
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Oncol. , 07 June 2024
Sec. Gastrointestinal Cancers: Gastric and Esophageal Cancers
Volume 14 - 2024 | https://doi.org/10.3389/fonc.2024.1367990
This article is part of the Research Topic Immune Checkpoint Inhibitors in Advanced Gastric and Esophageal Cancers View all 12 articles
Objectives: The prognostic relevance of the platelet-to-lymphocyte ratio (PLR) in gastric cancer (GC) patients undergoing immune checkpoint inhibitor (ICI) treatment remains unclear. This meta-analysis aimed to determine the prognostic impact of PLR in this specific patient cohort.
Methods: We searched the PubMed, Cochrane Library, CNKI, and EMBASE databases, including literature published up to September 2023, to investigate the prognostic implications of PLR in patients with gastric cancer undergoing immune checkpoint inhibitor therapy. Outcome measures encompassed overall survival (OS), progression-free survival (PFS), objective response rate (ORR), and disease control rates (DCR).
Results: Nine studies from seven articles comprising 948 eligible patients were selected. The results revealed a significant correlation between elevated PLR and poorer OS and progression-free survival (PFS) (OS: HR 1.67, 95% CI 1.39–2.00, p < 0.001; PFS: HR 1.51, 95% CI 1.29–1.76, p < 0.001). Subgroup analyses were performed to validate the robustness of the results. Moreover, a meta-analysis of four studies investigating the correlation between the PLR in gastric cancer (GC) patients and the objective response rate/disease control rate (ORR/DCR), showed no significant association between the PLR and ORR/DCR (ORR: RR = 1.01, p = 0.960; DCR: RR = 0.96, p = 0.319).
Conclusions: This meta-analysis indicates that elevated PLR in GC patients undergoing ICI treatment is significantly linked to worse OS and PFS. Therefore, PLR can serve as a prognostic indicator of post-treatment outcomes in patients with GC receiving ICIs. Further prospective studies are required to assess the reliability of these findings.
Systematic review registration: https://inplasy.com/, identifier INPLASY2023120103.
Gastric cancer (GC) is a malignancy that accounts for 7.7% of cancer-related fatalities. Globally, it is the fifth most frequently diagnosed cancer and ranks third in cancer-related mortality (1, 2). Although the incidence rate has declined in recent years, there has been an increasing trend in younger populations. GC is characterized by insidious onset, rapid progression, high malignancy, and a poor prognosis (3). Most patients with gastric cancer are already in the advanced stages of the disease at the time of their initial consultation and have lost the opportunity for curative surgery. This implies that more intricate and comprehensive treatment approaches aimed at disease control, symptom alleviation, and improvement in survival quality may need to be developed. Although traditional treatments, such as chemotherapy, can improve overall survival and quality of life, their overall clinical efficacy is limited. The RAINBOW-Asia trial (4) reported a median overall survival of only 7.92 months in patients undergoing chemotherapy alone. Since the latter part of the 20th century, significant progress has been made in leveraging immunotherapy for the management of gastric cancer, particularly through the use of immune checkpoint inhibitors (ICIs). ICIs have become the mainstream treatment for many types of malignant tumors, revolutionizing cancer therapy (5–11). Blocking the PD-1/PD-L1 signaling pathway with immune checkpoint inhibitors plays a crucial role in identifying and preventing the escape of tumor cells. This mechanism improves the tumor microenvironment, stimulates the body’s immune system, enhances immune responses, and effectively eliminates tumor cells. Numerous studies have suggested ICI therapy as a promising therapeutic strategy. Whether used alone or in combination with other treatments such as chemotherapy, ICIs show significant potential in improving the survival of patients with advanced gastric cancer patients (12–15). Tumor mutational burden (TMB) (16), tumor-infiltrating lymphocytes (17), microsatellite instability (MSI) (18), and other tumor biomarkers have been widely studied as predictive biomarkers for PD-1/L1 inhibitor therapy. However, their application in clinical settings is limited because of the relatively complex detection process and the lack of consensus on numerical thresholds. Since Virchow first proposed a potential link between inflammation and tumors, abundant experimental and clinical data have unequivocally established a clear correlation between inflammatory responses and the initiation and progression of tumors (19–21). During chronic inflammation, inflammatory cells release numerous cytokines, chemotactic proteins, and other inflammatory mediators. By activating endogenous or exogenous signals, they alter the cellular microenvironment and promote tumor growth, proliferation, and metastasis. Systemic inflammation is a prominent manifestation of host-tumor interactions in cancer (22, 23) and has been acknowledged as the 7th hallmark of malignant tumors. Building upon this, more scholars have investigated the prognostic value of systemic inflammatory biomarkers, such as the neutrophil-to-lymphocyte ratio (NLR) and monocyte-to-lymphocyte ratio (MLR), in various types of cancer (24–26). The platelet-to-lymphocyte ratio (PLR) is associated with prognosis in many malignant tumors, including non-small cell lung cancer (NSCLC) and breast cancer. The platelet-to-lymphocyte ratio (PLR) has been proven to be associated with prognosis (27, 28). As a straightforward and easily accessible biomarker, PLR can be used to gauge the inflammatory status of the immune system. However, there is currently a lack of meta-analyses on the predictive significance of the PLR and its variations in gastric cancer (GC) patients undergoing immune checkpoint inhibitor (ICI) therapy. Therefore, we included relevant cohort studies to compare the prognosis and treatment response differences in patients with GC with different PLR values after ICI treatment. This study aimed to investigate the prognostic value of the PLR in this patient cohort.
This systematic review and meta-analysis was carried out according to the guidelines outlined in the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) (29). Two independent researchers systematically searched PubMed, Embase, CNKI and the Cochrane Library to identify relevant studies concerning the prognostic significance of the Platelet-to-Lymphocyte Ratio (PLR) in patients (GC) patients undergoing treatment with immune checkpoint inhibitors (ICIs). The search encompassed the period from the inception of these databases until September 30, 2023. It utilizes the following terms to investigate the predictive significance of PLR and ICIs in patients with gastric cancer: “Platelet-Lymphocyte ratio” or “Platelet-to-Lymphocyte ratio” or PLR, “gastric cancer” or “gastric adenocarcinoma”, and “PD-L1 inhibitors” or “immune checkpoint inhibitors” or “programmed cell death ligand-1 inhibitors” or “immunotherapy”. In addition to utilizing free search terms and Medical Subject Headings (MeSH) for searching within titles or abstracts, we screened the references of selected articles to ensure comprehensive retrieval.
Inclusion criteria: (1) Patients with gastric cancer confirmed through pathological examination, in advanced or locally advanced stages, and receiving immune checkpoint inhibitor (ICI) treatment; (2) Studies providing long-term survival data, including overall survival (OS) or progression-free survival (PFS); (3) Studies providing hazard ratio (HR) or relative risk (RR) with a 95% confidence interval (CI).
The exclusion criteria were as follows: (1) reviews, case reports, case series, conference abstracts, or commentaries; (2) studies lacking sufficient data; (3) nonclinical or nonhuman studies; and (4) data overlap or duplication.
