
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Oncol., 19 April 2024
Sec. Breast Cancer
Volume 14 - 2024 | https://doi.org/10.3389/fonc.2024.1372710
Background: Phyllodes tumors (PTs), which account for less than 1% of mammary gland tumors, composed of both epithelial and stromal components. If a malignant heterologous component is encountered, PT is considered malignant. Malignant phyllodes tumors (MPTs) only account for 8% to 20% of PTs. We report a case of MPT with osteosarcoma and chondrosarcoma differentiation and review the literature to discuss the differential diagnosis and therapy.
Case presentation: A 59-year-old Chinese woman come to our hospital because of a palpable mass she had had for 1 months in the left breast. Preoperative core needle biopsy (CNB) was performed on the left breast mass on January 11, 2023. Pathological diagnosis was malignant tumor, the specific type was not clear. Mastectomy and sentinel lymph node biopsy of the left breast was performed. No metastasis was found in 3 sentinel lymph nodes identified by carbon nanoparticles and methylene blue double staining. Heterologous osteosarcoma and chondrosarcomatous differentiation of phyllodes tumor were observed. Immunohistochemistry: spindle tumor cells ER(-), PR(-), HER-2(-), CK-pan(-), CK7(-), CK8(-), SOX10(-), S100(-), and MDM2(-), CK5/6(-), P63(-), P40(-) were all negative. CD34:(+), SATB2(+), P53(90% strong), CD68 (+), Ki-67(LI: about 60%). No ductal carcinoma in situ was found in the breast. Fluorescence in situ hybridization (FISH) indicated USP6 was negatively expressed on formalin-fixed, paraffin-embedded (FFPE) tissue sections.
Conclusion: MPTs are rare, and heterologous differentiation in MPTs is exceedingly rare. It could be diagnosed by pathology when metaplastic carcinoma, primary osteosarcoma, or myositis ossificans were excluded. This case could help clinicians to improve the prognosis and treatment of this disease.
Phyllodes tumors (PTs), which account for less than 1% of mammary gland tumors, composed of both epithelial and stromal components (1). Malignant phyllodes tumors (MPTs) only account for 8% to 20% of PTs (2). The World Health Organization has subcategorized PTs into benign, borderline, and malignant categories on the basis of 5 histological parameters: stromal cellularity, stromal atypia, tumor margins, mitotic activity, and stromal overgrowth (3). MPTs are characterized by marked stromal cellularity, stromal growth, nuclear atypia, increased mitotic activity (≥10 per 10 high power fields), and infiltrative tumor margins (4). Moreover, the presence of heterologous sarcomatous elements such as osteosarcoma, chondrosarcoma, or liposarcoma within the tumor were frequently observed (5). Due to the limitation of rare incidence, it is difficult to proceed randomized trials and prospective cohort studies for MPTs with heterologous sarcomatous elements (6).
This study describes a case/patient with osteosarcomatous and chondrosarcomatous heterologous elements within a MPT based on detailed imaging and histopathologic records, and we review the literature to describe the characteristics and therapy of MPT with osteosarcoma and chondrosarcomatous differentiation.
Patient, ×××, female, 59 years old, due to “presented with a mass in the left breast for more than 1 month”. On January 7, 2023, she was admitted to the breast surgery Department of Taihe Hospital, Hubei University of Medicine. In the past 1 month, she felt a mass in the left breast, and the nipple was not bleeding or leaking. The patient had a history of hypertension for 30 years and cerebral hemorrhage for more than 1 year. In 2013, she underwent “left breast mass resection” in our hospital, and the pathological report was cystic hyperplasia (left breast). Clinical physical examination: a hard mass of about 3.0×2.0cm in size could be detected at 4cm from the nipple in the upper outer quadrant of the left breast, with no obvious tenderness, non-smooth surface, unclear boundary, and limited motion. No obvious abnormalities in the contralateral breast were found. There is no obvious mass in the bilateral axilla. The color ultrasound of the breast showed that there was a low-echo mass located at the edge of the mammary gland at 2 points in the left upper quadrant, with a size of 23×25×19mm, the boundary was not clear, and the shape was irregular, and no strong punctate echo was observed (Figure 1A). The combination of contrax-enhanced ultrasound suggested hypoechoic mass in the left breast, uneven enhancement in the arterial phase, unsmooth boundary, slow regression in the venous phase, and obvious enlargement in the lesion area identified as BI-RADS Category V. Reactive hyperplasia of bilateral axillary lymph nodes.
