- Department of Dermatology and Venerology, University Hospital of Bern, University Bern, Bern, Switzerland
The importance of eosinophilic granulocytes in cancer has been widely discussed in recent years. The current study reviews the evidence on the role of eosinophilic granulocytes in melanoma as a prognostic marker for cancer progression and the efficacy of treatment with modern immune checkpoint inhibitors. A total of 33 human clinical studies were included in the review, with heterogeneous data due to differences in patients populations, study design and inclusion of small study groups. However, 28 of the 33 studies suggested that eosinophilic granulocytes could be used as a prognostic biomarker for outcome and/or potential response to systemic treatment and/or occurrence of adverse events in melanoma patients. Nevertheless, the exact role of eosinophils remains to be elucidated. Further prospective, larger and better controlled studies are warranted to clarify the significance of eosinophilic granulocytes in patients with melanoma, in more details.
1 Introduction
In contrast to many other cancers, the global prevalence of melanoma continues to increase (1, 2). Early detection is crucial for a successful treatment (1). In recent years, there have been significant developments in therapeutic options. Immune checkpoint inhibitor (ICI) therapies, such as anti-cytotoxic T-lymphocyte-associated protein 4 (CTLA4) and anti-programmed cell death protein 1 (PD1), have shown promising results (3).
In the era of personalized medicine, there is great interest in finding prognostic markers that can predict survival, outcome, or response to therapy (4). The potential prognostic biomarkers in melanoma regarding overall survival (OS) are melanoma-inhibitory activity (MIA), S100 protein, lactate dehydrogenase (LDH), and possibly eosinophilic granulocytes (5–7). In the last decade, the role of eosinophils in malignant melanoma has been increasingly discussed. Eosinophilic granulocytes, identified histologically by their acidophilic staining pattern and heavy cytoplasmic granules, are primarily recognized for their immune function against helminths, parasites and during an allergic reactions (8–13). In addition, eosinophils may play a role in modulating the tumor microenvironment (TME) and immune response, and probably influencing the outcome of ICI therapies in melanoma patients, making them a potential biomarker to predict response to therapy. In the present study, we focused on the current knowledge of the role of eosinophilic granulocytes as a potential prognostic marker for melanoma progression, with a focus on the efficacy of treatment with modern ICIs.
2 Methods
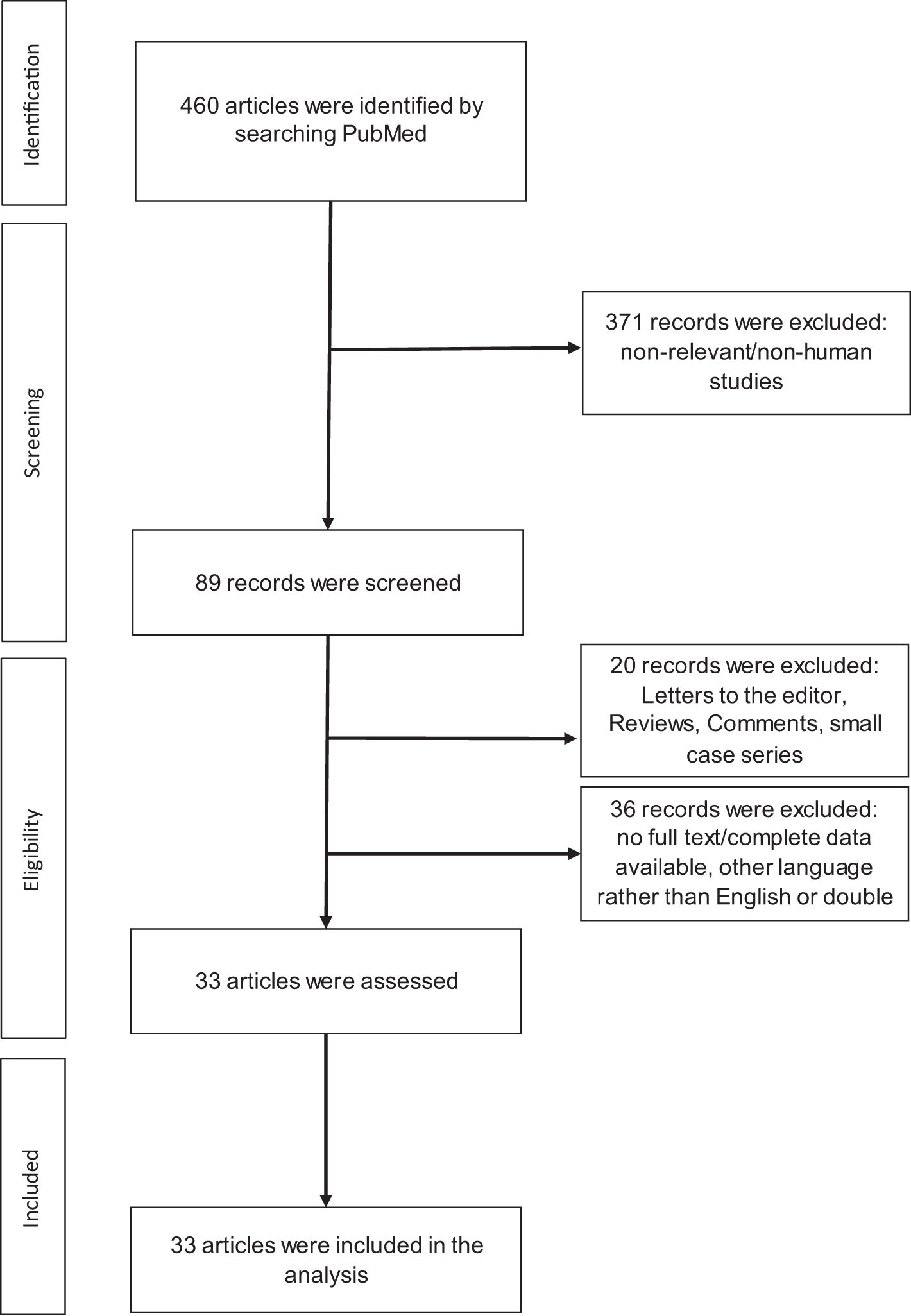
This review was conducted based on the PRISMA group statement. The systematic literature search was performed in the PubMed Library from January 2000 to December 2023 using the following search terms or respective combinations: “melanoma [Title] AND (eosinophils or eosinophil)” and “melanoma [Title] AND (eosinophils or eosinophilic or tumor associated blood eosinophilia (TABE) or tumor associated tissue eosinophilia (TATE) or tissue eosinophilia (TE)) AND (prognosis or prognostic or outcome or overall survival (OS))”. The publication had to be a human study. Review articles, case reports, case series (with fewer than 15 patients), meta-analyses, and animal studies were excluded.
3 Results