Two researchers independently extracted data from the eligible studies, and discrepancies were resolved through discussions or consultations with a third researcher. The extracted data included the first author’s name, publication year, study country, study design, sample size, average or median age of the included patients, sex distribution, treatment modalities, survival analysis (including hazard ratios with corresponding 95% confidence intervals for overall survival and progression-free survival), and objective response rate (ORR) or disease control rate (DCR). The Newcastle-Ottawa Scale (NOS) was used to assess the quality of the studies, covering three aspects: selection (0–4 points), comparability (0–2 points), and outcome assessment (0–3 points). Two researchers individually scored each of the eight questions on these three aspects, with a total score range of 0–9 points. Studies with scores exceeding 6 points were considered high quality (30). The results of another bias risk assessment tool, ROBINS-I (31), can be found in the Supplementary Materials.
This study used Stata SE (version 12.0; StataCorp, College Station, Texas, USA) for statistical analysis. The RR was used to assess the relationship between PLR, ORR, and DCR in patients with gastric cancer undergoing ICI treatment. HR and its associated 95% confidence interval (CI) were used to evaluate the potential association of PLR with OS and PFS. Cochran’s Q-test and I2 statistics were used to assess heterogeneity among the studies; based on this, an appropriate effect model was selected. A random-effects model was selected if I2 was > 50% or the p-value was < 0.10 (Q-test), indicating significant heterogeneity. Otherwise, a fixed effects model was used. Publication bias was evaluated by observing the symmetry of the funnel plot and utilizing methods such as Egger’s linear regression, Begg regression, where a P-value < 0.05 was considered indicative of publication bias. A sensitivity analysis was performed to explore the impact of different studies on OS and PFS. Subgroup analyses were conducted based on treatment methods, sample size, cutoff values, and analysis models to further investigate the sources of heterogeneity.
A comprehensive depiction of the literature selection process is shown in Figure 1. Following the previously outlines the search strategy and 114 articles were initially retrieved. After excluding duplicate studies, 96 studies were retained. After meticulous screening of titles and abstracts per the predefined inclusion and exclusion criteria, 88 studies were excluded. One study was excluded due to the unavailability of its full text. Ultimately, we identified seven articles covering nine observational cohort studies (32–38). Table 1 summarizes the characteristics of the included studies. All these studies were published between 2021 and 2022, with eight studies conducted in China from six articles and one study from Japan. All seven articles were retrospective studies. The sample sizes ranged from 45 to 238, with a total of 948 patients. Among them, six studies employed ICIs alone, whereas the remaining two studies utilized combination chemotherapy, including ICIs. One study focused solely on Progression-Free Survival (PFS), whereas seven studies concurrently documented Overall Survival (OS) and PFS. Based on the Newcastle-Ottawa Quality Assessment Scale (NOS), the scores for all included studies ranged from 7 to 8, indicating relatively high data quality. The Newcastle-Ottawa Quality Assessment Scale (NOS) scores of all included articles are shown in Table 2. In addition, following the detailed guidelines of ROBINS-I (30), we employed the ROBINS tool to assess each study as if it were a hypothetical target randomized controlled trial, with deviations from this target trial considered biases. We evaluated seven bias domains, each categorized as “low,” “moderate,” “serious,” or “critical,” or marked as “no information” for judgment (Supplementary Table 1).
Eight studies, including 878 patients, investigated the relationship between PLR and OS in patients with GC receiving Immune Checkpoint Inhibitors (ICIs). Heterogeneity testing indicated non-significant heterogeneity (P=0.812 > 0.1, I2 = 0.0% < 50%), suggesting that the fixed-effects model was suitable for the meta-analysis. The combined Hazard Ratio (HR) and its 95% Confidence Interval (CI) were as follows: HR=1.67, 95% CI=1.39–2.00, P<0.001, indicating that a higher PLR predicts poorer OS in GC patients undergoing ICIs (Figure 2A). In addition, nine studies involving 948 patients explored the relationship between the PLR and Progression-Free Survival (PFS) in patients with GC receiving ICIs. Heterogeneity testing revealed no significant heterogeneity (P=0.416 > 0.1, I2 = 2.2%, < 50%). The combined results showed that HR=1.51, 95% CI= 1.29–1.76, and P<0.001, indicating that higher PLR values are associated with worse PFS in GC patients (Figure 2B). Subgroup analyses, considering treatment (ICIs or ICIs + chemotherapy), sample size (≥100 or <100), cutoff (>214.08 or ≤214.08), and analysis (multivariate or univariate), consistently revealed that a higher PLR predicts poorer OS and PFS in patients (Tables 3, 4).
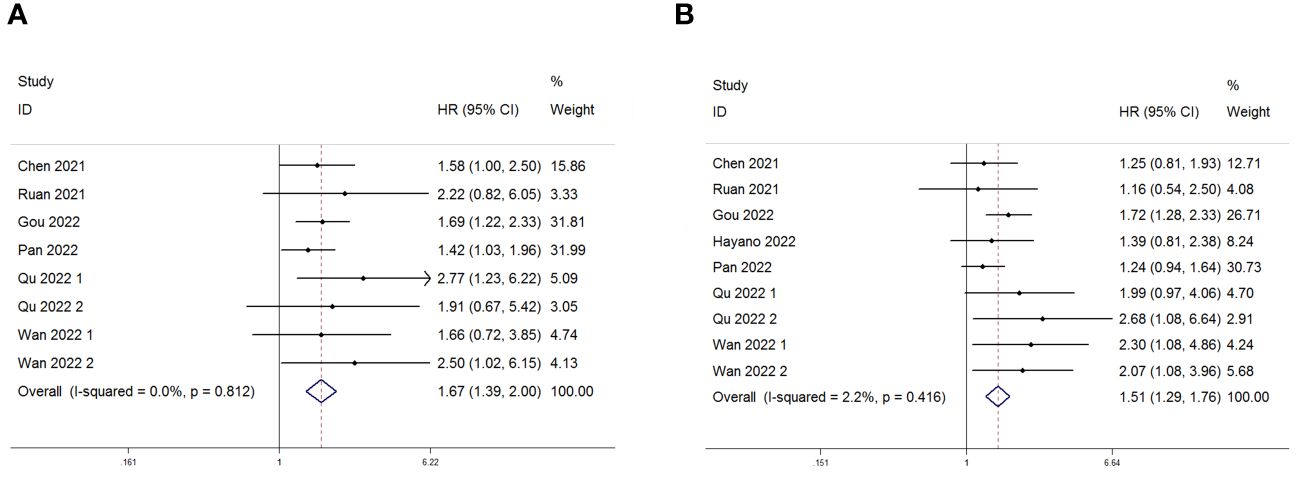
Figure 2 Forest plot for the association between Platelet-to-Lymphocyte Ratio (PLR) expression and (A) overall survival (OS) and (B) progression-free survival (PFS) in gastric cancer patients receiving Immune Checkpoint Inhibitors (ICIs).
