
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Oncol., 22 April 2024
Sec. Thoracic Oncology
Volume 14 - 2024 | https://doi.org/10.3389/fonc.2024.1377451
Leptomeningeal metastasis (LM) is a complication of non-small cell lung cancer (NSCLC) characterized by poor prognosis and short survival. A variety of therapeutic approaches have been sought to improve the efficacy of LM. Here we present a clinical case and conduct a literature review to investigate the effectiveness and safety of double-dose osimertinib combined with a pemetrexed intrathecal injection. This is an older man who underwent thoracoscopic pneumonectomy and was diagnosed with stage IIA lung adenocarcinoma with EGFR21 L858R mutation. He experienced thoracic vertebral metastases 33 months postoperatively and received first-line treatment with gefitinib combined with radiotherapy for vertebral metastases. However, the patient developed a grade 3 rash with unacceptable toxicity and his CEA levels were significantly increased 22 months later, leading to a targeted treatment adjustment to 80 mg of osimertinib orally once daily. Four months later, the patient developed LM and osimertinib dosage was increased to 160 mg once daily; however, neurological symptoms did not improve, and cerebrospinal fluid (CSF) tumor cells remained detected. Accordingly, the patient received an intrathecal injection of pemetrexed (dose 30 mg) every 2-3 months, 2-3 times per course (4-6 days each time), and continued to receive a double dose of osimertinib. After three courses of intrathecal chemotherapy, CSF tumor cells were eliminated, and neurological symptoms significantly improved. During the treatment, he experienced a one-degree rash, leukopenia, thrombocytopenia, and fatigue. This patient has been alive and well with disease control for 28 months since the diagnosis of meningeal metastases. Combining double-dose osimertinib and an intrathecal injection of pemetrexed demonstrated therapeutic efficacy and manageable adverse effects in this patient with advanced NSCLC with EGFR-mutant and LM.
Leptomeningeal metastasis (LM) is a rare and devastating complication of metastatic non-small cell lung cancer (NSCLC). The incidence of LM in patients is approximately 3-5%, and patients harboring epidermal growth factor receptor (EGFR) mutations and anaplastic lymphoma kinase (ALK) rearrangements are more likely to progress to LM (1–3). Owing to the lack of specific clinical manifestations, early diagnosis of LM remains difficult and challenging, often leading to misdiagnosis or missed diagnosis. In central nervous system metastases, the median overall survival (OS) is approximately 12 months in patients with brain metastases (4), whereas the median OS is only 3 months in patients with LM (2). Treatment options include radiotherapy, chemotherapy, intrathecal treatment, molecular targeted therapy, and immunotherapy. However, owing to the blood-brain barrier (BBB), most drugs face challenges in penetrating the meningeal cavity, resulting in limited treatment efficacy and a median survival of only a few months. Therefore, tyrosine kinase inhibitors (TKIs) with enhanced BBB permeability and more effective drugs are urgently needed to enhance the therapeutic efficacy against LM.
Osimertinib is a third-generation irreversible epidermal growth factor receptor tyrosine kinase inhibitor (EGFR-TKI) that effectively and selectively inhibits EGFR-TKI-sensitizing and EGFR T790M mutations. High doses of osimertinib exhibit high BBB penetration rates. The BLOOM phase I study demonstrated that a doubled dose of osimertinib (160 mg/day) exhibits significant efficacy in NSCLC patients with EGFR-mutant LM, with an objective response rate of 41%, a median progression-free survival (PFS) of 8.6 months and a median OS of 11 months (5). Although the incidence of adverse reactions has increased, osimertinib has shown significant therapeutic efficacy and manageable safety. Pemetrexed is a cell cycle-specific antimetabolite antineoplastic drug that inhibits folate metabolism and is a multitarget antifolate agent. It is considered the primary treatment option for advanced lung adenocarcinomas. However, the CSF penetration rate of pemetrexed is extremely low, accounting for less than 2% of plasma levels (6). Intrathecal chemotherapy (IC) is a viable approach for treating LM; its advantage is that it can directly penetrate the blood-CSF barrier and maximize drug exposure in the CSF. A direct intrathecal injection of pemetrexed has demonstrated effectiveness in the treatment of LM (7). Currently, there is limited literature available regarding the intrathecal injection of pemetrexed for the treatment of LM in NSCLC. Here we present a case report of lung adenocarcinoma with an EGFR mutation, who developed meningeal metastasis and received double-dose osimertinib combined with intrathecal injection of pemetrexed to enhance therapeutic efficacy.
