
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Oncol., 18 March 2024
Sec. Surgical Oncology
Volume 14 - 2024 | https://doi.org/10.3389/fonc.2024.1366607
This article is part of the Research TopicLiver Cancer Awareness Month 2023: Current Progress and Future Prospects on Advances in Primary Liver Cancer Investigation and TreatmentView all 21 articles
 Alessandro Martinino1
Alessandro Martinino1 Angela Bucaro2
Angela Bucaro2 Francesca Cardella3
Francesca Cardella3 Ishaan Wazir4
Ishaan Wazir4 Francesco Frongillo2
Francesco Frongillo2 Francesco Ardito5
Francesco Ardito5 Francesco Giovinazzo2*
Francesco Giovinazzo2*Background: HCC is a major global health concern, necessitating effective treatment strategies. This study conducts a meta-analysis of meta-analyses comparing liver resection (LR) and liver transplantation (LT) for HCC.
Methods: The systematic review included meta-analyses comparing liver resection vs. liver transplantation in HCC, following PRISMA guidelines. Primary outcomes included 5-year overall survival (OS) and disease-free survival (DFS). AMSTAR-2 assessed study quality. Citation matrix and hierarchical clustering validated the consistency of the included studies.
Results: A search identified 10 meta-analyses for inclusion. The median Pearson correlation coefficient for citations was 0.59 (IQR 0.41-0.65). LT showed better 5-year survival and disease-free survival in all HCC (OR): 0.79; 95% CI: 0.67-0.93, I^2:57% and OR: 0.44; 95% CI: 0.25-0.75, I^2:96%). Five-year survival in early HCC and ITT was 0.63 (95% CI: 0.50-0.78, I^2:0%) and 0.60 (95% CI: 0.39-0.92, I^2:0%). Salvage LT vs. Primary LT did not differ between 5-year survival and disease-free survival (OR: 0.62; 95% CI: 0.33-1.15, I^2:0% and 0.93; 95% CI: 0.82-1.04, I^2:0%).
Conclusion: Overall, the study underscores the superior survival outcomes associated with LT over LR in HCC treatment, supported by comprehensive meta-analysis and clustering analysis. There was no difference in survival or recurrence rate between salvage LT and primary LT. Therefore, considering the organ shortage, HCC can be resected and transplanted in case of recurrence.
Hepatocellular carcinoma (HCC), with 782000 cases diagnosed and 746 000 deaths in 2012 and an age-adjusted worldwide incidence of 10·1 cases per 100 000 person-years (1), is the sixth most common cancer and the third-leading cause of cancer-related mortality in the world (1, 2).
HCC usually develops in the setting of chronic liver diseases, such as cirrhosis, infections like hepatitis B or C, non-alcoholic fatty liver disease, or alcohol-related liver disease (1–3). Most HCCs (80%) occur in sub-Saharan Africa and eastern Asia, where the main risk factors are chronic hepatitis B and aflatoxin B1 exposure. Instead, in the USA, Europe, and Japan, hepatitis C is the leading risk factor, together with excessive alcohol intake (1, 4, 5).
The management of HCC depends on several factors, including the size and number of tumours, the underlying liver function, and the patient’s overall health status (6, 7). Liver resection (LR) and transplantation (LT) are the most effective curative treatments for HCC, with promising outcomes in survival and disease-free survival (DFS) (1, 8–10). In patients without clinically significant portal hypertension (CSPH), compensated liver function, and early HCC stages, LR achieves 70% 5-year survival in HCC. However, the survival rate decreases by 50% when those adverse factors are present (1). On the other hand, 5-year survival in HCC after LT is more than 70% with a recurrence rate of less than 10–15% (1) (11). However, the choice of the two treatments is also limited by the availability of donor organs. Therefore, choosing between LT and LR for HCC in several cases is still controversial (7, 10).
As robust evidence is missing with contrasting results, the objective of the present study was to perform a survival meta-analysis of meta-analyses to compare LT and LR in HCC. The primary outcomes were 5-year overall and disease-free survival after the two different types of treatment.
The systematic review and meta-analysis were conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.
A computerised search of PubMed, Scopus and Cochrane Library was carried out. Reference lists of all obtained and relevant articles were screened manually and cross-referenced to identify any additional studies. Articles published from the time of inception to June 2023 were included. An advanced search was performed using the following terms: [(transplant) OR (transplantation)] AND (hepatocellular) OR (HCC) OR (liver cancer).
The primary outcomes were 5-year graft overall (OS) and disease-free survival (DFS) in liver resection vs. liver transplantation in all HCCs. The secondary outcomes were OS and DSF in early HCC, Intention to treat, and salvage liver transplantation for HCC.
The systematic review included meta-analyses comparing liver resection vs. liver transplantation in HCC and reporting the primary and secondary outcomes. Abstracts, letters, comments, editorials and expert opinions, unpublished articles and abstracts, reviews without original data, and case reports were excluded from the analysis. Studies were included only when reporting the number or the rate of events (deaths or recurrences). Two reviewers (AM and IW) independently screened the titles and abstracts of all retrieved articles. The full texts of articles that could fulfil the inclusion criteria were obtained and checked for eligibility.
The internal validity of the meta-analyses was assessed by the Assessment of Multiple Systematic Reviews 2 (AMSTAR-2) method. AMSTAR is a standardised and reliable method for assessing the quality of systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. FG and FC completed the AMSTAR proforma for all included reviews, and discrepancies were discussed to reach a consensus. Studies were, finally, classified on the level of quality through the online tool calculator (12).
A sample citation matrix was created by measuring the primary overlap of every included study (Supplementary Table 1), and the Pearson correlation coefficient (r) was calculated. r was visualised through a heatmap. A hierarchical cluster analysis of the r was visualised through a dendrogram clusterisation and a silhouette analysis used to identify the number of clusters (13).
The results of the meta-analyses were combined using a summary meta-analysis model for odds ratios (OR) and hazard ratio (HR) with 95% confidence intervals.
The fixed-effect method was used to combine the results without statistically significant heterogeneity. The random-effect method was used when heterogeneity was confirmed (p ≤0·10). Potential publication bias was investigated by funnel plot. Egger’s and Begger’s tests were used to assess funnel plot asymmetry and biases [12], and Makaskill’s test was used to quantify the bias (14). P <0·05 (two-tailed) was considered to indicate statistical significance [13]. Trim-and-fill method was used to adjust for the publication biases.
The meta-analysis of meta-analyses and hierarchical analysis was performed using the R software suite (v3.4.0, https://www.R-project.org). Statistical heterogeneity between metanalysis was evaluated by χ2 and I2, with significance set at p ≤0,10 (14–16).
The PRISMA flow diagram reports the number of studies screened, assessed, and excluded (Figure 1). 19 full-text articles were assessed for eligibility, and 10 meta-analyses comparing an overall 105 studies were included in the umbrella review (11, 17–25). The characteristics of the included meta-analyses are shown in Table 1.
Authors of five of the eight meta-analyses cited the previously published meta-analyses, and only one study had no prior studies available to cite (Table 2). Every included study used Medline/PubMed as part of the literature search, and nine studies also used Embase (Table 3). There was variation in the utilisation of other databases, but every study (excluding two) used at least two electronic databases. According to the AMSTAR quality assessment, four studies rated low quality and six critically low quality (Table 4). The median Pearson correlation coefficient was 0.59 (IQR 0.41-0.65) for all the included studies (Figure 2A). Hierarchical clustering of the r identified 3 clusters after silhouette analysis (Cluster Sizes and Average Silhouette Widths: Cluster 1 (26 data points): Average Silhouette Width of 0.443; Cluster 2 (62 data points): Average Silhouette Width of 0.724; Cluster 3 (12 data points): Average Silhouette Width of 0.909); (Median: 0.7341 IQR: 0.5543- 0.8369; Mean: 0.6731 Range 0.1244-0.9534. (Figure 2B).
