
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Oncol., 15 July 2024
Sec. Thoracic Oncology
Volume 14 - 2024 | https://doi.org/10.3389/fonc.2024.1380453
This article is part of the Research TopicInnovative Approaches to Overcome Resistance and Toxicities of Anti-Cancer DrugsView all 21 articles
 David O’Reilly1,2,3*
David O’Reilly1,2,3* Caroline L. O’Leary1
Caroline L. O’Leary1 Aislinn Reilly1
Aislinn Reilly1 Min Yuen Teo4
Min Yuen Teo4 Grainne O’Kane5
Grainne O’Kane5 Lizza Hendriks6
Lizza Hendriks6 Kathleen Bennett7
Kathleen Bennett7 Jarushka Naidoo1,2,8
Jarushka Naidoo1,2,8The combination of immune checkpoint inhibitors (ICIs) and tyrosine kinase inhibitors (TKIs) can be associated with significant toxicity. We performed a systematic review and meta-analysis of the toxicity of combination treatment of ICIs with TKIs (ICI + TKI) in clinical trials with solid organ malignancies. Our primary endpoint explored the incidence of grade 3 - 5 (G3-5) treatment-related toxicity and our secondary endpoints included the incidence of toxicity by treatment type, disease type and studies with run-in strategies. A total of 9750 abstracts were identified, of which 72 eligible studies were included. The most common disease types were non-small cell lung cancer (n=8, 11.1%), renal cell carcinoma (n=10, 13.8%) and hepatobiliary cancers (n=10, 13.8%). The overall incidence of G3-5 toxicity was 56% (95% CI = 50% – 61%). The most common TKIs combined with ICIs in this analysis were multi-targeted TKIs (n = 52, 72%), VEGF specific (n = 9, 12.5%), or oncogene-targeting TKIs (EGFR, ALK, BRAF, MEK) (n =11, 15.3%). Oncogene-targeted TKIs were associated a higher incidence of rashes and immune related adverse events (irAEs) and lower incidence of hypertension. In studies which used a TKI ‘run-in’ to mitigate toxicity, the pooled estimate of G3-5 toxicity was 71% (95% CI 57-81%). Almost half of studies (48%) omitted the incidence of G3-5 irAEs. Our work suggests that the majority of patients who receive ICI-TKI combinations will experience high grade toxicity (G3-G5) and that toxicity may be specific to TKI partner (Oncogene targeted TKIs: Rash, irAEs; VEGF/Multitargeted: Hypertension). These data did not suggest that a TKI ‘run-in’ was associated with a lower incidence of G3-5 toxicity. Reporting of irAEs was inconsistent supporting the need for harmonisation of adverse event reporting to include onset, duration and treatment.
Systematic review registration: https://www.crd.york.ac.uk/prospero/, identifier CRD42022367416.
Immune checkpoint inhibitors (ICIs) have resulted in improved outcomes for patients with solid organ tumours. However, long-term survival ranges from over 50% amongst patients with advanced melanoma treated with nivolumab plus ipilimumab, to 10 – 30% among those with advanced non-small cell lung cancer (NSCLC) (1–4). Consequently a focus of research is to incorporate novel targeted therapies in combination with ICIs in order to improve response rates and patient outcomes.
Tyrosine kinase inhibitors (TKIs) incorporate a broad range of small molecule therapeutics which may target oncogenes (e.g. Epidermal Growth Factor Receptor, EGFR) or other targets in the tumour microenvironment (e.g. Vascular Endothelial Growth Factor, VEGF). Oncogenic driver alterations are often associated with suppressive immune microenvironments (5). It is therefore postulated that TKIs may induce anti-tumour immune responses by increasing tumour immunogenicity (5). For these reasons, combination strategies with ICIs and TKIs to maximize therapeutic efficacy, have been investigated in a number of diseases including melanoma, renal cell carcinoma (RCC) and NSCLC (6–8). Additional to approaches involving TKI/ICI combinations include biomarker discovery to identify ICI efficacy and novel immunotherapy approach which may augment ICI efficacy (7, 8).
However, the goal of therapeutic synergy can be complicated by treatment related toxicity. Reports have previously outlined notable toxicities include severe hepatotoxicity with sequential ICI and KRASG12C inhibitors (sotorasib) and endocrinopathies when ICIs are combined with lenvatinib (9, 10). Additionally, it has been shown that the combination of Lenvatinib and pembrolizumab is associated with a high incidence of fatigue/diarrhoea (11). In clinical practice, it can be difficult to differentiate between a non-immune related adverse event and an immune related AE when patients are receiving ICI/TKI combinations. Given that TKIs can inactivate tumour-associated immunosuppression, this may be the mechanism by which there is an increase in immune-related adverse events (irAEs). However, strategies aimed at minimising toxicity remain ill-defined. Run-in periods with TKIs prior to ICIs or using lower doses of TKIs have been investigated in prospective studies, with a limited biologic basis for this approach (9, 12).
Taken together, there is limited prospective data available to determine the optimal strategy of combining ICIs with TKIs. Given the paucity of data, we seek to assess the safety of TKI/ICI combinations by assessing the spectrum of toxicities when ICIs are combined with TKI’s across tumour types, the toxicities that occur by tumour types and regimen, and the evidence to date involving run-in strategies. These data would then contribute to the optimum combining of ICIs with TKIs, based on toxicity considerations.
In this review, the Preferred Reporting items for Systematic Reviews and Meta-analyses (PRISMA) guidelines were used and a study protocol (PROSPERO, CRD42022367416) uploaded to an international registry (13).
The primary endpoint of the study was the incidence of grade 3 - 5 toxicities (G3-5 toxicity) by Common Terminology Criteria for Adverse Events (14). Secondary objectives include identifying factors that associate with high incidences of toxicity including disease type, choice of combination, and utilizing novel approaches including run-in strategies to mitigate toxicity.
Clinical trials which included anti-cancer treatment with a TKI and ICI for a solid tumour malignancy, were eligible for inclusion. Patients in the included studies may have received >1 TKI or >1 ICI (e.g. vemurafenib plus cobimetinib). Studies in which patients were treated with TKI + ICI + other agent (e.g. cytotoxic therapy) were excluded. Lastly, phase I studies involving dose-escalation cohorts were also excluded.
The search strategy utilised the following search terms; Immune checkpoint inhibitors OR immune checkpoint inhibitor, Tyrosine kinase inhibitor OR protein-tyrosine kinases and Neoplasms OR carcinoma OR cancer. In addition to these terms, we also used the MESH terms; Humanized/adverse effects Antineoplastic Agents/therapeutic use Antineoplastic Combined Chemotherapy Protocols/adverse effects. Citations from published work were imported and de-duplicated using Endnote. Forward and backward citation chasing was completed to minimize the possibility of missing relevant studies. MEDLINE, EMBASE, Cochrane Database of Systematic Review and Central Registry of Clinical Trials were searched for publications from 16/8/2002 to 16/8/2022. Conference proceedings (abstracts) were considered eligible and included in our search.
Titles and abstract screening were performed independently by two review authors (COL; AR) to identify potentially eligible studies. Full-text manuscripts identified as potentially eligible were retrieved and independently assessed for eligibility by two review authors (COR, AR). Any disagreement between reviewers over the eligibility of studies were resolved by consensus after discussion with a third reviewer (DOR).
Extracted information included study setting; study population and participant demographics, study methodology; sample size; inclusion and exclusion criteria; details of the intervention and control conditions; recruitment and study completion rates; incidence of symptom toxicity (e.g. diarrhoea, shortness of breath (SOB), rash, liver enzyme abnormalities, fatigue) and irAEs in both intervention and control arms.
To facilitate the assessment of possible risk of bias for each study, we collected information regarding bias of included studies using the Crowe Critical Appraisal Tool (CAT) (15). Domains included in the CAT tool include Introduction, Design, Sampling, Data Collection, Ethical Matters, Results and Discussion.
We performed descriptive statistics on our study results. Given that some studies did not report all adverse events, the denominator reflects the total number of patients in which the specific toxicity was reported and is different for different toxicities.