A total of 460 articles were initially identified. After a thorough review and screening of all abstracts, 427 articles were excluded. Finally, 33 clinical studies met the search terms and inclusion criteria, as shown in Figure 1. A summary of the selected publications is presented in Table-1, in chronological order. Most of the studies were retrospective in design. 28 of the 33 studies suggested that eosinophilic granulocytes could be used as a prognostic biomarker for outcome and/or potential response to systemic treatment and/or occurrence of adverse events in melanoma patients.
3.1 Role of eosinophils independent of therapy type or treatment initiation
Four publications have investigated the prognostic value of eosinophils, independent of patient therapy. In a recent study by Zhang et al. (25) 80 uveal melanoma patients in The Cancer Genome Atlas (TCGA) were classified into two immune subgroups of the tumor microenvironment. Class 1 has low immune infiltration, contains memory B-cells, T helper-2 cells, T helper-17 cells, natural killer cells and eosinophilic granulocytes, and has a better prognosis. CD8+ T cells, T helper-1 cells, myeloid-derived suppressor cells, and dendritic cells are enriched in class 2, which has strong cytolytic activity, high expression of immune checkpoint genes and poor outcome (25). In another study, Wagner et al. (28) evaluated the impact of peripheral blood leukocytes on OS in 1412 patients with melanoma (stage I-II) who underwent sentinel lymph node biopsy. They concluded that peripheral blood leukocytes are independently associated with OS in patients with stage I-II melanoma and should be considered as a prognostic marker. An absolute eosinophil count ≤200/µL was associated with a decreased OS in their study (28). In addition, Krückel et al. (5) investigated eosinophil cationic protein (ECP) as an early prognostic marker in 56 patients with metastatic melanoma. This marker mediates anti-cancer effects, such as tissue remodeling and cytotoxic activity. Therefore, they concluded that ECP is a novel prognostic serum marker for the outcome of melanoma patients that is independent of LDH and easy to perform in clinical practice (5). Similarly, Moreira et al. (35) investigated whether eosinophilia is a prognostic marker in 173 patients with metastatic melanoma. They observed that melanoma patients with eosinophilia at any point in the course of their disease showed a trend toward longer survival, regardless of their therapy (35).
3.2 Eosinophilic granulocytes and anti-PD1 monotherapy (pembrolizumab, nivolumab)
In a recent study by Bai et al. (26), pre-treatment eosinophilic blood counts were negatively correlated with OS in 89 patients with advanced melanoma treated with the anti-PD-1 monotherapy from two prospective clinical trials. In contrast, Swame et al. and Nakamura et al. (30, 31) failed to demonstrate an association between OS and eosinophilic blood counts. Similarly, in the study by Kurzhals et al. (7), lymphocyte and eosinophil counts at baseline and during immunotherapy were not associated with disease recurrence. However, in three further studies, high relative eosinophil blood counts correlated with an improved OS (3, 6, 35). Similarly, Amman et al. (20) found a positive correlation between increased tumor-infiltrating eosinophils and T cells and delayed melanoma progression. Furthermore, high baseline eosinophil count, serum ECP, and eosinophil peroxidase levels were associated with prolonged progression-free survival (PFS) in metastatic melanoma under immune checkpoint inhibition (20). Pozorski et al. (15) also recently demonstrated that the baseline neutrophil/eosinophil ratio may be a novel prognostic marker for advanced melanoma patients receiving anti-PD-1-based therapies.
