
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol., 23 April 2024
Sec. Hematologic Malignancies
Volume 14 - 2024 | https://doi.org/10.3389/fonc.2024.1380492
 Dong Won Baek1
Dong Won Baek1 Ga-Young Song2
Ga-Young Song2 Ho Sup Lee3
Ho Sup Lee3 Young Rok Do4
Young Rok Do4 Ji Hyun Lee5
Ji Hyun Lee5 Ho-Young Yhim6
Ho-Young Yhim6 Joon Ho Moon1
Joon Ho Moon1 Deok-Hwan Yang2*
Deok-Hwan Yang2*Background: Elderly patients diagnosed with diffuse large B-cell lymphoma (DLBCL) undergoing reduced intensity R-CHOP therapy are at a heightened risk of acquiring infections, notably coronavirus disease 2019 (COVID-19) infection. This study aimed to evaluate the efficacy of intravenous immunoglobulin (IVIG) as prophylaxis against COVID-19 in this vulnerable population.
Methods: A total of 125 elderly patients with DLBCL undergoing reduced intensity R-CHOP therapy were analyzed in this prospective, multicenter study. Patients with hypogammaglobulinemia were categorized into IVIG and non-IVIG groups, while those with normal immunoglobulin levels constituted the observation group. The study evaluated COVID-19 infection rates, therapy response, and safety outcomes.
Results: Among the enrolled patients (median age: 77 years), 89 patients (71.2%) presented with hypogammaglobulinemia at diagnosis, and 56 patients enrolled in the IVIG administration group. IVIG administration remarkably reduced COVID-19 infection rates compared to non-IVIG recipients (8.9% vs. 24.6%; p =0.040). Notably, patients over 80 years old were more susceptible to COVID-19. Patients on IVIG exhibited good tolerance with manageable adverse events. Among patients with hypogammaglobulinemia who received IVIG, 40.5% of patients developed additional immunoglobulin deficiencies during chemotherapy. One or more new hypogammaglobulinemia occurred during chemotherapy in 72% of patients with hypogammaglobulinemia who did not receive IVIG, and in 61.3% of patients who did not have hypogammaglobulinemia at diagnosis.
Conclusion: IVIG showed promise in reducing COVID-19 infections among elderly patients with DLBCL receiving reduced intensity R-CHOP therapy. This highlights IVIG’s potential as a prophylactic measure, necessitating further investigation to optimize dosing, administration schedules, and potential interactions with vaccination strategies.
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has spread worldwide since December 2019, causing coronavirus disease 2019 (COVID-19) in numerous individuals. Notably, patients with hematologic malignancies have exhibited increased vulnerability to COVID-19 infection, leading to higher incidence rates and more adverse treatment outcomes compared to the general population (1–3). A Spanish research group reported that individuals with lymphoma over 70 years old faced a higher risk of mortality to COVID-19. Moreover, an active disease status considerably escalated the risk of death (4). Particularly, the mortality risk associated with COVID-19 in patients with diffuse large B-cell lymphoma (DLBCL) was noted to be higher than in other B-cell lymphomas such as follicular lymphoma (4).
SARS-CoV-2 continues to cause a global pandemic, and it has become evident that COVID-19’s clinical course is notably severe among patients with DLBCL (5, 6). With the introduction of vaccines targeting SARS-CoV-2, recommendations have been made for the vaccination of elderly patients with DLBCL (5). Vaccination is beneficial in preventing hospitalizations and deaths post-COVID-19 infection (7). However, most trials excluded individuals with malignancies, resulting in limited data concerning vaccine safety and efficacy for patients with DLBCL (8, 9). The immune response to SARS-CoV-2 vaccines may be significantly impaired, especially in patients undergoing B cell–depleting treatments (10). Moreover, there are still no clear standard guidelines for DLBCL patients infected with SARS-CoV-2 during immunochemotherapy (11, 12).
Meanwhile, approximately 15%–20% of patients with DLBCL display a reduction in at least one immunoglobulin class at diagnosis (13–15). Moreover, during B cell–depleting therapies such as rituximab, over 30% of patients with B-cell lymphoma newly develop some form of immunoglobulin deficiency (14). Hypogammaglobulinemia, characterized by reduced serum immunoglobulin G (IgG), immunoglobulin A (IgA), or immunoglobulin M (IgM) levels, is commonly observed in chronic lymphocytic leukemia (CLL) with an incidence ranging from 20% to 70% (16). IgM is a critical primary responder to viral pathogens causing major pandemics, and IgG responses are fundamental to adaptive immunity (17, 18). Emerging research is shedding light on the diverse functions of IgA in mucosal defense against viral infections, while the role of IgD in viral infections remains poorly elucidated (19, 20). Intravenous immunoglobulin (IVIG), primarily containing over 95% IgG and minute amounts of IgM or IgA derived from human plasma, may potentially offer clinical benefits in preventing or treating viral infections (21). However, the COVID-19 treatment guidelines currently discourage the use of IVIG in adults due to uncertainties regarding whether IVIG products contain SARS-CoV-2 neutralizing antibodies from pooled donor plasma. Moreover, clinical trials evaluating the therapeutic effect of SARS-CoV-2 hyperimmunoglobulin excluded patients with cancer (22, 23). Nonetheless, some positive findings regarding IVIG against COVID-19 infections have emerged. A British study examining anti-SARS-CoV-2-spike antibody titers before and after IVIG infusion in 35 patients with primary immunodeficiencies who regularly received IVIG demonstrated an increase in antibody titers and serum neutralization capacity post-IVIG infusion in most patients (24). However, a paucity of clinical studies exploring the effects of IVIG on the treatment or prevention of COVID-19 in patients with hematologic malignancies remains.