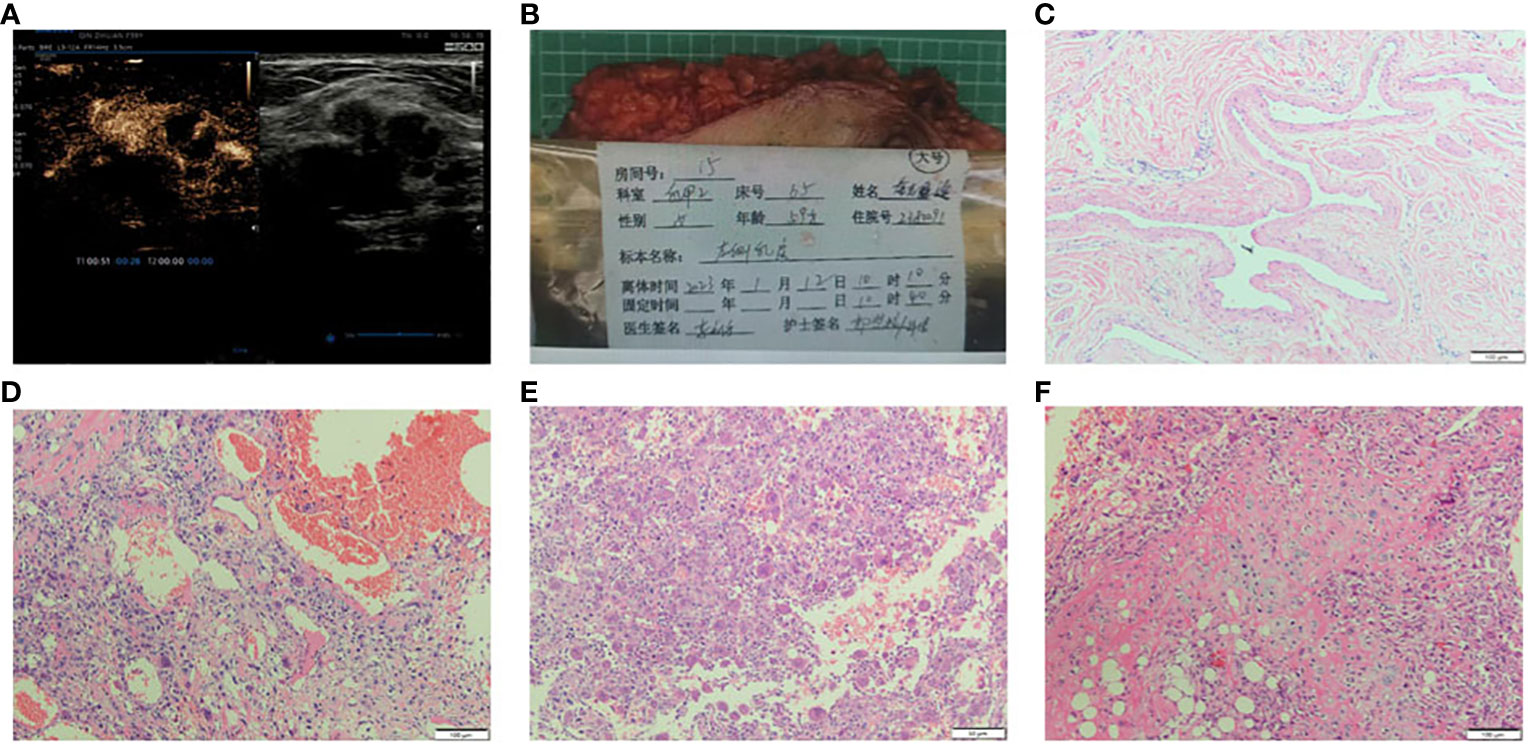
Figure 1 (A) Breast US images revealed a hypoechoic irregular mass in the breast left upper quadrant, with a size of about 23×25×19mm, the boundary was not uneven and no strong punctate echo was observed. The combination of contrax-enhanced ultrasound suggested the mass uneven enhancement in the arterial phase, unsmooth boundary, slow regression in the venous phase, and obvious enlargement in the lesion area. (B) The whole excised tissue of the left breast. (C) The resected specimen showed leaf-like (phyllodes) epithelial pattern (HE×100). (D) Stromal overgrowth, tumor giant cells and many abnormal mitosis are noted (HE×100). (E) The stroma showed a tumor osteoid rimmed by tumor cells along with osteoclastic giant cells, osteosarcoma differentiation (HE×100). (F) The section showed tumor cells with chondrosarcomatous differentiation, the chondrocyte density is different, the cell atypia is obvious, the nuclear mitosis and cartilage islands can be observed (HE×100).
Preoperative core needle biopsy (CNB) was performed on the left breast mass on January 11, 2023. Pathological diagnosis was malignant tumor, the specific type was not clear, and further diagnosis was to be made after surgery. The patient given up breast conserve surgery. Mastectomy and sentinel lymph node biopsy of the left breast was performed. No metastasis was found in 3 sentinel lymph nodes identified by carbon nanoparticles and methylene blue double staining. Pathological examination results: the size of the left breast was 23×18×3.0cm, and the fusiform skin was attached, the size of which was 19.5×7.0cm (Figure 1B). The size of the tumor was 3.0cm×2.5cm×2.2cm. The section was grayish-white and slightly hard in nature, and the boundary was poorly defined or poorly circumscribed. The tumor was 2.2cm away from the skin and 0.5cm away from the deep margin. TNM stage given was pT2N0M0.
