- 1Department of Medical Imaging, The Third Affiliated Hospital of Zhengzhou University, Zhengzhou, China
- 2Department of Medical Imaging, Zhengzhou People’s Hospital, Zhengzhou, China
- 3Department of Medical Imaging, The First Affiliated Hospital of Zhengzhou University, Zhengzhou, China
Objectives: The safety and feasibility of repeat biopsy after systemic treatment for non-small cell lung cancer have received extensive attention in recent years. The purpose of this research was to compare complication rates between initial biopsy and rebiopsy in non-small cell lung cancer patients with progressive disease and to assess complication risk factors and clinical results after rebiopsy.
Methods: The study included 113 patients initially diagnosed with non-small cell lung cancer who underwent lung biopsy at initial biopsy and rebiopsy after progression while on epidermal growth factor receptor tyrosine kinase inhibitors (EGFR-TKIs) and/or chemotherapy from January 2018 to December 2021. We compared the incidence of complications between the initial biopsy and rebiopsy and analyzed the predictors factors that influenced complications in patients who underwent rebiopsy.
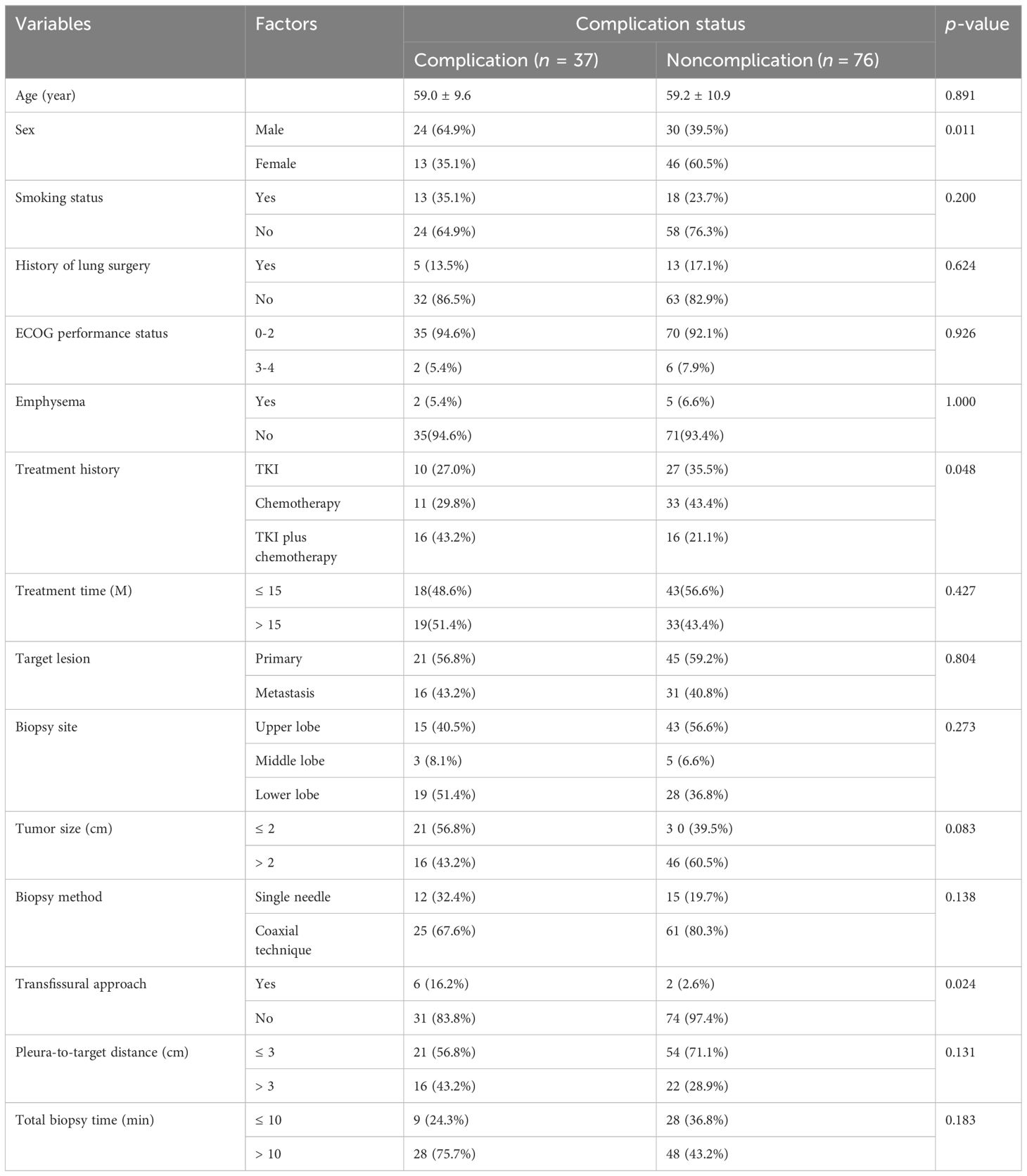
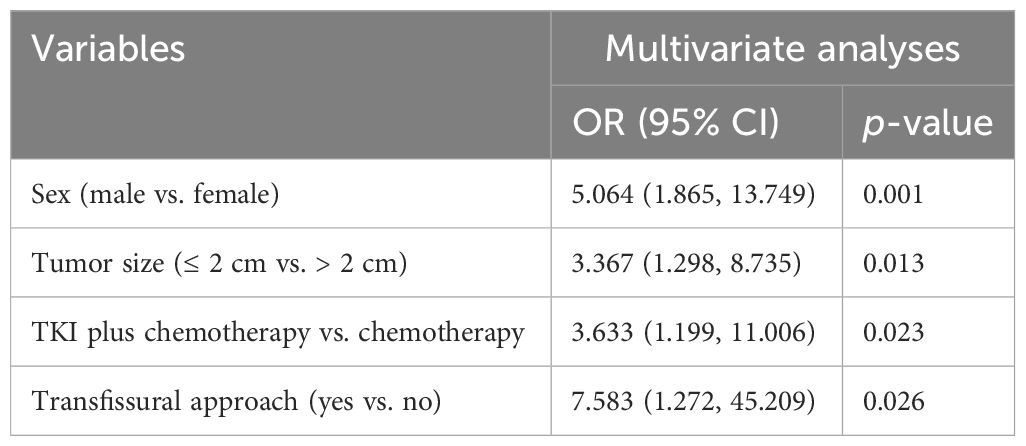
Results: The successful rate of rebiopsy was 88.5% (100/113). With the exception of two cases where lung adenocarcinoma changed into small cell lung cancer with gefitinib treatment, 98 individuals retained their initial pathological type. The secondary EGFR T790M mutation accounts for 55.6% of acquired resistance. The total number of patients with complications in initial biopsy was 25 (22.1%) and 37 (32.7%) in the rebiopsy. The incidence of pulmonary hemorrhage increased from 7.1% at the initial biopsy to 10.6% at rebiopsy, while the incidence of pneumothorax increased from 14.2% to 20.4%. Compared with the initial biopsy, the incidence of overall complications, parenchymal hemorrhage, and pneumothorax increased by 10.6%, 3.5%, and 6.2%, respectively. In all four evaluations (pneumorrhagia, pneumothorax, pleural reaction, and overall complication), there were no significant differences between the rebiopsy and initial biopsy (all p > 0.05). The multivariate logistic regression analysis suggested that male sex (odds ratio [OR] = 5.064, p = 0.001), tumor size ≤ 2 cm (OR = 3.367, p = 0.013), EGFR-TKIs with chemotherapy (OR = 3.633, p =0.023), and transfissural approach (OR = 7.583, p = 0.026) were independent risk factors for overall complication after rebiopsy.
