
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Oncol., 16 April 2024
Sec. Thoracic Oncology
Volume 14 - 2024 | https://doi.org/10.3389/fonc.2024.1370901
Background: The c-met proto-oncogene (MET) serves as a significant primary oncogenic driver in non-small cell lung cancer (NSCLC) and has the potential to fuse with other genes, such as KIF5B, although it occurs infrequently. Only a limited number of reported cases have examined the clinical efficacy of crizotinib in patients with KIF5B-MET gene fusion, with no known data regarding acquired resistance to crizotinib and its potential mechanisms. In this report, we present the clinical progression of a female patient diagnosed with NSCLC and harboring a KIF5B-MET gene fusion.
Case description: The patient initially exhibited partial response to first-line crizotinib treatment, albeit for a short duration and with limited efficacy. Subsequent disease progression revealed the emergence of a secondary MET mutation, specifically MET Y1230H, leading to acquired resistance to crizotinib.
Conclusion: The reporting of this case is imperative for informing clinical practice, given the uncommon occurrence of NSCLC with MET fusion, displaying responsiveness to MET tyrosine kinase inhibitor therapy, as well as the emergence of the secondary Y1230H alteration as a potential resistance mechanism.
MET is an important primary oncogenic driver gene in non-small cell lung cancer (NSCLC). The most common MET aberrations are gene amplifications and exon 14 splice variants. MET fusion is a rare type of structural rearrangement; nine MET fusion partner genes have been identified, namely: PRKAR2B (1), KIF5B (2–4), STARD3NL (5), CDR2 (6), UBE2H (7), HLA-DRB1 (8), ATXN7L1 (9), CD47 (10), and SPECC1L (11). To date, eight cases of KIF5B-MET gene fusion have been reported in the literature, of which only a few have described the efficacy of crizotinib (Table 1). Continued reporting of these cases is necessary to inform clinical practice. Herein, we present the case of a patient who presented with neck pain and limb myasthenia persisting for more than 6 months. She was diagnosed with advanced lung adenocarcinoma with KIF5B-MET fusion and achieved partial response to crizotinib for 4 months. In addition, we identified a secondary MET mutation (MET Y1230H) after disease progression during crizotinib therapy. To the best of our knowledge, this is the first clinical report of a MET Y1230H mutation arising in a patient with KIF5B-MET fusion.
In May 2020, a 59-year-old woman who had never smoked presented to our hospital with neck pain and limb myasthenia persisting for more than 6 months. Her medical history was unremarkable. However, computed tomography and magnetic resonance imaging revealed a tumor in the lower lobe of her right lung with pleural effusion (Figure A1) and multi-organ metastasis, including the brain (Figure B1), vertebrae, and bone. Histopathological analysis of tissue biopsy samples obtained from the lower lobe of the right lung using bronchoscopy revealed poorly differentiated adenocarcinoma; the immunohistochemistry results were as follows: napsin A(-), TTF-1(-), CK5/6(-), P40(-), CgA(-), Syn(-), CK7(+), and Ki67(70%+). Next-generation sequencing of the lung biopsy tissue showed KIF5B-MET (K24::M15) fusion without other targeted oncogenic alterations such as EGFR, ALK, ROS1, BRAF, HER2, RET, and KRAS (Table 2). In addition, the programmed death-ligand 1(PD-L1) expression analysis revealed a tumor proportion score of 40%.