The tumor is composed of two components: (1) The normal breast lobular structure is disordered or disappeared. The tumor has a lobulated mass (Figure 1C), which is composed of epithelial and stromal components. The epithelial cells are columnar or cuboidal without obviously atypia. Stromal cells are fusiform, the cytological atypia was obvious (Figure 1D), with 10 mitotic images/10HPF. And the epithelioid stromal cells are interspersed with short fusiform plump cells and mononuclear/multinucleoma giant cells. Local stromal cells form pseudoadenoid or clumps or nests. Mesenchymal myxoid changes in some areas of the tumor, the mesenchymal cells are sparse, epithelioid or spindle, and scattered tumor giant cells are also seen. About 1/5 of the tumor interstitial tissues showed fibrosis or hyalinoid degeneration. (2) Heterogenic components of tumor mesenchyma were observed: cartilage and bone tissue. The cartilage tissue presented cartilaginous islands of different sizes. The stroma showed a tumor osteoid rimmed by tumor cells along with osteoclastic giant cells, osteosarcoma differentiation. Tumor cells surrounded trabeculae, and the cell atypia was significant, showing mitotic images (Figure 1E). Chondrocytes of different density were observed with obvious cell atypia, and mitotic images (Figure 1F). ③ Immunohistochemistry: Spindle tumor cells ER(-), PR(-), HER-2(-), CK-pan(-), CK7(-), CK8(-), SOX10(-), S100(-), and MDM2(-), CK5/6(-), P63(-), P40(-) were all negative. CD34:(+), SATB2(+), P53(90% strong), CD68 (+), Ki-67(LI: about 60%) (Figure 2). ④ No ductal carcinoma in situ was found in the breast, and no metastasis were found in axillary lymph nodes. Pathological consultation advice of Union Hospital Affiliated to Tongji Medical College of Huazhong University of Science and Technology: Malignant (breast) phyllodes tumor with osteosarcoma and chondrosarcoma differentiation was considered.
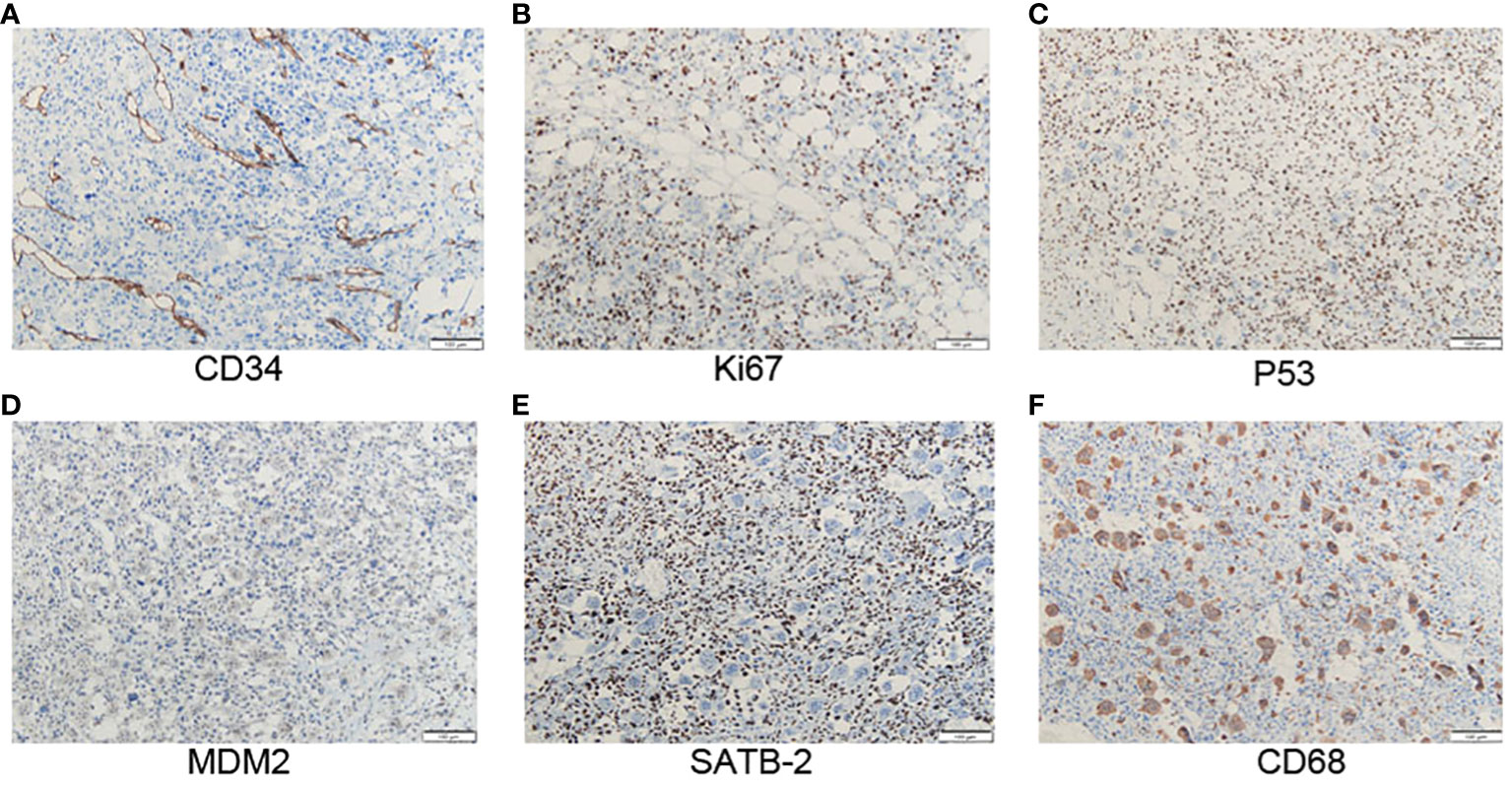
Figure 2 (A) Positive staining with CD34 in atypical stromal cells (x100). (B) The positive staining with Ki-67 in atypical stromal cells is about 60% (x100). (C) The positive staining with p53 in atypical stromal cells is about 90% (x100). (D) MDM2 is negative expressed in stromal cells (x100). (E) SATB2 is positively stained in atypical stromal cells (x100). (F) CD68 is expressed in osteoclastic giant cells (x100).
