- 1Department of Oncology, First Teaching Hospital of Tianjin University of Traditional Chinese Medicine, Tianjin, China
- 2Department of Oncology, National Clinical Research Center for Chinese Medicine Acupuncture and Moxibustion, Tianjin, China
Pancreatic cancer is one of the deadliest malignant tumors, which is a serious threat to human health and life, and it is expected that pancreatic cancer may be the second leading cause of cancer death in developed countries by 2030. Claudin18.2 is a tight junction protein expressed in normal gastric mucosal tissues, which is involved in the formation of tight junctions between cells and affects the permeability of paracellular cells. Claudin18.2 is highly expressed in pancreatic cancer and is associated with the initiation, progression, metastasis and prognosis of cancer, so it is considered a potential therapeutic target. Up to now, a number of clinical trials for Claudin18.2 are underway, including solid tumors such as pancreatic cancers and gastric cancers, and the results of these trials have not yet been officially announced. This manuscript briefly describes the Claudia protein, the dual roles of Cluadin18 in cancers, and summarizes the ongoing clinical trials targeting Claudin18.2 with a view to integrating the research progress of Claudin18.2 targeted therapy. In addition, this manuscript introduces the clinical research progress of Claudin18.2 positive pancreatic cancer, including monoclonal antibodies, bispecific antibodies, antibody-drug conjugates, CAR-T cell therapy, and hope to provide feasible ideas for the clinical treatment of Claudin18.2 positive pancreatic cancer.
1 Introduction
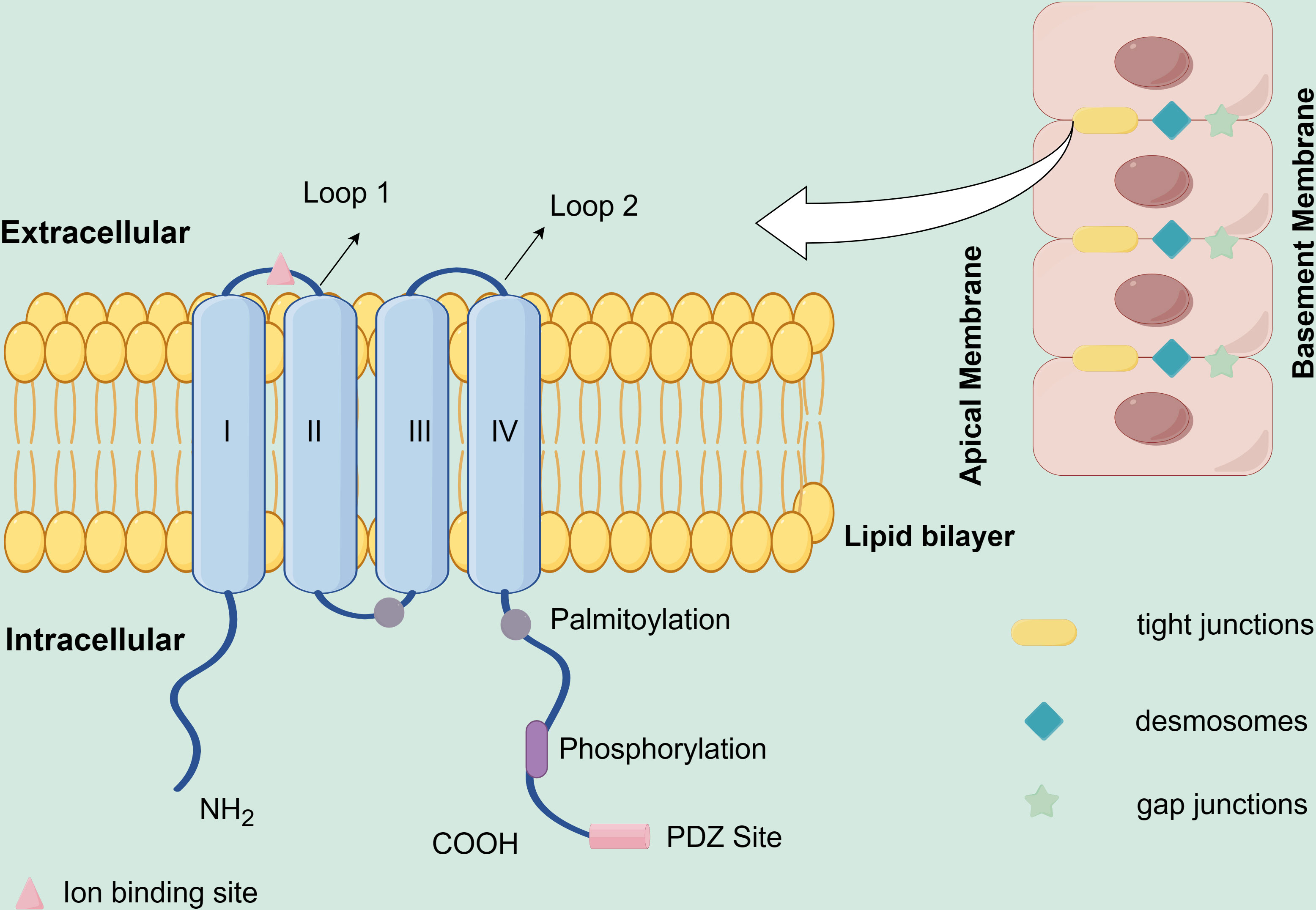
Claudins are important proteins in normal tissue tight junctions (TJ), which are related to the formation of epithelial and epidermal tight junction proteins (1), and were first discovered and named by researchers Mikio Furuse and Shoichiro Tsukita of Kyoto University in Japan in 1998. The name “Claudin” originated from the Latin word “claudere (to close)” (2). Three main functions of Claudins are intracellular signaling, fence, and barrier. While the function of fence divides the apical and basolateral domains and controls substance mobility in the plasma membrane, the function of barrier is the capacity to control the paracellular permeation of ions, water, immune cells, and macromolecules, selectively (3–5). Claudins include at least 27 family members, 4 transmembrane structural domains, and 2 extracellular loops (Schematic diagram of Claudins protein structure, Figure 1), which allow the Claudins proteins to effectively sustain the polarity of epithelial and endothelial cells, thereby effectively regulating paracellular permeability and conductance (6). Claudin18 is one of the Claudins (CLDN) proteins family, which has two different isoforms, Claudin18.1 and Claudin18.2, due to the optional exons 1a and 1b (7). Claudin18.2 is the highly targeted marker protein with tissue-specific expression. The expression of Claudin18.2 in normal tissues is restricted to differentiated gastric mucosal epithelial cells, which results that it is masked and is mostly untouched by intravenous antibodies. However, if the tissues are cancerous, changes in cell polarity expose the epitope of Claudin18.2 to the cell surface and increase the expression (8). Claudin18.2 is expressed in many tumors, including gastric cancer, pancreatic cancer, esophageal cancer, ovarian cancer, etc. (8–11). In a study of 414 cancer patients with immunohistochemistry (IHC) analysis to assess Claudin18.2 expression in different tumor types, a total of 4.1% (N=17) of subjects were Claudin18.2 positive, including pancreatic cancer (16.7%, 1/6), gastric cancer (14.1%, 12/85), colorectal cancer (0.9%, 2/203), biliary tract cancer (6.3%, 1/16), and genitourinary/miscellaneous (2.2%, 1/46) (12). The expression of Claudin18.2 is not only in the primary tumor, but also in metastases. In one study, pancreatic ductal adenocarcinoma (PDAC), which is the most common kind of pancreatic cancers, showed 59.2% (103/174) Claudin18.2 expression, of which 54.6% were strongly expressed (N=95). In the lymphatic metastasis, 69.4% (N=34) expressed Claudin18.2 with strong staining. Whereas the number of tumor cells in the samples expressing Claudin 18.2 was positively linked with the staining intensity (RS = 0.871, p<0.001). The results are similar in liver metastasis samples. 