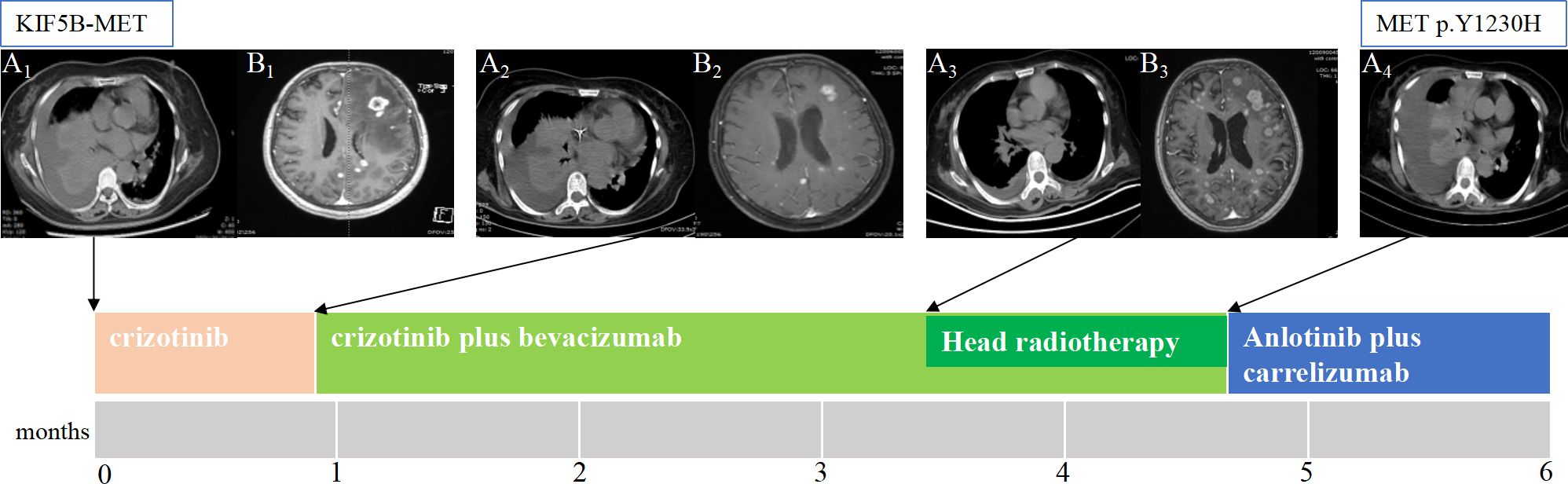
Figure 1 The patient’s clinical course illustrated using lung computed tomography and brain magnetic resonance imaging. (A1) Baseline imaging demonstrating abnormal lung mass and pleural effusion in the right lung. (A2) After 3 weeks of only crizotinib treatment, the chest CT scan shows that the lung mass and pleural fluid did not change significantly. (A3) After crizotinib plus three cycles of bevacizumab, the lung mass shrank, the pleural fluid decreased. (A4) After crizotinib plus bevacizumab brain radiotherapy, the chest CT scan shows disease progression, and next-generation sequencing detected the resistance mutation as METp.Y1230H. (B1) Brain MRI showing brain metastasis. (B2) After 3 weeks of only crizotinib treatment, most of the lesions in the brain slightly decreased, and edema around the lesions decreased, achieving partial response (PR). (B3) After crizotinib plus three cycles of bevacizumab, the head lesions grew. CT, computed tomography; MRI, magnetic resonance imaging .
The patient refused chemotherapy; thus, crizotinib (250 mg, po. bid) was initiated as the first-line treatment in June 2020 without adverse effects.
After 3 weeks of crizotinib treatment alone, a reduction in the size of most lesions in the brain was observed, accompanied by a decrease in edema around the lesions (Figure B2). However, the lung mass and pleural fluid did not significantly change (Figure A2). Thus, crizotinib was continued owing to its clinical benefits, and bevacizumab was initiated in July 2020.
After crizotinib plus three cycles of bevacizumab, the lung mass shrank, and the pleural fluid decreased (Figure A3), but the brain lesions grew (Figure B3). Therefore, brain radiation therapy was started in September 2020 (gross tumor volume: 50 Gy/2.5 Gy/20 F, planning target volume: 36 Gy/1.8 Gy/20 F).
By October 2020, the disease had progressed; compared to that in the previous evaluation, the pleural fluid increased, the previously decreased tumor grew, and new lesions appeared in the liver (Figure A4). Targeted sequencing was performed on new blood samples using a panel of 483 cancer-related genes to identify new actionable mutations, revealing a novel point mutation in MET exon 19 (c.3688T> C; p. Y1230H) (Table 2).
The patient again refused chemotherapy after crizotinib plus bevacizumab failure. The MET Y1230H mutant may be sensitive to the type II MET TKI, cabozantinib; however, cabozantinib has not been listed in China. Therefore, we chose anlotinib [a vascular endothelial growth factor receptor-2 tyrosine kinase inhibitor (VEGFR-2 TKI)] plus carrelizumab [an immune checkpoint inhibitor (ICI)] as salvage therapy, from which the patient did not benefit, and her condition worsened. The patient died of multiple organ dysfunction thereafter, surviving 6 months after the diagnosis.
The KIF5B-MET fusion gene was originally reported in one of 513 lung adenocarcinoma (LADC) samples (12). There are two known types of KIF5B-MET fusions in LADC – K24::M15 and K24::M14. We identified eight cases in the literature; six involved K24::M15 and two involved K24::M14 (Table 1). Thus, our patient with a gene fusion between exon 24 of KIF5B and exon 15 of MET is the ninth case to be reported.