Two-color separation probe kit was purchased from Lbp Medicine Science & Technology (Guangzhou, China) which was adopted to detect USP6 on formalin-fixed, paraffin-embedded (FFPE) tissue sections. The probe is located on chromosome 17p13.2, the proximal (proximal centromere) probe marks red fluorescence, and the distal (proximal telomere) probe marks green fluorescence. Fluorescence in situ hybridization (FISH) testing procedure was in strict accordance with the standardized steps: The 3-5μm thick tissue was sliced, incubated for 2 h at (65 ± 5)°C, dewaxed and hydrated, deionized boiled at (100 ± 5)°C for 20 min, digested by pepsin for 15 min, dripped with a probe, denatured at 85°C for 5 min, and hybridized at 37°C for 10-18 h. Nuclear restaining of 4 ‘, 6⁃diamidino⁃2 ‘phenylindole (DAPI) under fluorescence microscope.
Results Interpretation: The FISH results were interpreted independently by two experienced pathologists: 200 neoplastic cells in a blind fashion using an Olympus BX53 fluorescence microscope (Japan) were counted, the distance between red signal and green signal was > 2 signal points as positive cells, and the proportion of positive cells was > 10% as positive for separation and rearrangement (7). FISH showed USP6 positively rate was 2% which indicated negative expression on sections (Figure 3).
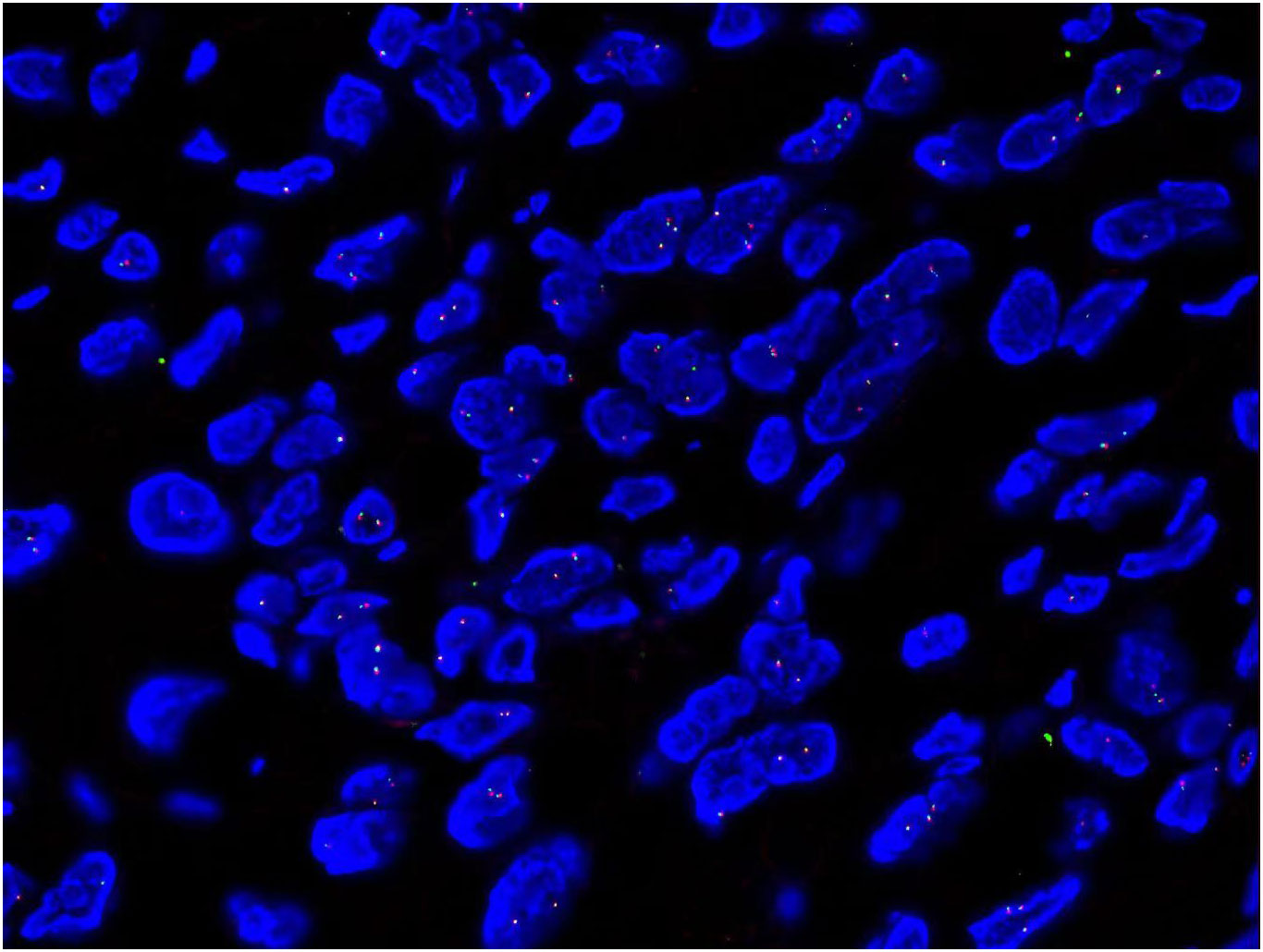
Figure 3 FISH showed USP6 positively rate was 2% which indicated negative expression on sections (x1000).
After radical resection of the left breast, anthracycline and ifosfamide chemotharapy were adopted for four cycles, and the patient still survived without any recurrence after eight months of follow up.
MPTs are identified when the tumor exhibits marked stromal nuclear pleomorphism, stromal overgrowth, with infiltrating borders, markedly increased stromal cellularity, stromal overgrowth with severe nuclear atypia (8). Higher malignancy grade, presence of heterologous elements, younger age, larger tumor size, and recent rapid tumor growth are poor prognostic factors for MPTs of the breast (9). Heterologous differentiation in MPTs is exceedingly rare, but there are MPTs with chondrosacomatous or osteosarcomatous differentiation reported (10). MPTs with the histological osteosarcomatous subtype increase mortality by 33% (11). MPTs accompanied by osteosarcoma only accounts for 1.3% of phyllodes tumors in the breast (12). According to Silver et al, MPTs with osteosarcomatous components are potentially more aggressive and could spread to the lung, bone, brain, contralateral breast. In our patient, we describe a MPT with osteosarcoma and chondrosarcomatous differentiation.