Previous research on CLL has highlighted the significant association between hypogammaglobulinemia and an increased risk of infectious events, a primary cause of death. Thus, patients with CLL with hypogammaglobulinemia might benefit from antibody replacement therapy using IVIG (16). Similarly, patients with hypogammaglobulinemia undergoing immunochemotherapy for DLBCL experience higher rates of severe infections, indicating potential benefits of IVIG for infection prevention (13, 25). Thus, this prospective interventional study aimed to investigate the clinical impact of prophylactic IVIG on elderly patients newly diagnosed with DLBCL receiving reduced intensity R -CHOP therapy, in the context of protection against COVID-19 infections.
This prospective, interventional, multicenter study was conducted from June 2021 to 2023 to evaluate the efficacy of IVIG in preventing COVID-19 infection in newly diagnosed elderly patients with DLBCL undergoing immunochemotherapy. Inclusion criteria encompassed patients aged 70 years or older, recently diagnosed with DLBCL, and suitable for reduced intensity R-CHOP therapy without contraindications for IVIG administration. The Ann Arbor staging system was used for staging, and risk stratification was determined using the international prognostic index (26, 27). Patients with primary central nervous system lymphoma, secondary transformed DLBCL, and human immunodeficiency virus-associated lymphoma were excluded. Serum IgG, IgA, IgM, and IgD levels were assessed at diagnosis, with any class falling below the reference range classified as hypogammaglobulinemia. Patients with hypogammaglobulinemia had the option to participate in a hypogammaglobulinemia group and select whether to receive IVIG as part of the study. The clinical courses of patients with hypogammaglobulinemia who did not receive IVIG and those with normal immunoglobulin levels were monitored as an observation group. All patients provided informed consent following the Declaration of Helsinki guidelines. The Institutional Review Board of Kyungpook National University Hospital (KNUH 2020-03-026) and each other participating center approved this study.
All enrolled patients underwent six cycles of reduced intensity R-CHOP therapy administered every 3 weeks. Treatment involved IV administration of rituximab at 375 mg/m2, cyclophosphamide at 600 mg/m2, doxorubicin at 30 mg/m2, vincristine at 1.0 mg on day 1, and oral prednisone at 40 mg/m2 on days 1–5. Pegylated granulocyte colony-stimulating factor was administered on day 2 of each chemotherapy cycle. Patients in the study group received 400 mg/kg of IVIG on days 3 and 11 during the first reduced intensity R-CHOP cycle, followed by 400 mg/kg of IVIG on day 3 from the second to the sixth cycle (Supplementary Table S1).
COVID-19 diagnosis was established when there is positivity for SARS-CoV-2 via reverse transcriptase-polymerase chain reaction (RT-PCR). Testing for COVID-19 was routinely performed before each chemotherapy for screening, and the test was repeated whenever there were unusual symptoms such as fever or sore throat. Serum immunoglobulin levels were assessed at interim and end-of-treatment evaluations, monitored at 3-month intervals post-treatment. Prophylactic agents for bacterial, viral, fungal, and pneumocystis pneumonia infections were permitted as per the investigator’s discretion. When patients required hospitalization and received intravenous antibiotic treatment, a severe infection was considered. Patients may withdraw from the study in cases of disease progression or upon personal request.
Patients were stratified into three groups: hypogammaglobulinemia with IVIG, hypogammaglobulinemia without IVIG, and normal immunoglobulin levels. The primary endpoint involved comparing COVID-19 infection rates during chemotherapy among these three groups. Safety assessments were conducted at each patient visit, and toxicity was evaluated as per the Common Terminology Criteria for Adverse Events version 5.0 (NIH, Bethesda, MD, USA). This clinical trial is registered as KCT0005626 in the clinical research information service (https://cris.nih.go.kr).
Using medians for continuous variables and frequency percentages for categorical variables, patient characteristics were summarized. Fisher’s exact test was employed for categorical variables, while the Wilcoxon rank-sum test was utilized for continuous variables in between-group comparisons. To identify risk factors for COVID-19 infection, Cox regression analysis was employed. Variables significantly associated with COVID-19 infection in univariate analysis (p ≤ 0.1) were included in the multivariate analysis. Hazard ratios and 95% confidence intervals were estimated for each factor, with statistical significance set at p < 0.05. Statistical analysis was conducted using R statistical software (version 4.3.1; R Foundation for Statistical Computing, Vienna, Austria, available at http://www.r-project.org).