The KIF5B-MET variant has oncogenic functions in cancer cells (4), but MET inhibitors have potential therapeutic effects on tumors expressing the KIF5B-MET fusion protein. Cho et al. (2) reported the first documented case of a KIF5B-MET (K24::M14) gene rearrangement in a patient with LADC who responded positively to treatment with crizotinib, a MET inhibitor. Plenker et al. (3) also reported positive results after 8 months of crizotinib treatment in a patient with a KIF5B-MET (K24::M15) gene fusion. In our case, the patient refused chemotherapy. Thus, crizotinib was taken orally with positive results after 3 weeks; most brain lesions decreased slightly. However, significant lung lesions or pleural fluid changes did not occur.
A multicenter Phase 3 study (CTONG1509) (13) reported that bevacizumab plus erlotinib provides superior progression-free survival compared to erlotinib alone in Chinese patients. In addition, bevacizumab with alectinib has been proven to be a safe and highly effective first-line therapy (14). Bevacizumab is a monoclonal antibody against VEGF; thus, VEGFR2 inhibition enhances the anti-tumor effect of molecularly targeted drugs in various oncogene-driven NSCLC models by inhibiting tumor angiogenesis and exerting a direct antiproliferative effect on cancer cells (15), which suggests that combination therapy with bevacizumab and molecularly targeted agents is a promising strategy for patients with NSCLC harboring oncogenic driver genes. Given the fact that the efficacy of using crizotinib alone was not very satisfactory, we also tried to administer bevacizumab. After treatment with crizotinib plus three cycles of bevacizumab, the lung lesions shrank and pleural fluid decreased; however, the brain lesions increased, possibly due to heterogeneity in the tumor’s response.
Unfortunately, disease progression occurred 1 month later. Therefore, to identify new actionable mutations, targeted sequencing was performed on fresh blood samples using a panel of 483 cancer-related genes, revealing a novel point mutation in MET exon 19 (c.3688T> C; p. Y1230H). MET Y1230H is a drug-resistance mutation in the MET activation loop, associated with secondary resistance mechanisms in preclinical studies (16). Clinical studies have shown that this mutation is associated with resistance to MET inhibitors (17), and structural analysis indicated that it destabilizes the auto-inhibitory conformation of MET and abrogates important aromatic stacking interactions with the inhibitor (18). Schrock et al. (19) reported that the MET Y1230 mutation is an acquired mechanism of crizotinib resistance in NSCLC with MET exon 14 skipping. Herein, we describe a similar case of a MET Y1230H mutation acquired after crizotinib treatment in a patient with NSCLC, driven by the KIF5B-MET (K24::M15) gene fusion. This patient developed resistance to crizotinib owing to an acquired MET Y1230H mutation. Based on the mechanism of action, MET TKIs are divided into two groups, type I and type II. As type I and II MET TKIs bind the ATP pocket of MET differently, they have distinct inhibition capacities for MET mutants. Engstrom and others confirmed that the MET Y1230H mutant was resistant to type I MET TKIs, such as capmatinib, lvotinib, and crizotinib, but remained sensitive to type II MET TKIs, glesatinib and cabozantinib (20). The switch from a type I MET TKI to a type II MET TKI, cabozantinib, can be an effective strategy to overcome acquired type I MET TKI resistance in NSCLC (21). Unfortunately, to date, cabozantinib has not been listed in China, and the patient was unable to purchase it in China in 2020.
Anlotinib is a small molecule multi-target TKI that can effectively inhibit kinases such as VEGFR, platelet-derived growth factor receptor (PDGFR), fibroblast growth factor receptor (FGFR), and C-Kit. It has anti-tumor angiogenesis and tumor growth inhibition effects. In May 2018, anlotinib hydrochloride capsules were approved for marketing by China National Medical Products Administration, making anlotinib hydrochloride the first drug in China to be approved for third-line treatment of advanced NSCLC. This drug is suitable for the treatment of patients with locally advanced or metastatic NSCLC who have experienced progression or recurrence after receiving at least two types of systemic chemotherapy in the past. The patient refused chemotherapy, hence we administered anlotinib as a third-line treatment. Meanwhile, an ICI was selected as salvage therapy but failed to yield positive outcomes. This is similar to the finding of previous studies that showed that ICIs are less effective for NSCLC with EGFR mutation or EML4-ALK fusion (22).