Stromal and epithelial cells of the breast tissues are the mainly components to develop malignancy. According to the different malignancy of both cells, it could been diagnosed as benign or malignant diseases (13). In the present case, most of the stromal cells were fibrosarcoma-like interlacing fascicles of spindle cells with stromal overgrowth. Multinucleated stromal giant cells have been rerorted in phyllodestumors. Focal areas of osteosarcoma and/or chondrosarcomatous differentiation were hemothera. MPT is also diagnosed when malignant heter-elements such as osteosarcoma, chondrosarcoma, and rhabdomyo-sarcoma are present even if the other features are absent. Chondrosarcomatous component even constituted over 80% of the tumor volume which is indeed rare (14).
In breast tumor, it is found that osteosarcoma or chondrosarcoma might occur in 3 different diseases: primary osteosarcoma of the breast as with a pure osteosarcoma or chondrosarcoma, as the stromal component of a histologically MPT, or as osteosarcomatous or chondrosarcomatous differentiation in a metaplastic carcinoma (15). Primary osteosarcoma of the breast is also a rare primary breast tumor which accounts for only 1% of breast tumors and < 5% of all osteosarcomas (16). The possibility of metastasis of osteosarcoma from other sites should be ruled out first, and it has the following two characteristics in histology: neoplastic osteogenesis or osteoid matrix; No epithelial component. The immunophenotype of primary osteosarcoma was strongly positive for vimentin, strongly positive for CD68 in osteoclastic multinucleated giant cells, and negative for ER, PR, Her-2 and epithelial markers (17). Some evidence suggests that MPTs with osteosarcomatous hemotherapy on are more aggressive, but compared with primary osteosarcomas in general, they have a much lower risk of metastasis (18).
Metaplastic carcinoma of the breast also have sarcoma like elements, including spindle cell sarcoma, chondrosarcoma, osteosarcoma, rhab-domyosarcoma, or a mixture of them (19). However, high molecular weight cytokeratin and p63 usually were positive in metaplastic carcinoma. These could be helpful in hemotherapyio these two tumors (20). It is also reported that p63 could also been diffusely and focally expressed in MPT (21). In this patient, we could also observe sporadic p63 expression, but lack of CK expression, these molecular markers help to eliminate metaplastic carcinoma or primary osteosarcoma. Special AT-rich sequence-binding protein 2 (SATB2) induces local chromatin loops to facilitate transcription. SATB2 immunostaining is commonly used as a marker for colorectal adenocarcinoma and osteosarcoma (22). In our patient, SATB2 expression was strong positive, which indicated osteosarcoma tissues. CD34 is also observed in MPT (23), this is in line with our case, that CD34 is positively expressed.
