- 1Department of Oncology, Affiliated Hospital of Guizhou Medical University, Guiyang, Guizhou, China
- 2School of Clinical Medicine, Guizhou Medical University, Guiyang, Guizhou, China
- 3Department of Oncology, The Affiliated Cancer Hospital of Guizhou Medical University, Guiyang, Guizhou, China
Purpose: This study aimed to evaluate 5-year outcomes and the late toxicity profile of chrono-chemotherapy with different infusion rates in patients with locally advanced nasopharyngeal carcinoma (NPC).
Methods and materials: Our retrospective analysis included 70 patients with locally advanced NPC stages III and IVB (according to the 2010 American Joint Committee on Cancer staging system). Patients were treated with two cycles of induction chemotherapy (IC) before concurrent chemoradiotherapy (CCRT) at Guizhou Cancer Hospital. The IC with docetaxel, cisplatin (DDP) and fluorouracil regimen. Patients were divided into two groups during CCRT. Using a “MELODIE” multi-channel programmed pump, DDP (100 mg/m2) was administered for 12 hours from 10:00 am to 10:00 pm and repeated every 3 weeks for 2-3 cycles. DDP was administered at the peak period of 4:00 pm in the sinusoidal chrono-modulated infusion group (Arm A, n=35). The patients in Arm B received a constant rate of infusion. Both arms received radiotherapy through the same technique and dose fraction. The long-term survival and disease progression were observed.
Results: After a median follow-up of 82.8 months, the 5-year progression-free survival rate was 81.3% in Arm A and 79.6% in Arm B (P = 0.85). The 5-year overall survival rate was not significantly different between Arm A and Arm B (79.6% vs 85.3%, P = 0.79). The 5-year distant metastasis-free survival rate was 83.6% in Arm A and 84.6% in Arm B (P = 0.75). The 5-year local recurrence-free survival rate was 88.2% in Arm A and 85.3% in Arm B (P = 0.16). There were no late toxicities of grade 3-4 in either group. Both groups had grade 1-2 late toxicities. Dry mouth was the most common late toxic side effect, followed by hearing loss and difficulty in swallowing. There was no statistically significant difference between Arm A and Arm B in terms of side effects.
Conclusion: Long-term analysis confirmed that in CCRT, cisplatin administration with sinusoidal chrono-modulated infusion was not superior to the constant infusion rate in terms of long-term toxicity and prognosis.
Introduction
Nasopharyngeal carcinoma (NPC) is considered a rare form of cancer globally. However, high incidence in specific geographic and ethnic populations is noteworthy (1, 2). NPC is endemic in Southeast Asia and Southern China, particularly in the Guangdong province (3). Platinum-based concurrent chemoradiotherapy (CCRT) combined with induction chemotherapy (IC) or adjuvant chemotherapy (AC) is the standard treatment regimen for locally advanced NPC. However, studies have shown that compared to CCRT-AC strategy, IC-CCRT offers the advantages of better tolerance and early eradication of micro metastases (4, 5). CCRT has been shown to have the highest benefit in patients with locally advanced NPC. The specific regimen includes commonly used DDP at 100 mg/m2 every 3 weeks during intensity modulated radiation therapy (IMRT) for 2-3 cycles. However, adding DDP chemotherapy to radiotherapy increases the incidence of treatment-related toxic side effects, which reduces patient treatment compliance and quality of life (6–8).