LT showed better 5-year survival in all HCC (Odd Ratio (OR): 0.79; 95% CI: 0.67-0.93, I^2:57%), (Figure 3A), Egger’s test showed a significant funnel plot asymmetry (t = -2.62, df = 5, p = 0.0468). Begg’s test did not find funnel plot asymmetry (z = -1.05, p = 0.2931) (Figure 3B). After the 5-year survival Trim-and-fill method, both Egger’s and Begg’s tests did not show evidence of publication bias (t = -0.07, df = 9, p-value = 0.9437 and z = -0.08, p-value = 0.9372, respectively) (Figure 3C).
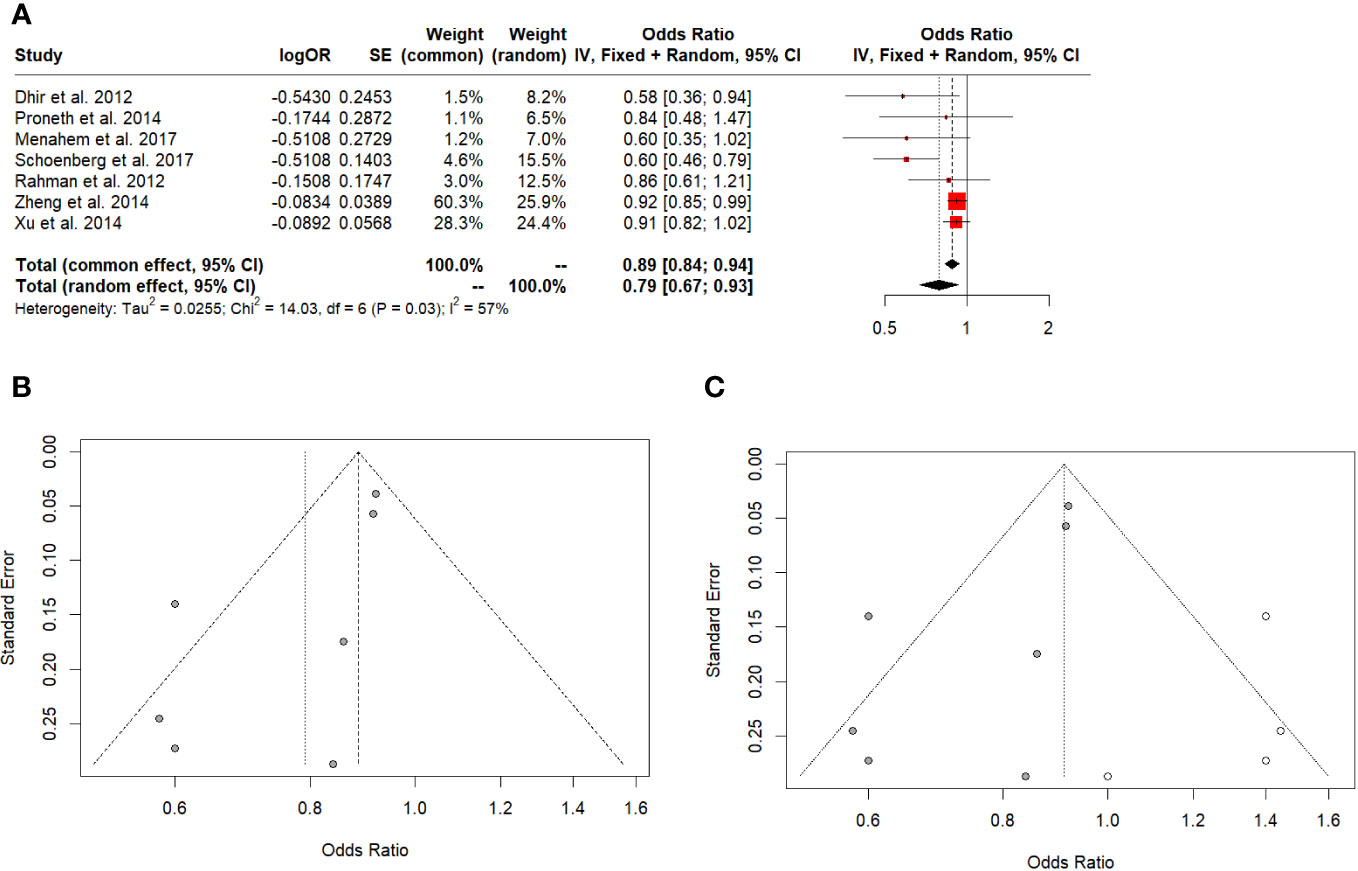
Figure 3 (A) 5-year overall survival in all HCC. (B) Funnel plot. (C) Funnel plot after Trim-and-fill.
DFS favoured LT for all HCC (OR: 0.44; 95% CI: 0.25-0.75, I^2:96%) (Figure 4A). The Egger’s test (t = 0.02, df = 3, p-value = 0.9879) and Begg’s test (z = -0.68, p-value = 0.4969) did not indicate significant publication bias in the original analysis (Figure 4B). After applying the Trim-and-fill method, the Egger’s test (t = 0.02, df = 3, p-value = 0.9879) and Begg’s test (z = -0.76, p-value = 0.4485) still did not show significant evidence of publication bias (Figure 4C).