Another differential diagnosis of MPT is breast myositis ossificans. Myositis ossificans is defined as a self-limiting pseudotumor composed of reactive hypercellular fibrous tissue and bone. USP6 rearrangements have been identified as a consistent genetic driving event in aneurysmal bone cyst and nodular fasciitis (24). It is therefore an integral part of the diagnostic workup when dealing with (myo)fibroblastic lesions of soft tissue and bone. USP6 rearrangement provided evidence of a relationship with nodular fasciitis and aneurysmal bone cyst (25). In our patient, the USP-6 is negative and supplies another strong proof to eliminate the diagnosis of myositis ossificans.
Recent next generation sequencing analyses had revealed novel genetic alterations in PT but lacked a further hemotherapyion of their relationship to different PT features and outcome (26). Malignant progression is associated with heterocytogenetic abnormalities, including MYC amplification, p53 mutation, increases in chromosomes 1q, 5p, 7 and 8, and loss of 6q, 9p, 10p, 13q, 16q and 19 (27). MED12 mutations are associated with alterations in related genes in the Wnt, TGFB, and THRA pathways (28, 29). Lin et al. demonstrated that ALDH1 and/or GD2 markers could be used for cancer stem cell research in patients with MPT (30).
The epithelial-mesenchymal transition (EMT) increased with the progression of MPTs tumor grade. Nuclear expression of the EMT proteins TWIST and Foxc2 is associated with increased tumor grade and deterioration of histological features (31, 32). Additional mutations or copy number alterations in known cancer driver genes NF1, RB1, TP53, PIK3CA, ERBB4, and EGFR have been identified in borderline and malignant MPTs through next-generation sequencing (33, 34). These molecules provide an important biological basis for the occurrence and development of MPTs, and provide a theoretical basis for molecular diagnosis and therapeutic targets in clinical practice.
Metastatectomy has been correlated with increased overall survival (of 25.9 versus 9.9 months; P = .01) in MPT (35). The definitive treatment for phyllodes tumor is wide surgical excision with at least 1-2 cm of negative margins, or mastectomy, depending on the size of the tumor and the patient’s breast size (36). Histological size ≥45 mm and dense stromal cellularity were demonstrated as histological risk factors of local recurrence of PT (37). Radiotherapy has often been associated with palliation and pain control in metastatic, malignant neoplasia. Anthracycline containing chemotherapy regimens has been associated with improved overall survival (22.4 months versus 13.2 months; P = .040). Anthracycline and alkylating agent-based combination regimens were most frequently administered (38). In the present case, metastatectomy was performed, anthracycline and ifosfamide hemotherapy were adopted, and the patient still survived without any recurrence.
Our case report illustrates that breast osteosarcoma and chondrosarcoma differentiation originating from an MPT is remarkably difficult to diagnose and manage. The standard treatment comprises complete excision of the tumor with wide margins or total mastectomy. A multiple oncology gene mutations happen and promote the malignant progression. The adjuvant therapy is still controversial due to the lack of multi-center large patients records and suitable clinical trials. Further research must be conducted to elucidate accurate diagnosis and clarify the best treatment for these tumors.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
The studies involving humans were approved by Ethics Committee of Shiyan Taihe Hospital (NO.2023KS55). The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
WL: Writing – review & editing, Writing – original draft, Resources, Funding acquisition. QO: Writing – original draft, Project administration, Methodology, Investigation, Data curation. YL: Writing – original draft, Methodology, Data curation. LY: Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was supported by the Shiyan City Scientific Research and Development Project (22Y32).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Bogach J, Sriskandarajah A, Wright FC, Look Hong N, Canadian Phyllodes Tumor Consensus Panel. Phyllodes tumors of the breast: Canadian national consensus document using modified delphi methodology. Ann Surg Oncol. (2023) 30:6386–6397. doi: 10.1245/s10434-023-13912-7