In this study, data from a total of 125 patients newly diagnosed with DLBCL were collected and analyzed. Table 1 summarizes the patient characteristics. The median age of all enrolled patients was 77 years, and 70 patients (56.0%) were male. A total of 89 patients (71.2%) exhibited decreased levels in one or more immunoglobulin classes at diagnosis, with 56 patients enrolled in the IVIG administration group. The median age of the IVIG group was 77 years, and 16 patients (28.6%) were over 80 years old. Before diagnosis, five patients in the IVIG group and four patients in the non-IVIG group had prior COVID-19 infections. In both groups, most patients received prophylactic antibiotics and antifungal agents during chemotherapy. Table 2 outlines the prevalence of hypogammaglobulinemia by class. In the IVIG group, decreased IgM and IgD levels were the most common, identified in 34 (60.7%) and 29 patients (51.8%), respectively. Sixteen patients (28.6%) demonstrated two or more overlapping decreased immunoglobulin levels. Thirty-three patients with hypogammaglobulinemia did not receive IVIG infusion, and in this non-IVIG group, decreased IgM and IgD levels were also prevalent, with five patients revealing two or more hypogammaglobulinemia classes.
In the IVIG administration group, 16 of 56 patients discontinued treatment. The reasons were as follows: nine patients withdrew due to severe general weakness, three patients discontinued reduced intensity R-CHOP due to disease progression, and two deaths were documented during chemotherapy (one cerebral hemorrhage associated with a fall and one liver failure from chronic hepatitis B). Furthermore, two patients could not continue immunochemotherapy due to aspiration pneumonia and cardiac issues (non-ST-elevation myocardial infarction [NSTEMI]), respectively. Eventually, 40 patients completed the scheduled reduced intensity R-CHOP and IVIG prophylaxis. A total of 40 patients achieved complete remission (CR). Adverse effects associated with IVIG included manageable symptoms such as fever, chills, nausea, and vomiting. One patient discontinued the study due to chest pain during IVIG administration (Supplementary Table S2). Among patients with hypogammaglobulinemia who did not receive IVIG, 24 completed six cycles of reduced intensity R-CHOP, with 19 achieving CR. Five patients discontinued treatment due to general weakness, and three had disease progression during chemotherapy. In the group with normal immunoglobulin levels at diagnosis, 26 of 36 patients achieved CR, while 10 patients discontinued treatment due to general weakness (n = 6) or disease progression (n = 4) (Figure 1).
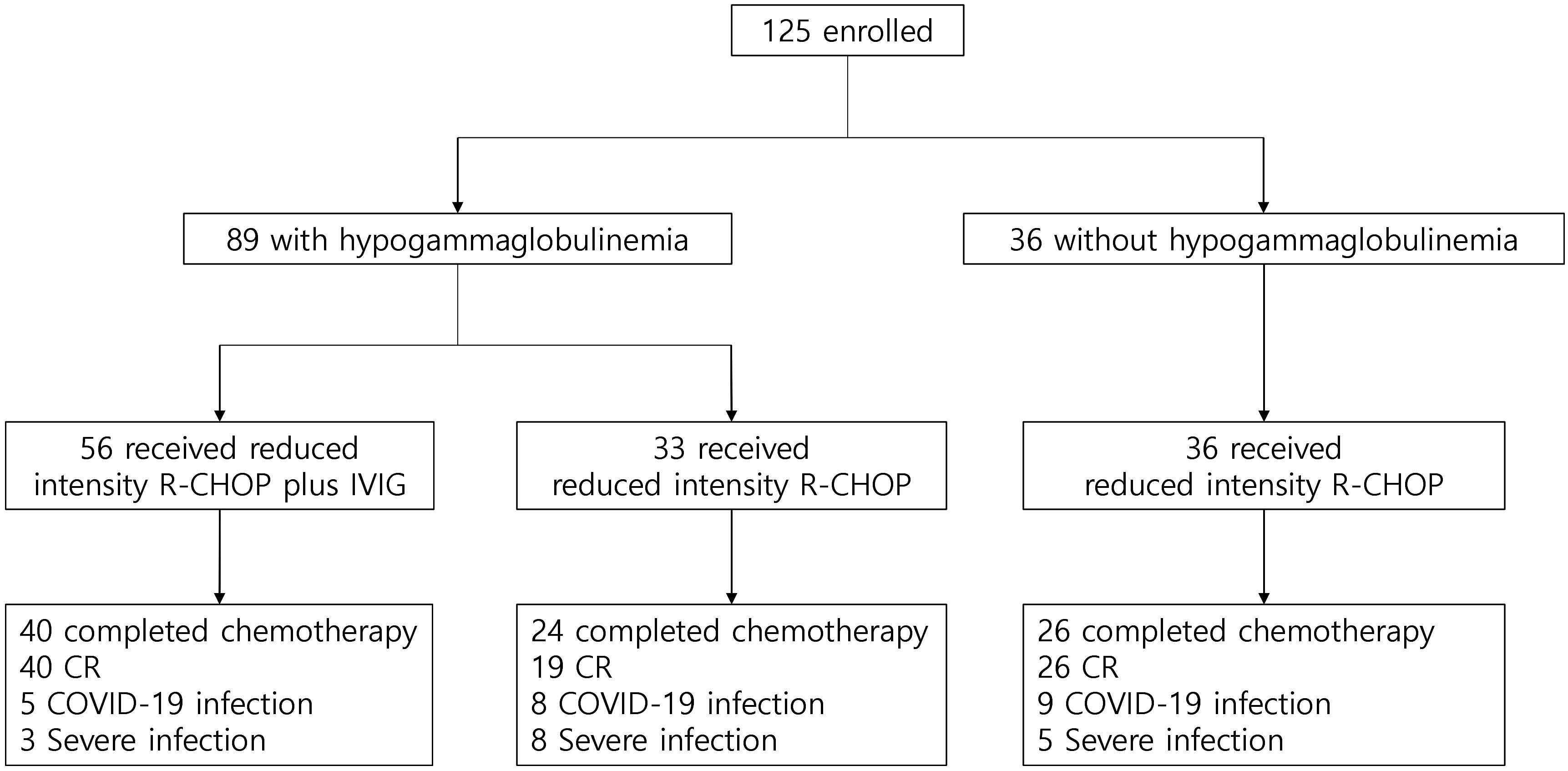
Figure 1 Study profile. COVID-19, coronavirus disease 2019; CR, complete response; IVIG, intravenous immunoglobulin; R-CHOP, immunochemotherapy including rituximab, cyclophosphamide, doxorubicin, vincristine, and oral prednisone.