The study results were synthesised using a random-effects meta-analysis, with standardised incidence rate ratios for binary outcomes. In reference to our choice of a random-effects meta-analysis, we chose this model (as opposed to a fixed effect model) given that we anticipated that there would be heterogeneity in the included studies. We calculated 95% confidence intervals (95% CI) and two-sided p-values. With sufficient studies available for pooling (minimum of five), we performed meta-analysis by tumour type/organ system (NSCLC, RCC, Hepatobiliary), and treatment regimen (Lenvatinib plus Pembrolizumab, Nivolumab plus Cabozantinib, Axitinib plus Avelumab, ICI + Oncogene-targeted TKI). Hepatobiliary (HPB) tumours include patients with primary hepatocellular carcinomas (HCC) or biliary tract cancers (BTCs). An oncogene-targeted TKI referred to TKIs directed at a specific oncogenic alteration known to be aberrant in the tumour type of interest [e.g. EGFR, Anaplastic Lymphoma Kinase (ALK)]. Heterogeneity was assessed using both the Cochran’s Q (chi-squared, χ2 statistic), H-squared (H2) and the I-squared (I2) statistic. H2 describes the relative excess in Q over its degrees of freedom as a measure of the extent of heterogeneity and H2= 1 indicates homogeneity of effect. We consider an I-squared value greater than 75% indicative of considerable heterogeneity (16). In a protocol-specified (pre-planned) analysis, we investigated the overall incidence of grade 3-5 (G3-5 as per CTCAE) toxicity with the use of concurrent versus TKIs with ICI run-in (14). We conducted sensitivity analyses based on study quality by excluding the poorer quality studies and repeating the analysis for our primary outcome (incidence of G3-5 toxicity in included studies). Finally, to describe heterogeneity across studies, a meta-regression analysis was conducted utilising the covariates of disease group, ICI target, and TKI target (See Supplementary Appendix for details on covariates included).
Our initial search yielded a total of 3348 titles for consideration of inclusion. With the addition of forward and backward citation chasing, a total of 9750 abstracts were identified for potential inclusion (See Figure 1: PRISMA flow chart). A total of 132 records were deemed appropriate for full-text review. Upon full-text review a significant number of records were excluded which resulted in a total of 72 eligible studies (See Supplementary Table 1 for included studies). Studies were excluded for the following reasons; insufficient adverse event reporting (n=14), dose-finding studies (n=28), duplicate studies (n=10) and other reasons (n= 8) (See Figure 1 for complete breakdown).
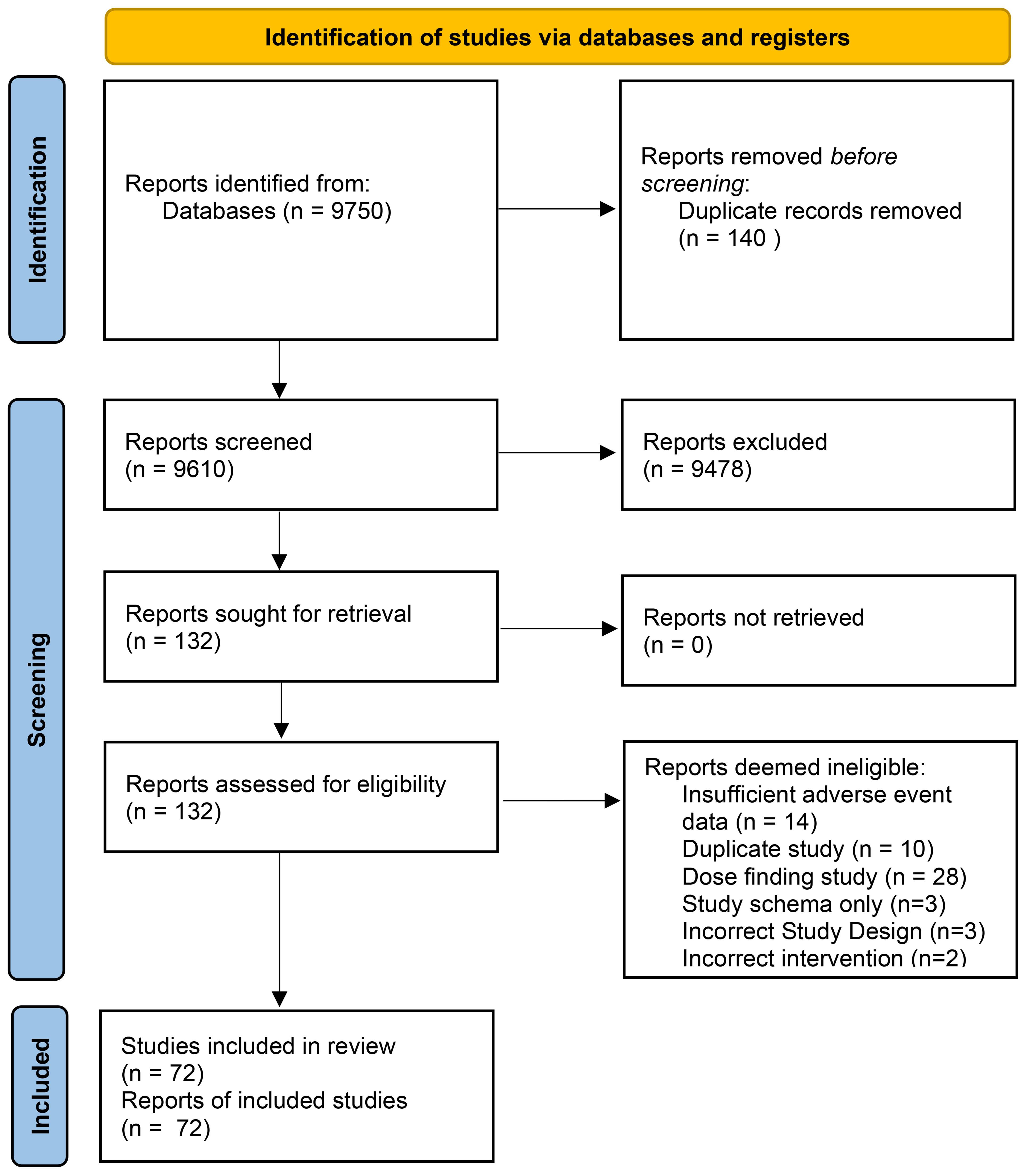
Figure 1 Systematic Review Search results and Eligibility assessment. PRISMA (Preferred Reporting Items for Systematic Reviews and Meta – Analyses) Flow Diagram describing the search results for abstracts & full – texts followed by eligibility assessment.
A total of 9404 patients were treated in 72 studies, of which 5860 (62.3%) received an ICI in combination with a TKI. The remainder of patients (37.4%, 3544/9404) in included studies were not included in our analysis since patients did not receive ICI/TKI (e.g. control arms with single agent TKI). In the identified 72 studies (See Supplementary Appendix – Table 1) a total of 20 (27.7%) studies represented abstracts from conference proceedings (See Table 1). The median score for the CAT (See Supplementary Appendix – Table 2) was 33 (Standard Deviation [SD]=9). For patients receiving ICIs, all studies involved drugs targeting either Programmed Cell Death Protein-1 (PD-1) or Programmed Cell Death Ligand-1 (PD-L1). In 65.2% of studies, patients were treated with a PD-1 inhibitor (47/72) and the remainder were treated with a PD-L1 inhibitor (25/72, 34.7%). In one study, patients received a Cytotoxic T-Lymphocyte Associated Protein 4 (CTLA-4) inhibitor in addition to a PD-1 inhibitor and TKI. The median number of participants in each study was 42 (Range: 10 – 1417). The majority of studies included patients receiving a multi-targeted TKI (52/72, 72.2%) or VEGF-specific TKI (9/72, 12.5%), with the remaining studies including patients receiving TKIs targeting an oncogenic driver alteration, either BRAF (B-raf), MEK (Mitogen Activated Protein Kinase), EGFR or ALK (11/72, 15.2%). The most commonly used regimens (by number of clinical trials and treated patients) were Lenvatinib plus Pembrolizumab (17 studies; n=1996), Avelumab plus Axitinib (5 studies; n=578) and Nivolumab plus Cabozantinib (5 studies; n =484).
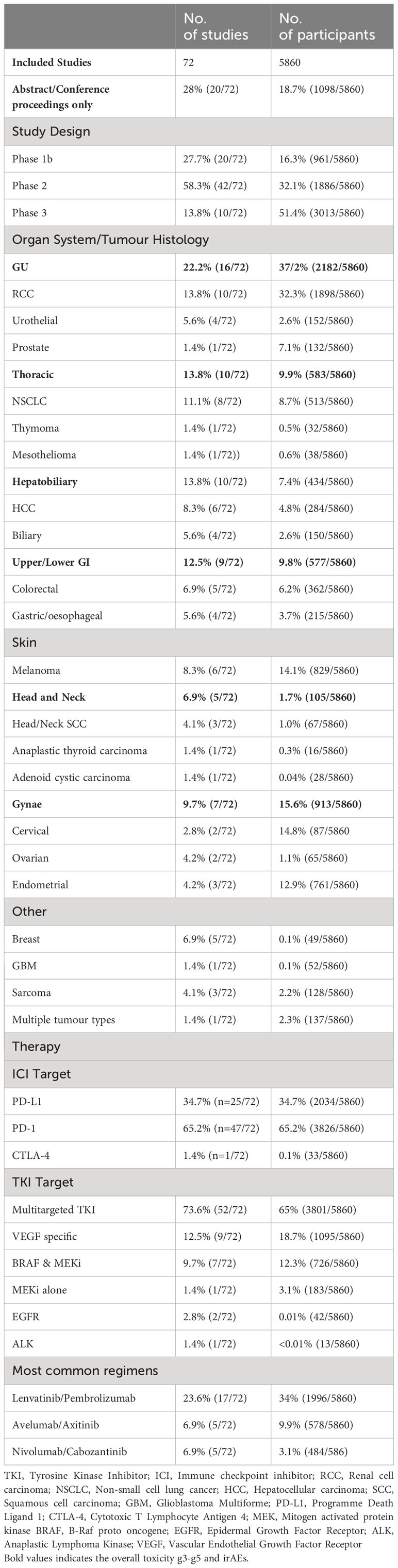
Table 1 Included studies in Meta-analysis of Immunotherapy and Targeted Therapy Combination Studies.