A 71-year-old male, with a smoking history of over 40 years, was admitted to The First Affiliated Hospital of Gannan Medical University (Ganzhou, China) on October 16, 2016, with a persistent cough lasting for 9 months and hemoptysis for 1 week. CT revealed a mass in the upper lobe of the left lung. On October 20, 2016, the patient underwent a total resection of the left lung and systemic lymph node dissection under general anesthesia. Postoperative pathology revealed lung adenocarcinoma, and one-third of the hilar lymph node metastases was positive. Immunohistochemistry revealed TTF-1 (+), CK7 (+), Napsin A (+), and Ki-67 expression (approximately 20%). The postoperative diagnosis was stage IIA (pT1bN1M0), according to the 7th edition of the TNM classification for lung cancer. Genetic testing using ARMS revealed the EGFR21 L858R mutation. After surgery, the patient received four cycles of adjuvant chemotherapy with docetaxel and cisplatin. On July 23, 2019, the patient presented with headache and pain in the neck and chest. MRI and CT scans indicated metastases in the T1 and T6 vertebrae (Figures 1A, B). Subsequently, the patient underwent radiotherapy for vertebral metastases at a dose of 30 Gy/10 fx along with molecular-targeted therapy using gefitinib (Iressa, 250 mg/day). During regular follow-up examinations, the disease remained stable, with osteogenic changes observed in the thoracic vertebral metastases (Figures 1C, D). In May 2021, CEA levels increased from 9.7 ng/ml to 214 ng/ml, accompanied by the onset of a grade 3 rash following the oral administration of gefitinib. Consequently, target therapy was switched to osimertinib at 80 mg/day. After one month, the CEA decreased to 161.5 ng/ml and the rash improved to grade 1. In September 2021, the patient presented with dizziness, headache, nausea, vomiting, and weakness in both lower extremities. Magnetic resonance imaging (MRI) revealed significant thickening and pronounced enhancement of the meninges in the cerebellum (Figures 2A–C). A lumbar puncture confirmed the presence of cancer cells in the cerebral effusion, suggesting meningeal metastasis (Figure 3A). Second-generation CSF sequencing (OncoDrug-Seq sequencing platform: Illumina NextSeq500vaSeq) identified an EGFR21 L858R mutation with an abundance of 38% in combination with TP53 and FGFR3 mutations. Subsequently, the patient received a double dose of osimertinib (160 mg/day) for 2 months; however, there was no significant improvement in the dizziness or headache. Starting in November 25, 2021, the patient received an intrathecal injection of 30 mg pemetrexed, administered 1-2 times per week, 2-3 times per course and repeated once every 2-3 months. Additionally, the patient was prescribed one centrum tablet daily and continued targeted therapy with osimertinib (160 mg/day). Following one course of intrathecal chemotherapy, the symptoms of dizziness and headache were alleviated, and after three courses of intrathecal chemotherapy, the thickening and pronounced enhancement of the meninges in the cerebellum disappeared (Figures 2D–F), and CSF cytology was negative (Figure 3B). The patient underwent a total of 12 intrathecal injections of pemetrexed from November 2021 to November 2022. The patient has a good quality of life, and no disease progression is observed. Adverse drug reactions include grade 1 rash, grade 1 leukopenia, grade 1 thrombocytopenia, and fatigue. Considering the absence of evident neurological symptoms and negative CSF cytology, intrathecal pemetrexed chemotherapy is discontinued, and only 160 mg osimertinib targeted therapy is currently being utilized. The patient exhibites mild weakness of the lower limbs as the main clinical symptom with no apparent adverse drug reactions. Since the diagnosis of LM, the patient has survived for 28 months (Figure 4).
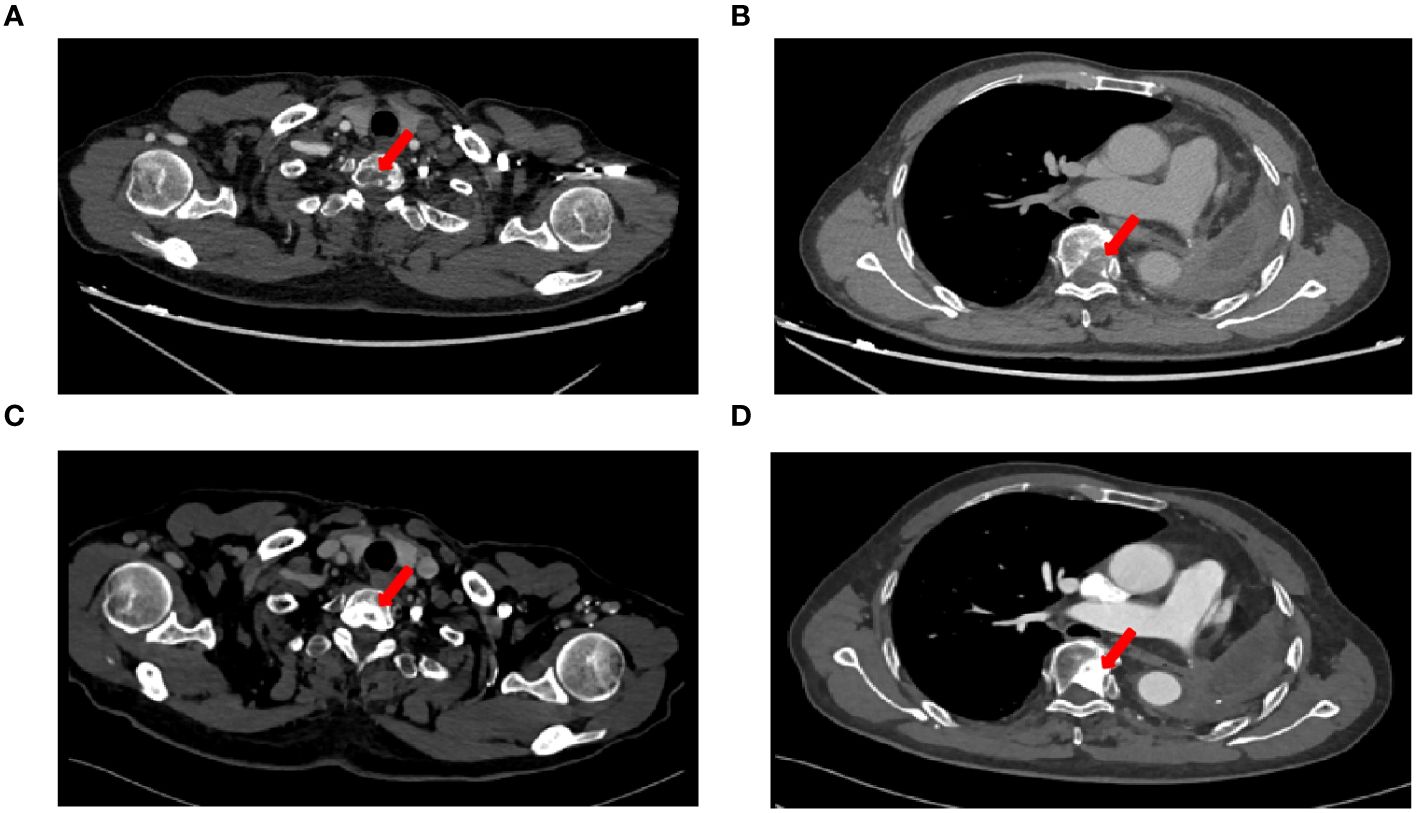
Figure 1 CT imaging changes before and after treatment. (A) Chest CT images on 23 July, 2019 showed T1 vertebral metastasis, (B) Chest CT images on 23 July, 2019 showed T6 vertebral metastasis, (C) Chest CT images showed osteogenic changes of T1 vertebral metastasis after radiotherapy, (D) Chest CT images showed osteogenic changes of T6 vertebral metastasis after radiotherapy.
