- 1The Second Hospital of Lanzhou University, Lanzhou, China
- 2The Second General Surgery Department, The Second Hospital of Lanzhou University, Lanzhou, China
- 3The Radiotherapy Department, The Second Hospital of Lanzhou University, Lanzhou, China
Hepatocellular carcinoma (HCC) is the most common primary malignant liver tumor and one of the leading causes of cancer-related deaths worldwide. The Wnt/β-Catenin signaling pathway is a highly conserved pathway involved in several biological processes, including the improper regulation that leads to the tumorigenesis and progression of cancer. New studies have found that abnormal activation of the Wnt/β-Catenin signaling pathway is a major cause of HCC tumorigenesis, progression, and resistance to therapy. New perspectives and approaches to treating HCC will arise from understanding this pathway. This article offers a thorough analysis of the Wnt/β-Catenin signaling pathway’s function and its therapeutic implications in HCC.
1 Introduction
Hepatocellular carcinoma (HCC) is the most prevalent primary malignant tumor of the liver and accounts for over 850,000 deaths from cancer-related causes globally each year (1, 2). In recent years, its morbidity and mortality have gradually increased (3). Despite significant advancements in HCC research, the prognosis of HCC is still terrible because the early symptoms of patients are not obvious and the disease has often progressed to the advanced stage when diagnosed (4). Radical hepatectomy is currently the first-line treatment for patients with HCC, but it is limited to patients with early-stage HCC who have good liver function, small masses, and no vascular invasion. However, due to its extremely high postoperative recurrence and metastasis rates, the 5-year survival rate of patients is only 19% (5). Liver transplantation offers another potential therapeutic option for patients with HCC, as it can remove the tumor while preventing the occurrence of postoperative liver stiffness in patients. However, when performing liver transplantation, it is extremely important to evaluate the patient’s stage, and the presence of extrahepatic metastases or vascular infiltration may lead to treatment failure. Moreover, due to the shortage of organ donors and the complexity of the procedure, its clinical implementation remains difficult. In addition, for some patients with intermediate to advanced HCC, chemotherapy offers a new option. First-line chemotherapy regimens based on sorafenib and lenvatinib are currently the treatment of choice for patients with advanced HCC, but the survival of most patients does not improve due to the resistance of HCC to chemotherapy and the development of cirrhosis in patients in the later stages of treatment. Furthermore, considering the complexity of HCC pathogenesis, targeted therapies may provide a more effective treatment option for HCC patients, which can specifically target key signaling pathways dysregulated during HCC pathogenesis through small molecules or monoclonal antibodies to achieve the goal of inhibiting tumor cell growth and inducing apoptosis. However, despite clinical trials of most drugs, there are still fewer targeted drugs approved for clinical use in HCC (6). Therefore, it is crucial to investigate the regulatory mechanisms behind the pathogenic process of HCC to identify novel biomarkers and therapeutic targets for the early diagnosis and treatment of HCC patients.
A highly conserved signaling pathway, the Wnt/β-Catenin signaling pathway, is also referred to as the typical Wnt signaling pathway. It is essential for liver development, metabolic zonation, and regeneration (7). It has been reported that abnormal activation of this pathway is a major carcinogen in liver cancer, and gene mutations encoding components of the pathway have been found in more than 80% of liver cancer patients (8). Mutations in the CTNNB1 gene, which encodes β-catenin, an essential constituent of this system, have been identified in various types of tumors, with HCC exhibiting the greatest frequency of such mutations (9). Recent studies have demonstrated that aberrant activation of the Wnt/β-Catenin signaling pathway is a significant contributor to the tumorigenesis, progression, and therapy resistance of HCC. Inhibiting this pathway holds promise as a hopeful therapeutic approach for HCC (10). In this review, we discuss the physiological role of the Wnt/β-Catenin signaling pathway in human liver and its mechanism in promoting the tumorigenesis, progression, and therapy resistance of HCC. In addition, we also explored the potential significance of this pathway in targeted therapy of HCC. In order to better understand the mechanism of action of this pathway in HCC and provide new directions for HCC-targeted treatment.
2 Overview of Wnt signaling pathway
The Wnt signaling pathway is an important signaling pathway for maintaining homeostasis from embryonic development to adulthood and plays a vital role in numerous biological processes (11). Typically, the canonical and non-canonical pathways make up the two kinds of Wnt pathways. Among them, the Wnt/β-Catenin signaling pathway is the canonical pathway, while the non-canonical pathway mainly includes the Wnt/PCP (Planar cell polarity) and Wnt/Ca2+ signaling pathways (12, 13).
2.1 Canonical Wnt/β-Catenin signaling pathway
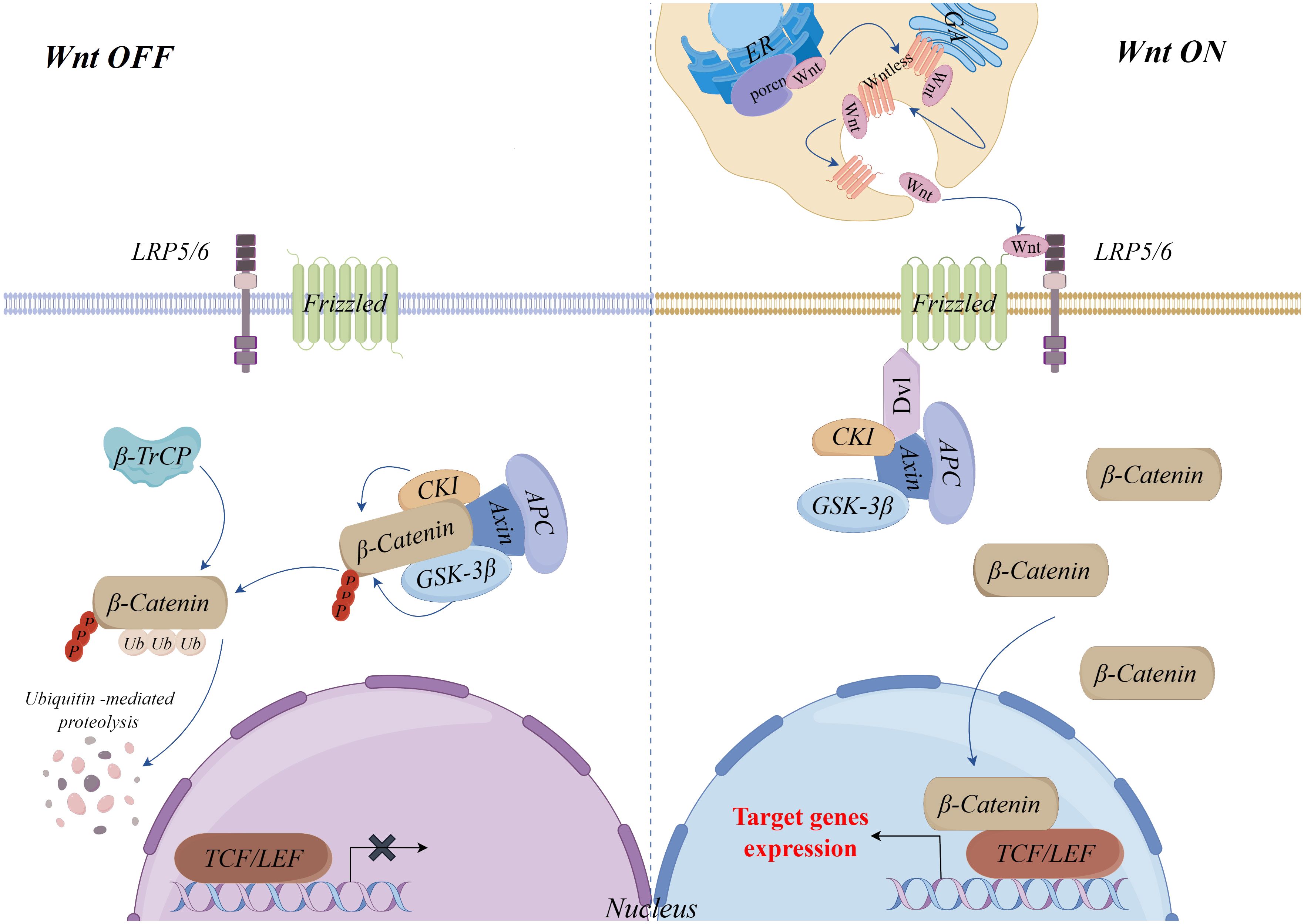
As an important transcription factor in the Wnt/β-Catenin signaling pathway, stabilization and nuclear translocation of β-Catenin mediated by Wnt ligands is a key mechanism of this pathway (14, 15). Without Wnt ligands, β-Catenin is phosphorylated and degraded by a complex consisting of Axin, Glycogen synthase kinase 3β (GSK-3β), Casein kinase I (CKI) and Adenomatous polyposis (APC). In this process, Axin serves as a scaffold protein, with CKI and GSK-3β sequentially phosphorylating β-Catenin, and APC-mediated recognition and eventual degradation of phosphorylated β-Catenin by the E3 ubiquitin ligase β-TrCP, which maintains the stability of intracellular cytoplasmic levels of β-Catenin and prevents the expression of target genes of the pathway (16–20).
Wnt ligands (Wnts) are secreted glycoproteins and there are 19 known Wnts (21). Before secretion, Wnts must be glycosylated and palmitoylated by the endoplasmic reticulum’s Porcupine O-acyltransferase (PORCN), a process that is essential for Wnts secretion and function (22). Additionally, the secretion of Wnts requires a carrier protein, Wntless, a multi-pass transmembrane protein. Wnts need to bind Wntless and are transported to the cell membrane for secretion via the Golgi vesicular system (23, 24). Once secreted, Wnts bind to the transmembrane Frizzled (FZD) receptors and coreceptors low-density lipoprotein receptor-related proteins 5/6 (LRP5/6), recruiting cytoplasmic Disheveled (Dvl) proteins, leading to the phosphorylation of LRP5/6 and attract the Axin complex to the plasma membrane. Through this mechanism, the Axin complex is unable to phosphorylate and destroy β-Catenin, which allows it to build up in the cytoplasm and then go into the nucleus. There, it binds to T-cell factor (TCF)/lymphoid enhancer factor (LEF) family transcription factors and coactivators, initiating the transcriptional expression of downstream target genes (25–27) (Figure 1).
2.2 Non−canonical Wnt signaling pathway
In addition to the canonical Wnt/β-Catenin signaling pathway, non-canonical Wnt pathways also play an important role, including Wnt/PCP and Wnt/Ca2+ signaling pathways. Studies have shown that non-canonical Wnt pathways are not only involved in the regulation of various cellular functions but also may be involved in the occurrence and progression of cancer when activated or inhibited abnormally (28). In the Wnt/PCP signaling pathway, Wnts bind to FZD receptors on the cell surface and further activate Jun N-terminal kinases (JNKs) by recruiting Dvl to activate the RAC and its downstream mitogen-activating protein 3 kinases (MAP3Ks) and mitogen-activated protein 2 kinases (MAP2Ks). In addition, Dvl can also activate its downstream Ras homolog gene-family members A (RhoA) and Rho-associated kinases (ROCK) through the Dvl-associated activator of morphogenesis 1 (Daam1), where RhoA can also activate JNK. This process regulates the cytoskeleton and initiates the expression of downstream target genes to facilitate cell motility (29, 30).
In the Wnt/Ca2+ signaling pathway, the interaction of Wnts with the FZD receptor leads to the activation of homotrimeric G proteins, which in turn activate phospholipase C (PLC). Activation of PLC leads to an increase in intracellular Ca2+, which inhibits the expression of cyclic guanosine monophosphate (cGMP) and promotes the activation of calmodulin-dependent protein kinase II (CaMKII) or calcineurin (CalN) and protein kinase C (PKC). Subsequently, cAMP response element binding protein (CREB) and Nuclear factor kappa-B (NF-κB) are activated by CaMKII and PKC, and the Nuclear factor of activated T cells (NFAT) in the cytoplasm is activated and dephosphorylated by CalN. Finally, CREB, NF-κB, and NFAT translocated into the nucleus and initiated the expression of target genes downstream of the Wnt/Ca2+ signaling pathway (13, 31, 32) (Figure 2).
3 Physiological role of the Wnt/β-catenin signaling pathway in the liver
3.1 Liver development
During different stages of embryonic development, the Wnt/β-catenin signaling pathway does not always promote liver development. In the gastrula and early somite formation stages, the endoderm along the anterior-posterior axis is divided into foregut, midgut, and hindgut, with the liver ultimately originating from the foregut (33). Homeobox (HHEX) gene, one of the earliest foregut markers, can induce the production of downstream transcription factors HHEX and Forkhead Box A2 (FOXA2), promoting foregut fate and future liver development (34). The Wnt/β-catenin signaling pathway is indispensable in the initial development of the hindgut. Wnts, Fibroblast Growth Factor 4 (FGF4), and Bone Morphogenetic Proteins (BMP) from the adjacent midgut endoderm promote hindgut development while also inhibiting foregut development by suppressing HHEX and FOXA2 expression (35, 36). Thus, there is a Wnt inhibitor, secreted FZD-related protein 5 (SFRP5), in the foregut endoderm, which inhibits this pathway conductance, thereby maintaining foregut features and promoting liver development (37, 38).
The activation of the Wnt/β-catenin signaling pathway is as crucial for liver specification in later development as is the repression of this pathway during early foregut development. Liver specification occurs around embryonic day 8.5, triggered by BMP signaling from mesenchymal cells of the septum transversum and FGF from the cardiac mesoderm, under the influence of transcription factors Hepatocyte Nuclear Factor-1β (HNF-1β), Forkhead box A1 (FOXA1), FOXA2, and GATA Binding Protein 4 (GATA4) (23, 39, 40). Research indicates that Wnt2b is essential for the induction of liver specialization, with reduced expression of liver-specific genes HHEX and Prospero homeobox 1 (PROX1), as well as transient deletion of liver specialization, observed in zebrafish embryos lacking Wnt2bb (Wnt2b homolog) (41). Furthermore, as liver specification occurs, liver buds consisting of hepatoblasts begin to develop, accompanied by hepatoblast proliferation (42). Wnts, FGF, and hepatocyte growth factor (HGF) have been shown to be involved in this process, which can promote the proliferation of hepatoblast by activating this pathway (43–45). Additionally, this pathway is equally significant in postnatal liver growth. It has been shown that mice with hepatic β-catenin-specific deletion or suppressed Wnt/β-catenin signaling show a notable reduction in liver weight relative to body weight (46, 47). The opposite was true in liver-specific non-mutated β-catenin-overexpressing transgenic mice (48).
3.2 Metabolic zonation of liver
As the smallest structural unit in the liver, hepatic lobules can be divided into three zones based on the different metabolic functions of hepatocytes at different locations in the liver lobules: the hepatocytes adjacent to the portal vein triad constitute Zone 1, the hepatocytes adjacent to the central vein constitute Zone 3, and the hepatocytes in between constitute Zone 2 (49).
Benhamouche and colleagues were the first to report the significant function of the Wnt/β-catenin signaling pathway in guiding liver metabolic zonation and proposed the concept of APC as the “zonal guardian” gene of the liver. Their research indicated high expression of APC in periportal areas without β-catenin activation, while an absence of APC expression was found in pericentral areas with β-catenin activation (50). This elucidates the distinct manifestation of this pathway targets in the pericentral areas, including Axin2, GS (Glutamine synthetase), and cytochrome P450 enzymes (CYP2E1 and CYP1A2). Inhibition of this pathway may lead to impairment of metabolic zonation of the liver. Recent studies have found that either liver-specific LRP5/6 deletion or LRP4/5 deletion results in loss of liver metabolic regions (47, 51). Additionally, mice with liver-specific β-catenin deletion also have liver metabolic zonation disorders and cause the liver to exhibit a periportal phenotype overall (52). Whereas β-catenin accumulation caused by APC-specific inactivation exhibits an overall pericentral phenotype (50). These results indicate that the Wnt/β-catenin signaling pathway is important for liver metabolic zonation and has distinct effects on the expression of genetic programs in the periportal and pericentral regions.
3.3 Liver regeneration
Liver regeneration is crucial for maintaining liver homeostasis and restoring the size and function of the damaged liver. Hepatocytes, as the main contributors to liver regeneration, mediate this process through their proliferation (53–57). Monga and colleagues found that in a rat model subjected to partial hepatectomy (PHx), β-catenin increased quickly in the first five minutes and subsequently moved into the nucleus (58). Similarly, Apet and colleagues found that β-catenin dramatically rose 1 to 6 hours after acetaminophen injection in mice with acute liver failure produced by the drug, and then increased again 24 hours later. Moreover, the expression of Wnt/β-catenin signaling pathway targets GS and cyclin-D1 (Cyclin-D1) also increased during these periods (59). These findings imply that liver regeneration may be significantly aided by this pathway.