The majority of the included studies were early phase (Phase Ib or II = 62/72, 87%) and the majority of studies included patients with metastatic disease (nstudies = 70/72, 97.2%). The most common tumour types in these studies (see Table 1) included RCC (10 studies, npatients=1898) melanoma (nstudies = 6, npatients = 829), NSCLC (nstudies = 8, npatients = 513) and HPB cancers (nstudies = 10, npatients = 434).
All studies reported the incidence of G3-5 toxicity and were included in our primary analysis. The duration of treatment exposure was reported in 64% (46/72) of studies, with a median duration of 6.2 months and an IQR of 3-9.2 months. In a random-effects model meta-analysis, the overall incidence of G3-5 toxicity (See Figure 2) was 56% (95% CI = 50% – 61%). In all included studies, the incidence of G3-5 toxicity ranged from 7 – 92% (IQR: 42 – 68%). We performed a sensitivity analysis (See Supplementary Appendix) excluding low-quality studies (CAT score <20, nstudies= 20/72, 27.7%), and a similar incidence of G3-5 toxicity was demonstrated across remaining studies (53.1%, 95% CI 45.0% – 61.0%). Significant heterogeneity was observed across studies (I2= 83.9%; H2= 2.65; Q = 193.92, p <0.01). There was no evidence from the meta-regression analysis that primary tumour subgroup (χ2= 14.07, p = 0.08), TKI target (χ2= 0.34, p = 0.84) or ICI target (χ2= 0.14, p = 0.7) accounted for the heterogeneity observed (See Supplementary Appendix for meta-regression analysis covariates). This was reported in the majority of studies (63/72, 87.5%). The overall incidence of discontinuation of ICI/TKI combinations due to toxicity was 16% (953/5218), based on reporting in 87.5% of studies (63/72). The overall incidence of G5 toxicity was 2% (95/4740), based on reporting in 77% (56/72) studies.
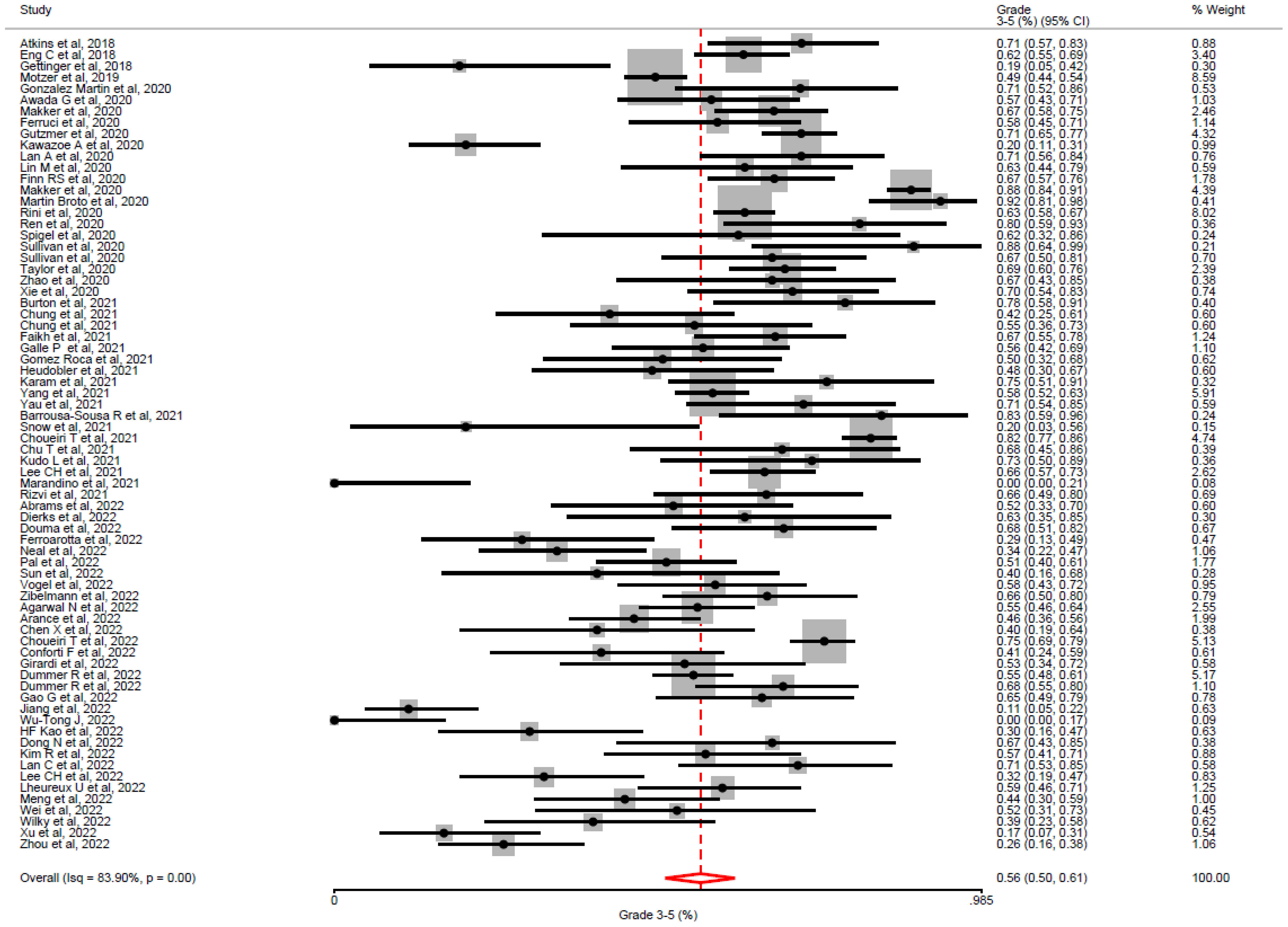
Figure 2 Overall incidence of grade 3-5 toxicity by Common toxicity Criteria for Adverse Events, in all included studies. In all studies utilising TKI-ICI combination, the overall incidence of grade 3 -5 toxicity was 56% (95% CI = 50–61%) with significant heterogeneity between studies (i2= 83.9%, p <0.01).
In pre-defined critical toxicities of interest (See Table 2), we report the incidences of: diarrhoea (7.1%, 393/5526); fatigue (4.1%, 213/5106); shortness of breath (1.4%, 34/2269); rash (3.6%, 129/4695); Alanine aminotransferase (ALT) increase (6.6%, 287/5088), Aspartate aminotransferase (AST) increase (5.7%, 236/4972). Notably, the incidence of G3-5 hypertension in all studies was 21.7% (935/4498), consistent with the significant number of patients in this analysis having received VEGF-targeted TKIs. There was significant variation in the missing data in specific symptom toxicity ranging from 5% – 62%.
There was unfortunately limited reporting on irAEs in included studies (See Table 2) with almost 50% of data missing on the overall incidence of G3-5 irAEs (2818/5860, 48.0%). In those studies in which the incidence and spectrum of irAEs was specifically annotated, the incidence of G3-5 ICI-associated toxicity was 12.8% (390/3042) – consistent with experience in the ICI monotherapy setting. The incidence of critical toxicities of G3-5 colitis, hepatitis and pneumonitis was 1.4% (42/3820), 2.1% (52/2470) and 1.1% (38/3471), respectively.
