
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol. , 02 July 2024
Sec. Gynecological Oncology
Volume 14 - 2024 | https://doi.org/10.3389/fonc.2024.1381268
This article is part of the Research Topic Tackling Breast, Brain, Lung and Ovarian Cancer with Nanomediated Delivery Systems: New Horizons in Cancer Treatment View all 8 articles
Background: This study aimed to determine the diagnostic accuracy of CA125, HE4, systemic immune-inflammation index (SII), prognostic nutritional index (PNI), fibrinogen-to-albumin ratio (FAR), neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR), monocyte-to-lymphocyte ratio (MLR), and the combination of the six inflammatory-nutritional markers for ovarian cancer (OC) to identify the best diagnostic indicator for OC early diagnosis. An extensive study was performed to establish the connection between these indicators and the pathological aspects of OC.
Methods: A total of 170 individuals were included in this study, with 87 diagnosed with OC and 83 with benign ovarian tumors (BOTs). The diagnostic abilities of the variables were evaluated by calculating sensitivity, specificity, and area under the ROC curves. Through the use of DCA, we evaluated the variables’ clinical value in the discrimination of ovarian masses.
Results: All markers showed significant diagnostic power for OC. CA125, HE4, SII, FAR, and MLR levels significantly increased from the BOTs group to the early-stage OC group. The advanced-stage OC group had significantly lower PNI values compared to the early-stage OC group but significantly higher levels of CA125, HE4, SII, NLR, and FAR. Moreover, the OC group with lymph node metastasis exhibited significantly higher levels of CA125, HE4, SII, NLR, PLR, and FAR, in contrast to the non-metastatic group, while PNI levels were significantly lower. Categorical factors, such as histological grade and pathological classification, showed noticeable discrepancies in CA125 and HE4 levels. NLR was significantly different among the pathological type groups. Among the six inflammatory-nutritional markers, the FAR displayed the greatest diagnostic value. In the analysis of logistic regression, it was observed that a combination marker containing all six inflammatory-nutritional markers exhibited a notably higher AUC value (0.881; 95% CI, 0.823 - 0.926) than any of the individual marker.
Conclusion: PNI, NLR, PLR, MLR, SII, and FAR showed excellent diagnostic performance for OC. The combination of these markers demonstrated a superior diagnostic capability compared to each individual one. The systemic inflammatory indicators may be helpful to diagnose OC.
Ovarian cancer (OC) is often cited as the leading cause of death among gynecologic cancers, with a five-year survival rate of 47% after diagnosis (1). Worldwide, each year approximately 230,000 women are diagnosed with epithelial ovarian cancer (EOC), and 150,000 die due to complications from the disease (2). Despite ongoing advancements in clinical diagnosis and treatment, the outlook for patients with OC remains unsatisfactory. Patients diagnosed with advanced OC have a much lower five-year survival rate (below 30%) than those in the early stages (95%) (3). Therefore, the early detection of OC is important for improving patient prognosis. However, the lack of appropriate screening methods and the asymptomatic growth of the cancer result in OC often being diagnosed only when it reaches an advanced stage. As such, there is a pressing need for dependable indicators to aid in the timely detection and treatment decisions for both patients and physicians. Until now, no single test has provided a reliable indicator of OC. The best biological diagnostic tool for OC will likely be a combination of biomarkers.
One of the most well-known uses for CA125 is as a tumor marker, commonly utilised for monitoring EOC and distinguishing malignant from benign pelvic masses (4, 5). Nonetheless, in the early stages of OC, this marker exhibits a low sensitivity (6). Furthermore, it is prone to producing a high number of false-positive results in non-cancerous gynecological issues like acute pelvic inflammation, adenomyosis, uterine myoma, and endometriosis (7). The possibility of false-positive results must be taken seriously, as it can cause a significant and unnecessary mental and treatment strain for women who are not diagnosed with OC.
In order to improve the precision of detecting OC, new biomarkers like HE4 have been introduced. The levels of HE4, a non-specific tumor marker, can vary in cervical, endometrial, ovarian, and nonepithelial tumors (8). It has been reported that OC tissues exhibit an increased expression of this biomarker (9). Although CA125 and HE4 are the leading markers, they are not powerful enough for early detection of OC (10–12). Extensive research is being conducted to identify new biomarkers that can improve the accuracy and speed of detecting OC for better treatment outcomes. In recent years, mounting evidence has shown that systemic inflammation is a major contributor to the development and advancement of cancer, resulting in a higher chance of metastasis and a weaker response to adjuvant chemotherapy (13). The presence of chronic inflammation is a major contributing factor in the growth and spread of multiple types of solid tumors, such as OC. The relationship between nutrition and inflammation in cancer patients has received considerable attention in the last several years. According to research, the inflammatory microenvironment of cancer is heavily influenced by certain components found in peripheral blood, such as neutrophils, lymphocytes, monocytes, platelets, albumin, globulin, and fibrinogen (14).
Preoperative inflammatory markers, such as systemic immune-inflammation index (SII), prognostic nutritional index (PNI), fibrinogen-to-albumin ratio (FAR), neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR) and monocyte-to-lymphocyte ratio (MLR) have been the subject of numerous studies on OC and Multiple studies have deemed them significant prognostic indicators in OC, although only a handful have specifically examined their role in predicting malignancy before surgery (15–18). As far as we know, there is a lack of published research that investigate the efficacy of CA125, HE4, SII, PNI, FAR, NLR, PLR, and MLR in predicting ovarian cancer before surgery. The main focus of this research was to assess the diagnostic potential of CA125, HE4, SII, PNI, FAR, NLR, PLR, MLR, and a combination of six inflammatory-nutritional markers, with the goal of creating a reliable diagnostic index for early detection of OC. In-depth research was performed to ascertain the connection between these markers and the pathological aspects of OC, offering valuable insights for the timely diagnosis and control of this disorder.
This is a retrospectively designed study performed in the Hebei General Hospital in HeBei, China. The study included the medical records of patients who had surgery in the Department of Gynecology from 1 January 2019 to 1 December 2022. The study was approved by the ethics committee of the Hebei General Hospital and followed the guidelines set forth in the Declaration of Helsinki.
In this study, 170 patients with OC or benign ovarian tumors (BOTs) were included. They were classified into two groups, with the assistance of two senior pathologists, based on the postoperative pathological results. The two groups consisted of 87 individuals with OC and 83 with BOTs. No chemotherapy or radiation therapy was given to patients with OC before their surgery. The OC clinical stage was established using the International Federation of Gynaecology and Obstetrics (FIGO) criteria. All enrolled individuals with OC underwent a thorough staging process, which consisted of a total hysterectomy, adnexectomy, complete pelvic/para-aortic lymphadenectomy, and peritoneal cytology. The study excluded individuals with infectious illnesses, autoimmune disorders, severe liver or kidney issues, thrombotic conditions, benign or malignant tumors, those who were pregnant, and individuals with preexisting blood disorders.
The data analysis included several clinical and laboratory parameters such as age, pathological type, FIGO staging, tissue differentiation degree, presence or absence of lymph node metastases, albumin, fibrinogen, neutrophil count, monocyte count, platelet count, lymphocyte count, CA125, and HE4. Prior to the operation, several tests were conducted and recorded within a week, including albumin, fibrinogen, neutrophil count, monocyte count, platelet count, lymphocyte count, and serum tumor biomarkers. The preoperative levels of CA125 and HE4 were measured using a COBAS E602 analyzer (Roche, Switzerland) and the supplied chemiluminescent reagent kit from Roche. Two expert pathologists conducted a thorough review of the pathological examinations. The ETHICS Committee of Hebei General Hospital has given approval for the study.
The equation used to calculate the serum inflammation-related markers was: FAR= fibrinogen(g/L)/albumin(g/L); PNI = albumin (g/L) + 5 × lymphocyte counts (109/L); SII= platelet count (109/L) × neutrophil count (109/L)/lymphocyte count (109/L); NLR= neutrophil count (109/L)/lymphocyte count (109/L); PLR= platelet count (109/L)/lymphocyte count (109/L); MLR= monocyte count (109/L)/lymphocyte count (109/L).