In summary, crizotinib can be effective for patients with KIF5B-MET fusion; however, cancer cells can develop resistance. In our patient with NSCLC with KIF5B-MET fusion, the MET Y1230H mutation was an acquired resistance mechanism to crizotinib. Preventing or overcoming resistance in these patients requires further exploration. ICIs may be ineffective for NSCLC with KIF5B-MET fusion.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article. Written informed consent was obtained from the participant/patient(s) for the publication of this case report.
SD: Conceptualization, Writing – original draft. WD: Conceptualization, Writing – original draft. YT: Data curation, Writing – review & editing. QX: Data curation, Writing – review & editing. TW: Supervision, Validation, Writing – review & editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
We thank the patient and her family. We also thank the whole project team who worked on this case. We are grateful to Liu Wei at Beijing Novogene Bioinformatics Technology Co for technical assistance. We would also like to thank Editage (www.editage.com) for English language editing.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
ICI, immune checkpoint inhibitor; LADC, lung adenocarcinoma; MET, c-met proto-oncogene; NSCLC, non-small cell lung cancer; TKI, tyrosine kinase inhibitor; VEGFR-2 TKI, vascular endothelial growth factor receptor-2 tyrosine kinase inhibitor.
1. Riedel R, Fassunke J, Tumbrink HL, Scheel AH, Heydt C, Hieggelke L, et al. Resistance to MET inhibition in MET-dependent NSCLC and therapeutic activity after switching from type I to type II MET inhibitors. Eur J Cancer. (2023) 179:124–35. doi: 10.1016/j.ejca.2022.11.010
2. Cho JH, Ku BM, Sun JM, Lee SH, Ahn JS, Park K, et al. KIF5B-MET gene rearrangement with robust antitumor activity in response to crizotinib in lung adenocarcinoma. J Thorac Oncol. (2018) 13:e29–31. doi: 10.1016/j.jtho.2017.10.014
3. Lin CY, Wei SH, Chen YL, Lee CT, Wu SY, Ho CL, et al. Case report: Salvage capmatinib therapy in KIF5B-MET fusion-positive lung adenocarcinoma with resistance to telisotuzumab vedotin. Front Oncol. (2022) 12:919123. doi: 10.3389/fonc.2022.919123
4. Gow CH, Liu YN, Li HY, Hsieh MS, Chang SH, Luo SC, et al. Oncogenic function of a KIF5B-MET fusion variant in non-small cell lung cancer. Neoplasia. (2018) 20:838–47. doi: 10.1016/j.neo.2018.06.007
5. Plenker D, Bertrand M, de Langen AJ, Riedel R, Lorenz C, Scheel AH, et al. Structural alterations of MET trigger response to MET kinase inhibition in lung adenocarcinoma patients. Clin Cancer Res. (2018) 24:1337–43. doi: 10.1158/1078-0432.CCR-17-3001
6. Liu LF, Deng JY, Lizaso A, Lin J, Sun S. Effective response to crizotinib of concurrent KIF5B-MET and MET-CDR2-rearranged non-small cell lung cancer: A case report. World J Clin cases. (2022) 10:2529–36. doi: 10.12998/wjcc.v10.i8.2529
7. Zhu YC, Wang WX, Song ZB, Zhang QX, Xu CW, Chen G, et al. MET-UBE2H fusion as a novel mechanism of acquired EGFR resistance in lung adenocarcinoma. J Thorac Oncol. (2018) 13:e202–4. doi: 10.1016/j.jtho.2018.05.009
8. Kunte S, Stevenson J. A case of HLA-DRB1-MET rearranged lung adenocarcinoma with rapid response to crizotinib. Clin Lung Cancer. (2021) 22:e298–300. doi: 10.1016/j.cllc.2020.05.005
9. Zhu YC, Wang WX, Xu CW, Zhang QX, Du KQ, Chen G, et al. Identification of a novel crizotinib-sensitive MET-ATXN7L1 gene fusion variant in lung adenocarcinoma by next generation sequencing. Ann Oncol. (2018) 29:2392–3. doi: 10.1093/annonc/mdy455
10. Liu J, Shen L, Qian Y, Liu Y, Su M, Yi L. Durable response to crizotinib in an advanced lung adenocarcinoma patient harboring rare CD47-MET fusion: A case report. Transl Cancer Res. (2022) 11:2931–5. doi: 10.21037/tcr-22-141
