- 1Department of Breast Surgery, Peking Union Medical College Hospital, Peking Union Medical College, Chinese Academy of Medical Sciences, Beijing, China
- 2Department of Pathology, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, China
Myofibroblastic sarcoma is a malignancy in which myofibroblasts are the main component, with a very low incidence. In this study, we report a case of low-grade myofibroblastic sarcoma (LGMS) in the breast. After the diagnosis of LGMS, the patient received a mastectomy. The patient showed no relapse or progression during the follow-up time of 3 months following the operation. LGMS in the breast is extremely rare, and the limited experience with its diagnosis and treatment brings obstacles to doctors. Therefore, this report summarizes the preoperative diagnosis, treatment, and prognosis of breast LGMS through a literature review.
1 Introduction
Myofibroblasts are primarily found in reactive granulation tissue, they are generally secondary components of malignancies, reflecting the response of the host stromal tissue to malignant cells. However, malignancies in which myofibroblasts are the main component are rare (1). Low-grade myofibroblastic sarcoma (LGMS) was first reported by Mentzel et al. in a case series of 18 cases in 1998, and it was classified as a distinct type of soft-tissue tumor by the World Health Organization in 2002 (2, 3). LGMS mainly occurs in the head and neck but can also occur in the bones, trunk, limbs, retroperitoneal, and other parts, LGMS in the breast is extremely rare (4–6). About 20 patients with LGMS of the breast have been previously reported in the literature. In this report, we report a rare case of breast LGMS. The patient underwent a right mastectomy, and no recurrence or metastasis has been found so far.
2 Case presentation
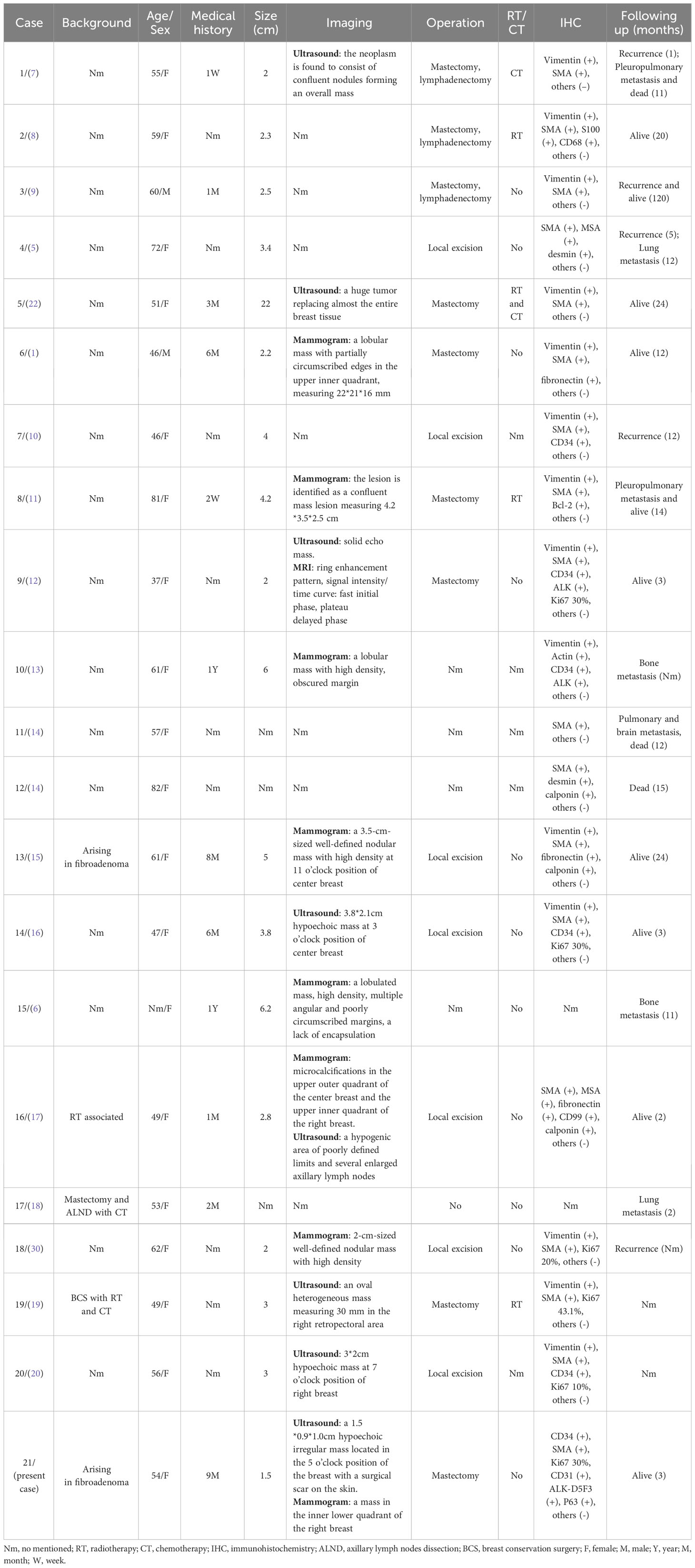