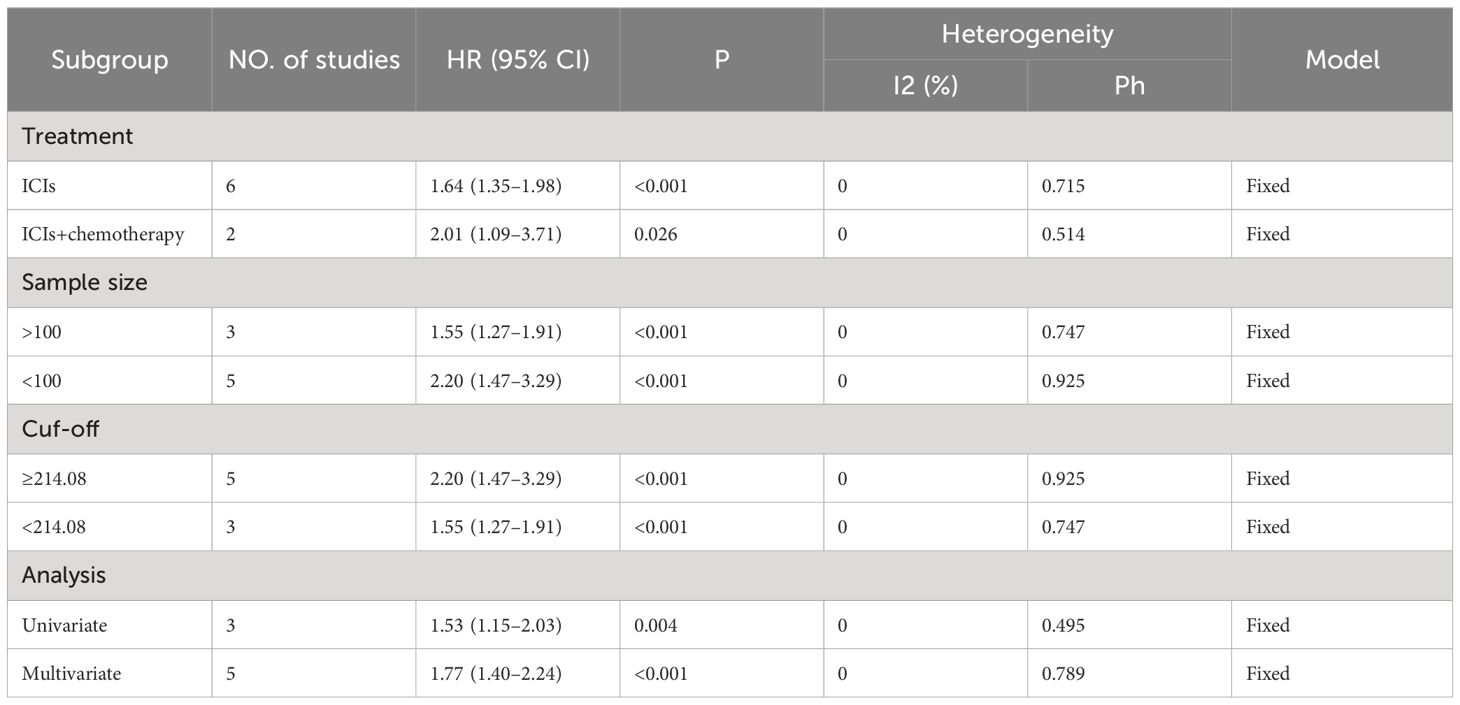
Table 3 Subgroup analysis evaluating the prognostic significance of PLR for OS in gastric cancer patients treated with ICIs.
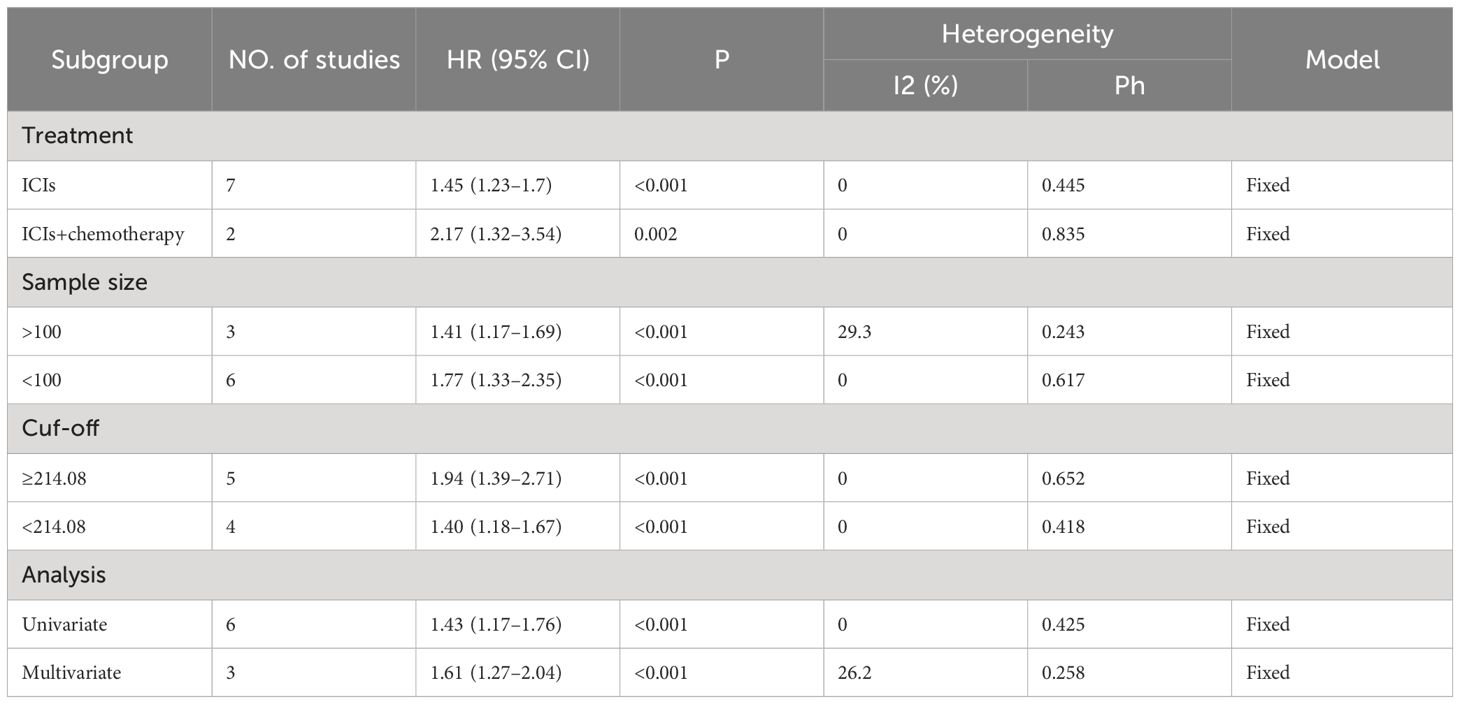
Table 4 Subgroup analysis evaluating the prognostic significance of PLR for PFS in gastric cancer patients treated with ICIs.