11. Nelson AW, Schrock AB, Pavlick DC, Ali SM, Atkinson EC, Chachoua A, et al. Novel SPECC1L-MET Fusion Detected in Circulating Tumor DNA in a Patient with Lung adenocarcinoma following Treatment with erlotinib and Osimertinib. J Thorac Oncol. (2019) 14:e27–9. doi: 10.1016/j.jtho.2018.10.160
12. Stransky N, Cerami E, Schalm S, Kim JL, Lengauer C. The landscape of kinase fusions in cancer. Nat Commun. (2014) 5:4846. doi: 10.1038/ncomms5846
13. Zhou Q, Xu CR, Cheng Y, Liu YP, Chen GY, Cui JW, et al. Bevacizumab plus erlotinib in Chinese patients with untreated, EGFR-mutated, advanced NSCLC (ARTEMIS-CTONG1509): A multicenter phase 3 study. Cancer Cell. (2021) 39:1279–1291.e3. doi: 10.1016/j.ccell.2021.07.005
14. Watanabe S, Sakai K, Matsumoto N, Koshio J, Ishida A, Abe T, et al. Phase II trial of the combination of alectinib with bevacizumab in alectinib refractory ALK-positive nonsquamous non-small-cell lung cancer (NLCTG1501). Cancers (Basel). (2022) 15:204. doi: 10.3390/cancers15010204
15. Watanabe H, Ichihara E, Kayatani H, Makimoto G, Ninomiya K, Nishii K, et al. VEGFR2 blockade augments the effects of tyrosine kinase inhibitors by inhibiting angiogenesis and oncogenic signaling in oncogene-driven non-small-cell lung cancers. Cancer Sci. (2021) 112:1853–64. doi: 10.1111/cas.14801
16. Tiedt R, Degenkolbe E, Furet P, Appleton BA, Wagner S, Schoepfer J, et al. A drug resistance screen using a selective MET inhibitor reveals a spectrum of mutations that partially overlap with activating mutations found in cancer patients. Cancer Res. (2011) 71:5255–64. doi: 10.1158/0008-5472.CAN-10-4433
17. Ou SI, Young L, Schrock AB, Johnson A, Klempner SJ, Zhu VW, et al. Emergence of preexisting MET Y1230C mutation as a resistance mechanism to crizotinib in NSCLC with MET Exon 14 skipping. J Thorac Oncol. (2017) 12:137–40. doi: 10.1016/j.jtho.2016.09.119
18. Qi J, McTigue MA, Rogers A, Lifshits E, Christensen JG, Jänne PA, et al. Multiple mutations and bypass mechanisms can contribute to development of acquired resistance to MET inhibitors. Cancer Res. (2011) 71:1081–91. doi: 10.1158/0008-5472.CAN-10-1623
19. Schrock AB, Lai A, Ali SM, Miller VA, Raez LE. Mutation of MET Y1230 as an acquired mechanism of crizotinib resistance in NSCLC with MET Exon 14 skipping. J Thorac Oncol. (2017) 12:e89–90. doi: 10.1016/j.jtho.2017.02.017
20. Engstrom LD, Aranda R, Lee M, Tovar EA, Essenburg CJ, Madaj Z, et al. Glesatinib exhibits antitumor activity in lung cancer models and patients harboring MET Exon 14 mutations and overcomes mutation-mediated resistance to Type I MET inhibitors in nonclinical models. Clin Cancer Res. (2017) 23:6661–72. doi: 10.1158/1078-0432.CCR-17-1192
21. Cai B, Li X, Huang X, Ma T, Qu B, Yu W, et al. Case report: Sequential combination targeted therapy with type I and II MET inhibitors in a metastatic EGFR-mutated, MET-amplified NSCLC patient with acquired MET Y1230H mutation. Front Oncol. (2021) 11:738832. doi: 10.3389/fonc.2021.738832
Keywords: acquired resistance, MET fusion, non-small cell lung cancer, gene mutations, crizotinib
Citation: Dong S-S, Dong W, Tan Y-F, Xiao Q and Wang T-L (2024) Case report: Acquired resistance to crizotinib from a MET Y1230H mutation in a patient with non-small cell lung cancer and KIF5B-MET fusion. Front. Oncol. 14:1370901. doi: 10.3389/fonc.2024.1370901
Received: 15 January 2024; Accepted: 29 March 2024;
Published: 16 April 2024.
Edited by:
Francesco Facchinetti, Dana–Farber Cancer Institute, United StatesReviewed by:
Seshiru Nakazawa, Dana–Farber Cancer Institute, United StatesCopyright © 2024 Dong, Dong, Tan, Xiao and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tian-Li Wang, dGlhbmx3NjZAMTYzLmNvbQ==
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.