Conclusion: Compared with the initial biopsy, the complication rates displayed a slight, but not significant, elevation in rebiopsy. Male sex, tumor size ≤ 2 cm, transfissural approach, and EGFR-TKIs combined with chemotherapy were independent risk factors for rebiopsy complications.
Introduction
Lung cancer is a significant global health issue as it is responsible for the majority of cancer-related deaths (1). Non-small cell lung cancer (NSCLC) is the primary type of lung cancer and constitutes around 85%–90% of all lung cancer (2). Unfortunately, due to a lack of symptoms and accurate detection at an early stage, a considerable proportion of lung cancer patients are diagnosed at an advanced stage and may only be treated with medications (3). In Chinese patients, 40% of lung cancer patients have epidermal growth factor receptor (EGFR) gene mutations, compared with only 15% in European patients, and these EGFR mutations confer sensitivity to tyrosine kinase inhibitors (TKIs) (4, 5). Indeed, the first-line treatment of advanced NSCLC has evolved from traditional chemotherapy to molecular target and immunotherapy, and the clinical results and use of EGFR-TKIs have significantly improved the overall response rate and progression-free survival of patients with advanced NSCLC (6). However, most patients who initially respond to EGFR-TKIs inevitably develop resistance and then relapse, ultimately leading to death (7). Survival is still seriously affected by cancer metastasis, therapeutic resistance, and relapse (8).
The secondary EGFR T790M mutation, identified in approximately 60% of cases, is the most common acquired resistance mechanism, thereby limiting progression-free survival to approximately 9–14 months (9). Hence, it is critical that the patient clearly understands the resistance mechanism of tissue biopsy in order to make individualized treatment decisions based on tumor-related molecular information. At the time of disease progression, several studies have reported that repeat biopsies were physically invasive, procedurally risky, expensive, and time-consuming, making it more technically difficult to obtain tissue than an initial biopsy and sometimes not feasible (10–12). Rebiopsy can be performed in 50% to 80% of NSCLC patients with progressive disease, and tissue samples for histology analysis were only available in 45% of the patients (12, 13). In most situations, a tissue sample for resistance is the test of first choice because tissue rebiopsy could produce more accurate, more reliable results than a liquid biopsy and is sufficient to predict drug susceptibility, and histopathological changes can be detected in tissue samples (14, 15). According to several previous reports, rebiopsy for patients with non‐small cell lung cancer treatment failure can be performed safely and accurately under CT guidance (10, 16, 17). However, no detailed investigation of the risk factors of NSCLC patients resistant to EGFR-TKIs and/or chemotherapy by CT-guided transthoracic needle biopsy has been reported until now. The purpose of this paper is to assess rebiopsy complication risk factors and clinical outcomes in NSCLC patients with progressive disease. Moreover, we also attempted to compare the different incidences of complications between initial biopsy and rebiopsy.
Materials and methods
Patient
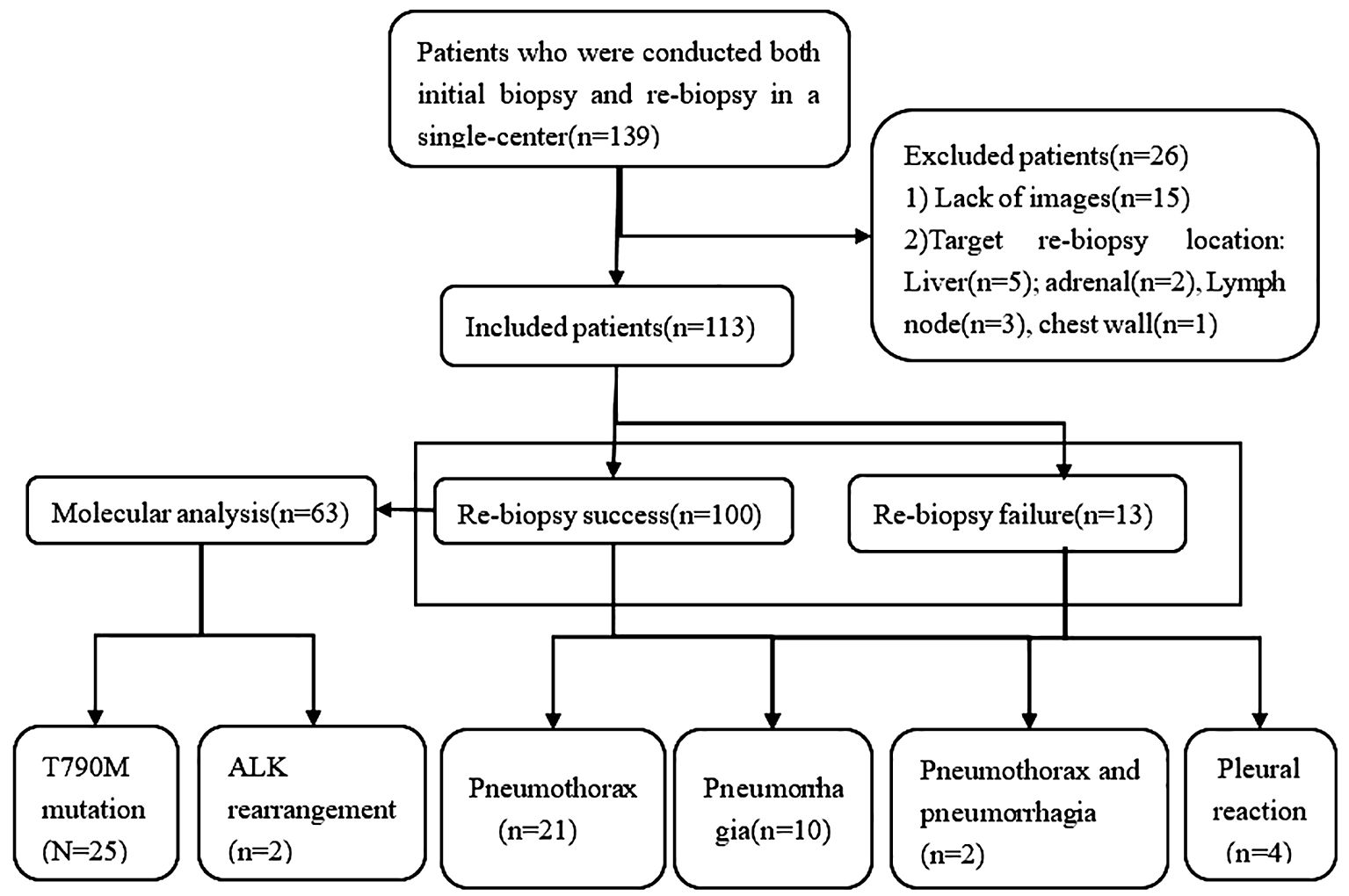
This is an observational, single-center, retrospective study approved by the institutional ethical committee. Patients enrolled in the current study were diagnosed pathologically with NSCLC stages IIIB–IV with progressive disease after failure of EGFR-TKIs and/or chemotherapy treatment between January 2019 and December 2022. All included patients underwent CT-guided transthoracic needle biopsy at the initial biopsy and rebiopsy by the same medical team at our hospital. In total, 139 patients were initially screened for inclusion, of whom 26 were excluded, and 113 patients were finally evaluated (Figure 1).