Publication bias was assessed using funnel plots, Egger’s linear regression and Begg regression. Funnel plots for Progression-Free Survival (PFS) exhibited favorable symmetry (Figure 3A). However, the symmetry of the funnel plot for overall survival (OS) is not as effective as that for progression-free survival (PFS) (Figure 3B).The Begg tests indicated no significant publication bias for OS or PFS (OS, p = 0.266; PFS, p = 0.118; Figure 4). The outcomes from the Egger (OS, p = 0.037; Figure 5A; PFS, p = 0.158; Figure 5B) tests revealed a probable publication bias inside the relevant OS investigations. Nevertheless, we obtained symmetrical funnel plots applying the cut-and-patch method (Figure 5C), showing that the results were still statistically significant and robust, without substantial interferences from publication bias, with an HR of 1.559 (95% CI: 1.316–1.847).
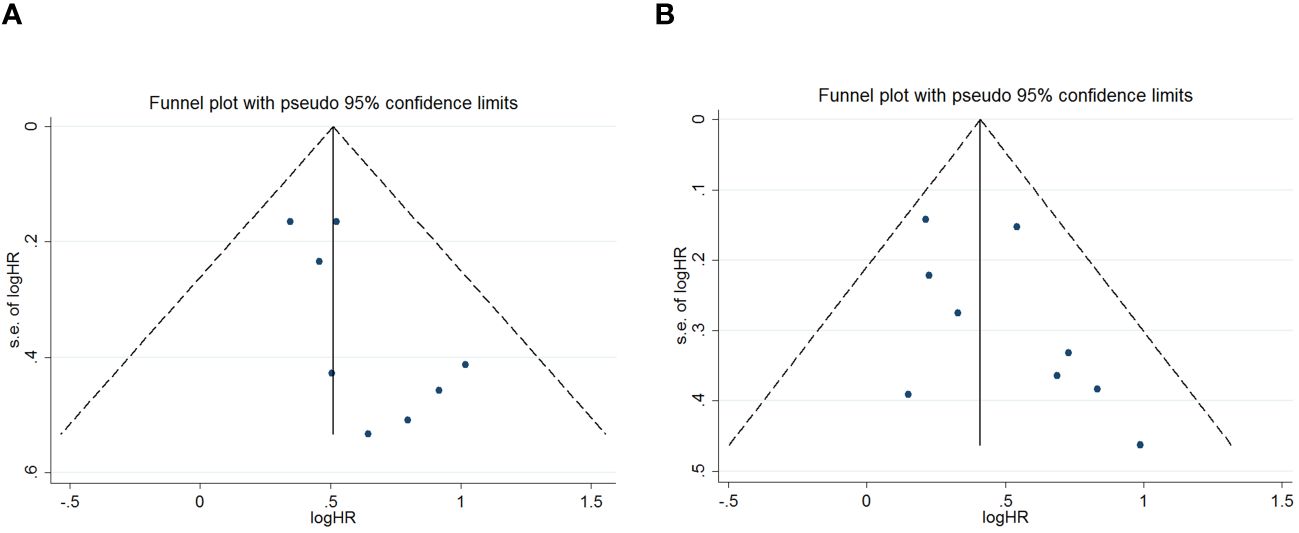
Figure 3 Funnel plots are utilized to assess the presence of publication bias in (A) overall survival (OS) and (B) progression-free survival (PFS).
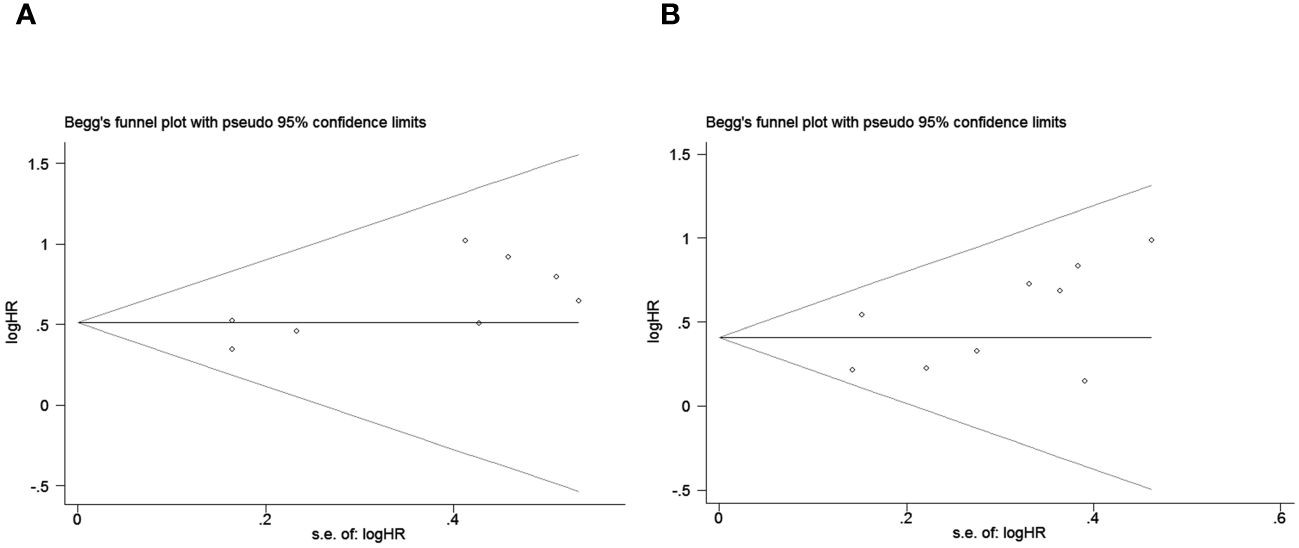
Figure 4 Publication bias test. (A) Begg tests for OS, p = 0.266; (B) Begg tests for PFS, p = 0.118.