Chrono-chemotherapy is based on the changes of the biological rhythm. If administered at appropriate times, not only can it reduce the adverse reactions of chemotherapy and improve the quality of life, it can also improve the immune function (9–12). Studies have shown that using a “MELODIE” multi-channel programmed pump during sinusoidal chrono-modulated infusion for IC in NPC, the DDP infusion time lasts from 10:00am to 10:00pm, and the peak delivery time occurs at 4:00 pm. The patients in the second group received infusions at a constant rate. As a result, chrono-chemotherapy significantly reduced stomatitis but did not show a superior therapeutic response (11, 13).
In our previous study, we compared the advantages and disadvantages of DDP administered through sinusoidal chrono-modulated infusion and at a constant rate of infusion to investigate the role of chrono-chemotherapy in CCRT for NPC. We found no significant difference in acute toxic side effects; efficacy; and the 2-year overall survival (OS), progression-free survival (PFS), and disease-free survival (DFS) between the two groups. However, the sinusoidal chrono-modulate infusion group showed improved T cell immunity (14). Herein, we aimed to report the updated 5-year detailed analyses of survival outcomes and late toxic effects to assess the ultimate therapeutic efficacy of sinusoidal chrono-modulated infusion and the constant infusion rate during CCRT.
Materials and methods
Patient selection
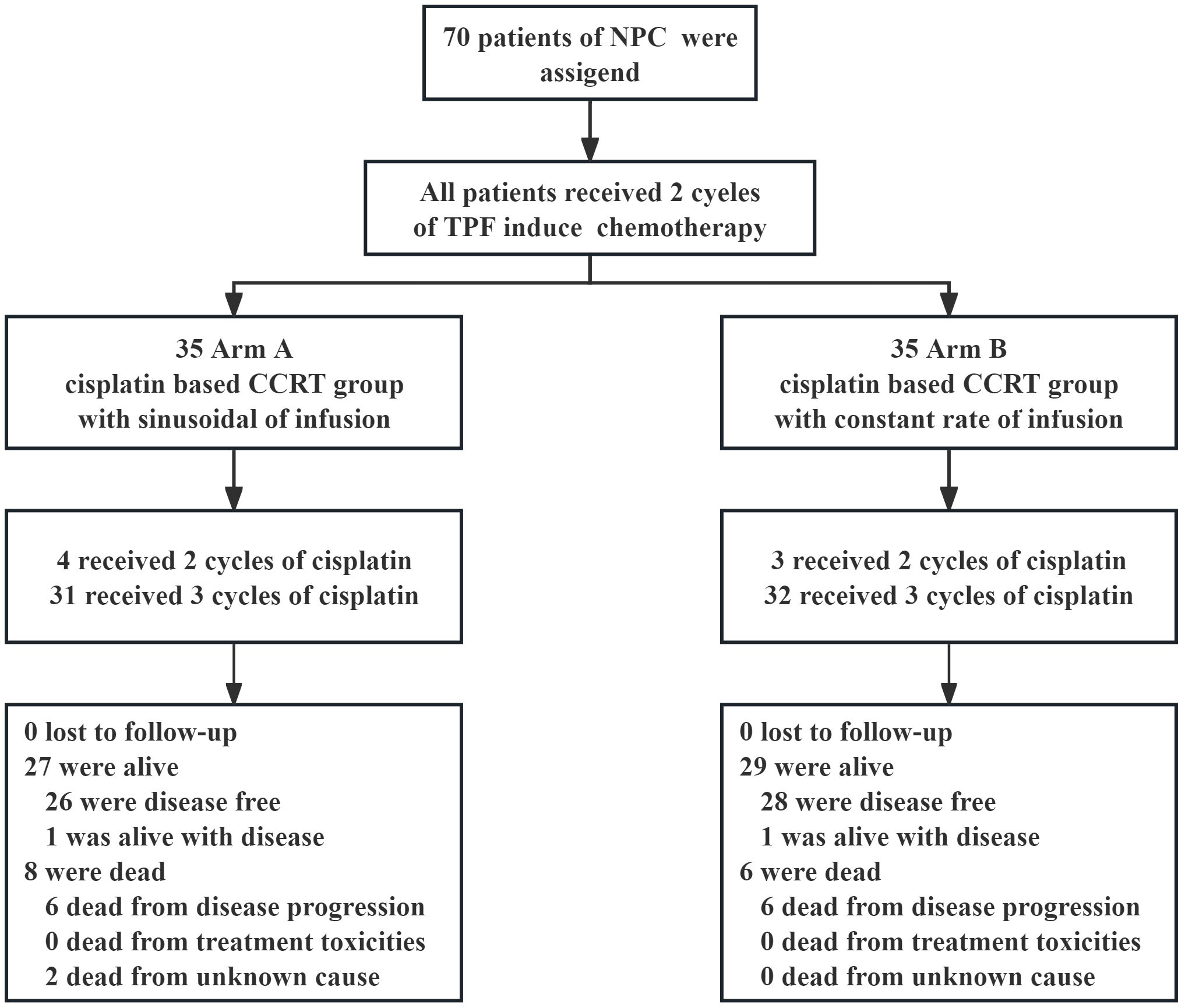
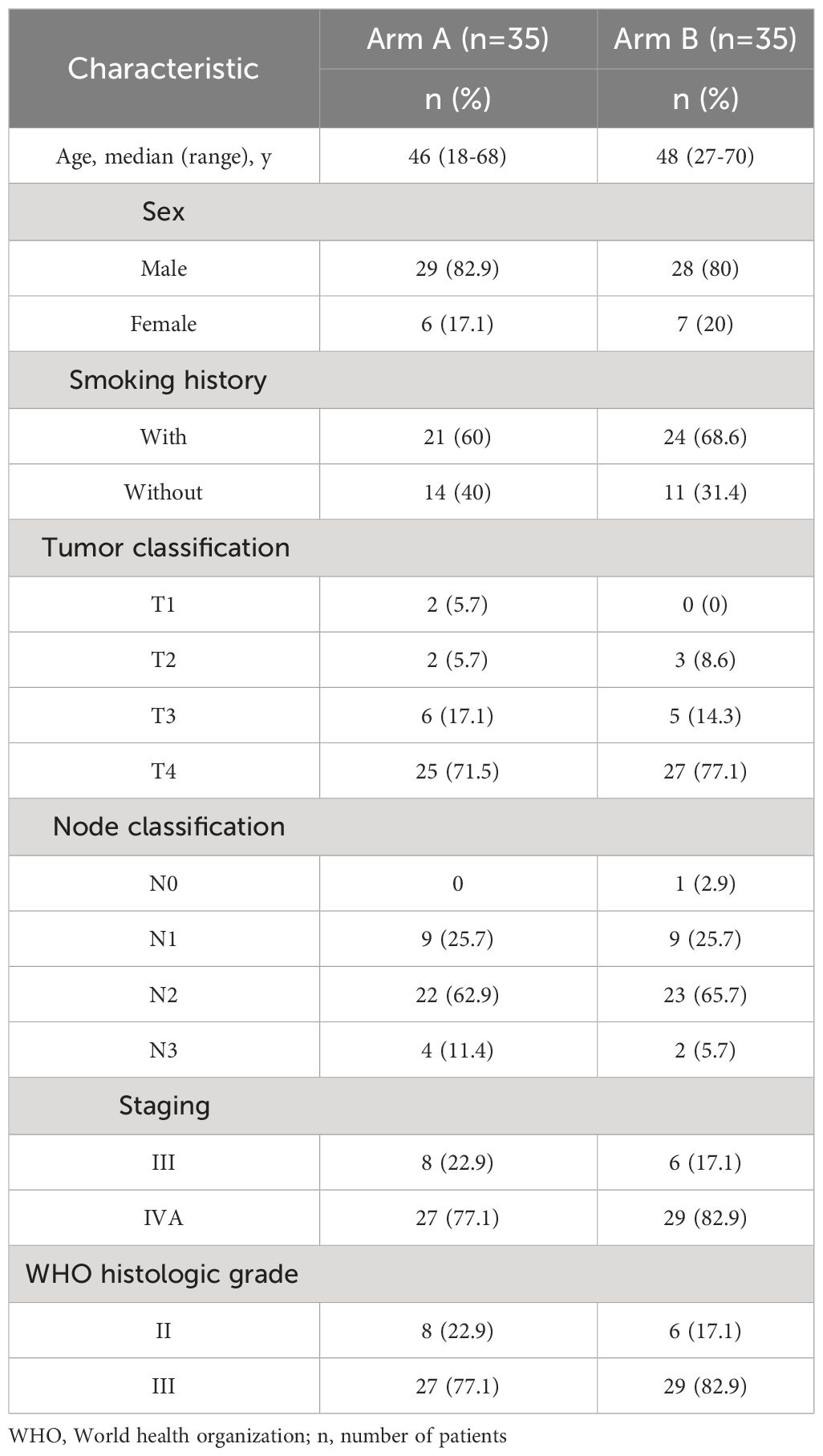
We included 70 patients with locally advanced non-keratinizing NPC (type II/III World Health Organization classification) who were treated in Guizhou Cancer Hospital between December 2013 and March 2017. NPC was newly diagnosed and confirmed by biopsy. Follow-up data were evaluated retrospectively. According to the different infusion rates of cisplatin during CCRT, the patients were divided into sinusoidal chrono-modulated infusion group (Arm A) and constant rate infusion group (Arm B).
The eligibility criteria were (1) newly diagnosed pathology stage III, IVa, and IVb NPC by (according to the 2010 American Joint Committee on Cancer [AJCC] staging system) and receiving initial treatment; (2) between 18 and 70 years old;(3) normal hematologic, kidney, and liver function; (4) the Karnofsky Performance Status (KPS) Scale score of 70 or higher and (5) no distant metastasis. The exclusion criteria were (1) contraindications to radiotherapy or chemotherapy; (2) previous treatment for NPC;(3) prior or synchronous malignant disease; (4) serious dysfunction of organs such as heart, liver, and kidney; (5) primary distant metastasis; and (6) pregnant and lactating mothers.