Subsequent experiments further confirmed the importance of this pathway in liver regeneration. The study found that after partial hepatectomy (PHx) in a β-catenin-specific knockout mouse model, the liver cell proliferation of mice in the experimental group was lower than that of the control group, and the liver weight/body weight ratio was significantly lower than that of the control group (60). This finding aligns with results from two other studies (46, 61). In contrast, a transgenic mouse model overexpressing β-catenin showed a significant increase in hepatocyte proliferation after receiving PHx. In addition, compared with the control group, exogenous activation of the Wnt/β-catenin signaling pathway in mice by the Wnt-1 gene can also increase the proliferation of liver cells (62). Furthermore, recent studies have shown that macrophages can regulate this pathway, upregulating important metabolic functions of non-proliferating hepatocytes in the compensatory phase of liver regeneration following acute liver injury, thereby preserving fundamental physiological functions of the liver (63). The above studies show that the Wnt/β-catenin signaling pathway is essential for the regeneration of the liver. Moreover, exogenous modification aimed at stimulating this pathway could potentially serve as a therapeutic agent to promote liver regeneration.
4 The role of the Wnt/β-Catenin signaling pathway in the tumorigenesis and progression of HCC
4.1 Wnt/β-Catenin signaling pathway and HCC tumorigenesis
Aberrant activation of the Wnt/β-catenin signaling pathway is one of the main driving factors in the tumorigenesis of HCC and is widely present in HCC patients, with their relationship being well established. Mutations in the CTNNB1 gene encoding β-catenin in Exon3 are the most common activation mechanism of this pathway (64). Research indicates that CTNNB1 gene mutations are present in about 20%-40% of HCC cases, and this mutation occurs more frequently in HCC cases related to Hepatitis C virus (HCV) infection than in HCC cases related to Hepatitis B virus (HBV) infection (65, 66). Second, loss of function or mutation of Axin, GSK-3β, and APC as members of the Axin complex can likewise activate the pathway and exert oncogenic effects. Axin1 mutations have been reported to account for approximately 3%-16% of all HCC cases, and Axin2 for approximately 3% (67). Additionally, studies have revealed that the proportions of phosphorylated GSK-3β and overexpressed β-catenin in HCC tissues are 52.2% and 56.5%, respectively, higher than in surrounding normal tissues. It is crucial to note that none of the HCC patients who exhibited phosphorylated GSK-3β possessed CTNNB1 gene mutations (68). Consistently, APC serves as one of the members of the Axin complex, and targeted inactivation of the hepatic APC gene similarly leads to overexpression of β-catenin and promotes HCC tumorigenesis (69). However, it’s noteworthy that in 40-60% of HCC cases, there are no mutations in CTNNB1, Axin1, or Axin2 (65). Based on this, another variation that controls the pathway was found in a recent genome-wide association study (GWAS) that specifically targeted alcohol-related HCC: the WNT3A-WNT9A gene variants are specifically linked to the occurrence of HCC in alcoholic liver disease patients (70, 71).
In the Wnt/β-catenin signaling pathway, 19 known Wnts that function by binding to one or more of the 10 types of FZD receptors (72). Secreted frizzled-related proteins (SFRPs) are antagonists of this pathway that bind Wnts, downregulate their ability to bind and activate FZD receptors, and inhibit this pathway (73). Studies have shown that in contrast to a normal liver, there is an upregulation of FZD3/6/7 and Wnt3/4/5a expression in 95% of HCC and 68% of the surrounding tumor tissues, along with downregulation of sFRP1/5, which accumulates progressively with tumor advancement and the severity of fibrosis in surrounding tissues (72). Among them, Wnt3 and FZD7 have been shown to activate this pathway through their interaction (74). Additionally, other studies have found that methylation of SFRP family genes is not only widely present in HCC tissues, but also in HBV- or HCV-related chronic hepatitis and cirrhosis tissues. It can lead to the down-regulation of SFRPs expression, which activates this pathway and may participate in promoting the occurrence of HCC. These events are considered early events in the tumorigenesis of HCC (75, 76).
In addition to the aforementioned mechanisms, activation of the Wnt/β-catenin signaling pathway involves various other mechanisms. It has been shown that TGF-β-dependent activation, and Receptor tyrosine kinase (RTK) activation in fibrotic laminar HCC are involved in this process (77, 78). However, the mechanisms of these two types of activation remain unclear. Epidermal Growth Factor (EGFR), a type of RTK, has been reported to be transcriptionally upregulated or aberrantly expressed in multiple cancers. Studies indicate that EGFR participates in regulating TCF-dependent β-catenin transcriptional activity in HCC through kinase-independent mechanisms, thereby participating in the regulation of the activity of the pathway (79). R-spondins (RSPOs) are secreted regulators of Wnt signaling and can enhance Wnt signaling. The RSPO2 gene encoding RSPOs has been confirmed to be an oncogene in colorectal cancer. Recent research has shown that RSPO2 is highly expressed in the CTNNB1 mutation subtype of HCC and can drive liver tumorigenesis by stimulating the activation of this pathway (80). Additionally, research indicates that various risk factors associated with HCC, including chronic HBV (81–85)or HCV (86–88) infection, Alcohol abuse (89, 90), Non-Alcoholic Fatty Liver Disease (NAFLD) (91, 92), and Aflatoxins Exposure (93, 94), can promote abnormal activation of this pathway through multiple mechanisms. This enhancement in the proliferation of affected hepatocytes and the overgrowth of adjacent normal hepatocytes can facilitate the progression of HCC precancerous lesions to HCC due to these factors.
4.2 Wnt/β-Catenin signaling pathway and HCC progression
4.2.1 Cancer stem cells
Cancer stem cells (CSCs), also known as tumor-initiating cells (TICs), possess self-renewal and differentiation abilities comparable to normal stem cells, and are pivotal in the initiation, recurrence, and metastasis of tumors (95). The Wnt/β-catenin signaling pathway is one of the important ways to maintain the stemness of CSCs/TICs (96). Long non-coding RNAs (LncRNAs), an emerging regulatory factor, have been implicated in the development of cancer (97). The study found that LncTCF7 (98), Lnc-β-Catm (99), LncAPC (100), LncFZD6 (101), and LncTIC1 (102) exhibit high expression in HCC cells and liver CSCs/TICs. They contribute to the promotion of hepatic CSCs/TICs self-renewal by activating the Wnt/β-catenin signaling pathway. Additionally, several microRNAs (miRNAs), including miRNA-1246 (103), miRNA-5188 (104), miRNA-452 (105), miRNA-217 (106), and miRNA-HCC2 (107), also contribute to hepatic CSC stemness through activating this pathway. Furthermore, a functional read-through rt-circRNA named rtcisE2 was found to be highly expressed in liver TICs. It can also activate this pathway and promote the self-renewal of liver TICs, initiating the occurrence and metastasis of liver tumors (108).
Research indicates that Protein tyrosine kinase 2 (PTK2) stimulates the accumulation of β-catenin in the nucleus of HCC cells, thereby increasing Wnt/β-catenin signaling pathway activity, and in this way promoting stemness of CSCs and enhancing the tumorigenicity of HCC cells (109). Additionally, Sirtuin1 or Silent mating–type information regulation 2 homolog-1 (SIRT1) has likewise been shown to promote the activity of this pathway in hepatic CSCs by maintaining the stability of β-catenin, and in this way promotes CSC self-renewal (110). In fact, Mitogen-activated protein kinase 1 (MAPK1/MEK1) was previously found to promote the proliferation and self-renewal of hepatic stem cells by maintaining the stability of the SIRT1 protein, but that study did not investigate the mechanisms involved (111). Furthermore, FZD10 was shown to be substantially expressed in liver cancer CSCs by recent research. The pathway can be activated by its overexpression, which encourages the stemness of liver cancer CSCs and might be a new prognostic biomarker for HCC (112).
Tumor-associated macrophages (TAMs) are one of the primary subtypes of tumor-infiltrating immune cells, usually classified into M1 and M2 macrophages. The former generally plays an anti-tumor role, while the latter promotes tumor occurrence, metastasis, and angiogenesis through cytokine secretion, leading to tumor progression (113). Recent research found that M2 macrophages can secrete Tumor necrosis factor-α (TNF-α) and promote Epithelial-mesenchymal transition (EMT) of HCC and CSC stemness by inducing the Wnt/β-catenin signaling pathway (114). Additionally, Reactive oxygen species (ROS) overproduction has been reported to inhibit this pathway in HCC and reduce liver CSC stemness (115, 116). Glutaminase 1 (GLS1) is highly expressed in HCC, and GLS1 overexpression has been found to decrease ROS levels, reduce the inhibitory effect of ROS on the pathway and enhance CSC stemness (117). Furthermore, recent studies have discovered that Secretory clusterin (sCLU) may promote CSC stemness by activating the AKT/GSK3β/β-catenin axis (118). Moreover, this pathway can be activated by Ring finger protein 1 (Ring1), which is highly expressed in HCC, and in this way contributes to promoting the transformation of Hepatic progenitor cells (HPC) into CSC (119). In summary, this pathway has a crucial role in regulating CSC stemness, and more studies will be conducted in the future to reveal the mechanisms involved in this phenomenon.
4.2.2 Proliferation, invasion, and metastasis of HCC
HCC cells exhibit strong capabilities in proliferation, invasion, and metastasis, which are important factors leading to poor prognosis of HCC patients (120). Numerous studies have shown that the Wnt/β-catenin signaling pathway plays an important role in regulating HCC cell proliferation, invasion and metastasis. Cripto-1 was found to be highly expressed in about 50% of HCC tissues (121). It can bind to the FZD7/LRP6 receptor and DVL3 and stabilize the expression of DVL3. In this way, it activates this pathway, which promotes the proliferation, invasion, and metastasis of HCC cells (122). Furthermore, the Tripartite motif (TRIM) protein family is reported to have extensive functions in tumor development, cell proliferation, and differentiation, although its effects vary across different tumors. Recent research has found that TRIM29 is downregulated in HCC tissue, potentially enhancing the activity of this pathway to promote the proliferation, invasion, and metastasis of HCC cells. Conversely, overexpression of TRIM29 inhibited this effect (123). However, this result is in stark contrast to previous studies, a discrepancy often attributable to genetic polymorphism and tumor complexity. Additionally, TRIM66 expression is upregulated in HCC compared to TRIM29. It can similarly activate this pathway and have the same effect on HCC cells (124). Similarly, overexpression of Ataxia telangiectasia group D complementing (ATDC) and Spindle and kinetochore-associated protein 2 (SKA2) in HCC similarly promotes HCC cell proliferation and invasion by activating this pathway (125, 126). Among the identified Wnts, Wnt7b has the ability to suppress Axin complex activity, stop β-catenin phosphorylation from being degraded, and facilitate its nuclear translocation, all of which contribute to the activation of the Wnt/β-catenin signaling pathway. It was found that TCP1 (also known as CCT1 subunit), which is overexpressed in HCC, can act as an upstream mediator of Wnt7b and increase Wnt7b expression, thus activating this pathway and enhancing the proliferation and metastasis of HCC cells (127). Furthermore, two deubiquitinating enzymes, USP9X and USP28, have significant expression in HCC and could similarly promote HCC cell proliferation by regulating the activity of this pathway (128, 129).
P62/IMP2, an oncofetal protein, was initially reported as a tumor-associated antigen in HCC. Research has established that p62/IMP2 is overexpressed in HCC tissues and may improve the invasion and metastasis capabilities of HCC by stimulating the Wnt/β-catenin signaling pathway (130). Additionally, Phosphatidylinositol 4-phosphate adaptor protein 2 (FAPP2) has also been identified as a tumor-associated regulatory factor related to tumorigenesis. It is reported to be highly expressed in HCC and can promote the proliferation and invasion of HCC cells by stimulating this pathway (131). SPINDOC (SPIN1 docking protein) and KIF18B (Kinesin family member 18B) have also been shown to be overexpressed in HCC and can similarly play a role in promoting the proliferative, invasive, and metastatic capacities of HCC cells through activation of this pathway (132, 133). ETS variant 4 (ETV4) is overexpressed in patients with HBV-related HCC and can activate the pathway, which promotes the proliferation, invasion, and metastasis of HCC cells, leading to the progression of HBV-associated HCC (134). Moreover, matrix metalloproteinases (MMPs) have been proven to be associated with HCC metastasis. Studies indicate that the expression of FBXO17 in HCC tissues was significantly higher than that in paracancerous tissues. It can promote HCC metastasis by down-regulating GSK-3β to mediate the activation of this pathway and increase the expression level of its downstream effector molecules (including MMP-2 and MMP-9) (135).
In addition, an increasing number of studies show that LncRNAs and miRNAs are equally involved in regulating this process and contributing to HCC progression. Research has discovered that LncRNA-miR194-2HG (136), LncRNA-DAW (137), LncRNA-NRAV (138), LncRNA-DUXAP10 (139), LncRNA-CRNDE (140), LncRNA OTUD6B-AS1 (141), and miR-550a-5p (142) are overexpression in HCC tissue. They can activate the Wnt/β-catenin signaling pathway to promote the proliferation, invasion, and metastasis of HCC cells. The inhibition of these genes’ expression may represent a viable therapeutic target for HCC.
4.2.3 Epithelial-mesenchymal transition
EMT is a major factor in HCC cell invasion and metastasis and is induced by EMT-related transcription factor (EMT-TF). Elevated levels of vimentin and N-cadherin and decreased levels of E-cadherin are the main features of EMT (143). Several investigations have shown a robust connection between EMT and the Wnt/β-catenin signaling pathway. Recent studies have found Hepatic stellate cells (HSCs) can induce overexpression of miRNA-1246 in HCC, which can activate this pathway by suppressing the expression of its target gene, RORα, and in this way promote EMT (144). The trans-activation response DNA-binding protein of 43 kDa (TDP-43), a nuclear protein, is highly expressed in HCC tissues and activates the pathway by targeting inhibition of GSK3β expression to induce EMT (145). NFE2L3 (Nuclear factor erythroid 2-like 3) is a member of the CNC family of proteins and has been shown to be highly expressed in HCC. It also can induce EMT by activating this pathway (146). Furthermore, Rho guanine nucleotide exchange factor 11 (ARHGEF11), which is also overexpressed in HCC, can activate this pathway by increasing the nuclear translocation of β-catenin, thereby inducing EMT (147). Another study showed that the GBA1 protein, catalyzing the conversion of glucosylceramide (GlcCer) into ganglioside, is downregulated in HCC tissues and stimulates this signaling pathway by mediating GlcCer reprogramming, thus promoting EMT and enhancing the metastatic capability of HCC. Targeting the upregulation of GBA1 could be a potential therapeutic strategy against HCC metastasis in the future (148). Moreover, it has been reported that cysteine-rich protein 1 (CRP-1) is extensively expressed in various cancers, including HCC, and similarly induces EMT in a manner that activates this pathway (149).
4.2.4 Glycolysis and angiogenesis
The proliferation, invasion, and metastasis of HCC cells is a complex process and requires a large amount of energy consumption, with glycolysis and angiogenesis being the main sources of energy in this process (150). Autophagy, as a programmed cell death mechanism, research has found that it can promote metastasis and glycolysis of HCC by increasing the expression of Monocarboxylate transporter 1 (MCT1) and activating the Wnt/β-catenin signaling pathway (151). As previously discussed, ROS produced by mitochondrial aerobic respiration can inhibit HCC progression by suppressing this pathway. Neoplastic cells opt for anaerobic glycolysis as their energy source, even when oxygen is present; this is referred to as the “Warburg effect” (152). Activation of the Wnt/β-catenin signaling pathway has been reported to stimulate the Warburg effect by up-regulating pyruvate dehydrogenase kinase isozyme 1 (PDK1), which promotes glycolysis in HCC cells, thereby supplying HCC cells with energy and enhancing HCC cell proliferation, invasion, and metastasis. Peroxisome proliferator-activated receptor-gamma (PPARγ) co-activator-1α (PGC-1α), a tumor suppressor, participates in cancer pathogenesis, progression, and metabolism. Based on research findings, it has been observed that PGC-1α inhibits the PDK1 pathway, thereby decreasing PDK1 expression and subsequently impeding the metastasis of HCC (153). Thus, the downregulation of PGC-1α expression in HCC may promote the Warburg effect and energize HCC progression by stimulating this pathway activity. Additionally, a recent study discovered that Galectin-3 is involved in HCC metastasis and activates this pathway by inducing Phosphatidylinositol 3-kinase (PI3K)/Akt axis-mediated degradation of GSK-3β. Finally, the β-catenin/TCF4 transcriptional complex directly targets IGFBP3 and waveform proteins, thereby promoting angiogenesis and EMT in HCC (154).
