- 1Adult Bone Marrow Transplant Service, Memorial Sloan Kettering Cancer Center, New York, NY, United States
- 2Cellular Therapy Service, Memorial Sloan Kettering Cancer Center, New York, NY, United States
- 3Department of Hematologic Oncology and Blood Disorders, Levine Cancer Institute, Atrium Health/Wake Forest Baptist, Charlotte, NC, United States
Chimeric antigen receptor (CAR) T cell therapy has revolutionized the management of relapsed and refractory myeloma, with excellent outcomes and a tolerable safety profile. High dose chemotherapy with autologous hematopoietic stem cell transplantation (AHCT) is established as a mainstream of newly diagnosed multiple myeloma (NDMM) management in patients who are young and fit enough to tolerate such intensity. This standard was developed based on randomized trials comparing AHCT to chemotherapy in the era prior to novel agents. More recently, larger studies have primarily shown a progression free survival (PFS) benefit of upfront AHCT, rather than overall survival (OS) benefit. There is debate about the significance of this lack of OS, acknowledging the potential confounders of the chronic nature of the disease, study design and competing harms and benefits of exposure to AHCT. Indeed upfront AHCT may not be as uniquely beneficial as we once thought, and is not without risk. New quadruple-agent regimens are highly active and effective in achieving a deep response as quantified by measurable residual disease (MRD). The high dose chemotherapy administered with AHCT imposes a burden of short and long-term adverse effects, which may alter the disease course and patient’s ability to tolerate future therapies. Some high-risk subgroups may have a more valuable benefit from AHCT, though still ultimately suffer poor outcomes. When compared to the outcomes of CAR T cell therapy, the question of whether AHCT can or indeed should be deferred has become an important topic in the field. Deferring AHCT may be a personalized decision in patients who achieve MRD negativity, which is now well established as a key prognostic factor for PFS and OS. Reserving or re-administering AHCT at relapse is feasible in many cases and holds the promise of resetting the T cell compartment and opening up options for immune reengagement. It is likely that personalized MRD-guided decision making will shape how we sequence in the future, though more studies are required to delineate when this is safe and appropriate.
1 Introduction
Multiple myeloma (MM) is a cancer of terminally differentiated plasma cells in the bone marrow. For 2023, an estimated 35,730 new cases will be diagnosed in the United States which represents 1.8% of all cancers and 19% of all hematologic malignancies (1). The advent of high doses of melphalan with autologous hematopoietic stem cell transplantation (AHCT) was a major advance and led to improved response rates (RR), progression free survival (PFS), and, in some trials, prolonged overall survival (OS) in patients with newly diagnosed MM (NDMM) and has been a cornerstone of treatment in eligible patients for the last 20 years (2–6). While there are no universally agreed upon transplant eligibility criteria and several risk stratification tools have been proposed, factors such as age, baseline performance status, and comorbidities are important tenets in determining a patient’s eligibility (7–10). Recently, adoptive cell therapy using BCMA-directed autologous chimeric antigen receptor (CAR) T therapies have been tested in patients with relapsed refractory multiple myeloma (RRMM) and have shown unprecedented response rates, depth of response, and improved PFS when compared to standard of care (SOC) regimens (11–14). Currently, CAR T cells are under investigation for use as consolidation after induction therapy in transplant ineligible patients and are being compared head-to-head against high-dose melphalan and AHCT in large phase III trials of transplant eligible patients (15, 16). While these trials are yet to report, there is significant reason to believe that CAR T therapy may lead to improved outcomes as T-cell fitness - which is a prime driver of CAR T success - has been shown to decline with increasing lines of MM directed treatment (17–20). This also leads to the question of whether these therapies should be sequenced or are mutually exclusive. In this manuscript, we review the current data for each of these modalities and discuss trials currently evaluating AHCT and CAR Ts for patients with MM. Finally, we provide our thoughts on the role of each of these treatments in MM therapy in the United States where both options are commercially available.
2 CAR T in MM
CAR T cell therapies have revolutionized the treatment of RRMM. Most CAR T target B cell maturation antigen (BCMA) also known as TNFRSF17 or CD269; a type III transmembrane glycoprotein and non-tyrosine kinase receptor in the tumor necrosis factor receptor (TNFR) superfamily (21, 22). Expression of BCMA is selectively induced during plasma cell differentiation. Expression is nearly absent on naïve and memory B cells but is ubiquitously expressed on plasmablasts and plasma cells (23–25). BCMA expression is rare in other tissues with only low-level BCMA mRNA and protein expression seen in areas with endogenous plasma cell populations (i.e., the testes, gastrointestinal tract and trachea) (25). Additionally, expression of plasma cell BCMA increases with progression from monoclonal gammopathy of undermaintained significance (MGUS), to smoldering multiple myeloma (SMM) and MM. Higher levels of soluble BCMA has been associated with shorter time to progression in MGUS and SMM patients, and higher levels surface BCMA is associated with worse prognosis in MM patients (26–31). Several different modalities (antibody drug conjugates, bispecific antibodies, and CAR T) have been designed to target BCMA. Two BCMA CAR T: idecabtagene vicleucel (ide-cel) and ciltacabtagene autoleucel (cilta-cel) have been FDA approved for RRMM based on outcomes seen in large phase 2 trials.
2.1 Idecabtagene vicleucel
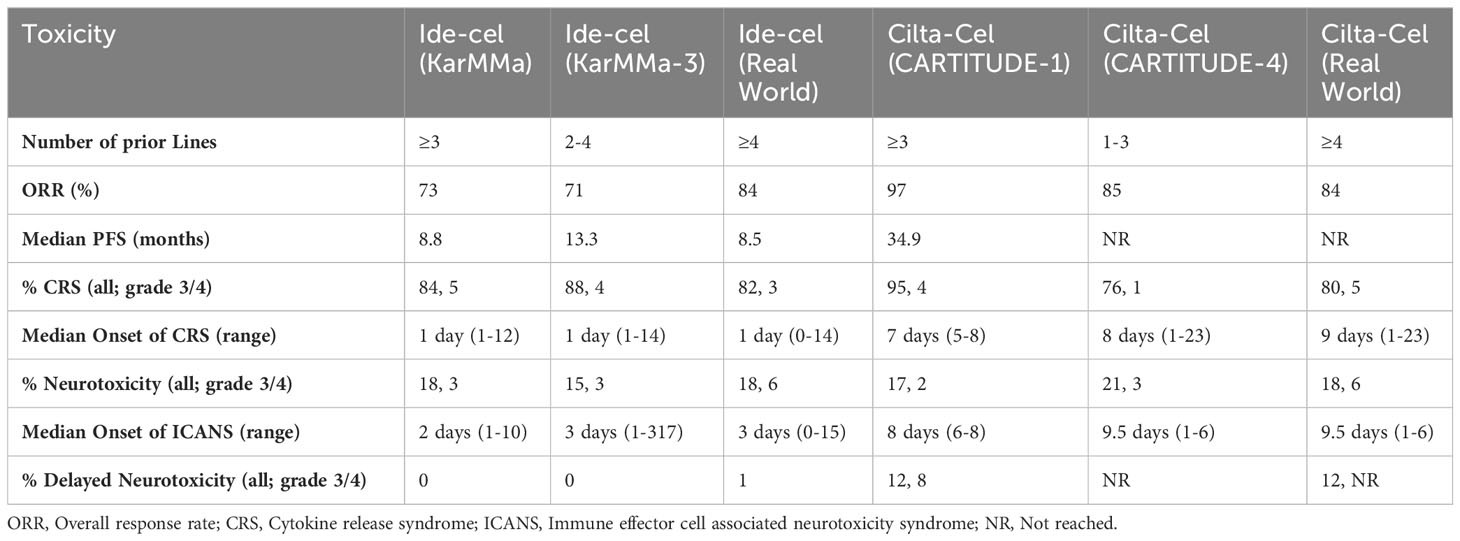
Ide-cel is a second-generation CAR which uses a lentiviral vector to transduce a BCMA targeting scFv fused to a 4-1BB co-stimulatory and CD3 signaling domains (32, 33). The pivotal phase II KarMMa study evaluated ide-cel at various doses in 128 RRMM patients who had previously received ≥ 3 prior lines of therapy including an immunomodulatory drug (IMiD), proteasome inhibitor (PI), and an anti-CD38 monoclonal antibody. Patients were infused with 150×106 to 450×106 CAR T cells. Overall response rate (ORR) was 73%, with 42 (33%) patients achieving a complete response (CR) or better. Measurable residual disease (MRD) negativity (at 10-5) was achieved in 26% of all patients, but 79% of patients who achieved a ≥ CR. Notably, these response rates were relatively preserved across patients with high-risk features including penta-refractory disease, extramedullary disease, and high-risk cytogenetics. Median PFS was 8.8 months, but was 20.2 months in patients who achieved ≥ CR (11). OS for the KarMMa study was 24.8 months, but interestingly, was lower in the cohort of patients who had previously been treated with 3 prior lines (median OS 22 months) versus those who had been treated with 4 or more prior lines (median OS 25.2 months) (34). Based on these findings, ide-cel was approved for the treatment of adults with RRMM after four or more prior lines of therapy including an IMiD, PI, and anti-CD38 monoclonal antibody by the US Food and Drug Administration (FDA) in March 2021. In a post-hoc analysis of the KarMMa trial, lower levels of serum soluble BCMA, more robust blood and bone marrow CAR T expansion, and an increased ratio of naive and early memory CD4 T cells compared to senescent CD3 and CD8 T cells in the apheresis product prior to manufacturing were associated with improved response to ide-cel (35). Subsequently, real-world data for patients treated with commercial ide-cel outside the context of a clinical trial showed very similar efficacy. ORR in this population was 84% with 42% achieving ≥ CR. Median PFS (8.5 months) at 6.1 months of follow-up was similar to the KarMMa data. Notably, 75% of the patients included in this cohort would have been deemed ineligible to enroll on the KarMMa trial (36). Predictors of poor response to ide-cel in the real-world cohort included prior BCMA therapy, high-risk cytogenetics, elevated baseline ferritin level, and younger age (36, 37).
