
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
EDITORIAL article
Front. Oncol. , 02 February 2024
Sec. Molecular and Cellular Oncology
Volume 14 - 2024 | https://doi.org/10.3389/fonc.2024.1369457
This article is part of the Research Topic New Insights into Fibrotic Signaling in Cancer View all 11 articles
Editorial on the Research Topic
New insights into fibrotic signaling in cancer
Cancer is still a top leading cause of death worldwide. Ineffective treatments, severe side effects, drug resistance, recurrence, and metastasis are major barriers to curing cancers with the conventional therapeutic methods. Immunotherapies show promise on blood cancers, but various response rates were observed on the treated patients with solid cancers, 30% of patients with non-small-cell lung cancer response to the T-cell based therapies including CAR-T and PD-1/L1 blockades in clinics.
Increasing evidence shows the importance of fibrotic signaling in solid cancers. For example, TGF-beta, a well-documented fibrotic cytokine, was first discovered as an anticancer regulator in cancer cells, but it has been found as a strong immunosuppressor for the host anticancer immunity (1, 2). Therefore, better understanding the potential roles of the fibrotic signaling in cancer cells as well as their microenvironment may eventually identify suitable strategies for improving the efficiency of cancer therapies in clinics.
This Research Topic serves as an interactive platform for sharing the latest insights of molecular mechanisms, translational potential, and clinical observations of the fibrotic singling in cancer. We have received manuscripts from research groups all over the world, and eventually accepted 6 original research and 4 review articles for publication. The articles widely covered findings from the clinical prognostic, molecular mechanisms, and therapeutic development based on the cancer cells as well as the tumor microenvironments.
The importance of fibrotic signaling in diseases beyond tissue fibrosis has been recognized. For examples, a well-documented phenomenon “Macrophage-Myofibroblast Transition” has been widely reported in kidney fibrosis (3), its implications in solid cancer has been started to be investigated nowadays (4). In this Research Topic, the clinical implications of fibrotic signaling in clinical solid cancers have been examined, where Chen et al. observed that intratumorally fibrosis and pseudo-capsule fibrosis are positively correlated to the disease progression of renal cell. While Shan et al. observed 10 fibrotic signaling (e.g. TLR4, Hedgehog, TGF-β, etc) are closely related to the biological activities of hepatocellular carcinoma cells after reviewing 264 related studies. Qin et al. has developed a new prognostic nomogram combined with desmoplastic reaction for predicting the progression of synchronous peritoneal metastasis in colorectal cancer patients.
Fibrotic signaling may contribute to pathogenesis that are beyond the end stage organ diseases. Myofibroblast formation is one of the critical steps for tissue scarring (5, 6) as well as tumor formation (4). Zhang et al. reported a pathogenic role of cancer-associated fibroblasts in the irradiation driven cancer progression. Cheng et al. further summarized the clinical implications of a myofibroblast-based targeting strategies for pancreatic cancer treatment. Besides, by transcriptome profiling of patient biopsies, Tulalamba et al. identified a Wnt signaling mediator FZD10 as potential biomarker for nasopharyngeal carcinoma recurrence and Chen et al. examined the clinical relevance of FGFR signaling in head and neck carcinoma. Sukphokkit et al. demonstrated a 3-dimensional culture system which can reformed the phenotype of cholangiocarcinoma cells compared to the conventional 2D system, may serve as an ideal platform for elucidating the underlying mechanisms as well as clinical potential of fibrotic signaling in cancer in vitro.
Indeed, a new study demonstrated that targeting of TGF-β/Smads signaling with a natural compound formula effectively overcome drug resistance of liver cancer cells via suppressing a multidrug resistant gene MDR1 (7). Here, Gao et al. reported Icaritin, an active component of the traditional Chinese herb Epimedium genus can inhibit cancer migration via targeting Akt/mTOR signaling of the cisplatin-resistant ovarian cancer cells in vitro. Ji et al. summarized the dynamics and therapeutic potentials of tumor-associated macrophages in solid cancer, such as a novel neuron type derived from “Macrophage to Neuron-like Cell Transition” in lung cancer (Figure 1) (8). Single-cell RNA-sequencing allows researchers to dissect the TME in a cell-type specific manner, therefore the contributions of fibrotic signaling in the cancer immunity which was hidden in the conventional bulk sequencing at population level can be unmasked (9).