4.2.5 Hypoxia
Hypoxia, a common feature of all solid tumors, results from an imbalance between oxygen supply and consumption in proliferative tumors. It is essential for the occurrence and progression of tumors (155). Like most solid tumors, HCC also exhibits a hypoxic microenvironment. Abundant evidence indicates that there is crosstalk between hypoxia-inducible factor hypoxia-inducible factor (HIF) and the Wnt/β-catenin signaling pathway and that it could contribute to the development of HCC (156–158). It has been found that hypoxia causes β-catenin to be expressed and accumulate in HCC cell lines, which facilitates invasion and metastasis (159). Further studies showed that crosstalk between hypoxia and this pathway is mediated through HIF-1α (160). However, the mechanisms involved remain unclear. BCL9, an essential co-activator of this pathway, is discovered to be overexpressed in HCC. It was found that hypoxia may cause BCL9 to be overexpressed in HCC via HIF-1α, activating this pathway and accelerating the growth, metastasis, and angiogenesis of HCC cells (156). Furthermore, this study provides evidence for the crosstalk between this pathway and the hypoxic and demonstrates that the specific regulation of BCL9 by HIF-1α may be a potential crosstalk mechanism between the two.
5 Role of Wnt/β catenin signaling pathway in HCC therapy resistance
Overcoming multidrug resistance (MDR) poses a substantial challenge in the management of hepatocellular carcinoma (HCC), among other malignancies, where it has emerged as a key obstacle. An important factor in MDR is the overexpression of ATP-binding cassette (ABC) transporters, which facilitates the expulsion of antitumor drugs from cells, thereby preventing their accumulation intracellularly and mitigating their cytotoxicity (161). It is reported that the Wnt/β-catenin signaling pathway can regulate tumor therapy resistance by modulating the expression of ABC transporters (162). FZD7 as an FZD receptor, ABC transporters (ABCB1, ABCC1, and ABCC2) can be upregulated in HCC cells by FZD7 overexpression via this pathway which results in increased therapy resistance in HCC. Quercetin can reverse this effect and enhance the drug sensitivity of HCC (163).
Additionally, a substantial amount of data indicates that this pathway is crucial in mediating chemoresistance in hepatocellular carcinoma. Gankyrin has been shown to be overexpressed in a variety of cancers. In HCC, it can activate this pathway to upregulate the expression of its target gene c-Myc, inducing metabolic reprogramming in HCC cells, and thus promoting HCC tumorigenesis, metastasis, and therapy resistance. Inhibiting c-Myc expression might be an optimal treatment strategy for HCC patients with high Gankyrin expression (164). As mentioned earlier, PROX1 is a specific gene in liver development. Studies have found that PROX1 is highly expressed in HCC, and it can activate this pathway by stimulating β-catenin transcription, promoting HCC cell proliferation and sorafenib resistance (165). Additionally, NIMA-related kinase 2 (Nek2) and LRP8 expression were found to be upregulated in HCC and contribute to sorafenib resistance in HCC by the same mechanism (166, 167). Recent studies have found that Src homolog and collagen homolog 3 (Shc3) are overexpressed in chemotherapy-resistant HCC and similarly activate this pathway, causing resistance to sorafenib and doxorubicin in HCC (168). Moreover, FZD10, found to be overexpressed in liver CSCs, activates this pathway to promote the resistance to Lenvatinib of HCC. Targeted knockdown of FZD10 can restore the sensitivity of lenvatinib-resistant HCC to lenvatinib (112). Furthermore, research has demonstrated that cisplatin-resistant HCC has substantially increased levels of miR-130a. Specifically, its overexpression inhibits the tumor suppressor gene RUNX3, which in turn activates this pathway and augments the HCC’s resistance to cisplatin. Knockdown of miR-130a can reverse the resistance of HCC to cisplatin (169). Additionally, the activation of this pathway is also facilitated by the overexpression of Krüppel-like factor 8 (KLF8) in HCC. This ultimately increases the chemoresistance of HCC to sorafenib and cisplatin. Compared to control HCC cells, the knockdown of KLF8 can significantly increase chemosensitivity (170). DVL1, an important component of the Wnt/β-catenin signaling pathway, stabilizes β-catenin activity and mediates Wnt signaling. DVL1 expression was found to be overexpressed in 5-FU-resistant HCC cells and may enhance HCC resistance to 5-FU by activating the Wnt/β-catenin signaling pathway (171).
Due to the high expression of various drug-resistant genes, HCC patients are often insensitive to chemotherapy. Consequently, for HCC patients who have lost the opportunity for surgery, radiation therapy has gradually become an important treatment method. However, some HCC patients still show resistance to radiation therapy. Mesenchymal stem cells (MSCs), as a crucial component of the tumor microenvironment, have been proven to participate in tumor therapy resistance (172). Research has found that Irradiated MSCs (IR-MSCs) can promote the maintenance of CSC stemness by activating the Wnt/β-catenin signaling pathway, leading to radiotherapy resistance in HCC (173). This suggests that the activation of this pathway under radiotherapy conditions might be responsible for this resistance.
In addition, with the continual advancements in HCC treatment modalities, immunotherapy has progressively emerged as a pivotal therapeutic approach. However, there is considerable variation in the response of HCC patients to immunotherapy. It is reported that cancer immune evasion and resistance to Immune checkpoint inhibitors (ICIs) are mediated by the Wnt/β-catenin signaling system (174). Research has found that in HCC, the activation of this pathway can compromise dendritic cell recruitment and reduce T cell activity, promoting immune evasion in HCC cells and inducing resistance to ICI drugs like PD-1 (Programmed cell death 1) (175). In syngeneic mouse models, using the chemically optimized RNAi trigger drug DCR-BCAT, targeting the CTNNB1 gene encoding β-catenin, it was found that DCR-BCAT could significantly increase T cell infiltration and enhance tumor sensitivity to ICIs (176). Additionally, a study using a biological nanoparticle delivery method delivered small interfering RNA (siRNA) targeting β-catenin directly into Extracellular vesicles (EVs), resulting in not only reduced growth of HCC cells but also enhanced responsiveness to PD-1 treatment (177). Combining these studies, it is possible to deduce that the Wnt/β-catenin signaling pathway significantly influences the immune evasion mechanism of HCC cells. Potentially, inhibiting this pathway could improve the efficacy of HCC immunotherapy.
6 Potential role of Wnt/β catenin signaling pathway in HCC targeted therapy
The incidence of HCC is increasing every year, with only a small proportion of patients eligible for surgical resection. Chemotherapy is the leading therapeutic approach for HCC patients who do not qualify for surgical resection. Significant advancements have been achieved in molecular targeted therapy in recent years, and the survival rate of HCC patients has been significantly increased through the combination of targeted therapy and chemotherapy. Given the important role of the Wnt/β-catenin signaling pathway in HCC tumorigenesis, progression, and therapy resistance, targeting this pathway may be a new potential therapeutic approach for HCC patients.
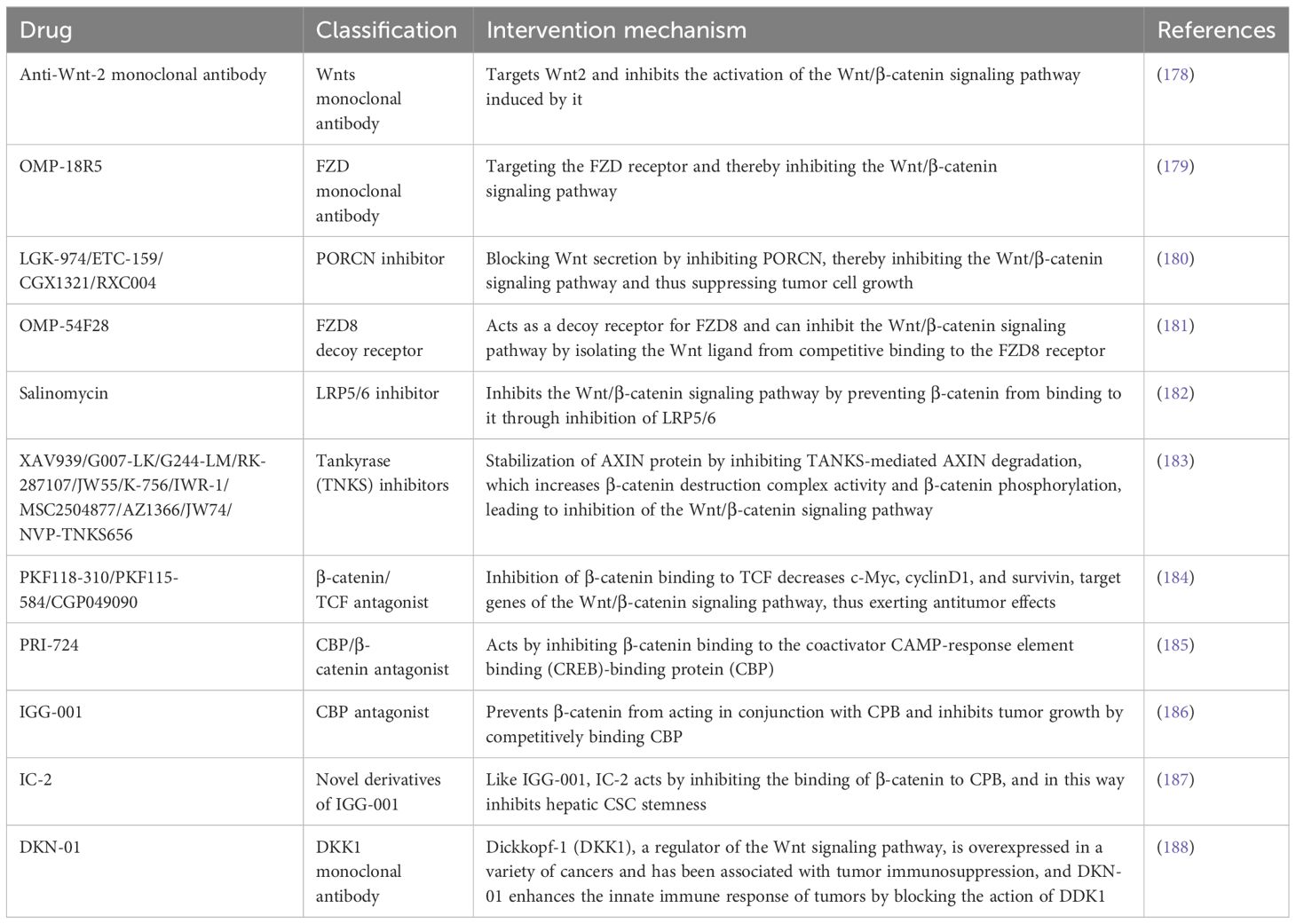
Numerous pharmaceuticals that inhibit this pathway have been developed as a result of years of research on this pathway. These drugs include monoclonal antibodies targeting Wnts and FZD receptors, molecules inhibiting their secretion and interaction, and small molecule inhibitors that stabilize the β-catenin destruction complex and block the binding of β-catenin to specific transcription co-activators (Table 1). Additionally, Glypican-3 (GPC3), a Heparan sulfate proteoglycan (HSPG) that is overexpressed in HCC, can recruit Wnts to the cell surface and stimulate cell proliferation. A monoclonal antibody targeting GPC3, HS20, has been reported, which can inhibit this pathway in HCC cells and exerts a potent antitumor effect by targeting GPC3 (189). Furthermore, several other drugs commonly used in clinical practice have been proven to have anti-Wnt/β-catenin signaling pathway activity, including indomethacin, pyrvinium, sulindac, aspirin, celecoxib, rofecoxib, peruvoside, and pirfenidone (183, 190, 191). However, whether these drugs have anti-tumor efficacy has not yet been determined in clinical settings.
Several inhibitors or modulators of this pathway are currently in clinical trials. CGX1321, a PORCN inhibitor, has been tested in phase I clinical trials in patients with HCC and Cholangiocarcinomas (CCA) (NCT03507998). OMP-18R5, a novel monoclonal antibody against FZD that can target the FZD receptor and thereby block the Wnt/β-catenin signaling pathway, has been evaluated for efficacy and safety in a clinical trial in relevant solid tumors (NCT01345201). In addition, OMP-54F28, an FZD8 decoy receptor, can competitively bind to Wnts to block this pathway, and its efficacy in combination with sorafenib was tested in patients with advanced HCC in a phase I clinical trial (NCT02069145). Dickkopf-1 (DKK1), a secreted regulator of the Wnt signaling pathway, is overexpressed in a variety of cancers and has been associated with tumor immunosuppression, and DKN-01 can act by blocking DKK1 and in so doing enhances the innate immune response of tumors. A clinical trial (NCT02375880) evaluated the clinical value of DKN-01 in combination with gemcitabine and cisplatin in the treatment of patients with biliary tract cancer, followed by another clinical trial (NCT03645980) evaluating the antitumor activity and safety of DKN-01 in combination with sorafenib in patients with advanced HCC. In addition, PRI-724, a Wnt signaling pathway inhibitor, has demonstrated its efficacy and safety in an earlier solid tumor clinical trial (NCT01302405), but no clinical trial has yet evaluated its clinical value in the treatment of HCC patients. Although several drugs have been shown to have antitumor activity in preclinical models of HCC, most of them have not yet entered clinical trials, and more clinical trials are still needed to evaluate their efficacy and safety in future studies.
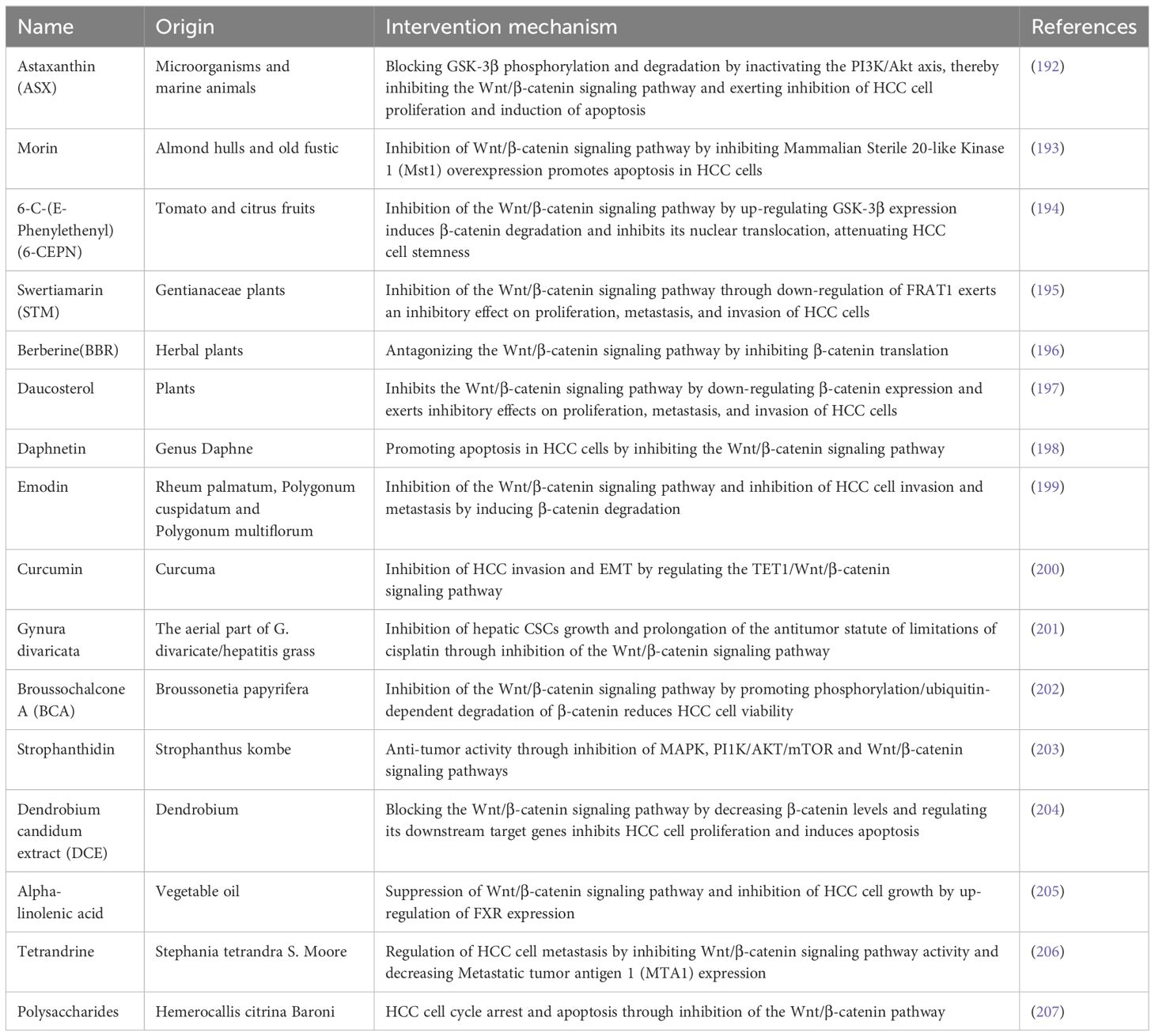
Diverse natural bioactive compounds derived from various dietary sources have been found to inhibit this pathway and demonstrate antitumor properties in HCC, according to recent research (Table 2). Compared to conventional chemotherapy drugs, these natural bioactive compounds have lower toxicity and are easily obtainable through diet, making them excellent adjuvant anti-cancer agents. However, it is still unclear if these chemicals can efficiently reach the tumor site and exert antitumor effects because the majority of them are derived from plants and have low bioavailability. Moreover, the majority of naturally occurring bioactive substances frequently influence additional molecular pathways in addition to this pathway. Future research should focus on addressing these issues.