Descriptive statistical analysis was carried out with the help of IBM SPSS statistics V26.0, GraphPad Prism 9.0 software, and R Environment for Statistical Computing software (R Foundation for Statistical Computing). Statistical significance was set at the 5% level and 2-sided p-values were calculated. Normal distribution of variables was assessed using the Shapiro-Wilk test and histograms. The variables that follow a normal distribution were expressed as mean ± standard deviation. For continuous variables, parameters that followed a normal distribution were analyzed by analysis of variance and reported as mean ± standard deviation (SD), and the median and interquartile range will be displayed for those who do not have a normal distribution. The Kruskal-Wallis test was utilized to compare variables across the groups, followed by the Mann-Whitney U test for conducting multiple comparisons. The area under the receiver operating characteristic (ROC), 95% confidence interval (CI), sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), positive likelihood ratio (LR), negative LR, and accuracy for the defined variables were calculated to test the diagnostic performance for OC prediction by ROC analysis. Based on Youden’s index from the ROC curve, the optimal cut-off value of the parameters was determined. The connections between important factors were examined through Spearman’s rank correlation and logistic regression analysis. Afterward, a decision curve analysis (DCA) was performed to identify the most effective single parameter for distinguishing between BOTs and OC. In conducting this visual analysis, we utilized specialized DCA software. All calculations were executed according to the method outlined by Vickers and Elkin (19).
The final analysis included a total of 87 patients with OC and 83 with BOTs. Table 1 contains comprehensive data on the laboratory measurements of the research participants.
The age range of patients with OC was 21‐76 years, and of patients with BOTs was 26‐78 years. The two groups did not differ significantly in age. Table 1 presents evidence of a notable difference in absolute neutrophil count, absolute lymphocyte count, absolute monocyte count, blood platelet count, albumin, fibrinogen, SII, PNI, FAR, NLR, PLR, MLR, CA125, and HE4 between BOTs and OC patients (P=0.001, P=0.001, P=0.009, P=0.001, P=0.001, P<0.001, P<0.001, P<0.001, P<0.001, P<0.001, P<0.001, P<0.001, P<0.001, P<0.001, respectively). Patients with OC showed significantly higher levels of the SII, FAR, NLR, PLR, MLR, CA125, and HE4 [884.86 (859.31), 0.087 (0.049), 3.10(2.14), 211.89(149.75), 0.21(0.15), 228.50 (777.87), 180.20 (308.80)], compared with the patients with BOTs [884.86 (859.31), 0.087 (0.049), 3.10(2.14), 211.89(149.75), 0.21(0.15), 228.50 (777.87), 180.20 (308.80)]. On the other hand, the PNI values were notably lower in the OC group [46.40 (8.10)] in comparison to the BOTs group [51.20 (5.10)]. All markers showed significant diagnostic utility. The PNI, NLR, and PLR levels in the BOTs group did not differ significantly from those of the early-stage OC (Stage I-II) group (P=0.578, P=0.102, P=0.059). Nevertheless, there was a significant increase in CA125, HE4, SII, FAR, and MLR levels observed from the BOTs group to the early-stage OC group (P<0.01, P<0.01, P=0.048, P=0.001) (Figure 1).
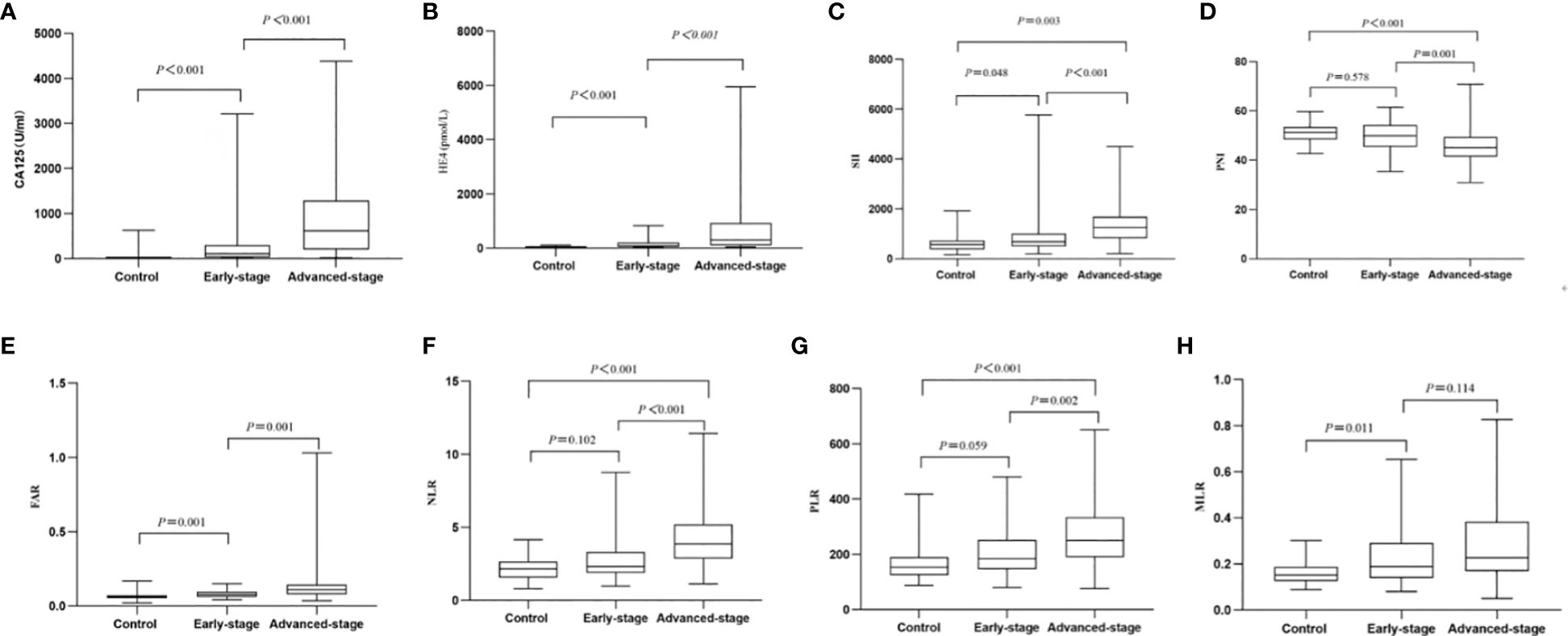
Figure 1 Box of the levels of the eight markers in BOTs group (controls) as well as early-stage OC (Stage I-II) group and advanced-stage OC (Stage III-IV) group. (A) CA125; (B) HE4; (C) SII; (D) PNI; (E) FAR; (F) NLR; (G) PLR; (H) MLR.
Table 2 contains a comprehensive analysis of the histopathology and characteristics of the OC-enrolled patients, incorporating differentiation grades and cancer stages. The advanced-stage OC (Stage III-IV) group showed significantly higher levels of CA125 [107.09(267.10)vs. 618.40(1086.20), (early-stage) vs. (advanced-stage), P<0.001], HE4 [82.80(153.37)vs. 299.00(816.95), (early-stage) vs. advanced-stage), P<0.001], SII [685.64(511.86)vs. 1252.37(857.18), (early-stage) vs. (advanced-stage), P<0.001], NLR [2.33(1.42)vs. 3.85(2.32), (early-stage) vs. (advanced-stage), P<0.001], PLR [184.19(107.27)vs. 251.25(144.11), (early-stage) vs. (advanced-stage), P= 0.002], and FAR [0.08(0.04)vs. 0.11(0.06), (Stage I-II) vs. (advanced-stage), P=0.001] compared to the early-stage OC group, while the levels of PNI [49.90(8.77)vs. 45.05(8.08), (early-stage) vs. (advanced-stage), P=0.001]were significantly lower in the advanced OC group. Nevertheless, there was a non-significant inclination for MLR levels to rise as OC progressed from early-stage to advanced-stage (Figure 1, Table 2).
