
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Oncol., 02 May 2024
Sec. Surgical Oncology
Volume 14 - 2024 | https://doi.org/10.3389/fonc.2024.1368996
Malignant peripheral nerve sheath tumors (MPNSTs) are a complex group of malignant tumors originating from nerve cells or benign peripheral nerve sheath tumors and are commonly found in major plexus/nerve root sites such as the limbs, head, and neck. Malignant peripheral nerve sheath tumors originating in the ureter are extremely rare. Herein, we report the case of a 63-year-old patient with a malignant peripheral nerve sheath tumor of the right ureter who underwent laparoscopic radical resection of the right kidney and ureter. The patient also had stage 5 chronic kidney disease (CKD). Therefore, chemotherapy and radiotherapy were not considered. No tumor recurrence was observed during the follow-up period.
Malignant peripheral nerve sheath tumors (MPNSTs) are complex malignant tumors of neural origin that account for no more than 10% of soft tissue sarcomas (1, 2). They are classified as neurofibromatosis type 1 (NF1) -associated MPNSTs and sporadic MPNSTs. MPNSTs can occur anywhere in the body but is more common at the location of major plexuses/nerve roots, with the most commonly involved sites being the extremities, followed by the trunk, head, and neck (3). It is rarely seen in the urinary system. This report retrospectively analyzes the management of a 63-year-old patient admitted to our hospital with a malignant peripheral nerve sheath tumor of ureteral origin.
A 63-year-old male patient was admitted to our hospital with a right ureteral tumor. He experienced pain in the right lumbar and back regions, with no signs of urinary tract irritation, hematuria, pyuria, or fever. There was no percussion pain in the renal region or ureteral tenderness, and no intra-abdominal or retroperitoneal masses were initially detected. Enhanced computed tomography (CT) of the urinary system in our hospital (Figure 1) showed a nodular soft tissue shadow filling the lumen of the upper section of the right ureter (approximately at the level of the flat lumbar 2-3 vertebrae), with a larger cross-section of approximately 18mm x 17mm, and upper and lower diameters of approximately 36mm. The lesion was mildly enhanced on contrast; the surrounding fat interstices were clear, and there was a slight dilatation of the upper part of the ureter on the right side of the lesion and the pelvic calyxes showed fluid accumulation; the left side did not show any abnormality. There were no obvious abnormal enhancing shadows in the parenchyma of either kidney and no signs of tumors in the bladder, prostate, retroperitoneum, or pelvis. Tumors in the right upper ureter were excluded. No tumors were observed on lung computed tomography. Routine urine examination revealed a strongly positive urinary occult blood test (3+) and a positive qualitative urine protein test. Tumor markers were as follows: carcinoembryonic antigen 9.4 ng/mL and alpha-fetoprotein 1.5 ng/mL. Multiple reviews showed serum creatinine greater than 700 μmol/L and glomerular filtration rate less than 10 ml/min/1.73m2; however, the patient denied a history of CKD. The patient was diagnosed with CKD stage 5 and was recommended long-term hemodialysis. The patient was severely anemic with a red blood cell count of 2.26 x 10^12/L and hemoglobin of 69.0 g/L; after a total transfusion of 6U of red cells suspensions, he underwent laparoscopic radical resection of the right kidney and ureter under general anesthesia on April 03, 2023. The intraoperative blood loss was approximately 50 ml.