65.7% of samples (N=23) expressed Claudin18.2 and all positive samples showed 2+ or higher staining intensity (13). Jiang H et al. finds that the expression of Claudin18.2 in the metastatic lesions is in line with the expression in the primary cancer tissues (14). And the expression of Claudin18.2 was more obvious in the disease progression. Samples from lymph nodes and liver metastases showed expression that was either identical to or higher than that in the original lesion, and the high expression of Claudin18.2 were related to the poor prognostic factors of positive lymph nodes (15). The longer median progression-free survival (mPFS) is related to the lower Claudin18.2 expression level in gastric cancer (p = 0.047) (16). Researchers also revealed that claudin18 expression correlates with poor survival in patients with CRC. And the prognosis of patients with positive claudin18 expression was significantly poorer than in negative cases (P= 0.0106) (17). While Jun et al. found that in patients with Claudin18 expression, OS was longer than those without Claudin18 expression (5-year survival rate, 90.5% vs. 64.8%) (18). Another study also found that patients with high expression levels of claudin18 have longer survival time than those with low claudin18 expression (19). Overexpression of Claudin18.2 may activate related signaling pathways, like SPAK-p38 MAPK signaling pathway, epidermal growth factor receptor (EGFR)/extracellular signal-regulated kinase (ERK) signaling et al., which leads to tumor cell proliferation (20–22). Another study on human pancreatic cancer cells showed Claudin18 is predominantly controlled at the transcriptional level by specific protein kinase C signaling pathways and modified by DNA methylation (23). At present, the exact mechanism that Claudin18.2 impacts on distant metastasis and tumor lymph node metastasis is unclear, and it is possible that its unusual expression changes the structure and function of tight junctions between cells. The studies have also found that different expressions of Claudin have been shown to be significant in the prognosis of cancer. For example, claudin-1 is associated with colon cancer prognosis (24), claudin-18 is related to gastric cancer (GC) prognosis (25), and claudin-10 is relevant to hepatocellular carcinoma prognosis (26). In summary, Claudin18.2 protein is involved in the migration, differentiation, and proliferation of tumor cells. And because of its specific expression it may be used as the target of precise attack on tumors, so the value of Claudin18.2 in the diagnosis, treatment and prognosis of tumors should be further explored.
2 The double-edged sword role of Claudin18 in cancers
There are many antigens with dual roles in tumor microenvironment, for example, TGF-β, desmosomes, HMGB1 (a nuclear DNA-binding protein) and cancer testis antigens etc. (27–30). Claudin18 also has different effects in tumors, acting as a tumor suppressant in gastric and lung cancers, but playing a promoting role in other gastrointestinal tumors, such as pancreatic cancer, colorectal cancer, and esophageal cancer (31). At present, only a few articles have elucidated the mechanism of action of Claudin18 in different tumors, which needs further summary.
2.1 Inhibition of tumors
Oshima et al. found that the Ki-67 index was inversely correlated with the level of Claudin18 in the front of gastric cancer invasion, and the loss of Claudin18 was also shown to be associated with the proliferation and invasion of GC cells in subsequent cell experiments (32). Helicobacter pylori infection in mice attenuated the expression of Claudin18 in the early stage of GC development, leading to rapid tumor development, which may be related to the involvement of Claudin18 in the regulation of cytokines, Wnt and Notch signaling pathways (33). Zhou et al. found that Claudin18 can inversely regulate the activity of Yes-associated protein (YAP), thereby promoting cell proliferation and tumorigenesis (34). Claudin18.1 can inhibit yes-associated protein/tafazzin (Yap/Taz) and insulin-like growth factor 1 receptor (IGF-1R) signaling pathway, resulting in AKT inhibition (35, 36). It proved that Claudins is an important signaling center involved in cell proliferation and tumorigenesis. In Claudin18-deficient mice, the frequency of lung adenocarcinoma also increased accordingly (37).
2.2 Promotion of tumors
The cancer-promoting effects of Claudin18 are mostly manifested in its ectopically activated cancers. For bile duct carcinoma cells, the overactivation of EGFR leads to the activation of the downstream Ras/Raf/MEK/ERK cascade, which induces the expression of Claudin18, and the expression of Claudin18 can further induce ERK1/2 phosphorylation, forming a positive feedback loop, and playing a role in promoting cancer (21). In the normal esophageal squamous epithelium, Claudin18 is not expressed, but the expression of Claudin18 in the columnar epithelium of Barrett’s esophagus is doubled, and Claudin18 can reduce the permeability of tightly junction H+ ions, prevent its attack and destroy the esophageal squamous epithelium, thereby promoting tumorigenesis (38). Claudin18.2 is mainly regulated by the hypomethylation gene sequence CpG island promoter and the transcription factor cAMP-response element blinding protein (CREB) in its genetic coding sequence (8), and its overexpression may be related to signaling pathways, like PKC pathway, ERK/MAPK pathway, and HER2/HER3 pathway, which promote the proliferation of tumor cells (31, 39, 40). In addition, the high expression of Claudin18.2 can regulate cell polarity, disrupt the tight junctions of tumor cells, change the adhesion and plasticity of tumor cells, increase their ability to metastasize and infiltration, and increase the degree of malignancy of tumors (41).