More recently ide-cel was evaluated against investigator’s choice of one of 5 SOC regimens: daratumumab, pomalidomide, and dexamethasone; daratumumab, bortezomib, and dexamethasone; ixazomib, lenalidomide, and dexamethasone; carfilzomib and dexamethasone; or elotuzumab, pomalidomide, and dexamethasone in RRMM patients who had received 2-4 prior lines of therapy including an IMiD, PI, and an anti-CD38 monoclonal antibody in the phase III KarMMa-3 trial. This trial enrolled a substantial population (43%) of patients with high-risk cytogenetics (defined as presence of del17p, t[4;14], or t[14;16]) which were evenly distributed across both arms. The ORR was 71% for ide-cel and 42% for SOC. Median PFS in the intention-to-treat population was substantially higher in the ide-cel arm (13.3 months vs 4.4 months). The hazard ratio for disease progression or death for ide-cel vs SOC was 0.49 (95% CI 0.38 - 0.65). Ide-cel showed similar improved hazard ratios for disease progression or death in patients with high-risk cytogenetics (HR 0.61; 95% CI 0.41-0.90), extramedullary disease (HR 0.40; 95% CI 0.25-0.65), and disease refractory to at least one IMiD, PI, and anti-CD38 monoclonal antibody (HR 0.46; 95% CI 0.34-0.62) (14, 38). The FDA is currently reviewing a supplemental biologics license for the approval of ide-cel in this less heavily pretreated population based on the data from the KarMMa-3 trial. Additionally, cohort 2c of the phase II KarMMa-2 trial is evaluating the efficacy of ide-cel in patients with newly diagnosed MM (NDMM) who had an inadequate response to frontline AHCT. In this population, 77% of patients achieved ≥ CR, and median PFS has not be reached at a median follow-up of 39.4 months (39).
2.2 Ciltacabtagene autoleucel
Similar to ide-cel, cilta-cel uses a lentiviral vector to create a construct with a CD3ζ activation domain, and 4-1BB costimulatory domain. Cilta-cel’s antigen binding domain contains bispecifc scFvs targeting two distinct BCMA epitopes, VHH1 and VHH2 (40). This bi-epitope binding confers higher avidity and specificity to BCMA. Cilta-cel was evaluated in the phase Ib/II CARTITUDE-1 trial RRMM patients who had previously been treated with ≥ 3 or were double refractory to an IMiD and a PI, and had previously received an anti-CD38 antibody. Ninety-seven patients were treated with cilta-cel (29 in the phase Ib portion, 68 in the phase II portion). The population was heavily pretreated (median of 6 prior lines; 84% penta-exposed) and included 42% who were penta-refractory (12). ORR was 98%, with 95% of patients achieving a VGPR or better and 82.5% of patients achieving a sCR. MRD negativity was evaluated in 61 patients at 10-5 and 52 patients at 10-6. MRD negativity rates were 92% and 75% at 10-5 and 10-6 respectively (41). Median PFS was 34.9 months; median OS has not been reached at 27.7 months of follow-up (42). Based on these data cilta-cel was approved for the treatment RRMM patients following 4 or more prior lines of therapy, including an IMiD, PI, and an anti-CD38 monoclonal antibody in Feb 2022. Real-world data for cilta-cel is not as mature as that for ide-cel. However, in an early analysis a multi-institutional cohort of 143 patients infused with commercial cilta-cel-of whom 57% would have been ineligible for participation in the CARTITUDE-1 trial-ORR was 84% with 53% ≥ CR. Notably, 22% of patients included in this dataset were infused with an out of specification (OOS) cilta-cel product. The presence of high-risk cytogenetics (defined as the presence of del17p, t[4;14)], or t[14;16]) was associated with poorer ORR, PFS, and OS in multivariate analysis (43).
Cilta-cel was also evaluated in less heavily pretreated patients in the phase III CARTITUDE-4 trial. This trial randomized 419 RRMM patients previously treated with 1-3 prior lines to either cilta-cel or investigator’s choice of 2 SOC regimens: pomalidomide, bortezomib, dexamethasone, or daratumumab, pomalidomide, dexamethasone. The trial strongly favored the cilta-cel arm with ORR of 84.6% vs 67.3% for SOC. Responses were deeper in the cilta-cel arm (73.1% vs 21.8% ≥ CR) which translated into improved PFS (median PFS not reached for cilta-cel, 11.8 months for SOC; HR 0.26; 95% CI, 0.18 -0.38). Subgroup analysis also favored cilta-cel for all parameters tested including high-risk cytogenetics, prior lines of therapy, degree of refractoriness, and presence of extramedullary disease (13, 44). Cilta-cel is currently under evaluation for patient with suboptimal response to frontline transplant, in treatment naïve high-risk and standard risk NDMM in cohorts D, E, F of the mutli-cohort CARTITUDE-2 trial.
2.3 CAR T toxicities
While these data illustrate the efficacy of both ide-cel and cilta-cel, both of these agents have important toxicities that need to be factored into any suitability discussion. Cytokine release syndrome (CRS) is a systemic inflammatory response thought to be secondary to activation of bystander immune and nonimmune cells resulting in significant cytokine release-especially IL-1, IL-2, IL-6, GM-CSF, and IFN-g (45–48). This inflammatory storm typically manifests as fever, fatigue, headache, arthralgias, and myalgias, but higher-grade manifestations including hypotension, shock, disseminated intravascular coagulation, and multiorgan system failure can occur (49, 50). Treatment of CRS ranges from supportive care with antipyretics, intravenous fluids, and supplemental oxygen for lower grade symptoms to vasopressors, the anti-IL-6 antibody tocilizumab, and high-dose corticosteroids for higher grade symptoms (50, 51). While low grade CRS is very common with both ide-cel and cilta-cel the timing of CRS onset varies for each CAR-T product (see Table 1). Ide-cel was associated with 84% CRS in the KarMMa trial with the majority being low grade (5% grade 3 or 4). Median onset of CRS with ide-cel in the KarMMa trial, the KarMMa-3 trial, and the real-world dataset was reliably 1 day (range 0-14) (11, 14, 36). In the CARTITUDE-1 trial 95% of patients experienced CRS with 4% grade 3 or 4. Median onset of CRS with cilta-cel in the CARTITUDE-1 trial was 7 days, while it was 8 days in the CARTITUDE-4 trial, and 9 days in the real-world cohort (12, 13, 43). These data are summarized in Table 1.
Immune effector cell-associated neurotoxicity syndrome (ICANS) is another adverse effect common to CAR-T therapy. ICANS is a toxic encephalopathy thought to be related to endothelial cell activation and disruption of the blood brain barrier mediated by inflammatory cytokines and chemokines, which results in direct neuronal cell injury. Mild symptoms include headache, confusion, focal neurologic deficits, and impaired fine motor skills. Higher grade ICANS can manifest with aphasia, seizure, cerebral edema, and coma (49). The mainstay of ICANS management is high-dose corticosteroids; additional supportive measures including mechanical ventilation (if evidence of airway compromise) may be needed. Tocilizumab is generally only used if patients have coexisting CRS (50, 51). ICANS typically manifests later than CRS (around day 2-3 with ide-cel and days 8-10 with cilta-cel) (11–14, 36, 43). An additional neurologic toxicity seemingly unique to cilta-cel is less well understood. Termed movement and neurocognitive treatment-emergent adverse events (MNTs), they compromise a cluster of movement (e.g., micrographia, tremors), cognitive (e.g., memory loss, disturbance in attention), and personality changes (e.g. reduced facial expression, flat affect) which typically manifest after symptoms of CRS and ICANS have resolved, and unlike ICANS, are generally not responsive to steroids (52, 53). While the unique AEs associated with CAR-Ts are certainly cause for concern, with increasing ubiquity of this class of therapy and earlier recognition of, and intervention for, CRS and ICANS the rates of higher-grade AEs are decreasing in more recent trials (summarized in Table 1). Ideally, less high-grade CRS and ICANS will lead to less delayed neurotoxicity and MNT events.
Finally, the risk of secondary primary malignancies, long known to be an adverse effect of several myeloma therapies is largely unknown post-CAR T. However, recent data does raise concern for a possible increased risk. Specifically, rare reports of T-cell lymphomas derived from CAR T cells have been reported in several CAR T recipients. To date, only 12 cases have been reported out of the 7946 patients infused with CAR Ts, indicated predominately for B-cell lymphomas (54). Only 1 case has been reported in a myeloma patient who was treated with cilta-cel, but analysis of the patient’s apheresis product (prior to CAR T manufacture) suggested that they had several genetic mutations present at baseline and the role of the CAR is unclear (55). Similarly, myeloid malignancies have been reported with post CAR T. In the long-term follow-up of the CARTITUDE-1 trial 9 patients (9%) have been diagnosed with MDS or AML (42). However, it is unclear whether this a result of the CAR T or lymphodepleting chemotherapy. To that effect, a recent analysis of 4 patients who developed MDS after treatment with an investigational anti-BCMA CAR T showed that while none of the patients had morphologic changes consistent with MDS prior to CAR T infusion, all four patients exhibited molecular alterations associated with MDS in their pre-CAR T as well as post-CAR T therapy bone marrow with no new mutations observed after CAR T (56). Clearly further follow-up is warranted.