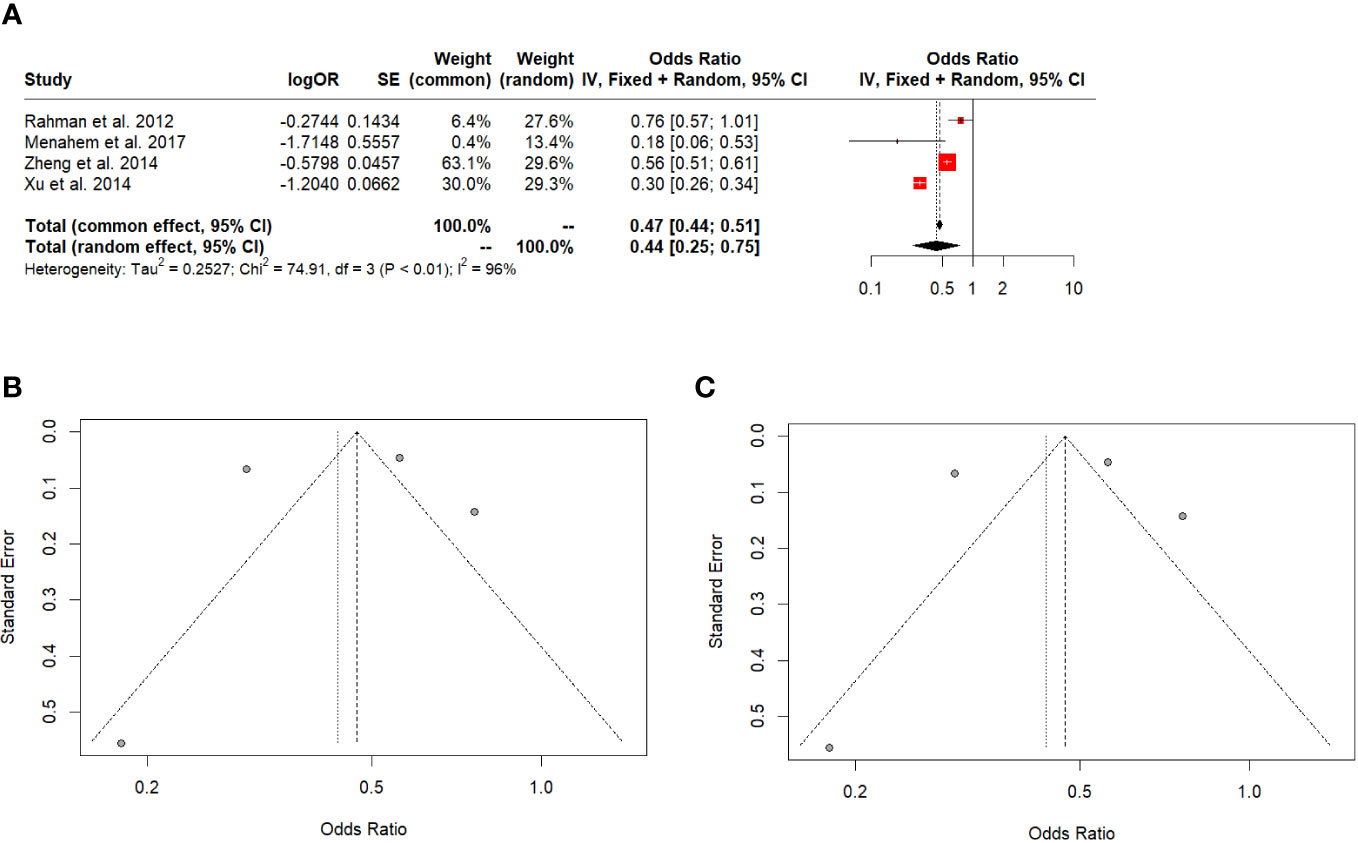
Figure 4 (A) 5-year disease-free survival in all HCC. (B) Funnel plot. (C) Funnel plot after Trim-and-fill.
Two studies reported the HR for overall and disease-free survival favouring LT over liver resection (1.30, 95% CI: 1.10-1.55, I^2: 24% and 2.46, 95% CI: 2.03-2.99, I^2: 47%) (Figures 5A, B).
Five-year survival in early HCC and ITT was 0.63 (95% CI: 0.50-0.78, I^2:0%), (Figure 6A) and 0.60 (95% CI: 0.39-0.92, I^2:0%), respectively (Figure 6B). Salvage LT vs. Primary LT did not differ between 5-year survival and DFS (OR: 0.62; 95% CI: 0.33-1.15, I^2:0% and 0.93; 95% CI: 0.82-1.04, I^2:0%) (Figures 7A, B).
Comparing the outcomes of LT and LR in HCC is crucial because it can inform the decision-making process for selecting the most appropriate treatment option for individual patients (1, 11, 21). By identifying the best treatment between LT and LR, healthcare providers improve the patient’s overall survival and quality of life. Furthermore, there is a shortage of donor organs worldwide, so optimising organ allocation is central to HCC. In some cases, LR may be a viable alternative to LT as a definitive treatment, especially for patients with early-stage HCC and those with limited underlying liver disease or bridge therapy in case of cancer recurrences (10, 27–29). The study included a large cohort of patients, which is a relatively large sample size and may increase the reliability of the findings.
Furthermore, the study conducted a systematic review and meta-analysis of multiple meta-analyses, which may provide a more comprehensive picture of the topic. Also, the study conducted subgroup analyses for different types of HCC and liver transplantation, which may help identify specific factors that influence outcomes.
LT showed better OS and DFS than LR for HCC. However, survival after retransplantation for cancer recurrences was equal to primary LT for HCC. The finding agreed with the included meta-analyses, independently from the correlation matrix and the cluster analysis.
The results of the meta-analysis provide valuable insights into the comparative effectiveness of liver transplantation (LT) and liver resection for hepatocellular carcinoma (HCC) in terms of 5-year overall survival, disease-free survival, and hazard ratio (HR) for overall survival. These findings align with the evolving body of research in the field, which examines the optimal treatment approaches for HCC patients.
While the meta-analysis indicates funnel plot asymmetry through Egger’s test, this could suggest the presence of publication bias that may skew the results. Using the Trim-and-fill method to address publication bias enhances the reliability of the findings. The favourable disease-free survival outcomes favouring LT over liver resection for all HCC cases align with previous research suggesting that LT can lead to more extended periods without recurrence (30, 31). The absence of significant publication bias in the initial analysis and after using the Trim-and-fill method adds confidence to these findings.
Furthermore, the HR analysis suggests that LT may be associated with better overall survival than liver resection, as the HR favours LT. The I^2 value of 24% suggests moderate heterogeneity, indicating relatively consistent results among the studies included.
The quality assessment of the included studies reveals that there was at least a critical flaw in the meta-analysis methodology. Many of the studies under consideration did not adequately address the potential risks of bias in their analyses, nor did they thoroughly discuss how these biases might influence the outcomes reported in the review. This oversight raises concerns about the robustness and reliability of the findings presented in these studies (32). Biases, whether related to study design, data collection, or reporting, can introduce systematic errors that may distort the overall conclusions of a meta-analysis. Failing to acknowledge and address these biases can undermine the validity and credibility of the study’s results. It is essential for future research to comprehensively evaluate and report on the potential biases and their potential impact to ensure the accuracy and reliability of the meta-analytic findings.
There was some heterogeneity in the data, particularly in the DFS analysis, possibly due to differences in study design and patient populations. Therefore, despite the present findings, individual patient factors and clinical considerations should still be considered when determining the most appropriate treatment approach for HCC (31).
The correlation analysis of the present study indicates a moderate association level between the variables, while hierarchical clustering identified three distinct clusters based on the correlation coefficients. The integration of hierarchical clustering analysis to validate the consistency of findings adds further strength to the results. The silhouette analysis suggests these clusters are well-defined, with different data points forming cohesive groups. The three clusters showed good separation and assignment of data points to clusters, confirming a consistent agreement among the meta-analyses about the advantage of LT over LR, independently from the included studies.
Several potential sources of bias in this study should be considered. While the results and conclusions of the study may provide valuable insights into the overall management of HCC, it is essential to consider the heterogeneity of the patient population and the specific clinical contexts when interpreting the findings for different subgroups of patients. A limitation of the present study was the difficulties in drawing the same conclusions for patients with HCC within or outside Milan criteria, undergoing a first or a salvage transplantation. Similarly, whether the manuscript included three meta-analyses, reporting outcomes in ITT patients, the lack of robust data may result in a positive outcome for the LT group and in a disadvantage in the LR group. Another potential source of bias is measurement bias, as the determination of survival and disease-free survival may be affected by factors such as follow-up time, surveillance protocols, and the definition of recurrence. Finally, there may be publication bias, as studies with negative or null findings may be less likely to be published or included in systematic reviews and meta-analyses (33, 34).
By systematically analysing the citation matrix, the authors identified clusters of meta-analysis indicating potential overlap or duplication. However, the association was moderate, and the primary outcomes results consistent. The integration of hierarchical clustering analysis to validate the consistency of findings added further strength to the results. The silhouette analysis suggested these clusters were well-defined, with different data points forming cohesive groups. The three clusters showed good separation and assignment of data points to clusters, confirming a consistent agreement among the meta-analyses about the advantage of LT over LR, independently from the included studies.