For patients with NSCLC (See Figure 3), most studies (5/8, 63%) involved treatment with a PD-(L)1 inhibitor and a multitargeted TKI (Targeting VEGF-1 and others). The three remaining studies involved TKIs targeting oncogenes [Erlotinib (EGFR), Alectinib & Crizotinib (ALK)]. The pooled incidence of grade 3-5 toxicity was 57% (95% CI = 43-69%;I2= 73.53%; H2= 1.04; Q = 8.23, p <0.01). The randomised phase III study of Lenvatinib plus pembrolizumab contributed the most patients (309/513) to the NSCLC subgroup. This study remains unpublished at the time of review and detailed data on adverse event reporting is unavailable. For this reason, when focusing on specific symptoms and irAEs, the analysis was limited by missing data (74.3%, 381/513). For patients receiving oncogene-targeting TKIs, a high burden of toxicities was observed in included studies (nstudies =3). This included severe hepatic toxicities - two patient deaths occurred with the combination of nivolumab plus crizotinib, and an incidence of G3-5 toxicity of 66.6% for patients receiving alectinib plus atezolizumab. In the NSCLC cohort, studies by Gettinger et al. (Nivolumab plus Erlotinib in EGFR-mutant NSCLC) and Neal et al. (Atezolizumab plus Cabozantinib had the lowest rates of G3-5 toxicity reported at 19% and 34% respectively (17, 18). Both of these studies (63% of patients, 50/79) included patients with EGFR-mutant lung cancer, unlike any other studies in the NSCLC cohort.
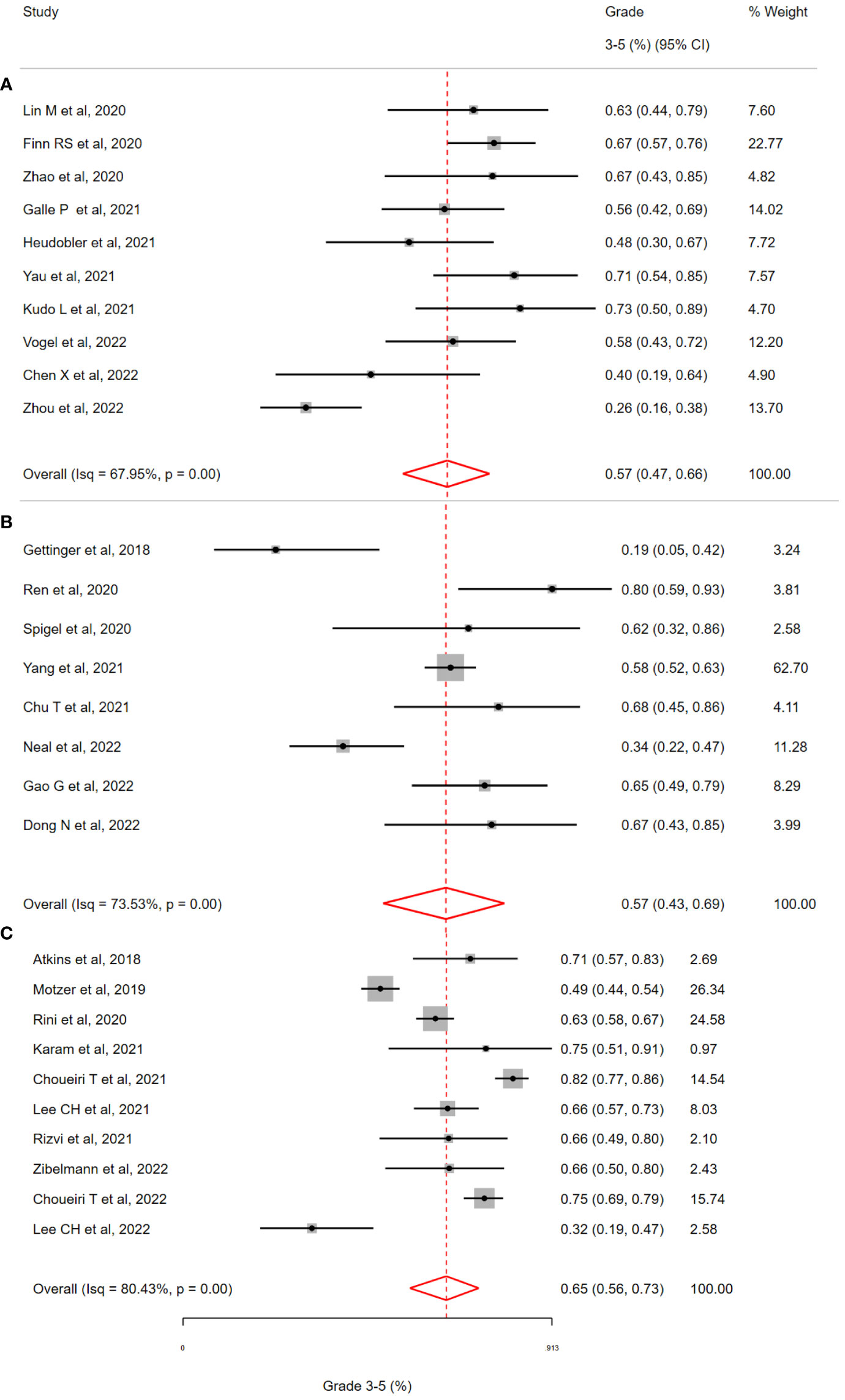
Figure 3 Incidence of grade 3-5 toxicity in selected clinical trials of patients with (A) Hepatobiliary Cancers (B) Non-small cell lung cancer (C) Renal cell cancer, treated with Immune Checkpoint Inhibitor and Targeted Therapy Combinations. (A) The overall incidence of grade 3-5 toxicity in patients with hepatobiliary (HCC & Biliary) cancer was 57 % (95% CI 47-66%, i2=68.0%). (B) The overall incidence of grade 3-5 toxicity in patients with non-small cell lung cancer was 57 % (95% CI 43-69%, i2=73.5%). (C) The overall incidence of grade 3-5 toxicity in patients with renal cell cancer was 65% (95% CI 56-73%, i2=80.4%).
In studies of RCC (n=10, see Figure 3), four randomised phase III studies (CLEAR, Checkmate9ER, Javelin Renal101, KEYNOTE-426) contributed significantly to the patients receiving ICI/TKI (n=1898). The pooled incidence of G3-5 toxicity was 58% (95% CI 40-73%) with observed heterogeneity (I2= 80.43%; H2= 3.52; Q = 30.19, p <0.01) All of these studies involved combining a PD-(L)1 inhibitor with a multi-targeted TKI (e.g. Cabozantinib) or a VEGF-specific TKI (e.g. Axitinib). Consistent with the overall population (all studies), G3-5 hypertension accounted for a significant burden of the G3-5 toxicity (392/1854, 21.1%). The overall incidence of G3-5 irAEs was 12.9%, based on reporting in >90% of patients (94.1%, 1787/1898). The incidence of G3-5 irAEs associated colitis, hepatitis and pneumonitis was low (1.7%, 2.0% and 1.0% respectively). The reported incidence of G3-5 toxicity was lowest in two studies; Motzer et al. (Avelumab plus Axitinib in RCC, G3-5 Toxicity of 49%) and Lee CH et al. (Lenvatinib plus Pembrolizumab in non-clear cell RCC, G3-5 Toxicity of 32%). This was the only study which included non-clear cell RCC in the cohort. The low toxicity observed with the combination of Avelumab plus Axitinib is consistent with this profile of this regimen across disease types (See section below: Toxicity by Specific TKI/ICI combination regimen).
For patients with hepatobiliary cancers (HCC = 6, BTC = 4, see Figure 3), a total of 434 patients received therapy with an ICI/TKI combination (See Table 2). In these studies, all patients received a PD-(L)1 inhibitor with a multitargeted TKI (except for one treated with Axitinib and another study evaluating a PD-1/CTLA-4 combination). The overall incidence of G3-5 toxicity in this group was 57% (95% CI = 47% – 66%) with heterogeneity observed (I2= 67.95%; H2= 1.28; Q = 9.78, p <0.01). Specific toxicities were reported in >80% (81.3%, 353/434) of patients except in the case of shortness of breath which was reported in <10% (7.3%, 32/434) of patients. The incidence of AST/ALT elevation was numerically higher for patients with HPB cancers than for all patients (ALT: 8.5% versus 5.6%; AST: 14.4% versus 4.7%). The incidence of irAEs was reported in just 20.2% of patients (88/434) A significant number of those patients (64.7%, n=57/88) received combination PD-1/CTLA-4 inhibition (the only study of 72 with this combination), thus comparison with other groups is limited. Studies by Chen et al. (Sintilimab plus Anlotinib in HCC) and Zhou et al. (Anlotinib plus TQB2450) demonstrated the lowest G3-5 toxicity of 40% and 26%, respectively (19, 20) et al. (Sintilimab plus Anlotinib in HCC). These two studies were the only trials in which patients received the TKI Anlotinib.