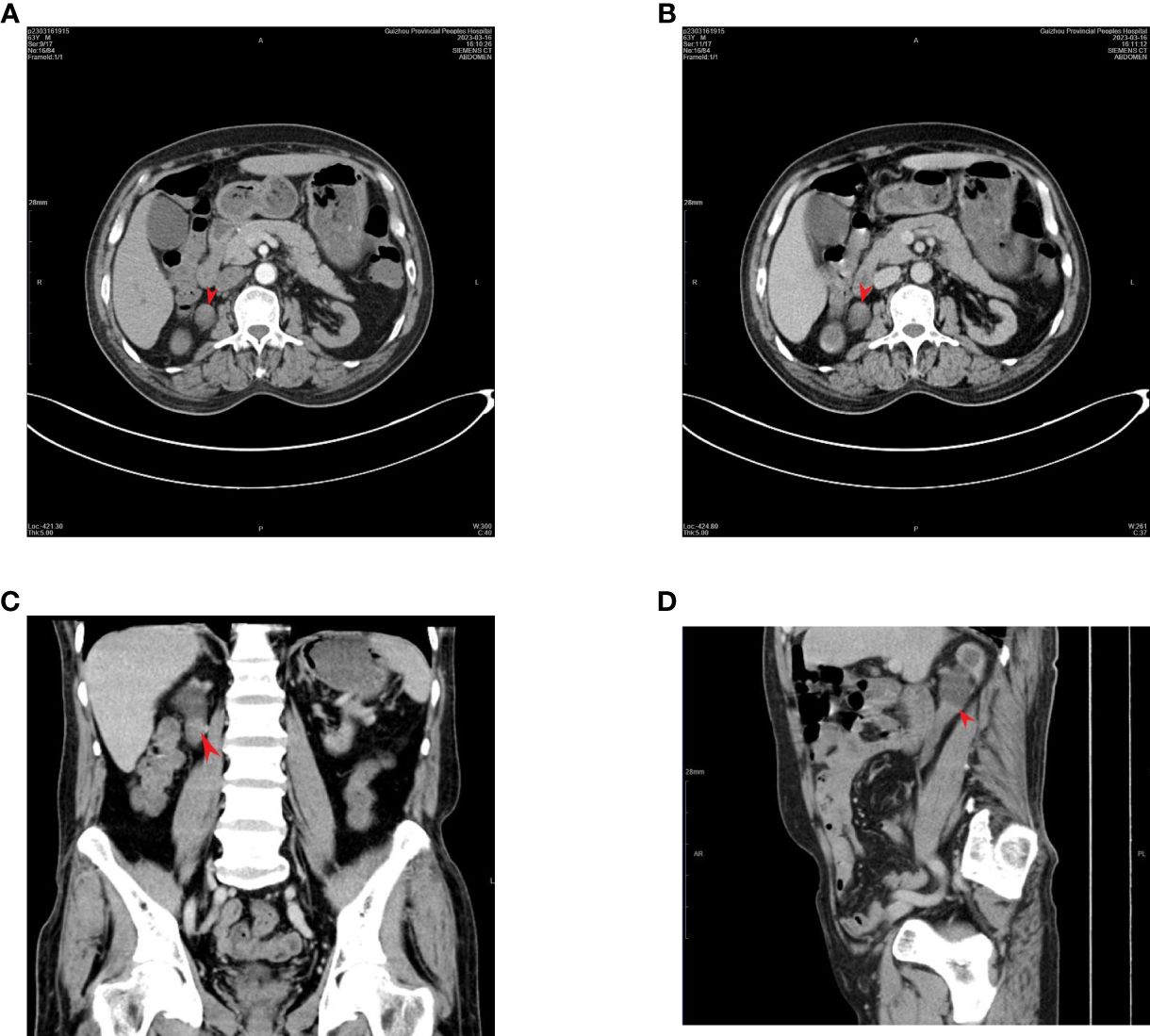
Figure 1 Enhanced CT of the urinary system: a soft tissue mass is visible within the right superior ureter, with mild enhancement noted on an enhanced scan. (tumor location flat against the 2nd-3rd lumbar vertebrae). The arrow points to the tumor: (A) arterial phase; (B) venous phase; (C) coronal plane; (D) sagittal plane.