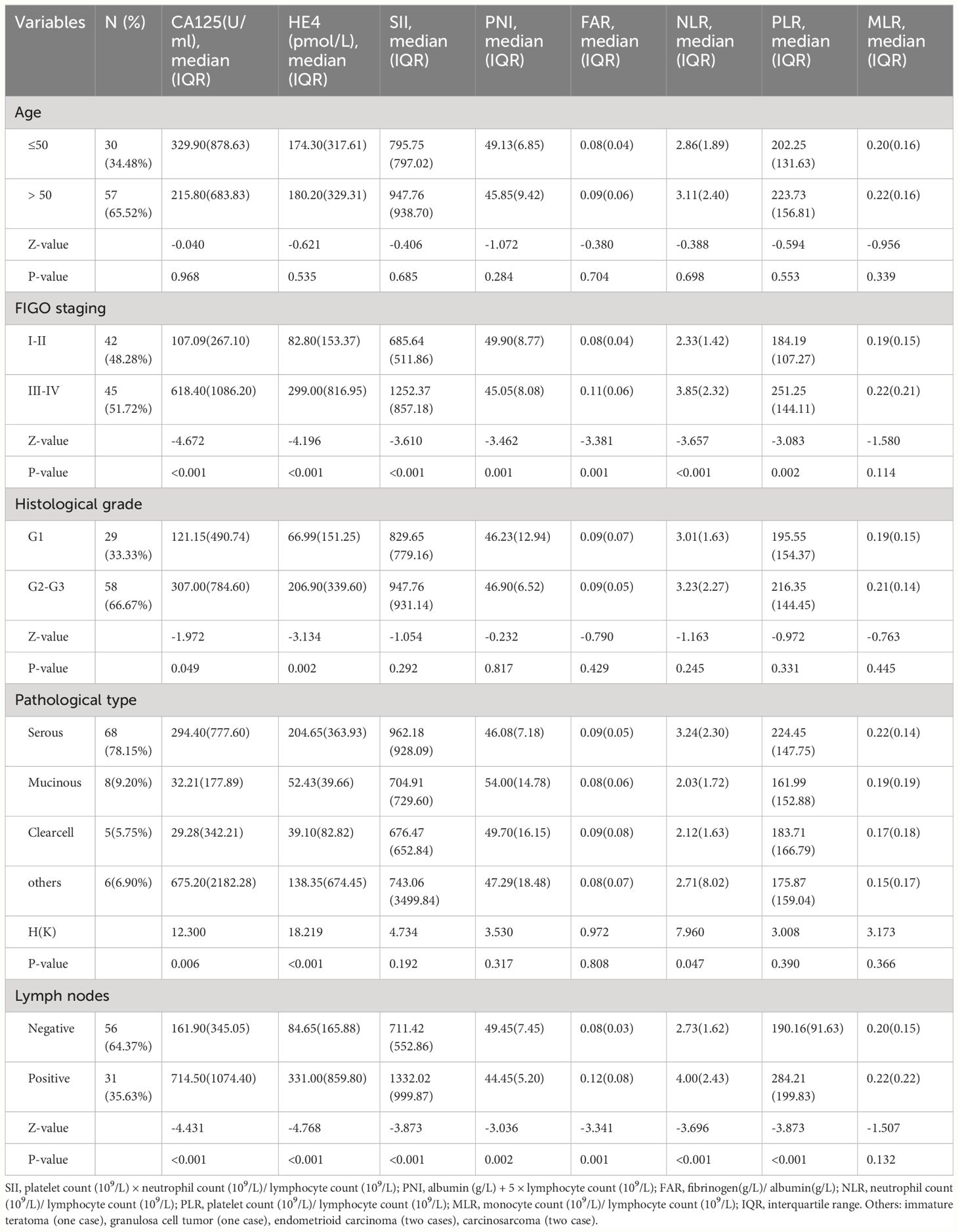
Table 2 Relationship between laboratory variables and clinic-pathological characteristics of OC patients.
Additionally, the lymph node metastasis OC group showed considerably higher levels of CA125 [714.50(1074.40)vs. 161.90(345.05), (lymph node metastasis) vs. (non-lymph node metastasis), P<0.001], HE4 [331.00(859.80)vs. 84.65(165.88), (lymph node metastasis) vs. (non-lymph node metastasis), P<0.001], SII [1332.02(999.87)vs. 711.42(552.86), (lymph node metastasis) vs. (non-lymph node metastasis), P<0.001], NLR[4.00(2.43)vs. 2.73(1.62), (lymph node metastasis) vs. (non-lymph node metastasis), P<0.001], PLR [284.21(199.83)vs. 190.16(91.63), (lymph node metastasis) vs. (non-lymph node metastasis), P<0.001], and FAR [0.12(0.08)vs. 0.08(0.03), (lymph node metastasis) vs. (non-lymph node metastasis), P=0.001] compared to the non-lymph node metastasis OC group, while PNI levels [44.45(5.20)vs. 49.45(7.45), (lymph node metastasis) vs. (non-lymph node metastasis), P=0.002] were significantly lower. Nevertheless, there were no notable differences in the MLR values between the two groups (Table 2). The findings implied that increased CA125, HE4, SII, NLR, PLR, and FAR levels before surgery, as well as decreased PNI levels, are indicative of a greater likelihood of advanced ovarian cancer progression and lymph node metastasis. Following that, we explored variances in the variables based on age, histological grade, and pathological category. No statistically significant disparities in the variables were observed among the different age categories, as shown in Table 2 (P= 0.968, P= 0.535, P= 0.685, P= 0.284, P= 0.704, P= 0.698, P= 0.553, P= 0.339, respectively). Nevertheless, there were notable discrepancies between CA125 and HE4 in relation to categorical variables such as histological grades (P=0.049, P=0.002, respectively) and pathological type (P=0.006, P<0.001, respectively). NLR was significantly different among the pathological type groups (P = 0.047). Further, the levels of SII, PNI, PLR, MLR, and FAR did not significantly differ according to the histological grades (P=0.292, P=0.817, P=0.331, P=0.445, P=0.429, respectively) or pathological types (P=0.192, P=0.317, P=0.390, P=0.366, P=0.808, respectively).
ROC curves were performed to determine the optimal cut-off values, sensitivity and specificity of an independent significant parameter (Figure 2). The primary statistics are shown in Table 3. The optimum cut-off value was chosen to maximize the Youden index (sensitivity + specificity − 1). The appropriate cut-off value of CA125 (AUC = 0.890, P<0.001), HE4 (AUC =0.859, P<0.001), FAR (AUC =0.793, P<0.001), SII (AUC =0.739, P<0.001), NLR (AUC =0.728, P<0.001), PLR (AUC =0.721, P<0.001), MLR (AUC =0.723, P<0.001),and PNI(AUC =0.692, P<0.001) for differentiating BOTs and OC were 79.89, 65.16, 0.08, 945.21, 2.92, 201.42, 0.21, and 46.90, respectively; with the corresponding sensitivity of 73.56%, 72.41%, 58.62%, 47.13%, 56.32%, 56.32%, 49.43%, and 54.02%, respectively; specificity of 97.59%, 92.77%, 91.57%, 92.77%,84.34%, 81.93%, 91.57%, and 87.95%, respectively; PPV of 95.52%, 90.00%, 88.14%, 85.42%, 77.78%, 75.38%, 86.00%, and 82.14%, respectively; NPV of 77.67%, 76.00%, 68.47%, 62.30%, 64.49%, 63.81%, 63.33%, and 62.39%, respectively; positive LR of 30.667, 10.056, 6.976, 6.542, 3.34, 2.92, 5.86,and 4.90, respectively; negative LR of 0.270, 0.297, 0.452, 0.570, 0.525, 0.541, 0.552, and 0.528, respectively; accuracy of 84.71, 81.76, 75.29, 68.82, 69.41, 68.24, 70.00, and 70.00 respectively. Among the six inflammatory-nutritional variables (FAR, PNI, NLR, PLR, MLR, and SII), the FAR test showed the greatest sensitivity (58.6%), PPV (88.14%), NPV (68.47%), positive LR (6.976), accuracy (75.29), and lowest negative LR (0.452) in distinguishing between BOTs and OC. Significantly, a logistic regression model incorporating all six inflammatory-nutritional markers demonstrated a higher diagnostic strength than each individual marker (AUC, 0.881; 95% [CI], 0.823 - 0.926).
