- Department of Pharmacy, Emergency General Hospital, Beijing, China
Objective: The optimal first-line immunotherapy regimen for patients with PD-L1 expression ≥50% in squamous non-small cell lung cancer (Sq-NSCLC) remains uncertain. This study utilized net-work meta-analysis (NMA) to indirectly compare the efficacy of various first-line immuno-therapy regimens in this patient subset.
Methods: Systematic searches were conducted across PubMed, the Cochrane Library, Web of Science, and Embase databases for randomized controlled trials reporting overall survival (OS) and progression-free survival (PFS) outcomes. The search spanned from database inception to November 3, 2023. Bayesian network meta-analysis was employed for a comprehen-sive analysis. To ensure scientific rigor and transparency, this study is registered in the Interna-tional Prospective Register of Systematic Reviews (PROSPERO) under the registration number CRD42022349712.
Results: The NMA encompassed 9 randomized controlled trials (RCTs), involving 2170 patients and investigating 9 distinct immunotherapy regimens. For OS, the combination of camrelizumab and chemotherapy demonstrated the highest probability (36.68%) of efficacy, fol-lowed by cemiplimab (33.86%) and atezolizumab plus chemotherapy (23.87%). Regarding PFS, the camrelizumab and chemotherapy combination had the highest probability (39.70%) of efficacy, followed by pembrolizumab (22.88%) and pembrolizumab plus chemotherapy (17.69%). Compared to chemotherapy, first-line treatment with immune checkpoint inhibitors (ICIs) in Sq-NSCLC pa-tients exhibited significant improvements in OS (HR 0.59, 95% CI 0.47-0.75) and PFS (HR 0.44, 95% CI 0.37-0.52).
Conclusion: This study suggests that, for Sq-NSCLC patients with PD-L1 expression ≥50%, the first-line immunotherapy regimen of camrelizumab plus chemotherapy provides superior OS and PFS outcomes. Furthermore, ICIs demonstrate enhanced efficacy compared to chemotherapy in this patient population.
Systematic review registration: https://www.crd.york.ac.uk/prospero/, identifier: CRD 42022349712.
1 Introduction
Globally, lung cancer presents a notable incidence and mortality, with approximately 2.2 million new cases and 1.8 million deaths reported annually, which raises significant public health concerns (1, 2). Non-small cell lung cancer (NSCLC), the most prevalent subtype, accounts for 80%-85% of all lung cancer cases (2).The diagnosis of a majority of NSCLC cases occurs at an advanced stage, leading to a dismal prognosis, characterized by a less than 5% five-year survival rate (3). Some patients suffer from locally advanced or metastatic NSCLC, making surgical resection impractical. Standard treatments for such cases often involve platinum-based paclitaxel chemotherapy and/or radiation therapy (4). However, despite the implementation of these therapeutic strategies, a significant proportion of patients fail to achieve favorable outcomes (5).
In the recent past, the advent of immune checkpoint inhibitors (ICIs) has extended the survival period of NSCLC patients with manageable adverse effects (6, 7). ICIs encompass inhibitors targeting programmed death-1 (PD-1), programmed death ligand-1 (PD-L1), and cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4) (8). Compared to ICIs, chemotherapy may more readily induce drug resistance in patients, potentially contributing to the superior efficacy observed with ICIs (9). This disparity in efficacy might be partly attributed to the differing mechanisms of action between the two treatment modalities. ICIs modulate the immune system by blocking the PD-1/PD-L1 pathway, thereby enhancing immune response (10). This immunomodulation may lead to a more sustained anti-cancer response, reducing adaptive resistance of tumor cells to the treatment (10). In contrast, chemotherapy primarily achieves therapeutic effects by directly destroying cancer cells, but its nonspecific nature might make patients more prone to developing drug resistance (8, 11). NSCLC can be pathologically categorized into squamous non-small cell lung cancer (Sq-NSCLC) and non-squamous cell lung cancer (NS-NSCLC). Patients with these subtypes demonstrate disparities in smoking history, tumor location, and clinical outcomes (12). Therefore, personalized treatment based on the distinct characteristics of each cancer subtype is imperative. In contrast to NS-NSCLC, Sq-NSCLC poses greater therapeutic challenges. Diagnosis often coincides with the presence of comorbidities such as chronic obstructive pulmonary disease and cardiovascular conditions, intensifying the complexity of treatment (13–15). PD-L1 has emerged as a potential prognostic factor and biomarker for predicting which patients are more likely to respond to immunotherapy in NSCLC, thereby refining the target population for potential benefits (16–18). Approximately 30% of advanced NSCLC patients exhibit positive PD-L1 expression (PD-L1 expression ≥50%) as detected by immunohistochemistry (IHC) testing (19).