Future research could explore the impact of patient-specific characteristics on treatment effectiveness, investigate new biomarkers for patient selection, develop individualised treatment algorithms, and assess novel therapies in combination with surgical interventions to improve outcomes.
In conclusion, the study’s findings consistently suggest that LT offers better 5-year and disease-free survival rates than LR for HCC. These results hold significance for clinical practice, as they provide insights into the most effective treatment approach for HCC patients. The study underscores the importance of addressing biases and limitations in meta-analyses and highlights potential areas for future research to enhance HCC treatment strategies.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
AM: Conceptualization, Formal analysis, Methodology, Writing – original draft, Writing – review & editing. AB: Writing – original draft, Writing – review & editing. FC: Writing – original draft, Writing – review & editing. IW: Writing – original draft, Writing – review & editing. FF: Writing – original draft, Writing – review & editing. FA: Writing – original draft, Writing – review & editing. FG: Writing – original draft, Writing – review & editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2024.1366607/full#supplementary-material
1. Forner A, Reig M, Bruix J. Hepatocellular carcinoma. Lancet (London England). (2018) 391:1301–14. doi: 10.1016/S0140-6736(18)30010-2
2. Konyn P, Ahmed A, Kim D. Current epidemiology in hepatocellular carcinoma. Expert Rev Gastroenterol hepatol. (2021) 15:1295–307. doi: 10.1080/17474124.2021.1991792
3. Massarweh NN, El-Serag HB. Epidemiology of hepatocellular carcinoma and intrahepatic cholangiocarcinoma. Cancer Control. (2017) 24:1073274817729245. doi: 10.1177/1073274817729245
4. Kulik L, El-Serag HB. Epidemiology and management of hepatocellular carcinoma. Gastroenterology. (2019) 156:477–491.e471.
5. Morgan TR, Mandayam S, Jamal MM. Alcohol and hepatocellular carcinoma. Gastroenterology. (2004) 127:S87–96. doi: 10.1053/j.gastro.2004.09.020
6. Yang JD, Heimbach JK. New advances in the diagnosis and management of hepatocellular carcinoma. BMJ. (2020) 371:m3544. doi: 10.1136/bmj.m3544
7. Yang JD, Hainaut P, Gores GJ, Amadou A, Plymoth A, Roberts LR. A global view of hepatocellular carcinoma: trends, risk, prevention and management. Nat Rev Gastroenterol hepatol. (2019) 16:589–604. doi: 10.1038/s41575-019-0186-y
8. Gilles H, Garbutt T, Landrum J. Hepatocellular carcinoma. Crit Care Nurs Clin North Am. (2022) 34:289–301. doi: 10.1016/j.cnc.2022.04.004
9. Zhang W, Zhang B, Chen XP. Adjuvant treatment strategy after curative resection for hepatocellular carcinoma. Front Med. (2021) 15:155–69. doi: 10.1007/s11684-021-0848-3
10. Vibert E, Schwartz M, Olthoff KM. Advances in resection and transplantation for hepatocellular carcinoma. J Hepatol. (2020) 72:262–76. doi: 10.1016/j.jhep.2019.11.017
11. Rahman A, Assifi MM, Pedroso FE, Maley WR, Sola JE, Lavu H, et al. Is resection equivalent to transplantation for early cirrhotic patients with hepatocellular carcinoma? A meta-analysis. J gastrointestinal Surg. (2012) 16:1897–909. doi: 10.1007/s11605-012-1973-8
12. Shea BJ, Reeves BC, Wells G, Thuku M, Hamel C, Moran J, et al. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ. (2017) 358:j4008. doi: 10.1136/bmj.j4008
13. Zhang Z, Murtagh F, Van Poucke S, Lin S, Lan P. Hierarchical cluster analysis in clinical research with heterogeneous study population: highlighting its visualization with R. Ann Trans Med. (2017) 5:75. doi: 10.21037/atm
14. Trikalinos TA, Salanti G, Zintzaras E, Ioannidis JP. Meta-analysis methods. Adv Genet. (2008) 60:311–34. doi: 10.1016/S0065-2660(07)00413-0
15. Lin L. Comparison of four heterogeneity measures for meta-analysis. J Eval Clin Pract. (2020) 26:376–84. doi: 10.1111/jep.13159
16. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. (2003) 327:557–60. doi: 10.1136/bmj.327.7414.557
17. Dhir M, Lyden ER, Smith LM, Are C. Comparison of outcomes of transplantation and resection in patients with early hepatocellular carcinoma: a meta-analysis. HPB (Oxford). (2012) 14:635–45. doi: 10.1111/j.1477-2574.2012.00500.x
18. Koh JH, Tan DJH, Ong Y, Lim WH, Ng CH, Tay PWL, et al. Liver resection versus liver transplantation for hepatocellular carcinoma within Milan criteria: a meta-analysis of 18,421 patients. Hepatobiliary Surg Nutr. (2022) 11:78–93. doi: 10.21037/hbsn
19. Li HY, Wei YG, Yan LN, Li B. Salvage liver transplantation in the treatment of hepatocellular carcinoma: a meta-analysis. World J Gastroenterol. (2012) 18:2415–22. doi: 10.3748/wjg.v18.i19.2415
20. Li W, Li L, Han J, Wu H. Liver transplantation vs liver resection in patients with HBV-related hepatocellular carcinoma beyond Milan criterion: A meta-analysis. Clin transpl. (2018) 32:e13193. doi: 10.1111/ctr.13193
21. Menahem B, Lubrano J, Duvoux C, Mulliri A, Alves A, Costentin C, et al. Liver transplantation versus liver resection for hepatocellular carcinoma in intention to treat: An attempt to perform an ideal meta-analysis. Liver Transplant. (2017) 23:836–44. doi: 10.1002/lt.24758
22. Proneth A, Zeman F, Schlitt HJ, Schnitzbauer AA. Is resection or transplantation the ideal treatment in patients with hepatocellular carcinoma in cirrhosis if both are possible? A systematic review and metaanalysis. Ann Surg Oncol. (2014) 21:3096–107. doi: 10.1245/s10434-014-3808-1
23. Schoenberg MB, Bucher JN, Vater A, Bazhin AV, Hao J, Guba MO, et al. Resection or transplant in early hepatocellular carcinoma. Deutsches Arzteblatt Int. (2017) 114:519–26. doi: 10.3238/arztebl.2017.0519
24. Xu XS, Liu C, Qu K, Song YZ, Zhang P, Zhang YL. Liver transplantation versus liver resection for hepatocellular carcinoma: a meta-analysis. Hepatobiliary pancreatic Dis Int. (2014) 13:234–41. doi: 10.1016/S1499-3872(14)60037-0
25. Zheng Z, Liang W, Milgrom DP, Zheng Z, Schroder PM, Kong NS, et al. Liver transplantation versus liver resection in the treatment of hepatocellular carcinoma: a meta-analysis of observational studies. Transplantation. (2014) 97:227–34. doi: 10.1097/TP.0b013e3182a89383
26. Kostakis ID, Machairas N, Prodromidou A, Stamopoulos P, Garoufalia Z, Fouzas I, et al. Comparison between salvage liver transplantation and repeat liver resection for recurrent hepatocellular carcinoma: A systematic review and meta-analysis. Transplant Proc. (2019) 51:433–6. doi: 10.1016/j.transproceed.2019.01.072
27. Vogel A, Martinelli E,Y2xpbmljYWxndWlkZWxpbmVzQGVzbW8ub3JnEGCEa, Committee EG. Updated treatment recommendations for hepatocellular carcinoma (HCC) from the ESMO Clinical Practice Guidelines. Ann Oncol. (2021) 32:801–5. doi: 10.1016/j.annonc.2021.02.014
28. Lee KK, Kim DG, Moon IS, Lee MD, Park JH. Liver transplantation versus liver resection for the treatment of hepatocellular carcinoma. J Surg Oncol. (2010) 101:47–53. doi: 10.1002/jso.21415
29. Krenzien F, Schmelzle M, Struecker B, Raschzok N, Benzing C, Jara M, et al. Liver transplantation and liver resection for cirrhotic patients with hepatocellular carcinoma: comparison of long-term survivals. J gastrointestinal Surg. (2018) 22:840–8. doi: 10.1007/s11605-018-3690-4
30. Kanneganti M, Mahmud N, Kaplan DE, Taddei TH, Goldberg DS. Survival benefit of liver transplantation for hepatocellular carcinoma. Transplantation. (2020) 104:104–12. doi: 10.1097/TP.0000000000002816
31. Di Sandro S, Sposito C, Ravaioli M, Lauterio A, Magistri P, Bongini M, et al. Surgical treatment of hepatocellular carcinoma: multicenter competing-risk analysis of tumor-related death following liver resection and transplantation under an intention-to-treat perspective. Transplantation. (2023) 107:1965–75. doi: 10.1097/TP.0000000000004593
32. Viswanathan M, Ansari MT, Berkman ND, Chang S, Hartling L, McPheeters M, et al. Risk of bias of individual studies in systematic reviews of health care interventions. In: Methods Guide for Effectiveness and Comparative Effectiveness Reviews. Agency for Healthcare Research and Quality (US, Rockville (MD (2012). p. 2008–.