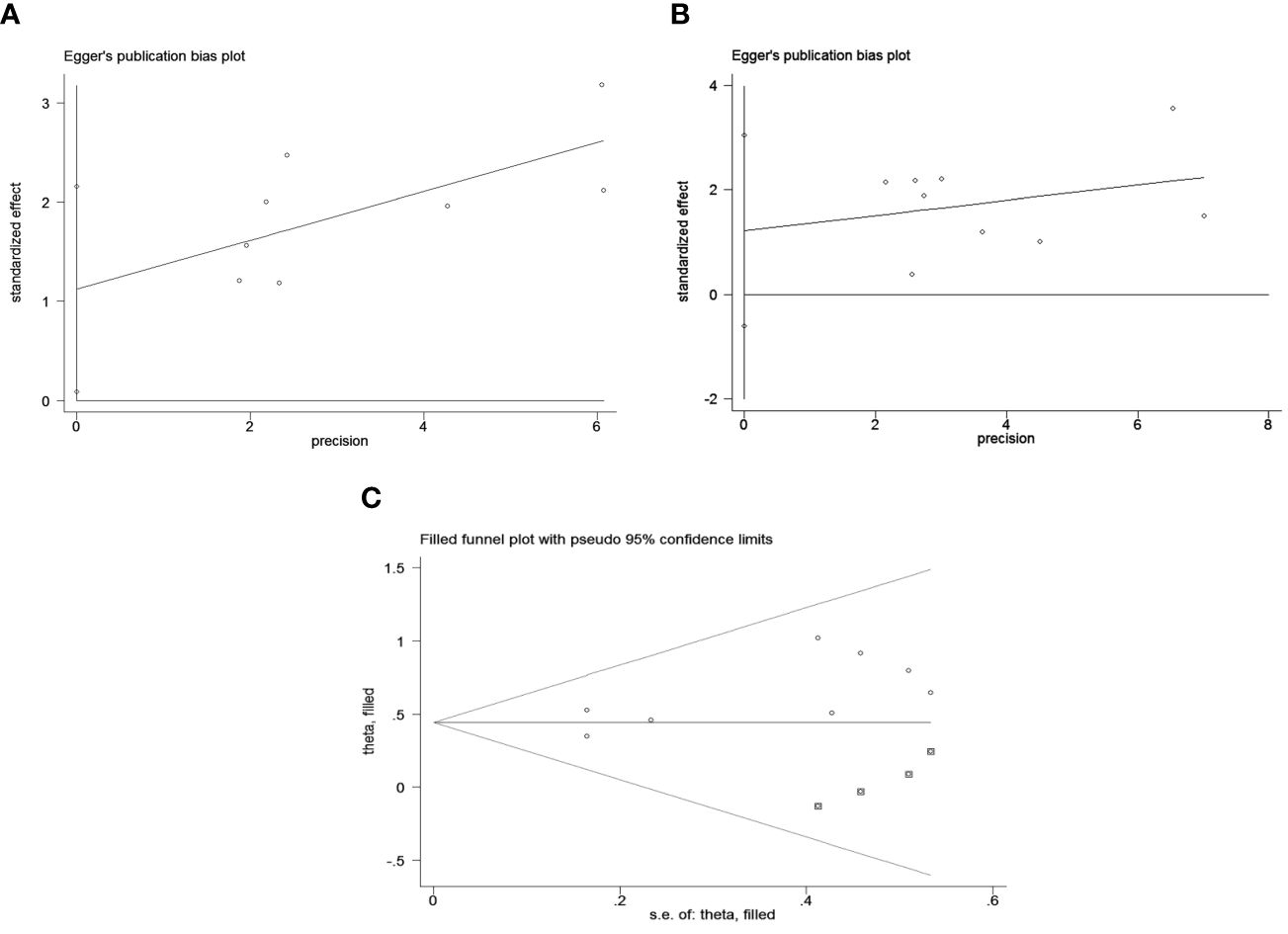
Figure 5 Publication bias test. (A) Egger’s test for OS, p = 0.037; (B) Egger’s test for PFS, p = 0.158; (C) The funnel diagram corrected by the cut-and-patch method in OS.
Sensitivity analysis revealed that no individual study significantly influenced the observed effect size of the association between the PLR and OS or PFS. In this study, removing a single article did not result in significant changes, indicating the reliability of the results (Figure 6).
As depicted in Figure 7, four studies examined the association between PLR and treatment outcomes (ORR or DCR) in patients (GC) undergoing Immune Checkpoint Inhibitors (ICIs). PLR showed no significant correlation with ORR or DCR (ORR: RR = 1.01, p = 0.960; DCR: RR = 0.96, p = 0.319).
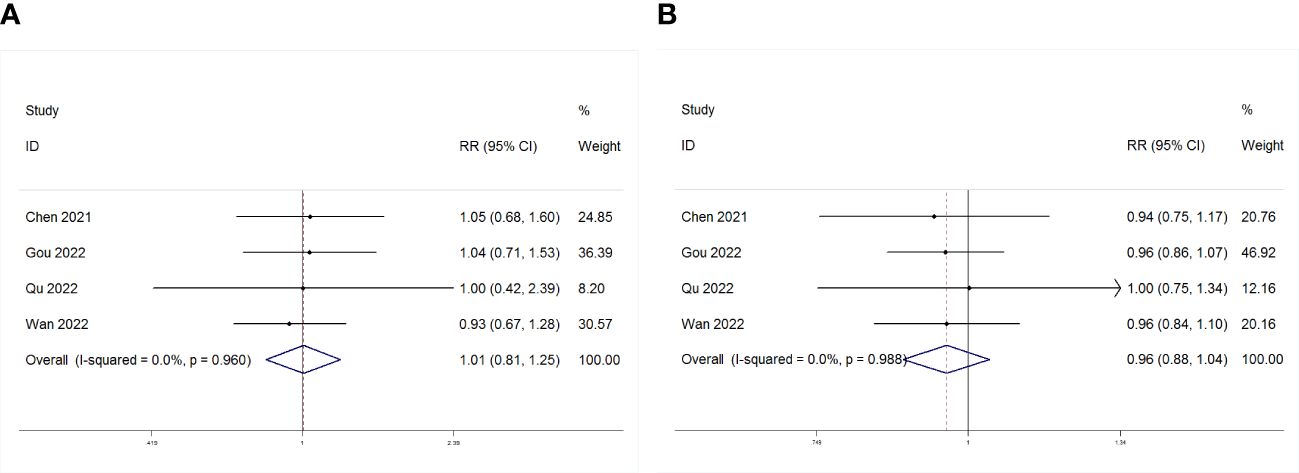
Figure 7 Forest plot for the association between PLR and (A) objective response rate (ORR) and (B) disease control rate (DCR).