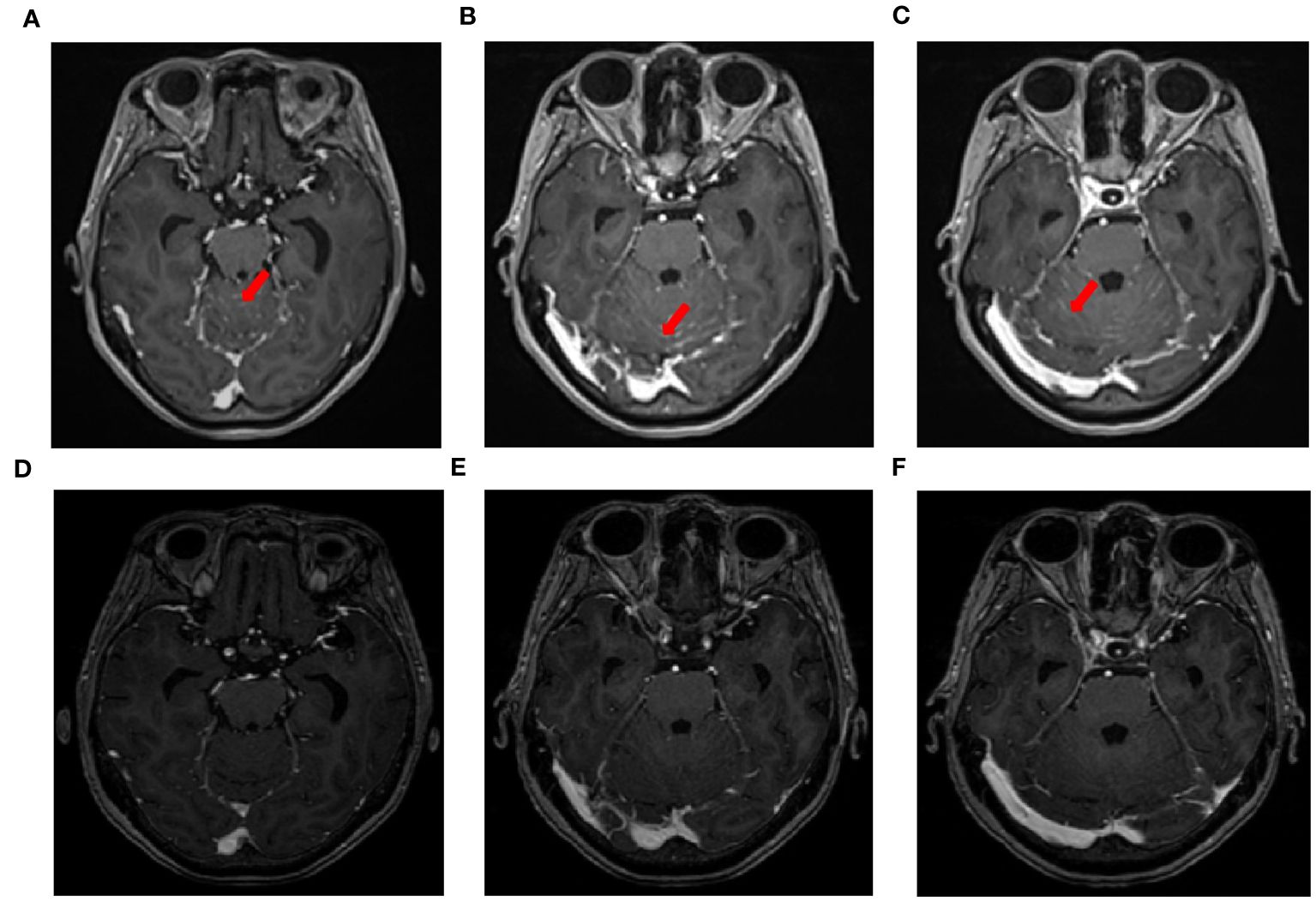
Figure 2 MRI changes in leptomeningeal metastasis before and after treatment. (A–C) MRI images of leptomeningeal metastasis in September 2021 showing significant thickening and pronounced enhancement of the meninges in the cerebellum. (D–F) After three courses of intrathecal chemotherapy, the thickening and pronounced enhancement of the meninges in the cerebellum disappeared.