In patients with hypogammaglobulinemia who received IVIG, five patients (8.9%) had COVID-19 infection during the study period. In patients with hypogammaglobulinemia who did not receive IVIG, COVID-19 infection was developed in 8 of 33 (24.2%). In patients without hypogammaglobulinemia, COVID-19 infection was developed in 9 of 36 patients (22.2%) (Supplementary Figure S1). Patients receiving IVIG infusion demonstrated a significantly lower incidence of COVID-19 infection compared to those who did not receive IVIG (8.9% vs. 24.6%; p = 0.040) (Figure 2). The clinical course of patients infected with COVID-19 is summarized in Supplementary Figure S2 and Supplementary Table S3. In the univariate analysis, age, ECOG performance status, Ann Arbor stage, IPI at diagnosis, hypogammaglobulinemia at any time during immunochemotherapy, and IVIG administration were associated with COVID-19 infection (Supplementary Table S4). However, in the multivariate analysis, only age > 80 years has a significantly increased risk of acquiring COVID-19 infection (hazard ratio [HR] 1.67, p = 0.041). Although IVIG administration demonstrated a protective tendency against COVID-19 infection, statistical significance was not reached (HR 0.40, p = 0.082) (Figure 3).
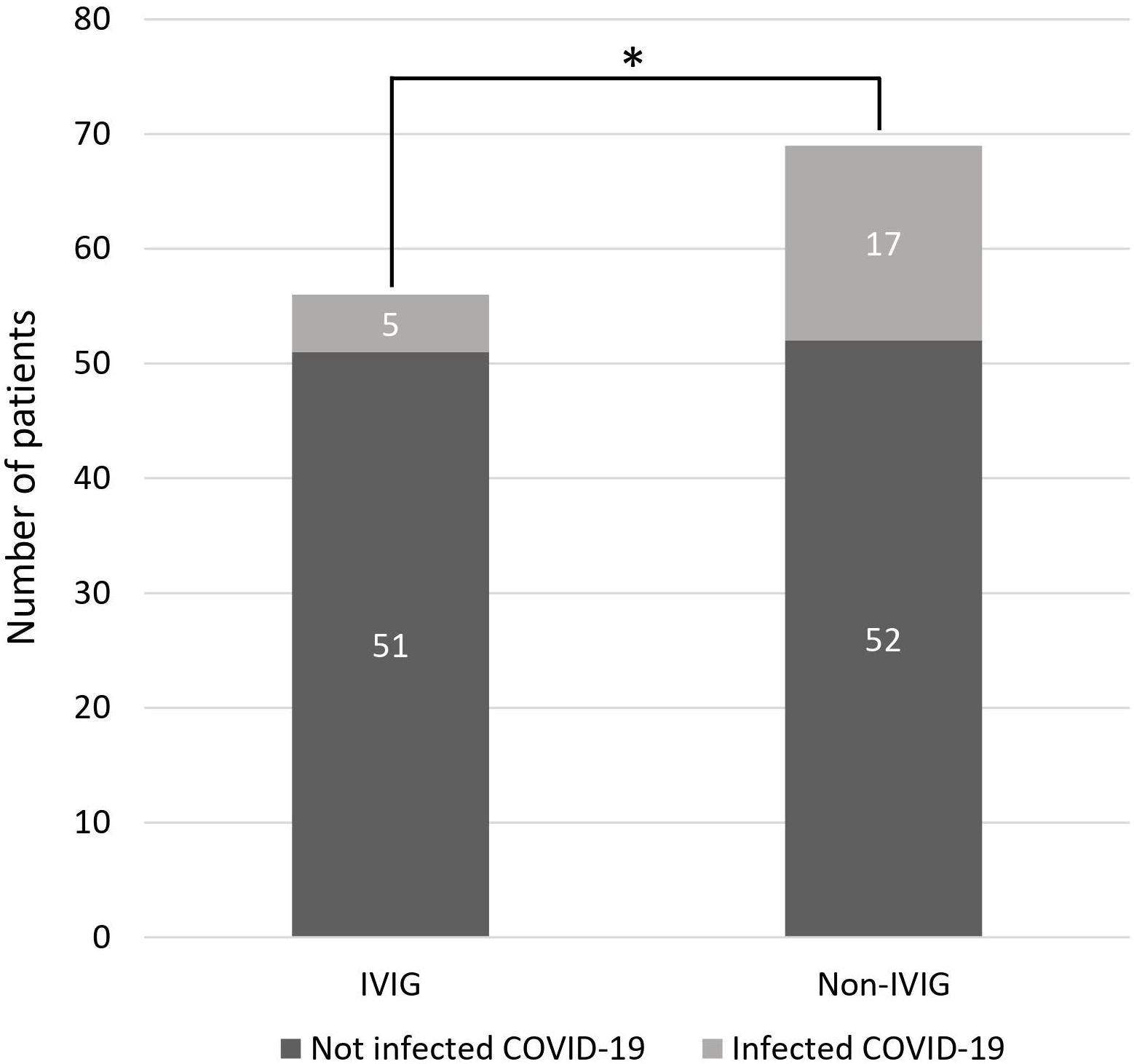
Figure 2 Comparison of COVID-19 infection between the IVIG and non-IVIG group (*p = 0.040). COVID-19, coronavirus disease 2019; IVIG, intravenous immunoglobulin.
