotherapy plus chemotherapy showed better treatment effects for gastric cancer patients (22). At the same time, immunotherapy plus chemotherapy can achieve a good prognosis of gastric cancer patients while reducing toxic side effects, which has also been reported in previous case reports (23–25). In our case, the patient suffered from gastrointestinal and myelosuppressive side effects due to intravenous oxaliplatin. However, the patient still achieved PR without the treatment with oxaliplatin. Therefore, for selective patients, cytotoxic drugs may be only needed in the early stages of treatment, and immunotherapy can be the primary option for subsequent stages. However, more clinical data are required to clarify the efficacy of this strategy.
Moreover, current ICIs primarily target specific populations (4). According to the ASCO guidelines, immunotherapy combined with chemotherapy was recommended when CPS is between 1 to 5 (9). In our case, considering that the patient has a CPS score of 1.7 and also has a high risk of DSF and a high TMB, the patient received perioperative chemo-immunotherapy first and achieved favorable response. The postoperative pathological showed it was MSS gastric cancer. In perspective of immunotherapeutic mechanism, an increase in TMB is associated with increased susceptibility of immune system recognition. Increased susceptibility allows specific patients to achieve better outcomes through ICIs (26). Our case reconfirms the possibility that MSS patients may derive benefits from immunotherapy when TMB is elevated.
This case highlights the benefits of nutrition therapy in perioperative chemo-immunotherapy for advanced GC with incomplete pyloric obstruction. Short-term of PN united with oral nutrition support and a tailored dietary plan can provide more effective support with fewer complications. Meanwhile we also explored combination of immunotherapy, targeted therapy and chemotherapy for new indications. Using fewer chemotherapy and more immunotherapy may benefit patients with advanced GC and incomplete pyloric obstruction, even in MSS. In the future, more clinical evidence is warranted.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
The studies involving humans were approved by the Ethics Committee and Institutional Review Boards of the Yantai Yuhuangding Hospital Affiliated to Medical College of Qingdao University. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
MJ: Writing – review & editing, Writing – original draft, Supervision, Methodology, Funding acquisition. ZY: Writing – original draft, Methodology, Investigation, Data curation, Conceptualization. XH: Writing – original draft, Supervision, Methodology, Investigation, Conceptualization. XW: Writing – review & editing, Validation, Supervision, Methodology. ZZ: Writing – review & editing, Validation, Supervision, Methodology. YZ: Writing – review & editing, Validation, Supervision, Methodology, Funding acquisition. XS: Writing – review & editing, Validation, Supervision, Methodology.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was supported by grants from the National Natural Science Foundation of China (No. 82102785), the Natural Science Foundation of Shandong Province (ZR2021QH314), the Natural Science Foundation of Yantai Yuhuangding Hospital (No.2021-36), the Natural Science Foundation of Yantai City (2023YTYD06000912) and the WU JIEPING MEDICAL FOUNDATION(No.320.6750.2023-07-4). The funding bodies did not have any role in the design of the study, data collection, and analysis, nor on the interpretation and dissemination of the results.
We are grateful for the support from our patient.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
GC, Gastric cancer; ICIs, immune checkpoint inhibitors; PR, partial response; AFP, alpha-fetoprotein; CEA, carcinoembryonic antigen; CA-125, carbohydrate antigen 125; MDT, multidisciplinary team; DSF, duodenal stump fistula; TMB, tumor mutation burden; PN, parenteral nutrition; BMI, body-mass index; S-1, oral tegafur–gimeracil–oteracil potassium; MSS, microsatellite stable.