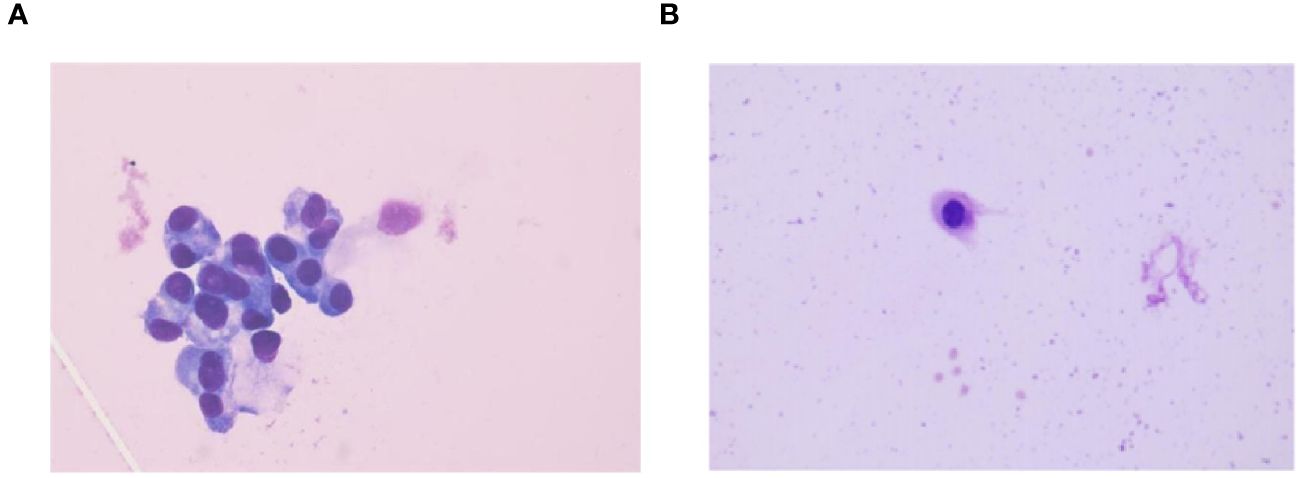
Figure 3 CSF cytology findings before and after treatment. (A) CSF cytology findings in September 2021 reveal tumor cells (HE staining,×100). (B) CSF cytology showing reactivity changes after three courses of intrathecal chemotherapy (HE staining,×100).
Patients with LM are primarily diagnosed based on clinical symptoms, MRI of the brain and spine, and CSF analysis. Typical cranial MRI enhancements include leptomeningeal, subependymal, dural, or cranial nerve enhancement; superficial cerebral lesions; and communicating hydrocephalus (8). The sensitivity and specificity of MRI for detecting LM in solid tumors are 70–87% and 75–94%, respectively (9, 10). In this patient, MRI revealed significant thickening and pronounced enhancement of the meninges in the cerebellum. The gold standard for LM diagnosis is the detection of tumor cells in the CSF. Initially, the CSF yielded a positive rate of approximately 50% upon the first examination, which increased to 80% with two consecutive tests. However, conducting more than three examinations within a short period does not increase the positivity rate (11). In addition, rapid in vitro fixation of lumbar puncture samples is crucial for enhancing the accuracy of the cytopathological diagnosis of CSF. An increasing number of molecular assays have been developed to enhance detection specificity and sensitivity. CSF analysis of epithelial cell adhesion molecules (EpCAMs), circulating tumor DNA (ctDNA), vascular endothelial growth factor (VEGF), and C3 mRNA can significantly improve the sensitivity and detection rate of LM (12–16).
Currently, no standard treatment options are available for lung cancer patients with LM. Common treatment options include radiotherapy, targeted therapy, immunotherapy, and chemotherapy, either as a monotherapy or in combination. However, the efficacy of these treatments is not ideal, resulting in a median survival time ranging from 3 to 11 months (2, 17). Radiotherapy is considered a pivotal therapy for LM, as it effectively diminishes the size of the LM, enhances CSF circulation, alleviates hydrocephalus, mitigates neurological symptoms, and ultimately ameliorates patients’ quality of life. Local radiotherapy is commonly employed to treat visible meningeal nodules, cranial nerve involvement at the skull base, and cauda equina syndrome. Whole brain radiotherapy (WBRT) is the primary treatment for patients with nodular leptomeningeal disease metastasis. However, the efficacy of WBRT remains unclear. Clinical studies have shown that the OS of patients who undergo WBRT and those who do not are 10.9 months and 2.4 months, respectively (P=0.002), which significantly prolonged the OS of patients (18). However, other studies have shown that WBRT can control craniocerebral symptoms but does not improve OS in patients with NSCLC-LM (19–21). As WBRT can lead to higher neurocognitive dysfunction, stereotactic radiosurgery or large-segmenting radiotherapy is currently preferred. WBRT can be considered in patients with neurological symptoms who are in good physical condition and ineligible for local stereotactic radiosurgery or large-segmenting radiotherapy (22). The general clinical consensus on the radiation dose and fractionation of WBRT is 30 Gy/10 fx and 40 Gy/20 fx. According to the guidelines of the National Comprehensive Cancer Network (NCCN), 37.5 Gy/15 fx was adopted as the fractionation method (23).