The inclusion and exclusion criteria were as follows: (1) patients who had available chest CT scan images on the Picture Archiving and Communication System (PACS); (2) patients with pathologically confirmed NSCLC with progressive disease after failure who had previously received EGFR‐TKIs and/or chemotherapy. We excluded patients who (1) lacked images on the PACS and complete procedural data; (2) the location of initial biopsy and repeat biopsy were extrapulmonary sites.
Biopsy procedure
All biopsy procedures were performed under local anesthesia by one of five interventional radiologists who had more than 10 years of clinical experience in CT-guided percutaneous lung biopsy. Patients were requested to stop taking any antiplatelet and anticoagulant medications at least 7 days before surgery. All the patients were informed of the procedure, all potential complications, and appropriate treatment and signed the operation informed consent. Prior to biopsy, a chest contrast-enhanced CT scan must be performed in all patients, which helps evaluate the precise location, size of lesions, shape, boundary, and proximity to neighboring organs at risk. Based on the imaging data of the patient, the radiologist determined the appropriate biopsy program.
The principles of biopsy were to choose the safest path to target the lesion, avoiding risk factors such as fissures, blood vessels, emphysema, and bullae. The patient was positioned appropriately according to the lesion location and instructed to breathe calmly. The puncture site was prepared using a sterile technique, and 5–10 ml of 2% lidocaine was used for local infiltration anesthesia to the parietal pleura. The operator subjectively determined the needle insertion angle and the needle insertion distance and then chose the appropriate biopsy method (single-needle or coaxial needle). The single-needle technique, with an 18-gauge biopsy needle, can be advanced into the lesion. When the needle tip was at the edge of the lesion, a tissue sample was obtained. The coaxial needle technique consists of a 17-gauge coaxial trocar together with an 18-gauge automatic biopsy needle. Once the 17-gauge coaxial needle enters the lesion, an automated 18-gauge biopsy needle passes through the lumen of the guide needle. The samples were obtained by core needle biopsy with a 1- or 2-cm needle to ensure safety. Once the specimen was obtained, the operator ensured that there was no blood in the needle channel, inserted the needle core, and withdrew the biopsy needle. It was important to note that there was no on-site pathologist available for immediate evaluation of the biopsy sample during the procedure. Subsequently, the tissue samples were placed in a 10% formalin solution for histopathological examination. Additionally, an immediate postprocedural CT scan was conducted to detect any potential complications.
Management of complications
Pneumothorax and pulmonary hemorrhage are found mainly on CT scans, while the diagnosis of pleural reaction is based on a series of clinical manifestations. A pneumothorax is the presence of air in the chest cavity. Pulmonary hemorrhage was defined as consolidation or ground glass opacity around the lesion and along the puncture path after biopsy, with or without hemoptysis. Pleural reaction refers to various reactions that occur when the lungs are punctured, such as dizziness, chest tightness, pale complexion, sweating, and even fainting.
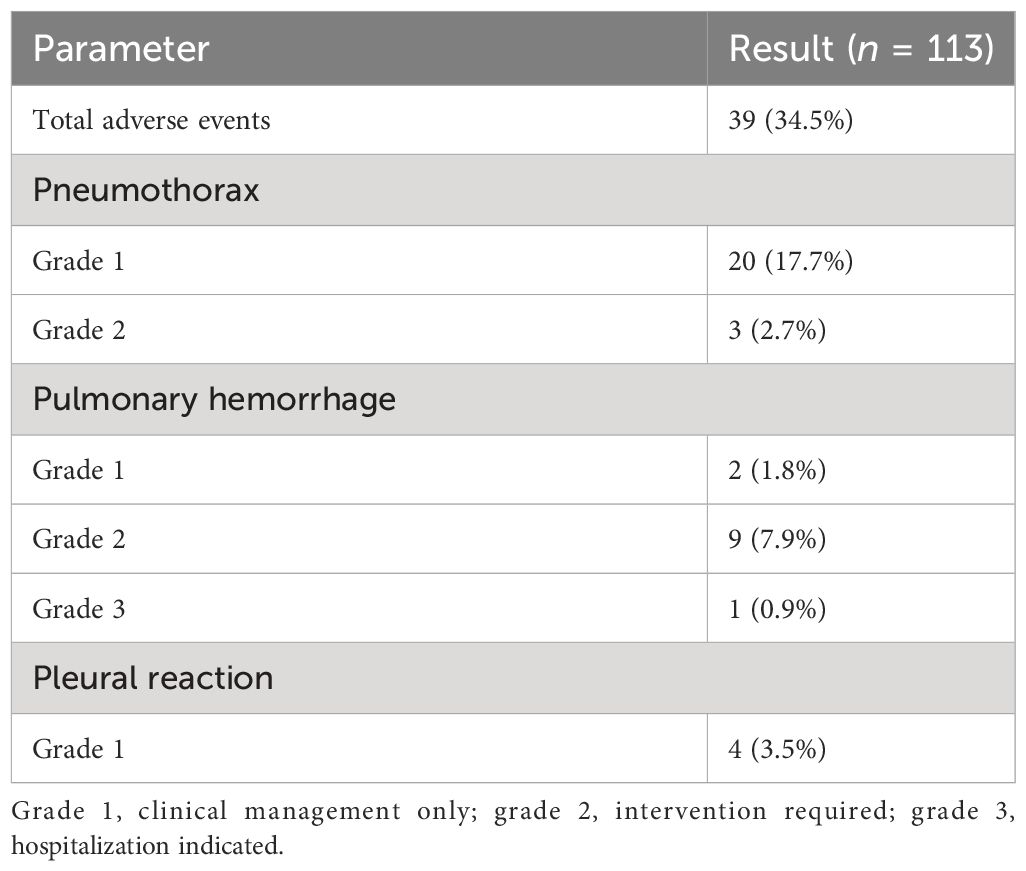
The Common Terminology Criteria for Adverse Events (CTCAE) version 5.0 was used to record adverse events. We classified grade 2 or higher complications as more serious complications because grade 1 adverse events are commonly seen based on past experience. Grade 1 pulmonary hemorrhage does not require specialized intervention, but for grade 2 or more severe cases, the patient should be positioned laterally with the puncture point facing downwards, receive prompt intravenous medication to stop bleeding, and undergo continuous monitoring of vital signs. Grade 1 pneumothorax does not require specific treatment. Grade 2 and above require a closed thoracic drainage tube.