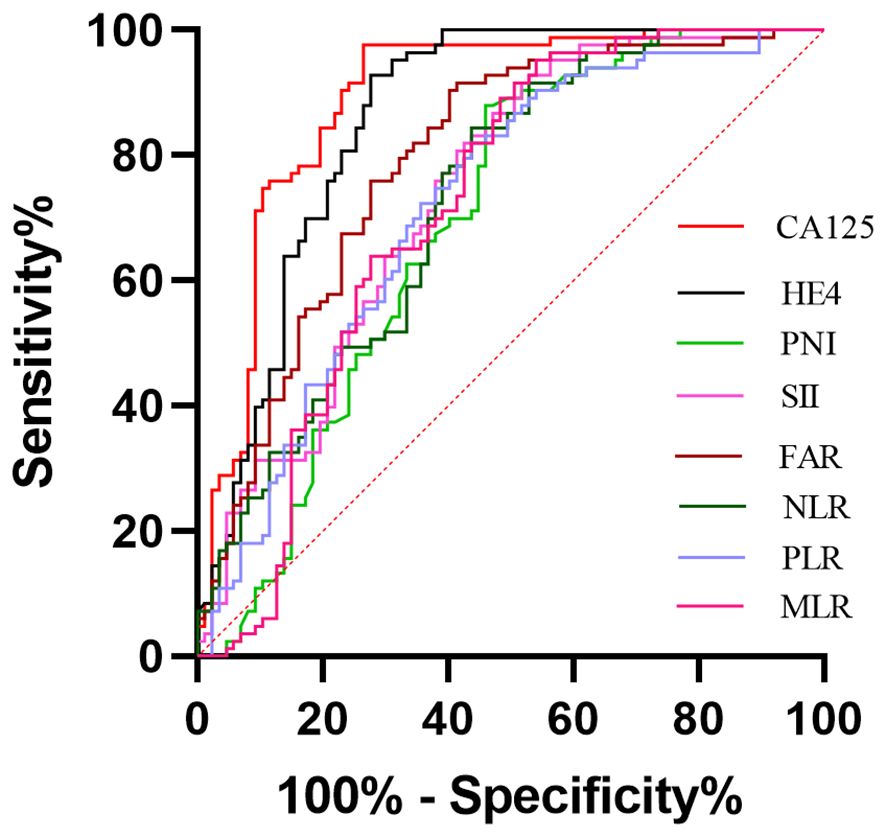
Figure 2 Evaluation of diagnostic performance of the markers for detecting ovarian cancer using receiver operating characteristic curve analysis.
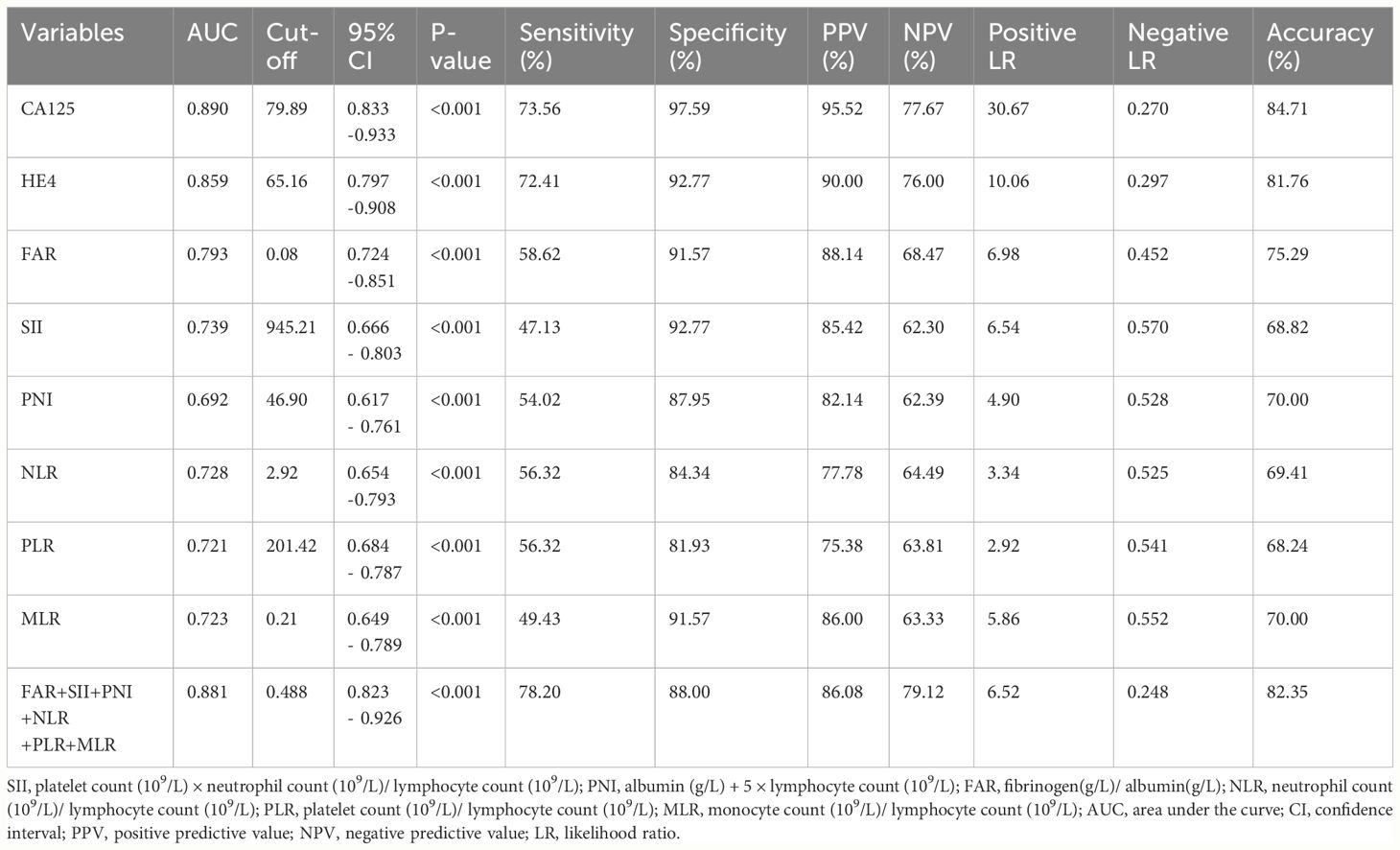
Table 3 Cut-off value and diagnostic value of CA125, HE4, SII, PNI, FAR, NLR, PLR, MLR, and the combination of the six inflammatory-nutritional indices in the diagnosis of OC.
In general, when evaluated individually, the CA125 examination exhibited the highest level of sensitivity (73.56%), specificity (97.59%), PPV (95.52%), NPV (77.67%), positive LR (30.667), accuracy (84.71%), and lowest negative LR (0.270) in distinguishing BOTs from OC.
Utilizing a combination of all six inflammatory-nutritional markers in the logistic regression analysis resulted in a significantly higher AUC value (0.881; 95% CI, 0.823 - 0.926) (Figure 3). Compared to CA125, the combination marker showed a slight increase in Sensitivity (78.20%), NPV (79.12%), and a decrease in negative LR (0.248) and Accuracy (82.35%), as seen in Table 3. The DCA for single CA125, HE4, FAR, PNI, NLR, PLR, MLR, and SII is shown in Figure 4. CA125 outperformed all other isolated parameters in terms of clinical utility, as shown in the graphic analysis.