Simon et al. (27) suggested that eosinophil levels may be a novel predictive marker for melanoma patients who may benefit from the immunotherapy, since clinical responses to immune checkpoint inhibitor treatment were associated with peripheral blood eosinophil accumulation (27). Similarly, Kartolo et al. (23) and Ohashi et al. (18) were able to show that eosinophilia on immunotherapy could be a favorable sign for advanced malignant melanoma. However, the retrospective study of melanoma patients receiving either nivolumab or pembrolizumab by Nakamura et al. (31) did not find a correlation between relative and absolute eosinophil blood counts and treatment response. In addition, the study by Bai et al. (26) found that the low early-on-/pre-treatment fold change in eosinophil count was not significantly associated with a better response to treatment.
Another retrospective study showed that patients with a high absolute pre-treatment eosinophilic count (EC) had a higher risk of immune-related adverse events (irAEs) (31). In line with this, Tasaki et al (14), recently showed that elevated eosinophils prior to two courses of treatment may be a predictor of immune-related adverse events in various cancers treated with different immune checkpoint inhibitors. However, Fujisawa et al. (36) found no significant correlation between the relative counts of eosinophils (REC) and irAEs, in their retrospective analysis of 101 patients with unresectable or stage IV melanoma, treated with nivolumab.
3.3 Eosinophilic granulocytes and CTLA4 monotherapy (ipilimumab)
In the study by Balatoni et al. (29) baseline absolute eosinophil counts >0.1 G/L were significantly associated with worse PFS in 47 patients with advanced melanoma treated with ipilimumab. However, Marlens et al. (39) reported that absolute and relative eosinophil counts were positively correlated with survival following ipilimumab treatment in their patients with advanced melanoma. In a similar retrospective study by Ferrucci et al. (38), a relative eosinophil count ≥ 1.5% was associated with a favorable outcome in patients receiving anti-CTLA4. However, in other studies, the baseline eosinophil count was not associated with OS (33, 42).
Machiraju et al. (24) observed a significant increase in absolute blood eosinophils with anti-CTLA4 treatment. Further studies reported that this increase in eosinophils during treatment with anti-CTLA4 monotherapy was a predictor of better OS (32, 41, 42).
In contrast, in a study of 43 patients with advanced melanoma receiving ipilimumab, absolute peripheral blood eosinophil counts at 3 and 9 weeks were significantly higher in patients who presented with irAEs of any grade (37). Nevertheless, Khoja et al. (40) reported that the eosinophil/lymphocyte ratio was not associated with toxicity or therapeutic response.
3.4 Eosinophilic granulocytes and anti-PD1/Anti-CTLA4 combination therapy
Some studies have shown a significant correlation between REC and OS in patients treated with an anti-PD1/anti-CTLA4 combination therapy (3, 27, 34). These studies showed that higher REC was as a prognostic marker for better survival (3, 27, 34). In addition, Machiraju et al. (24) observed in their retrospective study that patients generally experience an increase in peripheral blood eosinophils during treatment. Interestingly, Simon et al. (27) showed that treatment responders had a significantly higher increase in peripheral blood eosinophils during treatment than non-responders.
3.5 Eosinophilic granulocytes and other melanoma therapies
Ferrucci et al. (38) showed that EC in patients underwent chemotherapy was not associated with PFS or OS in patients with advanced melanoma.
Lang et al. (32) retrospectively investigated the role of eosinophils during treatment with B-Raf proto-oncogene, serine/threonine kinase (BRAF) inhibitors. No significant association was found between the eosinophil count and OS. They also found no correlation between eosinophils and long-term survival (32). Wendlinger et al. (21) demonstrated that high pre-treatment eosinophil counts in patients with advanced melanoma were associated with a significantly improved response to MAPK signaling pathway inhibitors (MAPKi). Functionally, eosinophils have potent cytotoxicity against melanoma cells that can be enhanced by MAPKi (21).
A single phase II cohort investigated the prognostic role of eosinophils in 41 patients with advanced melanoma treated with a combination therapy of BEMPEG (a PEGylated interleukin-2 [IL-2] prodrug) and nivolumab (22). The findings showed that a strong increase in eosinophils during the first eight days of treatment was a positive prognostic marker for better response to therapy, but not for a better PFS (22). In another study, prolonged PFS and OS were significantly associated with an increase in absolute peripheral blood eosinophil count during additional intralesional treatment with IL-2 in patients with locoregional progression on immunotherapy (19).