Targeted drugs have significantly improved the OS of patients with NSCLC harboring LM and driver gene mutations. The ability of small-molecule TKIs to penetrate the BBB is greater than that of large-molecule chemotherapy drugs. However, it should be noted that many TKIs are substrates of P-glycoprotein, which serves as a protein pump responsible for drug efflux at the BBB. Consequently, most TKIs still encounter limitations in traversing the BBB owing to their interaction with P-glycoproteins. Drugs, such as gefitinib, erlotinib, afatinib, and crizotinib, have low BBB penetration; therefore, the pia is a frequent site of disease progression in many TKIs. Studies have revealed that during treatment, a significant proportion of patients (up to 45%) receiving EGFR-TKI therapy develop central nervous system (CNS) metastases, encompassing both brain metastases and LM (4, 24). Osimertinib demonstrated superior CNS efficacy compared with platinum-pemetrexed in T790M-positive advanced NSCLC (25). A retrospective analysis of studies across the AURA program reported that the objective LM response rate to osimertinib (80 mg/day) was 55%. Median LM PFS and OS were 11.1 months and 18.8 months, respectively (26). In an EGFR mutant, PC9 mouse brain metastasis model, osimertinib demonstrated greater penetration than other first- or second-generation TKIs (27). Therefore, osimertinib is the preferred treatment for NSCLC patients with EGFR mutations with or without the T790M mutation. In the ALEX study, alectinib exhibited a median PFS of 25.4 months for patients with baseline CNS metastasis, compared to 7.4 months in the crizotinib group, resulting in a significant 40% reduction in the risk of mortality (28).
Immune checkpoint inhibitors (ICIs) have changed outcomes for locally advanced or metastatic NSCLC patients with negative driver genes by improving PFS, OS, and quality of life. Traditionally, the brain has been regarded as an immune-privileged organ; however, emerging evidence suggests that ICIs can penetrate the BBB when brain tumors or metastases disrupt their integrity, leading to alterations in the local tumor microenvironment (29). ICIs themselves do not directly target cancer cells; instead, they enhance the immune activity of T cells, facilitating their infiltration into the brain and pial metastasis tumors (30, 31), thereby exerting an immunotherapeutic effect. Owing to the exclusion of patients with LM in most clinical studies, limited data are available on the treatment of LM with ICIs. The brain response rate to Nivolumab in programmed death-1 (PD-1) positive NSCLC patients has been reported to be 33% (32). A retrospective analysis was conducted on a cohort of 1,288 patients with prior immunotherapy, among whom 19 (1.5%) had NSCLC-LM and received immunotherapy. The PFS rates at 6 and 12 months were 21.0% and 0%, respectively, and the OS rates were 36.8% and 21.1%, respectively (33). Combined immunotherapies have yielded promising results. A Phase II study investigated the efficacy of atezolizumab in combination with carboplatin and pemetrexed as first-line treatment for patients with initial brain metastases. This study included 40 patients with a median follow-up period of 30 months. The median intracranial PFS and median OS were 6.9 months and 11.8 months, respectively. The response rate was 42.7%, and the 2-year OS rate was 27.5% (34). Furthermore, clinical studies have supported the combination of immunotherapy and radiotherapy, demonstrating synergistic antitumor activities (35, 36). An initial Phase I trial (KEYNOTE-001) revealed that among 97 patients with advanced NSCLC who received pembrolizumab after radiotherapy, longer PFS and OS rates were observed, along with an acceptable safety profile (37).
Systemic chemotherapy is the primary treatment for driver gene mutation-negative NSCLC patients with meningeal metastasis. Pemetrexed, an anti-metabolic type of cancer drug, exerts its mechanism of action by inhibiting the synthesis of purines and pyrimidines through the blockade of three essential enzymes: thymidylate synthetase (TS), dihydrofolate reductase (DHFR), and glycinamide ribronucleotide formyltransferase(GARFT). This inhibition leads to cell cycle arrest in the S phase, effectively suppressing tumor cell growth (38). Pemetrexed demonstrates potent antitumor activity against non-squamous NSCLC. A retrospective study revealed that the median OS was 13.7 months for patients treated with pemetrexed after LM, compared to 4.0 months for those not treated with pemetrexed. Furthermore, multivariate analysis demonstrated that pemetrexed use after LM was independently associated with longer survival (39). However, because of the presence of the BBB, most chemotherapeutic drugs face challenges in penetrating the leptomeningeal cavity, thereby limiting their therapeutic efficacy. The key to treating LM is penetrating the BBB and reaching an effective concentration in the CSF. The intrathecal injection of chemotherapeutic agents avoids the “hurdle” posed by the BBB, directly delivers the drug to the subarachnoid space, and is considered the most direct treatment. Intrathecally injected drugs include cytarabine, methotrexate, and thiotepa; however, their therapeutic effects remain unsatisfactory. Professor Pan (40) was the first to carry out a phase I clinical study of intrathecal chemotherapy with pemetrexed as a salvage therapy for NSCLC-LM. The study results demonstrated that the clinical response rate to 10 mg pemetrexed was 31% (4/13), and the disease control rate was 54% (7/13). Moreover, this treatment exhibited excellent tolerability and offers a novel and efficacious therapeutic approach for patients with NSCLC-LM. The intravaginal administration of pemetrexel enables the drug to bypass the BBB and directly enter the CSF, achieving effective concentrations with minimal systemic toxicity. Similarly, Professor Xin Tao’s team (7) also conducted a phase I/II clinical trial to evaluate the efficacy of intrathecal chemotherapy with pemetrexed for LM. In the Phase I study, six patients were enrolled, and the dose of intrathecal pemetrexed was escalated from 15 mg to 80 mg, ultimately recommending a dose of 50 mg. Thirty patients diagnosed with EGFR mutation-positive LM-NSCLC were enrolled in the Phase II study, and 26 patients were included in the efficacy evaluation. Each patient received 2–12 intrathecal injections, resulting in a median OS of 9.0 months. The clinical response rate was 84.6% (22/26), with 2 patients achieving a complete response and 7 achieving a partial response. Most adverse events observed in all patients were mild and predominantly myelo-suppressed. This trial demonstrated the efficacy of intrathecal pemetrexel injections in patients with EGFR-mutant NSCLC-LM.