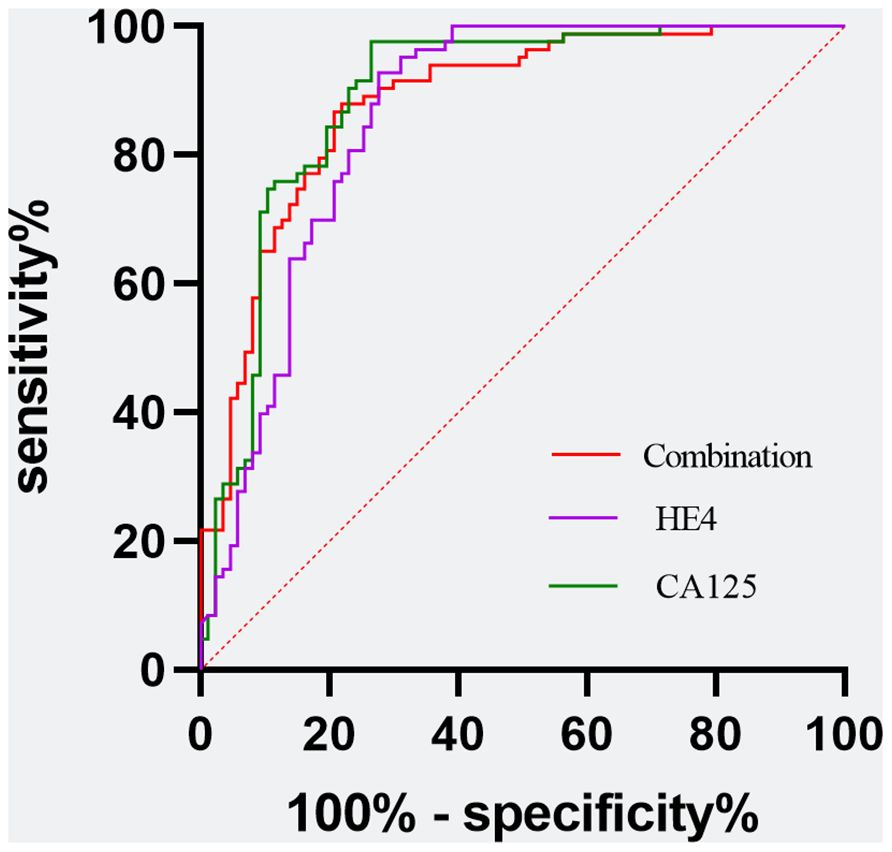
Figure 3 Evaluation of diagnostic performance of the CA125, HE4, and the combination marker for detecting ovarian cancer using receiver operating characteristic curve analysis. Combination marker indicates a logistic regression-based model of SII, PNI, FAR, NLR, PLR, and MLR.
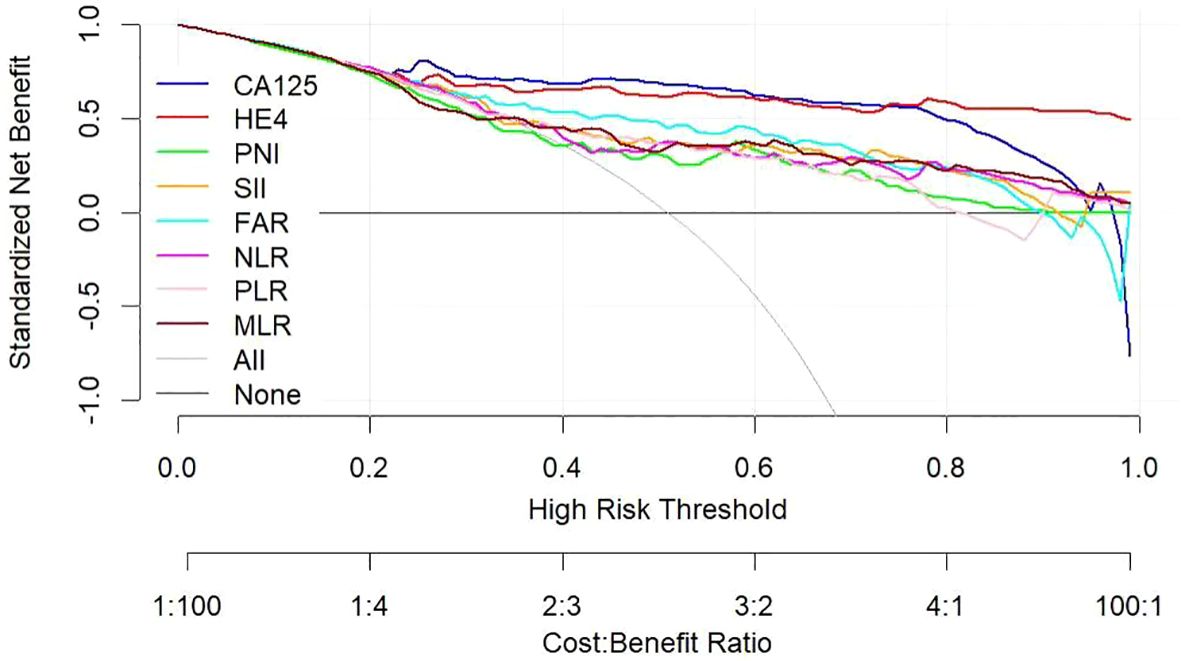
Figure 4 Decision curve showing the net benefit of CA125, HE4, SII, PNI, FAR, NLR, PLR, and MLR in women at risk of developing ovarian cancer. CA125 dosage, positive if ≥79.89 U/mL, negative if <79.89 U/mL; HE4, HE4 dosage, positive if ≥65.16 pmol/L, negative if < 65.16 pmol/L; SII, systemic immune-inflammation index, positive if ≥945.21, negative if <945.21; PNI, prognostic nutritional index, positive if ≤46.9, negative if >46.9; FAR, fibrinogen-to-albumin ratio, positive if ≥0.08, negative if <0.08; NLR, neutrophil-to-lymphocyte ratio, positive if ≥2.92, negative if <2.92; PLR, platelet-to-lymphocyte ratio, positive if ≥201.42, negative if <201.42; MLR, monocyte-to-lymphocyte ratio, positive if ≥0.21, negative if <0.21.
The advanced OC group exhibited significantly higher levels of CA125, HE4, SII, NLR, PLR, and FAR, and significantly lower levels of PNI, when compared to the early-stage OC group. The CA125 test showed the strongest ability to differentiate between BOTs and OC when the defined variables were evaluated individually. By combining all six inflammatory-nutritional indices, the AUC was found to be higher than that of any one index alone. The combination marker showed a slight advantage over CA125, with higher sensitivity and NPV, and lower negative LR and accuracy. The CA125 produced a more favorable result in terms of clinical outcome compared to either HE4 or the combined six inflammatory-nutritional indices. Increased levels of CA125, HE4, SII, NLR, PLR, and FAR prior to surgery may indicate an increased risk of advanced ovarian cancer progression and lymph node metastasis. When compared to other inflammation markers, FAR demonstrated a superior application value.
OC, a highly lethal gynecological cancer, poses a significant global challenge. Due to the absence of distinct early symptoms and reliable screening techniques, OC is typically detected during its advanced stages, leading to relatively high recurrence and mortality rates despite treatment. Timely identification and precise prediction play a crucial role in enhancing survival rates for individuals with OC (20). The use of serum CA125 levels as diagnostic and prognostic biomarkers for OC is common, but solely relying on them may not be sufficient for detecting early OC due to their limited diagnostic ability (21). In various levels, HE4 can be detected as a non-specific tumor marker in cervical, endometrial, ovarian, and nonepithelial tumors (8). According to reports, this biomarker is known to be highly expressed in OC tissues (9). Despite their widespread use, CA125 and HE4 are not reliable enough to detect early-stage OC (10–12). As a result, there has been a significant amount of research focused on discovering new biomarkers for OC.
The initiation and progression of various tumors rely heavily on the immune response and systemic inflammatory processes. They play a crucial role in every aspect of cancer development, from initiating tumors to promoting their spread throughout the body (22). Stephen Paget, a British surgeon, was the first to propose the “seed-soil” theory, which forms the basis of the tumor microenvironment concept. The cellular network is highly intricate, with multiple inflammatory factors produced by both tumor and stromal cells creating an inflammatory microenvironment. The presence of an inflammatory microenvironment significantly impacts the malignant properties of tumors, controlling the biological processes responsible for their growth (23, 24). The inflammatory response is heavily influenced by the body’s immune and nutritional status. Systemic inflammation induced by the progression of cancer is a major contributor to malnutrition and weakened immunity, both of which can significantly decrease survival (25–27). According to studies, malnutrition has been linked to a higher risk of postoperative complications, greater susceptibility to infection, and even a potential increase in tumor recurrence due to weakened tumor immunity (25, 28, 29).