Data collection
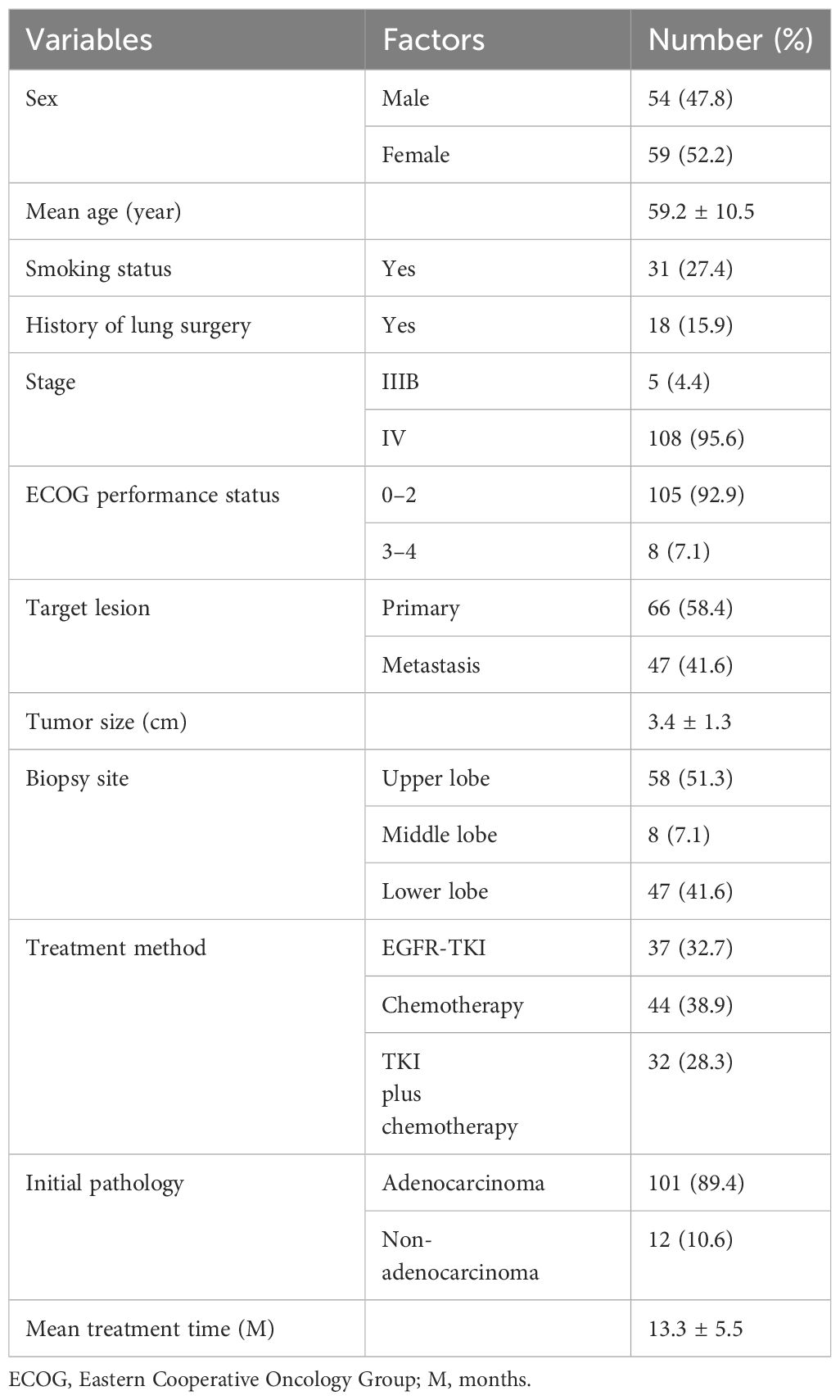
All clinical and demographic data were mainly collected from hospital records. The medical records provided information on the patient’s clinical and demographic features, including sex, age, smoking status (yes or no), history of lung surgery (yes or no), cancer stage, Eastern Cooperative Oncology Group (ECOG) performance status, emphysema (yes or no), initial pathology, treatment history (EGFR-TKIs, chemotherapy, and EGFR-TKI combined with chemotherapy), and treatment time.
Additionally, various biopsy-related features were collected from our radiology databases, such as tumor size, tumor location (lobe), target lesion (primary tumor or metastasis), transfissural approach (yes or no), biopsy method (single-needle or coaxial technique), total biopsy time, and pleura-to-target distance.
As a result of reviewing procedure records, medical charts, and follow-up dates, complications relating to the procedure, such as pneumothorax, pneumothorax requiring chest tube placement, pulmonary hemorrhage (with or without hemoptysis), and pleural reaction, were noted.
A successful rebiopsy was defined as obtaining sufficient malignant tissue in biopsy samples. Otherwise, it was defined as failure.
Statistical analysis
Statistical analysis uses the SPSS 21.0 software. Continuous variables are presented as mean ± standard deviation, while categorical variables are as frequencies (percentages). We counted the incidence of overall complication, pulmonary hemorrhage, pneumothorax, and pleural reaction, respectively, after the initial biopsy and rebiopsy puncture. The McNemar test was used to compare the incidence of complications during the initial biopsy and rebiopsy, and a p-value of < 0.05 was considered statistically significant. We then performed comparisons between the complications group and the no complications group to analyze independent risk factors associated with complications. Categorical factors were performed by Pearson Chi‐squared or Fisher exact tests. Continuous data were made using the Mann–Whitney U test or independent samples t-test.
To identify the risk factors for complications, we performed a binary logistic regression analysis. All covariates with p < 0.10 in univariate analysis were included in multivariate analysis. The backward stepwise selection method was used to generate a final reduced model. p < 0.05 was considered statistically significant; otherwise, there was no significance.
Results
Demographic and clinical characteristics of initial and rebiopsy patients
The population characteristics are shown in Table 1. From January 2019 to December 2022, there were 113 patients (mean age: 59.2 years ± 10.5 years, range: 33 to 83 years) who received rebiopsy. There were 54 male (47.8%) patients. Of these patients, 31 (27.4%) were smokers. Adenocarcinomas were found in 101 (89.4%) cases of tumor histology. Regarding treatment history, 37 (32.7%) patients received TKIs, 44 (38.9%) patients received chemotherapy, and 32 (28.3%) patients had received EGFR-TKIs combined with chemotherapy. Primary lesion was biopsied in 66 (58.4%) cases, and the mean tumor size was 3.4 cm ±1.3 cm.
Results of rebiopsy
The successful rate of rebiopsy was 88.5% (100/113). Of the patients, 98 maintained the original pathological type, including adenocarcinoma (n = 89), squamous cell carcinoma (n = 6), and large cell lung cancer (n = 3). We identified two cases of lung adenocarcinoma transformation to small cell lung cancer after treatment with gefitinib. The reason for the failure of rebiopsy was the absence of malignant cells or insufficient malignant cells (n = 13). The pathologies of unsuccessful biopsies included lung tissue, fibrocytes, and inflammatory cells. The examination of the driver gene mutation was performed in 45 patients with non-small cell lung cancer who had received the first-generation EGFR-TKIs, and 25 (55.6%) were positive for EGFR T790M mutation.