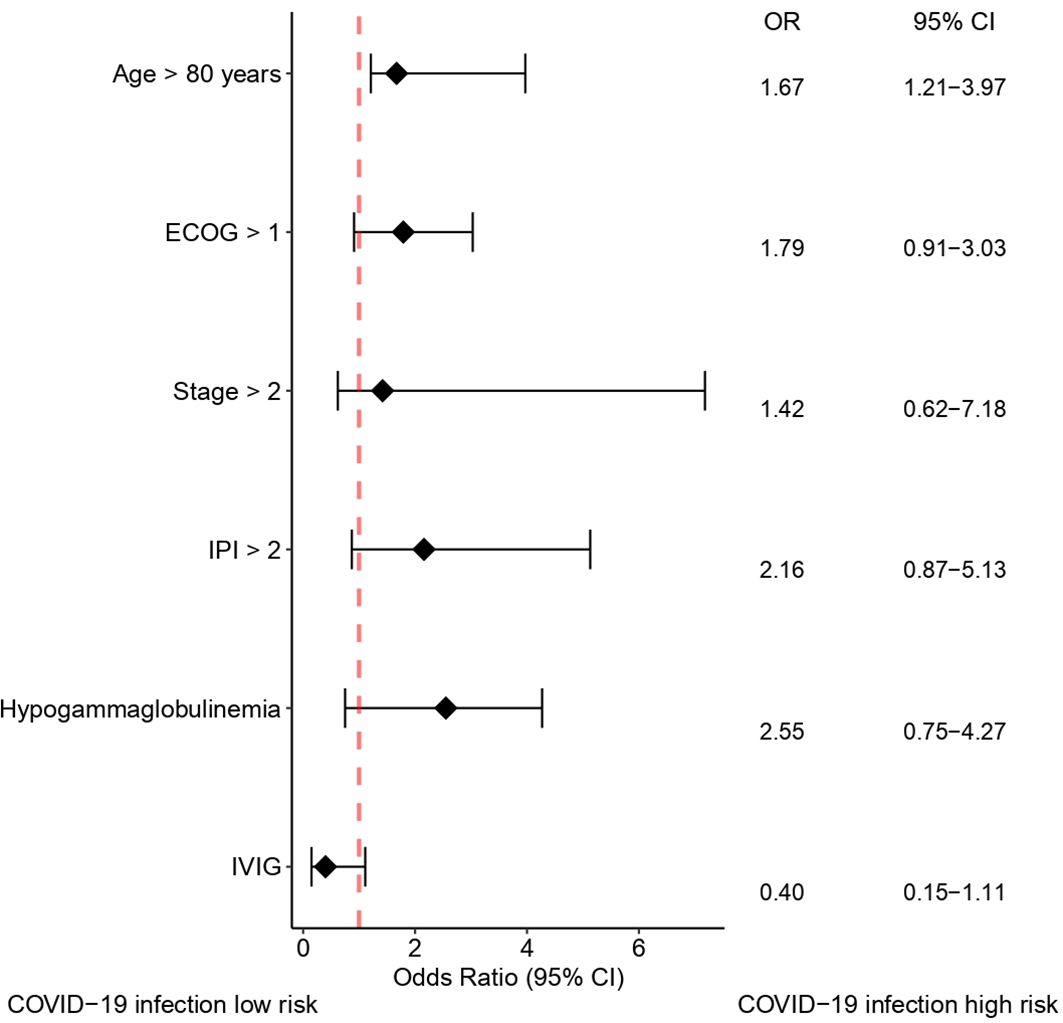
Figure 3 Forest plot showing factors affecting COVID-19 infection. COVID-19, coronavirus disease 2019; CI, confidence interval; ECOG, Eastern Cooperative Oncology Group; IVIG, intravenous immunoglobulin; IPI, International Prognostic Index; OR, odds ratio.