Treatment regimen
All patients received 2 cycles of docetaxel, DDP, and fluorouracil (TPF) based IC in 21 day cycles followed by CCRT. The IC with docetaxel and DDP at 75mg/m2 administered through bolus infusion on the first day. Further, fluorouracil at 750 mg/m2 for 5 days was administered as continuous intravenous pumping. IMRT was administered once a day, five times a week (Monday to Friday) for 6 to 7 weeks. The radiation dose of target areas were set as GTVnx (gross tumor volume of the nasopharynx): 69.96Gy-73.92Gy/33 fraction (fr), 2.12-2.24Gy/1fr; PTVnx (planning target volume of the nasopharynx): 69.96 Gy/33fr, 2.12 Gy/1fr; GTVnd (gross tumor volume of the involved lymph nodes): 69.96 Gy/33fr, 2.12 Gy/1fr; CTV1 (clinical volume 1, high-risk clinical target volume): 60.06 Gy/33 fr, 1.82 Gy/1fr; and CTV2 (low-risk clinical target volume): 50.96 Gy/28 f,1.82 Gy/1fr. Chrono-chemotherapy was carried out during radiotherapy. Patients in Arm A were given 100 mg/m2 cisplatin from 10:00 am to 10:00 pm (peaked at 04:00 pm) on Day 1. With sinusoidal administration, the maximum velocity (Vmax) of administration during peak time was 0.65ml/min. Patients in Arm B received conventional intravenous infusion of 100 mg/m2 cisplatin from 10:00 am to 10:00 pm on Day 1. The uniform administration velocity was 0.42ml/min. The CCRT was administered in 21-day cycles for 2-3 cycles. The administration modes of the two groups are shown in Figure 1.

Figure 1 Different administration methods of cisplatin in two groups. Patients in Arm A underwent sinusoidal chrono-modulated infusion, and those in Arm B underwent flat intermittent constant rate infusion.
Follow-up
All patients were followed up every 3 months during the first 2 years, every 6 months during the third to fifth year, and then annually thereafter. During follow-up, the data was reviewed for blood and biochemistry profiles, magnetic resonance imaging of nasopharynx and neck, fibrous nasopharyngoscopy, chest computed tomography (CT) or chest X-ray, upper abdominal CT or abdominal ultrasound, and emission CT based on the patient’s clinical symptoms. Patients were followed up on an outpatient basis and their survival and long-term toxic side effects were recorded. Patients who did not return for follow-up were contacted by phone to assess their survival and side effects. Adverse reactions were evaluated by Common Terminology Criteria for Adverse Events (CTCAE 3. 0) (15). All patients were followed up until November 30, 2021, or death from any cause. The primary endpoint of the study was progression-free survival (PFS) calculated from the time of enrollment until first recurrence at any site, death from any cause, or patient examination at last follow-up. The secondary endpoints were OS defined as the time from registration to death from any cause, distant metastasis–free survival (DMFS), and local recurrence–free survival (LRFS). DMFS and LRFS were defined as the time from patient admission until first distant metastasis and local recurrence, respectively. Late toxic side effects were defined as those that occurred 6 months after completion of radiotherapy. Side effects were assessed and graded based on the Radiation Therapy Oncology Group/European Organization for Research and Treatment of Cancer morbidity-scoring schema.
Statistical analysis
The Statistical Package for Social Sciences (SPSS) software, version 24.0 (SPSS Inc), was used for statistical analyses. The incidence of late toxic side effects and other categorical variables were compared using the χ2 test or Fisher’s exact test as appropriate. Survival curves were plotted using Kaplan–Meier method, and log-rank test was conducted. Hazard ratios (HRs) with 95% confidence intervals (CIs) were calculated using a Cox proportional hazards regression model. Measurement data were expressed as mean ± standard deviation, and t-test was used for comparison between the two groups. Interaction and stratified analyses were conducted based on age, sex, WHO histologic grade, smoking status and cancer stage. Interaction is the situation wherein the association of one risk factor with a certain outcome variable differs across the strata of another risk factor (8). In this study, treatment methods and other potential prognostic factors (age [>47 or ≤47], sex [female or male], cancer stage [III or IVA], WHO histologic grade [II or III],smoking status[without or with]) were entered into the multivariate Cox proportional hazards regression model to test for their main effects, and an interaction term between treatment methods and the potential prognostic factors was then added into the model to test their interaction effect on survival. All statistical tests were two-sided, and a P value <0.05 was deemed statistically significant.
Results
Patient characteristics
In the initial and updated analyses, 70 patients were evaluated retrospectively. There were 35 cases each Arm A and Arm B. The patient selection profile is shown in Figure 2. The two treatment groups were well matched on baseline demographic and clinical characteristics (Table 1). All patients were diagnosed with non-keratinizing differentiated or undifferentiated NPC (WHO type II and III). The median patient age was 47 years (range, 18 to 70 years), and 81.4% of the patients were male; 45 (64.3%) patients had a history of smoking; and 63 (90%) patients had T3 or T4 primary tumors. Most patients had a nodal status of either N1 (25.7%) or N2 (64.3%).
Efficacy
The follow-up period ended on November 30, 2021, with a median follow-up time of 82.8 months. The 5-year PFS rate was 81.3% (95% CI, 76.4-93.6) in Arm A and 79.6% (95% CI, 75.2-94.1) in Arm B (log-rank P = 0.85). No statistically significant difference was found in the 5-year OS between Arm A and Arm B (79.6% vs 85.3%; 95% CI, 72.6-92.5 vs 84.9-98.5; log-rank P = 0.79). Distant metastasis and local recurrence represented a major failure pattern. The 5-year DMFS rate was 83.6% (95% CI, 76.5-95.1) in Arm A and 84.6%(95% CI, 76.5-95.1)in Arm B (P = 0.75). The 5-year LRFS rate was 88.2% (95% CI, 85.1-98.6) in Arm A and 85.3% (95% CI, 74.0-93.9) in Arm B (P = 0.16). As shown in Figure 3, the PFS, OS, DMFS, and LRFS curves were plotted using the Kaplan–Meier method, and log-rank test showed no statistically significant differences between Arm A and Arm B.
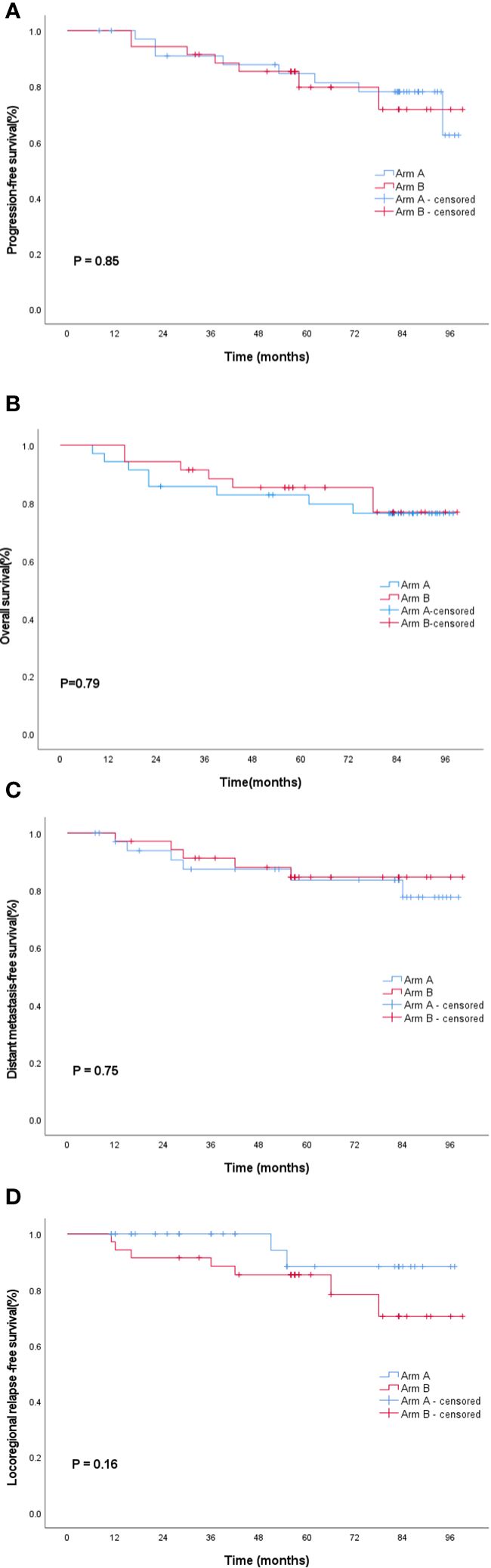
Figure 3 Survival curves of the entire cohort: (A) progression-free survival, (B) overall survival, (C) distant failure-free survival and, and (D) local recurrence-free survival. Blue lines indicate Arm A; red lines indicate the Arm B. Arm A, sinusoidal chrono-modulated infusion group; Arm B, constant rate infusion group.