Based on available studies (>5) and total patients (>450), three regimens (See Figure 4) were selected for subgroup meta-analysis (Lenvatinib/Pembrolizumab, L/P; Avelumab/Axitinib, A/A; Nivolumab/Cabozantinib, N/C). Across these studies, the overall incidence of G3-5 toxicity was consistent with the overall population (L/P = 56% 95% CI = 43-68%; A/A = 49% 95% CI = 39%-69%; N/C = 61% 95% CI 44%-75%). Despite receiving the same drug therapies, significant heterogeneity was observed in G3-5 toxicity associated with these regimens (L/P, I2= 82.66%, H2= 5.37; Q = 103.89, p <0.01; N/C, I2= 79.71%, H2= 2.21; Q = 9.10, p <0.01) with the exception of A/A (I2= 56.67%, H2= 1.0; Q = 3.01, p =0.34). For patients receiving L/P (See Table 2), the most commonly recorded G3-5 toxicities included diarrhoea (9.9%, 152/1521) and hypertension (16.9%, 258/1519). A/A was associated with the lowest overall G3-5 toxicities with hypertension the most commonly reported (22.5%, 130/578). Hypertension was numerically lower in the N/C cohort (14.2%, 49/343). In the L/P cohort, the incidence of G3-5 irAEs was low (2.3%, 18/759). However, >50% (61.9%, 1237/1996) of patient data was not reported in this cohort so these results may underestimate this toxicity. The incidence of G3-5 ICI irAEs was 11.0% (49/444) and 14.3% (49/343) in the A/A & N/C cohorts respectively.
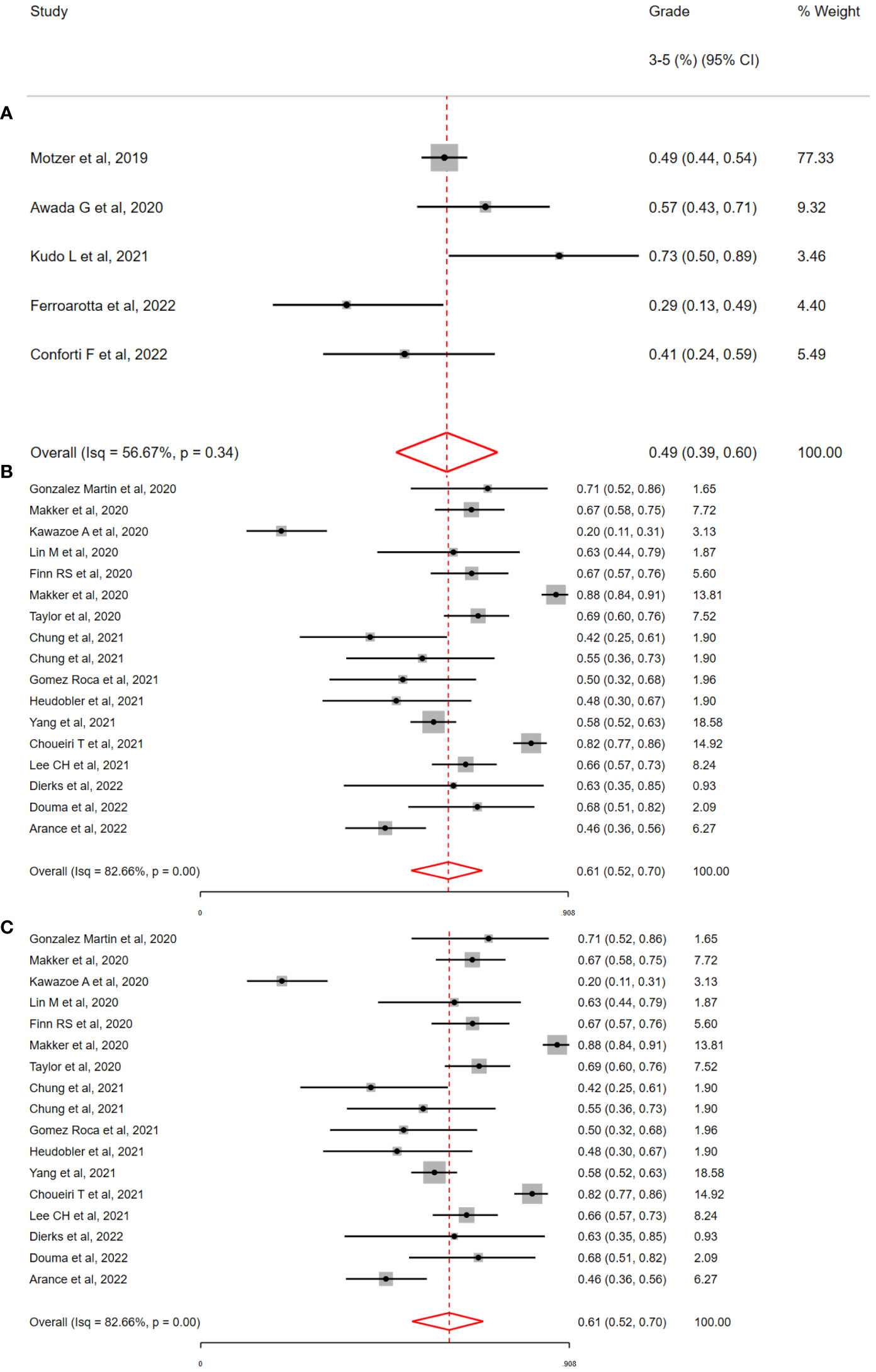
Figure 4 Incidence of grade 3-5 toxicity by immunotherapy and tyrosine kinase inhibitor regimen, by (A) Avelumab & Axitinib (A/A) (B) Lenvatinib & Pembrolizumab (L/P) (C) Nivolumab & Cabozantinib (N/C). The overall incidence of grade 3-5 toxicity was high with all three combinations; 49% with A/A, 56% with L/P and 61% with N/C. Significant interstudy heterogeneity was observed with the combination of L/P & N/C but not with A/A (i2=56%, p =0.37).
For patients who received ICI with TKIs targeting specific oncogenes (BRAF, MEK, EGFR, ALK; nstudies = 11), the incidence of G3-5 toxicity ranged from 18% to 88% (Interquartile range = 58% - 71%). In a random effects meta-analysis, the overall incidence of G3-5 toxicity was 61% (95% CI = 53% - 71%;I2 = 62.19%, H2= 1.09; Q = 10.71, p <0.01). The most common G3-5 toxicities included diarrhoea 4.8% (47/964), Rash 7.1% (68/964), AST increase 6.3% (61/964), ALT increase 5.6% (54/964) with less common toxicities of Fatigue 2.5% (23/904); SOB 2.2% (12/540); Hypertension 1.9% (6/312). Notably but not unexpectedly, the overall incidence of G3-5 hypertension was low at 1.9% (6/312). This is in contrast to the overall study population where hypertension occurred in 21.1% of patients treated with TKI-ICI combination therapy. Notably, the incidence of rash was higher (7.1% with oncogene targeted TKIs versus 2.7% in the overall study population). The incidence of irAEs was also higher (41.8% with oncogene targeting TKIs versus 12.8% in the overall study population), although small numbers in the oncogene group may confound results.
A total of 8 studies with a run-in period of between 1 and 4 weeks were included. These studies included a run-in with Vemurafenib (n=1), Cobimetanib plus Vemurafenib (n=3), Alectinib (n=1), Sunitinib (n=1), Axitinib (n=1) or Sitravatinib (n=1). The overall toxicity of studies employing a TKI-run-in was 71% (95% CI 57-81%;I2 = 70.84%, H2 = 1.00; Q = 5.57, p =0.01) with just one study reporting an overall incidence of G3-5 toxicity of <60% (See Figure 5).