3 Current research of Claudin18.2
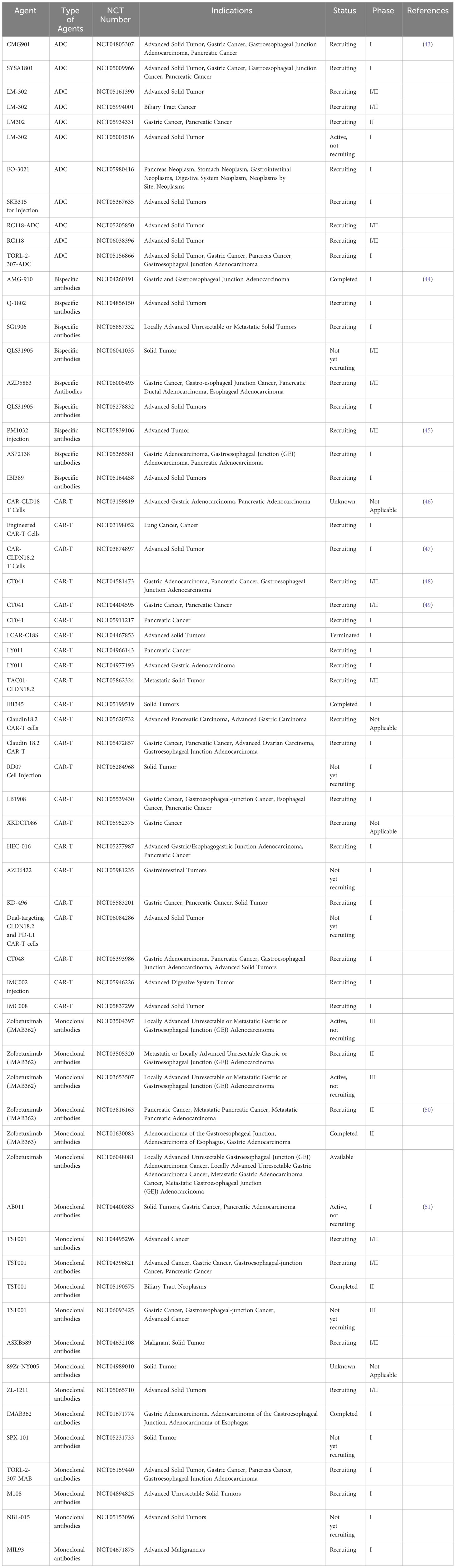
At present, there are no products targeting Claudin18.2 on the market, but research on various types of drugs targeting Claudin18.2 is in full swing, of which the indications are mostly focusing on Claudin18.2 positive gastric cancer and pancreatic cancer. Immunotherapy for solid tumor targeting Claudin18.2 includes monoclonal antibody, bispecific antibody, antibody-drug conjugate (ADC), and CAR-T cell. For example, the first targeted development drug, Zolbetuximab (IMAB362), is an IgG1 monoclonal antibody that specifically links structurally chimeric to Clauidn18.2 on the surface of tumor cells. It causes antibody-dependent cellular cytotoxicity (ADCC), complement-dependent cytotoxicity (CDC), apoptosis and inhibits cell proliferation. Its powerful capacity to eradicate cancer cells and regulate illness has been effectively confirmed by preclinical research (42). CMG901, monoclonal antibody + monomethyl auristatin E (MMAE) drug conjugates, can efficiently target tumor cells by anti-Claudin18.2 antibodies and trigger endocytosis, which allows the small molecule toxin MMAE to enter tumor cells to provide antitumor effects (14). Here, we list the latest clinical trials in Claudin18.2-targeted agents developed in ClinicalTrials.gov (Table 1) for further research.
4 Application of Claudin18.2 in pancreatic cancer
Pancreatic cancer (PC) is one of the deadliest tumors, which seriously threatens human health. Given that pancreatic cancer has no special clinical manifestations and its unique anatomical location, it is considered to be a “silent killer”. In 2020, there were more than 490,000 new cases and more than 460,000 deaths worldwide. Pancreatic cancer kills almost as many people as it does, and the highest incidence rates are found in Europe and Northern America (26). With the lowest 5-year survival rate of any cancer type (10%), PC is also expected to become the second leading cause of cancer-related mortality in the United States by 2030 (52, 53). As the population grows, the aging process accelerates and the westernized lifestyles become more prevalent, in the coming years the incidence of pancreatic cancer is likely to continue to rise. Currently, surgery treatment is the only promising method for pancreatic cancer, usually followed by postoperative adjuvant radiotherapy and chemotherapy, and the survival rate of five year in patients is only 30% (54). Exocrine cell tumors account for 95% of pancreatic cancer cases, and the most common of these is PDAC. However, PDAC is characterized by early invasive metastases, with more than 50% of patients presenting with distant metastases, only 15%-20% of patients with PDAC can perform the surgery and most surgical patients also experience metastases within four years (55, 56). At present, the survival status of pancreatic cancer patients is not optimistic, and the treatment choices are limited. The median overall survival (mOS) of patients with advanced pancreatic cancer is only 11.1 months (41), so there is an imperative need to find new targets and new therapies to bring new hope to patients. In both pancreatic adenocarcinoma and its metastases, claudin18.2 is heavily expressed. Patients who have high expression of claudin18 live longer than individuals without this target (57). So, at present, the research on claudin18 monoclonal antibodies, bispecific antibodies, antibody-drug conjugates, and CAR-T cell therapy are in full swing, which may offer fresh ideas to the treatment of pancreatic cancer.
4.1 Monoclonal antibodies – the potential of Claudin18.2 is emerging
4.1.1 Zolbetuximab (IMAB362)
Zolbetuximab (IMAB362) is one of monoclonal antibody drugs targeting Claudin 18.2. It can trigger ADCC and CDC by specifically connecting to Claudin18.2 on the surface of tumor cells, which may result in tumor cell lysis and death. The extent of cytotoxic effects induced by Zolbetuximab was found to be related to the expression of Claudin18.2 on the surface of cells (58). The study (NCT03816163) to evaluate the safety and effectiveness of the combination of Nab-Paclitaxel and Gemcitabine (Nab-P + GEM) with Zolbetuximab (IMAB362) as the first-line therapy for patients with metastatic pancreatic adenocarcinoma who are also Claudin18.2 positive is ongoing (50). The study is predicted to include 369 pancreatic cancer patients with high expression of Claudin18.2 (IHC staining intensity ≥75%). In order to assess the safety and tolerability of Zolbetuximab + GN, the trial involved a safety lead-in that enrolled 3-12 patients. And after 28 days, dose-limiting toxicities (DLTs) will be evaluated. About 357 patients will be assigned 2:1 randomly to receive either GN alone on Days 1, 8, and 15 of each cycle or Zolbetuximab Q2W on Days 1 and 15 plus GN on Days 1, 8, and 15 of each cycle, according to the recommended phase 2 dosage (RP2D), which was confirmed during the safety lead-in period. Liver metastases and ECOG performance status will be used to determine the randomization process. Patients will have the baseline MRI or CT every eight weeks until the beginning of another systemic anticancer treatment, or until the period that investigators assessed the disease progression, whichever occurs first. The main goals are to ascertain the safety and tolerability of Zolbetuximab + GN, and whether the treatment with Zolbetuximab + GN compared to GN alone improves overall survival (OS). Progression-free survival (PFS), objective response rate (ORR), disease control rate (DCR), pharmacokinetics, duration of response (DOR), and health-related quality of life are secondary endpoints. We are now looking forward to the final results of this experiment. Up to now, Zolbetuximab (IMAB362) has been approved for the first-line treatment for metastatic pancreatic cancer in China, and is expected to become the first marketed Claudin18.2 monoclonal antibody in the world.