A 54-year-old woman found a palpable, non-tender mass of the right breast, which she first noted on self-examination 9 months earlier. She denied any nipple discharge, itching, skin redness, and swelling of her breast. She underwent a right breast mass excision in July 2019, and the biopsy was fibroadenoma. She had no family history of breast cancer. On physical examination, a 1.5cm nodule was palpable at the 5 o’clock position of the right breast, with the obscured margin and the local skin retraction (Figure 1). There was no evidence of lymphadenopathy. Her laboratory tests are normal, and the tumor marker showed no abnormalities. Ultrasound reported a 1.5 ×0.9×1.0cm hypoechoic irregular mass located in the 5 o’clock position of the breast with a surgical scar on the skin. The aspect ratio of the node was greater than 1, and no clear blood flow signal was seen (Figure 2A). A mammogram confirmed the presence of a mass in the inner lower quadrant of the right breast (Figures 2B, C). Ultrasound-guided core biopsy of the lesion revealed a low-grade malignant spindle cell tumor with necrosis and peripheral lymphocyte infiltration. She underwent a right mastectomy on July 06, 2023. The patient’s postoperative recovery was uneventful, and she was discharged home after three days. Examination of the resected specimens revealed 1.8cm gray and white nodules with unclear boundaries. Under hematoxylin-eosin (HE) staining, spindle tumor cells can be seen as arranged in sweeping fascicles (Figures 3A, B). Combined with immunohistochemistry, LGMS was diagnosed, and the surgical margins were negative. Immunohistochemical (IHC) staining showed (Figure 4): CD34 (Vascular+) (Figure 4A), CK5/6 (-) (Figure 4B), CK14 (-) (Figure 4C), Desmin (-) (Figure 4D), Ki-67 (index 30%) (Figure 4E), SMA (partial+) (Figure 4F), AE1/AE3 (-), CD31 (Vascular+), ALK-D5F3 (-), ALK-D5F3 (NC) (-), ALK-D5F3 (PC) (+), β-catenin (-), S-100 (-), STAT6 (-), P63 (scattered +), EGFR (-), GFAP (-). Following mastectomy, the patient received no chemotherapy or radiation therapy. Furthermore, it is noteworthy that the patient maintained a positive attitude and collaborative spirit throughout the entire treatment process. Due to concerns about recurrence, the patient strongly requested a mastectomy, demonstrating resilience and optimism despite the challenging nature of the procedure, which played a pivotal role in the successful management of her condition. Additionally, we utilized physical examinations and imaging studies (ultrasound and mammography) during subsequent follow-up visits to ensure treatment continuity. She exhibits no evidence of metastasis or local recurrence after 3 months of follow-up.
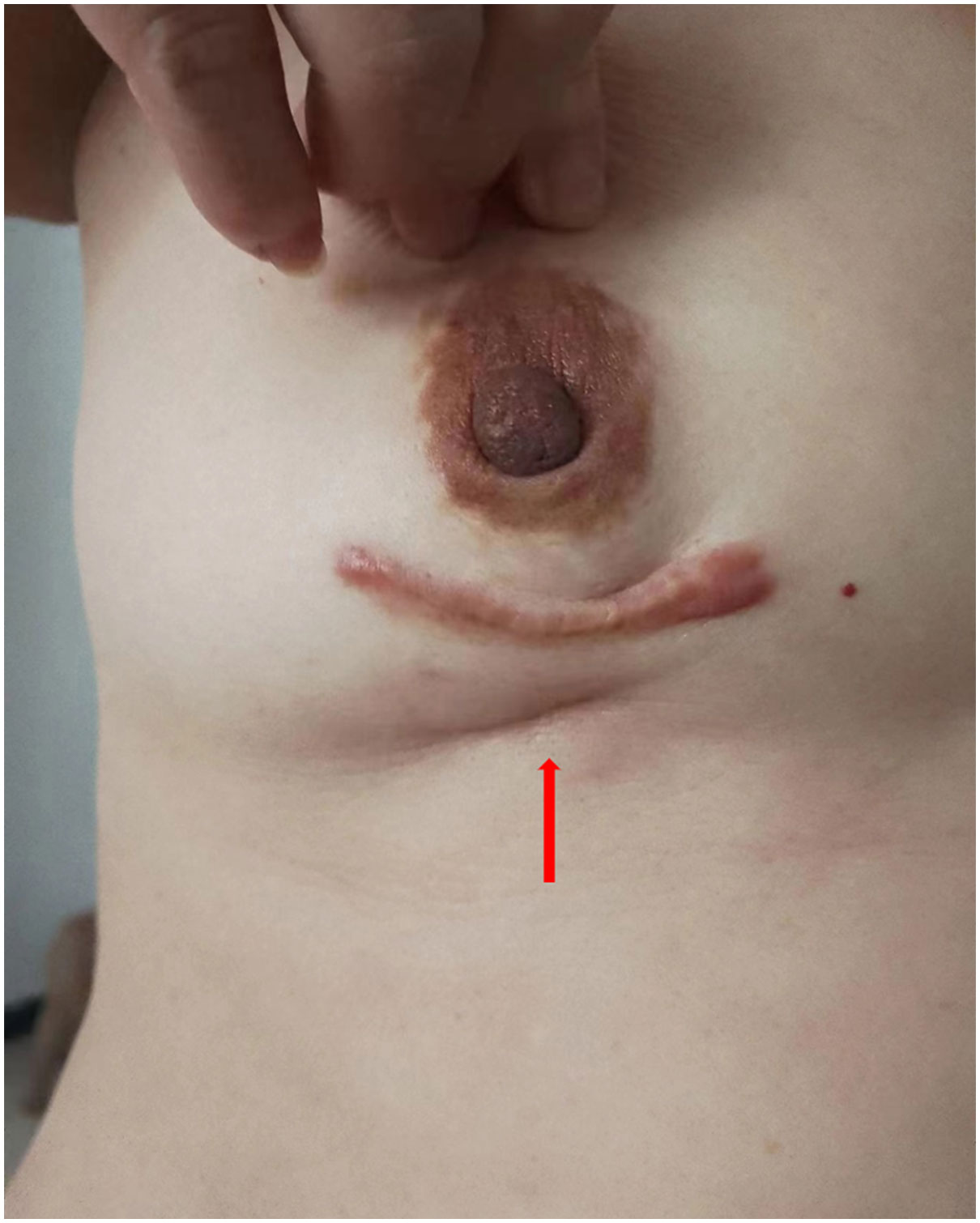
Figure 1 A 1.5cm mass at the 5 o’clock position of the right breast, with the boundary unclear and the local skin sunken (shown by arrow).
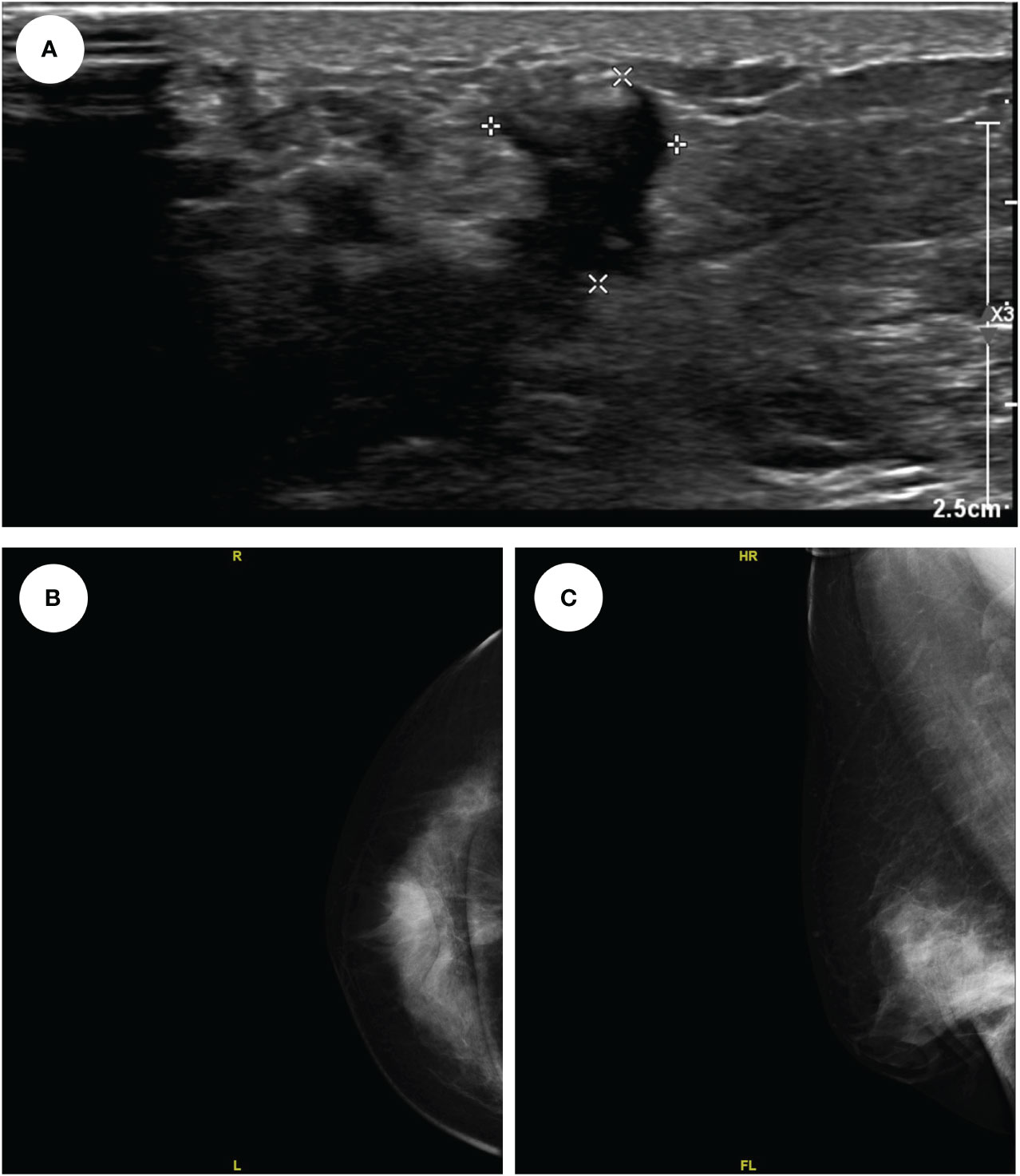
Figure 2 The ultrasound showed a 1.5*0.9*1.0cm hypo echoes irregular mass located in the 5 o’clock position of the right breast (A); The mammogram showed a mass in the inner lower quadrant of the right breast (CC, B); (MLO, C).
Figure 3 Microscopy showed that the spindle tumor cells were arranged in sweeping fascicles (HE, × 10, A); (HE, × 40, B).
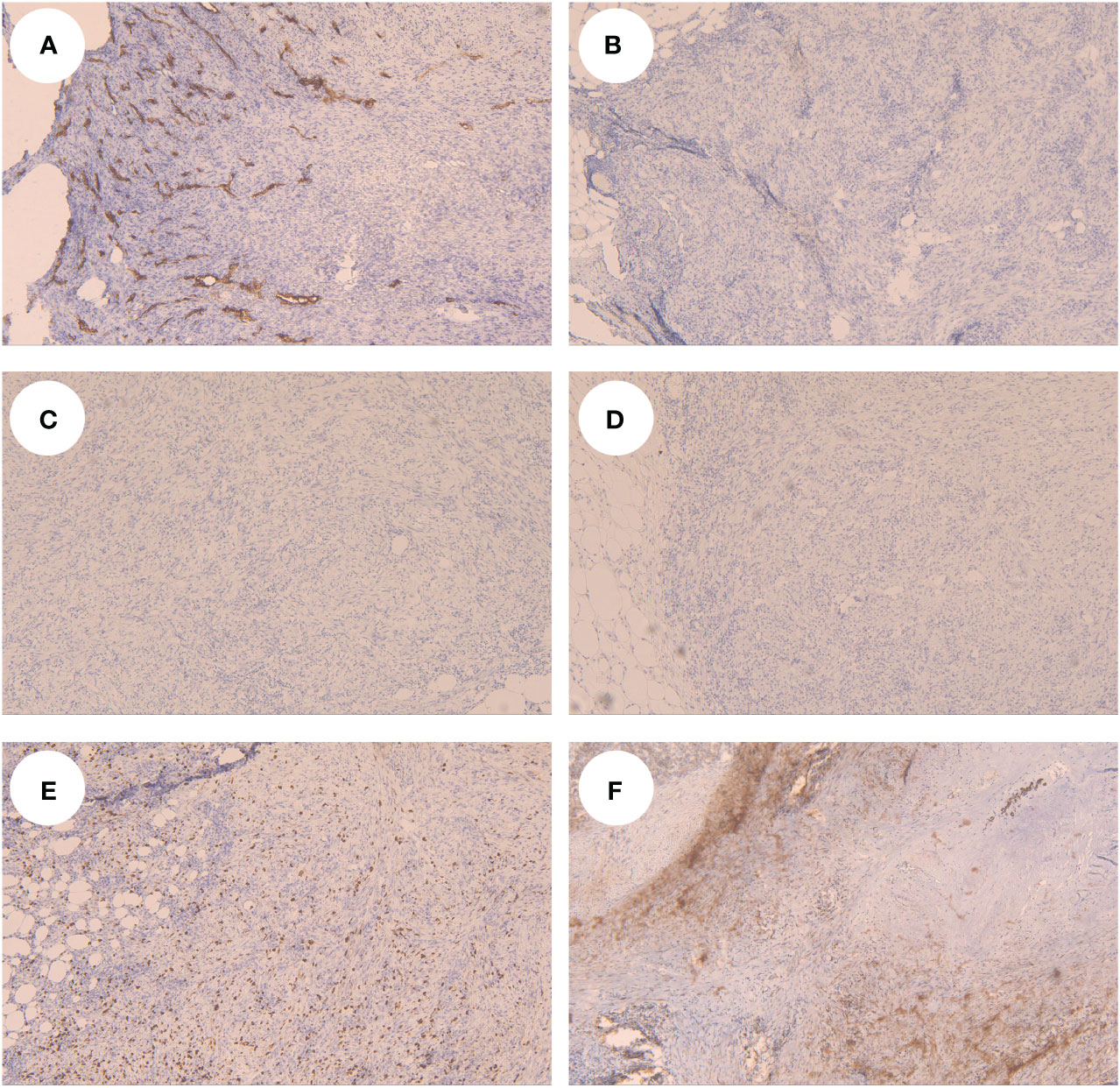
Figure 4 Tumor cells showed CD34 positive in the vessel in IHC (A), showed CK5/6 (B), CK14 (C) and Desmin (D) negative, Ki-67 (E) and SMA (F) showed partial positive.
3 Discussion