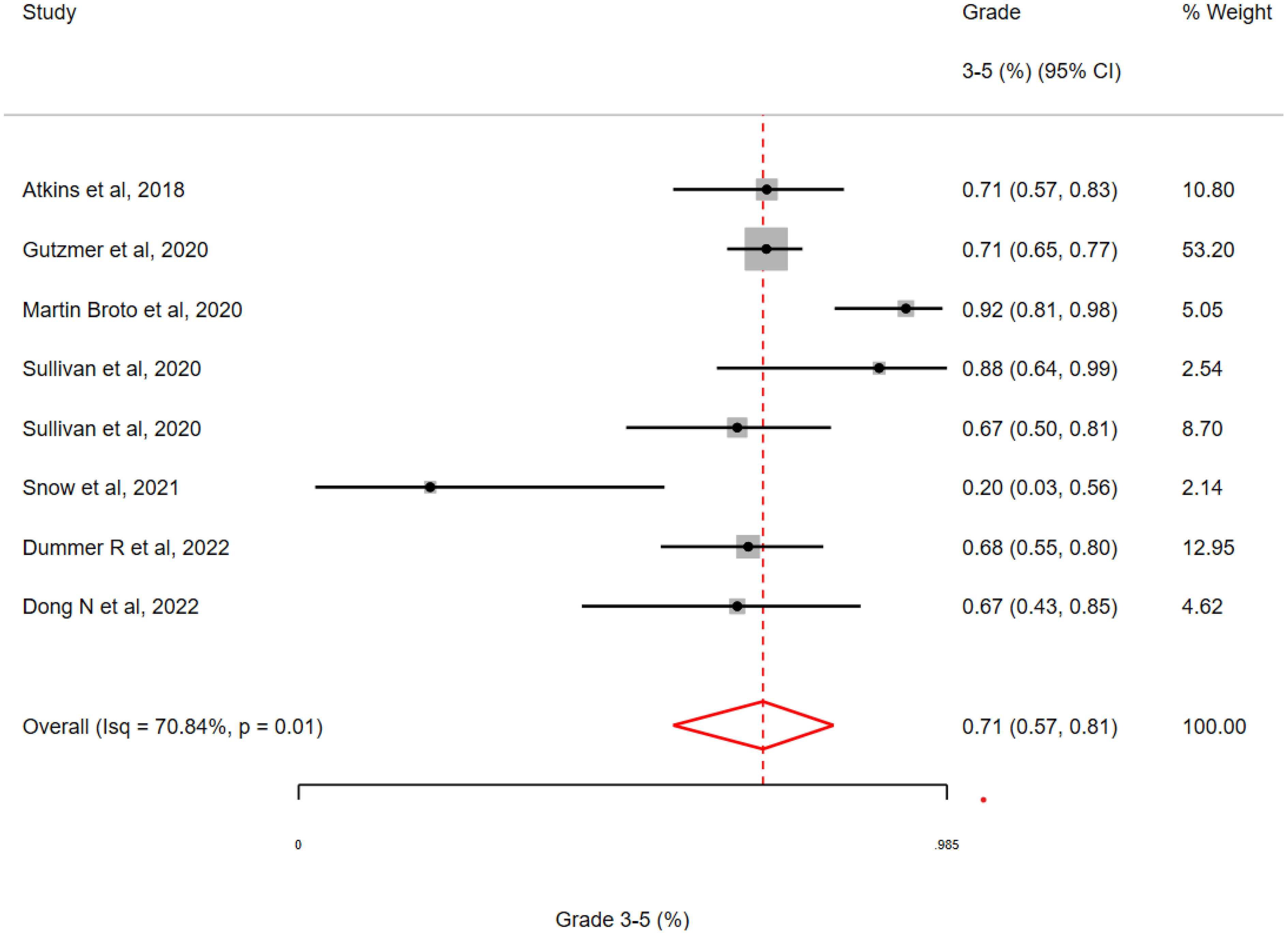
Figure 5 Incidence of grade 3-5 toxicity in clinical trials with a ‘run-in’ period involving a targeted therapy followed by an immune checkpoint inhibitor. The pooled estimate of grade 3-5 toxicity was 71% for all studies with a range of 20 – 92% (Interquartile Range 67-73%.
Combining ICIs with TKIs represents an opportunity for therapeutic synergy to improve outcomes from ICIs alone, yet can be associated with high-grade toxicity. In this comprehensive meta-analysis of 72 Phase Ib – III studies of TKI/ICI combinations across solid tumour types, the observed overall incidence of G3-5 toxicity (56%) was apparent across subgroups of the meta-analysis by tumour type (NSCLC, RCC, HPB) and treatment regimen (Lenvatinib plus Pembrolizumab, Avelumab plus Axitinib, Nivolumab plus Cabozantinib). Although the overall burden of toxicity was similar with different regimens, the incidence of specific toxicities observed varied with different TKI partners (VEGF/Multitargeted; Hypertension; Oncogene targeted TKIs; Rash, irAEs) and there was a high degree of heterogeneity between studies. Finally, while a TKI run-in strategy has been purported to potentially mitigate the toxicity of TKI/ICI combinations, a high incidence of G3-5 toxicity was observed in our included studies.
Our data suggests that the incidence of irAEs is higher for patients who receive oncogene targeting TKIs versus multitargeted/VEGF specific TKIs. In a retrospective analysis of patients who received the TKI, Sotorasib, the incidence of G3-5 toxicity was significantly higher in the group which had received an ICI in the preceding 30 days (33% versus 11%) (21). Specifically, it has been described that sotorasib in combination/sequential with PD-(L)1 inhibition is associated with a high-incidence of immune mediated hepatotoxicity (10, 21). Similarly, this has been demonstrated with the combination of crizotinib plus PD(L)-1 inhibition (22). In pre-clinical models, treatment with sotorasib has been shown to induce a pro-inflammatory tumour microenvironment, which may contribute to the synergistic toxicity of ICI/TKI combinations (23). A similar effect of the tumour immune microenvironment has been demonstrated with alectinib and osimertinib (24, 25). Our data supports this potential shared mechanism across oncogene-targeted TKIs which may underlie the high incidence of irAEs with these combinations.
A significant challenge in interpretation of irAE events in our study is the often absent reporting. The FDA recommends reporting of all irAEs including the duration, outcome, therapy if commenced and duration of irAE (26). There was very limited adherence to these guidelines in our included studies. A clinical challenge may be the differentiation of an irAE versus an AE for patients receiving combination therapy (eg ICI/TKI combination). Attention to this challenge is of increasing relevance with increased use of ICIs in combination with TKIs, chemotherapies and antibody drug conjugates (ADCs). The limited data presented in combination studies with regards to irAEs impairs clinicians and patients ability to make informed treatment decisions with the best available evidence. Our study contributes an analysis of ICI/TKI combination studies which raises a concerning trend of limited reporting of irAEs. A framework is needed to guide investigators in determining the aetiology of an AE and to ensure comprehensive reporting.
Our data would suggest that the studies with a TKI run-in were associated with what is generally considered an unacceptable incidence of G3-5 toxicity (G3-5 toxicity = 71%). Atezolizumab plus Vemurafenib was one of the earliest combinations explored in a run-in strategy (27). However, this was modified after only 3 initial patients experienced G3 toxicity. In a retrospective analysis of patients who received Osimertinib before or after PD-(L)1 inhibition, the authors discovered a high incidence of severe irAEs when PD-(L)1 inhibition was followed by Osimertinib (within 20 days) but not when Osimertinib was followed by PD-(L)1 (25). Our data contributes prospective data to the run-in approach and suggests that a run-in does not consistently mitigate the overall incidence of G3-5 toxicity. However, to conclusively address this question, randomised data would be needed in specific disease and treatment settings.
Our work has several limitations. Firstly, the studies included were associated with clinical heterogeneity which limits our interpretation of the pooled estimates, meaning our results are exploratory and hypothesis generating. However, we included different ICI/TKI combinations and diseases to ensure a comprehensive review. Despite our broad inclusion criteria, all studies involved an ICI targeting PD-1/PD-L1 and the vast majority of studies included a TKI targeting VEGF (VEGF specific or multitargeted; 85%, 61/72). We also saw in our work that there is significant heterogeneity in the incidence of G3-5 toxicity even when we focused on one regimen (eg Lenvatinib and Pembrolizumab). This is also apparent in other published works which focus on PD-1/PD-L1 inhibitors alone, where significant heterogeneity was also observed (28, 29). Our and others work are indicative that heterogeneity observed in toxicity meta-analysis may occur even when ICI/TKI combinations and diseases are homogenous. This supports our conclusions that further work is needed to harmonise reporting of AEs, which may contribute to observed heterogeneity. Nonetheless, the clinical heterogeneity and statistical heterogeneity observed is a limitation. Potential bias that may have reflected our results include publication bias. Given that we were assessing toxicity, rather than efficacy, it was our expectation that publication bias would be less likely to be a significant counfounder. Potential other biases include search and selection bias. We mitigated these biases with the designing of a search strategy with an information specialist and multiple reviewers of abstracts/full-texts. Furthermore, the exclusion of phase 1a studies (to avoid dose-finding studies) resulted in a significant number of excluded studies which may have had clinically relevant results (30–40). We did not have access to patient-level data, therefore results we included were limited to that available in publications or conference proceedings. This resulted in missing data (Range of 10.5% - 61.5%) which may have introduced bias to our symptom specific (e.g. diarrhoea) and toxicity specific (eg irAE) data. We were not able to provide an overall incidence of toxicity in a ‘Control Arm’ in our work given that the majority of included studies were not randomised and those that were may have had a control arm which did not include either therapy in the investigational arm (eg Checkmate9ER – Nivolumab & Cabozantinib versus Sunitinib) (41). If included studies included a control arm, it would have allowed us to compare relatively toxicity across studies potentially mitigating some of the challenges observed with heterogenous results. Studies which closed early due to excess toxicity are less likely to be published and as such there may be a reporting bias in included studies. Finally, the inclusion of studies which have not been peer-reviewed but data available as conference proceedings is a limitation but was intended to ensure a broad group of studies was included.