2. Chen K, Xu J, Wang W, Jiang R, Zhang H, Wang X, et al. Clinical outcomes and biomarkers of phyllodes tumors of the breast: a single-center retrospective study. Cancer Med. (2023) 12:11363–74. doi: 10.1002/cam4.5849
3. Tan BY, Fox SB, Lakhani SR, Tan PH. Survey of recur rent diagnostic challenges in breast phyllodes tumours. Histopathology. (2023) 82:95–105. doi: 10.1111/his.14730
4. Sars C, Sackey H, Frisell J, Dickman PW, Karlsson F, Kindts I, et al. Current clinical practice in the management of phyllodes tumors of the breast: an international cross-sectional study among surgeons and oncologists. Breast Cancer Res Treat. (2023) 199:293–304. doi: 10.1007/s10549-023-06896-1
5. Louie AD, Rosenberger LH. Phyllodes tumors of the breast: addressing the gaps in consensus recommendations for clinical management. Ann Surg Oncol. (2023) 30:6296–8. doi: 10.1245/s10434-023-14147-2
6. Md Nasir ND, Koh VCY, Cree IA, Ruiz B, Águila JD, Armon S. Phyllodes tumour evidence gaps mapped from the 5th edition of the WHO classification of tumours of the breast. Histopathology. (2023) 82:704–12. doi: 10.1111/his.14856
7. Zhang Y, Qiu Y, Zhang X, He X, Chen C, Chen M, et al. USP6-associated soft tissue tumors with bone metaplasia: clinicopathologic and genetic analysis and the identification of novel USP6 fusion partners. Front Oncol. (2023) 12:1065071. doi: 10.3389/fonc.2022.1065071
8. Wei Y, Dai Y, Guan Q, Min N, Geng R, Hu H, et al. Predicting the recurrence-free survival of phyllodes tumor of the breast: a nomogram based on clinicopathology features, treatment, and surgical margin. Gland Surg. (2023) 12:152–64. doi: 10.21037/gs-22-542
9. Liu J, Li F, Liu X, Lang R, Liang R, Lu H. Malignant phyllodes tumors of the breast: the Malignancy grading and associations with prognosis. Breast Cancer Res Treat. (2023) 199:435–44. doi: 10.1007/s10549-023-06933-z
10. Ko SY. Malignant phyllodes tumor of the breast with heterologous osteosarcoma and chondrosarcomatous differentiation: a rare case report with imaging findings. Radiol Case Rep. (2023) 18:1982–8. doi: 10.1016/j.radcr.2023.02.039
11. Tan BY, Acs G, Apple SK, Badve S, Bleiweiss IJ, Brogi E, et al. Phyllodes tumours of the breast: a consensus review. Histopathology. (2016) 68:5–21. doi: 10.1111/his.12876
12. Silver SA, Tavassoli FA. Osteosarcomatous differentiation in phyllodes tumors. Am J Surg Pathol. (1999) 23:81 5–21. doi: 10.1097/00000478-199907000-00010
13. Ni Y, Tse GM. Spindle cell lesions of the breast: a diagnostic algorithm. Arch Pathol Lab Med. (2023) 147:30–7. doi: 10.5858/arpa.2022-0048-RA
14. Vera-Sempere F, García-Martínez A. Malignant phyllodes tumor of the breast with predominant chondrosarcomatous differentiation. Pathol Res Pract. (2003) 199:841–5. doi: 10.1078/0344-0338-00505
15. Hall RR, Schammel CM, Devane AM, Scopteuolo A, Schammel DP. High grade phyllodes tumor with osteosarcomatous differentiation: case report and review of the literature. Radiol Case Rep. (2023) 18:3127–34. doi: 10.1016/j.radcr.2023.05.074
16. Hu QC, Mei X, Feng Y, Ma JL, Zhao ZY, Shao ZM, et al. Early local recurrence presents adverse effect on outcomes of primary breast sarcoma[J]. Medicine(Baltimore). (2016) 95:e2422. doi: 10.1097/MD.0000000000002422
17. Gutnik L, Ren Y, Thomas SM, Plichta JK, Greenup RA, Fayanju OM, et al. Malignant phyllodes tumor and primary breast sarcoma; distinct rare tumors of the breast. J Surg Oncol. (2022) 125:947–57. doi: 10.1002/jso.26820
18. Laforga JB. Malignant giant phyllodes tumor with heterologous osteosarcomatous differentiation and aneurysmatic bone cyst-like features. Breast J. (2020) 26:1387–8. doi: 10.1111/tbj.13788
19. Chaudhary D, Balhara K, Mandal S, Mallya V, Tomar R, Khurana N, et al. Metaplastic breast carcinoma: analysis of clinical and pathologic features, a five-year study. J Cancer Res Ther. (2023) 19:1226–30. doi: 10.4103/jcrt.jcrt_1229_21
20. Yang X, Chen L, Shen Y. Breast metaplastic carcinoma with osteosarcomatous differentiation: a case report and literature review. Clin Pathol. (2022) 15. doi: 10.1177/2632010X221118056
21. Chu X, Wu M, Yang J, Fu Y, Wang X, Wang H, et al. Organoid models derived from patients with Malignant phyllodes tumor of the breast. Breast Cancer Res Treat. (2023) 200:193–201. doi: 10.1007/s10549-023-06973-5
22. Dum D, Kromm D, Lennartz M, De Wispelaere N, Büscheck F, Luebke AM, et al. SATB2 expression in human tumors: a tissue microarray study on more than 15 000 tumors. Arch Pathol Lab Med. (2023) 147:451–64. doi: 10.5858/arpa.2021-0317-OA