33. Joober R, Schmitz N, Annable L, Boksa P. Publication bias: what are the challenges and can they be overcome? J Psychiatry Neurosci. (2012) 37:149–52. doi: 10.1503/jpn.110175
34. Nair AS. Publication bias - Importance of studies with negative results! Indian J anaesthesia. (2019) 63:505–7. doi: 10.4103/ija.IJA_142_19
35. Adam R, Azoulay D, Castaing D, et al. Liver resection as a bridge to transplantation for hepatocellular carcinoma on cirrhosis: a reasonable strategy? Ann Surg. (2003) 238:508–518; discussion 518-509. doi: 10.1097/01.sla.0000090449.87109.44
36. Adam R, Bhangui P, Vibert E, et al. Resection or transplantation for early hepatocellular carcinoma in a cirrhotic liver: does size define the best oncological strategy? Ann Surg. (2012) 256:883–91. doi: 10.1097/SLA.0b013e318273bad0
37. Aksoy SO, Unek T, Sevinc AI, et al. Comparison of resection and liver transplant in treatment of hepatocellular carcinoma. Exp Clin Transplant. (2020) 18:712–8. doi: 10.6002/ect
38. Baccarani U, Isola M, Adani GL, et al. Superiority of transplantation versus resection for the treatment of small hepatocellular carcinoma. Transplant Int. (2008) 21:247–54. doi: 10.1111/j.1432-2277.2007.00597.x
39. Belghiti J, Cortes A, Abdalla EK, et al. Resection prior to liver transplantation for hepatocellular carcinoma. Ann Surg. (2003) 238:885–892; discussion 892-883. doi: 10.1097/01.sla.0000098621.74851.65
40. Bellavance EC, Lumpkins KM, Mentha G, et al. Surgical management of early-stage hepatocellular carcinoma: resection or transplantation? J gastrointestinal Surg. (2008) 12:1699–708. doi: 10.1007/s11605-008-0652-2
41. Bigourdan JM, Jaeck D, Meyer N, et al. Small hepatocellular carcinoma in Child A cirrhotic patients: hepatic resection versus transplantation. Liver Transplant. (2003) 9:513–20. doi: 10.1053/jlts.2003.50070
42. Bismuth H, Chiche L, Adam R, Castaing D, Diamond T, Dennison A. Liver resection versus transplantation for hepatocellular carcinoma in cirrhotic patients. Ann Surg. (1993) 218:145–51. doi: 10.1097/00000658-199308000-00005
43. Borie F, Bouvier AM, Herrero A, et al. Treatment and prognosis of hepatocellular carcinoma: a population based study in France. J Surg Oncol. (2008) 98:505–9. doi: 10.1002/jso.21159
44. Bronowicki JP, Boudjema K, Chone L, et al. Comparison of resection, liver transplantation and transcatheter oily chemoembolization in the treatment of hepatocellular carcinoma. J Hepatol. (1996) 24:293–300. doi: 10.1016/S0168-8278(96)80007-9
45. Canter RJ, Patel SA, Kennedy T, et al. Comparative analysis of outcome in patients with hepatocellular carcinoma exceeding the milan criteria treated with liver transplantation versus partial hepatectomy. Am J Clin Oncol. (2011) 34:466–71. doi: 10.1097/COC.0b013e3181ec63dd
46. Cha CH, Ruo L, Fong Y, et al. Resection of hepatocellular carcinoma in patients otherwise eligible for transplantation. Ann Surg. (2003) 238:315–321; discussion 321-313. doi: 10.1097/01.sla.0000086548.84705.ef
47. Chan SC, Fan ST, Chok KS, et al. Survival advantage of primary liver transplantation for hepatocellular carcinoma within the up-to-7 criteria with microvascular invasion. Hepatol Int. (2012) 6:646–56. doi: 10.1007/s12072-011-9318-3
48. Chan AC, Chan SC, Chok KS, et al. Treatment strategy for recurrent hepatocellular carcinoma: salvage transplantation, repeated resection, or radiofrequency ablation? Liver Transplant. (2013) 19:411–9. doi: 10.1002/lt.v19.4
49. Chapman WC, Klintmalm G, Hemming A, et al. Surgical treatment of hepatocellular carcinoma in North America: can hepatic resection still be justified? J Am Coll Surg. (2015) 220:628–37. doi: 10.1016/j.jamcollsurg.2014.12.030
50. Choi GH, Kim DH, Kang CM, et al. Prognostic factors and optimal treatment strategy for intrahepatic nodular recurrence after curative resection of hepatocellular carcinoma. Ann Surg Oncol. (2008) 15:618–29. doi: 10.1245/s10434-007-9671-6
51. Chuan W, Li C, Wen TF, et al. Short-term and long-term outcomes of surgical treatment for HCC within Milan criteria with cirrhotic portal hypertension. Hepatogastroenterology. (2014) 61:2185–90.
52. Cillo U, Vitale A, Brolese A, et al. Partial hepatectomy as first-line treatment for patients with hepatocellular carcinoma. J Surg Oncol. (2007) 95:213–20. doi: 10.1002/jso.20641
53. Closset J, Van de Stadt J, Delhaye M, El Nakadi I, Lambilliotte JP, Gelin M. Hepatocellular carcinoma: surgical treatment and prognostic variables in 56 patients. Hepatogastroenterology. (1999) 46:2914–8.