In conclusion, this study aims to address the underrepresented topic of the nuances of toxicity of ICI/TKI combinations - a growing set of oncology regimens used across tumours - in the immunotherapy armamentarium. These data are in fact critical to clinical decision-making, particularly when multiple treatment options exist- and when toxicity becomes a key deciding factor when clinicians select appropriate therapy in partnership with patients. We have identified that more than half of patients receiving these therapies will experience a diverse range of G3-5 toxicity which does not appear to be mitigated by a run-in strategy. Reporting of irAEs in ICI/TKI studies is limited and a framework is needed to ensure adequate reporting of incidence, duration and treatment of AEs in studies. Future directions to compliment comprehensive reporting may include use of patient reported outcomes, collection of financial and time toxicity data, and novel clinical trial designs employing metronomic dosing, and other toxicity mitigation approaches.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
DO’R: Data curation, Formal analysis, Investigation, Methodology, Project administration, Software, Writing – original draft, Writing – review & editing. CO’L: Formal analysis, Writing – original draft, Writing – review & editing. AR: Formal analysis, Writing – original draft, Writing – review & editing. MT: Formal analysis, Methodology, Writing – original draft, Writing – review & editing. GO’K: Writing – original draft, Writing – review & editing. LH: Formal analysis, Methodology, Writing – original draft, Writing – review & editing. KB: Formal analysis, Methodology, Writing – original draft, Writing – review & editing. JN: Conceptualization, Data curation, Formal analysis, Methodology, Supervision, Validation, Writing – original draft, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was funded through the Royal College of Surgeons of Ireland StAR MD Programme.
All authors would like to thank all of the patients and their families for their involvement in the included clinical trials. We would also like to thank Mr Paul Murphy (Information Specialist) at the Royal College of Surgeons of Ireland for his guidance on the search strategy.
DO’R: Conference attendance; Pfizer, MSD, Servier. LH: Research funding Roche Genentech, AstraZeneca, Boehringer Ingelheim, Takeda, Merck, Pfizer, Novartis; Gilead under negotiation; Speaker educationals/webinars: AstraZeneca, Bayer, Lilly, MSD, high5oncology, Takeda, Janssen, GSK, Sanofi, Pfizer Inst, Medtalks, Benecke, VJOncology, Medimix self; All payments were paid to the institution with the exception of Medtalks, Benecke, VJOncology, Medimix; Advisory boards: Amgen, Boehringer Ingelheim, Lilly, Novartis, Pfizer, Takeda, Merck, Janssen, MSD, Anheart; All payments were paid to the institution. KB: Research funding from IQVIA and Novartis. JN: AstraZeneca: Research funding, Consulting/Advisory Board, Data Safety Monitoring Board, Honoraria; Bristol Myers Squibb: Research funding, Consulting/Advisory Board, Honoraria; Roche/Genentech: Research funding, Consulting/Advisory Board, Honoraria; Amgen: Research funding, Consulting/Advisory Board; NGM Pharmaceuticals: Consulting/Advisory Board; Takeda: Consulting/Advisory Board; Pfizer: Consulting/Advisory Board; Elevation Oncology: Consulting/Advisory Board; Abbvie: Consulting/Advisory Board; Kaleido Biosciences: Consulting/Advisory Board; Mirati: Research funding; Daiichi Sankyo: Consulting/Advisory Board Data Safety Monitoring Board, Honoraria.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer SP declared a shared affiliation with the author MT to the handling editor at the time of review.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2024.1380453/full#supplementary-material
1. Larkin J, Chiarion-Sileni V, Gonzalez R, Grob JJ, Rutkowski P, Lao CD, et al. Five-year survival with combined nivolumab and ipilimumab in advanced melanoma. New Engl J Med. (2019) 381:1535–46. doi: 10.1056/NEJMoa1910836
2. Castro Gd, Kudaba I, Wu YL, Lopes G, Kowalski DM, Turna HZ, et al. 363 KEYNOTE-042 5-year survival update: pembrolizumab versus chemotherapy in patients with previously untreated, PD-L1–positive, locally advanced or metastatic non–small-cell lung cancer. J Immunother Cancer. (2021) 9:A390. doi: 10.1136/jitc-2021-SITC2021.363
3. Garon EB, Hellmann MD, Rizvi NA, Carcereny E, Leighl NB, Ahn MJ, et al. Five-year overall survival for patients with advanced non-Small-cell lung cancer treated with pembrolizumab: results from the phase I KEYNOTE-001 study. J Clin Oncol. (2019) 37:2518–27. doi: 10.1200/JCO.19.00934
4. Paz-Ares L, Vicente D, Tafreshi A, Robinson A, Soto Parra H, Mazières J, et al. A randomized, placebo-controlled trial of pembrolizumab plus chemotherapy in patients with metastatic squamous NSCLC: protocol-specified final analysis of KEYNOTE-407. J Thorac Oncol. (2020) 15:1657–69. doi: 10.1016/j.jtho.2020.06.015
5. Ahn R, Ursini-Siegel J. Clinical potential of kinase inhibitors in combination with immune checkpoint inhibitors for the treatment of solid tumors. Int J Mol Sci. (2021) 22:2608. doi: 10.3390/ijms22052608
6. Rassy E, Flippot R, Albiges L. Tyrosine kinase inhibitors and immunotherapy combinations in renal cell carcinoma. Ther Adv Med Oncol. (2020) 12:1758835920907504–1758835920907504. doi: 10.1177/1758835920907504
7. Appleman LJ. Multifactorial, biomarker-based predictive models for immunotherapy response enter the arena. JNCI: J Natl Cancer Institute. (2021) 113:7–8. doi: 10.1093/jnci/djaa077
8. Looi CK, Chung FFL, Leong CO, Wong SF, Rosli R, Mai CW. Therapeutic challenges and current immunomodulatory strategies in targeting the immunosuppressive pancreatic tumor microenvironment. J Exp Clin Cancer Res. (2019) 38:162. doi: 10.1186/s13046-019-1153-8
9. Sasaki K, Kobayashi S, Kudo M, Sugimoto M, Takahashi S, Nakamura Y, et al. Hypothyroidism and hypopituitarism as immune-related adverse events due to lenvatinib plus pembrolizumab therapy in the immediate postoperative period after laparoscopic hepatectomy for liver metastases from gastric cancer: a case report. Surg Case Rep. (2021) 7:267. doi: 10.1186/s40792-021-01346-w
10. Begum P, Goldin RD, Possamai LA, Popat S. Severe immune checkpoint inhibitor hepatitis in KRAS G12C-mutant NSCLC potentially triggered by sotorasib: case report. JTO Clin Res Rep. (2021) 2:100213. doi: 10.1016/j.jtocrr.2021.100213
11. Shalata W, Iraqi M, Bhattacharya B, Fuchs V, Roisman LC, Cohen AY, et al. Rapid response to the combination of lenvatinib and pembrolizumab in patients with advanced carcinomas (Lung adenocarcinoma and Malignant pleural mesothelioma). Cancers. (2021) 13:3630. doi: 10.3390/cancers13143630
12. How JA, Patel S, Fellman B, Lu KH, Hwu P, Ramondetta LM, et al. Toxicity and efficacy of the combination of pembrolizumab with recommended or reduced starting doses of lenvatinib for treatment of recurrent endometrial cancer. Gynecol Oncol 2021/05/03. (2021) 162:24–31. doi: 10.1016/j.ygyno.2021.04.034
13. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Syst Rev. (2021) 10:89. doi: 10.1186/s13643-021-01626-4
14. US Department of Health and Human Services NI of HNCInstitute. Common Terminology Criteria for Adverse Events (CTCAE) Version 5. United States (2017).
15. Crowe M, Sheppard L, Campbell A. Reliability analysis for a proposed critical appraisal tool demonstrated value for diverse research designs. J Clin Epidemiol. (2012) 65:375–83. doi: 10.1016/j.jclinepi.2011.08.006
16. Cochrane Reviews. 9.5.2, Identifying and measuring heterogenity, Section 9.5 Heterogenity. In: General Methods for Cochrane Reviews (2023).