4 Discussion
The potential importance of baseline biomarkers present in both the blood and the tumor microenvironment for guiding pretreatment selection and predicting prognosis in melanoma patients has received considerable attention in the literature (19). The important beneficial effect of eosinophils and recent clinical studies suggest that eosinophils may have a significant impact on tumor progression in several types of cancer (43–45). Studies have reported that, depending on the type of cancer, the presence of eosinophils may either be beneficial for survival, or worsen the outcome. For example, some studies suggest a beneficial effect of high eosinophil blood counts in colon cancer, prostate cancer, breast cancer and melanoma (35, 46–49). In contrast, patients with Hodgkin’s lymphoma who have an increase in eosinophils seem to have a worse survival rate (50). Eosinophils are capable of phagocytosis and can express MHC-II on their surface, and can migrate to regional lymph nodes (51–53). Depending on the stimulus, they can activate innate and adaptive immunity, communicate with T cells and mast cells, and participate in the initiation and maintenance of an inflammatory state (54). In addition to the known and described properties of eosinophilic granulocytes, their effect and role in malignancies has mostly been studied in vitro or in animal models. Eosinophils play a role in tissue remodeling and cell turnover in both homeostasis and disease (45). In the context of tumors, eosinophils are often found in areas of tissue necrosis, and there is evidence that eosinophils can exert a cytotoxic effect on tumor cells, both in vitro and in vivo. Finally, tumor-associated tissue eosinophilia (TATE) appears to be generally protective (45). Several studies have shown an improved prognosis with TATE or evidence of eosinophil degranulation in various types of solid tumors (35, 45–47). In a large national cohort of metastatic solid tumor could show better OS correlated with increased eosinophil count (16). Moreira et al. and Wagner et al. (28, 35) supported this statement specifically for melanoma by demonstrating defined baseline eosinophilic granulocytes as a positive predictive marker for improved OS, independent of therapy. Zhang et al. (25) also showed that the presence of eosinophilic granulocytes in TME was associated with a better prognosis of choroidal melanoma.
Several factors might generally influence the response to ICI treatment, including tumor mutational burden, tumor microenvironment, and stool microbiome (55). Serum markers such as lactate dehydrogenase, PD-L1 expression on melanoma cells, microsatellite instability, and the composition of circulating blood cells such as lymphocytes, neutrophils, and eosinophils, either individually or in combination, have potential predictive value (56). However, studies evaluating the prognostic value of peripheral eosinophilic blood count in patients treated with various systemic therapies for OS have shown heterogeneous results. In addition, there were significant differences in the design and analytic methods of the available studies. They included patients with various melanoma subtypes at different melanoma stages, making it difficult to compare their results. There were also significant differences among studies concerning the association between eosinophilic granulocyte count and treatment response. Despite the extremely heterogeneous data, eosinophils could serve as a prognostic marker in immunotherapy of melanoma, given their effects on the TME and the relationship between TME and ICI-therapy response (27, 57). In a retrospective cohort based on a pharmacovigilance registry that included 909 patients with various tumors receiving anti-PD-1 or anti-PD-L1 therapy, 2.8% of patients were found to have immune-related eosinophilia, the majority of whom were being treated for advanced melanoma (58). Analysis of inflammatory mediators showed in a recent study that IL-16 levels tend to be associated with the frequency of circulating eosinophils (27). Eosinophils are a source of IL-16, which is a chemoattractant for both lymphocytes and eosinophils (27, 59). Furthermore, eosinophils have been shown to attract CD8+ T cells to the tumor microenvironment in the absence of regulatory T cells, in a melanoma mouse model (27). In a similar study, eosinophils were shown to enhance CD8+ T cells activation and improve the response to immunotherapy in breast cancer (60). This is important because previous publications found that tumor infiltration by CD8+ T cells was enhanced in responders before and during ICI treatment (27, 61). In addition, eosinophils from ICI-treated patients were shown to be enriched for IFN-γ response signatures and IL-2 signaling (27). IFN-γ signaling was found to be essential for the beneficial effect of PD-1 inhibition (27, 61). Nevertheless, not all eosinophil effects are solely due to ICI therapy. Future studies should include melanoma patients undergoing different treatments to better understand the exact role of eosinophils (27).
In spite of possible association between the occurrence of immune-related adverse events and improved OS (17), irAEs can profoundly affect nearly every organ system, resulting in severe to fatal toxicities that require discontinuation of ICI therapy in a significant proportion of patients (15). Several factors have been implicated in the occurrence of irAEs in patients receiving ICIs, including younger age, higher BMI, gender, smoking history, the presence of multiple chronic diseases, pre-existing autoimmune conditions and chronic use of certain drugs (15, 62). Potential biomarkers for irAEs include circulating blood counts, cytokines, autoantibodies, HLA genotypes, microRNA, gene expression profiling, and serum proteins (62). Although pre-treatment eosinophil count was not associated with the occurrence of irAE, in the study by Mehra et al. (17), elevated eosinophils prior to treatment have recently been shown to be a predictor of immune-related adverse events in several cancers treated with different ICIs (14). Similarly, in a recent cohort of patients with metastatic renal cell carcinoma treated with ipilimumab and nivolumab, an elevated eosinophil level 2 weeks after treatment may be an effective biomarker for ≥grade 2 irAEs (63).