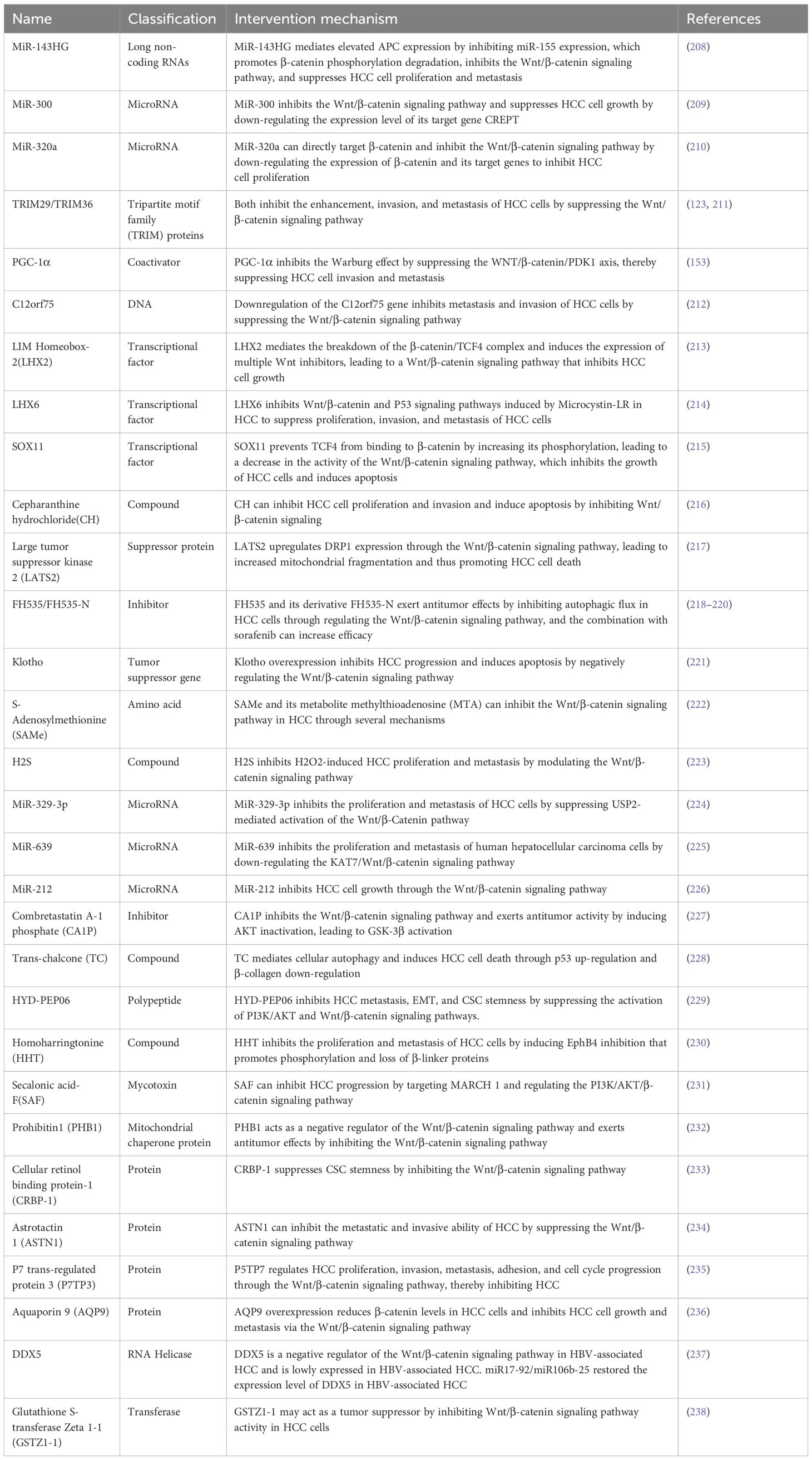
In addition, some bioactive molecules have been demonstrated to inhibit this pathway and exert anti-tumor effects (Table 3). Several anesthetics commonly used in clinical settings have been found to exhibit antitumor properties through the inhibition of the Wnt/β-catenin signaling pathway. Sevoflurane (Sevo), an inhalational anesthetic, has been demonstrated to inhibit tumor growth of HCC (239). Mechanistic studies indicate that it can regulate the PTEN/Akt/GSK-3β/β-catenin axis by down-regulating miR-25-3p expression, inhibit the Wnt/β-catenin signaling pathway, and exert anti-tumor effects (240). Like Sevo, propofol is an intravenous anesthetic commonly used in surgery, and it similarly inhibits this pathway, exerting an inhibitory effect on the growth and invasion of HCC cells (241, 242).
siRNA and antisense RNA are currently the most commonly used genetic tools, known for their specificity and ease of operation. They treat diseases caused by gene mutations or overexpression by reducing the expression of target genes and have been widely used in cancer therapy (243–245). Gene therapy based on siRNA or antisense RNA is considered another method to inhibit the Wnt/β-catenin signaling pathway. Studies have utilized siRNA targeting β-catenin to explore its value in HCC treatment. siRNA-CTNNB1 targeting to reduce β-catenin expression can inhibit this pathway and decrease the production of target genes cyclin-D1 and GS, impairing the proliferation and survival of HCC cells (246). Another study demonstrated that using siRNA to target β-catenin expression could arrest tumor cells in the G0/G1 phase of the cell cycle, thus inhibiting HCC cell proliferation (247). Furthermore, a CTNNB1 mutant mouse HCC model induced by Phenobarbital (PB) and Diethylnitrosamine (DEN) demonstrated that inhibiting β-catenin expression with locked nucleic acid (LNA) antisense oligonucleotides resulted in decreased HCC cell proliferation and increased apoptosis. In contrast, this effect was not observed in a rodent HCC model lacking CTNNB1 (248).
Existing studies have shown the importance of targeting Wnt/β-catenin signaling pathway transduction in the treatment of HCC, and a large number of preclinical studies have provided sufficient evidence for this. Various therapeutic strategies targeting this pathway have been developed. Nevertheless, due to the complex function of this pathway in the human body, these inhibitors exert anti-tumor activity while also inhibiting the Wnt/β-catenin signaling pathway in other normal tissues, resulting in toxic effects on normal tissues. This has severely limited the development of current therapeutic strategies targeting this pathway.
7 Conclusions and prospects
The role of the Wnt/β-catenin signaling pathway in the tumorigenesis, progression, and therapy resistance of HCC is indisputable. Targeting this pathway is an attractive target in HCC treatment. Increasingly studies have proven that drug or molecular targeting can block this pathway, which ultimately reduces tumor growth and improves therapeutic efficacy. With the increasing research on the Wnt/β-catenin signaling pathway, various inhibitors have been developed that can target this pathway. Unfortunately, owing to the toxicology of these inhibitors, there aren’t any authorized medications for the clinical therapy of HCC at this time.
In conclusion, targeting the Wnt/β-catenin signaling pathway still remains a significant challenge. Future studies should further deepen our understanding of the regulatory mechanisms of this pathway in HCC and guide the development of new HCC-targeted therapeutic strategies. Additionally, the development of specific targeted drugs that can selectively inhibit this pathway in tumor tissues remains a focus of future research.
Author contributions
ZKZ: Writing – original draft, Writing – review & editing. TC: Software, Writing – review & editing. FW: Writing – review & editing. ZMZ: Writing – review & editing. YS: Writing – review & editing. CG: Supervision, Writing – review & editing. XX: Funding acquisition, Writing – review & editing. HZ: Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. The Natural Science Foundation of Gansu Province (21JR11RA103) Gansu Provincial Youth Science and Technology Fund(21JR1RA161).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2021) 71:209–49. doi: 10.3322/caac.21660
2. Craig AJ, von Felden J, Garcia-Lezana T, Sarcognato S, Villanueva A. Tumour evolution in hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol. (2020) 17:139–52. doi: 10.1038/s41575-019-0229-4
3. Cao W, Chen HD, Yu YW, Li N, Chen WQ. Changing profiles of cancer burden worldwide and in China: a secondary analysis of the global cancer statistics 2020. Chin Med J. (2021) 134:783–91. doi: 10.1097/CM9.0000000000001474
4. Forner A, Reig M, Bruix J. Hepatocellular carcinoma. Lancet. (2018) 391:1301–14. doi: 10.1016/S0140-6736(18)30010-2
5. Thandra KC, Barsouk A, Saginala K, Aluru JS, Rawla P, Barsouk A. Epidemiology of non-alcoholic fatty liver disease and risk of hepatocellular carcinoma progression. Clin Exp Hepatol. (2020) 6:289–94. doi: 10.5114/ceh.2020.102153
6. Suresh D, Srinivas AN, Prashant A, Harikumar KB, Kumar DP. Therapeutic options in hepatocellular carcinoma: a comprehensive review. Clin Exp Med. (2023) 23:1901–16. doi: 10.1007/s10238-023-01014-3
7. Liu J, Xiao Q, Xiao J, Niu C, Li Y, Zhang X, et al. Wnt/β-catenin signalling: function, biological mechanisms, and therapeutic opportunities. Signal Transduct Target Ther. (2022) 7:3. doi: 10.1038/s41392-021-00762-6
8. Han SI, Lim SC. Expression and prognostic significance of CDK8 and β-catenin in hepatocellular carcinoma. In Vivo. (2020) 34:1387–94. doi: 10.21873/invivo.11918
9. Li YH, Yang SL, Zhang GF, Wu JC, Gong LL. Ming-Zhong; Lin, R.X. Mefloquine targets β-catenin pathway and thus can play a role in the treatment of liver cancer. Microb Pathog. (2018) 118:357–60. doi: 10.1016/j.micpath.2018.03.042
10. Mohapatra P, Chandrasekaran N. Wnt/β-catenin targeting in liver carcinoma through nanotechnology-based drug repurposing: A review. BioMed Pharmacother. (2022) 155:113713. doi: 10.1016/j.biopha.2022.113713
11. Nusse R, Clevers H. Wnt/β-catenin signaling, disease, and emerging therapeutic modalities. Cell. (2017) 169:985–99. doi: 10.1016/j.cell.2017.05.016
12. Clevers H, Nusse R. Wnt/β-catenin signaling and disease. Cell. (2012) 149:1192–205. doi: 10.1016/j.cell.2012.05.012
13. Zhu Y, Li X. Advances of wnt signalling pathway in colorectal cancer. Cells. (2023) 12:447. doi: 10.3390/cells12030447
14. He K, Gan WJ. Wnt/β-catenin signaling pathway in the development and progression of colorectal cancer. Cancer Manag Res. (2023) 15:435–48. doi: 10.2147/CMAR.S411168
15. Chen Y, Chen M, Deng K. Blocking the Wnt/β−catenin signaling pathway to treat colorectal cancer: Strategies to improve current therapies (Review). Int J Oncol. (2023) 62(2):24. doi: 10.3892/ijo.2022.5472
16. Wang D, Zhang Q, Li F, Wang C, Yang C, Yu H. β-TrCP-mediated ubiquitination and degradation of Dlg5 regulates hepatocellular carcinoma cell proliferation. Cancer Cell Int. (2019) 19:298. doi: 10.1186/s12935-019-1029-1
17. Li VS, Ng SS, Boersema PJ, Low TY, Karthaus WR, Gerlach JP, et al. Wnt signaling through inhibition of β-catenin degradation in an intact Axin1 complex. Cell. (2012) 149:1245–56. doi: 10.1016/j.cell.2012.05.002
18. Kim NG, Xu C, Gumbiner BM. Identification of targets of the Wnt pathway destruction complex in addition to beta-catenin. Proc Natl Acad Sci USA. (2009) 106:5165–70. doi: 10.1073/pnas.0810185106
19. Liu C, Li Y, Semenov M, Han C, Baeg GH, Tan Y, et al. Control of beta-catenin phosphorylation/degradation by a dual-kinase mechanism. Cell. (2002) 108:837–47. doi: 10.1016/S0092-8674(02)00685-2
20. Amit S, Hatzubai A, Birman Y, Andersen JS, Ben-Shushan E, Mann M, et al. Axin-mediated CKI phosphorylation of beta-catenin at Ser 45: a molecular switch for the Wnt pathway. Genes Dev. (2002) 16:1066–76. doi: 10.1101/gad.230302
21. Kusserow A, Pang K, Sturm C, Hrouda M, Lentfer J, Schmidt HA, et al. Unexpected complexity of the Wnt gene family in a sea anemone. Nature. (2005) 433:156–60. doi: 10.1038/nature03158
22. Zhai L, Chaturvedi D, Cumberledge S. Drosophila wnt-1 undergoes a hydrophobic modification and is targeted to lipid rafts, a process that requires porcupine. J OF Biol Chem. (2004) 279:33220–7. doi: 10.1074/jbc.M403407200
23. Bänziger C, Soldini D, Schütt C, Zipperlen P, Hausmann G, Basler K. Wntless, a conserved membrane protein dedicated to the secretion of Wnt proteins from signaling cells. Cell. (2006) 125:509–22. doi: 10.1016/j.cell.2006.02.049
24. Bartscherer K, Pelte N, Ingelfinger D, Boutros M. Secretion of Wnt ligands requires Evi, a conserved transmembrane protein. Cell. (2006) 125:523–33. doi: 10.1016/j.cell.2006.04.009
25. Zhao H, Ming T, Tang S, Ren S, Yang H, Liu M, et al. Wnt signaling in colorectal cancer: pathogenic role and therapeutic target. Mol Cancer. (2022) 21:144. doi: 10.1186/s12943-022-01616-7
26. Cadigan KM, Waterman ML. TCF/LEFs and Wnt signaling in the nucleus. Cold Spring Harb Perspect Biol. (2012) 4:a007906. doi: 10.1101/cshperspect.a007906
27. MacDonald BT, Tamai K, He X. Wnt/beta-catenin signaling: components, mechanisms, and diseases. Dev Cell. (2009) 17:9–26. doi: 10.1016/j.devcel.2009.06.016
28. Zhan T, Rindtorff N, Boutros M. Wnt signaling in cancer. Oncogene. (2017) 36:1461–73. doi: 10.1038/onc.2016.304
29. Pataki CA, Couchman JR, Brábek J. Wnt signaling cascades and the roles of syndecan proteoglycans. J Histochem Cytochem. (2015) 63:465–80. doi: 10.1369/0022155415586961
30. Wang H, Zhang R, Wu X, Chen Y, Ji W, Wang J, et al. The wnt signaling pathway in diabetic nephropathy. Front Cell Dev Biol. (2021) 9:701547. doi: 10.3389/fcell.2021.701547
31. Kohn AD, Moon RT. Wnt and calcium signaling: beta-catenin-independent pathways. Cell Calcium. (2005) 38:439–46. doi: 10.1016/j.ceca.2005.06.022
32. Molkentin JD. Calcineurin-NFAT signaling regulates the cardiac hypertrophic response in coordination with the MAPKs. Cardiovasc Res. (2004) 63:467–75. doi: 10.1016/j.cardiores.2004.01.021
33. Russell JO, Monga SP. Wnt/β-catenin signaling in liver development, homeostasis, and pathobiology. Annu Rev Pathol. (2018) 13:351–78. doi: 10.1146/annurev-pathol-020117-044010
34. Wild SL, Elghajiji A, Grimaldos Rodriguez C, Weston SD, Burke ZD, Tosh D. The canonical wnt pathway as a key regulator in liver development, differentiation and homeostatic renewal. Genes (Basel). (2020) 11:1163. doi: 10.3390/genes11101163
35. Zorn AM, Butler K, Gurdon JB. Anterior endomesoderm specification in Xenopus by Wnt/beta-catenin and TGF-beta signalling pathways. Dev Biol. (1999) 209:282–97. doi: 10.1006/dbio.1999.9257
36. McLin VA, Rankin SA, Zorn AM. Repression of Wnt/beta-catenin signaling in the anterior endoderm is essential for liver and pancreas development. Development. (2007) 134:2207–17. doi: 10.1242/dev.001230
37. Li Y, Rankin SA, Sinner D, Kenny AP, Krieg PA, Zorn AM. Sfrp5 coordinates foregut specification and morphogenesis by antagonizing both canonical and noncanonical Wnt11 signaling. Genes Dev. (2008) 22:3050–63. doi: 10.1101/gad.1687308
38. Pilcher KE, Krieg PA. Expression of the Wnt inhibitor, sFRP5, in the gut endoderm of Xenopus. Gene Expr. Patterns. (2002) 2:369–72. doi: 10.1016/S1567-133X(02)00023-6
39. Rossi JM, Dunn NR, Hogan BL, Zaret KS. Distinct mesodermal signals, including BMPs from the septum transversum mesenchyme, are required in combination for hepatogenesis from the endoderm. Genes Dev. (2001) 15:1998–2009. doi: 10.1101/gad.904601
40. Jung J, Zheng M, Goldfarb M, Zaret KS. Initiation of mammalian liver development from endoderm by fibroblast growth factors. Science. (1999) 284:1998–2003. doi: 10.1126/science.284.5422.1998
41. Ober EA, Verkade H, Field HA, Stainier DY. Mesodermal Wnt2b signalling positively regulates liver specification. Nature. (2006) 442:688–91. doi: 10.1038/nature04888
42. Yang L, Li LC, Lamaoqiezhong, Wang X, Wang WH, Wang YC, et al. The contributions of mesoderm-derived cells in liver development. Semin Cell Dev Biol. (2019) 92:63–76. doi: 10.1016/j.semcdb.2018.09.003
43. Tan X, Yuan Y, Zeng G, Apte U, Thompson MD, Cieply B, et al. Beta-catenin deletion in hepatoblasts disrupts hepatic morphogenesis and survival during mouse development. Hepatology. (2008) 47:1667–79. doi: 10.1002/hep.22225
44. Berg T, Rountree CB, Lee L, Estrada J, Sala FG, Choe A, et al. Fibroblast growth factor 10 is critical for liver growth during embryogenesis and controls hepatoblast survival via beta-catenin activation. Hepatology. (2007) 46:1187–97. doi: 10.1002/(ISSN)1527-3350
45. Monga SP, Mars WM, Pediaditakis P, Bell A, Mulé K, Bowen WC, et al. Hepatocyte growth factor induces Wnt-independent nuclear translocation of beta-catenin after Met-beta-catenin dissociation in hepatocytes. Cancer Res. (2002) 62:2064–71.