Toxicity
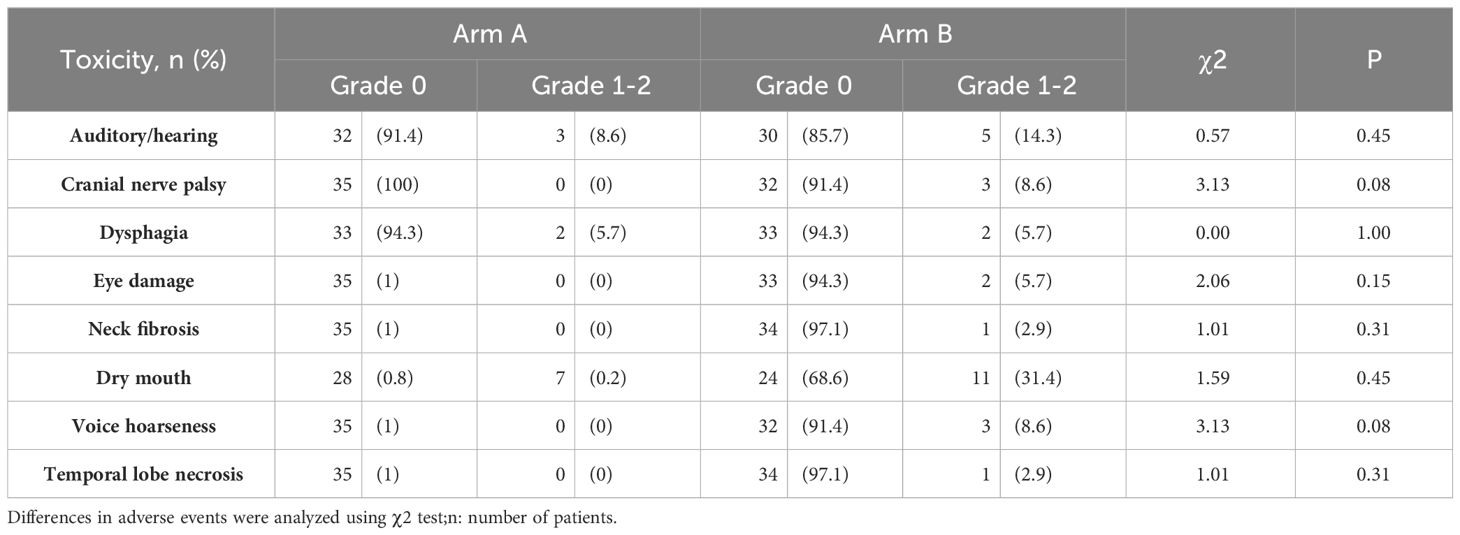
Publication of the early results of this trial included details of adverse events during treatment (14). In this long-term analysis, we evaluated the late toxic side effects that occurred after CCRT in Arm A and Arm B. There was no late toxicity of grade 3 to 4 in either group. Dry mouth was the most common late toxic side effect, followed by hearing loss and difficulty in swallowing. Other late toxic side effects included cranial neuropathy, eye damage, neck fibrosis, voice hoarseness, and brain radiation injury (Table 2). Although the incidence of 1-2 grade late toxicity in Arm B was higher than that in Arm A, there was no statistically significant difference.
Subgroup analyses
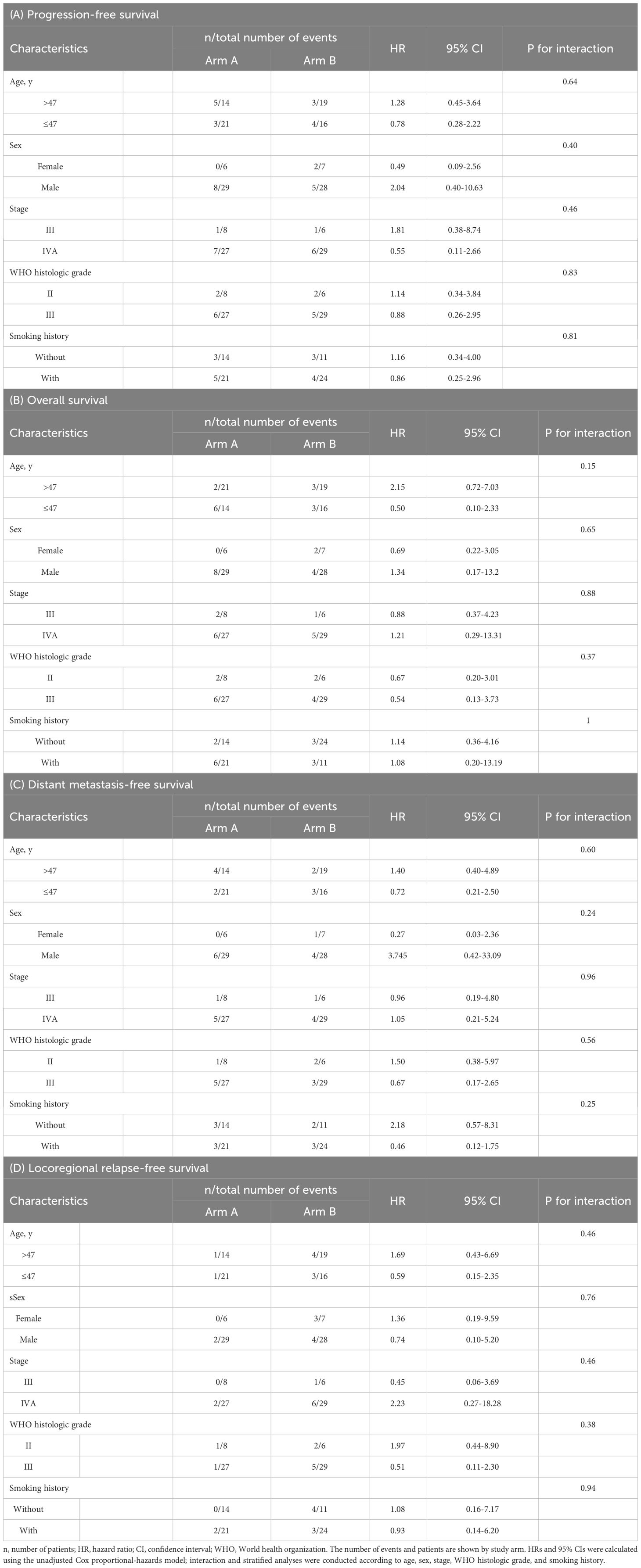
We further performed subgroup analyses for PFS, OS, DMFS, and LRFS in patients stratified by the following covariates: age (≤47 or>47), sex (female or male), disease stage (III or IVA), WHO histologic grade (type II/III), and smoking history (with or without). No interaction was observed between these covariates and the treatment group of PFS (age ≤47 years: HR, 0.78; 95% CI, 0.26-2.22; age >47: HR, 1.28; 95% CI, 0.45-3.64; P = 0.64 for interaction; female sex: HR, 0.49; 95% CI, 0.09-2.56 and male sex: HR, 2.04; 95% CI, 0.39-10.62; P = 0.40 for interaction; stage III disease: HR, 1.81; 95% CI, 0.38-8.74 and stage IVA disease: HR, 0.56; 95% CI, 0.11-2.66; P = 0.46 for interaction; WHO histologic grade type II: HR, 1.14; 95% CI, 0.34-3.84 and grade type III: HR, 0.88; 95% CI, 0.26-2.95; P = 0.83 for interaction; smoking history: HR, 0.86; 95% CI, 0.25-2.96; no smoking history: HR, 1.16; 95% CI, 0.34-4.00; P = 0.81 for interaction; Table 3). Similarly, no interaction was observed between these covariates in the two Arms of OS, DMFS, and LRFS. This indicated that the non-inferiority of sinusoidal chrono-modulated infusion group did not differ among specific populations.