Complications of initial biopsy and rebiopsy
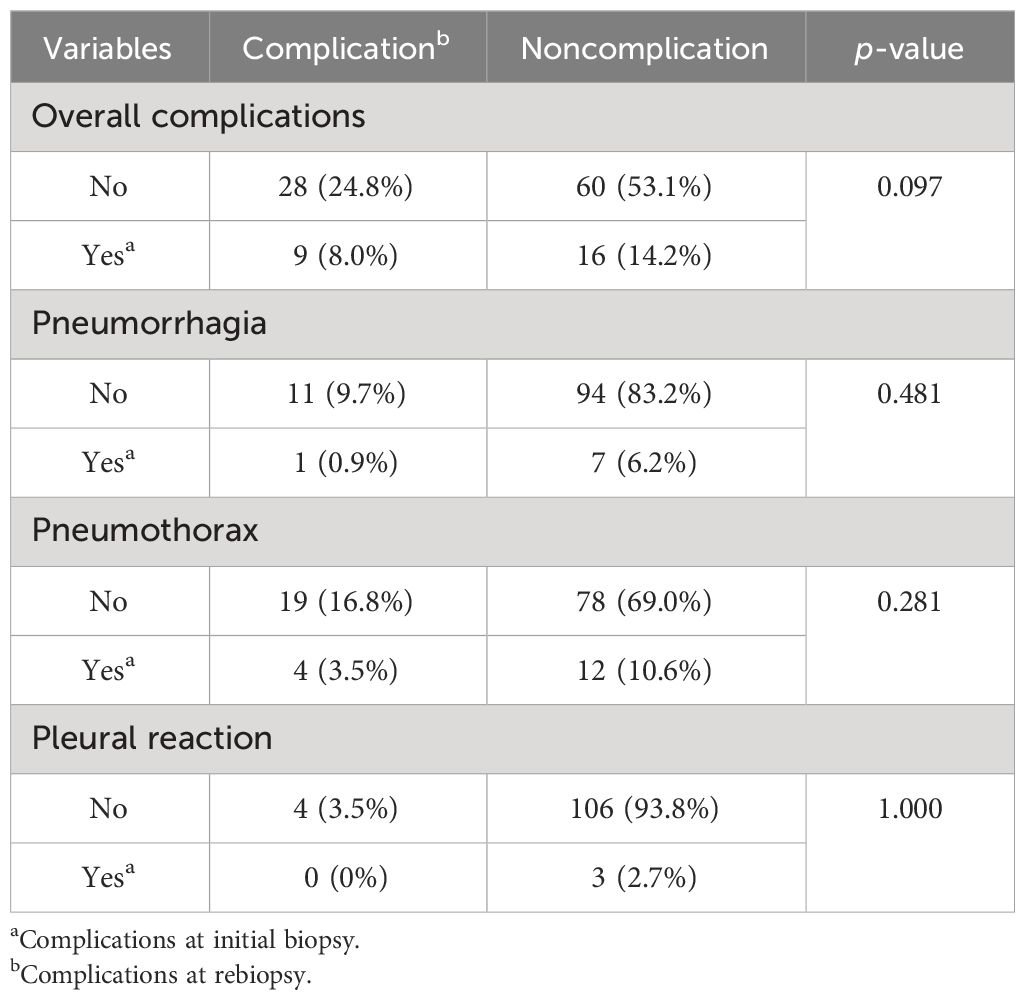
Rates of adverse events are shown in Table 2, and a comparison of complication probability between initial biopsy and rebiopsy is shown in Table 3. The number of patients with complications in the initial biopsy was 25 (22.1%) and 37 (32.7%) in the rebiopsy (two women each had two complications; in total, 39 complications). The main complications of rebiopsy were pulmonary hemorrhage and pneumothorax. Although the incidence of pulmonary hemorrhage and pneumothorax increased from 7.1% to 10.6% and 14.2% to 20.4%, respectively, there were no significant differences in overall complications, pulmonary hemorrhage, pneumothorax, or pleural reaction between initial biopsy and rebiopsy (all p > 0.05).
Pulmonary hemorrhage was observed in eight patients during the initial biopsy (Figure 2), including hemoptysis in four patients. During the rebiopsy, pulmonary hemorrhage occurred in 12 patients, including three cases of hemoptysis. However, only one patient experienced pulmonary hemorrhage in both biopsies. Pneumothorax was observed in 16 patients during the initial biopsy and in 23 patients during the rebiopsy. However, only four patients experienced pneumothorax in both biopsies. Three patients required the placement of a chest tube during the rebiopsy, and X-ray results showed complete absorption of pneumothorax before discharge. Two patients experienced both pneumothorax and pulmonary hemorrhage after the postprocedural biopsy. None of the patients experienced pleural reaction in both biopsies. No serious complications occurred in rebiopsy, such as air embolism, cardiac tamponade, hemodynamic instability, or death.
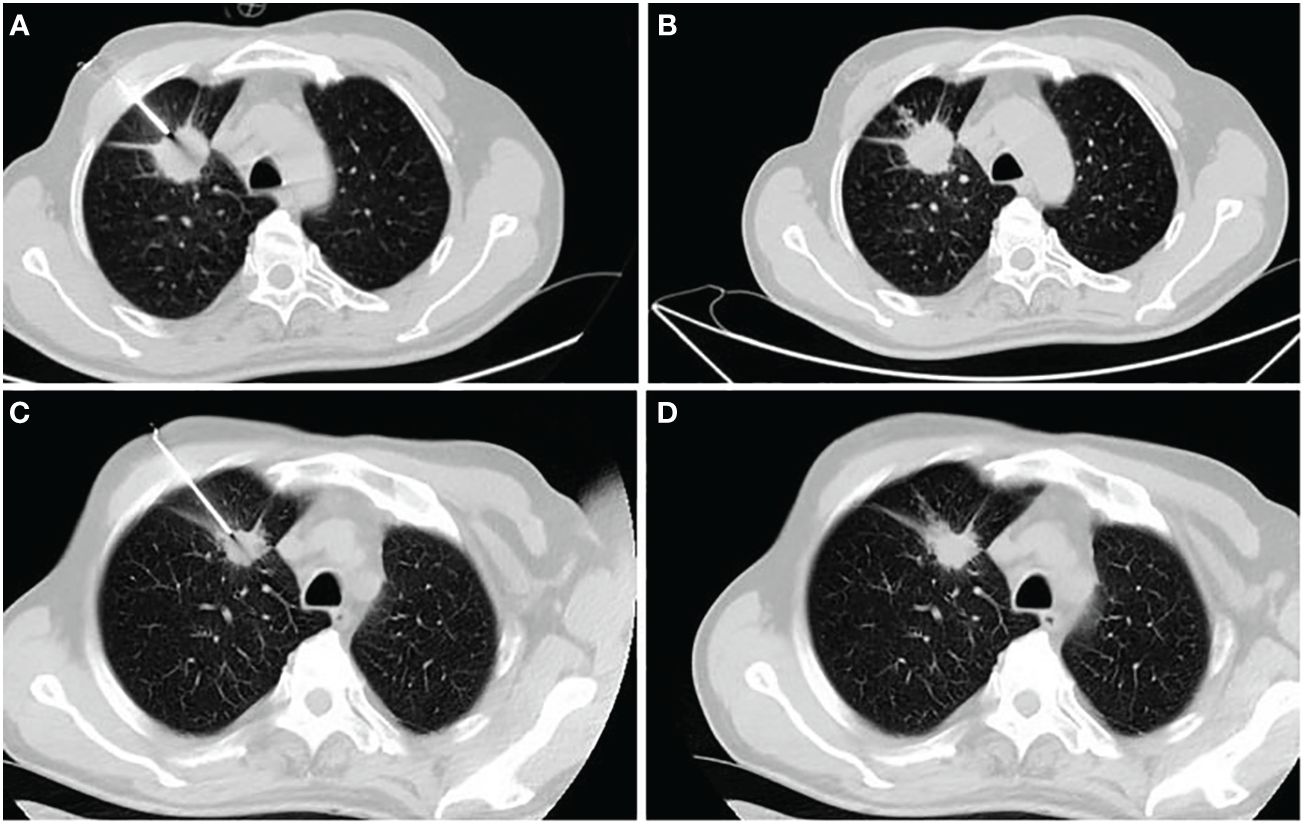
Figure 2 A 62-year-old man receiving his first biopsy (A, B) and rebiopsy at age 63 (C, D). (A) The needle enters the lesion through coaxial needles at first biopsy. (B) CT showed small amounts of bleeding along the needle path. (C) Disease progression after 10 months of gefitinib monotherapy and rebiopsy of the primary lesion using a coaxial biopsy needle. (D) There were no significant complications after rebiopsy.