Among patients with hypogammaglobulinemia who received IVIG, 19 of 47 patients (40.5%) developed additional immunoglobulin deficiencies during chemotherapy, primarily IgG (n = 10) and IgM (n = 10). In patients with hypogammaglobulinemia who did not receive IVIG, 18 out of 25 patients (72.0%) showed one or more new hypogammaglobulinemia, and IgG (n = 13), and IgM (n = 8) were most commonly identified. In patients who did not show hypogammaglobulinemia at diagnosis, 19 of 31 patients (61.3%) newly developed one or more classes of hypogammaglobulinemia during immunochemotherapy, and decreased IgG (n = 13), IgM (n = 10), IgD (n = 9), and IgA (n = 3) were identified.
Severe infection rates during reduced intensity R-CHOP therapy were considerably lower in patients receiving IVIG (p = 0.031). In the IVIG group, severe infections occurred in three patients (5.4%), while in the non-IVIG hypogammaglobulinemia group, eight patients (25.0%) experienced severe infections, and in patients without hypogammaglobulinemia, five patients (13.9%) had severe infections.
In this study, the incidence of COVID-19 infection was relatively higher in patients who did not receive IVIG during reduced intensity R-CHOP therapy than in patients with IVIG. IVIG administration was generally well-tolerated, with majority of the adverse events being mild and manageable. Notably, only one patient discontinued IVIG infusion due to NSTEMI.
While numerous studies have investigated hypogammaglobulinemia in CLL, limited research has investigated its clinical significance in patients with DLBCL. Hypogammaglobulinemia, associated with compromised T- and nonclonal CD5- B cell functions, significantly impacts infection frequency and survival rates in patients with CLL (28, 29). Studies in DLBCL, such as those of Singh et al. and Pan et al., have reported lower rates of hypogammaglobulinemia (22.1% and 19%, respectively) and highlighted its association with unfavorable survival and increased infection risks (13, 15). Our study noted a higher prevalence of hypogammaglobulinemia (71.2%) at diagnosis, which could be attributed to the advanced median age of our targeted patient group (77 years) compared to previous studies (62–65 years). Furthermore, the inclusion of IgD deficiency, documented in approximately 50% of cases, might have contributed to a higher prevalence. Hypogammaglobulinemia incidence and subsequent infection rates appeared to increase with B-cell depletion induced by rituximab-containing chemoimmunotherapy (30, 31). Importantly, more than half of the patients, irrespective of their immunoglobulin levels at diagnosis, developed additional immunoglobulin deficiencies, mainly IgG and IgM, during reduced intensity R-CHOP therapy.
During chemotherapy, COVID-19 infection negatively impacted DLBCL treatment outcomes, often leading to treatment postponement or discontinuation. While our study rarely indicated the normalization of serum immunoglobulin levels with 400 mg/kg IVIG replacement during chemotherapy, multivariate analysis indicated a potential preventive role against COVID-19 infection. Previous randomized clinical trials in CLL also supported the efficacy of prophylactic IVIG in remarkably reducing infection rates compared to placebo (32, 33).
The introduction of SARS-CoV-2 vaccines was ongoing during patient enrollment. Owing to safety and efficacy concerns, elderly patients initially exhibited hesitancy toward vaccination, resulting in incomplete vaccination. Consequently, this study could not confirm the preventive effect of SARS-CoV-2 vaccination in patients with DLBCL or assess the advantages and disadvantages of IVIG administration alongside vaccination. While our data do not conclusively establish the clinical benefit of IVIG, this study underscores the promising potential of IVIG as prophylaxis in elderly patients with DLBCL. However, the results of the present data should be interpreted cautiously due to certain limitations. First, the current study was not randomized. There may be a risk of bias. Second, this study could not address data analysis of anti-SARS-CoV-2 titers before and after IVIG replacement. Third, sample size and number of events were relatively small for between-group comparisons. Plus, follow-up duration was short. Lastly, this study was conducted on elderly patients, and the number of cases in which the study was discontinued due to various factors was greater than estimated. Given the susceptibility of patients with malignant lymphoma receiving B cell–depleting therapy to COVID-19 and their higher mortality rates, a comprehensive randomized controlled study with appropriate IVIG dosing and administration schedules is warranted to further define IVIG’s role in preventing COVID-19 infections.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
The studies involving humans were approved by The Institutional Review Board of Kyungpook National University Hospital (KNUH 2020-03-026). The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin.
DB: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. G-YS: Data curation, Investigation, Writing – original draft, Writing – review & editing. HL: Conceptualization, Investigation, Writing – original draft, Writing – review & editing. YD: Conceptualization, Investigation, Writing – original draft, Writing – review & editing. JL: Conceptualization, Investigation, Writing – original draft, Writing – review & editing. H-YY: Conceptualization, Investigation, Writing – original draft, Writing – review & editing. JM: Investigation, Methodology, Writing – original draft, Writing – review & editing. D-HY: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This research was supported by a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (grant number: HR20C0021).
IVIG used in this study was fully supported by Green Cross Biopharma.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2024.1380492/full#supplementary-material.