We reviewed previous case reports of breast LGMS, and Table 1 shows the relevant studies we reviewed, in which one case focused on the imaging features of breast LGMS (case 15), and the rest of the cases described the histological features, treatment and prognosis. LGMS consists of fibrosarcoma-like spindle tumor cells arranged in sweeping fascicles, sheets, or storiform whorls (21). Tapered or ovoid nuclei with deep staining have small nucleoli surrounding homogenous chromatin and display light to moderate atypia with slightly increased proliferative activity. The tumor cells have small or moderate amounts of eosinophilic cytoplasm with distinct cell boundaries. The peripheral stroma is collagenous and/or has a myxoid background, and necrosis occurs in a minority of cases. The mitotic activity ranged from 8 to 35 per 10 high power field (HPF), but abnormal mitotic figures are rare (22). A superficial biopsy may have a strong resemblance to a myofibroma. However, the histological features of invasive growth of tumor cells are different from those of myofibromatosis and similar diseases such as nodular fasciitis or inflammatory myofibroblastic tumor (IMT) and more similar to the growth pattern of fibromatosis (23).
Widely accepted for diagnosing LGMS, an electron microscope is required to demonstrate specialized ultrastructural organelles, including abundant rough endoplasmic reticulum, fibronexus junctions, and fibronectin fibrils (5). Among them, fibronexus was not found in smooth muscle cells and fibroblasts. It has been regarded as the most specific ultrastructural marker for myofibroblasts, connecting the myofibroblast to the extracellular matrix (1). The cell-to-matrix junction consists of myofilament and fibronectin filament systems converging on a discrete cell-surface plaque. Calponin and caldesmon, the main components of smooth muscle filaments, are regulators of smooth muscle contraction, and they can interact with actin to inhibit the ATPase activity of muscle actomyosin, thereby regulating muscle