Discussion
More than 70% of cases of newly diagnosed NPCs present with locally advanced disease (stage III/IV according to the sixth AJCC staging system) (16). CCRT followed by IC is the standard treatment mode for locally advanced NPC (17–20). Studies have shown that radiotherapy using IMRT can prolong the long-term survival in NPC compared with 2-dimensional radiotherapy (2DRT) (21, 22). The effects of concurrent radio- and chemotherapy drugs complement each other; however, although chemotherapy drugs can improve radiosensitivity, radiotherapy can enhance cytotoxicity. Therefore, the efficacy of concurrent radio- and chemotherapy in locally advanced NPC is better than that of radiotherapy alone (20, 23, 24). The standard chemotherapeutic regimen for NPC is DDP (100 mg/m2 in 21-day cycles). Although the efficacy of CCRT has improved, the incidence of adverse reactions has also increased, particularly the acute toxic reaction of DDP (25–27). Therefore, it is important to seek an effective treatment scheme for reducing the toxicity and side effects of cisplatin during CCRT.
The cell rhythm in malignant tumors is significantly different compared to normal cells (28). Disrupted biorhythms or circadian clock genes that are suppressed or mutated can trigger a variety of diseases including malignant tumors (29–33). Similar to most biological functions that are subject to circadian changes (34), pharmacodynamics and pharmacokinetics are influenced by circadian rhythms (35). Pharmacokinetics determines the optimal drug concentration required to produce a balance between efficacy and toxicity (36, 37). Drugs, such as anti-mitotic agents, anti-metabolites, alkylating agents, or inserters, usually achieve an optimal anti-tumor efficacy when used at the time of day when they are best tolerated, but this property is not always used for our own characteristic benefits (38). In contrast, levels of glutathione, an antioxidant molecule involved in drug withdrawal, peak at 04:00 pm. It has been reported that toxicities of certain drugs were decreased when those drugs were administered during the glutathione time of action (32). Therefore, chronotherapy or the pharmacology of clinical chronotherapy study the impact circadian rhythms have on the response to a drug to optimize its action, maximize health benefits, and minimize possible adverse effects on patients (39).
Chrono-chemotherapy is precisely based on the biological rhythm differences of human tumor tissue, normal tissue, and drug metabolism. It involves selecting the time period when chemotherapy drugs have the optimal efficacy on tumor tissue and the lowest toxicity to normal tissue and allows choosing the time of peak drug concentration with the help of a multi-channel programming infusion pump (40). In recent years, chrono-chemotherapy, as a part of the comprehensive treatment of cancer, has been applied in clinical practice locally and internationally and has shown good therapeutic effect in different tumor types. Such as a study showed that irinotecan tolerability was better after morning administration in men and afternoon administration women with metastatic colorectal cancer (41). Studies have shown that in colorectal cancer, the optimal time for oxaliplatin administration is 04:00 pm. In addition, the combination of oxaliplatin, 5-fluorouracil, and calcium folinate (ChronoFLO4) has a survival advantage fewer adverse side effects to the digestive tract in men with colorectal cancer and has (42–46). In non-small cell lung cancer, chronotherapy with DDP decreases hematological and gastrointestinal adverse effects (47). In studies of renal cell carcinoma, administration in accordance with circadian rhythm regulation (68% of the daily dose administered in the evening) induced a durable tumor response with less drug toxicity (47). Morning administration of temozolomide in glioblastoma increased OS in O6-Methylguanine-DNA-methyltransferase (MGMT) methylated patients, which was consistent with the peak expression of the clock gene BMAL1. Patients with glioblastoma may benefit from chrono-chemotherapy (48). Studies have shown that in the treatment of ovarian cancer, DDP administered from 04:00 pm to 08:00 pm and doxorubicin administered at 06:00 am showed minimal drug toxicity and side effects and high tumor response (32, 38). In NPC, induced chrono-chemotherapy combined with radiotherapy enhances tolerance during treatment and reduces treatment-related side effects including thrombocytopenia, leukopenia, nausea, and vomiting (10, 11). The above studies illustrate the superiority of chronotherapy.
The purpose of this study was to evaluate the difference between sinusoidal administration and conventional uniform administration of DDP for an optimal duration in CCRT after IC. In this long-term follow-up analysis, the median follow-up duration was 82.8 months. The 5-year survival results were consistent with those at 2 years. Patients from Arm A achieved comparable 5-year PFS, OS, DMFS, and LRFS rates as those in Arm B. The two groups in this study were well balanced in terms of patient characteristics, tumor factors, and treatment parameters. In the subgroup analysis, no interaction was observed between these covariates and the groups, indicating that the non-inferiority of Arm A did not differ among specific populations. This also suggests that there is no survival benefit to patients with different infusion rates of chrono-chemotherapy during long-term follow-up.