17. Rizvi NA, Chow LQM, Borghaei H, Shen Y, Harbison C, Alaparthy S, et al. Safety and response with nivolumab (anti-PD-1; BMS-936558, ONO-4538) plus erlotinib in patients (pts) with epidermal growth factor receptor mutant (EGFR MT) advanced NSCLC. J Clin Oncol. United States: ASCO (2014) 32. doi: 10.1200/jco.2014.32.15_suppl.8022
18. Neal JW, Lim FL, Aix SP, Viteri S, Santoro A, Spencer K, et al. EP08.02-081 cabozantinib plus atezolizumab in first or second-line advanced NSCLC and previously-treated EGFR mutant advanced NSCLC. J Thorac Oncol. Elsevier (2022) 17:S439. doi: 10.1016/j.jtho.2022.07.763
19. Zhou J, Sun Y, Zhang W, Yuan J, Peng Z, Wang W, et al. Phase Ib study of anlotinib combined with TQB2450 in pretreated advanced biliary tract cancer and biomarker analysis. Hepatology. (2022) n/a. doi: 10.1002/hep.32548
20. Chen X, Li W, Wu X, Zhao F, Wang D, Wu H, et al. Safety and efficacy of sintilimab and anlotinib as first line treatment for advanced hepatocellular carcinoma (KEEP-G04): A single-arm phase 2 study. Front Oncol. (2022) 12:909035. doi: 10.3389/fonc.2022.909035
21. Chour A, Denis J, Mascaux C, Zysman M, Bigay-Game L, Swalduz A, et al. Brief report: severe sotorasib-related hepatotoxicity and non-liver adverse events associated with sequential anti–programmed cell death (Ligand)1 and sotorasib therapy in KRASG12C-mutant lung cancer. J Thorac Oncol. (2023). doi: 10.1016/j.jtho.2023.05.013
22. Lin JJ, Chin E, Yeap BY, Ferris LA, Kamesan V, Lennes IT, et al. Increased hepatotoxicity associated with sequential immune checkpoint inhibitor and crizotinib therapy in patients with non–small cell lung cancer. J Thorac Oncol. (2019) 14:135–40. doi: 10.1016/j.jtho.2018.09.001
23. Canon J, Rex K, Saiki AY, Mohr C, Cooke K, Bagal D, et al. The clinical KRAS(G12C) inhibitor AMG 510 drives anti-tumour immunity. Nature. (2019) 575:217–23. doi: 10.1038/s41586-019-1694-1
24. Xiao X, Wu Y, Shen F, MuLaTiAize Y, Xinhua N. Osimertinib improves the immune microenvironment of lung cancer by downregulating PD-L1 expression of vascular endothelial cells and enhances the antitumor effect of bevacizumab. J Oncol. (2022) 2022:1531353. doi: 10.1155/2022/1531353
25. Kleczko EK, Hinz TK, Nguyen TT, Gurule NJ, Navarro A, Le AT, et al. Durable responses to alectinib in murine models of EML4-ALK lung cancer requires adaptive immunity. NPJ Precis Oncol. (2023) 7:15. doi: 10.1038/s41698-023-00355-2
26. US Food and Drug Administration. Characterizing, Collecting, and Reporting Immune-Mediated Adverse Reactions in Cancer Immunotherapeutic Clinical Trials. (2022).
27. Sullivan RJ, Hamid O, Gonzalez R, Infante JR, Patel MR, Hodi FS, et al. Atezolizumab plus cobimetinib and vemurafenib in BRAF-mutated melanoma patients. Nat Med. (2019) 25:929–935. doi: 10.1038/s41591-019-0474-7
28. Fujiwara Y, Horita N, Adib E, Zhou S, Nassar AH, Asad ZULA, et al. Treatment-related adverse events, including fatal toxicities, in patients with solid tumours receiving neoadjuvant and adjuvant immune checkpoint blockade: a systematic review and meta-analysis of randomised controlled trials. Lancet Oncol. (2024) 25:62–75. doi: 10.1016/S1470-2045(23)00524-7
29. Song P, Zhang D, Cui X, Zhang L. Meta-analysis of immune-related adverse events of immune checkpoint inhibitor therapy in cancer patients. Thorac Cancer. (2020) 11:2406–30. doi: 10.1111/1759-7714.13541
30. Wang F, He MM, Yao YC, Zhao X, Wang ZQ, Jin Y, et al. Regorafenib plus toripalimab in patients with metastatic colorectal cancer: a phase Ib/II clinical trial and gut microbiome analysis. Cell Rep Med. (2021) 2. doi: 10.1016/j.xcrm.2021.100383
31. Nadal RM, Mortazavi A, Stein M, Pal SK, Davarpanah NN, Parnes HL, et al. Results of phase I plus expansion cohorts of cabozantinib (Cabo) plus nivolumab (Nivo) and CaboNivo plus ipilimumab (Ipi) in patients (pts) with with metastatic urothelial carcinoma (mUC) and other genitourinary (GU) Malignancies. J Clin Oncol. (2018) 36:515. doi: 10.1200/JCO.2018.36.6_suppl.515
32. Mei K, Qin S, Chen Z, Liu Y, Wang L, Zou J. Camrelizumab in combination with apatinib in second-line or above therapy for advanced primary liver cancer: cohort A report in a multicenter phase Ib/II trial. J Immunother Cancer. (2021) 9:e002191. doi: 10.1136/jitc-2020-002191
33. Shaikh S, Zang Y, Hanmer J, Wang H, Lin Y, Davar D, et al. A phase I trial of pembrolizumab plus vemurafenib and cobimetinib in patients with advanced melanoma. J Clin Oncol. (2021) 39. doi: 10.1200/JCO.2021.39.15_suppl.e21506
34. Yang JCH, Gadgeel SM, Sequist LV, Wu CL, Papadimitrakopoulou VA, Su WC, et al. Pembrolizumab in combination with erlotinib or gefitinib as first-line therapy for advanced NSCLC with sensitizing EGFR mutation. J Thorac Oncol. (2019) 14:553–9. doi: 10.1016/j.jtho.2018.11.028
35. Karam JA, Msaouel P, Matin SF, Campbell MT, Zurita AJ, Shah AY, et al. A phase II study of sitravatinib (Sitra) in combination with nivolumab (Nivo) in patients (Pts) undergoing nephrectomy for locally-advanced clear cell renal cell carcinoma (accRCC). J Clin Oncol. (2021) 39. doi: 10.1200/JCO.2021.39.6_suppl.312
36. Choueiri TK, Plimack ER, Gupta S, Puzanov I, McDermott DF, Tarazi J, et al. Phase lb dose-finding study of axitinib plus pembrolizumab in treatment-naive patients with advanced renal cell carcinoma. BJU Int. (2015) 116:5.
37. Taylor MH, Rasco DW, Brose MS, Vogelzang NJ, Richey SL, Cohen AL, et al. A phase 1b/2 trial of lenvatinib plus pembrolizumab in patients with squamous cell carcinoma of the head and neck. Asia Pac J Clin Oncol. (2018) 14:174. doi: 10.1200/JCO.2018.36.15_suppl.6016
38. Chalmers AW, Patel S, Boucher K, Cannon L, Esplin M, Luckart J, et al. Phase I trial of targeted EGFR or ALK therapy with ipilimumab in metastatic NSCLC with long-term follow-up. Target Oncol. (2019) 14:417–21. doi: 10.1007/s11523-019-00658-0
39. Felip E, de Braud FG, Maur M, Loong HH, Shaw AT, Vansteenkiste JF, et al. Ceritinib plus nivolumab in patients with advanced ALK-rearranged non-small cell lung cancer: results of an open-label, multicenter, phase 1B study. J Thorac Oncol. (2020) 15:392–403. doi: 10.1016/j.jtho.2019.10.006
40. Ren S, Xiong X, You H, Shen J, Zhou P. The combination of immune checkpoint blockade and angiogenesis inhibitors in the treatment of advanced non-small cell lung cancer. Front Immunol. (2021) 12:689132. doi: 10.3389/fimmu.2021.689132
41. Choueiri TK, Powles T, Burotto M, Bourlon MT, Zurawski B, Oyervides Juárez VM, et al. Nivolumab + cabozantinib vs sunitinib in first-line treatment for advanced renal cell carcinoma: First results from the randomized phase III CheckMate 9ER trial. Ann Oncol. (2020) 31:S1159–. doi: 10.1016/j.annonc.2020.08.2257
Keywords: tyrosine kinase inhibitors, immunotherapy, toxicity, immune related adverse events, immune checkpoint blockade
Citation: O’Reilly D, O’Leary CL, Reilly A, Teo MY, O’Kane G, Hendriks L, Bennett K and Naidoo J (2024) Toxicity of immune checkpoint inhibitors and tyrosine kinase inhibitor combinations in solid tumours: a systematic review and meta-analysis. Front. Oncol. 14:1380453. doi: 10.3389/fonc.2024.1380453
Received: 01 February 2024; Accepted: 19 June 2024;
Published: 15 July 2024.
Edited by:
Awanish Mishra, National Institute of Pharmaceutical Education and Research, IndiaReviewed by:
Magdalena Knetki-Wróblewska, Maria Sklodowska-Curie National Research Institute of Oncology, PolandCopyright © 2024 O’Reilly, O’Leary, Reilly, Teo, O’Kane, Hendriks, Bennett and Naidoo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: David O’Reilly, ZGF2aWRvcmVpbGx5MjJAcmNzaS5pZQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.