In conclusion, eosinophilic granulocytes and their secreted proteins can be used as prognostic biomarkers for patients with melanoma. The role of eosinophils in melanoma is being elucidated. Eosinophilic granulocytes appear to play an important role in melanoma and cancer and are a potentially valuable biomarker for predicting response to ICIs, but their exact role remains unclear. This is because current knowledge in this area is based on mostly retrospective studies with heterogeneous study designs (including patient populations, treatment regimens, follow-up protocols, statistical analysis methods and cut-off values), leading to inconsistent and sometimes even controversial results. In addition, the other possible cause of hypereosinophilia should be considered in more detail. We hope that the current review will encourage cancer specialists to investigate the impact of local and/or peripheral eosinophilia on survival in melanoma patients in a well-designed, standardized prospective study.
Author contributions
CB: Conceptualization, Data curation, Writing – original draft, Writing – review & editing. RH: Conceptualization, Data curation, Methodology, Writing – original draft, Writing – review & editing. SS: Conceptualization, Data curation, Methodology, Supervision, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. MacKie RM, Hauschild A, Eggermont AMM. Epidemiology of invasive cutaneous melanoma. Ann Oncol. (2009) 20 Suppl 6:vi1–7. doi: 10.1093/annonc/mdp252
2. Seyed Jafari SM, Folini-Huesser F, Cazzaniga S, Hunger RE. Long-term follow-up of lentigo Maligna patients rreated with imiquimod 5% Cream. Cancers (Basel). (2023) 15:1546. doi: 10.3390/cancers15051546
3. Heppt MV, Heinzerling L, Kähler KC, Forschner A, Kirchberger MC, Loquai C, et al. Prognostic factors and outcomes in metastatic uveal melanoma treated with programmed cell death-1 or combined PD-1/cytotoxic T-lymphocyte antigen-4 inhibition. Eur J Cancer. (2017) 82:56–65. doi: 10.1016/j.ejca.2017.05.038
4. Seyed Jafari SM, Wiedmer C, Cazzaniga S, Frangež Ž, Shafighi M, Beltraminelli H, et al. Correlation of Vascular Endothelial Growth Factor subtypes and their receptors with melanoma progression: a next-generation Tissue Microarray (ngTMA) automated analysis. PloS One. (2018) 13:e0207019. doi: 10.1371/journal.pone.0207019
5. Krückel A, Moreira A, Fröhlich W, Schuler G, Heinzerling L. Eosinophil-cationic protein - a novel liquid prognostic biomarker in melanoma. BMC Cancer. (2019) 19:207. doi: 10.1186/s12885-019-5384-z
6. Weide B, Martens A, Hassel JC, Berking C, Postow MA, Bisschop K, et al. Baseline biomarkers for outcome of melanoma patients treated with pembrolizumab. Clin Cancer Res. (2016) 22:5487–96. doi: 10.1158/1078-0432.CCR-16-0127
7. Kurzhals JK, Klee G, Hagelstein V, Zillikens D, Terheyden P, Langan EA. Disease recurrence during adjuvant immune checkpoint inhibitor treatment in metastatic melanoma: clinical, laboratory, and radiological characteristics in patients from a single tertiary referral Center. Int J Mol Sci. (2022) 23:10723. doi: 10.3390/ijms231810723
8. Rothenberg ME, Hogan SP. The eosinophil. Annu Rev Immunol. (2006) 24:147–74. doi: 10.1146/annurev.immunol.24.021605.090720
9. Rosenberg HF, Dyer KD, Foster PS. Eosinophils: changing perspectives in health and disease. Nat Rev Immunol. (2013) 13:9–22. doi: 10.1038/nri3341
10. Akuthota P, Weller PF. Eosinophils and disease pathogenesis. Semin Hematol. (2012) 49:113–9. doi: 10.1053/j.seminhematol.2012.01.005
11. Gleich GJ. Mechanisms of eosinophil-associated inflammation. J Allergy Clin Immunol. (2000) 105:651–63. doi: 10.1067/mai.2000.105712
12. Jacobsen EA, Taranova AG, Lee NA, Lee JJ. Eosinophils: singularly destructive effector cells or purveyors of immunoregulation? J Allergy Clin Immunol. (2007) 119:1313–20. doi: 10.1016/j.jaci.2007.03.043
13. Munitz A, Levi-Schaffer F. Eosinophils: “new” roles for “old” cells. Allergy. (2004) 59:268–75. doi: 10.1111/j.1398-9995.2003.00442.x
14. Tasaki Y, Sugiyama Y, Hamamoto S, Naiki T, Uemura T, Yokota K, et al. Eosinophil may be a predictor of immune-related adverse events induced by different immune checkpoint inhibitor types: A retrospective multidisciplinary study. Cancer Med. (2023) 12:21666–79. doi: 10.1002/cam4.6724