In terms of long-term side effects, there were no grade 3-4 side effects in both groups. In Arm A and Arm B, the long-term toxic side effects were grade 1-2, including dry mouth, dysphagia, and hearing loss. Patients in group B also developed grade 1-2 cranial neuropathy, eye damage, neck fibrosis, voice hoarseness, and radiation brain radiation injury. Although there was no statistically significant difference between the two groups in the long-term toxic and side effects, it can be seen that the number of cases of long-term toxic and side effects in Arm B was higher than that in Arm A. This may be because the number of T4 stage cases in Arm B was slightly more compared to Arm A. The relatively large radiotherapy target area of T4 stage patients was possibly related to the differences in tolerance among patients. In the previous reports of this study, there was no statistically significant difference in acute toxic and side effects between the two groups during CCRT. Although studies have reported that sine administration of DDP and fluorouracil in TPF regimen induction chemotherapy has no survival advantage, it can reduce the incidence of stomatitis (11). The difference between our results and the above results is considered to be due to the different treatment stages. The effect of stomatitis caused by radiotherapy was more prominent than that caused by chemotherapy during CCRT. In addition, fluorouracil was the main factor leading to oral mucositis during IC, and there was no fluorouracil drug involved in the CCRT. Therefore, compared to the constant infusion rate of chemoradiotherapy, the sinusoidal form of administration during the CCRT did not improve the efficacy or reduce the adverse reactions related to chemoradiotherapy. However, we could not compare the sine administration and constant infusion rate of IC and CCRT, which will be considered in our future study. Besides, further research should be conducted to determine the optimal chronotherapy schedule for NPC.
T lymphocytes are a type of lymphocyte that plays a central role in cell-mediated immunity. Chemotherapy may have an inhibitory effect on immune cells, leading to immune dysfunction (49).In the previous report of this study, the CD3+ value of the sine group was higher than that of the constant infusion rate group after treatment, and the difference was statistically significant. It indicates that sine rate administration could improve the T-cell immune function of patients compared with constant infusion (14). Unfortunately, in this long-term follow-up, due to the limited inspection conditions, we could not detect the immune lymphocyte subsets in some patients visiting their hometown hospital for review and telephone follow-up. Therefore, we could not further analyze the long-term detection results of the immune lymphocyte subsets of these patients. At present, immune checkpoint therapy targeting Programmed death 1 (PD-1) receptor and its ligand PD-L1 has been approved for the treatment of patients with certain types of malignancies (50).Immunotherapy drugs have shown good anti-tumor activity in patients with advanced NPC or with recurrence or metastasis after failure of standard therapy (51). In recent findings, the antitumor efficacy of PD-1/PD-L1 inhibitor varies according to its administration time. This suggests that the selection of the most appropriate dosing time for PD-1/PD-L1 inhibitors is helpful to improve the efficacy of immunotherapy (52). Whether chronotherapy can be used in immunotherapy is also a question worth exploring.
This study was retrospective with a limited number of patients enrolled and without a comprehensive follow-up. In future, more rigorous multicenter prospective randomized studies with a large sample size should be designed to confirm the research conclusions.
Conclusions
In this retrospective analysis, long-term analysis confirmed that in the concurrent chemoradiotherapy, cisplatin administration with sinusoidal chronomodulated infusion group was not superior to constant rate of infusion in terms of Long-term toxicity and prognosis.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Ethics Committee of Affiliated Cancer Hospital of Guizhou Medical University. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements.
Author contributions
LL: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Writing – original draft, Writing – review & editing. XYL: Data curation, Formal analysis, Writing – original draft. WW: Validation, Writing – review & editing. YL: Validation, Writing – review & editing. JL: Validation, Writing – review & editing. XLL: Data curation, Investigation, Writing – review & editing. XC: Data curation, Investigation, Writing – review & editing. XG: Data curation, Investigation, Writing – review & editing. CZ: Data curation, Software, Writing – original draft. QH: Data curation, Software, Writing – original draft. ZL: Data curation, Software, Writing – original draft. KS: Data curation, Investigation, Methodology, Writing – original draft. YC: Data curation, Investigation, Methodology, Writing – original draft. XX: Data curation, Investigation, Writing – original draft. FJ: Supervision, Validation, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by Guizhou Provincial Health Commission. Project number: gzwkj2022-021. This research was supported in part by grants from the Guizhou Medical University 2021 National Foundation Cultivation Project (grant number 20NSP041).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. (2015) 65:87–108. doi: 10.3322/caac.21262
2. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2021) 71:209–49. doi: 10.3322/caac.21660
3. Chen YP, Chan ATC, Le QT, Blanchard P, Sun Y, Ma J. Nasopharyngeal carcinoma. Lancet. (2019) 394:64–80. doi: 10.1016/S0140-6736(19)30956-0
4. Chua DT, Ma J, Sham JS, Mai HQ, Choy DT, Hong MH, et al. Long-term survival after cisplatin-based induction chemotherapy and radiotherapy for nasopharyngeal carcinoma: a pooled data analysis of two phase III trials. J Clin Oncol. (2005) 23:1118–24. doi: 10.1200/JCO.2005.12.081
5. Lee AW, Lau KY, Hung WM, Ng WT, Lee MC, Choi CW, et al. Potential improvement of tumor control probability by induction chemotherapy for advanced nasopharyngeal carcinoma. Radiother Oncol. (2008) 87:204–10. doi: 10.1016/j.radonc.2008.02.003
6. Blanchard P, Lee A, Marguet S, Leclercq J, Ng WT, Ma J, et al. et al: Chemotherapy and radiotherapy in nasopharyngeal carcinoma: an update of the MAC-NPC meta-analysis. Lancet Oncol. (2015) 16:645–55. doi: 10.1016/S1470-2045(15)70126-9
7. Chan AT, Teo PM, Ngan RK, Leung TW, Lau WH, Zee B, et al. Concurrent chemotherapy-radiotherapy compared with radiotherapy alone in locoregionally advanced nasopharyngeal carcinoma: progression-free survival analysis of a phase III randomized trial. J Clin Oncol. (2002) 20:2038–44. doi: 10.1200/JCO.2002.08.149
8. Tang QN, Liu LT, Qi B, Guo SS, Luo DH, Sun R, et al. Effect of concurrent chemoradiotherapy with nedaplatin vs cisplatin on the long-term outcomes of survival and toxic effects among patients with stage II to IVB nasopharyngeal carcinoma: A 5-Year follow-up secondary analysis of a randomized clinical trial. JAMA Netw Open. (2021) 4:e2138470. doi: 10.1001/jamanetworkopen.2021.38470
9. Zhang S, Liu T. Efficacy of induction chemotherapy combined with chrono-chemotherapy and intensity-modulated radiotherapy on locally advanced nasopharyngeal carcinoma. J BUON. (2021) 26:774–80.