contraction (24). Some studies have also found that IMT and LGMS cells, following immunohistochemical staining, suggest diffusely positive for actin-associated proteins and fibronectin (25). These proteins were noted to be mainly distributed in the cytoplasm of tumor cells, with a tendency to extend into the extracellular space. Actin-associated proteins generally include α-smooth muscle actin (α-SMA), muscle-specific actin (MSA), and calponin, etc. As mentioned above, actin exists in myofilaments to regulate the contractile function of muscles. Proteins that are stained positive may be able to prove that myofibroblasts contain myofilaments. Fibronectin staining of spindle cells with extracellular extension might be equivalent to fibronectin fibrils known at the ultrastructure of myofibroblasts (25). Therefore, the combined expression of these markers may immunohistochemically reflect the characteristic cell-to-matrix ultrastructural components of myofibroblasts.
In immunohistochemical studies of LGMS, α-SMA and vimentin can be positive in most cases, but desmin can be both positive and negative (21). In this case, α-SMA staining showed positive, vimentin and desmin staining showed negative. Although SMA is regarded as the most specific and frequently expressed marker of myofibroblasts, a small percentage of cases express only SMA or desmin. In addition, calponin can often be positive, and CK/CD34/S-100 is often negative, as in this case. Focal expression of S-100 protein and CD34 has been observed in a minority of cases (23). Furthermore, H-caldesmon is usually expressed positively in leiomyosarcoma but negatively in LGMS, which plays a vital role in the differential diagnosis of the two. Moreover, CK expression is commonly seen in other myofibroblastic proliferations, like the inflammatory myofibroblastic tumor (4).