3 Autologous hematopoietic stem cell transplant
High-dose chemotherapy with melphalan followed by AHCT is well established as standard of care for patients with NDMM who are sufficiently fit to tolerate intensive therapy, ie, are transplant eligible. The principle of this practice is to induce disease control and collect a clean stem cell product following a limited induction, then administer powerful anti-myeloma therapy which would only be feasible with a stem cell rescue. It was theorized and then confirmed that this would provide a PFS benefit if offered early in the treatment course, as opposed to deferring it until a later relapse. Foundational studies showed improved PFS and OS and were a gamechanger in the natural history of myeloma (2, 57, 58). Short-term treatment related adverse events include obligate cytopenias, as well as infections, gastrointestinal upset, and mucositis (5, 59, 60). Despite these acute effects, treatment-related mortality remains low, and though quality of life is impacted in the short-term, these effects appear to be transient and recover post AHCT (59, 61, 62).
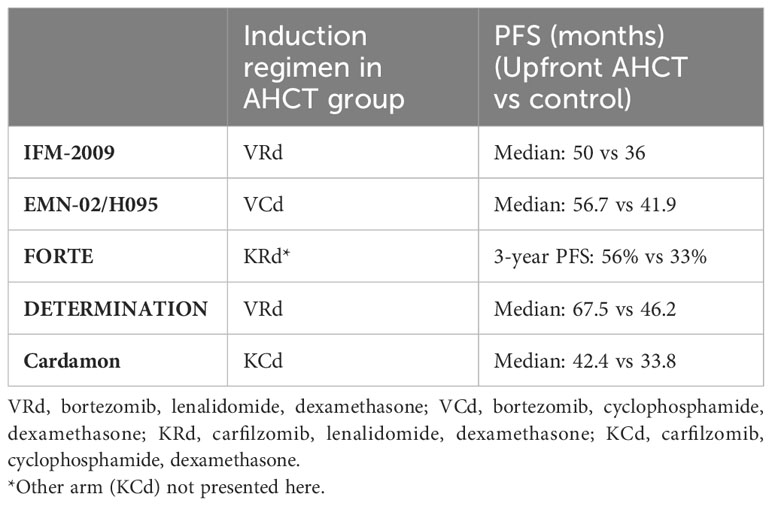
When PI-IMiD combination therapy became standard care (63), new studies were required to update our understanding of the true benefit of upfront AHCT with modern regimens and are summarized in Table 2. The IFM 2009 trial published in 2017 (5), included induction with three cycles of lenalidomine, bortezomib, dexamethasone (RVd) and 1 year of maintenance lenalidomide after consolidation and demonstrated a PFS benefit, which was sustained in the updated long-term follow up data (64). No difference in OS was noted at 4 years. The DETERMINATION trial included maintenance until disease progression and demonstrated a greater PFS than its IFM precursor, and confirmed the PFS benefit of AHCT when added to RVd, but again did not show an OS advantage at 72 months follow-up (59). The FORTE study looked at new generation PI carfilzomib(K)-based regimens as induction and confirmed the PFS benefit of upfront AHCT with both KRd and KCd induction (65). In the CARDAMON trial published in 2022, investigators determined that PFS of KCd alone was not non-inferior to upfront AHCT following KCd induction at 2 years (66).
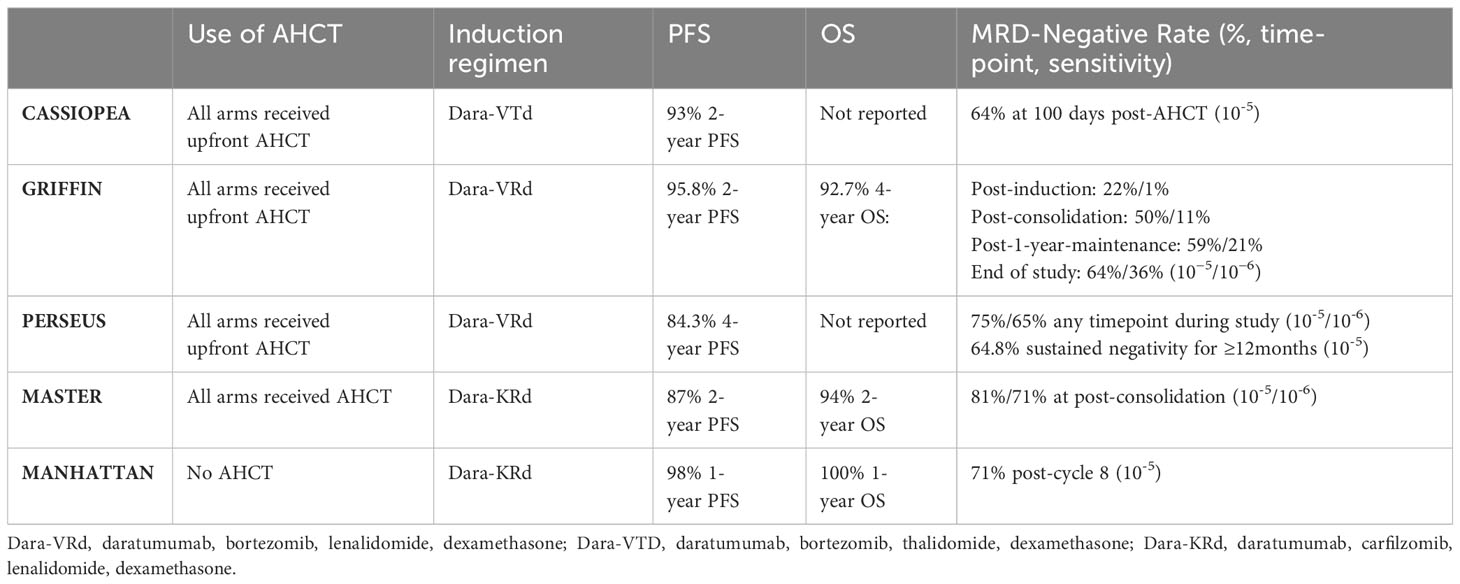
Newer studies which have included the addition of anti-CD38 antibody agents to a triplet backbone have led to deep responses when used together with AHCT and are summarized in Table 3. Of note, none of these quad-therapy studies have compared upfront AHCT to a deferred- or non-AHCT control arm. In CASSIOPEA, Dara-DTd plus AHCT induced excellent response rates and MRD negativity compared to DTd plus AHCT alone, with a 93% 2-year PFS in the quad-containing arm (67). The phase II GRIFFIN trial tested Dara-VRd as induction with AHCT and showed similarly excellent 2-year PFS at 95.8% (68). The recently published phase III PERSEUS trial compared Dara-VRd vs VRd in induction and post-AHCT consolidation, and demonstrated a significant improvement in PFS with the addition of Dara (84.3% 4-year PFS, vs 67.7% in the VRd only group) (6). Importantly PERSEUS reported a powerful depth of response with 75.2% of patients in the D-VRd group achieving MRD-negativity. Similarly, the phase III IsKia trial compared the combination of Isatuximab-KRd to KRd alone with AHCT and consolidation showed significant improvement in MRD negativity rates with the quadruplet (MRD at 10-5 77% vs 67%; MRD at 10-6 67% vs 48% respectively) (69). It is important to consider whether the benefits seen from these therapies are attributable more to the addition of the anti-CD38 agent itself, as opposed to the quad-agent nature of combination. Indeed, more drugs are not necessarily better, as demonstrated in trials of other four-drug inductions outside the anti-CD38 setting, suggesting they may perform as well as three-drug combinations (70). Additionally, the MAIA trial showed a significant PFS and OS with the addition of daratumumab to Rd in transplant ineligible patients (71), though this abridged regimen has not been studied in the AHCT setting.
3.1 Impact of disease risk
Cytogenetics, and more recently MRD status, have been shown to correlate with survival. In a 2022 evaluation of the impact of AHCT with quad-therapy induction in NDMM, MRD was assessed by NGS pre and post AHCT, and the group with the greatest reduction in MRD burden had high-risk cytogenetics (HRCG) demonstrating a ‘dose effect’ with stepwise greater reduction in those with 0, 1 or 2+ HRCG abnormalities (72). Those with more than 2 HRCG abnormalities – so called ultra-high risk – have worse outcomes as demonstrated in subgroup analysis of MASTER and GRIFFIN trials (73). Though among ultra-high risk patients, those who achieve MRD negativity prior to or after AHCT have improved outcomes (74). In IFM 2009 long term follow up subgroup analysis, PFS (HR 0.28, p<0001) and OS (HR 0.35, p<0.001) was longer in patients who became MRD negative (64), and in DETERMINATION, there was no PFS difference between AHCT and non-AHCT therapy in patients who achieved MRD negativity (59). (59). In the CARDAMON trial, of the 22.8% of patients who achieved MRD negativity following induction, analysis suggested there was no benefit from AHCT gained in this group (66). A large retrospective study of NDMM patients who achieved a VGPR or greater after induction therapy assessed the MRD status by next-generation flow cytometry and found pre-AHCT MRD positivity was associated with a shorter PFS (48.2 months vs 80.1 months, p<0.001) (75). Finally, the single-arm MASTER trial attempted to use MRD negativity to guide decision making in patients receiving Dara-KRd induction followed by upfront AHCT and Dara-KRd consolidation, ceasing treatment when a patient achieved two consecutive MRD-negative readings. This strategy showed promising PFS and rates of MRD negativity (76). HRCG patients in the MASTER trial had far poorer PFS, especially when their therapy was stopped-and achievement of MRD negativity.