10. Gou XX, Jin F, Wu WL, Long JH, Li YY, Gong XY, et al. Induction chronomodulated chemotherapy plus radiotherapy for nasopharyngeal carcinoma: A Phase II prospective randomized study. J Cancer Res Ther. (2018) 14:1613–9. doi: 10.4103/jcrt.JCRT_883_17
11. Lin HX, Hua YJ, Chen QY, Luo DH, Sun R, Qiu F, et al. Randomized study of sinusoidal chronomodulated versus flat intermittent induction chemotherapy with cisplatin and 5-fluorouracil followed by traditional radiotherapy for locoregionally advanced nasopharyngeal carcinoma. Chin J Cancer. (2013) 32:502–11. doi: 10.5732/cjc.013.10004
12. Printezi MI, Kilgallen AB, Bond MJG, Stibler U, Putker M, Teske AJ, et al. Toxicity and efficacy of chronomodulated chemotherapy: a systematic review. Lancet Oncol. (2022) 23:e129–43. doi: 10.1016/S1470-2045(21)00639-2
13. Focan C, Demolin G, Kreutz F, Graas MP, Longree L, Matus G, et al. Chronotherapy with 5-fluorouracil folinic acid and oxaliplatin delivered over 48 hours every second week in colorectal cancer. CHC-Liege Exp (Belgium). Pathol Biol (Paris). (2013) 61:e71–74. doi: 10.1016/j.patbio.2011.02.002
14. Wan S JF, Wu WL, Li YY, Long JH, Chen GY, Gan JY, et al. YuF An analysis on the combination of chrono-chemotherapy with different speed rate and concomitant intensity-modulated radiotherapy in the treatment of locally advanced nasopharyngeal carcinoma. Chin J Radiological Med Prot. (2018) 38:278–83. doi: 10.3760/cma.j
15. Cox JD, Stetz J, Pajak TF. Toxicity criteria of the radiation therapy oncology group (RTOG) and the european organization for research and treatment of cancer (EORTC). Int J Radiat Oncol Biol Phys. (1995) 31:1341–6. doi: 10.1016/0360-3016(95)00060-C
16. Mao YP, Xie FY, Liu LZ, Sun Y, Li L, Tang LL, et al. Re-evaluation of 6th edition of AJCC staging system for nasopharyngeal carcinoma and proposed improvement based on magnetic resonance imaging. Int J Radiat Oncol Biol Phys. (2009) 73:1326–34. doi: 10.1016/j.ijrobp.2008.07.062
17. Cao SM, Yang Q, Guo L, Mai HQ, Mo HY, Cao KJ, et al. Neoadjuvant chemotherapy followed by concurrent chemoradiotherapy versus concurrent chemoradiotherapy alone in locoregionally advanced nasopharyngeal carcinoma: A phase III multicenter randomized controlled trial. Eur J Cancer. (2017) 75:14–23. doi: 10.1016/j.ejca.2016.12.039
18. Sun Y, Li WF, Chen NY, Zhang N, Hu GQ, Xie FY, et al. Induction chemotherapy plus concurrent chemoradiotherapy versus concurrent chemoradiotherapy alone in locoregionally advanced nasopharyngeal carcinoma: a phase 3, multicenter, randomized controlled trial. Lancet Oncol. (2016) 17:1509–20. doi: 10.1016/S1470-2045(16)30410-7
19. Hui EP, Ma BB, Leung SF, King AD, Mo F, Kam MK, et al. Randomized phase II trial of concurrent cisplatin-radiotherapy with or without neoadjuvant docetaxel and cisplatin in advanced nasopharyngeal carcinoma. J Clin Oncol. (2009) 27:242–9. doi: 10.1200/JCO.2008.18.1545
20. Yang Q, Cao SM, Guo L, Hua YJ, Huang PY, Zhang XL, et al. Induction chemotherapy followed by concurrent chemoradiotherapy versus concurrent chemoradiotherapy alone in locoregionally advanced nasopharyngeal carcinoma: long-term results of a phase III multicenter randomized controlled trial. Eur J Cancer. (2019) 119:87–96. doi: 10.1016/j.ejca.2019.07.007
21. Zhang MX, Li J, Shen GP, Zou X, Xu JJ, Jiang R, et al. Intensity-modulated radiotherapy prolongs the survival of patients with nasopharyngeal carcinoma compared with conventional two-dimensional radiotherapy: A 10-year experience with a large cohort and long follow-up. Eur J Cancer. (2015) 51:2587–95. doi: 10.1016/j.ejca.2015.08.006
22. Lai SZ, Li WF, Chen L, Luo W, Chen YY, Liu LZ, et al. How does intensity-modulated radiotherapy versus conventional two-dimensional radiotherapy influence the treatment results in nasopharyngeal carcinoma patients? Int J Radiat Oncol Biol Phys. (2011) 80:661–8. doi: 10.1016/j.ijrobp.2010.03.024
23. Ma BB, Chan AT. Recent perspectives in the role of chemotherapy in the management of advanced nasopharyngeal carcinoma. Cancer. (2005) 103:22–31. doi: 10.1002/cncr.20768
24. Chan AT, Leung SF, Ngan RK, Teo PM, Lau WH, Kwan WH, et al. Overall survival after concurrent cisplatin-radiotherapy compared with radiotherapy alone in locoregionally advanced nasopharyngeal carcinoma. J Natl Cancer Inst. (2005) 97:536–9. doi: 10.1093/jnci/dji084
25. Lee AW, Lau WH, Tung SY, Chua DT, Chappell R, Xu L, et al. Preliminary results of a randomized study on therapeutic gain by concurrent chemotherapy for regionally-advanced nasopharyngeal carcinoma: NPC-9901 Trial by the Hong Kong Nasopharyngeal Cancer Study Group. J Clin Oncol. (2005) 23:6966–75. doi: 10.1200/JCO.2004.00.7542
26. Demizu Y, Sasaki R, Soejima T, Maruta T, Okamoto Y, Yamada K, et al. Efficacy and feasibility of cisplatin-based concurrent chemoradiotherapy for nasopharyngeal carcinoma. Jpn J Clin Oncol. (2006) 36:620–5. doi: 10.1093/jjco/hyl083
27. Zhang Y, Chen M, Chen C, Kong L, Lu JJ, Xu B. The efficacy and toxicities of intensive induction chemotherapy followed by concurrent chemoradiotherapy in nasopharyngeal carcinoma patients with N(3) disease. Sci Rep. (2017) 7:3668. doi: 10.1038/s41598-017-03963-8
28. Greene MW. Circadian rhythms and tumor growth. Cancer Lett. (2012) 318:115–23. doi: 10.1016/j.canlet.2012.01.001
29. Takahashi JS, Hong HK, Ko CH, McDearmon EL. The genetics of mammalian circadian order and disorder: implications for physiology and disease. Nat Rev Genet. (2008) 9:764–75. doi: 10.1038/nrg2430
30. Haus EL, Smolensky MH. Shift work and cancer risk: potential mechanistic roles of circadian disruption, light at night, and sleep deprivation. Sleep Med Rev. (2013) 17:273–84. doi: 10.1016/j.smrv.2012.08.003
31. Schernhammer ES, Feskanich D, Liang G, Han J. Rotating night-shift work and lung cancer risk among female nurses in the United States. Am J Epidemiol. (2013) 178:1434–41. doi: 10.1093/aje/kwt155