The management of LM should be personalized, based on clinical manifestations, imaging, and CSF analysis, to guide the systemic treatment plan in combination with radiotherapy, immunotherapy, targeted therapy, or intrathecal therapy. In this case, after the occurrence of meningeal metastases, monotherapy with osimertinib at a dosage of 160 mg did not yield significant improvements in the symptoms of dizziness and headache; however, when combined with intrathecal injection of pemetrexel, cranial symptoms were effectively controlled while exhibiting controllable toxicity. Given the patient’s advanced age, pemetrexed was intrathecally administered at a dose of 30 mg. The BLOOM study indicates that osimertinib 160 mg was generally well tolerated, and the adverse drug reactions included rash, acne, diarrhea, nausea, and paronychia and were predominantly grade 1-2 (5). Osimertinib (160 mg/day) combined with intrathecal injection of pemetrexed chemotherapy did not significantly increase the adverse drug events, and the adverse drug reactions mainly were grade 1 rash, grade 1 leukopenia, grade 1 thrombocytopenia, and fatigue. Since the diagnosis of LM, the patient has undergone 12 intrathecal injections and has survived for 28 months, experiencing a significantly improved quality of life. We will continue to follow-up the patient. Intrathecal injection of pemetrexed may be a promising treatment strategy for patients with NSCLC-LM.
The combination of double-dose osimertinib and an intrathecal injection of pemetrexed demonstrated meaningful therapeutic efficacy and manageable adverse effects in this advanced NSCLC patient with EGFR-mutant and LM. However, its efficacy and safety require further validation and more clinical data.
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary Material.
The studies involving humans were approved by Ethics Committee of the First Affiliated Hospital of Gannan Medical University. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
WZ: Conceptualization, Data curation, Writing – original draft, Writing – review & editing. LW: Methodology, Supervision, Writing – review & editing. LH: Formal Analysis, Writing – review & editing, Investigation. JW: Software, Writing – review & editing. HS: Supervision, Validation, Writing – review & editing. SW: Conceptualization, Methodology, Validation, Writing – review & editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2024.1377451/full#supplementary-material
Supplementary Material 1 | The trend of changes in CEA(ng/ml) in serum (normal levels, <5 ng/ml).
1. Cheng H, Perez-Soler R. Leptomeningeal metastases in non-small-cell lung cancer. Lancet Oncol. (2018) 19:e43–55. doi: 10.1016/S1470-2045(17)30689-7
2. Li YS, Jiang BY, Yang JJ, Tu HY, Zhou Q, Guo WB, et al. Leptomeningeal metastases in patients with NSCLC with EGFR mutations. J Thorac oncology: Off Publ Int Assoc Study Lung Cancer. (2016) 11:1962–9. doi: 10.1016/j.jtho.2016.06.029
3. Gainor JF, Ou SH, Logan J, Borges LF, Shaw AT. The central nervous system as a sanctuary site in ALK-positive non-small-cell lung cancer. J Thorac oncology: Off Publ Int Assoc Study Lung Cancer. (2013) 8:1570–3. doi: 10.1097/JTO.0000000000000029
4. Rangachari D, Yamaguchi N, VanderLaan PA, Folch E, Mahadevan A, Floyd SR, et al. Brain metastases in patients with EGFR-mutated or ALK-rearranged non-small-cell lung cancers. Lung Cancer (Amsterdam Netherlands). (2015) 88:108–11. doi: 10.1016/j.lungcan.2015.01.020
5. Yang JCH, Kim SW, Kim DW, Lee JS, Cho BC, Ahn JS, et al. Osimertinib in patients with epidermal growth factor receptor mutation-positive non-small-cell lung cancer and leptomeningeal metastases: the BLOOM study. J Clin oncology: Off J Am Soc Clin Oncol. (2020) 38:538–47. doi: 10.1200/JCO.19.00457
6. Sørensen JB. Pharmacokinetic evaluation of pemetrexed. Expert Opin Drug Metab toxicology. (2011) 7:919–28. doi: 10.1517/17425255.2011.587411
7. Fan C, Zhao Q, Li L, Shen W, Du Y, Teng C, et al. Efficacy and safety of intrathecal pemetrexed combined with dexamethasone for treating tyrosine kinase inhibitor-failed leptomeningeal metastases from EGFR-mutant NSCLC-a prospective, open-label, single-arm phase 1/2 clinical trial (Unique identifier: chiCTR1800016615). J Thorac oncology: Off Publ Int Assoc Study Lung Cancer. (2021) 16:1359–68. doi: 10.1016/j.jtho.2021.04.018
8. Freilich RJ, Krol G, DeAngelis LM. Neuroimaging and cerebrospinal fluid cytology in the diagnosis of leptomeningeal metastasis. Ann neurology. (1995) 38:51–7. doi: 10.1002/ana.410380111
9. Seong M, Park S, Kim ST, Park SG, Kim YK, Kim HJ, et al. Diagnostic accuracy of MR imaging of patients with leptomeningeal seeding from lung adenocarcinoma based on 2017 RANO proposal: added value of contrast-enhanced 2D axial T2 FLAIR. J neuro-oncology. (2020) 149:367–72. doi: 10.1007/s11060-020-03617-2
10. Singh SK, Leeds NE, Ginsberg LE. MR imaging of leptomeningeal metastases: comparison of three sequences. AJNR Am J neuroradiology. (2002) 23:817–21.