Gastric cancer (GC) is the leading cause of cancer-related fatalities worldwide. Despite notable progress in GC treatment in recent years, persistently high rates of recurrence and mortality prevail (39, 40). The primary challenge stems from the nonspecific nature of early-stage GC detection, with most patients being diagnosed during the advanced stages of cancer. Thus, the identification of biomarkers capable of predicting post-treatment prognosis is of paramount significance for GC treatment. Lymphocytes and platelets play pivotal roles in the systemic inflammatory response, demonstrating crucial functions in tumor development, infiltration, and metastasis (19, 41, 42). Tumor cells employ various mechanisms to activate platelets, leading to the direct release of factors such as IL-1, thrombin, and endothelin, thereby promoting tumor angiogenesis and enhancing tumor migration and dissemination (43). Platelet aggregates encapsulate circulating tumor cells, bolstering their ability to evade host immune attacks (44). Lymphocytes show robust antitumor activity and effectively hinder tumor cell proliferation and metastasis throughout cancer progression (45). A reduced lymphocyte count could result in an inadequate immune response, consequently exerting an adverse impact on the prognosis of patients with solid tumors (46). Platelet-to-lymphocyte ratio (PLR), characterized by its cost-effectiveness and ready accessibility, has garnered widespread attention among scholars. Numerous studies have demonstrated an association between PLR and the prognosis of patients with gastric cancer, including overall survival and progression-free survival (47–50). Tomás and Tiago Cruz (51) affirmed that patients with an elevated PLR have a heightened risk of progression and mortality. Hirahara (52) identified the predictive potential of the combined NLR and platelet-to-lymphocyte ratio (PLR) for treatment outcomes and prognosis in patients with advanced gastric cancer. Tang, Cheng (53) corroborated a substantial association between high PLR and a diminished objective response rate (ORR) in advanced gastric cancer patients following neoadjuvant chemotherapy. Although the Platelet-to-Lymphocyte Ratio has shown prognostic value in diverse cancers, research specifically investigating its prognostic significance in patients undergoing immune checkpoint inhibitor (ICI) therapy is still somewhat limited. A recent retrospective study by Wan M (33), et al. suggested that the PLR and systemic inflammation markers are associated with PFS in patients with gastric cancer receiving immune checkpoint inhibitors (ICIs), while showing no correlation with overall survival (OS). Through univariate and multivariate analyses, Gou (35) concluded that PLR was an independent prognostic factor for both OS and PFS. Additionally, findings from Ziting Qu’s study (37) indicated that PLR acts as an independent prognostic factor for overall survival (OS) in patients with advanced gastric cancer (AGC) undergoing first-line immunotherapy and for PFS in patients undergoing second-line or subsequent immunotherapy. Chen Y (34) suggested a correlation between high PLR, low DCR and ORR. To address this confusion and fill the knowledge gap, we conducted a meticulous meta-analysis of data from nine relevant trials involving 948 patients across two nations. Our study aimed to elucidate whether PLR values could predict the survival outcomes of patients with gastric cancer undergoing immune checkpoint inhibitor (ICI) treatment. In comparison with these studies, our research is the first meta-analysis to incorporate PLR into the analysis of patients with gastric cancer undergoing ICI treatment. Our analysis revealed a discernible trend; elevated PLR values were significantly correlated with reduced survival rates, indicating a significant association between increased PLR and diminished OS and PFS. The combined hazard ratio (HR) for PLR and OS was 1.67, and for PLR and PFS, it stood at 1.51. Furthermore, our subgroup analyses indicated that, irrespective of treatment type (ICIs or ICIs + chemotherapy), sample size (≥100 or <100), cutoff value (>214.08 or ≤214.08), and analysis type (multivariate or univariate), higher PLR values were consistently associated with poorer OS and PFS. The sensitivity analysis and assessment of publication bias also demonstrated the robustness of the results of this meta-analysis. However, there was no statistically significant correlation between PLR and treatment response (ORR/DCR) (ORR: RR = 1.01, p = 0.960; DCR: RR = 0.96, p = 0.319). It is crucial to note that certain limitations should not be overlooked when interpreting our findings. Firstly, the reduction in evidence quality is attributed to the observational and retrospective nature of all included studies, as well as the small sample sizes. Secondly, immune-related adverse events (irAEs) are an inevitable issue that needs to be discussed in the context of immunotherapy. The use of immune checkpoint inhibitors (ICIs) increases the occurrence of irAEs, leading to off-target immune activation and inflammatory reactions. Severe irAEs may necessitate the temporary suspension or dose adjustment of immunotherapy agents, and severe or fatal toxicities pose significant challenges to immunotherapeutic approaches. Due to limitations in the original literature, this study only elucidates the relationship between PLR and overall survival (OS) and progression-free survival (PFS), without revealing the correlation between PLR and irAEs (54, 55). Lastly, since the synthesis of samples is based on studies meeting search criteria, selection bias may be present.
For gastric cancer patients receiving treatment with immune checkpoint inhibitors (ICI), our meta-analysis showed a significant correlation between a higher Platelet-to-Lymphocyte Ratio (PLR) and worse Overall Survival (OS) and Progression-Free Survival (PFS). PLR is a promising prospective biomarker for the prognostication of patients with gastric cancer receiving ICIs. However, considering the limitations of our meta-analysis, larger-scale, multicenter, high-quality prospective trials are needed to validate our results.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author/s.
SH: Writing – review & editing, Writing – original draft, Methodology, Formal analysis, Conceptualization. DS: Writing – review & editing, Writing – original draft, Methodology, Conceptualization. YZ: Writing – original draft, Project administration, Data curation. RH: Writing – original draft, Project administration, Data curation. LL: Writing – review & editing, Formal analysis. JZ: Writing – review & editing, Supervision.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the Youth Science Foundation Funding Program of Shandong First Medical University (Project No. 2022002) and the 2023 Youth Talent Promotion Project of Shandong Medical Association (Project No. 2023_LC_0228).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2024.1367990/full#supplementary-material