1. Li K, Zhang A, Li X, Zhang H, Zhao L. Advances in clinical immunotherapy for gastric cancer. Biochim Biophys Acta Rev Cancer. (2021) 1876:188615. doi: 10.1016/j.bbcan.2021.188615
2. Wang FH, Zhang XT, Li YF, Tang L, Qu XJ, Ying JE, et al. The Chinese Society of Clinical Oncology (CSCO): Clinical guidelines for the diagnosis and treatment of gastric cancer, 2021. Cancer Commun (Lond). (2021) 41:747–95. doi: 10.1002/cac2.12193
3. Troncone E, Fugazza A, Cappello A, Del Vecchio Blanco G, Monteleone G, Repici A, et al. Malignant gastric outlet obstruction: Which is the best therapeutic option? World J Gastroenterol. (2020) 26:1847–60. doi: 10.3748/wjg.v26.i16.1847
4. Joshi SS, Badgwell BD. Current treatment and recent progress in gastric cancer. CA Cancer J Clin. (2021) 71:264–79. doi: 10.3322/caac.21657
5. US Food and Drug Administration approves first cancer treatment for any solid tumors with specific biomarker. Cancer. (2017) 123:3652–3. doi: 10.1002/cncr.30992
6. Schutte K, Schulz C, Middelberg-Bisping K. Impact of gastric cancer treatment on quality of life of patients. Best Pract Res Clin Gastroenterol. (2021) 50-51:101727. doi: 10.1016/j.bpg.2021.101727
7. Guo ZQ, Yu JM, Li W, Fu ZM, Lin Y, Shi YY, et al. Survey and analysis of the nutritional status in hospitalized patients with Malignant gastric tumors and its influence on the quality of life. Support Care Cancer. (2020) 28:373–80. doi: 10.1007/s00520-019-04803-3
8. Cho JW, Youn J, Kim EM, Choi MG, Lee JE. Associations of patient-generated subjective global assessment (PG-SGA) and NUTRISCORE with survival in gastric cancer patients: timing matters, a retrospective cohort study. BMC Gastroenterol. (2022) 22:468. doi: 10.1186/s12876-022-02515-3
9. Shah MA, Kennedy EB, Alarcon-Rozas AE, Alcindor T, Bartley AN, Malowany AB, et al. Immunotherapy and targeted therapy for advanced gastroesophageal cancer: ASCO guideline. J Clin Oncol. (2023) 41:1470–91. doi: 10.1200/JCO.23.00441
10. Dong Z, Ni B, Yang L, Guan Y, Zhu C, Zhao E, et al. Efficacy and safety of camrelizumab in combination with docetaxel + S-1 sequenced by camrelizumab + S-1 for stage III (PD-1+/MSI-H/EBV+/dMMR) gastric cancer: study protocol for a single-center, prospective, open-label, single-arm trial. Front Surg. (2022) 9:917352. doi: 10.3389/fsurg.2022.917352
11. Parisi A, Porzio G, Ficorella C. Multimodality treatment in metastatic gastric cancer: from past to next future. Cancers (Basel). (2020) 12(9):2598. doi: 10.3390/cancers12092598
12. Correia M, Moreira I, Cabral S, Castro C, Cruz A, Magalhães B, et al. Neoadjuvant gastric cancer treatment and associated nutritional critical domains for the optimization of care pathways: A systematic review. Nutrients. (2023) 15(10):2241. doi: 10.3390/nu15102241
13. Parisi A, Cortellini A, Roberto M, Venditti O, Santini D, Dell'Aquila E, et al. Weight loss and body mass index in advanced gastric cancer patients treated with second-line ramucirumab: a real-life multicentre study. J Cancer Res Clin Oncol. (2019) 145:2365–73. doi: 10.1007/s00432-019-02971-7
14. Fearon K, Strasser F, Anker SD, Bosaeus I, Bruera E, Fainsinger RL, et al. Definition and classification of cancer cachexia: an international consensus. Lancet Oncol. (2011) 12:489–95. doi: 10.1016/S1470-2045(10)70218-7
15. Okamoto Y, Okano K, Izuishi K, Usuki H, Wakabayashi H, Suzuki Y. Attenuation of the systemic inflammatory response and infectious complications after gastrectomy with preoperative oral arginine and omega-3 fatty acids supplemented immunonutrition. World J Surg. (2009) 33:1815–21. doi: 10.1007/s00268-009-0140-1