The mammograms of breast LGMS are generally irregular shapes and high density, with a lack of encapsulation (6). LGMS of the breast are tumors of stromal origin and are etiologically more likely to have hematologic metastases than to have lymphoid metastases compared to epithelial original breast cancers. Therefore, mammograms of advanced-stage breast cancers usually find interstitial edema and opacity of the subcutaneous fat layer (6). On the other hand, there are no signs of interstitial edema and swollen axillary lymph nodes in LGMS. We reviewed cases of breast LGMS, and a few patients underwent computed tomography (CT) and magnetic resonance imaging (MRI), so they could not provide sufficient imaging evidence for diagnosis.
Breast LGMS seems to have a higher incidence of distant metastasis. In the cases we reviewed, almost all patients underwent surgical treatment, with 31.3% of postoperative recurrences and 18.8% of distant metastases. In terms of LGMS at other sites, previous studies have calculated a local recurrence rate of 13.3% to 44.4% (5, 26, 27). In the previous study by Munehisa Kito, 24 patients with LGMS were counted and analyzed statistically (4). Among them, 22 patients underwent surgical treatment, the results showed that the local recurrence rate was 20%, and the incidence of distant metastasis was 4.2%. In a population-based study on LGMS, 49 LGMS patients in the Surveillance, Epidemiology, and End Results Program (SEER) database had survival data with 3-year and 5-year overall survival (OS) of 75.0% and 71.6%, and disease-specific survival (DSS) of 80.0% and 76.3%, respectively. Of these, 93.9% underwent surgery (28). Additionally, a univariate analysis of prognostic outcomes was performed, and the results showed that only tumors larger than 5cm in diameter had a higher local recurrence rate (29). However, this conclusion may not apply to breast LGMS, as shown in Table 1, patients with local recurrence had tumors smaller than 5 cm. Tumor size is not one of the critical factors affecting the long-term prognosis of breast LGMS patients. Therefore, for the prognostic analysis of breast LGMS, more cases and data analysis are still needed.
As for the treatment of LGMS, the benefits of radiotherapy and chemotherapy for patients after surgery are unclear (28). Almost all patients have undergone surgery, and no patients have received radiotherapy or chemotherapy alone. Thus, the therapeutic effect of radiotherapy or chemotherapy cannot be evaluated separately (22, 26, 30). In Y. Xu’s study, 96 patients with LGMS were included in the SEER database, of whom 89.6% had received surgery, 29.2% had received radiotherapy, and 10.4% had received chemotherapy (31). According to the results, chemotherapy and radiotherapy had no significant benefit on patients’ OS and DSS. Only For unresectable cases, radiotherapy may be a treatment option (4). However, there have been reports of cases of recurrence outside the irradiated area after radiotherapy in LGMS patients, likely related to the pattern of invasive tumor growth. Thus, the choice of radiotherapy may be considered in some cases. In summary, surgery may currently be the primary treatment for LGMS patients, while the prognosis and benefits of radiotherapy and chemotherapy are still unknown. This study comes with its limitations, a three-month follow-up period may not be sufficient to fully ascertain the long-term prognosis of LGMS. Future studies with extended follow-up periods are imperative to gain a more comprehensive understanding of the recurrence patterns and overall prognosis for patients with LGMS.
4 Conclusions
The diagnosis of LGMS still relies on discovering special ultrastructure under electron microscopy, and the specificity of immunohistochemistry and imaging methods is still insufficient. According to the current literature, surgery is the most important treatment. Notably, LGMS occurring in the breast is a tumor with a high rate of recurrence and a higher incidence of distant metastasis relative to other sites. The currently summarized data on the clinical manifestations, treatment, and prognosis of LGMS mainly come from other sites, and the data of LGMS in the breast need to be further accumulated.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
Ethical approval was not required for the study involving humans in accordance with the local legislation and institutional requirements. Written informed consent to participate in this study was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and the institutional requirements. Written informed consent was obtained from the individual(s), and minor(s)’ legal guardian/next of kin, for the publication of any potentially identifiable images or data included in this article.
Author contributions
ZD: Writing – original draft, Methodology, Conceptualization. CX: Writing – original draft, Resources. YLi: Writing – review & editing. YLu: Writing – review & editing. SS: Writing – review & editing, Project administration, Methodology, Conceptualization.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was supported by National High Level Hospital Clinical Research Funding (2022-PUMCH-B-038).