46. Tan X, Behari J, Cieply B, Michalopoulos GK, Monga SP. Conditional deletion of beta-catenin reveals its role in liver growth and regeneration. Gastroenterology. (2006) 131:1561–72. doi: 10.1053/j.gastro.2006.08.042
47. Planas-Paz L, Orsini V, Boulter L, Calabrese D, Pikiolek M, Nigsch F, et al. The RSPO-LGR4/5-ZNRF3/RNF43 module controls liver zonation and size. Nat Cell Biol. (2016) 18:467–79. doi: 10.1038/ncb3337
48. Tan X, Apte U, Micsenyi A, Kotsagrelos E, Luo JH, Ranganathan S, et al. Epidermal growth factor receptor: a novel target of the Wnt/beta-catenin pathway in liver. Gastroenterology. (2005) 129:285–302. doi: 10.1053/j.gastro.2005.04.013
49. Gayden J, Hu S, Joseph PN, Delgado E, Liu S, Bell A, et al. Spatial atlas of wnt receptors in adult mouse liver. Am J Pathol. (2023) 193:558–66. doi: 10.1016/j.ajpath.2023.01.011
50. Benhamouche S, Decaens T, Godard C, Chambrey R, Rickman DS, Moinard C, et al. Apc tumor suppressor gene is the "zonation-keeper" of mouse liver. Dev Cell. (2006) 10:759–70. doi: 10.1016/j.devcel.2006.03.015
51. Yang J, Mowry LE, Nejak-Bowen KN, Okabe H, Diegel CR, Lang RA, et al. β-catenin signaling in murine liver zonation and regeneration: a Wnt-Wnt situation. Hepatology. (2014) 60:964–76. doi: 10.1002/hep.v60.3
52. Gougelet A, Torre C, Veber P, Sartor C, Bachelot L, Denechaud PD, et al. T-cell factor 4 and β-catenin chromatin occupancies pattern zonal liver metabolism in mice. Hepatology. (2014) 59:2344–57. doi: 10.1002/hep.v59.6
53. Bellanti F, Vendemiale G. The aging liver: Redox biology and liver regeneration. Antioxid Redox Signal. (2021) 35:832–47. doi: 10.1089/ars.2021.0048
54. Sun T, Pikiolek M, Orsini V, Bergling S, Holwerda S, Morelli L, et al. AXIN2(+) pericentral hepatocytes have limited contributions to liver homeostasis and regeneration. Cell Stem Cell. (2020) 26:97–107.e6. doi: 10.1016/j.stem.2019.10.011
55. Chen F, Jimenez RJ, Sharma K, Luu HY, Hsu BY, Ravindranathan A, et al. Broad distribution of hepatocyte proliferation in liver homeostasis and regeneration. Cell Stem Cell. (2020) 26:27–33.e4. doi: 10.1016/j.stem.2019.11.001
56. Matsumoto T, Wakefield L, Tarlow BD, Grompe M. In vivo lineage tracing of polyploid hepatocytes reveals extensive proliferation during liver regeneration. Cell Stem Cell. (2020) 26:34–47.e3. doi: 10.1016/j.stem.2019.11.014
57. Lin S, Nascimento EM, Gajera CR, Chen L, Neuhöfer P, Garbuzov A, et al. Distributed hepatocytes expressing telomerase repopulate the liver in homeostasis and injury. Nature. (2018) 556:244–8. doi: 10.1038/s41586-018-0004-7
58. Monga SP, Pediaditakis P, Mule K, Stolz DB, Michalopoulos GK. Changes in WNT/beta-catenin pathway during regulated growth in rat liver regeneration. Hepatology. (2001) 33:1098–109. doi: 10.1053/jhep.2001.23786
59. Apte U, Singh S, Zeng G, Cieply B, Virji MA, Wu T, et al. Beta-catenin activation promotes liver regeneration after acetaminophen-induced injury. Am J Pathol. (2009) 175:1056–65. doi: 10.2353/ajpath.2009.080976
60. Sodhi D, Micsenyi A, Bowen WC, Monga DK, Talavera JC, Monga SP. Morpholino oligonucleotide-triggered beta-catenin knockdown compromises normal liver regeneration. J Hepatol. (2005) 43:132–41. doi: 10.1016/j.jhep.2005.02.019
61. Preziosi M, Okabe H, Poddar M, Singh S, Monga SP. Endothelial Wnts regulate β-catenin signaling in murine liver zonation and regeneration: A sequel to the Wnt-Wnt situation. Hepatol Commun. (2018) 2:845–60. doi: 10.1002/hep4.1196
62. Nejak-Bowen KN, Thompson MD, Singh S, Bowen WC. Jr; Dar, M.J.; Khillan, J.; Dai, C.; Monga, S.P. Accelerated liver regeneration and hepatocarcinogenesis in mice overexpressing serine-45 mutant beta-catenin. Hepatology. (2010) 51:1603–13. doi: 10.1002/hep.23538
63. Walesky CM, Kolb KE, Winston CL, Henderson J, Kruft B, Fleming I, et al. Functional compensation precedes recovery of tissue mass following acute liver injury. Nat Commun. (2020) 11:5785. doi: 10.1038/s41467-020-19558-3
64. Monga SP. β-catenin signaling and roles in liver homeostasis, injury, and tumorigenesis. Gastroenterology. (2015) 148:1294–310. doi: 10.1053/j.gastro.2015.02.056
65. Waisberg J, Saba GT. Wnt-/-β-catenin pathway signaling in human hepatocellular carcinoma. World J Hepatol. (2015) 7:2631–5. doi: 10.4254/wjh.v7.i26.2631
66. Khalaf AM, Fuentes D, Morshid AI, Burke MR, Kaseb AO, Hassan M, et al. Role of Wnt/β-catenin signaling in hepatocellular carcinoma, pathogenesis, and clinical significance. J Hepatocell Carcinoma. (2018) 5:61–73. doi: 10.2147/JHC
67. Taniguchi K, Roberts LR, Aderca IN, Dong X, Qian C, Murphy LM, et al. Mutational spectrum of beta-catenin, AXIN1, and AXIN2 in hepatocellular carcinomas and hepatoblastomas. Oncogene. (2002) 21:4863–71. doi: 10.1038/sj.onc.1205591
68. Ban KC, Singh H, Krishnan R, Seow HF. GSK-3beta phosphorylation and alteration of beta-catenin in hepatocellular carcinoma. Cancer Lett. (2003) 199:201–8. doi: 10.1016/S0304-3835(03)00421-X
69. Colnot S, Decaens T, Niwa-Kawakita M, Godard C, Hamard G, Kahn A, et al. Liver-targeted disruption of Apc in mice activates beta-catenin signaling and leads to hepatocellular carcinomas. Proc Natl Acad Sci USA. (2004) 101:17216–21. doi: 10.1073/pnas.0404761101
70. Trépo E, Caruso S, Yang J, Imbeaud S, Couchy G, Bayard Q, et al. Common genetic variation in alcohol-related hepatocellular carcinoma: a case-control genome-wide association study. Lancet Oncol. (2022) 23:161–71. doi: 10.1016/S1470-2045(21)00603-3
71. Nahon P, Bamba-Funck J, Layese R, Trépo E, Zucman-Rossi J, Cagnot C, et al. Integrating genetic variants into clinical models for hepatocellular carcinoma risk stratification in cirrhosis. J Hepatol. (2023) 78:584–95. doi: 10.1016/j.jhep.2022.11.003
72. Bengochea A, de Souza MM, Lefrançois L, Le Roux E, Galy O, Chemin I, et al. Common dysregulation of Wnt/Frizzled receptor elements in human hepatocellular carcinoma. Br J Cancer. (2008) 99:143–50. doi: 10.1038/sj.bjc.6604422
73. Jones SE, Jomary C. Secreted Frizzled-related proteins: searching for relationships and patterns. Bioessays. (2002) 24:811–20. doi: 10.1002/bies.10136
74. Kim M, Lee HC, Tsedensodnom O, Hartley R, Lim YS, Yu E, et al. Functional interaction between Wnt3 and Frizzled-7 leads to activation of the Wnt/beta-catenin signaling pathway in hepatocellular carcinoma cells. J Hepatol. (2008) 48:780–91. doi: 10.1016/j.jhep.2007.12.020
75. Shih YL, Shyu RY, Hsieh CB, Lai HC, Liu KY, Chu TY, et al. Promoter methylation of the secreted frizzled-related protein 1 gene SFRP1 is frequent in hepatocellular carcinoma. Cancer. (2006) 107:579–90. doi: 10.1002/cncr.22023
76. Takagi H, Sasaki S, Suzuki H, Toyota M, Maruyama R, Nojima M, et al. Frequent epigenetic inactivation of SFRP genes in hepatocellular carcinoma. J Gastroenterol. (2008) 43:378–89. doi: 10.1007/s00535-008-2170-0
77. Hoshida Y, Nijman SM, Kobayashi M, Chan JA, Brunet JP, Chiang DY, et al. Integrative transcriptome analysis reveals common molecular subclasses of human hepatocellular carcinoma. Cancer Res. (2009) 69:7385–92. doi: 10.1158/0008-5472.CAN-09-1089
78. Cieply B, Zeng G, Proverbs-Singh T, Geller DA, Monga SP. Unique phenotype of hepatocellular cancers with exon-3 mutations in beta-catenin gene. Hepatology. (2009) 49:821–31. doi: 10.1002/hep.22695
79. Singh G, Hossain MM, Bhat AQ, Ayaz MO, Bano N, Eachkoti R, et al. Identification of a cross-talk between EGFR and Wnt/beta-catenin signaling pathways in HepG2 liver cancer cells. Cell Signal. (2021) 79:109885. doi: 10.1016/j.cellsig.2020.109885
80. Conboy CB, Vélez-Reyes GL, Tschida BR, Hu H, Kaufmann G, Koes N, et al. R-spondin 2 drives liver tumor development in a yes-associated protein-dependent manner. Hepatol Commun. (2019) 3:1496–509. doi: 10.1002/hep4.1422
81. Xie Q, Chen L, Shan X, Shan X, Tang J, Zhou F, et al. Epigenetic silencing of SFRP1 and SFRP5 by hepatitis B virus X protein enhances hepatoma cell tumorigenicity through Wnt signaling pathway. Int J Cancer. (2014) 135:635–46. doi: 10.1002/ijc.v135.3
82. Hsieh A, Kim HS, Lim SO, Yu DY, Jung G. Hepatitis B viral X protein interacts with tumor suppressor adenomatous polyposis coli to activate Wnt/β-catenin signaling. Cancer Lett. (2011) 300:162–72. doi: 10.1016/j.canlet.2010.09.018
83. Daud M, Rana MA, Husnain T, Ijaz B. Modulation of Wnt signaling pathway by hepatitis B virus. Arch Virol. (2017) 162:2937–47. doi: 10.1007/s00705-017-3462-6
84. Tian X, Li J, Ma ZM, Zhao C, Wan DF, Wen YM. Role of hepatitis B surface antigen in the development of hepatocellular carcinoma: regulation of lymphoid enhancer-binding factor 1. J OF Exp Clin Cancer Res. (2009) 28:58. doi: 10.1186/1756-9966-28-58
85. Tran BM, Flanagan DJ, Ebert G, Warner N, Tran H, Fifis T, et al. The hepatitis B virus pre-core protein p22 activates wnt signaling. Cancers (Basel). (2020) 12:1435. doi: 10.3390/cancers12061435
86. Liu J, Ding X, Tang J, Cao Y, Hu P, Zhou F, et al. Enhancement of canonical Wnt/β-catenin signaling activity by HCV core protein promotes cell growth of hepatocellular carcinoma cells. PLoS One. (2011) 6:e27496. doi: 10.1371/journal.pone.0027496
87. Umer M, Qureshi SA, Hashmi ZY, Raza A, Ahmad J, Rahman M, et al. Promoter hypermethylation of Wnt pathway inhibitors in hepatitis C virus - induced multistep hepatocarcinogenesis. Virol J. (2014) 11:117. doi: 10.1186/1743-422X-11-117
88. Park CY, Choi SH, Kang SM, Kang JI, Ahn BY, Kim H, et al. Nonstructural 5A protein activates beta-catenin signaling cascades: implication of hepatitis C virus-induced liver pathogenesis. J Hepatol. (2009) 51:853–64. doi: 10.1016/j.jhep.2009.06.026
89. Mercer KE, Hennings L, Ronis MJ. Alcohol consumption, Wnt/β-catenin signaling, and hepatocarcinogenesis. Adv Exp Med Biol. (2015) 815:185–95. doi: 10.1007/978-3-319-09614-8_11
90. Lai K, Kweon SM, Chi F, Hwang E, Kabe Y, Higashiyama R, et al. Stearoyl-coA desaturase promotes liver fibrosis and tumor development in mice via a wnt positive-signaling loop by stabilization of low-density lipoprotein-receptor-related proteins 5 and 6. Gastroenterology. (2017) 152:1477–91. doi: 10.1053/j.gastro.2017.01.021
91. Debebe A, Medina V, Chen CY, Mahajan IM, Jia C, Fu D, et al. Wnt/β-catenin activation and macrophage induction during liver cancer development following steatosis. Oncogene. (2017) 36:6020–9. doi: 10.1038/onc.2017.207
92. Tian Y, Wong VW, Wong GL, Yang W, Sun H, Shen J, et al. Histone deacetylase HDAC8 promotes insulin resistance and β-catenin activation in NAFLD-associated hepatocellular carcinoma. Cancer Res. (2015) 75:4803–16. doi: 10.1158/0008-5472.CAN-14-3786
93. Wang W, Smits R, Hao H, He C. Wnt/β-catenin signaling in liver cancers. Cancers (Basel). (2019) 11(7):926. doi: 10.3390/cancers11070926
94. Devereux TR, Stern MC, Flake GP, Yu MC, Zhang ZQ, London SJ, et al. CTNNB1 mutations and beta-catenin protein accumulation in human hepatocellular carcinomas associated with high exposure to aflatoxin B1. Mol Carcinog. (2001) 31:68–73. doi: 10.1002/mc.1041
95. Ma Z, Wang YY, Xin HW, Wang L, Arfuso F, Dharmarajan A, et al. The expanding roles of long non-coding RNAs in the regulation of cancer stem cells. Int J Biochem Cell Biol. (2019) 108:17–20. doi: 10.1016/j.biocel.2019.01.003
96. Pandit H, Li Y, Li X, Zhang W, Li S, Martin R. Enrichment of cancer stem cells via β-catenin contributing to the tumorigenesis of hepatocellular carcinoma. BMC Cancer. (2018) 18:783. doi: 10.1186/s12885-018-4683-0
97. Tan YT, Lin JF, Li T, Li JJ, Xu RH, Ju HQ. LncRNA-mediated posttranslational modifications and reprogramming of energy metabolism in cancer. Cancer Commun (London England). (2021) 41:109–20. doi: 10.1002/cac2.12108