3.2 Role of autograft at relapse
There have been several retrospective studies evaluating responses to a second or third AHCT in the setting of RRMM, demonstrating this a feasible and safe approach, which may provide PFS benefit (77–82). Further retrospective subgroup analysis studies have demonstrated the benefit of salvage AHCT is greater in those who had a longer duration of response with their first AHCT (83, 84). This was recently called into question when long term follow-up of the GMMG ReLApsE trial did not show a difference in PFS or OS, but patients were not allowed onto the study if lenalidomide refractory, and therefore likely not generalizable to the current RRMM population (85). The interim analysis of the prospective single arm Second Chance trial shows deep responses with median PFS not reached when using Dara-KRD with salvage AHCT in the early relapse setting (86).
Melphalan retains its potent disease control even in the post CAR T setting. In a recent assessment of salvage therapies after relapse following BCMA-directed CAR T cell treatment, there appeared to be a reasonable response with 71.4% ORR, and an OS of 23.2 months in those who underwent AHCT or allogeneic HCT. Many of these patients were refractory to multiple lines of therapy (median 5 lines prior to CAR T) and the vast majority (94.9%) had had a prior AHCT (87). Salvage AHCT holds theoretical appeal in augmenting the biology of relapsed myeloma to wipe the slate clean of a heavily exposed patient. The rationale here is twofold: to gain clonal control and reset the immune milieu (88). There is a pattern of immune dysregulation and microenvironment abnormalities seen in myeloma patients with reduced NK and T cells and increased immunosuppressive cells, particularly T regulatory cells (89). This dysfunction worsens with exposure to anti-myeloma agents (90, 91). With an infusion of relatively chemotherapy naïve autologous stem cells, there opens up an opportunity for myeloma-specific immunity to be regained. In particular, the pattern of dynamics of T cell reconstitution after AHCT with a favorable ratio of T regulatory to T effector cells (92–94), may be able to be harnessed to leverage the sensitivity to immune therapies including CAR T (95). This is being tested prospectively prior to CAR T cells in NCT05393804 with the hypothesis that “fresh” non-exhausted T cells will lead to better expansion and persistence of the CAR T cell made from these cells. Furthermore, the early recovery of NK cells after AHCT may provide an opportunity to maximize potency of NK cell-therapies in this window (96).
4 Discussion: CAR T or AHCT or both?
It remains very difficult to show OS benefit in any modern comparative trial for MM given the median 7-10 year survival quoted for standard risk patients and significant crossover that occurs in many trials. Increasingly, patients’ OS is based on sequential progression free intervals in which the optimal sequence is unclear and ever changing due to newer data, approvals, and guidelines (97–99). The considerations we present in this section presume the indications approved in the United States in early 2024, and we acknowledge that in other parts of the world, these discussions differ based on availability and cost (100–102).
Firstly, studies of delayed AHCT, performed >12 months after diagnosis, suggested a reasonable response to this approach with a similar median time to progression and no difference in OS rates (5, 59). A major issue seen in the IFM2009 study was that 21% of those randomized to delayed AHCT -and deemed transplant eligible at randomization - were not able to later receive a salvage AHCT (5). A more recent retrospective comparison of upfront or delayed AHCT, found that delayed AHCT did not result in worse OS or PFS even when adjusting for age, disease risk, or depth of response at time of collection, but interestingly highlighted that those who underwent delayed AHCT frequently received a lower melphalan dose, reflective of mounting medical complexity with the passage of time and disease evolution (103). Data on the outcomes and safety of CAR T in frail patients suggests a relatively tolerable profile in this group, giving some weight to the argument that reserving CAR T for later in a patient’s course may be a more deliverable sequence (104, 105).
Second, some believe that the post CAR T cell journey is much easier than after AHCT, but this may not always be the case. Prolonged cytopenias, immune compromise, CRS, ICANS, MNTs, and infection risk, and the requirements to stay within a certain distance of the treating facility can impact qualify of life (QoL) after CAR T infusion. Comparisons show that the recovery to baseline may not be that different between the two modalities (106, 107).
Increasingly concerning is the risk of secondary malignancies. A CIBMTR analysis recently reported a risk of 4% at a median of 37 months of follow-up after AHCT, and though most of these patients eventually died from their myeloma rather than the secondary malignancy, these patients had a reduced PFS and OS (108). However, studies have also demonstrated that melphalan exposure and AHCT (+/- lenalidomide exposure) increase the mutational burden in patients with MM (109, 110). On the CAR T side, the updated analysis of CARTITUDE-1 showed 16/97 (16%) had a secondary malignancy with 9 (9%) being myelodysplastic syndrome or acute myeloid leukemia (42). It true risk of CAR T derived T cell lymphoma is not yet clear, and impacts on monitoring guidelines yet to be established (55, 111). The etiology of these findings, and whether it may manifest with earlier use of CAR T are not yet known.
Practical and financial considerations will inevitably shape the uptake of these therapies, and incremental cost effectiveness analysis should be factored into paradigm development. CAR T therapy costs are known to be dependent on rates of CRS and ICANS, and resource requirements may be prohibitive in some settings (112, 113). AHCT and CAR T costs may be reduced with utilization of outpatient care packages, however institutions need to have the resources and quality systems in place in order to safely facilitate the delivery of outpatient care, which can be a limiting factor particularly in low- and middle-income countries (LMIC) (114). In the LMIC setting, uptake of more efficacious practice may be limited at least in the short-term by costs, and we should be mindful of the increasing gap of resource-intensive and high-cost practices between high-, middle- and low-income settings (115, 116). Short-term focus can be premature however, and recent analyses have suggested more intensive therapies upfront may not only offset costs but leads to a long-term cost savings (117). Given the chronic nature of MM, our continued improvement in managing side effects, shortening hospital length of stay, and generally improving safety of both AHSCT and CAR T will be increasingly important to consider when evaluating the economic and quality of life impact. This will be especially important as CAR T migrates into less academic institutions where the systems to ensure adequate supportive care may need to be optimized. Additionally, when considering the prospect of bringing CAR T therapy to earlier lines of treatment, we will need to understand the value beyond traditional efficacy alone, with demonstration of quality-adjusted life years and other patient-reported outcomes, and the cost (both short- and long-term) to the healthcare system (97).
Overall, patients with MM will likely have both CAR T and AHCT during their treatment course. Sequencing depends on approvals and availability of the options, and will change over time as more treatments are available in earlier lines and with the results for the frontline prospective studies mentioned above. Prior toxicities and comorbidities, as well as concerns for future determents to quality of life and risk of secondary malignancies, allow for discussion and personalization of treatment. Optimizing both of these very effective modalities can allow patients to have long progression free remissions, which may even allow for a yet undescribed curative mechanism of action therapy to be approved.
Author contributions
CH: Writing – original draft, Writing – review & editing. GS: Conceptualization, Supervision, Writing – review & editing. BP: Conceptualization, Supervision, Writing – review & editing, Writing – original draft.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
GS received research funding from Janssen, Amgen, Bristol Myers Squibb, Beyond Spring, and GPCR, and served on the data safety monitoring board for Arcellx. BP has research funding from Bristol Myers Squibb, and served as a consultant or in an advisory role for Janssen and Abbvie.