Tumors are often triggered and advanced by uncontrollable inflammation, with changes in inflammatory markers in the blood serving as a reflection of this inflammatory state. Critical markers of inflammation include pro-inflammatory blood cells such as white blood cells (WBC), lymphocytes, monocytes, neutrophils, platelets, and, even more relevant, the MLR, NLR, and PLR. The progression of tumors can be influenced by the release of tumor necrosis factors, interleukin-1, and interleukin-6 from neutrophils (30). Lymphocytes play a vital role in targeting tumors through triggering cytotoxic cell death and hindering the growth and movement of malignant cells (23). Monocytes play a crucial role in the process of tumor occurrence, growth, migration, vascularization, invasion, and metastasis (31). In circulation, platelets can prompt the transformation of tumor cells into an epithelial-mesenchymal state, aiding in their spread to the metastatic site (32). The presence of neutropenia, lymphopenia, monocytosis, and thrombocytosis in cancer leads to inflammation, which can speed up the progression of the disease by promoting angiogenesis, invasion, metastasis, and paraneoplastic phenomena (33–35).
The SII takes into consideration the levels of platelets, neutrophils, and lymphocytes in the blood, serving as an indicator of the multiple Inflammatory and immunological pathways at play in the body. It has outstanding stability (36). It has been utilized in the diagnosis and treatment of various malignant tumors. It is linked to the prediction of patients’ outcomes, reflecting the immune and inflammatory conditions of patients with cancerous tumors (37–39). Although SII is considered an important prognostic factor for OC, its potential as a preoperative indicator of malignancy has not been thoroughly explored in existing research. Albumin is an important marker for both acute-phase proteins and chronic inflammation throughout the body (40). It is frequently utilized to evaluate the body’s nutritional status and is considered a possible prognostic factor for various types of cancer (41, 42). A decrease in serum albumin levels could suggest that the individual is suffering from malnutrition, which can have adverse effects on their overall physical condition. This state of malnourishment can weaken the body’s defense systems, such as cellular immunity, humoral immunity, and phagocytic functioning (43). Preoperative serum albumin levels have been recognized as a significant independent predictor of OS for those with EOC, according to a meta-analysis (44). Elevated levels of plasma fibrinogen, an acute-phase protein, are commonly seen during periods of system-wide inflammation (45). Through its effects on the multiplication and movement of cancer cells, as well as the stimulation of blood vessel growth, it is commonly recognized as a crucial factor in regulating inflammation and cancer progression (46). Numerous studies have consistently demonstrated a correlation between high levels of fibrinogen before treatment and a negative prognosis in a variety of cancers (47).. PNI serves as an indicator for overall inflammatory condition in the body. The combination of serum albumin levels and peripheral blood lymphocyte count, referred to as PNI, provides a comprehensive assessment of the host’s nutritional and immunological conditions. The effectiveness of this approach has been confirmed in accurately predicting both short and long-term prognosis (48). Studies are increasingly showing that preoperative PNI can be served as a prognostic factor for OC patients (48, 49). The results of Miao et al.’s research revealed that PNI is a significant predictor for the overall survival (OS) and progression-free survival (PFS) of OC patients (50). However, it is rarely reported as an indicator of the diagnosis of OC. Researchers have found that FAR, an indicator that takes both fibrinogen and albumin into consideration, can be used to predict outcomes for various malignancies (16, 51). A different strategy for improving the predictive ability of inflammation and nutritional status in patients is to combine fibrinogen and albumin in the FAR. Elevated levels of serum fibrinogen and decreased levels of serum albumin are commonly accepted as reliable indicators of heightened systemic inflammation (52, 53). According to Xie H et al.’s investigation, a high FAR could be an indication of more aggressive tumor characteristics and the development of systemic inflammation (54). Additionally, FAR was observed to heighten the sensitivity of inflammation and nutrition levels in EOC patients, proving to be a superior predictor of EOC prognosis than individual markers like fibrinogen or albumin.
Our study examined the diagnostic utilities of CA125, HE4, and six inflammatory-nutritional markers (PNI, NLR, PLR, MLR, SII, and FAR) in patients with OC. All markers demonstrated significant diagnostic value in identifying patients with OC. CA125, HE4, SII, FAR, and MLR levels significantly increased from the BOTs to early-stage OC groups. Nevertheless, there was no significant difference in PNI, NLR, and PLR levels between the BOTs and early-stage OC groups. The advanced-stage OC group had notably elevated levels of CA125, HE4, SII, NLR, and FAR, in contrast to the early-stage OC group. On the other hand, the advanced OC group had significantly lower PNI values. There was a slight, non-significant trend of MLR levels increasing from the early stages to the advanced stages. Additionally, the presence of lymph node metastasis in the OC group was associated with significantly elevated levels of CA125, HE4, SII, NLR, PLR, and FAR when compared to the OC group without lymph node involvement. Conversely, the PNI values were notably decreased in the OC group with lymph node metastasis. There were no notable differences in the MLR values among the two groups. The findings indicate that elevated preoperative levels of CA125, HE4, SII, NLR, PLR, and FAR, as well as decreased PNI levels, are associated with a higher probability of advanced ovarian cancer progression and lymph node metastasis. Significant variations were observed in CA125 and HE4 levels among categorical variables such as histological grades and pathological type. NLR was significantly different among the pathological type groups. However, the SII, PNI, PLR, MLR, and FAR levels did not significantly differ according to the histological grades or pathological types. Based on the six inflammatory-nutritional indices (FAR, PNI, NLR, PLR, MLR, and SII), the FAR test was identified as the top performer in distinguishing between BOTs and OC, exhibiting the highest sensitivity (58.6%), PPV (88.14%), NPV (68.47%), positive LR (6.976), accuracy (75.29), and lowest negative LR (0.452).
Additionally, the ROC curve analysis revealed significant AUC values for all eight markers, ranked in the following order: CA125 (0.890; 95% CI,0.833 -0.933), HE4 (0.859; 95% CI, 0.797 -0.908), FAR (0.793, 95% CI 0.724 -0.851), SII (0.739, 95% CI 0.666 - 0.803), NLR (0.728, 95% CI 0.654 -0.793), MLR (0.723, 95% CI 0.649 - 0.789), PLR (0.721, 95% CI 0.684 - 0.787), and PNI (0.692, 95% CI, 0.617 - 0.761). The logistic regression analysis revealed that a composite marker comprising all six inflammatory-nutritional markers exhibited a significantly greater AUC value (0.881; 95% CI, 0.823 - 0.926) compared to each individual marker. The combination marker model displayed the most sensitivity (78.20%) compared with CA125 (73.56%), HE4 (72.41%), FAR (58.62%), SII (47.13%), PNI (54.02%), NLR (56.32%), PLR (56.32%), and MLR (49.43%).
Our research revealed that incorporating inflammatory biomarkers alongside CA125 and HE4 is an effective method for differentiating between BOTs and OC. Before surgery, biomarkers can be helpful, but imaging tests are usually the main method of managing abnormalities found in serum biomarkers. By further exploring the inflammatory pathways linked to tumors, we may uncover improved combinations of tumor and inflammatory biomarkers.
This study has a number of shortcomings that warrant mentioning. First, this was a retrospective study conducted at a single medical center, and further research on a larger scale is needed to validate our findings. Secondly, only patients with BOTs were included in the control group. In future studies, it is recommended to include both individuals without health issues and those with the disease as controls. Despite this, our research provides robust evidence base for the reliability and reproducibility of the diagnostic traits under study, as a result of our meticulous inclusion and exclusion standards and highly significant statistical results.
In conclusion, systemic inflammatory indicators (PNI, NLR, PLR, MLR, SII, and FAR) showed excellent diagnostic performance for OC. Significantly, the combination of these markers demonstrated a superior diagnostic capability compared to each individual one. Furthermore, FAR exhibited a greater application value than other inflammation-related markers, including PNI, NLR, PLR, MLR, and SII. Our findings suggest systemic inflammatory indicators may be helpful to diagnose OC. In the near future, we aim to carry out an external validation study.
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
The studies involving humans were approved by the ethics committee of the Hebei General Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. The human samples used in this study were acquired from a by- product of routine care or industry. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements.