4.1.2 TST001
TST001 is a monoclonal antibody targeting Claudin18.2 that was developed globally following IMAB362. Compared to IMAB362, TST001 exhibits better affinity and Fc fragment receptor (FcR) binding activity because of its enhanced natural killer cell-mediated ADCC and fewer fucose content (59). Preclinical studies found that TST001 shows strong anti-tumor abilities in tumor models, with moderate to high levels of Claudin18.2 expression and synergistic anti-cancer effects with immune checkpoint inhibitors (ICIs) (60). The study has found that the combination of TST001 (Osemitamab) and atezolizumab has better anti-tumor activity than the single agent (61). There is an ongoing trial (NCT04396821) about TST001 to study the safety and tolerability in advanced or metastatic solid tumors. In cohort C of the trial, subjects with previously untreated, locally advanced, unresectable, or metastatic pancreatic adenocarcinoma will be enrolled. These participants will be given TST001 at a dose of 2 mg/kg or 4 mg/kg Q2W together with albumin-bound paclitaxel AND gemcitabine. The key observational indicators comprise the frequency and severity of adverse events, maximum tolerated dosage, safety and tolerability. And the secondary observational indicators include immunogenicity, PFS, ORR, DOR et al. We are awaiting the latest research progress.
4.1.3 AB011
A humanized recombinant monoclonal antibody injection against Claudin18.2 is known as AB011. which is the first monoclonal antibody against Claudin18.2 independently developed in China and the first humanized monoclonal antibody against this target in the world. Currently, AB011 is in the phase I clinical trial (NCT04400383) for the treatment of Claudin18.2 positive solid tumors (pancreatic adenocarcinoma, gastric cancer, and solid tumors). The study is consisted of two phases: the first phase is a single treatment and the second phase is a combination therapy. From Aug 2020 to Aug 2021, in Part 1, 14 patients in the dose-escalation stage were given AB011 at different dosage and 21 patients in the dose-expansion stage (11 at 30 mg/kg, 10 at 20 mg/kg). 77.1% have had at least two lines of treatment before. Twelve subjects (12/46, 46.2%) of GC/gastroesophageal junction (GEJ) adenocarcinoma had three or more metastatic organs, of which fifteen (56.7%) had peritoneal metastases. Six out of nine (66.7%) pancreatic cancer subjects had two or more metastatic organs. Eight patients (3 in 20 mg/kg, 5 in 30 mg/kg) suffered treatment-related adverse events (TRAEs) of grade 3, with grade 1-2 accounting for the majority of TRAEs. One case of DLT (grade 3 dyspnea) was reported in the 30 mg/kg group. Twelve patients achieved disease control out of the twenty patients with evaluable disease and at least one tumor assessment, and one GC without target lesions was shown to be complete response (CR) (51). There are some other monoclonal antibodies against Claudin18.2 that are being studied, like ASKB589 (NCT04632108), M108 (NCT04894825), MIL93 (NCT04671875), NBL-015 (NCT05153096), ZL-1211 (NCT05065710).
4.2 Bispecific antibodies – Claudin 18.2 is a strong combination
Two distinct epitopes on the same or different antigens are bound by bispecific antibodies. Bispecific antibodies exert activity that exceeds that of beyond natural antibodies due to the dual specificity of soluble or cell surface antigens, which may present additional therapeutic application potential (62). Currently, bispecific antibodies that have entered clinical trials include: Claudin18.2/CD3 (NCT04260191) (44), Claudin18.2/4-1BB (NCT05839106) (45), Claudin18.2/PD-L1 (NCT04856150) et al. These bispecific antibodies have stronger specificity and lower off-target toxicity than some monoclonal antibodies, and have more therapeutic potential for gastric and pancreatic cancers. AZD5863 is a bispecific antibody which targets Claudin18.2 and CD3 T cells. The safety, pharmacokinetics, pharmacodynamics, and effectiveness of AZD5863 in adult patients who have advanced or metastatic solid tumors are being investigated in the trial (NCT06005493). In this experiment, the AZD5863 will be infused in two different forms, intravenous and subcutaneous, to observe the number of adverse reactions and ORR. Clinical studies of other bispecific antibodies are ongoing, for example, IBI389 (NCT05164458), QLS31905 (NCT05278832), SG1906 (NCT05857332) et al.
4.3 Antibody-drug conjugates – Claudin 18.2 is getting better
4.3.1 CMG901
The most common form of antibody-drug conjugates (ADC) is monoclonal antibodies (mAbs) linked to cytotoxic medicines via chemical linkers by covalent bonds. Because of the benefits of strong killing power and high specificity targeting ability, it removes cancer cells effectively, making it a hotspot in the production of anticancer medications (63). CMG901 is the first ADC drug for Claudin18.2 in the world, which has shown strong anti-cancer efficacy through CDC, ADCC, and MMAE-mediated cytotoxicity with bystander killing. The phase I trial (NCT04805307) in patients who have advanced solid tumors is ongoing. As of August 4, 2022, CMG901 had been administered to 27 participants (14 patients with pancreatic cancer and 13 patients with gastric/GEJ cancer) at doses of 3.4 mg/kg. The median line of prior systemic treatment was 2, with the range of 1 to 5. 3/27 (11.1%) of the patients experienced grade 3 adverse events (AEs) as a result of the medicine. There were no documented grade 4 or 5 AEs linked to the drug. MTD wasn’t accomplished. The ORR and DCR in patients with gastric/GEJ cancer who were positive for Claudin18.2 were 75.0% and 100%, respectively. In the 2.6, 3.0, and 3.4 mg/kg Q3W dosage groups, ORR was 100%. The mOS and mPFS had not yet been reached. Patients exhibited little exposure to unconjugated MMAE systemically, according to the data. The results will be reported on an ongoing basis (43).