54. Colella G, Bottelli R, De Carlis L, et al. Hepatocellular carcinoma: comparison between liver transplantation, resective surgery, ethanol injection, and chemoembolization. Transplant Int. (1998) 11 Suppl 1:S193–196. doi: 10.1111/j.1432-2277.1998.tb01113.x
55. Concejero A, Chen CL, Wang CC, et al. Living donor liver transplantation for hepatocellular carcinoma: a single-center experience in Taiwan. Transplantation. (2008) 85:398–406. doi: 10.1097/TP.0b013e3181622ff8
56. Dai Y, Li C, Wen TF, Yan LN. Comparison of liver resection and transplantation for Child-pugh A cirrhotic patient with very early hepatocellular carcinoma and portal hypertension. Pak J Med Sci. (2014) 30:996–1000. doi: 10.12669/pjms.305.5038
57. De Carlis L, Sammartino C, Giacomoni A, et al. [Surgical treatment of hepatocellular carcinoma: resection or transplantation? Results of a multivariate analysis]. Chirurgia italiana. (2001) 53:579–86.
58. De Carlis L, Giacomoni A, Pirotta V, et al. Surgical treatment of hepatocellular cancer in the era of hepatic transplantation. J Am Coll Surg. (2003) 196:887–97. doi: 10.1016/S1072-7515(03)00140-6
59. Del Gaudio M, Ercolani G, Ravaioli M, et al. Liver transplantation for recurrent hepatocellular carcinoma on cirrhosis after liver resection: University of Bologna experience. Am J Transplant. (2008) 8:1177–85. doi: 10.1111/j.1600-6143.2008.02229.x
60. Dima SO, Iacob S, Botea F, et al. Multimodal treatment of hepatocellular carcinoma: an eastern European experience. Hepatogastroenterology. (2009) 56:1696–703.
61. El-Gazzaz G, Wong W, El-Hadary MK, et al. Outcome of liver resection and transplantation for fibrolamellar hepatocellular carcinoma. Transplant Int. (2000) 13 Suppl 1:S406–409. doi: 10.1111/tri.2000.13.issue-S1
62. Facciuto ME, Koneru B, Rocca JP, et al. Surgical treatment of hepatocellular carcinoma beyond Milan criteria. Results of liver resection, salvage transplantation, and primary liver transplantation. Ann Surg Oncol. (2008) 15:1383–91. doi: 10.1245/s10434-008-9851-z
63. Fan HL, Chen TW, Hsieh CB, et al. Liver transplantation is an alternative treatment of hepatocellular carcinoma beyond the Milan criteria. Am J Surg. (2010) 200:252–7. doi: 10.1016/j.amjsurg.2009.07.049
64. Fan ST, Poon RT, Yeung C, et al. Outcome after partial hepatectomy for hepatocellular cancer within the Milan criteria. Br J Surg. (2011) 98:1292–300. doi: 10.1002/bjs.7583
65. Farinati F, Gianni S, Marin G, Fagiuoli S, Rinaldi M, Naccarato R. Does the choice of treatment influence survival of patients with small hepatocellular carcinoma in compensated cirrhosis? Eur J Gastroenterol Hepatol. (2001) 13:1217–24. doi: 10.1097/00042737-200110000-00015
66. Figueras J, Jaurrieta E, Valls C, et al. Resection or transplantation for hepatocellular carcinoma in cirrhotic patients: outcomes based on indicated treatment strategy. J Am Coll Surg. (2000) 190:580–7. doi: 10.1016/S1072-7515(00)00251-9
67. Foltys D, Zimmermann T, Kaths M, et al. Hepatocellular carcinoma in Child's A cirrhosis: a retrospective analysis of matched pairs following liver transplantation vs. liver resection according to the intention-to-treat principle. Clin transpl. (2014) 28:37–46. doi: 10.1111/ctr.12273
68. Franssen B, Alshebeeb K, Tabrizian P, et al. Differences in surgical outcomes between hepatitis B- and hepatitis C-related hepatocellular carcinoma: a retrospective analysis of a single North American center. Ann Surg. (2014) 260:650–656; discussion 656-658. doi: 10.1097/SLA.0000000000000917
69. Fuks D, Dokmak S, Paradis V, Diouf M, Durand F, Belghiti J. Benefit of initial resection of hepatocellular carcinoma followed by transplantation in case of recurrence: an intention-to-treat analysis. Hepatology. (2012) 55:132–40. doi: 10.1002/hep.24680
70. Graham JA, Newman DA, Smirniotopolous J, Shetty K, Slidell MB, Johnson LB. Transplantation for hepatocellular carcinoma in younger patients has an equivocal survival advantage as compared with resection. Transplant Proc. (2013) 45:265–71. doi: 10.1016/j.transproceed.2012.07.151
71. Harada N, Shirabe K, Ikeda Y, Korenaga D, Takenaka K, Maehara Y. Surgical management of hepatocellular carcinoma in Child-Pugh class B cirrhotic patients: hepatic resection and/or microwave coagulation therapy versus living donor liver transplantation. Ann transpl. (2012) 17:11–20. doi: 10.12659/AOT.883689
72. Ho CM, Lee PH, Chen CL, Ho MC, Wu YM, Hu RH. Long-term outcomes after resection versus transplantation for hepatocellular carcinoma within UCSF criteria. Ann Surg Oncol. (2012) 19:826–33. doi: 10.1245/s10434-011-1975-x
73. Hsueh KC, Lee TY, Kor CT, et al. The role of liver transplantation or resection for patients with early hepatocellular carcinoma. Tumour Biol. (2016) 37:4193–201. doi: 10.1007/s13277-015-4243-z
74. Huang ZY, Liang BY, Xiong M, et al. Severity of cirrhosis should determine the operative modality for patients with early hepatocellular carcinoma and compensated liver function. Surgery. (2016) 159:621–31. doi: 10.1016/j.surg.2015.09.002
75. Hwang S, Lee SG, Moon DB, et al. Salvage living donor liver transplantation after prior liver resection for hepatocellular carcinoma. Liver Transpl. (2007) 13:741–6. doi: 10.1002/(ISSN)1527-6473
76. Iwatsuki S, Starzl TE, Sheahan DG, et al. Hepatic resection versus transplantation for hepatocellular carcinoma. Ann Surg. (1991) 214:221–228; discussion 228-229. doi: 10.1097/00000658-199109000-00005
77. Jiang L, Liao A, Wen T, Yan L, Li B, Yang J. Living donor liver transplantation or resection for Child-Pugh A hepatocellular carcinoma patients with multiple nodules meeting the Milan criteria. Transplant Int. (2014) 27:562–9. doi: 10.1111/tri.2014.27.issue-6
78. Kaido T, Morita S, Tanaka S, et al. Long-term outcomes of hepatic resection versus living donor liver transplantation for hepatocellular carcinoma: a propensity score-matching study. Dis Markers. (2015) 2015:425926. doi: 10.1155/2015/425926
79. Kim BW, Park YK, Kim YB, Wang HJ, Kim MW. Salvage liver transplantation for recurrent hepatocellular carcinoma after liver resection: feasibility of the Milan criteria and operative risk. Transplant Proc. (2008) 40:3558–61. doi: 10.1016/j.transproceed.2008.03.175
80. Koniaris LG, Levi DM, Pedroso FE, et al. Is surgical resection superior to transplantation in the treatment of hepatocellular carcinoma? Ann Surg. (2011) 254:527–537; discussion 537-528. doi: 10.1097/SLA.0b013e31822ca66f
81. Kooby DA, Egnatashvili V, Graiser M, et al. Changing management and outcome of hepatocellular carcinoma: evaluation of 501 patients treated at a single comprehensive center. J Surg Oncol. (2008) 98:81–8. doi: 10.1002/jso.21049
82. Kuroda S, Tashiro H, Kobayashi T, Oshita A, Amano H, Ohdan H. Selection criteria for hepatectomy in patients with hepatocellular carcinoma classified as Child-Pugh class B. World J surg. (2011) 35:834–41. doi: 10.1007/s00268-010-0929-y
83. Langer B, Greig PD, Taylor BR. Surgical resection and transplantation for hepatocellular carcinoma. Cancer Treat Res. (1994) 69:231–40.