1. Yang K, Sheng Y, Huang C, Jin Y, Xiong N, Jiang K, et al. Clinical characteristics, outcomes, and risk factors for mortality in patients with cancer and COVID-19 in Hubei, China: a multicentre, retrospective, cohort study. Lancet Oncol. (2020) 21:904–13. doi: 10.1016/S1470-2045(20)30310-7
2. Liang W, Guan W, Chen R, Wang W, Li J, Xu K, et al. Cancer patients in SARS-CoV-2 infection: a nationwide analysis in China. Lancet Oncol. (2020) 21:335–7. doi: 10.1016/S1470-2045(20)30096-6
3. Onder G, Rezza G, Brusaferro S. Case-fatality rate and characteristics of patients dying in relation to COVID-19 in Italy. JAMA. (2020) 323:1775–6. doi: 10.1001/jama.2020.4683
4. Regalado-Artamendi I, Jiménez-Ubieto A, Hernández-Rivas JA, Navarro B, Núñez L, Alaez C, et al. Risk factors and mortality of COVID-19 in patients with lymphoma: a multicenter study. Hemasphere. (2021) 5:e538. doi: 10.1097/HS9.0000000000000538
5. Langerbeins P, Hallek M. COVID-19 in patients with hematologic Malignancy. Blood. (2022) 140:236–52. doi: 10.1182/blood.2021012251
6. He W, Chen L, Chen L, Yuan G, Fang Y, Chen W, et al. COVID-19 in persons with haematological cancer. Leukemia. (2020) 34:1637–45. doi: 10.1038/s41375-020-0836-7
7. Zheng C, Shao W, Chen X, Zhang B, Wang G, Zhang W, et al. Real-world effectiveness of COVID-19 vaccines: a literature review and meta-analysis. Int J Infect Dis. (2022) 114:252–60. doi: 10.1016/j.ijid.2021.11.009
8. Baden LR, El Sahly HM, Essink B, Kotloff K, Frey S, Novak R, et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. (2021) 384:403–16. doi: 10.1056/NEJMoa2035389
9. Voysey M, Clemens SA, Madhi SA, Weckx LY, Folegatti PM, Aley PK, et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. (2021) 397:99–111. doi: 10.1016/S0140-6736(20)32661-1
10. Bilich T, Roerden M, Maringer Y, Nelde A, Heitmann JS, Dubbelaar ML, et al. Preexisting and post-COVID-19 immune responses to SARS-CoV-2 in patients with cancer. Cancer Discovery. (2021) 11:1982–95. doi: 10.1158/2159-8290.CD-21-0191
11. Pasquini Z, Toschi A, Casadei B, Pellegrini C, D’Abramo A, Vita S, et al. Dual combined antiviral treatment with remdesivir and nirmatrelvir/ritonavir in patients with impaired humoral immunity and persistent SARS-CoV-2 infection. Hematol Oncol. (2023) 41:904–11. doi: 10.1002/hon.3206
12. D’Abramo A, Vita S, Nicastri E. The unmet need for COVID-19 treatment in immunocompromised patients. BMC Infect Dis. (2022) 22:930. doi: 10.1186/s12879-022-07918-x
13. Pan BH, Kong YL, Wang L, Zhu HY, Li XT, Jiang JH, et al. The prognostic roles of hypogammaglobulinemia and hypocomplementemia in newly diagnosed diffuse large B-cell lymphoma. Leuk Lymphoma. (2021) 62:291–9. doi: 10.1080/10428194.2020.1832673
14. Casulo C, Maragulia J, Zelenetz AD. Incidence of hypogammaglobulinemia in patients receiving rituximab and the use of intravenous immunoglobulin for recurrent infections. Clin Lymphoma Myeloma Leuk. (2013) 13:106–11. doi: 10.1016/j.clml.2012.11.011
15. Singh N, Mott SL, McCarthy AN, Syrbu S, Habermann TM, Feldman AL, et al. Prevalence and the impact of hypogammaglobulinemia in newly diagnosed, untreated diffuse large B cell lymphoma. Blood. (2019) 134:1604. doi: 10.1182/blood-2019-122737
16. Noto A, Cassin R, Mattiello V, Bortolotti M, Reda G, Barcellini W. Should treatment of hypogammaglobulinemia with immunoglobulin replacement therapy (IgRT) become standard of care in patients with chronic lymphocytic leukemia? Front Immunol. (2023) 14:1062376. doi: 10.3389/fimmu.2023.1062376
17. Gong S, Ruprecht RM. Immunoglobulin M: an ancient antiviral weapon – rediscovered. Front Immuno. (2020) 11:1943. doi: 10.3389/fimmu.2020.01943
18. Corrales-Aguilar E, Trilling M, Reinhard H, Falcone V, Zimmermann A, Adams O, et al. Highly individual patterns of virus-immune IgG effector responses in humans. Med Microbiol Immunol. (2016) 205:409–24. doi: 10.1007/s00430-016-0457-y
19. Yan H, Lamm ME, Björling E, Huang YT. Multiple functions of immunoglobulin A in mucosal defense against viruses: an in vitro measles virus model. J Virol. (2002) 76:10972–9. doi: 10.1128/JVI.76.21.10972-10979.2002