The remaining author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Siegel RL, Miller KD, Wagle NS, Jemal A. Cancer statistics, 2023. CA Cancer J Clin. (2023) 73:17–48. doi: 10.3322/caac.21763
2. Child JA, Morgan GJ, Davies FE, Owen RG, Bell SE, Hawkins K, et al. High-dose chemotherapy with hematopoietic stem-cell rescue for multiple myeloma. N Engl J Med. (2003) 348:1875–83. doi: 10.1056/NEJMoa022340
3. Blade J, Rosinol L, Sureda A, Ribera JM, Diaz-Mediavilla J, Garcia-Larana J, et al. High-dose therapy intensification compared with continued standard chemotherapy in multiple myeloma patients responding to the initial chemotherapy: long-term results from a prospective randomized trial from the Spanish cooperative group PETHEMA. Blood. (2005) 106:3755–9. doi: 10.1182/blood-2005-03-1301
4. Palumbo A, Cavallo F, Gay F, Di Raimondo F, Ben Yehuda D, Petrucci MT, et al. Autologous transplantation and maintenance therapy in multiple myeloma. N Engl J Med. (2014) 371:895–905. doi: 10.1056/NEJMoa1402888
5. Attal M, Lauwers-Cances V, Hulin C, Leleu X, Caillot D, Escoffre M, et al. Lenalidomide, bortezomib, and dexamethasone with transplantation for myeloma. New Engl J Med. (2017) 376:1311–20. doi: 10.1056/NEJMoa1611750
6. Sonneveld P, Dimopoulos MA, Boccadoro M, Quach H, Ho PJ, Beksac M, et al. Daratumumab, bortezomib, lenalidomide, and dexamethasone for multiple myeloma. N Engl J Med. (2023) 390(4):301–13. doi: 10.1056/NEJMoa2312054
7. Saad A, Mahindra A, Zhang MJ, Zhong X, Costa LJ, Dispenzieri A, et al. Hematopoietic cell transplant comorbidity index is predictive of survival after autologous hematopoietic cell transplantation in multiple myeloma. Biol Blood Marrow Transplant. (2014) 20:402–8.e1. doi: 10.1016/j.bbmt.2013.12.557
8. Palumbo A, Bringhen S, Mateos MV, Larocca A, Facon T, Kumar SK, et al. Geriatric assessment predicts survival and toxicities in elderly myeloma patients: an International Myeloma Working Group report. Blood. (2015) 125:2068–74. doi: 10.1182/blood-2014-12-615187
9. Engelhardt M, Dold SM, Ihorst G, Zober A, Moller M, Reinhardt H, et al. Geriatric assessment in multiple myeloma patients: validation of the International Myeloma Working Group (IMWG) score and comparison with other common comorbidity scores. Haematologica. (2016) 101:1110–9. doi: 10.3324/haematol.2016.148189
10. Larocca A, Dold SM, Zweegman S, Terpos E, Wasch R, D'Agostino M, et al. Patient-centered practice in elderly myeloma patients: an overview and consensus from the European Myeloma Network (EMN). Leukemia. (2018) 32:1697–712. doi: 10.1038/s41375-018-0142-9
11. Munshi NC, Anderson LD Jr., Shah N, Madduri D, Berdeja J, Lonial S, et al. Idecabtagene vicleucel in relapsed and refractory multiple myeloma. N Engl J Med. (2021) 384:705–16. doi: 10.1056/NEJMoa2024850
12. Berdeja JG, Madduri D, Usmani SZ, Jakubowiak A, Agha M, Cohen AD, et al. Ciltacabtagene autoleucel, a B-cell maturation antigen-directed chimeric antigen receptor T-cell therapy in patients with relapsed or refractory multiple myeloma (CARTITUDE-1): a phase 1b/2 open-label study. Lancet. (2021) 398:314–24. doi: 10.1016/S0140-6736(21)00933-8
13. San-Miguel J, Dhakal B, Yong K, Spencer A, Anguille S, Mateos MV, et al. Cilta-cel or standard care in lenalidomide-refractory multiple myeloma. N Engl J Med. (2023) 389:335–47. doi: 10.1056/NEJMoa2303379
14. Rodriguez-Otero P, Ailawadhi S, Arnulf B, Patel K, Cavo M, Nooka AK, et al. Ide-cel or standard regimens in relapsed and refractory multiple myeloma. N Engl J Med. (2023) 388:1002–14. doi: 10.1056/NEJMoa2213614
15. Dytfeld D, Dhakal B, Agha M, Manier S, Delforge M, Kuppens S, et al. Bortezomib, lenalidomide and dexamethasone (VRd) followed by ciltacabtagene autoleucel versus vrd followed by lenalidomide and dexamethasone (Rd) maintenance in patients with newly diagnosed multiple myeloma not intended for transplant: A randomized, phase 3 study (CARTITUDE-5). Blood. (2021) 138:1835–. doi: 10.1182/blood-2021-146210
16. Boccadoro M, San-Miguel J, Suzuki K, Van De Donk NWCJ, Cook G, Jakubowiak A, et al. DVRd followed by ciltacabtagene autoleucel versus DVRd followed by ASCT in patients with newly diagnosed multiple myeloma who are transplant eligible: A randomized phase 3 study (EMagine/CARTITUDE-6). Blood. (2022) 140:4630–2. doi: 10.1182/blood-2022-157021
17. Neri P, Maity R, Tagoug I, McCulloch S, Duggan P, Jimenez-Zepeda V, et al. Immunome single cell profiling reveals T cell exhaustion with upregulation of checkpoint inhibitors LAG3 and tigit on marrow infiltrating T lymphocytes in daratumumab and IMiDs resistant patients. Blood. (2018) 132:242–. doi: 10.1182/blood-2018-99-117531
18. Rytlewski J, Madduri D, Fuller J, Campbell TB, Mashadi-Hossein A, Thompson EG, et al. Effects of prior alkylating therapies on preinfusion patient characteristics and starting material for CAR T cell product manufacturing in late-line multiple myeloma. Blood. (2020) 136:7–8. doi: 10.1182/blood-2020-134369
19. Cooke RE, Quinn KM, Quach H, Harrison S, Prince HM, Koldej R, et al. Conventional treatment for multiple myeloma drives premature aging phenotypes and metabolic dysfunction in T cells. Front Immunol. (2020) 11:2153. doi: 10.3389/fimmu.2020.02153
20. Rytlewski J, Fuller J, Mertz DR, Freeman C, Manier S, Shah N, et al. Correlative analysis to define patient profiles associated with manufacturing and clinical endpoints in relapsed/refractory multiple myeloma (RRMM) patients treated with idecabtagene vicleucel (ide-cel; bb2121), an anti-BCMA CAR T cell therapy. J Clin Oncol. (2022) 40:8021. doi: 10.1200/JCO.2022.40.16_suppl.8021
21. Tai YT, Acharya C, An G, Moschetta M, Zhong MY, Feng X, et al. APRIL and BCMA promote human multiple myeloma growth and immunosuppression in the bone marrow microenvironment. Blood. (2016) 127:3225–36. doi: 10.1182/blood-2016-01-691162
22. Madry C, Laabi Y, Callebaut I, Roussel J, Hatzoglou A, Le Coniat M, et al. The characterization of murine BCMA gene defines it as a new member of the tumor necrosis factor receptor superfamily. Int Immunol. (1998) 10:1693–702. doi: 10.1093/intimm/10.11.1693
23. Ryan MC, Hering M, Peckham D, McDonagh CF, Brown L, Kim KM, et al. Antibody targeting of B-cell maturation antigen on Malignant plasma cells. Mol Cancer Ther. (2007) 6:3009–18. doi: 10.1158/1535-7163.MCT-07-0464
24. O'Connor BP, Raman VS, Erickson LD, Cook WJ, Weaver LK, Ahonen C, et al. BCMA is essential for the survival of long-lived bone marrow plasma cells. J Exp Med. (2004) 199:91–8. doi: 10.1084/jem.20031330
25. Carpenter RO, Evbuomwan MO, Pittaluga S, Rose JJ, Raffeld M, Yang S, et al. B-cell maturation antigen is a promising target for adoptive T-cell therapy of multiple myeloma. Clin Cancer Res. (2013) 19:2048–60. doi: 10.1158/1078-0432.CCR-12-2422
26. Novak AJ, Darce JR, Arendt BK, Harder B, Henderson K, Kindsvogel W, et al. Expression of BCMA, TACI, and BAFF-R in multiple myeloma: a mechanism for growth and survival. Blood. (2004) 103:689–94. doi: 10.1182/blood-2003-06-2043
27. Lee L, Bounds D, Paterson J, Herledan G, Sully K, Seestaller-Wehr LM, et al. Evaluation of B cell maturation antigen as a target for antibody drug conjugate mediated cytotoxicity in multiple myeloma. Br J Haematol. (2016) 174:911–22. doi: 10.1111/bjh.14145
28. Bujarski S, Soof C, Chen H, Li M, Sanchez E, Wang CS, et al. Serum b-cell maturation antigen levels to predict progression free survival and responses among relapsed or refractory multiple myeloma patients treated on the phase I IRUX trial. J Clin Oncol. (2018) 36:e24313–e. doi: 10.1200/JCO.2018.36.15_suppl.e24313
29. Ghermezi M, Li M, Vardanyan S, Harutyunyan NM, Gottlieb J, Berenson A, et al. Serum B-cell maturation antigen: a novel biomarker to predict outcomes for multiple myeloma patients. Haematologica. (2017) 102:785–95. doi: 10.3324/haematol.2016.150896
30. Sanchez E, Li M, Kitto A, Li J, Wang CS, Kirk DT, et al. Serum B-cell maturation antigen is elevated in multiple myeloma and correlates with disease status and survival. Br J Haematol. (2012) 158:727–38. doi: 10.1111/j.1365-2141.2012.09241.x
31. Visram A, Soof C, Rajkumar SV, Kumar SK, Bujarski S, Spektor TM, et al. Serum BCMA levels predict outcomes in MGUS and smoldering myeloma patients. Blood Cancer J. (2021) 11:120. doi: 10.1038/s41408-021-00505-4