11. Le Rhun E, Massin F, Tu Q, Bonneterre J, Bittencourt Mde C, Faure GC. Development of a new method for identification and quantification in cerebrospinal fluid of Malignant cells from breast carcinoma leptomeningeal metastasis. BMC Clin pathology. (2012) 12:21. doi: 10.1186/1472-6890-12-21
12. Thakkar JP, Kumthekar P, Dixit KS, Stupp R, Lukas RV. Leptomeningeal metastasis from solid tumors. J neurological Sci. (2020) 411:116706. doi: 10.1016/j.jns.2020.116706
13. Milojkovic Kerklaan B, Pluim D, Bol M, Hofland I, Westerga J, van Tinteren H, et al. EpCAM-based flow cytometry in cerebrospinal fluid greatly improves diagnostic accuracy of leptomeningeal metastases from epithelial tumors. Neuro-oncology. (2016) 18:855–62. doi: 10.1093/neuonc/nov273
14. Li YS, Jiang BY, Yang JJ, Zhang XC, Zhang Z, Ye JY, et al. Unique genetic profiles from cerebrospinal fluid cell-free DNA in leptomeningeal metastases of EGFR-mutant non-small-cell lung cancer: a new medium of liquid biopsy. Ann oncology: Off J Eur Soc Med Oncol. (2018) 29:945–52. doi: 10.1093/annonc/mdy009
15. Sharma A, Low JT, Kumthekar P. Advances in the diagnosis and treatment of leptomeningeal disease. Curr Neurol Neurosci Rep. (2022) 22:413–25. doi: 10.1007/s11910-022-01198-3
16. Boire A, Zou Y, Shieh J, Macalinao DG, Pentsova E, Massagué J. Complement component 3 adapts the cerebrospinal fluid for leptomeningeal metastasis. Cell. (2017) 168:1101–13.e13. doi: 10.1016/j.cell.2017.02.025
17. Remon J, Le Rhun E, Besse B. Leptomeningeal carcinomatosis in non-small cell lung cancer patients: A continuing challenge in the personalized treatment era. Cancer Treat Rev. (2017) 53:128–37. doi: 10.1016/j.ctrv.2016.12.006
18. Liao BC, Lee JH, Lin CC, Chen YF, Chang CH, Ho CC, et al. Epidermal growth factor receptor tyrosine kinase inhibitors for non-small-cell lung cancer patients with leptomeningeal carcinomatosis. J Thorac oncology: Off Publ Int Assoc Study Lung Cancer. (2015) 10:1754–61. doi: 10.1097/JTO.0000000000000669
19. Kuiper JL, Hendriks LE, van der Wekken AJ, de Langen AJ, Bahce I, Thunnissen E, et al. Treatment and survival of patients with EGFR-mutated non-small cell lung cancer and leptomeningeal metastasis: A retrospective cohort analysis. Lung Cancer (Amsterdam Netherlands). (2015) 89:255–61. doi: 10.1016/j.lungcan.2015.05.023
20. Mulvenna P, Nankivell M, Barton R, Faivre-Finn C, Wilson P, McColl E, et al. Dexamethasone and supportive care with or without whole brain radiotherapy in treating patients with non-small cell lung cancer with brain metastases unsuitable for resection or stereotactic radiotherapy (QUARTZ): results from a phase 3, non-inferiority, randomised trial. Lancet (London England). (2016) 388:2004–14. doi: 10.1016/S0140-6736(16)30825-X
21. Morris PG, Reiner AS, Szenberg OR, Clarke JL, Panageas KS, Perez HR, et al. Leptomeningeal metastasis from non-small cell lung cancer: survival and the impact of whole brain radiotherapy. J Thorac oncology: Off Publ Int Assoc Study Lung Cancer. (2012) 7:382–5. doi: 10.1097/JTO.0b013e3182398e4f
22. Glatzer M, Faivre-Finn C, De Ruysscher D, Widder J, Van Houtte P, Troost EGC, et al. Role of radiotherapy in the management of brain metastases of NSCLC - Decision criteria in clinical routine. Radiotherapy oncology: J Eur Soc Ther Radiol Oncol. (2021) 154:269–73. doi: 10.1016/j.radonc.2020.10.043
23. Shi Y, Sun Y, Yu J, Ding C, Ma Z, Wang Z, et al. [China experts consensus on the diagnosis and treatment of brain metastases of lung cancer (2017 version)]. Zhongguo fei ai za zhi = Chin J Lung cancer. (2017) 20:1–13. doi: 10.3779/j.issn.1009-3419.2017.01.01
24. Wu YL, Zhao Q, Deng L, Zhang Y, Zhou XJ, Li YY, et al. Leptomeningeal metastasis after effective first-generation EGFR TKI treatment of advanced non-small cell lung cancer. Lung Cancer (Amsterdam Netherlands). (2019) 127:1–5. doi: 10.1016/j.lungcan.2018.11.022
25. Wu YL, Ahn MJ, Garassino MC, Han JY, Katakami N, Kim HR, et al. CNS efficacy of osimertinib in patients with T790M-positive advanced non-small-cell lung cancer: data from a randomized phase III trial (AURA3). J Clin oncology: Off J Am Soc Clin Oncol. (2018) 36:2702–9. doi: 10.1200/JCO.2018.77.9363
26. Ahn MJ, Chiu CH, Cheng Y, Han JY, Goldberg SB, Greystoke A, et al. Osimertinib for patients with leptomeningeal metastases associated with EGFR T790M-positive advanced NSCLC: the AURA leptomeningeal metastases analysis. J Thorac oncology: Off Publ Int Assoc Study Lung Cancer. (2020) 15:637–48. doi: 10.1016/j.jtho.2019.12.113