1. Smyth EC, Nilsson M, Grabsch HI, van Grieken NC, Lordick F. Gastric cancer. Lancet (London England). (2020) 396:635–48. doi: 10.1016/S0140-6736(20)31288-5
2. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: Cancer J Clin. (2021) 71(3):209–49. doi: 10.3322/caac.21660
3. López MJ, Carbajal J, Alfaro AL, Saravia LG, Zanabria D, Araujo JM, et al. Characteristics of gastric cancer around the world. Crit Rev Oncol Hematol. (2023) 181:103841. doi: 10.1016/j.critrevonc.2022.103841
4. Xu RH, Zhang Y, Pan H, Feng J, Zhang T, Liu T, et al. Efficacy and safety of weekly paclitaxel with or without ramucirumab as second-line therapy for the treatment of advanced gastric or gastroesophageal junction adenocarcinoma (RAINBOW-Asia): a randomised, multicentre, double-blind, phase 3 trial. Lancet Gastroenterol Hepatol. (2021) 6:1015–24. doi: 10.1016/S2468-1253(21)00313-7
5. Alsina M, Arrazubi V, Diez M, Tabernero J. Current developments in gastric cancer: from molecular profiling to treatment strategy. Nat Rev Gastroenterol Hepatol. (2023) 20(3):155–70. doi: 10.1038/s41575-022-00703-w
6. Schoenfeld AJ, Hellmann MD. Acquired resistance to immune checkpoint inhibitors. Cancer Cell. (2020) 37(4):443–55. doi: 10.1016/j.ccell.2020.03.017
7. Keenan TE, Tolaney SM. Role of immunotherapy in triple-negative breast cancer. J Natl Compr Canc Netw. (2020) 18(4):479–89. doi: 10.6004/jnccn.2020.7554
8. Reda M, Ngamcherdtrakul W, Nelson MA, Siriwon N, Wang R, Zaidan HY, et al. Development of a nanoparticle-based immunotherapy targeting PD-L1 and PLK1 for lung cancer treatment. Nat Commun. (2022) 13(1):4261. doi: 10.1038/s41467-022-31926-9
9. Fennell DA, Dulloo S, Harber J. Immunotherapy approaches for Malignant pleural mesothelioma. Nat Rev Clin Oncol. (2022) 19(9):573–84. doi: 10.1038/s41571-022-00649-7
10. Boukouris AE, Theochari M, Stefanou D, Papalambros A, Felekouras E, Gogas H, et al. Latest evidence on immune checkpoint inhibitors in metastatic colorectal cancer: A 2022 update. Crit Rev Oncol Hematol. (2022) 173:103663. doi: 10.1016/j.critrevonc.2022.103663
11. Li S, Yu W, Xie F, Luo H, Liu Z, Lv W, et al. Neoadjuvant therapy with immune checkpoint blockade, antiangiogenesis, and chemotherapy for locally advanced gastric cancer. Nat Commun. (2023) 14(1):8. doi: 10.1038/s41467-022-35431-x
12. Zhao JJ, Yap DWT, Chan YH, Tan BKJ, Teo CB, Syn NL, et al. Low programmed death-ligand 1-expressing subgroup outcomes of first-line immune checkpoint inhibitors in gastric or esophageal adenocarcinoma. J Clin oncology: Off J Am Soc Clin Oncol. (2022) 40(4):392–402. doi: 10.1200/JCO.21.01862
13. Janjigian YY, Shitara K, Moehler M, Garrido M, Salman P, Shen L, et al. First-line nivolumab plus chemotherapy versus chemotherapy alone for advanced gastric, gastro-oesophageal junction, and oesophageal adenocarcinoma (CheckMate 649): a randomised, open-label, phase 3 trial. Lancet (London England). (2021) 398:27–40. doi: 10.1016/S0140-6736(21)00797-2
14. Chen Y, Bai B, Ying K, Pan H, Xie B. Anti-PD-1 combined with targeted therapy: Theory and practice in gastric and colorectal cancer. Biochim Biophys Acta Rev Cancer. (2022) 1877(5):188775. doi: 10.1016/j.bbcan.2022.188775
15. Vafaei S, Zekiy AO, Khanamir RA, Zaman BA, Ghayourvahdat A, Azimizonuzi H, et al. Combination therapy with immune checkpoint inhibitors (ICIs); a new frontier. Cancer Cell Int. (2022) 22(1):2. doi: 10.1186/s12935-021-02407-8
16. Cristescu R, Mogg R, Ayers M, Albright A, Murphy E, Yearley J, et al. Pan-tumor genomic biomarkers for PD-1 checkpoint blockade-based immunotherapy. Sci (New York N.Y.). (2018) 362:eaar3593. doi: 10.1126/science.aar3593
17. Anraku M, Cunningham KS, Yun Z, Tsao MS, Zhang L, Keshavjee S, et al. Impact of tumor-infiltrating T cells on survival in patients with Malignant pleural mesothelioma. J Thorac Cardiovasc Surg. (2008) 135(4):823–9. doi: 10.1016/j.jtcvs.2007.10.026
18. Luchini C, Bibeau F, Ligtenberg MJL, Singh N, Nottegar A, Bosse T, et al. ESMO recommendations on microsatellite instability testing for immunotherapy in cancer, and its relationship with PD-1/PD-L1 expression and tumour mutational burden: a systematic review-based approach. Ann Oncol. (2019) 30(8):1232–43. doi: 10.1093/annonc/mdz116
19. Balkwill F, Mantovani A. Inflammation and cancer: back to virchow. Lancet. (2001) 357:539–45. doi: 10.1016/S0140-6736(00)04046-0
20. Hussain SP, Harris CC. Inflammation and cancer: An ancient link with novel potentials. Int J Cancer. (2007) 121:2373–2380. doi: 10.1002/ijc.23173
21. Crusz SM, Balkwill FR. Inflammation and cancer: Advances and new agents. Nat Rev Clin Oncol. (2015) 12:584–96. doi: 10.1038/nrclinonc.2015.105
22. Xie H, Ruan G, Ge Y, Zhang Q, Zhang H, Lin S, et al. Inflammatory burden as a prognostic biomarker for cancer. Clin Nutr (Edinburgh Scotland). (2022) 41(6):1236–43. doi: 10.1016/j.clnu.2022.04.019
23. Ding P, Wu H, Liu P, Sun C, Yang P, Tian Y, et al. The inflammatory burden index: A promising prognostic predictor in patients with locally advanced gastric cancer. Clin Nutr. (2023) 42(2):247–8. doi: 10.1016/j.clnu.2023.01.005
24. Mei Z, Shi L, Wang B, Yang J, Xiao Z, Du P, et al. Prognostic role of pretreatment blood neutrophil-to-lymphocyte ratio in advanced cancer survivors: A systematic review and meta-analysis of 66 cohort studies. Cancer Treat Rev. (2017) 58:1–13. doi: 10.1016/j.ctrv.2017.05.005
25. Li MX, Liu XM, Zhang XF, Zhang JF, Wang WL, Zhu Y, et al. Prognostic role of neutrophil-to-lymphocyte ratio in colorectal cancer: a systematic review and meta-analysis. Int J Cancer. (2014) 134(10):2403–13. doi: 10.1002/ijc.v134.10
26. Wang J, Li H, Xu R, Lu T, Zhao J, Zhang P, et al. NLR, PLR and D-dimer are associated with clinical outcome in lung cancer patients treated with surgery. BMC pulmonary Med. (2022) 22(1):104. doi: 10.1186/s12890-022-01901-7
27. Diem S, Schmid S, Krapf M, Flatz L, Born D, Jochum W, et al. Neutrophil-to-Lymphocyte ratio (NLR) and Platelet-to-Lymphocyte ratio (PLR) as prognostic markers in patients with non-small cell lung cancer (NSCLC) treated with nivolumab. Lung Cancer (Amsterdam Netherlands). (2017) 111:176–81. doi: 10.1016/j.lungcan.2017.07.024
28. Cho U, Park HS, Im SY, Yoo CY, Jung JH, Suh YJ, et al. Prognostic value of systemic inflammatory markers and development of a nomogram in breast cancer. PloS One. (2018) 13:e0200936. doi: 10.1371/journal.pone.0200936
29. Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the prisma statement. J Clin Epidemiol. (2009) 62:1006–12. doi: 10.1016/j.jclinepi.2009.06.005