Acknowledgments
The authors thank AiMi Academic Services (www.aimieditor.com) for English language editing and review services.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Morgan PB, Chundru S, Hatch SS, Hawkins HK, Adegboyega PA, Eltorky MA. Uncommon Malignancies: case 1. Low-grade myofibroblastic sarcoma of the breast. J Clin Oncol. (2005) 23:6249–51. doi: 10.1200/jco.2005.06.213
2. Fletcher C, Bridge JA, Hogendoorn PCW, Mertens F. WHO classification of tumours of soft tissue and bone: WHO classification of tumours Vol. 5. France: World Health Organization (2013).
3. Mentzel T, Dry S, Katenkamp D, Fletcher CD. Low-grade myofibroblastic sarcoma: analysis of 18 cases in the spectrum of myofibroblastic tumors. Am J Surg Pathol. (1998) 22:1228–38. doi: 10.1097/00000478-199810000-00008
4. Kito M, Ae K, Okamoto M, Endo M, Ikuta K, Takeuchi A, et al. Clinical outcome of low-grade myofibroblastic sarcoma in Japan: A multicenter study from the Japanese musculoskeletal oncology group. Cancers (Basel). (2023) 15(8):2314. doi: 10.3390/cancers15082314
5. Montgomery E, Goldblum JR, Fisher C. Myofibrosarcoma: a clinicopathologic study. Am J Surg Pathol. (2001) 25:219–28. doi: 10.1097/00000478-200102000-00010
6. Wang L, Li LX, Chen DQ, Yang L, Li SK, Cheng C. Low-grade Myofibroblastic sarcoma: clinical and imaging findings. BMC Med Imaging. (2019) 19:36. doi: 10.1186/s12880-018-0287-z
7. Taccagni G, Rovere E, Masullo M, Christensen L, Eyden B. Myofibrosarcoma of the breast: review of the literature on myofibroblastic tumors and criteria for defining myofibroblastic differentiation. Am J Surg Pathol. (1997) 21:489–96. doi: 10.1097/00000478-199704000-00017
8. Gocht A, Bösmüller HC, Bässler R, Tavassoli FA, Moinfar F, Katenkamp D, et al. Breast tumors with myofibroblastic differentiation: clinico-pathological observations in myofibroblastoma and myofibrosarcoma. Pathol Res Pract. (1999) 195:1–10. doi: 10.1016/s0344-0338(99)80087-9
9. Gonzalez-Palacios F, Enriquez JL, Miguel P, Vazzquez R, Carcia-Cosio M. Myofibroblastic tumors of the breast: A histologic spectrum with a case of recurrent male breast myofibrosarcoma. Int J Surg Pathol. (1999) 7:11–7. doi: 10.1177/106689699900700102
10. Jie W. Clinical and pathological analysis of breast fibroblastic/myofibroblastic tumors. Chin J Clin Exp Pathol. (2009) 25:33–6. doi: 10.13315/j.cnki.cjcep.2009.01.015
11. Stark M, Hoffmann A, Xiong Z. Mammary myofibrosarcoma: case report and literature review. Breast J. (2011) 17:300–4. doi: 10.1111/tbj.2011.17.issue-3
12. Anfei W, Yi Z, Wugan Z, Ying H, Yanping J, Xiaoyan W. A case of myofibroblastic sarcoma of the breast diagnosed by MRI. J Clin Radiology. (2011) 30:1186–7. doi: 10.13437/j.cnki.jcr.2011.08.028
13. Weiwei Z, Yikai X. Imaging findings of low-grade myofibroblastic sarcoma. Chin J Med Imaging Technology. (2012) 28:1591–5. doi: 10.13929/j.1003-3289.2012.08.029
14. Shenjere P, Eyden B, Banerjee SS, Chakrabarty B, Shanks JH, Sikand KA, et al. Ultrastructurally confirmed myofibrosarcoma: a series of 10 new cases, with a discussion on diagnostic criteria. Int J Surg Pathol. (2013) 21:29–36. doi: 10.1177/1066896912454568
15. Myong NH, Min JW. Low-grade myofibroblastic sarcoma arising in fibroadenoma of the breast-A case report. Diagn Pathol. (2016) 11:33. doi: 10.1186/s13000-016-0480-8
16. Ming Z, Xiangru W, Jian Z, Yipeng Y, Yunshu L, Baosan H, et al. A case of low-grade Malignant myofibroblastic sarcoma originating from the breast and review of the literature. Chin J Breast Disease(Electronic Edition). (2017) 11:255–6. doi: CNKI:SUN:ZHRD.0.2017-04-015
17. Val-Bernal JF, Hermana S, Alonso-Bartolomé MP. Myofibroblastic sarcoma of the breast. Report of a case induced by radiotherapys. Pathol Res Pract. (2019) 215:152664. doi: 10.1016/j.prp.2019.152664