15. Pozorski V, Park Y, Mohamoud Y, Tesfamichael D, Emamekhoo H, Birbrair A, et al. Neutrophil-to-eosinophil ratio as a biomarker for clinical outcomes in advanced stage melanoma patients treated with anti-PD-1 therapy. Pigment Cell Melanoma Res. (2023) 36:501–11. doi: 10.1111/pcmr.13109
16. Goldschmidt JH, Chou LN, Chan PK, Chen L, Robert N, Kinsey J, et al. Real-world outcomes of 18,186 metastatic solid tumor outpatients: Baseline blood cell counts correlate with survival after immune checkpoint inhibitor therapy. Cancer Med. (2023) 12:20783–97. doi: 10.1002/cam4.6645
17. Mehra T, Dongre K, Boesing M, Frei P, Suenderhauf C, Zippelius A, et al. Pre-treatment comorbidities, C-reactive protein and eosinophil count, and immune-related adverse events as predictors of survival with checkpoint inhibition for multiple tumour entities. Cancer Med. (2023) 12:12253–62. doi: 10.1002/cam4.5919
18. Ohashi H, Takeuchi S, Miyagaki T, Kadono T. Increase of lymphocytes and eosinophils, and decrease of neutrophils at an early stage of anti-PD-1 antibody treatment is a favorable sign for advanced Malignant melanoma. Drug Discovery Ther. (2020) 14:11721. doi: 10.5582/ddt.2020.03043
19. Rafei-Shamsabadi D, Lehr S, Behrens M, Meiss F. Additive intralesional interleukin-2 improves progression-free survival in a distinct subgroup of melanoma patients with prior progression under immunotherapy. Cancers (Basel). (2022) 14:540. doi: 10.3390/cancers14030540
20. Ammann NL, Schwietzer YF, Mess C, Stadler JC, Geidel G, Kött J, et al. Activated eosinophils predict longer progression-free survival under immune checkpoint inhibition in melanoma. Cancers (Basel). (2022) 14:5676. doi: 10.3390/cancers14225676
21. Wendlinger S, Wohlfarth J, Kreft S, Siedel C, Kilian T, Dischinger U, et al. Blood eosinophils are associated with efficacy of targeted therapy in patients with advanced melanoma. Cancers (Basel). (2022) 14:2294. doi: 10.3390/cancers14092294
22. Diab A, Tykodi SS, Daniels GA, Maio M, Curti BD, Lewis KD, et al. Bempegaldesleukin plus nivolumab in first-line metastatic melanoma. J Clin Oncol. (2021) 39:2914–25. doi: 10.1200/JCO.21.00675
23. Kartolo A, Holstead R, Hopman W, Baetz T. Prognosticating role of serum eosinophils on immunotherapy efficacy in patients with advanced melanoma. Immunotherapy. (2021) 13:217–5. doi: 10.2217/imt-2020-0265
24. Machiraju D, Wiecken M, Lang N, Hülsmeyer I, Roth J, Schank TE, et al. Soluble immune checkpoints and T-cell subsets in blood as biomarkers for resistance to immunotherapy in melanoma patients. Oncoimmunology. (2021) 10:1926762. doi: 10.1080/2162402X.2021.1926762
25. Zhang Z, Su J, Li L, Du W. Identification of precise therapeutic targets and characteristic prognostic genes based on immune gene characteristics in uveal melanoma. Front Cell Dev Biol. (2021) 9:666462. doi: 10.3389/fcell.2021.666462
26. Bai X, Dai J, Li C, Cui C, Mao L, Wei X, et al. Risk models for advanced melanoma patients under anti-PD-1 monotherapy-ad hoc analyses of pooled data from two clinical trials. Front Oncol. (2021) 11:639085. doi: 10.3389/fonc.2021.639085
27. Simon SCS, Hu X, Panten J, Grees M, Renders S, Thomas D, et al. Eosinophil accumulation predicts response to melanoma treatment with immune checkpoint inhibitors. Oncoimmunology. (2020) 9:1727116. doi: 10.1080/2162402X.2020.1727116
28. Wagner NB, Luttermann F, Gassenmaier M, Forschner A, Leiter U, Garbe C, et al. Absolute and relative differential blood count predicts survival of AJCC stage I-II melanoma patients scheduled for sentinel lymph node biopsy. Australas J Dermatol. (2020) 61:e310–8. doi: 10.1111/ajd.13248
29. Balatoni T, Ladányi A, Fröhlich G, Czirbesz K, Kovács P, Pánczél G, et al. Biomarkers associated with clinical outcome of advanced melanoma patients treated with ipilimumab. Pathol Oncol Res. (2020) 26:317–25. doi: 10.1007/s12253-018-0466-9
30. Swami U, Chennamadhavuni A, Borcherding N, Bossler AD, Mott SL, Garje R, et al. Multivariable analysis of 169 cases of advanced cutaneous melanoma to evaluate antibiotic exposure as predictor of survival to anti-PD-1 based immunotherapies. Antibiotics (Basel). (2020) 9:E740. doi: 10.3390/antibiotics9110740