30. Moskalewicz A, Oremus M. No clear choice between Newcastle-Ottawa scale and appraisal tool for cross-sectional studies to assess methodological quality in cross-sectional studies of health-related quality of life and breast cancer. J Clin Epidemiol. (2020) 120:94–103. doi: 10.1016/j.jclinepi.2019.12.013
31. Sterne JA, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. (2016) 355:i4919. doi: 10.1136/bmj.i4919
32. Pan Y, Si H, Deng G, Chen S, Zhang N, Zhou Q, et al. A composite biomarker of derived neutrophil-lymphocyte ratio and platelet-lymphocyte ratio correlates with outcomes in advanced gastric cancer patients treated with anti-PD-1 antibodies. Front Oncol. (2022) 11:798415. doi: 10.3389/fonc.2021.798415
33. Wan M, Ding Y, Mao C, Ma X, Li N, Xiao C, et al. Association of inflammatory markers with survival in patients with advanced gastric cancer treated with immune checkpoint inhibitors combined with chemotherapy as first line treatment. Front Oncol. (2022) 12:1029960. doi: 10.3389/fonc.2022.1029960
34. Chen Y, Zhang C, Peng Z, Qi C, Gong J, Zhang X, et al. Association of lymphocyte-to-monocyte ratio with survival in advanced gastric cancer patients treated with immune checkpoint inhibitor. Front Oncol. (2021) 11:589022. doi: 10.3389/fonc.2021.589022
35. Gou M, Zhang Y. Pretreatment platelet-to-lymphocyte ratio (PLR) as a prognosticating indicator for gastric cancer patients receiving immunotherapy. Discover. Oncol. (2022) 13(1):118. doi: 10.1007/s12672-022-00571-5
36. Hayano K, Ohira G, Kano M, Suito H, Matsumoto Y, Kurata Y, et al. Prognostic impact of hepatic steatosis evaluated by CT on immunotherapy for gastric cancer: associations with sarcopenia, systemic inflammation, and hormones. Oncology. (2023) 101(3):185–92. doi: 10.1159/000528005
37. Qu Z, Wang Q, Wang H, Jiao Y, Li M, Wei W, et al. The effect of inflammatory markers on the survival of advanced gastric cancer patients who underwent anti-programmed death 1 therapy. Front Oncol. (2022) 12:783197. doi: 10.3389/fonc.2022.783197
38. Ruan DY, Chen YX, Wei XL, Wang YN, Wang ZX, Wu HX, et al. Elevated peripheral blood neutrophil-to-lymphocyte ratio is associated with an immunosuppressive tumour microenvironment and decreased benefit of PD-1 antibody in advanced gastric cancer. Gastroenterol Rep (Oxf). (2021) 9(6):560–70. doi: 10.1093/gastro/goab032
39. O’Connor A, O’Morain CA, Ford AC. Population screening and treatment of helicobacter pylori infection. Nat Rev Gastroenterol Hepatol. (2017) 14:230–40. doi: 10.1038/nrgastro.2016.195
40. O’Connor A, O’Morain C. Helicobacter pylori infection in Europe: current perspectives. Expert Rev Gastroenterol Hepatol. (2013) 7(6):541–8. doi: 10.1586/17474124.2013.824707
41. Paijens ST, Vledder A, de Bruyn M, Nijman HW. Tumor-infiltrating lymphocytes in the immunotherapy era. Cell Mol Immunol. (2021) 18:842–59. doi: 10.1038/s41423-020-00565-9
42. Haemmerle M, Stone RL, Menter DG, Afshar-Kharghan V, Sood AK. The platelet lifeline to cancer: challenges and opportunities. Cancer Cell. (2018) 33(6):965–83. doi: 10.1016/j.ccell.2018.03.002
43. Lazar S, Goldfinger LE. Platelet microparticles and miRNA transfer in cancer progression: many targets, modes of action, and effects across cancer stages. Front Cardiovasc Med. (2018) 5. doi: 10.3389/fcvm.2018.00013
44. Schlesinger M. Role of platelets and platelet receptors in cancer metastasis. J Hematol Oncol. (2018) 11:125. doi: 10.1186/s13045-018-0669-2
45. Kim MR, Kim AS, Choi HI, Jung JH, Park JY, Ko HJ. Inflammatory markers for predicting overall survival in gastric cancer patients: A systematic review and meta-analysis. PloS One. (2020) 15(7):e0236445. doi: 10.1371/journal.pone.0236445
46. Väyrynen JP, Tuomisto A, Klintrup K, Mäkelä J, Karttunen TJ, Mäkinen MJ.. Detailed analysis of inflammatory cell infiltration in colorectal cancer. Br J Cancer. (2013) 109(7):1839–47. doi: 10.1038/bjc.2013.508
47. Wang W, Tong Y, Sun S, Tan Y, Shan Z, Sun F, et al. Predictive value of NLR and PLR in response to preoperative chemotherapy and prognosis in locally advanced gastric cancer. Front Oncol. (2022) 12:936206. doi: 10.3389/fonc.2022.936206
48. Zhang Y, Lu JJ, Du YP, Feng CX, Wang LQ, Chen MB. Prognostic value of neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio in gastric cancer. Med (Baltimore). (2018) 97(12):e0144. doi: 10.1097/MD.0000000000010144
49. Zhao G, Liu N, Wang S, Guo J, Song X, Qi Y, et al. Prognostic significance of the neutrophil-to-lymphocyte and platelet-to-lymphocyte ratio in patients with metastatic gastric cancer. Med (Baltimore). (2020) 99(10):e19405. doi: 10.1097/MD.0000000000019405
50. Mungan İ, Dicle ÇB, Bektaş Ş, Sarı S, Yamanyar S, Çavuş M, et al. Does the preoperative platelet-to-lymphocyte ratio and neutrophil-to-lymphocyte ratio predict morbidity after gastrectomy for gastric cancer? [published correction appears in Mil Med Res. 2020 Mar 23;7(1):12]. Mil Med Res. (2020) 7(1):9. doi: 10.1186/s40779-020-00234-y
51. Tomás TC, Eiriz I, Vitorino M, Vicente R, Gramaça J, Oliveira AG, et al. Neutrophile-to-lymphocyte, lymphocyte-to-monocyte, and platelet-to-lymphocyte ratios as prognostic and response biomarkers for resectable locally advanced gastric cancer. World J gastrointestinal Oncol. (2022) 14(7):1307–23. doi: 10.4251/wjgo.v14.i7.1307
52. Hirahara T, Arigami T, Yanagita S, Matsushita D, Uchikado Y, Kita Y, et al. Combined neutrophil-lymphocyte ratio and platelet-lymphocyte ratio predicts chemotherapy response and prognosis in patients with advanced gastric cancer. BMC Cancer. (2019) 19(1):672. doi: 10.1186/s12885-019-5903-y
53. Tang C, Cheng X, Yu S, Wang Y, Hou J, Li Q, et al. Platelet-to-lymphocyte ratio and lymphocyte-to-white blood cell ratio predict the efficacy of neoadjuvant chemotherapy and the prognosis of locally advanced gastric cancer patients treated with the oxaliplatin and capecitabine regimen. OncoTargets Ther. (2018) 11:7061–75. doi: 10.2147/OTT
54. Solimando AG, Crudele L, Leone P, Argentiero A, Guarascio M, Silvestris N, et al. Immune checkpoint inhibitor-related myositis: from biology to bedside. Int J Mol Sci. (2020) 21(9):3054. doi: 10.3390/ijms21093054
Keywords: platelet-to-lymphocyte ratio, overall survival, progression-free survival, gastric cancer, immune checkpoint inhibitors
Citation: Hou S, Song D, Zang Y, Hao R, Li L and Zhu J (2024) Prognostic relevance of platelet lymphocyte ratio (PLR) in gastric cancer patients receiving immune checkpoint inhibitors: a systematic review and meta-analysis. Front. Oncol. 14:1367990. doi: 10.3389/fonc.2024.1367990
Received: 09 January 2024; Accepted: 21 May 2024;
Published: 07 June 2024.
Edited by:
Bianca Mostert, Erasmus Medical Center, NetherlandsReviewed by:
Hasan Cagri Yildirim, Niğde Ömer Halisdemir University Training and Research Hospital, TürkiyeCopyright © 2024 Hou, Song, Zang, Hao, Li and Zhu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jiankang Zhu, MjQ5MjE3NTIzQHFxLmNvbQ==
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.