32. Friedman KM, Garrett TE, Evans JW, Horton HM, Latimer HJ, Seidel SL, et al. Effective targeting of multiple B-cell maturation antigen-expressing hematological Malignances by anti-B-cell maturation antigen chimeric antigen receptor T cells. Hum Gene Ther. (2018) 29:585–601. doi: 10.1089/hum.2018.001
33. Raje N, Berdeja J, Lin Y, Siegel D, Jagannath S, Madduri D, et al. Anti-BCMA CAR T-cell therapy bb2121 in relapsed or refractory multiple myeloma. N Engl J Med. (2019) 380:1726–37. doi: 10.1056/NEJMoa1817226
34. Larry D, Anderson J, Munshi NC, Shah N, Jagannath S, Berdeja JG, et al. Idecabtagene vicleucel (ide-cel, bb2121), a BCMA-directed CAR T cell therapy, in relapsed and refractory multiple myeloma: Updated KarMMa results. J Clin Oncol. (2021) 39:8016. doi: 10.1200/JCO.2021.39.15_suppl.8016
35. Lin Y, Raje NS, Berdeja JG, Siegel DS, Jagannath S, Madduri D, et al. Idecabtagene vicleucel for relapsed and refractory multiple myeloma: post hoc 18-month follow-up of a phase 1 trial. Nat Med. (2023) 29:2286–94. doi: 10.1038/s41591-023-02496-0
36. Hansen DK, Sidana S, Peres LC, Colin Leitzinger C, Shune L, Shrewsbury A, et al. Idecabtagene vicleucel for relapsed/refractory multiple myeloma: real-world experience from the myeloma CAR T consortium. J Clin Oncol. (2023) 41:2087–97. doi: 10.1200/JCO.22.01365
37. Hashmi H, Hansen DK, Peres LC, Castaneda Puglianini OA, Freeman CL, De Avila G, et al. Factors associated with refractoriness or early progression after idecabtagene vicleucel (Ide-cel) in patients with relapsed/refractory multiple myeloma (RRMM): U.S. Myeloma CAR T consortium real world experience. Blood. (2022) 140:4642–5. doi: 10.1182/blood-2022-164828
38. Patel K, Rodríguez-Otero P, Manier S, Baz R, Raab MS, Cavo M, et al. S195: Idecabtagene vicleucel (ide-cel) vs standard regimens in patients with triple-class–exposed (tce) relapsed and refractory multiple myeloma (rrmm): a karmma-3 analysis in high-risk subgroups. HemaSphere. (2023) 7:e369897b. doi: 10.1097/01.HS9.0000967692.36989.7b
39. Dhodapkar MV, Alsina M, Berdeja JG, Patel KK, Richard S, Vij R, et al. Efficacy and safety of idecabtagene vicleucel (ide-cel) in patients with clinical high-risk newly diagnosed multiple myeloma (NDMM) with an inadequate response to frontline autologous stem cell transplantation (ASCT): karMMa-2 cohort 2c extended follow-up. Blood. (2023) 142:2101. doi: 10.1182/blood-2023-173970
40. Xu J, Chen LJ, Yang SS, Sun Y, Wu W, Liu YF, et al. Exploratory trial of a biepitopic CAR T-targeting B cell maturation antigen in relapsed/refractory multiple myeloma. Proc Natl Acad Sci U S A. (2019) 116:9543–51. doi: 10.1073/pnas.1819745116
41. Martin T, Usmani SZ, Berdeja JG, Agha M, Cohen AD, Hari P, et al. Ciltacabtagene autoleucel, an anti-B-cell maturation antigen chimeric antigen receptor T-cell therapy, for relapsed/refractory multiple myeloma: CARTITUDE-1 2-year follow-up. J Clin Oncol. (2023) 41:1265–74. doi: 10.1200/JCO.22.00842
42. Lin Y, Martin TG, Usmani SZ, Berdeja JG, Jakubowiak AJ, Agha ME, et al. CARTITUDE-1 final results: Phase 1b/2 study of ciltacabtagene autoleucel in heavily pretreated patients with relapsed/refractory multiple myeloma. J Clin Oncol. (2023) 41:8009. doi: 10.1200/JCO.2023.41.16_suppl.8009
43. Hansen DK, Patel KK, Peres LC, Kocoglu MH, Shune L, Simmons G, et al. Safety and efficacy of standard of care (SOC) ciltacabtagene autoleucel (Cilta-cel) for relapsed/refractory multiple myeloma (RRMM). J Clin Oncol. (2023) 41:8012. doi: 10.1200/JCO.2023.41.16_suppl.8012
44. Dhakal B, Yong K, Harrison SJ, Mateos M-V, Moreau P, van de Donk NWCJ, et al. First phase 3 results from CARTITUDE-4: Cilta-cel versus standard of care (PVd or DPd) in lenalidomide-refractory multiple myeloma. J Clin Oncol. (2023) 41:LBA106–LBA. doi: 10.1200/JCO.2023.41.17_suppl.LBA106
45. Wang Z, Han W. Biomarkers of cytokine release syndrome and neurotoxicity related to CAR-T cell therapy. biomark Res. (2018) 6:4. doi: 10.1186/s40364-018-0116-0
46. Norelli M, Camisa B, Barbiera G, Falcone L, Purevdorj A, Genua M, et al. Monocyte-derived IL-1 and IL-6 are differentially required for cytokine-release syndrome and neurotoxicity due to CAR T cells. Nat Med. (2018) 24:739–48. doi: 10.1038/s41591-018-0036-4
47. Shimabukuro-Vornhagen A, Godel P, Subklewe M, Stemmler HJ, Schlosser HA, Schlaak M, et al. Cytokine release syndrome. J Immunother Cancer. (2018) 6:56. doi: 10.1186/s40425-018-0343-9
48. Cosenza M, Sacchi S, Pozzi S. Cytokine release syndrome associated with T-cell-based therapies for hematological Malignancies: pathophysiology, clinical presentation, and treatment. Int J Mol Sci. (2021) 22(14):7652. doi: 10.3390/ijms22147652
49. Lee DW, Santomasso BD, Locke FL, Ghobadi A, Turtle CJ, Brudno JN, et al. ASTCT consensus grading for cytokine release syndrome and neurologic toxicity associated with immune effector cells. Biol Blood Marrow Transplant. (2019) 25:625–38. doi: 10.1016/j.bbmt.2018.12.758
50. Neelapu SS, Tummala S, Kebriaei P, Wierda W, Gutierrez C, Locke FL, et al. Chimeric antigen receptor T-cell therapy - assessment and management of toxicities. Nat Rev Clin Oncol. (2018) 15:47–62. doi: 10.1038/nrclinonc.2017.148
51. Santomasso BD, Nastoupil LJ, Adkins S, Lacchetti C, Schneider BJ, Anadkat M, et al. Management of immune-related adverse events in patients treated with chimeric antigen receptor T-cell therapy: ASCO guideline. J Clin Oncol. (2021) 39:3978–92. doi: 10.1200/JCO.21.01992
52. Cohen AD, Parekh S, Santomasso BD, Gallego Perez-Larraya J, van de Donk N, Arnulf B, et al. Incidence and management of CAR-T neurotoxicity in patients with multiple myeloma treated with ciltacabtagene autoleucel in CARTITUDE studies. Blood Cancer J. (2022) 12:32. doi: 10.1038/s41408-022-00629-1
53. Van Oekelen O, Aleman A, Upadhyaya B, Schnakenberg S, Madduri D, Gavane S, et al. Neurocognitive and hypokinetic movement disorder with features of parkinsonism after BCMA-targeting CAR-T cell therapy. Nat Med. (2021) 27:2099–103. doi: 10.1038/s41591-021-01564-7
54. Adminstration FaD. FDA adverse event reporting system (FAERS) public dashboard. (2023). Available at: https://www.fda.gov/Drugs/GuidanceComplianceRegulatoryInformation/Surveillance/AdverseDrugEffects/ucm070093.htm.
55. Harrison SJ, Nguyen T, Rahman M, Er J, Li J, Li K, et al. CAR+ T-cell lymphoma post ciltacabtagene autoleucel therapy for relapsed refractory multiple myeloma. Blood. (2023) 142:6939. doi: 10.1182/blood-2023-178806
56. Vainstein V, Avni B, Grisariu S, Kfir-Erenfeld S, Asherie N, Nachmias B, et al. Clonal myeloid dysplasia following CAR T-cell therapy: chicken or the egg? Cancers (Basel). (2023) 15(13):3471. doi: 10.3390/cancers15133471
57. Attal M, Harousseau JL, Stoppa AM, Sotto JJ, Fuzibet JG, Rossi JF, et al. A prospective, randomized trial of autologous bone marrow transplantation and chemotherapy in multiple myeloma. Intergroupe Francais du Myelome. N Engl J Med. (1996) 335:91–7. doi: 10.1056/NEJM199607113350204
58. Koreth J, Cutler CS, Djulbegovic B, Behl R, Schlossman RL, Munshi NC, et al. High-dose therapy with single autologous transplantation versus chemotherapy for newly diagnosed multiple myeloma: A systematic review and meta-analysis of randomized controlled trials. Biol Blood Marrow Transplant. (2007) 13:183–96. doi: 10.1016/j.bbmt.2006.09.010
59. Richardson PG, Jacobus SJ, Weller EA, Hassoun H, Lonial S, Raje NS, et al. Triplet therapy, transplantation, and maintenance until progression in myeloma. N Engl J Med. (2022) 387:132–47. doi: 10.1056/NEJMoa2204925
60. Grazziutti ML, Dong L, Miceli MH, Krishna SG, Kiwan E, Syed N, et al. Oral mucositis in myeloma patients undergoing melphalan-based autologous stem cell transplantation: incidence, risk factors and a severity predictive model. Bone Marrow Transplant. (2006) 38:501–6. doi: 10.1038/sj.bmt.1705471
61. Chakraborty R, Hamilton BK, Hashmi SK, Kumar SK, Majhail NS. Health-related quality of life after autologous stem cell transplantation for multiple myeloma. Biol Blood Marrow Transplant. (2018) 24:1546–53. doi: 10.1016/j.bbmt.2018.03.027