4.3.2 LM-302
LM-302 can specifically target Claudin18.2-positive tumor cells and enter tumor cells through endocytosis, releasing small molecule toxins, thus exerting anti-tumor effects. Preclinical studies of LM-302 (TPX-4589) have shown favorable safety profile and activity in vitro and in vivo, including good tumor suppression and decreasing tumor size in tumor models with high- or low-expression of Claudin18.2 (64). The Food and Drug Administration (FDA) has declared the medicine as the orphan drug (ODD) in 2021 for pancreatic cancer, gastric cancer and gastric junction cancer. Currently, experiments on LM-302 are underway, like NCT05161390, NCT05994001, NCT05934331, NCT05001516.
4.3.3 EO-3021/SYSA1801
A monoclonal antibody specific to the Claudin18.2 target and an MMAE payload site-specifically attached by cleavable linkers make up EO-3021/SYSA1801. The trial found that EO-3021 exhibits antitumor activity by inducing tumor regression with the single dosage in the models of gastric cancers, pancreatic cancers, and lung cancers with low, medium, and high Claudin18.2 expression (65). SYSA1801 is being assessed in patients with advanced solid tumors who are Claudin18.2 positive for the safety, anticancer efficacy, tolerability, and pharmacokinetics in the phase 1 trial (NCT05009966).
4.4 CAR-T cell – there is a breakthrough targeting Claudin18.2
4.4.1 CT041
Artificial receptor molecule made by genetic engineering technology, CAR can direct lymphocytes, most often T cells, to identify and destroy cells that express particular target antigens. Regardless of the major histocompatibility complex (MHC) receptor, the CAR connects to the target antigens that presented on the cell surface, leading to strong T cell activity and powerful anti-tumor effect (66). As the first CAR-T cell drug targeting Claudin18.2 in the world, CT041 appeared in 2019, and its remarkable efficacy shows good prospects for the treatment of digestive system tumors. Twelve patients-seven with gastric cancer and five with pancreatic cancer-were offered with CAR-positive T cell infusions in the open-label, single-arm, phase I study (NCT03159819). The results were found that among the 11 evaluable individuals one had CR who had a gastric adenocarcinoma, three had partial response (PR) (two gastric and one pancreatic adenocarcinoma), five had stable disease (SD), and two had progressive disease (PD). There was a 33.3% ORR, with a 130 days mPFS. This suggests that patients with advanced gastric cancer and pancreatic cancer may benefit from CAR- Claudin18.2 T cells (46). Qi C et al. reported two cases (NCT04581473 and NCT03874897) of CT041 in patients who had failed standard therapy with Claudin18.2 positive metastatic pancreatic cancer (48). After lymphocyte depletion, CAR-T cells were injected in the example 1, where the expression of Claudin18.2 was 2+, 70%. On day 1, there was grade 1 cytokine release syndrome (CRS) appeared, which was handled successfully by tocilizumab. PR was reached with a significant reduction in lung metastases, based on RECIST v1.1. There was a rise in CD8+ T cells and Treg cells and a decrease in CD4+ T cells and B cells. The IHC in the example 2 for Claudin18.2 was 3+, 60%. After that, Claudin18.2 CAR-T cells were given. The patient had grade 2 CRS, which was treated by tocilizumab. In addition, CR was attained in the lung metastatic target lesions. From peripheral blood, similar results that there were rises in both CD8+ T cells and Treg cells. Furthermore, decreasing transforming growth factor-β1 (TGF-β1) and increasing interleukin-8 (IL-8) were also reported. Until the final follow-up on July 18, 2023, the tumor was remained under control. In the interim results of a phase I trial (NCT03874897), the total of 37 patients with metastatic gastrointestinal tumors (five patients with pancreatic cancer) were included to receive CT041 infusion. For all patients, the ORR was 48.6% (95% confidence interval (CI); 31.9, 65.6) and the DCR was 73.0% (95% CI; 55.9, 86.2). The mPFS was 3.7 months (95% CI; 2.6, 5.4), and the OS rate was 80.1% (95% CI; 62.5, 90.0) at 6 months. In pancreatic cancer and other cancers group (n=9), 2 had PR, 4 had SD. The ORR and DCR were 22.2% (95% CI; 2.8,60.0) and 66.7% (95% CI; 29.9,92.5), respectively. The mPFS was 2.6 months (95% CI; 1.8,3.5) (47). In the single-arm, open-label, phase Ib study (NCT04404595), at the end of February 15, 2022, eleven participators (5 had gastric cancer and 6 had pancreatic cancer) were treated with CT041 at the dose between 2.5 and 4 × 108 cells. Two patients with pancreatic cancer were in stable disease with reduced tumor size, and there were also 3 patients with pancreatic cancer who progressed on the disease. The ORR was 37.5% (3/8) in patients (49). There are also experiments (NCT05911217) on CT041 that are in progress. The studies above have demonstrated that CT041, a Claudin18.2-targeted CAR-T cell drug, has good anti-tumor activity in advanced gastrointestinal tumors, including pancreatic cancer, and is safe and reliable.
4.4.2 Other CAR-T drugs
LY011 is one of the third generation of CAR-T cell products against Claudin18.2. Currently, two phase I trials (NCT04977193, NCT04966143) are being conducted in patients with Claudin18.2 positive pancreatic cancer and advanced gastric cancer, and as of now the results have not been officially reported. In addition, LB1908 (NCT05539430), HEC-016 (NCT05277987), KD-496 (NCT05583201), and CT048 (NCT05393986) have entered clinical trials for the treatment of pancreatic cancer, gastric cancer, and other solid tumors to verify their safety, tolerability and efficacy. The results of these clinical studies are awaiting.
More and more evidence suggest that pancreatic cancer has the firm barrier, deep invasion of immunosuppressive cells, and deficiency of effector T cells in the suppressive tumor microenvironment, which is considered as the major factor in chemotherapy resistance, immunotherapy insensitivity, and recurrence and metastasis (67). Therefore, in order to improve the anti-tumor effect of Claudin18.2-targeted CAR-T cell in the pancreatic cancer, a feasible strategy may be to remodel the immune microenvironment. This also means that there is still room for further advancement and promotion of the clinical use of Claudin18.2 targets. In addition, the combination of other immunomodulators, targeted tumor angiogenesis drugs, or improvement of CAR-T cells may also provide more effective treatments for pancreatic cancer patients.
5 Summary and outlook
In summary, Claudin18.2 is expected to become a “dark horse” target due to its high selectivity for pancreatic cancer in the future, and its targeted drugs include monoclonal antibodies, bispecific antibodies, ADC, CAR-T and other mainstream directions. And a number of ongoing clinical trials are also expected to offer more choices for patients who have Claudin18.2 positive pancreatic cancer. At present, the research and development of medicine which target Claudin18.2 is mostly based on IHC, but there are differences in the detection antibodies used by different institutions and the patient populations included, resulting in differences in the positive rate of Claudin18.2. Therefore, accurate diagnosis and multi-dimensional screening methods are essential for the detection of Claudin18.2-positive patients, while screening for beneficiary populations is also a key issue in accurately defining Claudin18.2-positive patients. But overall, targeted Claudin18.2 therapy has a “bright future”.