84. Launois B, Chauvin J, MaChado ML, Bourdonnec P, Campion JP, Bardaxoglou E. [Surgical treatment of hepatocarcinoma in cirrhosis]. Annales gastroenterologie d'hepatologie. (1996) 32:35–39; discussion 39-40.
85. Lei JY, Yan LN, Wang WT. Transplantation vs resection for hepatocellular carcinoma with compensated liver function after downstaging therapy. World J Gastroenterol. (2013) 19:4400–8. doi: 10.3748/wjg.v19.i27.4400
86. Li C, Zhu WJ, Wen TF, et al. Child-Pugh A hepatitis B-related cirrhotic patients with a single hepatocellular carcinoma up to 5 cm: liver transplantation vs. resection. J gastrointestinal Surg. (2014) 18:1469–76. doi: 10.1007/s11605-014-2550-0
87. Li C, Liu JY, Peng W, et al. Liver resection versus transplantation for multiple hepatocellular carcinoma: a propensity score analysis. Oncotarget. (2017) 8:81492–500. doi: 10.18632/oncotarget.v8i46
88. Lim C, Shinkawa H, Hasegawa K, et al. Salvage liver transplantation or repeat hepatectomy for recurrent hepatocellular carcinoma: An intent-to-treat analysis. Liver Transplant. (2017) 23:1553–63. doi: 10.1002/lt.24952
89. Llovet JM, Fuster J, Bruix J. Intention-to-treat analysis of surgical treatment for early hepatocellular carcinoma: resection versus transplantation. Hepatology. (1999) 30:1434–40. doi: 10.1002/(ISSN)1527-3350
90. Margarit C, Escartin A, Castells L, Vargas V, Allende E, Bilbao I. Resection for hepatocellular carcinoma is a good option in Child-Turcotte-Pugh class A patients with cirrhosis who are eligible for liver transplantation. Liver Transplant. (2005) 11:1242–51. doi: 10.1002/(ISSN)1527-6473
91. Mazziotti A, Grazi GL, Cavallari A. Surgical treatment of hepatocellular carcinoma on cirrhosis: a Western experience. Hepatogastroenterology. (1998) 45 Suppl 3:1281–7.
92. Meyerovich G, Goykhman Y, Nakache R, et al. Resection vs transplant listing for hepatocellular carcinoma: an intention-to-treat analysis. Transplant Proc. (2019) 51:1867–73. doi: 10.1016/j.transproceed.2019.02.030
93. Michel J, Suc B, Montpeyroux F, et al. Liver resection or transplantation for hepatocellular carcinoma? Retrospective analysis of 215 patients with cirrhosis. J Hepatol. (1997) 26:1274–80.
94. Michelakos T, Xourafas D, Qadan M, et al. Hepatocellular carcinoma in transplantable child-Pugh A cirrhotics: should cost affect resection vs transplantation? J gastrointestinal Surg. (2019) 23:1135–42. doi: 10.1007/s11605-018-3946-z
95. Moon DB, Lee SG, Hwang S. Liver transplantation for hepatocellular carcinoma: single nodule with Child-Pugh class A sized less than 3 cm. Dig Dis. (2007) 25:320–8. doi: 10.1159/000106912
96. Ng KK, Lo CM, Liu CL, Poon RT, Chan SC, Fan ST. Survival analysis of patients with transplantable recurrent hepatocellular carcinoma: implications for salvage liver transplant. Arch Surg. (2008) 143:68–74; discussion 74. doi: 10.1001/archsurg.2007.15
97. Obed A, Tsui TY, Schnitzbauer AA, et al. Liver transplantation as curative approach for advanced hepatocellular carcinoma: is it justified? Langenbeck's Arch Surg / Deutsche Gesellschaft fur Chirurgie. (2008) 393:141–7. doi: 10.1007/s00423-007-0250-x
98. Otto G, Heuschen U, Hofmann WJ, Krumm G, Hinz U, Herfarth C. Survival and recurrence after liver transplantation versus liver resection for hepatocellular carcinoma: a retrospective analysis. Ann Surg. (1998) 227:424–32. doi: 10.1097/00000658-199803000-00015
99. Park MS, Lee KW, Kim H, et al. Primary living-donor liver transplantation is not the optimal treatment choice in patients with early hepatocellular carcinoma with poor tumor biology. Transplant Proc. (2017) 49:1103–8. doi: 10.1016/j.transproceed.2017.03.016
100. Perry JF, Charlton B, Koorey DJ, et al. Outcome of patients with hepatocellular carcinoma referred to a tertiary centre with availability of multiple treatment options including cadaveric liver transplantation. Liver Int. (2007) 27:1240–8. doi: 10.1111/j.1478-3231.2007.01569.x
101. Peters NA, Javed AA, He J, Wolfgang CL, Weiss MJ. Association of socioeconomics, surgical therapy, and survival of early stage hepatocellular carcinoma. J Surg Res. (2017) 210:253–60. doi: 10.1016/j.jss.2016.11.042
102. Philosophe B, Greig PD, Hemming AW, et al. Surgical management of hepatocellular carcinoma: resection or transplantation? J gastrointestinal Surg. (1998) 2:21–7. doi: 10.1016/S1091-255X(98)80099-1
103. Poon RT, Fan ST, Lo CM, Liu CL, Wong J. Difference in tumor invasiveness in cirrhotic patients with hepatocellular carcinoma fulfilling the Milan criteria treated by resection and transplantation: impact on long-term survival. Ann Surg. (2007) 245:51–8. doi: 10.1097/01.sla.0000225255.01668.65
104. Rayya F, Harms J, Bartels M, Uhlmann D, Hauss J, Fangmann J. Results of resection and transplantation for hepatocellular carcinoma in cirrhosis and noncirrhosis. Transplant Proc. (2008) 40:933–5. doi: 10.1016/j.transproceed.2008.03.045
105. Ringe B, Pichlmayr R, Wittekind C, Tusch G. Surgical treatment of hepatocellular carcinoma: experience with liver resection and transplantation in 198 patients. World J surg. (1991) 15:270–85. doi: 10.1007/BF01659064
106. Ruzzenente A, Capra F, Pachera S, et al. Is liver resection justified in advanced hepatocellular carcinoma? Results of an observational study in 464 patients. J gastrointestinal Surg. (2009) 13:1313–20. doi: 10.1007/s11605-009-0903-x
107. Sangro B, Herraiz M, Martinez-Gonzalez MA, et al. Prognosis of hepatocellular carcinoma in relation to treatment: a multivariate analysis of 178 patients from a single European institution. Surgery. (1998) 124:575–83. doi: 10.1016/S0039-6060(98)70105-9
108. Sapisochin G, Castells L, Dopazo C, et al. Single HCC in cirrhotic patients: liver resection or liver transplantation? Long-term outcome according to an intention-to-treat basis. Ann Surg Oncol. (2013) 20:1194–202. doi: 10.1245/s10434-012-2655-1
109. Sapisochin G, Bilbao I, Balsells J, et al. Optimization of liver transplantation as a treatment of intrahepatic hepatocellular carcinoma recurrence after partial liver resection: experience of a single European series. World J surg. (2010) 34:2146–54. doi: 10.1007/s00268-010-0583-4
110. Scatton O, Zalinski S, Terris B, et al. Hepatocellular carcinoma developed on compensated cirrhosis: resection as a selection tool for liver transplantation. Liver Transplant. (2008) 14:779–88. doi: 10.1002/(ISSN)1527-6473
111. Seshadri RM, Besur S, Niemeyer DJ, et al. Survival analysis of patients with stage I and II hepatocellular carcinoma after a liver transplantation or liver resection. HPB (Oxford). (2014) 16:1102–9. doi: 10.1111/hpb.12300
112. Shabahang M, Franceschi D, Yamashiki N, et al. Comparison of hepatic resection and hepatic transplantation in the treatment of hepatocellular carcinoma among cirrhotic patients. Ann Surg Oncol. (2002) 9:881–6. doi: 10.1007/BF02557525
113. Shah SA, Cleary SP, Tan JC, et al. An analysis of resection vs transplantation for early hepatocellular carcinoma: defining the optimal therapy at a single institution. Ann Surg Oncol. (2007) 14:2608–14. doi: 10.1245/s10434-007-9443-3
114. Shao Z, Lopez R, Shen B, Yang GS. Orthotopic liver transplantation as a rescue operation for recurrent hepatocellular carcinoma after partial hepatectomy. World J Gastroenterol. (2008) 14:4370–6. doi: 10.3748/wjg.14.4370
115. Shen BY, Li HW, Regimbeau JM, Belghiti J. Recurrence after resection of hepatocellular carcinoma. Hepatobiliary pancreatic Dis Int. (2002) 1:401–5.