98. Wang Y, He L, Du Y, Zhu P, Huang G, Luo J, et al. The long noncoding RNA lncTCF7 promotes self-renewal of human liver cancer stem cells through activation of Wnt signaling. Cell Stem Cell. (2015) 16:413–25. doi: 10.1016/j.stem.2015.03.003
99. Zhu P, Wang Y, Huang G, Ye B, Liu B, Wu J, et al. lnc-β-Catm elicits EZH2-dependent β-catenin stabilization and sustains liver CSC self-renewal. Nat Struct Mol Biol. (2016) 23:631–9. doi: 10.1038/nsmb.3235
100. Fu X, Lin J, Qin F, Yang Z, Ding Y, Zhang Y, et al. LncAPC drives Wnt/β-catenin activation and liver TIC self-renewal through EZH2 mediated APC transcriptional inhibition. Mol Carcinog. (2018) 57:408–18. doi: 10.1002/mc.22764
101. Chen Z, Gao Y, Yao L, Liu Y, Huang L, Yan Z, et al. LncFZD6 initiates Wnt/β-catenin and liver TIC self-renewal through BRG1-mediated FZD6 transcriptional activation. Oncogene. (2018) 37:3098–112. doi: 10.1038/s41388-018-0203-6
102. Chen Z, Yao L, Liu Y, Zhu P. LncTIC1 interacts with β-catenin to drive liver TIC self-renewal and liver tumorigenesis. Cancer Lett. (2018) 430:88–96. doi: 10.1016/j.canlet.2018.05.023
103. Chai S, Ng KY, Tong M, Lau EY, Lee TK, Chan KW, et al. Octamer 4/microRNA-1246 signaling axis drives Wnt/β-catenin activation in liver cancer stem cells. Hepatology. (2016) 64:2062–76. doi: 10.1002/hep.28821
104. Lin X, Zuo S, Luo R, Li Y, Yu G, Zou Y, et al. HBX-induced miR-5188 impairs FOXO1 to stimulate β-catenin nuclear translocation and promotes tumor stemness in hepatocellular carcinoma. Theranostics. (2019) 9:7583–98. doi: 10.7150/thno.37717
105. Zheng Z, Liu J, Yang Z, Wu L, Xie H, Jiang C, et al. MicroRNA-452 promotes stem-like cells of hepatocellular carcinoma by inhibiting Sox7 involving Wnt/β-catenin signaling pathway. Oncotarget. (2016) 7:28000–12. doi: 10.18632/oncotarget.v7i19
106. Jiang C, Yu M, Xie X, Huang G, Peng Y, Ren D, et al. miR-217 targeting DKK1 promotes cancer stem cell properties via activation of the Wnt signaling pathway in hepatocellular carcinoma. Oncol Rep. (2017) 38:2351–9. doi: 10.3892/or.2017.5924
107. Gao H, Fan H, Xie H. The regulatory effect of the YY1/miR−HCC2/BAMBI axis on the stemness of liver cancer cells. Int J Oncol. (2023) 62:59. doi: 10.3892/ijo
108. Chen Z, Huang L, Wang K, Zhang L, Zhong X, Yan Z, et al. rtcisE2F promotes the self-renewal and metastasis of liver tumor-initiating cells via N(6)-methyladenosine-dependent E2F3/E2F6 mRNA stability. Sci China Life Sci. (2022) 65:1840–54. doi: 10.1007/s11427-021-2038-5
109. Fan Z, Duan J, Wang L, Xiao S, Li L, Yan X, et al. PTK2 promotes cancer stem cell traits in hepatocellular carcinoma by activating Wnt/β-catenin signaling. Cancer Lett. (2019) 450:132–43. doi: 10.1016/j.canlet.2019.02.040
110. Chen X, Huan H, Liu C, Luo Y, Shen J, Zhuo Y, et al. Deacetylation of β-catenin by SIRT1 regulates self-renewal and oncogenesis of liver cancer stem cells. Cancer Lett. (2019) 463:1–10. doi: 10.1016/j.canlet.2019.07.021
111. Cheng J, Liu C, Liu L, Chen X, Shan J, Shen J, et al. MEK1 signaling promotes self-renewal and tumorigenicity of liver cancer stem cells via maintaining SIRT1 protein stabilization. Oncotarget. (2016) 7:20597–611. doi: 10.18632/oncotarget.v7i15
112. Wang J, Yu H, Dong W, Zhang C, Hu M, Ma W, et al. N6-methyladenosine-mediated up-regulation of FZD10 regulates liver cancer stem cells' Properties and lenvatinib resistance through WNT/β-catenin and hippo signaling pathways. Gastroenterology. (2023) 164:990–1005. doi: 10.1053/j.gastro.2023.01.041
113. Pan Y, Yu Y, Wang X, Zhang T. Tumor-associated macrophages in tumor immunity. Front Immunol. (2020) 11:583084. doi: 10.3389/fimmu.2020.583084
114. Chen Y, Wen H, Zhou C, Su Q, Lin Y, Xie Y, et al. TNF-α derived from M2 tumor-associated macrophages promotes epithelial-mesenchymal transition and cancer stemness through the Wnt/β-catenin pathway in SMMC-7721 hepatocellular carcinoma cells. Exp Cell Res. (2019) 378:41–50. doi: 10.1016/j.yexcr.2019.03.005
115. Kirtonia A, Sethi G, Garg M. The multifaceted role of reactive oxygen species in tumorigenesis. Cell Mol Life Sci. (2020) 77:4459–83. doi: 10.1007/s00018-020-03536-5
116. Wang JR, Li TZ, Wang C, Li SM, Luo YH, Piao XJ, et al. Liquiritin inhibits proliferation and induces apoptosis in HepG2 hepatocellular carcinoma cells via the ROS-mediated MAPK/AKT/NF-κB signaling pathway. Naunyn-Schmiedeberg's Arch Pharmacol. (2020) 393:1987–99. doi: 10.1007/s00210-019-01763-7
117. Li B, Cao Y, Meng G, Qian L, Xu T, Yan C, et al. Targeting glutaminase 1 attenuates stemness properties in hepatocellular carcinoma by increasing reactive oxygen species and suppressing Wnt/beta-catenin pathway. EBioMedicine. (2019) 39:239–54. doi: 10.1016/j.ebiom.2018.11.063
118. Zheng W, Yao M, Wu M, Yang J, Yao D, Wang L. Secretory clusterin promotes hepatocellular carcinoma progression by facilitating cancer stem cell properties via AKT/GSK-3β/β-catenin axis. J Transl Med. (2020) 18:81. doi: 10.1186/s12967-020-02262-7
119. Zhu K, Li J, Li J, Sun J, Guo Y, Tian H, et al. Ring1 promotes the transformation of hepatic progenitor cells into cancer stem cells through the Wnt/β-catenin signaling pathway. J Cell Biochem. (2020) 121:3941–51. doi: 10.1002/jcb.29496
120. Raghunath A, Sundarraj K, Arfuso F, Sethi G, Perumal E. Dysregulation of nrf2 in hepatocellular carcinoma: Role in cancer progression and chemoresistance. Cancers (Basel). (2018) 10:481. doi: 10.3390/cancers10120481
121. Wang JH, Wei W, Xu J, Guo ZX, Xiao CZ, Zhang YF, et al. Elevated expression of Cripto-1 correlates with poor prognosis in hepatocellular carcinoma. Oncotarget. (2015) 6:35116–28. doi: 10.18632/oncotarget.v6i33
122. Lo RC, Leung CO, Chan KK, Ho DW, Wong CM, Lee TK, et al. Cripto-1 contributes to stemness in hepatocellular carcinoma by stabilizing Dishevelled-3 and activating Wnt/β-catenin pathway. Cell Death Differ. (2018) 25:1426–41. doi: 10.1038/s41418-018-0059-x
123. Xu M, Hu J, Zhou B, Zhong Y, Lin N, Xu R. TRIM29 prevents hepatocellular carcinoma progression by inhibiting Wnt/β-catenin signaling pathway. Acta Biochim Biophys Sin (Shanghai). (2019) 51:68–77. doi: 10.1093/abbs/gmy151
124. Fan W, Du F, Liu X. TRIM66 confers tumorigenicity of hepatocellular carcinoma cells by regulating GSK-3β-dependent Wnt/β-catenin signaling. Eur J Pharmacol. (2019) 850:109–17. doi: 10.1016/j.ejphar.2019.01.054
125. Li W, Xue H, Li Y, Li P, Ma F, Liu M, et al. ATDC promotes the growth and invasion of hepatocellular carcinoma cells by modulating GSK-3β/Wnt/β-catenin signalling. Clin Exp Pharmacol Physiol. (2019) 46:845–53. doi: 10.1111/1440-1681.13119
126. Jiang J, Xu B, Zheng Y, Guo X, Chen F. Spindle and kinetochore-associated protein 2 facilitates the proliferation and invasion of hepatocellular carcinoma via the regulation of Wnt/β-catenin signaling. Exp Cell Res. (2020) 395:112181. doi: 10.1016/j.yexcr.2020.112181
127. Tang N, Cai X, Peng L, Liu H, Chen Y. TCP1 regulates Wnt7b/β-catenin pathway through P53 to influence the proliferation and migration of hepatocellular carcinoma cells. Signal Transduct Target Ther. (2020) 5:169. doi: 10.1038/s41392-020-00278-5
128. Chen MY, Li ZP, Sun ZN, Ma M. USP9X promotes the progression of hepatocellular carcinoma by regulating beta-catenin. Ir J Med Sci. (2020) 189:865–71. doi: 10.1007/s11845-020-02199-2
129. Sun X, Cai M, Wu L, Zhen X, Chen Y, Peng J, et al. Ubiquitin-specific protease 28 deubiquitinates TCF7L2 to govern the action of the Wnt signaling pathway in hepatic carcinoma. Cancer Sci. (2022) 113:3463–75. doi: 10.1111/cas.15509
130. Xing M, Li P, Wang X, Li J, Shi J, Qin J, et al. Overexpression of p62/IMP2 can Promote Cell Migration in Hepatocellular Carcinoma via Activation of the Wnt/β-Catenin Pathway. Cancers (Basel). (2019) 12(1):7. doi: 10.3390/cancers12010007
131. Fan W, Du F, Liu X. Phosphatidylinositol 4-phosphate adaptor protein 2 accelerates the proliferation and invasion of hepatocellular carcinoma cells by enhancing Wnt/β-catenin signaling. J Bioenerg. Biomembr. (2020) 52:301–9. doi: 10.1007/s10863-020-09852-6
132. Tong W, Yang L, Liu L, Liu X, Luo N. SPINDOC is highly expressed in pan-cancer samples and can promote the proliferation, invasion and migration of hepatocellular carcinoma cells by activating wnt/β-catenin signaling pathway. Onco Targets Ther. (2022) 15:555–70. doi: 10.2147/OTT.S348843
133. Yang B, Wang S, Xie H, Wang C, Gao X, Rong Y, et al. KIF18B promotes hepatocellular carcinoma progression through activating Wnt/β-catenin-signaling pathway. J Cell Physiol. (2020) 235:6507–14. doi: 10.1002/jcp.29444
134. Sun T, Zhang J. ETV4 mediates the Wnt/β-catenin pathway through transcriptional activation of ANXA2 to promote hepatitis B virus-associated liver hepatocellular carcinoma progression. J Biochem. (2021) 170:663–73. doi: 10.1093/jb/mvab088
135. Liu FH, Cui YP, He YK, Shu RH. FBXO17 promotes Malignant progression of hepatocellular carcinoma by activating wnt/β-catenin pathway. Eur Rev Med Pharmacol Sci. (2019) 23:8265–73. doi: 10.26355/eurrev_201910_19137
136. Xu G, Zhu Y, Liu H, Liu Y, Zhang X. LncRNA MIR194-2HG Promotes Cell Proliferation and Metastasis via Regulation of miR-1207-5p/TCF19/Wnt/β-Catenin Signaling in Liver Cancer. Onco Targets Ther. (2020) 13:9887–99. doi: 10.2147/OTT.S264614
137. Liang W, Shi C, Hong W, Li P, Zhou X, Fu W, et al. Super-enhancer-driven lncRNA-DAW promotes liver cancer cell proliferation through activation of Wnt/β-catenin pathway. Mol Ther Nucleic Acids. (2021) 26:1351–63. doi: 10.1016/j.omtn.2021.10.028
138. Wang Q, Tang Y, Ge Y, Zhang S, Zheng M. Long non-coding RNA NRAV enhances proliferation and invasion of hepatocellular carcinoma cells by modulating the Wnt/β-catenin signaling pathway. Bioengineered. (2022) 13:10026–37. doi: 10.1080/21655979.2022.2062977
139. Han K, Li C, Zhang X, Shang L. DUXAP10 inhibition attenuates the proliferation and metastasis of hepatocellular carcinoma cells by regulation of the Wnt/β-catenin and PI3K/Akt signaling pathways. Biosci Rep. (2019) 39(5):BSR20181457. doi: 10.1042/BSR20181457
140. Zhu L, Yang N, Du G, Li C, Liu G, Liu S, et al. LncRNA CRNDE promotes the epithelial-mesenchymal transition of hepatocellular carcinoma cells via enhancing the Wnt/β-catenin signaling pathway. J Cell Biochem. (2019) 120:1156–64. doi: 10.1002/jcb.26762
141. Kong S, Xue H, Li Y, Li P, Ma F, Liu M, et al. The long noncoding RNA OTUD6B-AS1 enhances cell proliferation and the invasion of hepatocellular carcinoma cells through modulating GSKIP/Wnt/β-catenin signalling via the sequestration of miR-664b-3p. Exp Cell Res. (2020) 395:112180. doi: 10.1016/j.yexcr.2020.112180
142. Li CM, Wu W, Zhu Z, Lu PL, Gong JP, Ma R. miR-550a-5p promotes the proliferation and migration of hepatocellular carcinoma by targeting GNE via the Wnt/β-catenin signaling pathway. Neoplasma. (2022) 69:1359–72. doi: 10.4149/neo_2022_220727N774
143. Deldar Abad Paskeh M, Mirzaei S, Ashrafizadeh M, Zarrabi A, Sethi G. Wnt/β-catenin signaling as a driver of hepatocellular carcinoma progression: An emphasis on molecular pathways. J Hepatocell Carcinoma. (2021) 8:1415–44. doi: 10.2147/JHC.S336858
144. Huang JL, Fu YP, Gan W, Liu G, Zhou PY, Zhou C, et al. Hepatic stellate cells promote the progression of hepatocellular carcinoma through microRNA-1246-RORα-Wnt/β-Catenin axis. Cancer Lett. (2020) 476:140–51. doi: 10.1016/j.canlet.2020.02.012
145. Guo F, Wang H, Jiang M, Yang Q, Xiang Q, Zhou H, et al. TDP-43 induces EMT and promotes hepatocellular carcinoma metastasis via activating Wnt/β-catenin signaling pathway. Am J Cancer Res. (2020) 10:3285–301.