20. Vladutiu AO. Immunoglobulin D: properties, measurement, and clinical relevance. Clin Diagn Lab Immunol. (2000) 7:131–40. doi: 10.1128/CDLI.7.2.131-140.2000
21. Rütter A, Luger TA. High-dose intravenous immunoglobulins: an approach to treat severe immune-mediated and autoimmune diseases of the skin. J Am Acad Dermatol. (2001) 44:1010–24. doi: 10.1067/mjd.2001.112325
22. Lai CC, Chen WC, Chen CY, Wei YF. The effect of intravenous immunoglobulins on the outcomes of patients with COVID-19: a systematic review and meta-analysis of randomized controlled trials. Expert Rev Anti Infect Ther. (2022) 20:1333–40. doi: 10.1080/14787210.2022.2098112
23. ITAC (INSIGHT 013) study group. Hyperimmune immunoglobulin for hospitalised patients with COVID-19 (ITAC): a double-blind, placebo-controlled, phase 3, randomised trial. Lancet. (2022) 399:530–40. doi: 10.1016/S0140-6736(22)00101-5
24. Upasani V, Townsend K, Wu MY, Carr EJ, Hobbs A, Dowgier G, et al. Commercial immunoglobulin products contain neutralizing antibodies against severe acute respiratory syndrome coronavirus 2 spike protein. Clin Infect Dis. (2023) 77:950–60. doi: 10.1093/cid/ciad368
25. Lindberg Å, Johansson Å, Kahn F, Jönsson G, Cederwall S, Jerkeman M. Hypogammaglobulinemia at diagnosis is associated with inferior survival and higher risk of infections in diffuse large B cell lymphoma: a retrospective study from Sweden. Hematol Oncol. (2023) 41:726. doi: 10.1002/hon.3165_578
26. Lister TA, Crowther D, Sutcliffe SB, Glatstein E, Canellos GP, Young RC, et al. Report of a committee convened to discuss the evaluation and staging of patients with Hodgkin’s disease: Cotswolds meeting. J Clin Oncol. (1989) 7:1630–6. doi: 10.1200/JCO.1989.7.11.1630
27. International Non-Hodgkin’s Lymphoma Prognostic Factors Project. A predictive model for aggressive non-Hodgkin’s lymphoma. N Engl J Med. (1993) 329:987–94. doi: 10.1056/NEJM199309303291402
28. Morrison VA. Infectious complications in patients with chronic lymphocytic leukemia: pathogenesis, spectrum of infection, and approaches to prophylaxis. Clin Lymphoma Myeloma. (2009) 9:365–70. doi: 10.3816/CLM.2009.n.071
29. Parikh SA, Leis JF, Chaffee KG, Call TG, Hanson CA, Ding W, et al. Hypogammaglobulinemia in newly diagnosed chronic lymphocytic leukemia: Natural history, clinical correlated, and outcomes. Cancer. (2015) 121:2883–91. doi: 10.1002/cncr.29438
30. Hainsworth JD, Lichy S, Barton JH, Houston GA, Hermann RC, Bradof JE, et al. Single-agent rituximab as first-line and maintenance treatment for patients with chronic lymphocytic leukemia or small lymphocytic lymphoma: a phase II trial of the Minnie Pearl Cancer Research Network. J Clin Oncol. (2003) 21:1746–51. doi: 10.1200/JCO.2003.09.027
31. Byrd JC, Peterson BL, Morrison VA, Park K, Jacobson R, Hoke E, et al. Randomized phase 2 study of fludarabine with concurrent versus sequential treatment with rituximab in symptomatic, untreated patients with B-cell chronic lymphocytic leukemia: results from Cancer and Leukemia Group B 9712 (CALGB 9712). Blood. (2003) 101:6–14. doi: 10.1182/blood-2002-04-1258
32. Khan S, Allsup D, Molica S. An updated perspective on immunoglobulin replacement in chronic lymphocytic leukaemia in the era of targeted therapies. Front Oncol. (2023) 13:1135812. doi: 10.3389/fonc.2023.1135812
Keywords: COVID-19, diffuse large B-cell lymphoma, elderly, hypogammaglobulinemia, immunochemotherapy
Citation: Baek DW, Song G-Y, Lee HS, Do YR, Lee JH, Yhim H-Y, Moon JH and Yang D-H (2024) Clinical efficacy of prophylactic intravenous immunoglobulin for elderly DLBCL patients with hypogammaglobulinemia in the COVID-19 pandemic era. Front. Oncol. 14:1380492. doi: 10.3389/fonc.2024.1380492
Received: 01 February 2024; Accepted: 09 April 2024;
Published: 23 April 2024.
Edited by:
Maria Ilaria Del Principe, University of Rome Tor Vergata, ItalyReviewed by:
Serena Vita, National Institute for Infectious Diseases Lazzaro Spallanzani (IRCCS), ItalyCopyright © 2024 Baek, Song, Lee, Do, Lee, Yhim, Moon and Yang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Deok-Hwan Yang, ZHJ5ZGgxNjg1QGhvdG1haWwuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.