16. Kubota T, Shoda K, Konishi H, Okamoto K, Otsuji E. Nutrition update in gastric cancer surgery. Ann Gastroenterol Surg. (2020) 4:360–8. doi: 10.1002/ags3.12351
17. Lu Z, Fang Y, Liu C, Zhang X, Xin X, He, et al. Early interdisciplinary supportive care in patients with previously untreated metastatic esophagogastric cancer: A phase III randomized controlled trial. J Clin Oncol. (2021) 39:748–56. doi: 10.1200/JCO.20.01254
18. Cao Y, Han D, Yang S, Shi Y, Zhao S, Jin Q, et al. Effects of pre-operative enteral immunonutrition for esophageal cancer patients treated with neoadjuvant chemoradiotherapy: protocol for a multicenter randomized controlled trial (point trial, pre-operative immunonutrition therapy). BMC Cancer. (2022) 22:650. doi: 10.1186/s12885-022-09721-y
19. Fong C, Johnston E, Starling N. Neoadjuvant and adjuvant therapy approaches to gastric cancer. Curr Treat Options Oncol. (2022) 23:1247–68. doi: 10.1007/s11864-022-01004-9
20. Li S, Yu W, Xie F, Luo H, Liu Z, Lv W, et al. Neoadjuvant therapy with immune checkpoint blockade, antiangiogenesis, and chemotherapy for locally advanced gastric cancer. Nat Commun. (2023) 14:8. doi: 10.1038/s41467-022-35431-x
21. Zheng Y, Wang Z, Yan C, Yan M, Hou Z, Zheng R, et al. Protocol for a randomized controlled trial of perioperative S-1 plus oxaliplatin combined with apatinib and camrelizumab in patients with resectable, locally advanced gastric or gastroesophageal junction adenocarcinoma. Ann Transl Med. (2020) 8:1684. doi: 10.21037/atm
22. Guan WL, He Y, Xu RH. Gastric cancer treatment: recent progress and future perspectives. J Hematol Oncol. (2023) 16:57. doi: 10.1186/s13045-023-01451-3
23. Lv W, Cheng K, Li X, Feng L, Li H, Li J, et al. Case report: favorable response and manageable toxicity to the combination of camrelizumab, oxaliplatin, and oral S-1 in a patient with advanced epstein-barr virus-associated gastric cancer. Front Oncol. (2021) 11:759652. doi: 10.3389/fonc.2021.759652
24. Jin H, Li P, Mao C, Zhu K, Chen H, Gao Y, et al. Pathological complete response after a single dose of anti-PD-1 therapy in combination with chemotherapy as a first-line setting in an unresectable locally advanced gastric cancer with PD-L1 positive and microsatellite instability. Onco Targets Ther. (2020) 13:1751–6. doi: 10.2147/OTT
25. Avgustinovich AV, Bakina OV, Afanas'ev SG, Spirina LV, Volkov AM. Safety and efficacy of neoadjuvant chemoimmunotherapy in gastric cancer patients with a PD-L1 positive status: A case report. Curr Issues Mol Biol. (2023) 45:7642–9. doi: 10.3390/cimb45090481
Keywords: nutritional intervention, gastric cancer, pyloric obstruction, immunotherapy, perioperative treatment
Citation: Jian M, Yang Z, Hu X, Wang X, Zhang Z, Zhang Y and Song X (2024) Case report: Nutritionally supported perioperative chemo-immunotherapy for advanced gastric cancer with incomplete pyloric obstruction. Front. Oncol. 14:1383076. doi: 10.3389/fonc.2024.1383076
Received: 06 February 2024; Accepted: 08 April 2024;
Published: 23 April 2024.
Edited by:
Ramon Andrade Bezerra De Mello, Universidade Nove de Julho, BrazilReviewed by:
Alessandro Parisi, AOU Ospedali Riuniti-Ancona, ItalyCopyright © 2024 Jian, Yang, Hu, Wang, Zhang, Zhang and Song. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yifei Zhang, enlmYWZAc2luYS5jb20=; Xinna Song, eXRzb25neGlubmFAMTYzLmNvbQ==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.