31. Nakamura Y, Tanaka R, Maruyama H, Ishitsuka Y, Okiyama N, Watanabe R, et al. Correlation between blood cell count and outcome of melanoma patients treated with anti-PD-1 antibodies. Jpn J Clin Oncol. (2019) 49:431–7. doi: 10.1093/jjco/hyy201
32. Lang BM, Peveling-Oberhag A, Faidt D, Hötker AM, Weyer-Elberich V, Grabbe S, et al. Long-term survival with modern therapeutic agents against metastatic melanoma-vemurafenib and ipilimumab in a daily life setting. Med Oncol. (2018) 35:24. doi: 10.1007/s12032-018-1084-9
33. Gambichler T, Brown V, Steuke A-K, Schmitz L, Stockfleth E, Susok L. Baseline laboratory parameters predicting clinical outcome in melanoma patients treated with ipilimumab: a single-centre analysis. J Eur Acad Dermatol Venereol. (2018) 32:972–7. doi: 10.1111/jdv.14629
34. Rosner S, Kwong E, Shoushtari AN, Friedman CF, Betof AS, Brady MS, et al. Peripheral blood clinical laboratory variables associated with outcomes following combination nivolumab and ipilimumab immunotherapy in melanoma. Cancer Med. (2018) 7:690–7. doi: 10.1002/cam4.1356
35. Moreira A, Leisgang W, Schuler G, Heinzerling L. Eosinophilic count as a biomarker for prognosis of melanoma patients and its importance in the response to immunotherapy. Immunotherapy. (2017) 9:115–21. doi: 10.2217/imt-2016-0138
36. Fujisawa Y, Yoshino K, Otsuka A, Funakoshi T, Fujimura T, Yamamoto Y, et al. Fluctuations in routine blood count might signal severe immune-related adverse events in melanoma patients treated with nivolumab. J Dermatol Sci. (2017) 88:225–31. doi: 10.1016/j.jdermsci.2017.07.007
37. de Coaña YP, Wolodarski M, Poschke I, Yoshimoto Y, Yang Y, Nyström M, et al. Ipilimumab treatment decreases monocytic MDSCs and increases CD8 effector memory T cells in long-term survivors with advanced melanoma. Oncotarget. (2017) 8:21539–53. doi: 10.18632/oncotarget.v8i13
38. Ferrucci PF, Gandini S, Cocorocchio E, Pala L, Baldini F, Mosconi M, et al. Baseline relative eosinophil count as a predictive biomarker for ipilimumab treatment in advanced melanoma. Oncotarget. (2017) 8:79809–15. doi: 10.18632/oncotarget.v8i45
39. Martens A, Wistuba-Hamprecht K, Geukes Foppen M, Yuan J, Postow MA, Wong P, et al. Baseline peripheral blood biomarkers associated with clinical outcome of advanced melanoma patients treated with ipilimumab. Clin Cancer Res. (2016) 22:2908–18. doi: 10.1158/1078-0432.CCR-15-2412
40. Khoja L, Atenafu EG, Templeton A, Qye Y, Chappell MA, Saibil S, et al. The full blood count as a biomarker of outcome and toxicity in ipilimumab-treated cutaneous metastatic melanoma. Cancer Med. (2016) 5:2792–9. doi: 10.1002/cam4.878
41. Gebhardt C, Sevko A, Jiang H, Lichtenberger R, Reith M, Tarnanidis K, et al. Myeloid cells and related chronic inflammatory factors as novel predictive markers in melanoma treatment with ipilimumab. Clin Cancer Res. (2015) 21:5453–9. doi: 10.1158/1078-0432.CCR-15-0676
42. Delyon J, Mateus C, Lefeuvre D, Lanoy E, Zitvogel L, Chaput N, et al. Experience in daily practice with ipilimumab for the treatment of patients with metastatic melanoma: an early increase in lymphocyte and eosinophil counts is associated with improved survival. Ann Oncol. (2013) 24:1697–703. doi: 10.1093/annonc/mdt027
43. Carretero R, Sektioglu IM, Garbi N, Salgado OC, Beckhove P, Hämmerling GJ. Eosinophils orchestrate cancer rejection by normalizing tumor vessels and enhancing infiltration of CD8(+) T cells. Nat Immunol. (2015) 6:609–17. doi: 10.1038/ni.3159
44. Varricchi G, Galdiero MR, Loffredo S, Lucarini V, Marone G, Mattei F, et al. Eosinophils: The unsung heroes in cancer? Oncoimmunology. (2018) 7:e1393134. doi: 10.1080/2162402X.2017.1393134
45. Davis BP, Marc E Rothenberg ME. Eosinophils and cancer. Cancer Immunol Res. (2014) 2:1–8. doi: 10.1158/2326-6066.CIR-13-0196
46. Pretlow TP, Keith EF, Cryar AK, Bartolucci AA, Pitts AM, Pretlow TG 2nd, et al. Eosinophil infiltration of human colonic carcinomas as a prognostic indicator. Cancer Res. (1983) 43:2997–3000.
47. Fernández-Aceñero MJ, Galindo-Gallego M, Sanz J, Aljama A. Prognostic influence of tumor-associated eosinophilic infiltrate in colorectal carcinoma. Cancer. (2000) 88:1544–8.