Analysis of biopsy-related complications
Statistical modeling can help determine the significant factors that affect the rate of complications for rebiopsy, as shown in Table 4. Regarding clinical features, age, smoking status, history of lung surgery, ECOG performance status, emphysema, and treatment time, there were no statistically significant differences (all p > 0.10). Male sex (p = 0.011) and treatment history (p = 0.048) were statistically different (p < 0.10). Among the rebiopsy-related factors, tumor size (p = 0.083) and transfissural approach (p = p0.024) showed p-values less than 0.10. Next, the above suggestive factors were included in the binary logistic regression analysis (Table 5). The independent risk factors of overall complications were male sex (odds ratio [OR], 5.064; 95% confidence interval [CI]: 1.865, 13.749; p = 0.001), tumor size ≤ 2 cm (OR, 3.367; 95% CI: 1.298, 8.735; p = 0.013), EGFR-TKIs combined with chemotherapy (OR, 3.633; 95% CI: 1.199, 11.006; p = 0.023), and transfissural approach (OR, 7.583; 95% CI: 1.272, 45.209; p =0.026).
Discussion
In this study, we have three main findings: First, CT-guided transthoracic needle rebiopsy was safe and feasible for patients with treatment failure and disease progression. Second, compared with the initial biopsy, the complication rates displayed a slight, but not significant, elevation in rebiopsy. Third, male sex, tumor size ≤ 2 cm, transfissural approach, and EGFR-TKIs combined with chemotherapy were independent risk factors for rebiopsy complications.
Currently, tumor heterogeneity is associated with therapy resistance, malignant progression, and recurrence in cancer. Hence, there is a strong demand for rebiopsy to assess the molecular and pathological characterization of NSCLC patients with progressive disease (18–20). A study of repeat biopsies after EGFR-TKI treatment found that repeat biopsies were technically more difficult than initial biopsies and procedures associated with higher complication rates and failure rates (21).
The rate of surgical resection increased from 1.8% at the initial biopsy to 7.8% at rebiopsy when compared to the biopsy collection method, while the rate of lung biopsy rose obviously from 7.6% to 29.1% (10). This evidence illustrates the technical difficulty in obtaining tissue samples for patients at a repeat biopsy. CT-guided transthoracic needle biopsy is one of the invasive diagnostic methods with highly sensitive and accurate procedures, and complications will inevitably occur during the operation. According to previous reports (22–27), the rate of pulmonary parenchymal hemorrhage ranged from 5% to 26.8%, severe pulmonary hemorrhage or hemoptysis was 0.06% to 7.0%, and the rate of pneumothorax was 12% to 45%, with 2.7% to 6.6% requiring chest tube placement. In the meantime, some interesting prior research has reported that the complication rates in patients with repeat biopsy were similar to those who had a first biopsy (16, 28, 29). Both pulmonary hemorrhage and pneumothorax in rebiopsy were accepted, and pneumothorax was the most common. Also, a meta-analysis (30) showed a slight but non-significant reduction in the pooled complication rate for the rebiopsies vs. the initial biopsies (16.8% vs. 22.2%). Although patients showed disease progression after receiving a previous systemic treatment, the overall complication rate of the biopsy was not statistically significant between the two groups. On the contrary, in our study, all patients underwent repeat biopsies according to disease progression, and the incidences of parenchymal hemorrhage and pneumothorax increased from 7.1% to 10.6% and 14.2% to 20.4%, respectively, from the first biopsy to the second biopsy. For CT-guided biopsy, the overall complication rate at the single center study improved from 22.1% in the initial biopsy to 32.7% in the rebiopsy. In all four evaluations (pneumorrhagia, pneumothorax, pleural reaction, and overall complication), there were no significant differences between the rebiopsy and initial biopsy (all p > 0.05). Compared with initial biopsy, the incidence of overall complications, parenchymal hemorrhage, and pneumothorax increased by 10.6%, 3.5%, and 6.2%, respectively, but no serious postoperative complications, including air embolism, severe hemodynamic changes, or death, occurred at the rebiopsy. These findings suggest that rebiopsy is both feasible and safe for NSCLC patients with treatment failure and disease progression.
Male sex, tumor size ≤ 2 cm, transfissural approach, and the use of EGFR-TKIs combined with chemotherapy were independent risk factors for complications even after multivariate analysis. There were several plausible explanations for this result. First, EGFR-TKIs combined with chemotherapy had significant therapeutic benefits, but they may cause or aggravate lung injury, which leads to an increased risk of biopsy-related complications. Previous research has shown that EGFR-TKI-induced lung injury has clinical risk factors, including male sex, smoking status, early treatment initiation after diagnosis, and prior treatment with chemotherapy or radiotherapy (31–33). Likewise, our research also confirmed clinical evidence of a higher risk of complication with concurrent use of EGFR-TKIs combined with chemotherapy relative to chemotherapy alone. Second, the size of the tumor is small, the operation is difficult, and the complication rate is relatively high (34). Notably, accuracy was significantly dependent on tumor size; the accuracy rate of the size of 21–30 mm vs. ≤ 20 mm was significantly higher (97% vs. 85%, p < 0.05) (35). Third, a transfissural approach was a significant predictor of pneumothorax, and the risk of parenchymal hemorrhage is also increased when the puncture needle breaks through the pleura more than two times. The more times of repeated punctures of the pleura, the greater the possibility of pneumothorax and parenchymal hemorrhage (36–38).
In addition, there were other factors that may influence the complications of lung rebiopsy. The main factors were as follows: (1) Sampling location: the volume of primary lesion increased, and a large number of tumor angiogenesis and cells were distributed in marginal areas after progression of treatment failure, where tumor cell growth was active, which was the ideal sampling target for rebiopsy, and biopsy increases the risk of complications (39, 40). (2) Patients with underlying disease and cooperation level: stage IIB/IV tumor patients with long-term systemic treatment, especially those men with a history of smoking, have reduced lung basal and vascular fragility, as well as reduced ability to contract after injury, which may increase the challenge of rebiopsies (41, 42). Therefore, accurate measurement of the depth of the needle during a biopsy, minimizing the number of punctures, and avoiding the needle path by passing through bullae, blood vessels, and interlobar fissures can effectively reduce the incidence of complications. In addition, it is necessary to actively communicate with the patient to alleviate their anxiety before the biopsy. The local anesthesia needle should be inserted as close as possible to the parietal pleura to reduce the occurrence of pleural reaction so that the patient can better cooperate during the biopsy.
Several previous studies have shown that the success rate of rebiopsy of advanced lung cancer by transthoracic puncture is about 82%–85% (10, 18, 29). Our slightly higher success rate (88.5%) may be due only to CT-guided biopsy samples compared to that in prior studies. Driven by drug resistance factors, tumor lesions were more advanced than before, the substance components were more complex and diverse, and the lesions in the central region may have a higher incidence of necrosis, resulting in sampling errors in small sample biopsy. For rebiopsy, combined with an enhanced CT scan or PET-CT scan, biopsy metabolically active lesions can improve the detection rate of malignant tumor cells.
In summary, in the real world, rebiopsy is safe after treatment failure in patients with non-small cell lung cancer. The most common complications are pulmonary parenchymal hemorrhage and pneumothorax. Adequate biopsy samples can be used to monitor disease progression, interpret drug resistance mechanisms, and provide a reference for subsequent treatment planning for patients with targeted drug therapy failure or drug resistance (43).