62. Roussel M, Hebraud B, Hulin C, Perrot A, Caillot D, Stoppa AM, et al. Health-related quality of life results from the IFM 2009 trial: treatment with lenalidomide, bortezomib, and dexamethasone in transplant-eligible patients with newly diagnosed multiple myeloma. Leuk Lymphoma. (2020) 61:1323–33. doi: 10.1080/10428194.2020.1719091
63. Durie BGM, Hoering A, Abidi MH, Rajkumar SV, Epstein J, Kahanic SP, et al. Bortezomib with lenalidomide and dexamethasone versus lenalidomide and dexamethasone alone in patients with newly diagnosed myeloma without intent for immediate autologous stem-cell transplant (SWOG S0777): a randomised, open-label, phase 3 trial. Lancet. (2017) 389:519–27. doi: 10.1016/S0140-6736(16)31594-X
64. Perrot A, Lauwers-Cances V, Cazaubiel T, Facon T, Caillot D, Clement-Filliatre L, et al. Early versus late autologous stem cell transplant in newly diagnosed multiple myeloma: long-term follow-up analysis of the IFM 2009 trial. Blood. (2020) 136:39. doi: 10.1182/blood-2020-134538
65. Gay F, Musto P, Rota-Scalabrini D, Bertamini L, Belotti A, Galli M, et al. Carfilzomib with cyclophosphamide and dexamethasone or lenalidomide and dexamethasone plus autologous transplantation or carfilzomib plus lenalidomide and dexamethasone, followed by maintenance with carfilzomib plus lenalidomide or lenalidomide alone for patients with newly diagnosed multiple myeloma (FORTE): a randomised, open-label, phase 2 trial. Lancet Oncol. (2021) 22:1705–20. doi: 10.1016/S1470-2045(21)00535-0
66. Yong K, Wilson W, De Tute RM, Camilleri M, Ramasamy K, Streetly M, et al. Upfront autologous haematopoietic stem-cell transplantation versus carfilzomib–cyclophosphamide–dexamethasone consolidation with carfilzomib maintenance in patients with newly diagnosed multiple myeloma in England and Wales (CARDAMON): a randomised, phase. Lancet Haematology. (2023) 10:e93–e106. doi: 10.1016/S2352-3026(22)00350-7
67. Moreau P, Attal M, Hulin C, Arnulf B, Belhadj K, Benboubker L, et al. Bortezomib, thalidomide, and dexamethasone with or without daratumumab before and after autologous stem-cell transplantation for newly diagnosed multiple myeloma (CASSIOPEIA): a randomised, open-label, phase 3 study. Lancet. (2019) 394:29–38. doi: 10.1016/S0140-6736(19)31240-1
68. Voorhees PM, Kaufman JL, Laubach J, Sborov DW, Reeves B, Rodriguez C, et al. Daratumumab, lenalidomide, bortezomib, and dexamethasone for transplant-eligible newly diagnosed multiple myeloma: the GRIFFIN trial. Blood. (2020) 136:936–45. doi: 10.1182/blood.2020005288
69. Gay F, Roeloffzen W, Dimopoulos MA, Rosiñol L, van der Klift M, Mina R, et al. Results of the phase III randomized iskia trial: isatuximab-carfilzomib-lenalidomide-dexamethasone vs carfilzomib-lenalidomide-dexamethasone as pre-transplant induction and post-transplant consolidation in newly diagnosed multiple myeloma patients. Blood. (2023) 142:4. doi: 10.1182/blood-2023-177546
70. Kumar S, Flinn I, Richardson PG, Hari P, Callander N, Noga SJ, et al. Randomized, multicenter, phase 2 study (EVOLUTION) of combinations of bortezomib, dexamethasone, cyclophosphamide, and lenalidomide in previously untreated multiple myeloma. Blood. (2012) 119:4375–82. doi: 10.1182/blood-2011-11-395749
71. Facon T, Kumar SK, Plesner T, Orlowski RZ, Moreau P, Bahlis N, et al. Daratumumab, lenalidomide, and dexamethasone versus lenalidomide and dexamethasone alone in newly diagnosed multiple myeloma (MAIA): overall survival results from a randomised, open-label, phase 3 trial. Lancet Oncol. (2021) 22:1582–96. doi: 10.1016/S1470-2045(21)00466-6
72. Bal S, Dhakal B, Silbermann RW, Schmidt TM, Dholaria B, Giri S, et al. Impact of autologous hematopoietic cell transplantation on disease burden quantified by next-generation sequencing in multiple myeloma treated with quadruplet therapy. Am J Hematol. (2022) 97:1170–7. doi: 10.1002/ajh.26640
73. Callander N, Silbermann R, Kaufman JL, Godby KN, Laubach JP, Schmidt TM, et al. Analysis of transplant-eligible patients (Pts) who received frontline daratumumab (DARA)-based quadruplet therapy for the treatment of newly diagnosed multiple myeloma (NDMM) with high-risk cytogenetic abnormalities (HRCA) in the griffin and master studies. Blood. (2022) 140:10144–7. doi: 10.1182/blood-2022-160451
74. Pasvolsky O, Ghanem S, Milton DR, Masood A, Tanner MR, Bashir Q, et al. Outcomes of autologous stem cell transplantation in patients with ultra-high-risk multiple myeloma. Transplant Cell Ther. (2023) 29:757–62. doi: 10.1016/j.jtct.2023.08.031
75. Pasvolsky O, Pasyar S, Bassett RL, Khan HN, Tanner MR, Bashir Q, et al. Impact of pretransplant minimal residual disease in patients with multiple myeloma and a very good partial response or better receiving autologous hematopoietic stem cell transplantation. Cancer. (2023). doi: 10.1002/cncr.35171
76. Costa LJ, Chhabra S, Medvedova E, Dholaria BR, Schmidt TM, Godby KN, et al. Minimal residual disease response-adapted therapy in newly diagnosed multiple myeloma (MASTER): final report of the multicentre, single-arm, phase 2 trial. Lancet Haematol. (2023) 10:e890–901. doi: 10.1016/S2352-3026(23)00236-3
77. Grovdal M, Nahi H, Gahrton G, Liwing J, Waage A, Abildgaard N, et al. Autologous stem cell transplantation versus novel drugs or conventional chemotherapy for patients with relapsed multiple myeloma after previous ASCT. Bone Marrow Transplant. (2015) 50:808–12. doi: 10.1038/bmt.2015.39
78. Garderet L, Iacobelli S, Koster L, Goldschmidt H, Johansson JE, Bourhis JH, et al. Outcome of a salvage third autologous stem cell transplantation in multiple myeloma. Biol Blood Marrow Transplant. (2018) 24:1372–8. doi: 10.1016/j.bbmt.2018.01.035
79. Manjappa S, Fiala MA, King J, Kohnen DA, Vij R. The efficacy of salvage autologous stem cell transplant among patients with multiple myeloma who received maintenance therapy post initial transplant. Bone Marrow Transplant. (2018) 53:1483–6. doi: 10.1038/s41409-018-0216-3
80. Khan AM, Ozga M, Bhatt H, Faisal MS, Ansari S, Zhao Q, et al. Outcomes after salvage autologous hematopoietic cell transplant for patients with relapsed/refractory multiple myeloma: A single-institution experience. Clin Lymphoma Myeloma Leuk. (2023) 23:e182–e9. doi: 10.1016/j.clml.2022.12.001
81. Tilmont R, Yakoub-Agha I, Eikema D-J, Zinger N, Haenel M, Schaap N, et al. Carfilzomib, lenalidomide and dexamethasone followed by a second ASCT is an effective strategy in first relapse multiple myeloma: a study on behalf of the Chronic Malignancies working party of the EBMT. Bone Marrow Transplantation. (2023) 58:1182–8. doi: 10.1038/s41409-023-02048-7
82. Hashmi H, Atrash S, Jain J, Khasawneh G, Mohan M, Mahmoudjafari Z, et al. Daratumumab, pomalidomide, and dexamethasone (DPd) followed by high dose chemotherapy-Autologous Stem Cell Transplantation leads to superior outcomes when compared to DPd-alone for patients with Relapsed Refractory Multiple Myeloma. Transplant Cell Ther. (2023) 29:262 e1– e6. doi: 10.1016/j.jtct.2023.01.013
83. Michaelis LC, Saad A, Zhong X, Le-Rademacher J, Freytes CO, Marks DI, et al. Salvage second hematopoietic cell transplantation in myeloma. Biol Blood Marrow Transplant. (2013) 19:760–6. doi: 10.1016/j.bbmt.2013.01.004
84. Lemieux E, Hulin C, Caillot D, Tardy S, Dorvaux V, Michel J, et al. Autologous stem cell transplantation: an effective salvage therapy in multiple myeloma. Biol Blood Marrow Transplant. (2013) 19:445–9. doi: 10.1016/j.bbmt.2012.11.013
85. Baertsch M-A, Schlenzka J, Hielscher T, Raab MS, Sauer S, Merz M, et al. Salvage autologous transplant and lenalidomide maintenance versus continuous lenalidomide/dexamethasone for relapsed multiple myeloma: long term follow up results of the randomized GMMG phase III multicenter trial relapse. Blood. (2023) 142:782. doi: 10.1182/blood-2023-178835
86. Shah GL, Bal S, Rodriguez C, Chhabra S, Bayer R-L, Costa LJ, et al. 534 - interim analysis of the 2nd chance protocol: A multicenter trial of daratumumab, carfilzomib, lenalidomide, & Dexamethasone for relapsed/refractory myeloma with salvage autologous hematopoietic cell transplantation. Transplant Cell Ther. (2022) 28:S415–S6. doi: 10.1016/S2666-6367(22)00693-5