146. Ren Y, Wang Y, Hao S, Yang Y, Xiong W, Qiu L, et al. NFE2L3 promotes Malignant behavior and EMT of human hepatocellular carcinoma (HepG2) cells via Wnt/β−catenin pathway. J Cancer. (2020) 11:6939–49. doi: 10.7150/jca.48100
147. Du J, Zhu Z, Xu L, Chen X, Li X, Lan T, et al. ARHGEF11 promotes proliferation and epithelial-mesenchymal transition of hepatocellular carcinoma through activation of β-catenin pathway. Aging. (2020) 12:20235–53. doi: 10.18632/aging.v12i20
148. Qiu Z, Wang X, Yang Z, Liao S, Dong W, Sun T, et al. GBA1-dependent membrane glucosylceramide reprogramming promotes liver cancer metastasis via activation of the Wnt/β-catenin signalling pathway. Cell Death Dis. (2022) 13:508. doi: 10.1038/s41419-022-04968-6
149. Lei S, Du X, Tan K, He X, Zhu Y, Zhao S, et al. CRP−1 promotes the Malignant behavior of hepatocellular carcinoma cells via activating epithelial−mesenchymal transition and Wnt/β−catenin signaling. Exp Ther Med. (2023) 26:314. doi: 10.3892/etm
150. Kee HJ, Cheong JH. Tumor bioenergetics: an emerging avenue for cancer metabolism targeted therapy. BMB Rep. (2014) 47:158–66. doi: 10.5483/BMBRep.2014.47.3.273
151. Fan Q, Yang L, Zhang X, Ma Y, Li Y, Dong L, et al. Autophagy promotes metastasis and glycolysis by upregulating MCT1 expression and Wnt/β-catenin signaling pathway activation in hepatocellular carcinoma cells. J OF Exp Clin Cancer Res. (2018) 37:9. doi: 10.1186/s13046-018-0673-y
152. Icard P, Shulman S, Farhat D, Steyaert JM, Alifano M, Lincet H. How the Warburg effect supports aggressiveness and drug resistance of cancer cells. Drug Resist Updat. (2018) 38:1–11. doi: 10.1016/j.drup.2018.03.001
153. Zuo Q, He J, Zhang S, Wang H, Jin G, Jin H, et al. PPARγ Coactivator-1α Suppresses metastasis of hepatocellular carcinoma by inhibiting warburg effect by PPARγ-dependent WNT/β-catenin/pyruvate dehydrogenase kinase isozyme 1 axis. Hepatology. (2021) 73:644–60. doi: 10.1002/hep.31280
154. Song M, Pan Q, Yang J, He J, Zeng J, Cheng S, et al. Galectin-3 favours tumour metastasis via the activation of β-catenin signalling in hepatocellular carcinoma. Br J Cancer. (2020) 123:1521–34. doi: 10.1038/s41416-020-1022-4
155. Harris AL. Hypoxia–a key regulatory factor in tumour growth. Nat Rev Cancer. (2002) 2:38–47. doi: 10.1038/nrc704
156. Xu W, Zhou W, Cheng M, Wang J, Liu Z, He S, et al. Hypoxia activates Wnt/β-catenin signaling by regulating the expression of BCL9 in human hepatocellular carcinoma. Sci Rep. (2017) 7:40446. doi: 10.1038/srep40446
157. Luo D, Wang Z, Wu J, Jiang C, Wu J. The role of hypoxia inducible factor-1 in hepatocellular carcinoma. BioMed Res Int. (2014) 2014:409272. doi: 10.1155/2014/409272
158. Menrad H, Werno C, Schmid T, Copanaki E, Deller T, Dehne N, et al. Roles of hypoxia-inducible factor-1alpha (HIF-1alpha) versus HIF-2alpha in the survival of hepatocellular tumor spheroids. Hepatology. (2010) 51:2183–92. doi: 10.1002/hep.23597
159. Liu L, Zhu XD, Wang WQ, Shen Y, Qin Y, Ren ZG, et al. Activation of beta-catenin by hypoxia in hepatocellular carcinoma contributes to enhanced metastatic potential and poor prognosis. Clin Cancer Res. (2010) 16:2740–50. doi: 10.1158/1078-0432.CCR-09-2610
160. Zhang Q, Bai X, Chen W, Ma T, Hu Q, Liang C, et al. Wnt/β-catenin signaling enhances hypoxia-induced epithelial-mesenchymal transition in hepatocellular carcinoma via crosstalk with hif-1α signaling. Carcinogenesis. (2013) 34:962–73. doi: 10.1093/carcin/bgt027
161. Zhang J, Zhang X, Li J, Song Z. Systematic analysis of the ABC transporter family in hepatocellular carcinoma reveals the importance of ABCB6 in regulating ferroptosis. Life Sci. (2020) 257:118131. doi: 10.1016/j.lfs.2020.118131
162. Chen Z, Pan T, Jiang D, Jin L, Geng Y, Feng X, et al. The lncRNA-GAS5/miR-221-3p/DKK2 Axis Modulates ABCB1-Mediated Adriamycin Resistance of Breast Cancer via the Wnt/β-Catenin Signaling Pathway. Mol Ther Nucleic Acids. (2020) 19:1434–48. doi: 10.1016/j.omtn.2020.01.030
163. Chen Z, Huang C, Ma T, Jiang L, Tang L, Shi T, et al. Reversal effect of quercetin on multidrug resistance via FZD7/β-catenin pathway in hepatocellular carcinoma cells. Phytomedicine. (2018) 43:37–45. doi: 10.1016/j.phymed.2018.03.040
164. Liu R, Li Y, Tian L, Shi H, Wang J, Liang Y, et al. Gankyrin drives metabolic reprogramming to promote tumorigenesis, metastasis and drug resistance through activating β-catenin/c-Myc signaling in human hepatocellular carcinoma. Cancer Lett. (2019) 443:34–46. doi: 10.1016/j.canlet.2018.11.030
165. Liu Y, Ye X, Zhang JB, Ouyang H, Shen Z, Wu Y, et al. PROX1 promotes hepatocellular carcinoma proliferation and sorafenib resistance by enhancing β-catenin expression and nuclear translocation. Oncogene. (2015) 34:5524–35. doi: 10.1038/onc.2015.7
166. Deng L, Sun J, Chen X, Liu L, Wu D. Nek2 augments sorafenib resistance by regulating the ubiquitination and localization of β-catenin in hepatocellular carcinoma. J OF Exp Clin Cancer Res. (2019) 38:316. doi: 10.1186/s13046-019-1311-z
167. Cai J, Chen J, Wu T, Cheng Z, Tian Y, Pu C, et al. Genome-scale CRISPR activation screening identifies a role of LRP8 in Sorafenib resistance in Hepatocellular carcinoma. Biochem Biophys Res Commun. (2020) 526:1170–6. doi: 10.1016/j.bbrc.2020.04.040
168. Liu Y, Zhuang H, Cao F, Li J, Guo Y, Zhang J, et al. Shc3 promotes hepatocellular carcinoma stemness and drug resistance by interacting with β-catenin to inhibit its ubiquitin degradation pathway. Cell Death Dis. (2021) 12:278. doi: 10.1038/s41419-021-03560-8
169. Xu N, Shen C, Luo Y, Xia L, Xue F, Xia Q, et al. Upregulated miR-130a increases drug resistance by regulating RUNX3 and Wnt signaling in cisplatin-treated HCC cell. Biochem Biophys Res Commun. (2012) 425:468–72. doi: 10.1016/j.bbrc.2012.07.127
170. Shen YN, He HG, Shi Y, Cao J, Yuan JY, Wang ZC, et al. Krüppel-like factor 8 promotes cancer stem cell-like traits in hepatocellular carcinoma through Wnt/β-catenin signaling. Mol Carcinog. (2017) 56:751–60. doi: 10.1002/mc.22532
171. Xu Y, Zhang C, Liang H, Hu S, Li P, Liu L, et al. Dishevelled 1, a pivotal positive regulator of the Wnt signalling pathway, mediates 5-fluorouracil resistance in HepG2 cells. Artif Cells Nanomed Biotechnol. (2018) 46:192–200. doi: 10.1080/21691401.2018.1453827
172. Wei X, Yang X, Han ZP, Qu FF, Shao L, Shi YF. Mesenchymal stem cells: a new trend for cell therapy. Acta Pharmacol Sin. (2013) 34:747–54. doi: 10.1038/aps.2013.50
173. Hou J, Zhao N, Zhu P, Chang J, Du Y, Shen W. Irradiated mesenchymal stem cells support stemness maintenance of hepatocellular carcinoma stem cells through Wnt/β-catenin signaling pathway. Cell Biosci. (2020) 10:93. doi: 10.1186/s13578-020-00449-5
174. Morita M, Nishida N, Aoki T, Chishina H, Takita M, Ida H, et al. Role of β-catenin activation in the tumor immune microenvironment and immunotherapy of hepatocellular carcinoma. Cancers (Basel). (2023) 15(8):2311. doi: 10.3390/cancers15082311
175. Ruiz de Galarreta M, Bresnahan E, Molina-Sánchez P, Lindblad KE, Maier B, Sia D, et al. β-catenin activation promotes immune escape and resistance to anti-PD-1 therapy in hepatocellular carcinoma. Cancer Discovery. (2019) 9:1124–41. doi: 10.1158/2159-8290.CD-19-0074
176. Ganesh S, Shui X, Craig KP, Park J, Wang W, Brown BD, et al. RNAi-mediated β-catenin inhibition promotes T cell infiltration and antitumor activity in combination with immune checkpoint blockade. Mol Ther. (2018) 26:2567–79. doi: 10.1016/j.ymthe.2018.09.005
177. Matsuda A, Ishiguro K, Yan IK, Patel T. Extracellular vesicle-based therapeutic targeting of β-catenin to modulate anticancer immune responses in hepatocellular cancer. Hepatol Commun. (2019) 3:525–41. doi: 10.1002/hep4.1311
178. You L, He B, Xu Z, Uematsu K, Mazieres J, Mikami I, et al. Inhibition of Wnt-2-mediated signaling induces programmed cell death in non-small-cell lung cancer cells. Oncogene. (2004) 23:6170–4. doi: 10.1038/sj.onc.1207844
179. Gurney A, Axelrod F, Bond CJ, Cain J, Chartier C, Donigan L, et al. Wnt pathway inhibition via the targeting of Frizzled receptors results in decreased growth and tumorigenicity of human tumors. Proc Natl Acad Sci USA. (2012) 109:11717–22. doi: 10.1073/pnas.1120068109
180. Shah K, Panchal S, Patel B. Porcupine inhibitors: Novel and emerging anti-cancer therapeutics targeting the Wnt signaling pathway. Pharmacol Res. (2021) 167:105532. doi: 10.1016/j.phrs.2021.105532
181. Zhang Y, Wang X. Targeting the Wnt/β-catenin signaling pathway in cancer. J Hematol Oncol. (2020) 13:165. doi: 10.1186/s13045-020-00990-3
182. Wang F, He L, Dai WQ, Xu YP, Wu D, Lin CL, et al. Salinomycin inhibits proliferation and induces apoptosis of human hepatocellular carcinoma cells. Vitro vivo. PLoS One. (2012) 7:e50638. doi: 10.1371/journal.pone.0050638
183. Xu C, Xu Z, Zhang Y, Evert M, Calvisi DF, Chen X. β-Catenin signaling in hepatocellular carcinoma. J Clin Invest. (2022) 132(4):e154515. doi: 10.1172/JCI154515
184. Wei W, Chua MS, Grepper S, So S. Small molecule antagonists of Tcf4/beta-catenin complex inhibit the growth of HCC cells. Vitro vivo. Int J Cancer. (2010) 126:2426–36. doi: 10.1002/ijc.24810
185. Gabata R, Harada K, Mizutani Y, Ouchi H, Yoshimura K, Sato Y, et al. Anti-tumor activity of the small molecule inhibitor PRI-724 against β-catenin-activated hepatocellular carcinoma. Anticancer Res. (2020) 40:5211–9. doi: 10.21873/anticanres.14524
186. Arensman MD, Telesca D, Lay AR, Kershaw KM, Wu N, Donahue TR, et al. The CREB-binding protein inhibitor ICG-001 suppresses pancreatic cancer growth. Mol Cancer Ther. (2014) 13:2303–14. doi: 10.1158/1535-7163.MCT-13-1005
187. Seto K, Sakabe T, Itaba N, Azumi J, Oka H, Morimoto M, et al. Novel small-molecule WNT inhibitor, IC-2, has the potential to suppress liver cancer stem cells. Anticancer Res. (2017) 37:3569–79. doi: 10.21873/anticanres
188. Haas MS, Kagey MH, Heath H, Schuerpf F, Rottman JB, Newman W. mDKN-01, a novel anti-DKK1 mAb, enhances innate immune responses in the tumor microenvironment. Mol Cancer Res. (2021) 19:717–25. doi: 10.1158/1541-7786.MCR-20-0799
189. Gao W, Kim H, Feng M, Phung Y, Xavier CP, Rubin JS, et al. Inactivation of Wnt signaling by a human antibody that recognizes the heparan sulfate chains of glypican-3 for liver cancer therapy. Hepatology. (2014) 60:576–87. doi: 10.1002/hep.v60.2
190. Reddy D, Kumavath R, Tan TZ, Ampasala DR, Kumar AP. Peruvoside targets apoptosis and autophagy through MAPK Wnt/β-catenin and PI3K/AKT/mTOR signaling pathways in human cancers. Life Sci. (2020) 241:117147. doi: 10.1016/j.lfs.2019.117147
191. Zou WJ, Huang Z, Jiang TP, Shen YP, Zhao AS, Zhou S, et al. Pirfenidone inhibits proliferation and promotes apoptosis of hepatocellular carcinoma cells by inhibiting the wnt/β-catenin signaling pathway. Med Sci MONITOR. (2017) 23:6107–13. doi: 10.12659/MSM.907891
192. Li J, Dai W, Xia Y, Chen K, Li S, Liu T, et al. Astaxanthin inhibits proliferation and induces apoptosis of human hepatocellular carcinoma cells via inhibition of nf-Κb P65 and wnt/Β-catenin in vitro. Mar Drugs. (2015) 13:6064–81. doi: 10.3390/md13106064
193. Perumal N, Perumal M, Kannan A, Subramani K, Halagowder D, Sivasithamparam N. Morin impedes Yap nuclear translocation and fosters apoptosis through suppression of Wnt/β-catenin and NF-κB signaling in Mst1 overexpressed HepG2 cells. Exp Cell Res. (2017) 355:124–41. doi: 10.1016/j.yexcr.2017.03.062
194. Kang Q, Gong J, Wang M, Wang Q, Chen F, Cheng KW. 6-C-(E-phenylethenyl)Naringenin attenuates the stemness of hepatocellular carcinoma cells by suppressing wnt/β-catenin signaling. J Agric Food Chem. (2019) 67:13939–47. doi: 10.1021/acs.jafc.9b05733
195. Xiao S, Tang H, Bai Y, Zou R, Ren Z, Wu X, et al. Swertiamarin suppresses proliferation, migration, and invasion of hepatocellular carcinoma cells via negative regulation of FRAT1. Eur J Histochem. (2020) 64:3169. doi: 10.4081/ejh.2020.3169
196. Vishnoi K, Ke R, Saini KS, Viswakarma N, Nair RS, Das S, et al. Berberine represses β-catenin translation involving 4E-BPs in hepatocellular carcinoma cells. Mol Pharmacol. (2021) 99:1–16. doi: 10.1124/molpharm.120.000029
197. Zeng J, Liu X, Li X, Zheng Y, Liu B, Xiao Y. Daucosterol inhibits the proliferation, migration, and invasion of hepatocellular carcinoma cells via wnt/β-catenin signaling. Molecules. (2017) 22(6):862. doi: 10.3390/molecules22060862
198. Liu C, Pan J, Liu H, Lin R, Chen Y, Zhang C. Daphnetin inhibits the survival of hepatocellular carcinoma cells through regulating Wnt/β-catenin signaling pathway. Drug Dev Res. (2022) 83:952–60. doi: 10.1002/ddr.21920
199. Qin B, Zeng Z, Xu J, Shangwen J, Ye ZJ, Wang S, et al. Emodin inhibits invasion and migration of hepatocellular carcinoma cells via regulating autophagy-mediated degradation of snail and β-catenin. BMC Cancer. (2022) 22:671. doi: 10.1186/s12885-022-09684-0