32. Lee Y. Roles of circadian clocks in cancer pathogenesis and treatment. Exp Mol Med. (2021) 53:1529–38. doi: 10.1038/s12276-021-00681-0
33. Droin C, Paquet ER, Naef F. Low-dimensional dynamics of two coupled biological oscillators. Nat Phys. (2019) 15:1086–94. doi: 10.1038/s41567-019-0598-1
34. Smolensky MH, Peppas NA. Chronobiology, drug delivery, and chronotherapeutics. Adv Drug Delivery Rev. (2007) 59:828–51. doi: 10.1016/j.addr.2007.07.001
35. Lee Y, Field JM, Sehgal A. Circadian rhythms, disease and chronotherapy. J Biol Rhythms. (2021) 36:503–31. doi: 10.1177/07487304211044301
36. Dong D, Yang D, Lin L, Wang S, Wu B. Circadian rhythm in pharmacokinetics and its relevance to chronotherapy. Biochem Pharmacol. (2020) 178:114045. doi: 10.1016/j.bcp.2020.114045
37. Lu D, Wang Z, Wu B. Pharmacokinetics-based chronotherapy. Curr Drug Metab. (2022) 23:2–7. doi: 10.2174/1389200223666220106124218
38. Levi F. Circadian chronotherapy for human cancers. Lancet Oncol. (2001) 2:307–15. doi: 10.1016/S1470-2045(00)00326-0
39. Amiama-Roig A, Verdugo-Sivianes EM, Carnero A, Blanco JR. Chronotherapy: circadian rhythms and their influence in cancer therapy. Cancers (Basel). (2022) 14. doi: 10.3390/cancers14205071
40. Levi F, Okyar A. Circadian clocks and drug delivery systems: impact and opportunities in chronotherapeutics. Expert Opin Drug Delivery. (2011) 8:1535–41. doi: 10.1517/17425247.2011.618184
41. Innominato PF, Ballesta A, Huang Q, Focan C, Chollet P, Karaboue A, et al. Sex-dependent least toxic timing of irinotecan combined with chronomodulated chemotherapy for metastatic colorectal cancer: Randomized multicenter EORTC 05011 trial. Cancer Med. (2020) 9:4148–59. doi: 10.1002/cam4.3056
42. Caussanel JP, Levi F, Brienza S, Misset JL, Itzhaki M, Adam R, et al. Phase I trial of 5-day continuous venous infusion of oxaliplatin at circadian rhythm-modulated rate compared with constant rate. J Natl Cancer Inst. (1990) 82:1046–50. doi: 10.1093/jnci/82.12.1046
43. Filipski E, King VM, Li X, Granda TG, Mormont MC, Liu X, et al. Host circadian clock as a control point in tumor progression. J Natl Cancer Inst. (2002) 94:690–7. doi: 10.1093/jnci/94.9.690
44. Giacchetti S, Bjarnason G, Garufi C, Genet D, Iacobelli S, Tampellini M, et al. Phase III trial comparing 4-day chronomodulated therapy versus 2-day conventional delivery of fluorouracil, leucovorin, and oxaliplatin as first-line chemotherapy of metastatic colorectal cancer: the European Organization for Research and Treatment of Cancer Chronotherapy Group. J Clin Oncol. (2006) 24:3562–9. doi: 10.1200/JCO.2006.06.1440
45. Levi F, Zidani R, Misset JL. Randomized multicenter trial of chronotherapy with oxaliplatin, fluorouracil, and folinic acid in metastatic colorectal cancer. International Organization for Cancer Chronotherapy. Lancet. (1997) 350:681–6. doi: 10.1016/s0140-6736(97)03358-8
46. evi F, Misset JL, Brienza S, Adam R, Metzger G, Itzakhi M, et al. A chronopharmacologic phase II clinical trial with 5-fluorouracil, folinic acid, and oxaliplatin using an ambulatory multichannel programmable pump High antitumor effectiveness against metastatic colorectal cancer. Cancer. (1992) 69:893–900. doi: 10.1002/1097-0142(19920215)69:4<893::aid-cncr2820690410>3.0.co;2-x
47. Kobayashi M, Wood PA, Hrushesky WJ. Circadian chemotherapy for gynecological and genitourinary cancers. Chronobiol Int. (2002) 19:237–51. doi: 10.1081/CBI-120002600
48. Damato AR, Luo J, Katumba RGN, Talcott GR, Rubin JB, Herzog ED, et al. Temozolomide chronotherapy in patients with glioblastoma: a retrospective single-institute study. Neurooncol Adv. (2021) 3:vdab041. doi: 10.1093/noajnl/vdab041
49. Zitvogel L, Apetoh L, Ghiringhelli F, Kroemer G. Immunological aspects of cancer chemotherapy. Nat Rev Immunol. (2008) 8:59–73. doi: 10.1038/nri2216
50. Ohdo S, Koyanagi S, Matsunaga N. Chronopharmacology of immune-related diseases. Allergol Int. (2022) 71:437–47. doi: 10.1016/j.alit.2022.06.006
51. Wang FH, Wei XL, Feng J, Li Q, Xu N, Hu XC, et al. Efficacy, safety, and correlative biomarkers of toripalimab in previously treated recurrent or metastatic nasopharyngeal carcinoma: A phase II clinical trial (POLARIS-02). J Clin Oncol. (2021) 39:704–12. doi: 10.1200/JCO.20.02712
52. Tsuruta A, Shiiba Y, Matsunaga N, Fujimoto M, Yoshida Y, Koyanagi S, et al. Diurnal expression of PD-1 on tumor-associated macrophages underlies the dosing time-dependent antitumor effects of the PD-1/PD-L1 inhibitor BMS-1 in B16/BL6 melanoma-bearing mice. Mol Cancer Res. (2022) 20:972–82. doi: 10.1158/1541-7786.MCR-21-0786
Keywords: nasopharyngeal carcinoma, chrono-chemotherapy, radiotherapy, intensity-modulated radiotherapy, late toxicity
Citation: Liu L, Luo X, Wu W, Li Y, Long J, Luo X, Chen X, Gong X, Zhao C, He Q, Li Z, Shang K, Chen Y, Xinyu X and Jin F (2024) Long-term survival, toxicities, and the role of chrono-chemotherapy with different infusion rates in locally advanced nasopharyngeal carcinoma patients treated with intensity-modulated radiation therapy: a retrospective study with a 5-year follow-up. Front. Oncol. 14:1371878. doi: 10.3389/fonc.2024.1371878
Received: 17 January 2024; Accepted: 01 March 2024;
Published: 22 March 2024.
Edited by:
Nerina Denaro, IRCCS Ca ‘Granda Foundation Maggiore Policlinico Hospital, ItalyReviewed by:
Guanzhong Gong, Shandong University, ChinaYijun Hua, Sun Yat-sen University Cancer Center (SYSUCC), China
Copyright © 2024 Liu, Luo, Wu, Li, Long, Luo, Chen, Gong, Zhao, He, Li, Shang, Chen, Xinyu and Jin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Feng Jin, dGp6bGsyMDIzQDE2My5jb20=
 Lina Liu
Lina Liu Xunyan Luo2
Xunyan Luo2 Qianyong He
Qianyong He Kai Shang
Kai Shang Yue Chen
Yue Chen