Post-operative pathology showed the kidney specimen with part of the ureter and bladder wall measuring 8.5 x 6 x 4 cm, with ureter measuring 21 cm in length and 0.5-1.5 cm in diameter. In the upper part of the ureter, a grayish-white striated mass was seen, measuring 3.6 x 1.8 x 1.6 cm (Classified by the American Joint Committee on Cancer [AJCC] as Class I), solid with medium texture and friable areas, invading the whole layer of the ureteral wall and not involving the renal pelvis, with dilated pelvic calyces and thinning of the renal parenchyma which measured 0.8-1.2 cm in thickness. The rest of the ureter showed no obvious mass. The upper right ureter tumor was a malignant mesenchymal tumor with necrosis, and considered to be MPNSTs measuring 3.6 x 1.8 x 1.6 cm, with vascular invasion, but without neural invasion or tumors elsewhere in the kidney or bladder wall. Chronic pyelonephritis with atrophy of the renal parenchyma, sclerosis of some glomeruli, significant interstitial fibrosis, a large number of lymphoid hyperplasia and lymphoid follicle formations, and unspecified renal hilar vessels was noted (Figure 2). Immunohistochemistry (IHC) results were as follows: S100 (+), SOX10 (+), SMA (+), Desmin (localized +), MyoD1 (cytoplasmic +), CD117 (-), CD34 (vascular +), CK20 (-), CK7 (-), panCK (localized +), Dog-1 (-), Melanoma (-), Myogenin (-), P63 (-), STAT-6 (-), and Ki67 (approximately 70%).
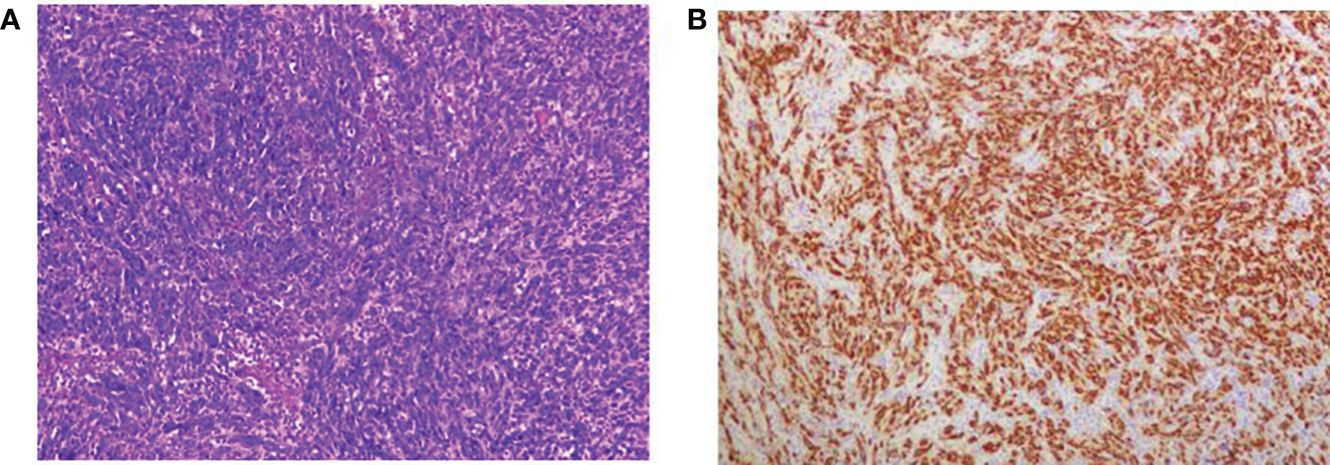
Figure 2 Microscopic view of tumor histopathological sections; (A) HE staining x100; (B) SOX10 histochemical staining.
The final diagnosis was malignant peripheral nerve sheath tumor of the ureter. The patient’s pelvic drain was removed 3 days after surgery, and the patient was followed up regularly for 8 months and did not receive regular dialysis treatment. There has been no local recurrence or distant metastasis of the tumor to date; however, non-specific clinical manifestations of renal failure such as obvious loss of appetite, poor natriuresis, and fatigue were observed.