The limitations of our study can be listed as follows: first, a single-center retrospective study fails to record and access some risk factors if those factors were not systematically reported, such as pain. Second, the relatively small sample size fails to reach statistical significance for a certain risk factor. Third, procedure techniques and operators’ experience may also affect the occurrence of complications.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by Ethics Committee of the Third Affiliated Hospital of Zhengzhou University. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin because this was a retrospective, single-center study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
YW: Writing – original draft, Writing – review & editing. YZ: Conceptualization, Data curation, Writing – review & editing. NR: Software, Writing – review & editing. FL: Formal analysis, Writing – review & editing. LL: Methodology, Writing – review & editing. ZZ: Data curation, Writing – original draft. MG: Investigation, Writing – original draft. MW: Investigation, Writing – original draft. XZ: Methodology, Writing – original draft.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study has received funding by Beijing Xisike Clinical Oncology Research Foundation(Grant Number Y-2019AZMS-0505).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2021. CA: A Cancer J Clin. (2021) 71:7–33. doi: 10.3322/caac.21654
2. Chen B, Zhao HY, Li M, She Q, Liu W, Zhang JY, et al. SHANK1 facilitates non-small cell lung cancer processes through modulating the ubiquitination of Klotho by interacting with MDM2. Cell Death Dis. (2022) 13:403. doi: 10.1038/s41419-022-04860-3
3. Ou JW, Zhu XY, Chen PF, Du YP, Lu YM, Peng XF, et al. A randomized phase II trial of best supportive care with or without hyperthermia and vitamin C for heavily pretreated, advanced, refractory non-small-cell lung cancer. J Adv Res. (2020) 24:175–82. doi: 10.1016/j.jare.2020.03.004
4. Tu E, McGlinchey K, Wang JX, Martin P, Ching SLK, Floc'h N, et al. Anti-PD-L1 and anti-CD73 combination therapy promotes T cell response to EGFR-mutated NSCLC. JCI Insight. (2022) 7:e142843. doi: 10.1172/jci.insight.142843
5. Weber R, Meister M, Muley T, Thomas M, Sultmann H, Warth A, et al. Pathways regulating the expression of the immunomodulatory protein glycodelin in non-small cell lung cancer. Int J Oncol. (2019) 54:515–26. doi: 10.3892/ijo.2018.4654
6. Sarne V, Huter S, Braunmueller S, Rakob L, Jacobi N, Kitzwogerer M, et al. Promoter methylation of selected genes in non-small-cell lung cancer patients and cell lines. Int J Mol Sci. (2020) 21:4595. doi: 10.3390/ijms21134595
7. Han JY, Lee KH, Kim SW, Min YJ, Cho E, Lee Y, et al. A phase II study of poziotinib in patients with epidermal growth factor receptor (EGFR)-mutant lung adenocarcinoma who have acquired resistance to EGFR tyrosine kinase inhibitors. Cancer Res Treat. (2017) 49:10–9. doi: 10.4143/crt.2016.058
8. Arroyo MM, Berral-Gonzalez A, Bueno-Fortes S, Alonso-Lopez D, De Las Rivas J. Mining drug-target associations in cancer: analysis of gene expression and drug activity correlations. Biomolecules. (2020) 10:667. doi: 10.3390/biom10050667
9. Brown BP, Zhang YK, Westover D, Yan YJ, Qiao H, Huang V, et al. On-target resistance to the mutant-selective EGFR inhibitor osimertinib can develop in an allele-specific manner dependent on the original EGFR-activating mutation. Clin Cancer Res. (2019) 25:3341–51. doi: 10.1158/1078-0432.Ccr-18-3829
10. Nosaki K, Satouchi M, Kurata T, Yoshida T, Okamoto I, Katakami N, et al. Re-biopsy status among non-small cell lung cancer patients in Japan: A retrospective study. Lung Cancer. (2016) 101:1–8. doi: 10.1016/j.lungcan.2016.07.007
11. Hong MH, Kim HR, Ahn BC, Heo SJ, Kim JH, Cho BC. Real-world analysis of the efficacy of rebiopsy and EGFR mutation test of tissue and plasma samples in drug-resistant non-small cell lung cancer. Yonsei Med J. (2019) 60:525–34. doi: 10.3349/ymj.2019.60.6.525
12. Kawamura T, Kenmotsu H, Taira T, Omori S, Nakashima K, Wakuda K, et al. Rebiopsy for patients with non-small-cell lung cancer after epidermal growth factor receptor-tyrosine kinase inhibitor failure. Cancer Science. (2016) 107:1001–5. doi: 10.1111/cas.12963
13. Sueoka-Aragane N, Katakami N, Satouchi M, Yokota S, Aoe K, Iwanaga K, et al. Monitoring EGFR T790M with plasma DNA from lung cancer patients in a prospective observational study. Cancer Science. (2016) 107:162–7. doi: 10.1111/cas.12847
14. Hsu PC, Chang JWC, Chang CF, Huang CY, Yang CT, Kuo CHS, et al. Sequential treatment in advanced non-small cell lung cancer harboring EGFR mutations. Ther Adv Respir Dis. (2022) 16:17534666221132731. doi: 10.1177/17534666221132731
15. Wei B, Zhao CZ, Li J, Zhao JZ, Ren PF, Yang K, et al. Combined plasma and tissue genotyping of EGFR T790M benefits NSCLC patients: a real-world clinical example. Mol Oncol. (2019) 13:1226–34. doi: 10.1002/1878-0261.12481
16. Tsai EB, Pomykala K, Ruchalski K, Genshaft S, Abtin F, Gutierrez A, et al. Feasibility and safety of intrathoracic biopsy and repeat biopsy for evaluation of programmed cell death ligand–1 expression for immunotherapy in non–small cell lung cancer. Radiology. (2018) 287:326–32. doi: 10.1148/radiol.2017170347
17. Yoon SH, Park CM, Lee KH, Lim KY, Suh YJ, Im DJ, et al. Analysis of complications of percutaneous transthoracic needle biopsy using CT-guidance modalities in a multicenter cohort of 10568 biopsies. Korean J Radiol. (2019) 20(2):323–31. doi: 10.3348/kjr.2018.0064
18. Yoon HJ, Lee HY, Lee KS, Choi YL, Ahn MJ, Park K, et al. Repeat biopsy for mutational analysis of non-small cell lung cancers resistant to previous chemotherapy: adequacy and complications. Radiology. (2012) 265:939–48. doi: 10.1148/radiol.12112613
19. Kirita K, Izumo T, Matsumoto Y, Hiraishi Y, Tsuchida T. Bronchoscopic re-biopsy for mutational analysis of non-small cell lung cancer. Lung. (2016) 194:371–8. doi: 10.1007/s00408-016-9864-5
20. Loi M, Franceschini D, Dominici L, Franzese C, Chiola I, Comito T, et al. Stereotactic radiotherapy for ultra-central lung oligometastases in non-small-cell lung cancer. Cancers. (2020) 12:885. doi: 10.3390/cancers12040885
21. Kim J, Kang HJ, Moon SH, Lee JM, Kim HY, Lee GK, et al. Endobronchial ultrasound-guided transbronchial needle aspiration for re-biopsy in previously treated lung cancer. Cancer Res Treat. (2019) 51:1488–99. doi: 10.4143/crt.2019.031