87. Van Oekelen O, Nath K, Mouhieddine TH, Farzana T, Aleman A, Melnekoff DT, et al. Interventions and outcomes of patients with multiple myeloma receiving salvage therapy after BCMA-directed CAR T therapy. Blood. (2023) 141:756–65. doi: 10.1182/blood.2022017848
88. Janakiram M, Arora N, Bachanova V, Miller JS. Novel cell and immune engagers in optimizing tumor- specific immunity post-autologous transplantation in multiple myeloma. Transplant Cell Ther. (2022) 28:61–9. doi: 10.1016/j.jtct.2021.10.001
89. Tamura H. Immunopathogenesis and immunotherapy of multiple myeloma. Int J Hematol. (2018) 107:278–85. doi: 10.1007/s12185-018-2405-7
90. Davies FE, Raje N, Hideshima T, Lentzsch S, Young G, Tai YT, et al. Thalidomide and immunomodulatory derivatives augment natural killer cell cytotoxicity in multiple myeloma. Blood. (2001) 98:210–6. doi: 10.1182/blood.V98.1.210
91. Krejcik J, Casneuf T, Nijhof IS, Verbist B, Bald J, Plesner T, et al. Daratumumab depletes CD38+ immune regulatory cells, promotes T-cell expansion, and skews T-cell repertoire in multiple myeloma. Blood. (2016) 128:384–94. doi: 10.1182/blood-2015-12-687749
92. Douek DC, Vescio RA, Betts MR, Brenchley JM, Hill BJ, Zhang L, et al. Assessment of thymic output in adults after haematopoietic stem-cell transplantation and prediction of T-cell reconstitution. Lancet. (2000) 355:1875–81. doi: 10.1016/S0140-6736(00)02293-5
93. Chung DJ, Pronschinske KB, Shyer JA, Sharma S, Leung S, Curran SA, et al. T-cell exhaustion in multiple myeloma relapse after autotransplant: optimal timing of immunotherapy. Cancer Immunol Res. (2016) 4:61–71. doi: 10.1158/2326-6066.CIR-15-0055
94. Rueff J, Medinger M, Heim D, Passweg J, Stern M. Lymphocyte subset recovery and outcome after autologous hematopoietic stem cell transplantation for plasma cell myeloma. Biol Blood Marrow Transplant. (2014) 20:896–9. doi: 10.1016/j.bbmt.2014.03.007
95. Garfall AL, Dancy EK, Cohen AD, Hwang WT, Fraietta JA, Davis MM, et al. T-cell phenotypes associated with effective CAR T-cell therapy in postinduction vs relapsed multiple myeloma. Blood Adv. (2019) 3:2812–5. doi: 10.1182/bloodadvances.2019000600
96. Porrata LF, Gastineau DA, Padley D, Bundy K, Markovic SN. Re-infused autologous graft natural killer cells correlates with absolute lymphocyte count recovery after autologous stem cell transplantation. Leuk Lymphoma. (2003) 44:997–1000. doi: 10.1080/1042819031000077089
97. Anderson LD Jr., Dhakal B, Jain T, Oluwole OO, Shah GL, Sidana S, et al. Chimeric antigen receptor T cell therapy for myeloma: where are we now and what is needed to move chimeric antigen receptor T cells forward to earlier lines of therapy? Expert panel opinion from the american society for transplantation and cellular therapy. Transplant Cell Ther. (2024) 30:17–37. doi: 10.1016/j.jtct.2023.10.022
98. mSMART. Treatment guidelines: multiple myeloma(2023). Available online at: https://www.msmart.org/mm-treatment-guidelines.
99. Dhakal B, Shah N, Kansagra A, Kumar A, Lonial S, Garfall A, et al. ASTCT clinical practice recommendations for transplantation and cellular therapies in multiple myeloma. Transplant Cell Ther. (2022) 28:284–93. doi: 10.1016/j.jtct.2022.03.019
100. Kumar SK, Lacy MQ, Dispenzieri A, Buadi FK, Hayman SR, Dingli D, et al. Early versus delayed autologous transplantation after immunomodulatory agents-based induction therapy in patients with newly diagnosed multiple myeloma. Cancer. (2012) 118:1585–92. doi: 10.1002/cncr.26422
101. Beinfeld M, Lee S, McQueen B, Fluetsch N, Pearson SD, Ollendorf DA. Anti B-cell maturation antigen CAR T-cell and antibody drug conjugate therapy for heavily pretreated relapsed and refractory multiple myeloma. J Manag Care Spec Pharm. (2021) 27:1315–20. doi: 10.18553/jmcp.2021.27.9.1315
102. Kapinos KA, Hu E, Trivedi J, Geethakumari PR, Kansagra A. Cost-effectiveness analysis of CAR T-cell therapies vs antibody drug conjugates for patients with advanced multiple myeloma. Cancer Control. (2023) 30:10732748221142945. doi: 10.1177/10732748221142945
103. Leng S, Moshier E, Tremblay D, Hu L, Biran N, Barman N, et al. Timing of autologous stem cell transplantation for multiple myeloma in the era of current therapies. Clin Lymphoma Myeloma Leuk. (2020) 20:e734–e51. doi: 10.1016/j.clml.2020.05.027
104. Davis JA, Dima D, Ahmed N, DeJarnette S, McGuirk J, Jia X, et al. Impact of frailty on outcomes after chimeric antigen receptor T cell therapy for patients with relapsed/refractory multiple myeloma. Transplant Cell Ther. (2023). doi: 10.1182/blood-2023-179981
105. Reyes KR, Huang CY, Lo M, Arora S, Chung A, Wong SW, et al. Safety and efficacy of BCMA CAR-T cell therapy in older patients with multiple myeloma. Transplant Cell Ther. (2023) 29:350–5. doi: 10.1016/j.jtct.2023.03.012
106. Sidana S, Dueck AC, Thanarajasingam G, Griffin JM, Thompson C, Durani U, et al. Longitudinal patient reported outcomes with CAR-T cell therapy versus autologous and allogeneic stem cell transplant. Transplant Cell Ther. (2022) 28:473–82. doi: 10.1016/j.jtct.2022.05.004
107. Delforge M, Shah N, Miguel JSF, Braverman J, Dhanda DS, Shi L, et al. Health-related quality of life with idecabtagene vicleucel in relapsed and refractory multiple myeloma. Blood Adv. (2022) 6:1309–18. doi: 10.1182/bloodadvances.2021005913
108. Ragon BK, Shah MV, D'Souza A, Estrada-Merly N, Gowda L, George G, et al. Impact of second primary Malignancy post-autologous transplantation on outcomes of multiple myeloma: a CIBMTR analysis. Blood Adv. (2023) 7:2746–57.doi: 10.1182/bloodadvances.2022009138
109. Maura F, Weinhold N, Diamond B, Kazandjian D, Rasche L, Morgan G, et al. The mutagenic impact of melphalan in multiple myeloma. Leukemia. (2021) 35:2145–50. doi: 10.1038/s41375-021-01293-3
110. Samur MK, Roncador M, Aktas Samur A, Fulciniti M, Bazarbachi AH, Szalat R, et al. High-dose melphalan treatment significantly increases mutational burden at relapse in multiple myeloma. Blood. (2023) 141:1724–36. doi: 10.1182/blood.2022017094
111. Levine BL, Pasquini MC, Connolly JE, Porter DL, Gustafson MP, Boelens JJ, et al. Unanswered questions following reports of secondary Malignancies after CAR-T cell therapy. Nat Med. (2024) 30:338–41. doi: 10.1038/s41591-023-02767-w
112. Hernandez I, Prasad V, Gellad WF. Total costs of chimeric antigen receptor T-cell immunotherapy. JAMA Oncol. (2018) 4:994–6. doi: 10.1001/jamaoncol.2018.0977
113. Hoda D, Richards R, Faber EA, Deol A, Hunter BD, Weber E, et al. Process, resource and success factors associated with chimeric antigen receptor T-cell therapy for multiple myeloma. Future Oncol. (2022) 18:2415–31. doi: 10.2217/fon-2022-0162
114. Lee SJ, McQueen RB, Beinfeld M, Fluetsch N, Whittington MD, Pearson SD, et al. Anti B-cell maturation antigen CAR T-cell and antibody drug conjugate therapy for heavily pre-treated relapsed and refractory multiple myeloma; final evidence report.: institute for clinical and economic review. (2021). Available at: https://icer.org/assessment/multiplemyeloma-2021/#timeline
115. Fiorenza S, Ritchie DS, Ramsey SD, Turtle CJ, Roth JA. Value and affordability of CAR T-cell therapy in the United States. Bone Marrow Transplantation. (2020) 55:1706–15. doi: 10.1038/s41409-020-0956-8
116. Choi G, Shin G, Bae S. Price and prejudice? The value of chimeric antigen receptor (CAR) T-cell therapy. Int J Environ Res Public Health. (2022) 19(19):12366. doi: 10.3390/ijerph191912366
Keywords: autologous transplant, CAR T cell therapy, multiple myeloma, newly diagnosed multiple myeloma, relapsed refractory multiple myeloma
Citation: Hughes CFM, Shah GL and Paul BA (2024) Autologous hematopoietic stem cell transplantation for multiple myeloma in the age of CAR T cell therapy. Front. Oncol. 14:1373548. doi: 10.3389/fonc.2024.1373548
Received: 19 January 2024; Accepted: 15 March 2024;
Published: 27 March 2024.
Edited by:
Pasquale Niscola, Sant’Eugenio Hospital of Rome, ItalyReviewed by:
Joselle Cook, Mayo Clinic, United StatesGuillermo José Ruiz-Argüelles, Clínica Ruiz, Mexico
Copyright © 2024 Hughes, Shah and Paul. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Barry A. Paul, QmFycnkucGF1bEBhdHJpdW1oZWFsdGgub3Jn
 Charlotte F. M. Hughes
Charlotte F. M. Hughes Gunjan L. Shah
Gunjan L. Shah Barry A. Paul
Barry A. Paul