MPNSTs are often defined as malignant tumors originating from a peripheral nerve or a preexisting benign nerve sheath tumor (usually neurofibroma) that has deteriorated or a tumor formed by the deterioration of neurofibroma in individuals with NF1 (2). They can occur in any age group, with children accounting for 10–20% of MPNSTs cases (4, 5). They arise from nerve cell components such as Schwann cells, perineuronal cells, or transformation of previously benign peripheral nerve sheath tumors (6); approximately 8–13% of NF1 cases can mutate to progress to MPNSTs, accounting for 50% of MPNSTs; while the remainder of cases are predominantly disseminated, with radiation induction and genetic factors also contributing to the occurrence of MPNSTs (2, 3, 5, 7). Importantly, NF1 is an inherited autosomal dominant disease, involving a tumor suppressor gene on chromosome 17 (2), and the international consensus of the latest revision of the NF1 diagnostic criteria for 2021 suggests that the diagnosis can be made definitively (8). In the current case, the patient’s medical and family histories were examined, and malignancies of NF1 origin were excluded.
Malignant peripheral nerve sheath tumor symptoms are non-specific and imaging is indispensable for tumor screening. Currently, a range of tests, such as CT, magnetic resonance imaging (MRI) and positron emission tomography (PET), are the main tools for evaluating and diagnosing MPNSTs. MRI features such as peritumoral edema, local infiltration with irregularities of margins, and heterogeneous enhancement after tumor contrast appear to be more indicative of MPNSTs (3, 9). Additionally, [18F]2-fluoro-2-deoxy-D-glucose (18F-FDG) PET is a sensitive and specific diagnostic tool for MPNSTs; FDG PET and PET CT identify primary and/or metastatic MPNSTs and provide an estimate of the tumor grade (3, 10–12). Although its sensitivity and specificity vary, FDG-PET (particularly in combination with CT) is a more reliable adjunctive diagnostic tool for MPNSTs and helps determine their prognosis. The diagnosis of MPNSTs requires a combination of clinical presentation, morphology, and IHC owing to a lack of definite immunohistochemical markers (13).
Currently, surgical resection remains the only treatment for limited MPNSTs (3, 6), and negative margins are critical for tumor recurrence and patient survival (14). Patients with completely resected tumors have a significantly lower risk of tumor recurrence and metastasis and a higher 5-year survival rate compared to patients with incomplete tumor resection (15). Negative margins, tumor location, size, grade, and metastasis are also important factors affecting patient survival (14, 15). Complete surgical resection is more difficult for MPNSTs, considering the high degree of malignancy, early metastasis, and high mortality; therefore, adjuvant radiotherapy is important to improve survival, especially for patients with positive margins (14). However, the decision to administer adjuvant radiotherapy must be evaluated individually according to the patient’s medical status and risk of tumor recurrence (7). Radiotherapy is generally considered most effective for patients with larger tumors (> 5cm), high-grade tumors, and/or positive resection margins (3). Our team did not recommend routine postoperative adjuvant radiotherapy for this patient owing to a lack of definitive evidence that adjuvant radiotherapy for low-grade tumors results in time-to-survival gains. Additionally, a higher tumor grade is associated with a greater risk of poor prognosis (14). Anthracyclines are the first-line treatment option for patients with unresectable, advanced, or metastatic MPNSTs; furthermore, other cytotoxic chemotherapeutic agents (including the alkylating agent cyclophosphamide and the topoisomerase II inhibitor etoposide) may also be chosen (6). Adjuvant chemotherapy is considered important to improve the survival rate of patients with MPNSTs (14); however, the sensitivity of the tumor to chemotherapeutic agents needs to be clarified. The patient’s tumor grade was low owing to early diagnosis and treatment; therefore, we do not recommend conventional adjuvant radiotherapy (RT) and adjuvant chemotherapy (even with normal renal function) to avoid potential adverse effects.
Our patient had extremely poor renal function, requiring dialysis and other therapeutic means. The management of patients undergoing chemotherapy must be taken seriously owing to the potential myelosuppressive and hepatic and renal function impairments caused by most chemotherapeutic agents. Recent studies show a gradual increase in the use of adjuvant RT in patients with MPNSTs (16) with the application of novel RT techniques similar to Image Guided Radiation Therapy (IGRT), intensity modulation RT, volumetric modulated arc therapy (VMAT), stereotactic RT, and proton-based RT can effectively control tumor recurrence (16–18). However, the effect of adjuvant RT on patient survival is uncertain, making it a highly debated treatment option. Additionally, the use of DNA-damaging agents may lead to tumor recurrence or additional mutations, further complicating treatment decisions (3, 6, 7). A main challenge in the current advancement of medical technology is determining how to maximize the control of the radiation dose distribution and minimize the radiation to adjacent organs.