22. Tai R, Dunne RM, Trotman-Dickenson B, Jacobson FL, Madan R, Kumamaru KK, et al. Frequency and severity of pulmonary hemorrhage in patients undergoing percutaneous CT-guided transthoracic lung biopsy: single-institution experience of 1175 cases. Radiology. (2016) 279:287–96. doi: 10.1148/radiol.2015150381
23. Zhu JB, Qu YM, Wang XL, Jiang CX, Mo JH, Xi JD, et al. Risk factors associated with pulmonary hemorrhage and hemoptysis following percutaneous CT-guided transthoracic lung core needle biopsy: a retrospective study of 1,090 cases. Quant Imag Med Surg. (2020) 10:1008–20. doi: 10.21037/qims-19-1024
24. Heerink WJ, de Bock GH, de Jonge GJ, Groen HJM, Vliegenthart R, Oudkerk M. Complication rates of CT-guided transthoracic lung biopsy: meta-analysis. Eur Radiology. (2017) 27:138–48. doi: 10.1007/s00330-016-4357-8
25. Wiener RS, Schwartz LM, Woloshin S, Welch HG. Population-based risk for complications after transthoracic needle lung biopsy of a pulmonary nodule: an analysis of discharge records. Ann Intern Med. (2011) 155:137–+. doi: 10.7326/0003-4819-155-3-201108020-00003
26. Rong ES, Hirschl DA, Zalta B, Shmukler A, Krausz S, Levsky JM, et al. A retrospective multi-site academic center analysis of pneumothorax and associated risk factors after CT-guided percutaneous lung biopsy. Lung. (2021) 199:299–305. doi: 10.1007/s00408-021-00445-7
27. Guo Z, Shi H, Li WT, Lin DM, Wang CL, Liu C, et al. Chinese multidisciplinary expert consensus: Guidelines on percutaneous transthoracic needle biopsy. Thorac Cancer. (2018) 9:1530–43. doi: 10.1111/1759-7714.12849
28. Fintelmann FJ, Troschel FM, Kuklinski MW, McDermott S, Petranovic M, Digumarthy SR, et al. Safety and success of repeat lung needle biopsies in patients with epidermal growth factor receptor-mutant lung cancer. Oncologist. (2019) 24:1570–6. doi: 10.1634/theoncologist.2019-0158
29. Tokaca N, Barth S, O'Brien M, Bhosle J, Fotiadis N, Wotherspoon A, et al. Molecular adequacy of image-guided rebiopsies for molecular retesting in advanced non-small cell lung cancer: A single-center experience. J Thorac Oncol. (2018) 13:63–72. doi: 10.1016/j.jtho.2017.09.1958
30. Nam BD, Yoon SH, Hong H, Hwang JH, Goo JM, Park S. Tissue adequacy and safety of percutaneous transthoracic needle biopsy for molecular analysis in non-small cell lung cancer: A systematic review and meta-analysis. Korean J Radiol. (2021) 22:2082–93. doi: 10.3348/kjr.2021.0244
31. Gào X, Xuan Y, Benner A, Anusruti A, Brenner H, Schöttker B. Nitric oxide metabolites and lung cancer incidence: A matched case-control study nested in the ESTHER cohort. Oxid Med Cell Longev. (2019) 2019:6470950. doi: 10.1155/2019/6470950
32. Ohmori T, Yamaoka T, Ando K, Kusumoto S, Kishino Y, Manabe R, et al. Molecular and clinical features of EGFR-TKI-associated lung injury. Int J Mol Sci. (2021) 22:792. doi: 10.3390/ijms22020792
33. Hirashima T, Satouchi M, Hida T, Nishio M, Kato T, Sakai H, et al. Osimertinib for Japanese patients with T790M-positive advanced non-small-cell lung cancer: A pooled subgroup analysis. Cancer Science. (2019) 110:2884–93. doi: 10.1111/cas.14120
34. Otto S, Mensel B, Friedrich N, Schäfer S, Mahlke C, von Bernstorff W, et al. Predictors of technical success and rate of complications of image-guided percutaneous transthoracic lung needle biopsy of pulmonary tumors. PloS One. (2015) 10:e0124947. doi: 10.1371/journal.pone.0124947
35. Zhao G, Shi XB, Sun W, Liang HY, Mao XN, Wen F, et al. Factors affecting the accuracy and safety of computed tomography-guided biopsy of intrapulmonary solitary nodules ≤30 mm in a retrospective study of 155 patients. Exp Ther Med. (2017) 13:1986–92. doi: 10.3892/etm.2017.4179
36. Moreland A, Novogrodsky E, Brody L, Durack J, Erinjeri J, Getrajdman G, et al. Pneumothorax with prolonged chest tube requirement after CT-guided percutaneous lung biopsy: incidence and risk factors. Eur Radiology. (2016) 26:3483–91. doi: 10.1007/s00330-015-4200-7
37. Khan MF, Straub R, Moghaddam SR, Maataoui A, Gurung J, Wagner TOF, et al. Variables affecting the risk of pneumothorax and intrapulmonal hemorrhage in CT-guided transthoracic biopsy. Eur Radiology. (2008) 18:1356–63. doi: 10.1007/s00330-008-0893-1
38. Kuban JD, Tam AL, Huang SY, Ensor JE, Philip AS, Chen GJ, et al. The effect of needle gauge on the risk of pneumothorax and chest tube placement after percutaneous computed tomographic (CT)-guided lung biopsy. Cardiovasc Inter Rad. (2015) 38:1595–602. doi: 10.1007/s00270-015-1097-0
39. Wu CC, Maher MM, Shepard JAO. Complications of CT-guided percutaneous needle biopsy of the chest: prevention and management. Am J Roentgenol. (2011) 196:W678–W82. doi: 10.2214/Ajr.10.4659
40. Beslic S, Zukic F, Milisic S. Percutaneous transthoracic CT guided biopsies of lung lesions; fine needle aspiration biopsy versus core biopsy. Radiol Oncol. (2012) 46:19–22. doi: 10.2478/v10019-012-0004-4
41. Hong D, Zhang G, Zhang X. Pulmonary toxicities of gefitinib in patients with advanced non-small-cell lung cancer: A meta-analysis of randomized controlled trials. Medicine. (2016) 95(9):e3008. doi: 10.1097/MD.0000000000003008
42. Shi L, Tang JF, Tong L, Liu Z. Risk of interstitial lung disease with gefitinib and erlotinib in advanced non-small cell lung cancer: A systematic review and meta-analysis of clinical trials. Lung Cancer. (2014) 83:231–9. doi: 10.1016/j.lungcan.2013.11.016
Keywords: non-small cell lung cancer, rebiopsy, computed tomography-guided, tyrosine kinase inhibitors, acquired drug resistance, chemotherapy
Citation: Wang Y, Zhang Y, Ren N, Li F, Lu L, Zhao X, Zhou Z, Gao M and Wang M (2024) Repeat biopsy versus initial biopsy in terms of complication risk factors and clinical outcomes for patients with non-small cell lung cancer: a comparative study of 113 CT-guided needle biopsy of lung lesions. Front. Oncol. 14:1367603. doi: 10.3389/fonc.2024.1367603
Received: 10 January 2024; Accepted: 17 April 2024;
Published: 13 May 2024.
Edited by:
Francesco Pepe, University of Naples Federico II, ItalyReviewed by:
He Chuang, Army Medical University, ChinaHsiao-Chi Chuang, Taipei Medical University, Taiwan
Copyright © 2024 Wang, Zhang, Ren, Li, Lu, Zhao, Zhou, Gao and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhigang Zhou, aG56emdAMTI2LmNvbQ==
 Yangyang Wang
Yangyang Wang Yongyuan Zhang1
Yongyuan Zhang1