Author contributions
QX: Writing – original draft, Writing – review & editing. CJ: Writing – original draft, Writing – review & editing. YO: Writing – original draft, Writing – review & editing. CZ: Visualization, Writing – review & editing. YJ: Validation, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
Thanks to Figdraw for the drawing support.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Niimi T, Nagashima K, Ward JM, Minoo P, Zimonjic DB, Popescu NC, et al. claudin-18, a novel downstream target gene for the T/EBP/NKX2.1 homeodomain transcription factor, encodes lung- and stomach-specific isoforms through alternative splicing. Mol Cell Biol. (2001) 21:7380–90. doi: 10.1128/MCB.21.21.7380-7390.2001
2. Furuse M, Fujita K, Hiiragi T, Fujimoto K, Tsukita S. Claudin-1 and -2: novel integral membrane proteins localizing at tight junctions with no sequence similarity to occludin. J Cell Biol. (1998) 141:1539–50. doi: 10.1083/jcb.141.7.1539
3. Zihni C, Mills C, Matter K, Balda MS. Tight junctions: from simple barriers to multifunctional molecular gates. Nat Rev Mol Cell Biol. (2016) 17:564–80. doi: 10.1038/nrm.2016.80
4. Tsukita S, Furuse M, Itoh M. Multifunctional strands in tight junctions. Nat Rev Mol Cell Biol. (2001) 2:285–93. doi: 10.1038/35067088
5. Zeisel MB, Dhawan P, Baumert TF. Tight junction proteins in gastrointestinal and liver disease. Gut. (2019) 68:547–61. doi: 10.1136/gutjnl-2018-316906
6. Brunner J, Ragupathy S, Borchard G. Target specific tight junction modulators. Adv Drug Delivery Rev. (2021) 171:266–88. doi: 10.1016/j.addr.2021.02.008
7. Türeci O, Koslowski M, Helftenbein G, Castle J, Rohde C, Dhaene K, et al. Claudin-18 gene structure, regulation, and expression is evolutionary conserved in mammals. Gene. (2011) 481:83–92. doi: 10.1016/j.gene.2011.04.007
8. Sahin U, Koslowski M, Dhaene K, Usener D, Brandenburg G, Seitz G, et al. Claudin-18 splice variant 2 is a pan-cancer target suitable for therapeutic antibody development. Clin Cancer Res. (2008) 14:7624–34. doi: 10.1158/1078-0432.CCR-08-1547
9. Lee JH, Kim KS, Kim TJ, Hong SP, Song SY, Chung JB, et al. Immunohistochemical analysis of claudin expression in pancreatic cystic tumors. Oncol Rep. (2011) 25:971–8. doi: 10.3892/or.2011.1132
10. Singh P, Toom S, Huang Y. Anti-claudin 18.2 antibody as new targeted therapy for advanced gastric cancer. J Hematol Oncol. (2017) 10:105. doi: 10.1186/s13045-017-0473-4
11. Hong JY, An JY, Lee J, Park SH, Park JO, Park YS, et al. Claudin 18.2 expression in various tumor types and its role as a potential target in advanced gastric cancer. Transl Cancer Res. (2020) 9:3367–74. doi: 10.21037/tcr-19-1876
12. Wöll S, Schlitter AM, Dhaene K, Roller M, Esposito I, Sahin U, et al. Claudin 18.2 is a target for IMAB362 antibody in pancreatic neoplasms. Int J Cancer. (2014) 134:731–9. doi: 10.1002/ijc.28400
13. Jiang H, Shi Z, Wang P, Wang C, Yang L, Du G, et al. Claudin18.2-specific chimeric antigen receptor engineered T cells for the treatment of gastric cancer. J Natl Cancer Inst. (2019) 111:409–18. doi: 10.1093/jnci/djy134
14. Wang C, Wu N, Pei B, Ma X, Yang W. Claudin and pancreatic cancer. Front Oncol. (2023) 13:1136227. doi: 10.3389/fonc.2023.1136227
15. Cao W, Xing H, Li Y, Tian W, Song Y, Jiang Z, et al. Claudin18.2 is a novel molecular biomarker for tumor-targeted immunotherapy. biomark Res. (2022) 10:38. doi: 10.1186/s40364-022-00385-1
16. Xu B, Chen F, Zhang X, Wang Z, Che K, Wu N, et al. Antigen-specific T cell immunotherapy targeting claudin18.2 in gastric cancer. Cancers (Basel). (2022) 14:2758. doi: 10.3390/cancers14112758
17. Matsuda M, Sentani K, Noguchi T, Hinoi T, Okajima M, Matsusaki K, et al. Immunohistochemical analysis of colorectal cancer with gastric phenotype: claudin-18 is associated with poor prognosis. Pathol Int. (2010) 60:673–80. doi: 10.1111/j.1440-1827.2010.02587.x
18. Jun KH, Kim JH, Jung JH, Choi HJ, Chin HM. Expression of claudin-7 and loss of claudin-18 correlate with poor prognosis in gastric cancer. Int J Surg. (2014) 12:156–62. doi: 10.1016/j.ijsu.2013.11.022
19. Karanjawala ZE, Illei PB, Ashfaq R, Infante JR, Murphy K, Pandey A, et al. New markers of pancreatic cancer identified through differential gene expression analyses: claudin 18 and annexin A8. Am J Surg Pathol. (2008) 32:188–96. doi: 10.1097/PAS.0b013e31815701f3
20. Shen CH, Lin JY, Lu CY, Yang SS, Peng CK, Huang KL. SPAK-p38 MAPK signal pathway modulates claudin-18 and barrier function of alveolar epithelium after hyperoxic exposure. BMC Pulm Med. (2021) 21:58. doi: 10.1186/s12890-021-01408-7
21. Takasawa K, Takasawa A, Osanai M, Aoyama T, Ono Y, Kono T, et al. Claudin-18 coupled with EGFR/ERK signaling contributes to the Malignant potentials of bile duct cancer. Cancer Lett. (2017) 403:66–73. doi: 10.1016/j.canlet.2017.05.033
22. Resnick MB, Konkin T, Routhier J, Sabo E, Pricolo VE. Claudin-1 is a strong prognostic indicator in stage II colonic cancer: a tissue microarray study. Mod Pathol. (2005) 18:511–8. doi: 10.1038/modpathol.3800301
23. Ito T, Kojima T, Yamaguchi H, Kyuno D, Kimura Y, Imamura M, et al. Transcriptional regulation of claudin-18 via specific protein kinase C signaling pathways and modification of DNA methylation in human pancreatic cancer cells. J Cell Biochem. (2011) 112:1761–72. doi: 10.1002/jcb.23095