LS: Investigation, Software, Supervision, Writing – original draft, Writing – review & editing, Conceptualization, Data curation, Formal analysis, Validation, Resources. QW: Writing – original draft, Writing – review & editing, Conceptualization, Investigation, Methodology, Resources. SB: Conceptualization, Writing – original draft, Formal analysis, Methodology, Project administration. JZ: Formal analysis, Methodology, Resources, Writing – original draft. JQ: Data curation, Investigation, Resources, Writing – original draft. JMZ: Data curation, Project administration, Resources, Writing – original draft.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
We would like to thank all doctors, nurses, patients, and their family members for their kindness to support our study.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Lheureux S, Braunstein M, Oza AM. Epithelial ovarian cancer: Evolution of management in the era of precision medicine. CA Cancer J Clin. (2019) 69:280–304. doi: 10.3322/caac.21559
2. Lheureux S, Gourley C, Vergote I, Oza AM. Epithelial ovarian cancer. Lancet. (2019) 393:1240–53. doi: 10.1016/S0140-6736(18)32552-2
3. Prat J. Pathology of borderline and invasive cancers. Best Pract Res Clin Obstet Gynaecol. (2017) 41:15–30. doi: 10.1016/j.bpobgyn.2016.08.007
4. Duffy MJ, Bonfrer JM, Kulpa J, Rustin GJ, Soletormos G, Torre GC, et al. CA125 in ovarian cancer: European Group on Tumor Markers guidelines for clinical use. Int J Gynecol Cancer. (2005) 15:679–91. doi: 10.1111/j.1525-1438.2005.00130.x
5. Sturgeon CM, Duffy MJ, Stenman UH, Lilja H, Brünner N, Chan DW, et al. National Academy of Clinical Biochemistry laboratory medicine practice guidelines for use of tumor markers in testicular, prostate, colorectal, breast, and ovarian cancers. Clin Chem. (2008) 54:e11–79. doi: 10.1373/clinchem.2008.105601
6. Urban N, McIntosh MW, Andersen M, Karlan BY. Ovarian cancer screening. Hematol Oncol Clin North Am. (2003) 17:989–1005. doi: 10.1016/S0889-8588(03)00063-7
7. Gu Z, He Y, Zhang Y, Chen M, Song K, Huang Y, et al. Postprandial increase in serum CA125 as a surrogate biomarker for early diagnosis of ovarian cancer. J Transl Med. (2018) 16:114. doi: 10.1186/s12967-018-1489-4
8. Huang C, Xiao L, Luo HL, Zhu ZM. Preoperative neutrophil-to-lymphocyte ratio combined with serum CEA, CA19-9, CA125 and CA72-4 levels in the clinical pathological staging of gastric cancer-based on propensity score matching. J Biol Regul Homeost Agents. (2020) 34:1111–6. doi: 10.23812/19-458-L-46
9. Drapkin R, von Horsten HH, Lin Y, Mok SC, Crum CP, Welch WR, et al. Human epididymis protein 4 (HE4) is a secreted glycoprotein that is overexpressed by serous and endometrioid ovarian carcinomas. Cancer Res. (2005) 65:2162–9. doi: 10.1158/0008-5472.CAN-04-3924
10. Jacobs IJ, Menon U, Ryan A, Gentry-Maharaj A, Burnell M, Kalsi JK, et al. Ovarian cancer screening and mortality in the UK Collaborative Trial of Ovarian Cancer Screening (UKCTOCS): a randomised controlled trial. Lancet. (2016) 387:945–56. doi: 10.1016/S0140-6736(15)01224-6
11. Terry KL, Schock H, Fortner RT, Hüsing A, Fichorova RN, Yamamoto HS, et al. A prospective evaluation of early detection biomarkers for ovarian cancer in the european EPIC cohort. Clin Cancer Res. (2016) 22:4664–75. doi: 10.1158/1078-0432.CCR-16-0316
12. Menon U, Gentry-Maharaj A, Burnell M, Singh N, Ryan A, Karpinskyj C, et al. Ovarian cancer population screening and mortality after long-term follow-up in the UK Collaborative Trial of Ovarian Cancer Screening (UKCTOCS): a randomised controlled trial. Lancet. (2021) 397:2182–93. doi: 10.1016/S0140-6736(21)00731-5
13. Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. (2008) 454:436–44. doi: 10.1038/nature07205
14. Cordeiro LAF, Silva TH, de Oliveira LC, Neto JFN. Systemic inflammation and nutritional status in patients on palliative cancer care: A systematic review of observational studies. Am J Hosp Palliat Care. (2020) 37:565–71. doi: 10.1177/1049909119886833
15. Yildirim M, Demir Cendek B, Filiz Avsar A. Differentiation between benign and Malignant ovarian masses in the preoperative period using neutrophil-to-lymphocyte and platelet-to-lymphocyte ratios. Mol Clin Oncol. (2015) 3:317–21. doi: 10.3892/mco.2014.481
16. Yu W, Ye Z, Fang X, Jiang X, Jiang Y. Preoperative albumin-to-fibrinogen ratio predicts chemotherapy resistance and prognosis in patients with advanced epithelial ovarian cancer. J Ovarian Res. (2019) 12:88. doi: 10.1186/s13048-019-0563-8
17. Xiang J, Zhou L, Li X, Bao W, Chen T, Xi X, et al. Preoperative monocyte-to-lymphocyte ratio in peripheral blood predicts stages, metastasis, and histological grades in patients with ovarian cancer. Transl Oncol. (2017) 10:33–9. doi: 10.1016/j.tranon.2016.10.006
18. Zhang CL, Jiang XC, Li Y, Pan X, Gao MQ, Chen Y, et al. Independent predictive value of blood inflammatory composite markers in ovarian cancer: recent clinical evidence and perspective focusing on NLR and PLR. J Ovarian Res. (2023) 16:36. doi: 10.1186/s13048-023-01116-2
19. Vickers AJ, Elkin EB. Decision curve analysis: a novel method for evaluating prediction models. Med Decis Making. (2006) 26:565–74. doi: 10.1177/0272989X06295361
20. Nossov V, Amneus M, Su F, Lang J, Janco JM, Reddy ST, et al. The early detection of ovarian cancer: from traditional methods to proteomics. Can we really do better than serum CA-125? Am J Obstet Gynecol. (2008) 199:215–23. doi: 10.1016/j.ajog.2008.04.009
21. Zhang M, Cheng S, Jin Y, Zhao Y, Wang Y. Roles of CA125 in diagnosis, prediction, and oncogenesis of ovarian cancer. Biochim Biophys Acta Rev Cancer. (2021) 1875:188503. doi: 10.1016/j.bbcan.2021.188503
22. Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. (2010) 140:883–99. doi: 10.1016/j.cell.2010.01.025
23. Arneth B. Tumor microenvironment. Medicina (Kaunas). (2019) 56:15. doi: 10.3390/medicina56010015
24. Kulbe H, Chakravarty P, Leinster DA, Charles KA, Kwong J, Thompson RG, et al. A dynamic inflammatory cytokine network in the human ovarian cancer microenvironment. Cancer Res. (2012) 72:66–75. doi: 10.1158/0008-5472.CAN-11-2178
25. Sasahara M, Kanda M, Ito S, Mochizuki Y, Teramoto H, Ishigure K, et al. The preoperative prognostic nutritional index predicts short-term and long-term outcomes of patients with stage II/III gastric cancer: analysis of a multi-institution dataset. Dig Surg. (2020) 37:135–44. doi: 10.1159/000497454
26. Shimizu T, Taniguchi K, Asakuma M, Tomioka A, Inoue Y, Komeda K, et al. Lymphocyte-to-monocyte ratio and prognostic nutritional index predict poor prognosis in patients on chemotherapy for unresectable pancreatic cancer. Anticancer Res. (2019) 39:2169–76. doi: 10.21873/anticanres.13331
27. Arends J, Baracos V, Bertz H, Bozzetti F, Calder PC, Deutz NEP, et al. ESPEN expert group recommendations for action against cancer-related malnutrition. Clin Nutr. (2017) 36:1187–96. doi: 10.1016/j.clnu.2017.06.017