23. Chu X, Wu M, Yang J, Fu Y, Wang X, Wang H, et al. Organoid models derived from patients with Malignant phyllodes tumor of the breast. Breast Cancer Res Treat. (2023) 200:193–201. doi: 10.1007/s10549-023-06973-5
24. Wang JC, Li WS, Kao YC, Lee JC, Lee PH, Huang SC, et al. Clinicopathological and molecular characterisation of USP6-rearranged soft tissue neoplasms: the evidence of genetic relatedness indicates an expanding family with variable bone-forming capacity. Histopathology. (2021) 78:676–89. doi: 10.1111/his.14268
25. Bekers EM, Eijkelenboom A, Grünberg K, Roverts RC, de Rooy JWJ, van der Geest ICM, et al. Myositis ossificans - another condition with USP6 rearrangement, providing evidence of a relationship with nodular fasciitis and aneurysmal bone cyst. Ann Diagn Pathol. (2018) 34:56–9. doi: 10.1016/j.anndiagpath.2018.01.006
26. Tsang JY, Shao Y, Poon IK, Ni Y-B, Kwan JS, Chow C, et al. Analysis of recurrent molecular alterations in phyllodes tumour of breast: insights into prognosis and pathogenesis. Pathology. (2022) 54:678–85. doi: 10.1016/j.pathol.2022.03.008
27. Kuijper A, Snijders M, Berns J, Kuenen-Boumeester V, Wall E, Albertson DG, et al. Genomio profiling by arary comparative genomic hybridization reveals novel DNA copy number changes in breast phyllodes tumours. Cell Oncol. (2009) 31:31–9. doi: 10.1155/2009/410672
28. Chang HY, Koh VCY, Md Nasir ND, Ng CCY, Guan P, Thike AA, et al. MED12, TERT and RARA in fibroepithelial tumours of the breast. J Clin Pathol. (2020) 73:51–6. doi: 10.1136/jclinpath-2019-206208
29. Piscuoglio S, Ng CK, Murray M, Burke KA, Edelweiss M, Geyer FC, et al. Massively parallel sequencing of phyllodes tumours of the breast reveals actionable mutations, and TERT promoter hotspot mutations and TERT gene amplification as likely drivers of progression. J Pathol. (2016) 238:508–18. doi: 10.1002/path.4672
30. Lin JJ, Huang CS, Yu J, Liao GS, Lien HC, Hung JT. Malignant phyllodes tumors display mesenchymal stem cell features and aldehyde dehydrogenase/disialoganglioside identify their tumor stem cells. Breast Cancer Res. (2014) 16:R29. doi: 10.1186/bcr3631
31. Wang J, Wang WL, Sun H, Huo L, Wu Y, Chen H, et al. Expression of TRPS1 in phyllodes tumor and sarcoma of the breast. Hum Pathol. (2022) 121:73–80. doi: 10.1016/j.humpath.2022.01.002
32. Chen K, Xu J, Wang W, Jiang R, Zhang H, Wang X, et al. Clinical outcomes and biomarkers of phyllodes tumors of the breast: a single-center retrospe ctive study. Cancer Med. (2023) 12:11363–74. doi: 10.1002/cam4.5849
33. Muller KE, Tafe LJ, de Abreu FB, Peterson JD, Wells WA, Barth RJ, et al. Benign phyllodes tumor of the breast recurring as a Malignant phyllodes tumor and spindle cell metaplastic carcinoma. Hum Pathol. (2015) 46:327–33. doi: 10.1016/j.humpath.2014.10.014
34. Tan J, Ong CK, Lim WK, Ng CCY, Thike AA, Ng LM, et al. Genomic landscapes of breast fibroepithelial tumors. Nat Genet. (2015) 47:1341–5. doi: 10.1038/ng.3409
35. Goodwin B, Oyinlola AF, Palhang M, Lehman D, Platoff R, Atabek U, et al. Metastatic and Malignant phyllodes tumors of the breast: an update for current management. Am Surg. (2023) 89(12):6190–6. doi: 10.1177/00031348231198114
36. Zervoudis S, Xepapadakis G, Psarros N, Bothou A, Tsikouras P, Galazios G, et al. Management of Malignant and borderline phyllodes tumors of the breast: our experience. J Buon. (2019) 24:1521–5.
37. Mimoun C, Legay L, Lorphelin H, Leveau-Vallier AS, Cornelis F, Miquel C, et al. Histological risk factors for local recurrence of phyllodes tumors of the breast. Anticancer Res. (2023) 43:143–7. doi: 10.21873/anticanres.16143
Keywords: malignant phyllodes tumors, breast tumor, osteosarcoma, chondrosarcoma, thoracic oncology
Citation: Li W, Ou Q, Li Y and Yuan LY (2024) Malignant phyllodes tumor of the breast with predominant osteosarcoma and chondrosarcomatous differentiation: a rare case report and review of literature. Front. Oncol. 14:1372710. doi: 10.3389/fonc.2024.1372710
Received: 18 January 2024; Accepted: 03 April 2024;
Published: 19 April 2024.
Edited by:
Dirk Geerts, University of Amsterdam, NetherlandsReviewed by:
Eunice Van Den Berg, University of the Witwatersrand, South AfricaCopyright © 2024 Li, Ou, Li and Yuan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qin Ou, b3VxaW4xOTgwQDE2My5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.