24. Sanada Y, Oue N, Mitani Y, Yoshida K, Nakayama H, Yasui W. Down-regulation of the claudin-18 gene, identified through serial analysis of gene expression data analysis, in gastric cancer with an intestinal phenotype. J Pathol. (2006) 208:633–42. doi: 10.1002/path.1922
25. Cheung ST, Leung KL, Ip YC, Chen X, Fong DY, Ng IO, et al. Claudin-10 expression level is associated with recurrence of primary hepatocellular carcinoma. Clin Cancer Res. (2005) 11:551–6. doi: 10.1158/1078-0432.551.11.2
26. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2021) 71:209–49. doi: 10.3322/caac.21660
27. Liu YQ, Zou HY, Xie JJ, Fang WK. Paradoxical roles of desmosomal components in head and neck cancer. Biomolecules. (2021) 11:914. doi: 10.3390/biom11060914
28. Zhang Z, Auyeung KK, Sze SC, Zhang S, Yung KK, Ko JK. The dual roles of calycosin in growth inhibition and metastatic progression during pancreatic cancer development: A “TGF-β paradox”. Phytomedicine. (2020) 68:153177. doi: 10.1016/j.phymed.2020.153177
29. Cebrián MJ, Bauden M, Andersson R, Holdenrieder S, Ansari D. Paradoxical role of HMGB1 in pancreatic cancer: tumor suppressor or tumor promoter? Anticancer Res. (2016) 36:4381–9. doi: 10.21873/anticanres.10981
30. Bose M. Preferentially expressed antigen in melanoma is a multifaceted cancer testis antigen with diverse roles as a biomarker and therapeutic target. Int J Transl Med. (2023) 3:334–59. doi: 10.3390/ijtm3030024
31. Chen J, Xu Z, Hu C, Zhang S, Zi M, Yuan L, et al. Targeting CLDN18.2 in cancers of the gastrointestinal tract: New drugs and new indications. Front Oncol. (2023) 13:1132319. doi: 10.3389/fonc.2023.1132319
32. Oshima T, Shan J, Okugawa T, Chen X, Hori K, Tomita T, et al. Down-regulation of claudin-18 is associated with the proliferative and invasive potential of gastric cancer at the invasive front. PloS One. (2013) 8:e74757. doi: 10.1371/journal.pone.0074757
33. Hagen SJ, Ang LH, Zheng Y, Karahan SN, Wu J, Wang YE, et al. Loss of tight junction protein claudin 18 promotes progressive neoplasia development in mouse stomach. Gastroenterology. (2018) 155:1852–67. doi: 10.1053/j.gastro.2018.08.041
34. Zhou B, Flodby P, Luo J, Castillo DR, Liu Y, Yu FX, et al. Claudin-18-mediated YAP activity regulates lung stem and progenitor cell homeostasis and tumorigenesis. J Clin Invest. (2018) 128:970–84. doi: 10.1172/JCI90429
35. Shimobaba S, Taga S, Akizuki R, Hichino A, Endo S, Matsunaga T, et al. Claudin-18 inhibits cell proliferation and motility mediated by inhibition of phosphorylation of PDK1 and Akt in human lung adenocarcinoma A549 cells. Biochim Biophys Acta. (2016) 1863:1170–8. doi: 10.1016/j.bbamcr.2016.02.015
36. Luo J, Chimge NO, Zhou B, Flodby P, Castaldi A, Firth AL, et al. CLDN18.1 attenuates Malignancy and related signaling pathways of lung adenocarcinoma in vivo and in vitro. Int J Cancer. (2018) 143:3169–80. doi: 10.1002/ijc.31734
37. Kotton DN. Claudin-18: unexpected regulator of lung alveolar epithelial cell proliferation. J Clin Invest. (2018) 128:903–5. doi: 10.1172/JCI99799
38. Jovov B, Van Itallie CM, Shaheen NJ, Carson JL, Gambling TM, Anderson JM, et al. Claudin-18: a dominant tight junction protein in Barrett’s esophagus and likely contributor to its acid resistance. Am J Physiol Gastrointest Liver Physiol. (2007) 293:G1106–13. doi: 10.1152/ajpgi.00158.2007
39. Yano K, Imaeda T, Niimi T. Transcriptional activation of the human claudin-18 gene promoter through two AP-1 motifs in PMA-stimulated MKN45 gastric cancer cells. Am J Physiol Gastrointest Liver Physiol. (2008) 294:G336–43. doi: 10.1152/ajpgi.00328.2007
40. Tanaka M, Shibahara J, Fukushima N, Shinozaki A, Umeda M, Ishikawa S, et al. Claudin-18 is an early-stage marker of pancreatic carcinogenesis. J Histochem Cytochem. (2011) 59:942–52. doi: 10.1369/0022155411420569
41. Kyuno D, Takasawa A, Takasawa K, Ono Y, Aoyama T, Magara K, et al. Claudin-18.2 as a therapeutic target in cancers: cumulative findings from basic research and clinical trials. Tissue Barriers. (2022) 10:1967080. doi: 10.1080/21688370.2021.1967080
42. Zhang J, Dong R, Shen L. Evaluation and reflection on claudin 18.2 targeting therapy in advanced gastric cancer. Chin J Cancer Res. (2020) 32:263–70. doi: 10.21147/j.issn.1000-9604.2020.02.13
43. Xu RH, Wei X, Zhang D, Qiu M, Zhang Y, Zhao H, et al. A phase 1a dose-escalation, multicenter trial of anti-claudin 18.2 antibody drug conjugate CMG901 in patients with resistant/refractory solid tumors. J Clin Oncol. (2023) 41:352. doi: 10.1200/JCO.2023.41.4_suppl.352
44. Zhu G, Foletti D, Liu X, Ding S, Melton Witt J, Hasa-Moreno A, et al. Targeting CLDN18.2 by CD3 bispecific and ADC modalities for the treatments of gastric and pancreatic cancer. Sci Rep. (2019) 9:8420. doi: 10.1038/s41598-019-44874-0
45. Gao J, Wang Z, Jiang W, Zhang Y, Meng Z, Niu Y, et al. CLDN18.2 and 4-1BB bispecific antibody givastomig exerts antitumor activity through CLDN18.2-expressing tumor-directed T-cell activation. J Immunother Cancer. (2023) 11:e006704. doi: 10.1136/jitc-2023-006704
46. Zhan X, Wang B, Li Z, Li J, Wang H, Chen L, et al. Phase I trial of Claudin 18.2-specific chimeric antigen receptor T cells for advanced gastric and pancreatic adenocarcinoma. J Clin Oncol. (2019) 37:2509. doi: 10.1200/JCO.2019.37.15_suppl.2509