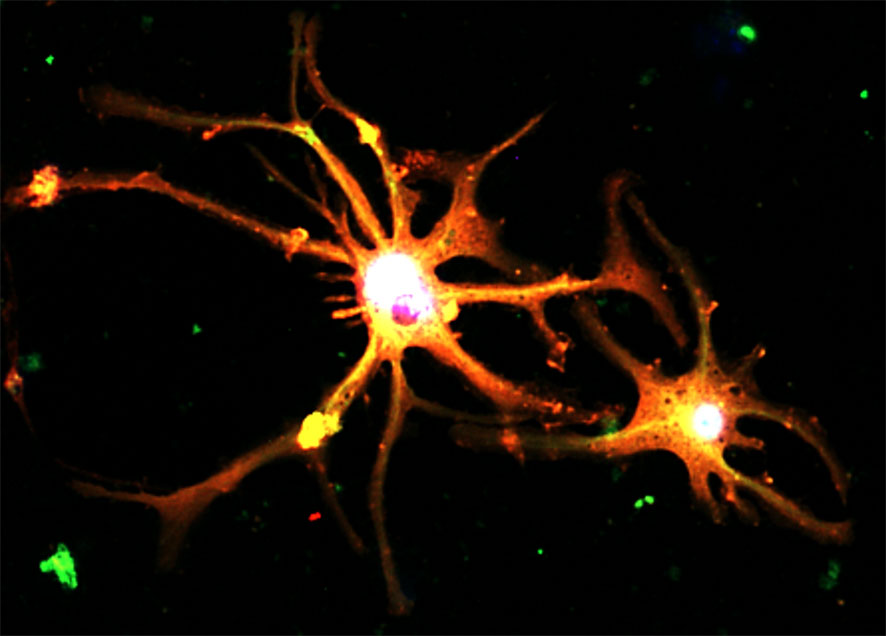
Figure 1 Cancer pain associated neurons formed by a TGF-β1-driven novel phenomenon “Macrophage to Neuron-like Cell Transition” in tumor microenvironment, expressing neuronal markers Synaptophysin (red) and Tubb3 (green) detecting by immunofluorescence in vitro (8).
In conclusion, this Research Topic gathered the latest findings about fibrotic signaling in solid cancer, highlighting their clinical relevance and translational potential beyond fibrosis. We hope the collected articles can inspire both pre-clinical and translational researchers, clinical strategy targeting fibrotic signaling may eventually be developed for cancer therapy.
PT: Conceptualization, Funding acquisition, Resources, Visualization, Writing – original draft, Writing – review & editing. EL: Validation, Writing – review & editing. FM: Writing – review & editing. DZ: Writing – review & editing. CL: Writing – review & editing.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was supported by the Research Grants Council of Hong Kong (14106518, 14111019, 14111720, 24102723); RGC Postdoctoral Fellowship Scheme (PDFS2122-4S06); Health and Medical Research Fund (10210726, 11220576); CU Medicine Passion for Perfection Scheme (PFP202210-004) and Faculty Innovation Award (4620528), CUHK Strategic Seed Funding for Collaborative Research Scheme (178896941), Direct Grant for Research (4054722), Postdoctoral Fellowship Scheme (NL/LT/PDFS2022/0360/22lt, WW/PDFS2023/0640/23en).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Chan MK, Chung JY, Tang PC, Chan AS, Ho JY, Lin TP, et al. TGF-beta signaling networks in the tumor microenvironment. Cancer Lett (2022) 550:215925. doi: 10.1016/j.canlet.2022.215925
2. Chung JY, Chan MK, Li JS, Chan AS, Tang PC, Leung KT, et al. TGF-beta signaling: from tissue fibrosis to tumor microenvironment. Int J Mol Sci (2021) 22(14):7575. doi: 10.3390/ijms22147575
3. Tang PC, Chan AS, Zhang CB, Garcia Cordoba CA, Zhang YY, To KF, et al. TGF-beta1 signaling: immune dynamics of chronic kidney diseases. Front Med (Lausanne) (2021) 8:628519. doi: 10.3389/fmed.2021.628519
4. Tang PC, Chung JY, Xue VW, Xiao J, Meng XM, Huang XR, et al. Smad3 promotes cancer-associated fibroblasts generation via macrophage-myofibroblast transition. Adv Sci (Weinh) (2022) 9:e2101235. doi: 10.1002/advs.202101235
5. Tang PM, Zhang YY, Xiao J, Tang PC, Chung JY, Li J, et al. Neural transcription factor Pou4f1 promotes renal fibrosis via macrophage-myofibroblast transition. Proc Natl Acad Sci U.S.A. (2020) 117:20741–52. doi: 10.1073/pnas.1917663117
6. Tang PM, Zhou S, Li CJ, Liao J, Xiao J, Wang QM, et al. The proto-oncogene tyrosine protein kinase Src is essential for macrophage-myofibroblast transition during renal scarring. Kidney Int (2018) 93:173–87. doi: 10.1016/j.kint.2017.07.026
7. Chung JY, Chan MK, Tang PC, Chan AS, Chung JS, Meng XM, et al. AANG: A natural compound formula for overcoming multidrug resistance via synergistic rebalancing the TGF-beta/Smad signalling in hepatocellular carcinoma. J Cell Mol Med (2021) 25:9805–13. doi: 10.1111/jcmm.16928
8. Tang PC, Chung JY, Liao J, Chan MK, Chan AS, Cheng G, et al. Single-cell RNA sequencing uncovers a neuron-like macrophage subset associated with cancer pain. Sci Adv (2022) 8:eabn5535. doi: 10.1126/sciadv.abn5535
Keywords: cancer, tumor microenvironment, fibrotic signaling, cancer therapy, macrophage-myofibroblast transition (MMT), macrophage to Neuron-like cell Transition (MNT)
Citation: Tang PM-K, Lam EW-F, Mussal F, Zhang D and Li C (2024) Editorial: New insights into fibrotic signaling in cancer. Front. Oncol. 14:1369457. doi: 10.3389/fonc.2024.1369457
Received: 12 January 2024; Accepted: 26 January 2024;
Published: 02 February 2024.
Edited and Reviewed by:
Tao Liu, University of New South Wales, AustraliaCopyright © 2024 Tang, Lam, Mussal, Zhang and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Patrick Ming-Kuen Tang, cGF0cmljay50YW5nQGN1aGsuZWR1Lmhr
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.