48. McNeel DG, Gardner TA, Higano CS, Kantoff PW, Small EJ, Wener MH, et al. A transient increase in eosinophils is associated with prolonged survival in men with metastatic castration-resistant prostate cancer who receive sipuleucel-T. Cancer Immunol Res. (2014) 2:988–99. doi: 10.1158/2326-6066.CIR-14-0073
49. Ownby HE, Roi LD, Isenberg RR, Brennan MJ. Peripheral lymphocyte and eosinophil counts as indicators of prognosis in primary breast cancer. Cancer. (1983) 52:126–30. doi: 10.1002/1097-0142(19830701)52:1<126::aid-cncr2820520123>3.0.co;2-y
50. von Wasielewski R, Seth S, Franklin J, Fischer R, Hübner K, Hansmann ML, et al. Tissue eosinophilia correlates strongly with poor prognosis in nodular sclerosing Hodgkin’s disease, allowing for known prognostic factors. Blood. (2000) 95:1207–13. doi: 10.1182/blood.V95.4.1207.004k34_1207_1213
51. Akuthota P, Wang HB, Spencer LA, Weller PF. Immunoregulatory roles of eosinophils: a new look at a familiar cell. Clin Exp Allergy. (2008) 38:1254–63. doi: 10.1111/j.1365-2222.2008.03037.x
52. Tamura N, Ishii N, Nakazawa M, Nagoya M, Yoshinari M, Amano T, et al. Requirement of CD80 and CD86 molecules for antigen presentation by eosinophils. Scand J Immunol. (1996) 44:229–38. doi: 10.1046/j.1365-3083.1996.d01-303.x
53. Akuthota P, Wang H, Weller PF. Eosinophils as antigen-presenting cells in allergic upper airway disease. Curr Opin Allergy Clin Immunol. (2010) 10:14–9. doi: 10.1097/ACI.0b013e328334f693
54. Hogan SP, Rosenberg HF, Moqbel R, Phipps S, Foster PS, Lacy P, et al. Eosinophils: biological properties and role in health and disease. Clin Exp Allergy. (2008) 38:709–50. doi: 10.1111/j.1365-2222.2008.02958.x
55. Buder-Bakhaya K, Hassel JC. Biomarkers for clinical benefit of immune checkpoint inhibitor treatment-A review from the melanoma perspective and beyond. Front Immunol. (2018) 9:1474. doi: 10.3389/fimmu.2018.01474
56. Kambayashi Y, Fujimura T, Hidaka T, Aiba S. Biomarkers for predicting efficacies of anti-PD1 antibodies. Front Med (Lausanne). (2019) 6:174. doi: 10.3389/fmed.2019.00174
57. Robinson I, Santa Lucia G, Li A, Oberholtzer N, Plante J, Quinn KM, et al. Eosinophils and melanoma: Implications for immunotherapy. Pigment Cell Melanoma Res. (2022) 35:192–202. doi: 10.1111/pcmr.13025
58. Bernard-Tessier A, Jeanville P, Champiat S, Lazarovici J, Voisin AL, Mateus C, et al. Immune-related eosinophilia induced by anti-programmed death 1 or death-ligand 1 antibodies. Eur J Cancer. (2017) 81:135–7. doi: 10.1016/j.ejca.2017.05.017
59. Lim KG, Wan HC, Bozza PT, Resnick MB, Wong DT, Cruikshank WW, et al. Human eosinophils elaborate the lymphocyte chemoattractants. IL-16 (lymphocyte hemoattractant factor) and RANTES. J Immunol. (1996) 156:2566–70. doi: 10.4049/jimmunol.156.7.2566
60. Blomberg OS, Spagnuolo L, Garner H, Voorwerk L, Isaeva OI, van Dyk E, et al. IL-5-producing CD4+ T cells and eosinophils cooperate to enhance response to immune checkpoint blockade in breast cancer. Cancer Cell. (2023) 41:106–23. doi: 10.1016/j.ccell.2022.11.014
61. Tumeh PC, Harview CL, Yearley JH, Shintaku IP, Taylor EJ, Robert L, et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature. (2014) 515:568–71. doi: 10.1038/nature13954
62. Chennamadhavuni A, Abushahin L, Jin N, Presley CJ, Manne A. Risk factors and biomarkers for immune-related adverse events: A practical guide to identifying high-risk patients and rechallenging immune checkpoint inhibitors. Front Immunol. (2022) 13:779691. doi: 10.3389/fimmu.2022.779691
Keywords: cancer, eosinophils, immune checkpoint inhibitors, immunology, melanoma
Citation: Brand CL, Hunger RE and Seyed Jafari SM (2024) Eosinophilic granulocytes as a potential prognostic marker for cancer progression and therapeutic response in malignant melanoma. Front. Oncol. 14:1366081. doi: 10.3389/fonc.2024.1366081
Received: 05 January 2024; Accepted: 17 April 2024;
Published: 02 May 2024.
Edited by:
Adil Daud, University of California, San Francisco, United StatesCopyright © 2024 Brand, Hunger and Seyed Jafari. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Seyed Morteza Seyed Jafari, bW9ydGV6YS5qYWZhcmlAaW5zZWwuY2g=
 Corsin Linard Brand
Corsin Linard Brand Robert Emil Hunger
Robert Emil Hunger Seyed Morteza Seyed Jafari
Seyed Morteza Seyed Jafari