Nowadays, targeted therapy is an attractive treatment option for patients with metastatic or unresectable MPNSTs (3, 7). Cell signaling pathways and the tumor microenvironment are important areas for future research (19). Some studies suggest that the Ras/Raf and mTOR signaling pathways may be important in the pathogenesis of MPNSTs; and new target sites such as CDKN2A, Hsp90, BRAF V600E, and NRAS Q61 have been identified (6, 19). Although targeted therapeutic agents are less commonly used in clinical practice and have yielded inconsistent results, several animal-based experimental studies have shown positive outcomes (6, 19). Sex hormones may be associated with primary MPNSTs in the urinary tract (13); however, there are very few relevant cases, and more experimental studies or clinical cases are needed to confirm this. The investigation of targeted drugs for MPNSTs is a current research hotspot that could result in a significant advancement in treating patients with MPNSTs in the near future.
Choosing the appropriate treatment for patients with tumors with poor renal function is difficult. More clinical evidence is required to determine whether adjuvant chemotherapy based on renal function control is possible. We will also follow this patient for an extended period to obtain information regarding disease progression and treatment.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
The studies involving humans were approved by Ethics Committee of The Guizhou Provincial People’s Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article. Written informed consent was obtained from the participant/patient(s) for the publication of this case report.
XY: Resources, Writing – original draft, Writing – review & editing. XW: Methodology, Writing – review & editing, Writing – original draft. ZH: Resources, Writing – review & editing. XC: Investigation, Writing – review & editing. QW: Conceptualization, Validation, Writing – review & editing. KJ: Conceptualization, Validation, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was funded by National Natural Science Foundation of China (Number: 82060462).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Fuchs B, Spinner RJ, Rock MG. Malignant peripheral nerve sheath tumors: an update. J Surg Orthop Adv. (2005) 14:168–74.
2. Le Guellec S, Decouvelaere AV, Filleron T, Valo I, Charon-Barra C, Robin YM, et al. Malignant peripheral nerve sheath tumor is a challenging diagnosis: A systematic pathology review, immunohistochemistry, and molecular analysis in 160 patients from the French sarcoma group database. Am J Surg Pathol. (2016) 40:896–908. doi: 10.1097/pas.0000000000000655
3. Knight SWE, Knight TE, Santiago T, Murphy AJ, Abdelhafeez AH. Malignant peripheral nerve sheath tumors-A comprehensive review of pathophysiology, diagnosis, and multidisciplinary management. Children (Basel). (2022) 9. doi: 10.3390/children9010038
4. Ferrari A, Bisogno G, Carli M. Management of childhood Malignant peripheral nerve sheath tumor. Paediatr Drugs. (2007) 9:239–48. doi: 10.2165/00148581-200709040-00005
5. Amirian ES, Goodman JC, New P, Scheurer ME. Pediatric and adult Malignant peripheral nerve sheath tumors: an analysis of data from the surveillance, epidemiology, and end results program. J Neurooncol. (2014) 116:609–16. doi: 10.1007/s11060-013-1345-6
6. Hassan A, Pestana RC, Parkes A. Systemic options for Malignant peripheral nerve sheath tumors. Curr Treat Options Oncol. (2021) 22:33. doi: 10.1007/s11864-021-00830-7