47. Qi C, Gong J, Li J, Liu D, Qin Y, Ge S, et al. Claudin18.2-specific CAR T cells in gastrointestinal cancers: phase 1 trial interim results. Nat Med. (2022) 28:1189–98. doi: 10.1038/s41591-022-01800-8
48. Qi C, Xie T, Zhou J, Wang X, Gong J, Zhang X, et al. CT041 CAR T cell therapy for Claudin18.2-positive metastatic pancreatic cancer. J Hematol Oncol. (2023) 16:102. doi: 10.1186/s13045-023-01491-9
49. Botta GP, Becerra CR, Jin Z, Kim DW, Zhao D, Lenz H-J. Multicenter phase Ib trial in the U.S. @ of salvage CT041 CLDN18.2-specific chimeric antigen receptor T-cell therapy for patients with advanced gastric and pancreatic adenocarcinoma. J Clin Oncol. (2022) 40:2538. doi: 10.1200/JCO.2022.40.16_suppl.2538
50. Park W, O’Reilly EM, Furuse J, Li CP, Oh DY, Garcia-Carbonero R, et al. Zolbetuximab plus gemcitabine and nab-paclitaxel (GN) in first-line treatment of claudin 18.2-positive metastatic pancreatic cancer (mPC): Phase 2, open-label, randomized study. J Clin Oncol. (2023) 41(4):782. doi: 10.1200/JCO.2023.41.4_suppl.TPS782
51. Li J, Pan H, Liu T, Xu N, Zhang Y, Qin Y, et al. A multicenter, phase 1 study of AB011, a recombinant humanized anti-CLDN18.2 monoclonal antibody, as monotherapy and combined with capecitabine and oxaliplatin (CAPOX) in patients with advanced solid tumors. J Clin Oncol. (2023) 41:391. doi: 10.1200/JCO.2023.41.4_suppl.391
52. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2021. CA Cancer J Clin. (2021) 71:7–33. doi: 10.3322/caac.21654
53. Rahib L, Smith BD, Aizenberg R, Rosenzweig AB, Fleshman JM, Matrisian LM. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. (2014) 74:2913–21. doi: 10.1158/0008-5472.CAN-14-0155
54. Zhu H, Li T, Du Y, Li M. Pancreatic cancer: challenges and opportunities. BMC Med. (2018) 16:214. doi: 10.1186/s12916-018-1215-3
55. Li D, Xie K, Wolff R, Abbruzzese JL. Pancreatic cancer. Lancet. (2004) 363:1049–57. doi: 10.1016/S0140-6736(04)15841-8
56. Grossberg AJ, Chu LC, Deig CR, Fishman EK, Hwang WL, Maitra A, et al. Multidisciplinary standards of care and recent progress in pancreatic ductal adenocarcinoma. CA Cancer J Clin. (2020) 70:375–403. doi: 10.3322/caac.21626
57. Conroy T, Desseigne F, Ychou M, Bouché O, Guimbaud R, Bécouarn Y, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. (2011) 364:1817–25. doi: 10.1056/NEJMoa1011923
58. Türeci Ö, Mitnacht-Kraus R, Wöll S, Yamada T, Sahin U. Characterization of zolbetuximab in pancreatic cancer models. Oncoimmunology. (2018) 8:e1523096. doi: 10.1080/2162402X.2018.1523096
59. Chen Y, Hou X, Li D, Ding J, Liu J, Wang Z, et al. Development of a CLDN18.2-targeting immuno-PET probe for non-invasive imaging in gastrointestinal tumors. J Pharm Anal. (2023) 13:367–75. doi: 10.1016/j.jpha.2023.02.011
60. Gabrail NY, Tolcher A, Alese OB, Cecchini M, Manish P, Park H, et al. A phase I clinical trial to evaluate the safety, tolerability, and pharmacokinetics of TST001 in patients with locally advanced or metastatic solid tumors. J Clin Oncol (2022) 40(4):375. doi: 10.1200/JCO.2022.40.4_suppl.TPS375
61. Qian X, Teng F, Guo H, Yao X, Shi L, Wu Y, et al. 1560P Osemitamab (TST001): An ADCC enhanced humanized anti-CLDN18.2 mab, demonstrated improved efficacy in combination with anti-PD-L1/PD-1 mab and oxaliplatin/5-FU in preclinical tumor models. Ann Oncol. (2023) 34:S873. doi: 10.1016/j.annonc.2023.09.1472
62. Brinkmann U, Kontermann RE. Bispecific antibodies. Science. (2021) 372:916–7. doi: 10.1126/science.abg1209
63. Fu Z, Li S, Han S, Shi C, Zhang Y. Antibody drug conjugate: the “biological missile” for targeted cancer therapy. Signal Transduct Target Ther. (2022) 7:93. doi: 10.1038/s41392-022-00947-7
64. Huang W, Li Y, Liu Z, Rodon L, Correia S, Li R. Preclinical activity for TPX-4589 (LM-302), an antibody-drug conjugate targeting tight junction protein CLDN18.2 in solid tumors. Eur J Cancer. (2022) 174:S41–2. doi: 10.1016/S0959-8049(22)00911-X
65. Dan M, Hui X, Wang Y, Yuan C, O’Hare T, Jansen VM, et al. Therapeutic potential of EO-3021/SYSA1801, a Claudin18.2 antibody-drug conjugate, for the treatment of CLDN18.2-expressing cancers. Cancer Res. (2023) 83:6300. doi: 10.1158/1538-7445.AM2023-6300
66. Sadelain M, Brentjens R, Rivière I. The basic principles of chimeric antigen receptor design. Cancer Discovery. (2013) 3:388–98. doi: 10.1158/2159-8290.CD-12-0548
Keywords: pancreatic cancer, Claudin18.2, targeted therapy, immunotherapy, trials
Citation: Xu Q, Jia C, Ou Y, Zeng C and Jia Y (2024) Dark horse target Claudin18.2 opens new battlefield for pancreatic cancer. Front. Oncol. 14:1371421. doi: 10.3389/fonc.2024.1371421
Received: 16 January 2024; Accepted: 23 February 2024;
Published: 06 March 2024.
Edited by:
Kathleen E. DelGiorno, Vanderbilt University, United StatesReviewed by:
Elva Morretta, Università degli Studi di Salerno, ItalyMukulika Bose, University of Texas MD Anderson Cancer Center, United States
Copyright © 2024 Xu, Jia, Ou, Zeng and Jia. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yingjie Jia, MTYxNDIzNjQwNUBxcS5jb20=
†These authors have contributed equally to this work
 Qian Xu
Qian Xu Caiyan Jia1,2†
Caiyan Jia1,2†