27. Ballard P, Yates JW, Yang Z, Kim DW, Yang JC, Cantarini M, et al. Preclinical comparison of osimertinib with other EGFR-TKIs in EGFR-mutant NSCLC brain metastases models, and early evidence of clinical brain metastases activity. Clin Cancer research: an Off J Am Assoc Cancer Res. (2016) 22:5130–40. doi: 10.1158/1078-0432.CCR-16-0399
28. Mok T, Camidge DR, Gadgeel SM, Rosell R, Dziadziuszko R, Kim DW, et al. Updated overall survival and final progression-free survival data for patients with treatment-naive advanced ALK-positive non-small-cell lung cancer in the ALEX study. Ann oncology: Off J Eur Soc Med Oncol. (2020) 31:1056–64. doi: 10.1016/j.annonc.2020.04.478
29. Fecci PE, Ochiai H, Mitchell DA, Grossi PM, Sweeney AE, Archer GE, et al. Systemic CTLA-4 blockade ameliorates glioma-induced changes to the CD4+ T cell compartment without affecting regulatory T-cell function. Clin Cancer research: an Off J Am Assoc Cancer Res. (2007) 13:2158–67. doi: 10.1158/1078-0432.CCR-06-2070
30. Berghoff AS, Fuchs E, Ricken G, Mlecnik B, Bindea G, Spanberger T, et al. Density of tumor-infiltrating lymphocytes correlates with extent of brain edema and overall survival time in patients with brain metastases. Oncoimmunology. (2016) 5:e1057388. doi: 10.1080/2162402X.2015.1057388
31. Zeng J, See AP, Phallen J, Jackson CM, Belcaid Z, Ruzevick J, et al. Anti-PD-1 blockade and stereotactic radiation produce long-term survival in mice with intracranial gliomas. Int J Radiat oncology biology physics. (2013) 86:343–9. doi: 10.1016/j.ijrobp.2012.12.025
32. Goldberg SB, Gettinger SN, Mahajan A, Chiang AC, Herbst RS, Sznol M, et al. Pembrolizumab for patients with melanoma or non-small-cell lung cancer and untreated brain metastases: early analysis of a non-randomised, open-label, phase 2 trial. Lancet Oncol. (2016) 17:976–83. doi: 10.1016/S1470-2045(16)30053-5
33. Hendriks LEL, Bootsma G, Mourlanette J, Henon C, Mezquita L, Ferrara R, et al. Survival of patients with non-small cell lung cancer having leptomeningeal metastases treated with immune checkpoint inhibitors. Eur J Cancer (Oxford England: 1990). (2019) 116:182–9. doi: 10.1016/j.ejca.2019.05.019
34. Nadal E, Rodríguez-Abreu D, Simó M, Massutí B, Juan O, Huidobro G, et al. Phase II trial of atezolizumab combined with carboplatin and pemetrexed for patients with advanced nonsquamous non-small-cell lung cancer with untreated brain metastases (Atezo-brain, GECP17/05). J Clin oncology: Off J Am Soc Clin Oncol. (2023) 41:4478–85. doi: 10.1200/JCO.22.02561
35. Herter-Sprie GS, Koyama S, Korideck H, Hai J, Deng J, Li YY, et al. Synergy of radiotherapy and PD-1 blockade in Kras-mutant lung cancer. JCI Insight. (2016) 1:e87415. doi: 10.1172/jci.insight.87415
36. De Ruysscher D. Radiotherapy and PD-L1 inhibition in metastatic NSCLC. Lancet Oncol. (2017) 18:840–2. doi: 10.1016/S1470-2045(17)30354-6
37. Shaverdian N, Lisberg AE, Bornazyan K, Veruttipong D, Goldman JW, Formenti SC, et al. Previous radiotherapy and the clinical activity and toxicity of pembrolizumab in the treatment of non-small-cell lung cancer: a secondary analysis of the KEYNOTE-001 phase 1 trial. Lancet Oncol. (2017) 18:895–903. doi: 10.1016/S1470-2045(17)30380-7
38. Rollins KD, Lindley C. Pemetrexed: a multitargeted antifolate. Clin Ther. (2005) 27:1343–82. doi: 10.1016/j.clinthera.2005.09.010
39. Choi M, Keam B, Ock CY, Kim M, Kim TM, Kim DW, et al. Pemetrexed in the treatment of leptomeningeal metastasis in patients with EGFR-mutant lung cancer. Clin Lung cancer. (2019) 20:e442–e51. doi: 10.1016/j.cllc.2019.03.005
Keywords: non-small cell lung cancer, leptomeningeal metastasis, osimertinib, pemetrexed, intrathecal chemotherapy
Citation: Zhong W, Wu L, Huang L, Wang J, Shi H and Wu S (2024) Double-dose osimertinib combined with intrathecal injection of pemetrexed improves the efficacy of EGFR-mutant non-small cell lung cancer and leptomeningeal metastasis: case report and literature review. Front. Oncol. 14:1377451. doi: 10.3389/fonc.2024.1377451
Received: 27 January 2024; Accepted: 08 April 2024;
Published: 22 April 2024.
Edited by:
Francesco Pepe, University of Naples Federico II, ItalyReviewed by:
Rachel E. Sanborn, Earle A. Chiles Research Institute, United StatesCopyright © 2024 Zhong, Wu, Huang, Wang, Shi and Wu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shugui Wu, amRkeGYyMDEyQDEyNi5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.