7. Somatilaka BN, Sadek A, McKay RM, Le LQ. Malignant peripheral nerve sheath tumor: models, biology, and translation. Oncogene. (2022) 41:2405–21. doi: 10.1038/s41388-022-02290-1
8. Legius E, Messiaen L, Wolkenstein P, Pancza P, Avery RA, Berman Y, et al. Revised diagnostic criteria for neurofibromatosis type 1 and Legius syndrome: an international consensus recommendation. Genet Med. (2021) 23:1506–13. doi: 10.1038/s41436-021-01170-5
9. Demehri S, Belzberg A, Blakeley J, Fayad LM. Conventional and functional MR imaging of peripheral nerve sheath tumors: initial experience. AJNR Am J Neuroradiol. (2014) 35:1615–20. doi: 10.3174/ajnr.A3910
10. Ferner RE, Golding JF, Smith M, Calonje E, Jan W, Sanjayanathan V, et al. [18F]2-fluoro-2-deoxy-D-glucose positron emission tomography (FDG PET) as a diagnostic tool for neurofibromatosis 1 (NF1) associated Malignant peripheral nerve sheath tumours (MPNSTs): a long-term clinical study. Ann Oncol. (2008) 19:390–4. doi: 10.1093/annonc/mdm450
11. Assadi M, Velez E, Najafi MH, Matcuk G, Gholamrezanezhad A. PET imaging of peripheral nerve tumors. PET Clin. (2019) 14:81–9. doi: 10.1016/j.cpet.2018.08.013
12. Widemann BC, Italiano A. Biology and management of undifferentiated pleomorphic sarcoma, myxofibrosarcoma, and Malignant peripheral nerve sheath tumors: state of the art and perspectives. J Clin Oncol. (2018) 36:160–7. doi: 10.1200/jco.2017.75.3467
13. Brentlinger MN, Padilla O, Qiao J. Primary urethral Malignant peripheral neural sheath tumor in a 58-year-old female in the absence of neurofibromatosis type 1. Cureus. (2022) 14:e32634. doi: 10.7759/cureus.32634
14. Cai Z, Tang X, Liang H, Yang R, Yan T, Guo W. Prognosis and risk factors for Malignant peripheral nerve sheath tumor: a systematic review and meta-analysis. World J Surg Oncol. (2020) 18:257. doi: 10.1186/s12957-020-02036-x
15. Dunn GP, Spiliopoulos K, Plotkin SR, Hornicek FJ, Harmon DC, Delaney TF, et al. Role of resection of Malignant peripheral nerve sheath tumors in patients with neurofibromatosis type 1. J Neurosurg. (2013) 118:142–8. doi: 10.3171/2012.9.Jns101610
16. Schaefer IM, Hong K, Kalbasi A. How technology is improving the multidisciplinary care of sarcoma. Am Soc Clin Oncol Educ Book. (2020) 40:445–62. doi: 10.1200/edbk_280729
17. Harikar MM, Ferini G, Palmisciano P, Shakir M, Amico P, Ferraresi S, et al. Case Report: Adjuvant image-guided radiation therapy reduces surgical invasiveness in Malignant peripheral nerve sheath tumors. Front Oncol. (2023) 13:1129537. doi: 10.3389/fonc.2023.1129537
18. Ferini G, Palmisciano P, Zagardo V, Viola A, Illari SI, Marchese V, et al. Combining a customized immobilization system with an innovative use of the exacTrac system for precise volumetric modulated arc therapy of challenging forearm sarcomas. Pract Radiat Oncol. (2023) 13:148–52. doi: 10.1016/j.prro.2022.10.005
Keywords: MPNSTs, malignant tumors, chronic kidney disease, NF1, chemotherapy, case report
Citation: Yin X, Wang X, He Z, Chen X, Wang Q and Jiang K (2024) Chronic kidney disease with malignant peripheral nerve sheath tumor of the ureter: a case report. Front. Oncol. 14:1368996. doi: 10.3389/fonc.2024.1368996
Received: 11 January 2024; Accepted: 22 April 2024;
Published: 02 May 2024.
Edited by:
Gianluca Ferini, Rem Radiotherapy, ItalyReviewed by:
Farzad Kakaee, Tabriz University of Medical Sciences, IranCopyright © 2024 Yin, Wang, He, Chen, Wang and Jiang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kehua Jiang, amlhbmdrZWh1YUBnejUwNTUuY29t; Qing Wang, d2FuZ3FpbmcxQGd6NTA1NS5jb20=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.