116. Shen JY, Li C, Wen TF, et al. Transplantation versus hepatectomy for HCC beyond the Milan criteria: A propensity score analysis. Int J Surg (London England). (2017) 44:33–42. doi: 10.1016/j.ijsu.2017.05.034
117. Sogawa H, Shrager B, Jibara G, Tabrizian P, Roayaie S, Schwartz M. Resection or transplant-listing for solitary hepatitis C-associated hepatocellular carcinoma: an intention-to-treat analysis. HPB (Oxford). (2013) 15:134–41. doi: 10.1111/j.1477-2574.2012.00548.x
118. Sotiropoulos GC, Druhe N, Sgourakis G, et al. Liver transplantation, liver resection, and transarterial chemoembolization for hepatocellular carcinoma in cirrhosis: which is the best oncological approach? Digestive Dis Sci. (2009) 54:2264–73. doi: 10.1007/s10620-008-0604-4
119. Squires MH 3rd, Hanish SI, Fisher SB, et al. Transplant versus resection for the management of hepatocellular carcinoma meeting Milan Criteria in the MELD exception era at a single institution in a UNOS region with short wait times. J Surg Oncol. (2014) 109:533–41. doi: 10.1002/jso.23531
120. Sung PS, Yang H, Na GH, et al. Long-term outcome of liver resection versus transplantation for hepatocellular carcinoma in a region where living donation is a main source. Ann transpl. (2017) 22:276–84. doi: 10.12659/AOT.904287
121. Tan KC, Rela M, Ryder SD, et al. Experience of orthotopic liver transplantation and hepatic resection for hepatocellular carcinoma of less than 8 cm in patients with cirrhosis. Br J Surg. (1995) 82:253–6. doi: 10.1002/bjs.1800820239
122. Tiao GM, Bobey N, Allen S, et al. The current management of hepatoblastoma: a combination of chemotherapy, conventional resection, and liver transplantation. J Pediatr. (2005) 146:204–11. doi: 10.1016/j.jpeds.2004.09.011
123. Vargas V, Castells L, Balsells J, et al. Hepatic resection or orthotopic liver transplant in cirrhotic patients with small hepatocellular carcinoma. Transplant Proc. (1995) 27:1243–4.
124. Vennarecci G, Ettorre GM, Antonini M, et al. First-line liver resection and salvage liver transplantation are increasing therapeutic strategies for patients with hepatocellular carcinoma and child a cirrhosis. Transplant Proc. (2007) 39:1857–60. doi: 10.1016/j.transproceed.2007.05.073
125. Weimann A, Schlitt HJ, Oldhafer KJ, Hoberg S, Tusch G, Raab R. Is liver transplantation superior to resection in early stage hepatocellular carcinoma? Transplant Proc. (1999) 31:500–1. doi: 10.1016/S0041-1345(98)01727-8
126. Wu Z, Chen W, Ouyang T, Liu H, Cao L. Management and survival for patients with stage-I hepatocellular carcinoma: An observational study based on SEER database. Med (Baltimore). (2020) 99:e22118. doi: 10.1097/MD.0000000000022118
127. Yamamoto J, Iwatsuki S, Kosuge T, et al. Should hepatomas be treated with hepatic resection or transplantation? Cancer. (1999) 86:1151–8. doi: 10.1002/(ISSN)1097-0142
128. Yamashita Y, Yoshida Y, Kurihara T, et al. Surgical results for recurrent hepatocellular carcinoma after curative hepatectomy: Repeat hepatectomy versus salvage living donor liver transplantation. Liver Transplant. (2015) 21:961–8. doi: 10.1002/lt.24111
129. Yang A, Ju W, Yuan X, et al. Comparison between liver resection and liver transplantation on outcomes in patients with solitary hepatocellular carcinoma meeting UNOS criteria: a population-based study of the SEER database. Oncotarget. (2017) 8:97428–38. doi: 10.18632/oncotarget.v8i57
130. Yokoi H, Isaji S, Yamagiwa K, et al. The role of living-donor liver transplantation in surgical treatment for hepatocellular carcinoma. J Hepatobiliary Pancreat Surg. (2006) 13:123–30. doi: 10.1007/s00534-005-1018-8
131. Zaydfudim VM, Vachharajani N, Klintmalm GB, et al. Liver resection and transplantation for patients with hepatocellular carcinoma beyond Milan criteria. Ann Surg. (2016) 264:650–8. doi: 10.1097/SLA.0000000000001866
Keywords: liver transplantation, liver resection, hepatocellular carcinoma, survival, meta-analysis
Citation: Martinino A, Bucaro A, Cardella F, Wazir I, Frongillo F, Ardito F and Giovinazzo F (2024) Liver transplantation vs liver resection in HCC: promoting extensive collaborative research through a survival meta-analysis of meta-analyses. Front. Oncol. 14:1366607. doi: 10.3389/fonc.2024.1366607
Received: 06 January 2024; Accepted: 13 February 2024;
Published: 18 March 2024.
Edited by:
Giorgio Treglia, Ente Ospedaliero Cantonale (EOC), SwitzerlandReviewed by:
Pierluigi Romano, University Hospital of Padua, ItalyCopyright © 2024 Martinino, Bucaro, Cardella, Wazir, Frongillo, Ardito and Giovinazzo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Francesco Giovinazzo, Z2lvdmluYXp6b19mcmFuY2VzY29AbGl2ZS5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.