28. Yoshida K, Yoshikawa N, Shirakawa A, Niimi K, Suzuki S, Kajiyama H, et al. Prognostic value of neutrophil-to-lymphocyte ratio in early-stage ovarian clear-cell carcinoma. J Gynecol Oncol. (2019) 30:e85. doi: 10.3802/jgo.2019.30.e85
29. Ryan AM, Reynolds JV, Healy L, Byrne M, Moore J, Brannelly N, et al. Enteral nutrition enriched with eicosapentaenoic acid (EPA) preserves lean body mass following esophageal cancer surgery: results of a double-blinded randomized controlled trial. Ann Surg. (2009) 249:355–63. doi: 10.1097/SLA.0b013e31819a4789
30. Hedrick CC, Malanchi I. Neutrophils in cancer: heterogeneous and multifaceted. Nat Rev Immunol. (2022) 22:173–87. doi: 10.1038/s41577-021-00571-6
31. Olingy CE, Dinh HQ, Hedrick CC. Monocyte heterogeneity and functions in cancer. J Leukoc Biol. (2019) 106:309–22. doi: 10.1002/JLB.4RI0818-311R
32. Cedervall J, Hamidi A, Olsson AK. Platelets, NETs and cancer. Thromb Res. (2018) 164 Suppl 1:S148–52. doi: 10.1016/j.thromres.2018.01.049
33. Stone RL, Nick AM, McNeish IA, Balkwill F, Han HD, Bottsford-Miller J, et al. Paraneoplastic thrombocytosis in ovarian cancer. N Engl J Med. (2012) 366:610–8. doi: 10.1056/NEJMoa1110352
34. Huang H, Liu Q, Zhu L, Zhang Y, Lu X, Wu Y, et al. Prognostic value of preoperative systemic immune-inflammation index in patients with cervical cancer. Sci Rep. (2019) 9:3284. doi: 10.1038/s41598-019-39150-0
35. Stoiber D, Assinger A. Platelet-leukocyte interplay in cancer development and progression. Cells. (2020) 9:855. doi: 10.3390/cells9040855
36. Wang Q, Zhu D. The prognostic value of systemic immune-inflammation index (SII) in patients after radical operation for carcinoma of stomach in gastric cancer. J Gastrointest Oncol. (2019) 10:965–78. doi: 10.21037/jgo.2019.05.03
37. Han R, Tian Z, Jiang Y, Guan G, Sun X, Yu Y, et al. Prognostic significance of systemic immune-inflammation index and platelet-albumin-bilirubin grade in patients with pancreatic cancer undergoing radical surgery. Gland Surg. (2022) 11:576–87. doi: 10.21037/gs-22-117
38. Xu S, Cao S, Yu Y. High systemic immune-inflammation index is a predictor of poor prognosis in patients with nonsmall cell lung cancer and bone metastasis. J Cancer Res Ther. (2021) 17:1636–42. doi: 10.4103/jcrt.jcrt_176_21
39. Zhu M, Chen L, Kong X, Wang X, Li X, Fang Y, et al. The systemic immune-inflammation index is an independent predictor of survival in breast cancer patients. Cancer Manag Res. (2022) 14:775–820. doi: 10.2147/CMAR.S346406
40. Wang Y, Li J, Chang S, Zhou K, Che G. Low albumin to fibrinogen ratio predicts poor overall survival in esophageal small cell carcinoma patients: A retrospective study. Cancer Manag Res. (2020) 12:2675–83. doi: 10.2147/CMAR.S250293
41. Fiala O, Pesek M, Finek J, Racek J, Minarik M, Benesova L, et al. Serum albumin is a strong predictor of survival in patients with advanced-stage non-small cell lung cancer treated with erlotinib. Neoplasma. (2016) 63:471–6. doi: 10.4149/318_151001N512
42. Ku JH, Kim M, Choi WS, Kwak C, Kim HH. Preoperative serum albumin as a prognostic factor in patients with upper urinary tract urothelial carcinoma. Int Braz J Urol. (2014) 40:753–62. doi: 10.1590/S1677-5538.IBJU.2014.06.06
43. Ataseven B, du Bois A, Reinthaller A, Traut A, Heitz F, Aust S, et al. Pre-operative serum albumin is associated with post-operative complication rate and overall survival in patients with epithelial ovarian cancer undergoing cytoreductive surgery. Gynecol Oncol. (2015) 138:560–5. doi: 10.1016/j.ygyno.2015.07.005
44. Ge LN, Wang F. Prognostic significance of preoperative serum albumin in epithelial ovarian cancer patients: a systematic review and dose-response meta-analysis of observational studies. Cancer Manag Res. (2018) 10:815–25. doi: 10.2147/CMAR.S161876
45. Ghanim B, Hoda MA, Klikovits T, Winter MP, Alimohammadi A, Grusch M, et al. Circulating fibrinogen is a prognostic and predictive biomarker in Malignant pleural mesothelioma. Br J Cancer. (2014) 110:984–90. doi: 10.1038/bjc.2013.815
46. Li W, Tang Y, Song Y, Chen SH, Sisliyan N, Ni M, et al. Prognostic role of pretreatment plasma D-dimer in patients with solid tumors: a systematic review and meta-analysis. Cell Physiol Biochem. (2018) 45:1663–76. doi: 10.1159/000487734
47. Qi Q, Geng Y, Sun M, Chen H, Wang P, Chen Z. Hyperfibrinogen is associated with the systemic inflammatory response and predicts poor prognosis in advanced pancreatic cancer. Pancreas. (2015) 44:977–82. doi: 10.1097/MPA.0000000000000353
48. Feng Z, Wen H, Ju X, Bi R, Chen X, Yang W, et al. The preoperative prognostic nutritional index is a predictive and prognostic factor of high-grade serous ovarian cancer. BMC Cancer. (2018) 18:883. doi: 10.1186/s12885-018-4732-8
49. Komura N, Mabuchi S, Yokoi E, Shimura K, Kawano M, Matsumoto Y, et al. Prognostic significance of the pretreatment prognostic nutritional index in patients with epithelial ovarian cancer. Oncotarget. (2019) 10:3605–13. doi: 10.18632/oncotarget.v10i38
50. Miao Y, Li S, Yan Q, Li B, Feng Y. Prognostic significance of preoperative prognostic nutritional index in epithelial ovarian cancer patients treated with platinum-based chemotherapy. Oncol Res Treat. (2016) 39:712–9. doi: 10.1159/000452263
51. Xu Q, Yan Y, Gu S, Mao K, Zhang J, Huang P, et al. A novel inflammation-based prognostic score: the fibrinogen/albumin ratio predicts prognoses of patients after curative resection for hepatocellular carcinoma. J Immunol Res. (2018) 2018:4925498. doi: 10.1155/2018/4925498
52. Perisanidis C, Psyrri A, Cohen EE, Engelmann J, Heinze G, Perisanidis B, et al. Prognostic role of pretreatment plasma fibrinogen in patients with solid tumors: A systematic review and meta-analysis. Cancer Treat Rev. (2015) 41:960–70. doi: 10.1016/j.ctrv.2015.10.002
53. Gupta D, Lis CG. Pretreatment serum albumin as a predictor of cancer survival: a systematic review of the epidemiological literature. Nutr J. (2010) 9:69. doi: 10.1186/1475-2891-9-69
Keywords: ovarian cancer, systemic immune-inflammation index, prognostic nutritional index, fibrinogen-to-albumin ratio, neutrophil-to-lymphocyte ratio, platelet-to-lymphocyte ratio, monocyte-to-lymphocyte ratio, diagnosis
Citation: Song L, Wu Q, Bai S, Zhao J, Qi J and Zhang J (2024) Comparison of the diagnostic efficacy of systemic inflammatory indicators in the early diagnosis of ovarian cancer. Front. Oncol. 14:1381268. doi: 10.3389/fonc.2024.1381268
Received: 03 February 2024; Accepted: 18 June 2024;
Published: 02 July 2024.
Edited by:
Sharon R. Pine, University of Colorado, United StatesReviewed by:
Daniele Vergara, University of Salento, ItalyCopyright © 2024 Song, Wu, Bai, Zhao, Qi and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Liyun Song, aGVoZTI3MTU2NTIyN0AxMjYuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.