18. Jiaxin Y, Yin T, Chunfu D, Yiou C. A case of new low-grade Malignant myofibroblastic sarcoma in the postoperative incision of a breast cancer patient. J Med Theory Pract. (2019) 32:2360. doi: 10.19381/j.issn.1001-7585.2019.15.015
19. Park SY, Kim HJ, Lee J, Jeong JY, Byun J, Kim WH, et al. A radiation induced low-grade myofibroblastic sarcoma in the retropectoral area after breast conserving surgery: A case report. J Breast Cancer. (2023) 26:397–402. doi: 10.4048/jbc.2023.26.e36
20. Zhe L, Meng Z, Jie Y, Chenmao X, Bin L, Yali S. Low-grade myofibroblastic sarcoma of mammary gland: A case report. J Clin Pathological Res. (2023) 43:634–8. doi: 10.3978/j.issn.2095-6959.2022
21. Fisher C. Myofibroblastic Malignancies. Adv Anat Pathol. (2004) 11:190–201. doi: 10.1097/01.pap.0000131773.16130.aa
22. Lucin K, Mustać E, Jonjić N. Breast sarcoma showing myofibroblastic differentiation. Virchows Arch. (2003) 443:222–4. doi: 10.1007/s00428-003-0853-8
23. Cai C, Dehner LP, El-Mofty SK. In myofibroblastic sarcomas of the head and neck, mitotic activity and necrosis define grade: a case study and literature review. Virchows Arch. (2013) 463:827–36. doi: 10.1007/s00428-013-1494-1
24. Makuch R, Birukov K, Shirinsky V, Dabrowska R. Functional interrelationship between calponin and caldesmon. Biochem J. (1991) 280:33–8. doi: 10.1042/bj2800033
25. Qiu X, Montgomery E, Sun B. Inflammatory myofibroblastic tumor and low-grade myofibroblastic sarcoma: a comparative study of clinicopathologic features and further observations on the immunohistochemical profile of myofibroblasts. Hum Pathol. (2008) 39:846–56. doi: 10.1016/j.humpath.2007.10.010
26. Kim JH, Choi W, Cho HS, Lee KS, Park JK, Kim BK. Surgical treatment and long-term outcomes of low-grade myofibroblastic sarcoma: a single-center case series of 15 patients. World J Surg Oncol. (2021) 19:339. doi: 10.1186/s12957-021-02454-5
27. Meng GZ, Zhang HY, Bu H, Zhang XL, Pang ZG, Ke Q, et al. Myofibroblastic sarcomas: a clinicopathological study of 20 cases. Chin Med J (Engl). (2007) 120:363–9. doi: 10.1097/00029330-200703010-00003
28. Chan JY, Gooi Z, Wong EW, Ng SK, Tong MC, Vlantis AC. Low-grade myofibroblastic sarcoma: A population-based study. Laryngoscope. (2017) 127:116–21. doi: 10.1002/lary.26146
29. Maki RG, Moraco N, Antonescu CR, Hameed M, Pinkhasik A, Singer S, et al. Toward better soft tissue sarcoma staging: building on american joint committee on cancer staging systems versions 6 and 7. Ann Surg Oncol. (2013) 20:3377–83. doi: 10.1245/s10434-013-3052-0
30. Scardina L, Franceschini G, Di Leone A, D'Archi S, Santoro A, Mulè A, et al. Low-grade myofibroblastic sarcoma of the breast. Breast J. (2020) 26:2077–8. doi: 10.1111/tbj.13956
Keywords: myofibroblastic sarcoma, myofibrosarcoma, breast, myofibroblast, immunohistochemistry
Citation: Deng Z, Xia C, Li Y, Luo Y and Shen S (2024) Myofibroblastic sarcoma in breast: a case report and literature review. Front. Oncol. 14:1366546. doi: 10.3389/fonc.2024.1366546
Received: 06 January 2024; Accepted: 25 April 2024;
Published: 13 May 2024.
Edited by:
Dragos Eugen Georgescu, Carol Davila University of Medicine and Pharmacy, RomaniaReviewed by:
Octav Ginghina, Carol Davila University of Medicine and Pharmacy, RomaniaAdelina Silvana Gheorghe, Carol Davila University of Medicine and Pharmacy, Romania
Iuliana Pantelimon, Carol Davila University of Medicine and Pharmacy, Romania
Copyright © 2024 Deng, Xia, Li, Luo and Shen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Songjie Shen, c2hlbnNqQHB1bWNoLmNu
†These authors have contributed equally to this work and share first authorship
 Zixi Deng1†
Zixi Deng1† Chuan Xia
Chuan Xia Songjie Shen
Songjie Shen