200. Zhu J, Qu J, Fan Y, Zhang R, Wang X. Curcumin inhibits invasion and epithelial-mesenchymal transition in hepatocellular carcinoma cells by regulating TET1/wnt/β-catenin signal axis. Bull Exp Biol Med. (2022) 173:770–4. doi: 10.1007/s10517-022-05629-6
201. Yen CH, Lai CC, Shia TH, Chen M, Yu HC, Liu YP, et al. Gynura divaricata attenuates tumor growth and tumor relapse after cisplatin therapy in HCC xenograft model through suppression of cancer stem cell growth and Wnt/β-catenin signalling. J Ethnopharmacol. (2018) 213:366–75. doi: 10.1016/j.jep.2017.07.019
202. Shin S, Son Y, Liu KH, Kang W, Oh S. Cytotoxic activity of broussochalcone a against colon and liver cancer cells by promoting destruction complex-independent β-catenin degradation. Food Chem Toxicol. (2019) 131:110550. doi: 10.1016/j.fct.2019.05.058
203. Reddy D, Ghosh P, Kumavath R. Strophanthidin attenuates MAPK, PI3K/AKT/mTOR, and wnt/β-catenin signaling pathways in human cancers. Front Oncol. (2019) 9:1469. doi: 10.3389/fonc.2019.01469
204. Guo Z, Zhou Y, Yang J, Shao X. Dendrobium candidum extract inhibits proliferation and induces apoptosis of liver cancer cells by inactivating Wnt/β-catenin signaling pathway. BioMed Pharmacother. (2019) 110:371–9. doi: 10.1016/j.biopha.2018.11.149
205. Feng S, Xie X, Chen C, Zuo S, Zhao X, Li H. Alpha-linolenic acid inhibits hepatocellular carcinoma cell growth through Farnesoid X receptor/β-catenin signaling pathway. Nutr Metab. (2022) 19:57. doi: 10.1186/s12986-022-00693-1
206. Zhang Z, Liu T, Yu M, Li K, Li W. The plant alkaloid tetrandrine inhibits metastasis via autophagy-dependent Wnt/β-catenin and metastatic tumor antigen 1 signaling in human liver cancer cells. J OF Exp Clin Cancer Res. (2018) 37:7. doi: 10.1186/s13046-018-0678-6
207. Sang T, Fu YJ, Song L. Polysaccharides from hemerocallis citrina baroni inhibit the growth of hepatocellular carcinoma cells by regulating the wnt/β-catenin pathway. Nutr Cancer. (2023) 75:1658–72. doi: 10.1080/01635581.2023.2216915
208. Lin X, Xiaoqin H, Jiayu C, Li F, Yue L, Ximing X. Long non-coding RNA miR143HG predicts good prognosis and inhibits tumor multiplication and metastasis by suppressing mitogen-activated protein kinase and Wnt signaling pathways in hepatocellular carcinoma. Hepatol Res. (2019) 49:902–18. doi: 10.1111/hepr.13344
209. Bai J, Gao Y, Du Y, Yang X, Zhang X. MicroRNA-300 inhibits the growth of hepatocellular carcinoma cells by downregulating CREPT/Wnt/β-catenin signaling. Oncol Lett. (2019) 18:3743–53. doi: 10.3892/ol
210. Lu C, Liao Z, Cai M, Zhang G. MicroRNA-320a downregulation mediates human liver cancer cell proliferation through the Wnt/β-catenin signaling pathway. Oncol Lett. (2017) 13:573–8. doi: 10.3892/ol.2016.5479
211. Tong Q, Yi M, Kong P, Xu L, Huang W, Niu Y, et al. TRIM36 inhibits tumorigenesis through the Wnt/β-catenin pathway and promotes caspase-dependent apoptosis in hepatocellular carcinoma. Cancer Cell Int. (2022) 22:278. doi: 10.1186/s12935-022-02692-x
212. Zhang X, Tao X, Feng F. Downregulation of C12orf75 gene inhibits migration and invasion of liver cancer cell via suppressing the Wnt/β-catenin signaling pathway in vitro. Biochem Biophys Res Commun. (2022) 614:92–9. doi: 10.1016/j.bbrc.2022.05.018
213. Mosca N, Khoubai FZ, Fedou S, Carrillo-Reixach J, Caruso S, Del Rio-Alvarez A, et al. LIM homeobox-2 suppresses hallmarks of adult and pediatric liver cancers by inactivating MAPK/ERK and wnt/beta-catenin pathways. Liver Cancer. (2022) 11:126–40. doi: 10.1159/000521595
214. Chen HQ, Zhao J, Li Y, Huang YJ, Chen DJ, He LX, et al. Epigenetic inactivation of LHX6 mediated microcystin-LR induced hepatocarcinogenesis via the Wnt/β-catenin and P53 signaling pathways. Environ pollut (Barking Essex 1987). (2019) 252:216–26. doi: 10.1016/j.envpol.2019.05.049
215. Liu Z, Zhong Y, Chen YJ, Chen H. SOX11 regulates apoptosis and cell cycle in hepatocellular carcinoma via Wnt/β-catenin signaling pathway. Biotechnol Appl Biochem. (2019) 66:240–6. doi: 10.1002/bab.1718
216. Su GF, Huang ZX, Huang DL, Chen PX, Wang Y, Wang YF. Cepharanthine hydrochloride inhibits the Wnt/β−catenin/Hedgehog signaling axis in liver cancer. Oncol Rep. (2022) 47(4):83. doi: 10.3892/or
217. Zhang L, Li S, Wang R, Chen C, Ma W, Cai H. Anti-tumor effect of LATS2 on liver cancer death: Role of DRP1-mediated mitochondrial division and the Wnt/β-catenin pathway. BioMed Pharmacother. (2019) 114:108825. doi: 10.1016/j.biopha.2019.108825
218. Turcios L, Chacon E, Garcia C, Eman P, Cornea V, Jiang J, et al. Autophagic flux modulation by Wnt/β-catenin pathway inhibition in hepatocellular carcinoma. PLoS One. (2019) 14:e0212538. doi: 10.1371/journal.pone.0212538
219. Gedaly R, Galuppo R, Daily MF, Shah M, Maynard E, Chen C, et al. Targeting the Wnt/β-catenin signaling pathway in liver cancer stem cells and hepatocellular carcinoma cell lines with FH535. PLoS One. (2014) 9:e99272. doi: 10.1371/journal.pone.0099272
220. Galuppo R, Maynard E, Shah M, Daily MF, Chen C, Spear BT, et al. Synergistic inhibition of HCC and liver cancer stem cell proliferation by targeting RAS/RAF/MAPK and WNT/β-catenin pathways. Anticancer Res. (2014) 34:1709–13.
221. Sun H, Gao Y, Lu K, Zhao G, Li X, Li Z, et al. Overexpression of Klotho suppresses liver cancer progression and induces cell apoptosis by negatively regulating wnt/β-catenin signaling pathway. World J Surg Oncol. (2015) 13:307. doi: 10.1186/s12957-015-0717-0
222. Li TW, Peng H, Yang H, Kurniawidjaja S, Panthaki P, Zheng Y, et al. Lu, S.C. S-Adenosylmethionine and methylthioadenosine inhibit β-catenin signaling by multiple mechanisms in liver and colon cancer. Mol Pharmacol. (2015) 87:77–86. doi: 10.1124/mol.114.095679
223. Zhao H, Zhao L, Wu L, Hu S, Huang Y, Zhao W. Hydrogen sulfide suppresses H2O2-induced proliferation and migration of HepG2 cells through Wnt/β-catenin signaling pathway. Med Oncol (Northwood London England). (2023) 40:214. doi: 10.1007/s12032-023-02091-w
224. Xin RQ, Li WB, Hu ZW, Wu ZX, Sun W. MiR-329-3p inhibits hepatocellular carcinoma cell proliferation and migration through USP22-Wnt/β-Catenin pathway. Eur Rev Med Pharmacol Sci. (2020) 24:9932–9. doi: 10.26355/eurrev_202010_23204
225. Bai Z, Xia X, Lu J. MicroRNA-639 is down-regulated in hepatocellular carcinoma tumor tissue and inhibits proliferation and migration of human hepatocellular carcinoma cells through the KAT7/wnt/β-catenin pathway. Med Sci MONITOR. (2020) 26:e919241. doi: 10.12659/MSM.919241
226. Jia P, Wei G, Zhou C, Gao Q, Wu Y, Sun X, et al. Upregulation of miR-212 inhibits migration and tumorigenicity and inactivates wnt/β-catenin signaling in human hepatocellular carcinoma. Technol Cancer Res Treat. (2018) 17:1533034618765221. doi: 10.1177/1533034618765221
227. Mao J, Wang D, Wang Z, Tian W, Li X, Duan J, et al. Combretastatin A-1 phosphate, a microtubule inhibitor, acts on both hepatocellular carcinoma cells and tumor-associated macrophages by inhibiting the Wnt/β-catenin pathway. Cancer Lett. (2016) 380:134–43. doi: 10.1016/j.canlet.2016.06.020
228. Siqueira E, Concato VM, Tomiotto-Pellissier F, Silva TF, Bortoleti B, Gonçalves MD, et al. Trans-chalcone induces death by autophagy mediated by p53 up-regulation and β-catenin down-regulation on human hepatocellular carcinoma HuH7.5 cell line. Phytomedicine. (2021) 80:153373. doi: 10.1016/j.phymed.2020.153373
229. Tian W, Li J, Wang Z, Zhang T, Han Y, Liu Y, et al. HYD-PEP06 suppresses hepatocellular carcinoma metastasis, epithelial-mesenchymal transition and cancer stem cell-like properties by inhibiting PI3K/AKT and WNT/β-catenin signaling activation. Acta Pharm Sin B. (2021) 11:1592–606. doi: 10.1016/j.apsb.2021.03.040
230. Zhu M, Gong Z, Wu Q, Su Q, Yang T, Yu R, et al. Homoharringtonine suppresses tumor proliferation and migration by regulating EphB4-mediated β-catenin loss in hepatocellular carcinoma. Cell Death Dis. (2020) 11:632. doi: 10.1038/s41419-020-02902-2
231. Xie L, Li M, Liu D, Wang X, Wang P, Dai H, et al. Secalonic acid-F, a novel mycotoxin, represses the progression of hepatocellular carcinoma via MARCH1 regulation of the PI3K/AKT/β-catenin signaling pathway. Molecules. (2019) 24(3):393. doi: 10.3390/molecules24030393
232. Mavila N, Tang Y, Berlind J, Ramani K, Wang J, Mato JM, et al. Prohibitin 1 acts as a negative regulator of wingless/integrated-beta-catenin signaling in murine liver and human liver cancer cells. Hepatol Commun. (2018) 2:1583–600. doi: 10.1002/hep4.1257
233. Liu X, Shan W, Li T, Gao X, Kong F, You H, et al. Cellular retinol binding protein-1 inhibits cancer stemness via upregulating WIF1 to suppress Wnt/β-catenin pathway in hepatocellular carcinoma. BMC Cancer. (2021) 21:1224. doi: 10.1186/s12885-021-08967-2
234. Chen QF, Shi F, Huang T, Huang C, Shen L, Wu P, et al. ASTN1 is associated with immune infiltrates in hepatocellular carcinoma, and inhibits the migratory and invasive capacity of liver cancer via the Wnt/β−catenin signaling pathway. Oncol Rep. (2020) 44:1425–40. doi: 10.3892/or
235. Zhao J, Wang Y, Han M, Lu H, Chen X, Liu S, et al. P7TP3 inhibits tumor development, migration, invasion and adhesion of liver cancer through the Wnt/β-catenin signaling pathway. Cancer Sci. (2020) 111:994–1007. doi: 10.1111/cas.14243
236. Liao S, Chen H, Liu M, Gan L, Li C, Zhang W, et al. Aquaporin 9 inhibits growth and metastasis of hepatocellular carcinoma cells via Wnt/β-catenin pathway. Aging. (2020) 12:1527–44. doi: 10.18632/aging.v12i2
237. Mani S, Yan B, Cui Z, Sun J, Utturkar S, Foca A, et al. Restoration of RNA helicase DDX5 suppresses hepatitis B virus (HBV) biosynthesis and Wnt signaling in HBV-related hepatocellular carcinoma. Theranostics. (2020) 10:10957–72. doi: 10.7150/thno.49629
238. Lei C, Wang Q, Tang N, Wang K. GSTZ1-1 downregulates Wnt/β-catenin signalling in hepatocellular carcinoma cells. FEBS Open Bio. (2020) 10:6–17. doi: 10.1002/2211-5463.12769
239. Song G, Tian L, Cheng Y, Liu J, Wang K, Li S, et al. Antitumor activity of sevoflurane in HCC cell line is mediated by miR-29a-induced suppression of Dnmt3a. J Cell Biochem. (2019) 120:18152–61. doi: 10.1002/jcb.29121
240. Cao Y, Lv W, Ding W, Li J. Sevoflurane inhibits the proliferation and invasion of hepatocellular carcinoma cells through regulating the PTEN/Akt/GSK−3β/β−catenin signaling pathway by downregulating miR−25−3p. Int J Mol Med. (2020) 46:97–106. doi: 10.3892/ijmm
241. Ou W, Lv J, Zou X, Yao Y, Wu J, Yang J, et al. Propofol inhibits hepatocellular carcinoma growth and invasion through the HMGA2-mediated Wnt/β-catenin pathway. Exp Ther Med. (2017) 13:2501–6. doi: 10.3892/etm.2017.4253
242. Gong T, Ning X, Deng Z, Liu M, Zhou B, Chen X, et al. Propofol-induced miR-219-5p inhibits growth and invasion of hepatocellular carcinoma through suppression of GPC3-mediated Wnt/β-catenin signalling activation. J Cell Biochem. (2019) 120:16934–45. doi: 10.1002/jcb.28952
243. Mirzaei S, Mahabady MK, Zabolian A, Abbaspour A, Fallahzadeh P, Noori M, et al. (siRNA) to target genes and molecular pathways in glioblastoma therapy: Current status with an emphasis on delivery systems. Life Sci. (2021) 275:119368. doi: 10.1016/j.lfs.2021.119368
244. Ashrafizadeh M, Delfi M, Hashemi F, Zabolian A, Saleki H, Bagherian M, et al. Biomedical application of chitosan-based nanoscale delivery systems: Potential usefulness in siRNA delivery for cancer therapy. Carbohydr Polym. (2021) 260:117809. doi: 10.1016/j.carbpol.2021.117809
245. Bajan S, Hutvagner G. RNA-based therapeutics: From antisense oligonucleotides to miRNAs. Cells. (2020) 9:137. doi: 10.3390/cells9010137
246. Zeng G, Apte U, Cieply B, Singh S, Monga SP. siRNA-mediated beta-catenin knockdown in human hepatoma cells results in decreased growth and survival. Neoplasia. (2007) 9:951–9. doi: 10.1593/neo.07469
247. Wang XH, Sun X, Meng XW, Lv ZW, Du YJ, Zhu Y, et al. Jin, S.Z. beta-catenin siRNA regulation of apoptosis- and angiogenesis-related gene expression in hepatocellular carcinoma cells: potential uses for gene therapy. Oncol Rep. (2010) 24:1093–9.
Keywords: Wnt pathway, hepatocellular carcinoma, tumorigenesis, progression, therapy resistance, mechanism, therapy
Citation: Zhao Z, Cui T, Wei F, Zhou Z, Sun Y, Gao C, Xu X and Zhang H (2024) Wnt/β-Catenin signaling pathway in hepatocellular carcinoma: pathogenic role and therapeutic target. Front. Oncol. 14:1367364. doi: 10.3389/fonc.2024.1367364
Received: 08 January 2024; Accepted: 19 March 2024;
Published: 02 April 2024.
Edited by:
Anurag Kumar Singh, Martin Luther University of Halle-Wittenberg, GermanyReviewed by:
Zaki A. Sherif, Howard University, United StatesAmrita Rai, Max Planck Institute for Molecular Physiology, Germany
Copyright © 2024 Zhao, Cui, Wei, Zhou, Sun, Gao, Xu and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Huihan Zhang, MTM5MTkyNDQ2NzNAMTYzLmNvbQ==
†These authors have contributed equally to this work
 Zekun Zhao
Zekun Zhao Tenglu Cui1,3†
Tenglu Cui1,3† Fengxian Wei
Fengxian Wei Xiaodong Xu
Xiaodong Xu