Presently, for stage IV NSCLC without driver gene mutations and with PD-L1 expression ≥50%, pembrolizumab stands as the preferred first-line treatment option (20). Additionally, due to the lack of randomized controlled trials (RCTs) directly comparing various immune checkpoint inhibitors, selecting the optimal immunotherapeutic approach remains challenging. Nevertheless, ambiguity remains regarding the optimal initial treatment for squamous NSCLC exhibiting PD-L1 expression ≥50%. To address this uncertainty, our study employs network meta-analysis (NMA) to compare the efficacy of various first-line immunotherapy approaches, aiming to identify the most effective treatment regimen. This research endeavors to furnish evidence-based guidance for clinical drug selection and offer improved treatment options for patients with squamous NSCLC.
2 Materials and methods
The NMA adhered to the guidelines outlined in the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) extension statement (21). Utilizing Bayesian methods, we conducted indirect comparisons of treatment modalities that lacked direct comparisons in clinical trials (22). This approach facilitated the generation of probabilistic predictions for treatment outcomes. For the sake of transparency, reliability, and originality, the study protocol has been prospectively registered in the International Prospective Register of Systematic Reviews (PROSPERO) under the reference number CRD42022349712.
2.1 Data sources and search strategy
To identify eligible studies, systematic searches were performed on PubMed, the Cochrane Library, Web of Science, and Embase databases. We utilized both medical subject headings and textwords in the search process. The search encompassed articles available in these databases from inception until November 3, 2023. The search terms included “immune checkpoint inhibitors,” “PD-1 inhibitor,” “PD-L1 inhibitor,” “CTLA-4 inhibitor,” “nivolumab,” “atezolizumab,” “durvalumab,” “tremelimumab,” “pembrolizumab,” “sintilimab,” “tislelizumab,” “camrelizumab,” “ipilimumab,” and “non-small-cell lung cancer.” The detailed search strategy is available in the Supplementary Document.
2.2 Inclusion and exclusion criteria
This analysis considered randomized controlled trials meeting the following criteria: (1) patients diagnosed with histologically or cytologically confirmed stage IV Sq-NSCLC, (2) the experimental group receiving immune checkpoint inhibitors, (3) availability of overall survival (OS) or progression-free survival (PFS) data for patients with squamous NSCLC and PD-L1 expression ≥50%, and (4) inclusion of randomized controlled trials specifically investigating first-line treatment regimens.
This study excluded the following: (1) editorials, observational studies, meta-analyse, and reviews, and (2) randomized controlled trials involving the same patient cohort.
2.3 Data extraction
Following the PRISMA guidelines, a meticulous process of data extraction was applied to the chosen RCTs. For precision and comprehensiveness, four researchers (W.C, H.L,Y.L. and W.X) autonomously extracted pertinent data, resolving any disparities through discussions with the fifth author(L.Z). The extracted information encompassed details such as the trial name, first author, publication year, trial phase, number of treatment lines, clinical trial identification number, sample size, age and gender distribution of patients, as well as comprehensive particulars about the experimental and control groups. Additionally, clinical outcomes such as OS and PFS were extracted, including the Hazard Ratios (HR) and 95% Confidence Intervals (CIs).
2.4 Statistical analysis
Within a Bayesian framework, we employed the “JAGS” and “GeMTC” packages in the R software for NMA (23, 24). This was undertaken to evaluate the effectiveness of different ICIs in the treatment of advanced Sq-NSCLC. A fixed-effects consistency model was utilized, with 25,000 iterations conducted simultaneously across three independent Markov chains. Each chain underwent 60,000 sample iterations. The NMA endpoints included OS and PFS, with effect sizes measured by HR and corresponding 95% CIs. For pairwise meta-analysis, the Revman software was used. Rank probability commands were employed to rank the treatments from best to worst, and differences were considered statistically significant at a two-sided α level < 0.05. One reviewer conducted the statistical analysis, and the results were cross-verified by three additional reviewers to ensure accuracy.
2.5 Quality assessment
To guarantee the inclusion of studies adhering to high-quality standards, we utilized the Cochrane Risk of Bias Tool (2.0) for the assessment of randomized controlled trials. This tool scrutinizes the risk of bias across five pivotal domains: the randomization process, potential bias in the implementation of the intended intervention, missing outcome data, measurement of outcomes, and the selection of reported results (25). Following the outcomes of the quality assessment, the included studies were classified as either low risk, high risk, or unclear risk. This categorization ensures the incorporation of only those studies that employ rigorous and reliable methodologies in the NMA.
2.6 Sensitivity analysis
Additionally, for optimal alignment with our analysis, model comparison was conducted using the Deviance Information Criterion (DIC). This criterion assesses the relative goodness of fit for fixed-effects and random-effects models, where a lower DIC value signifies superior model fit. Consistency between the fixed-effects and random-effects models is affirmed if the DIC difference is below 5. This method contributed to the selection of the most suitable model for each analysis cohort, ensuring precision in our approach (26).
2.7 Heterogeneity analysis
The “anote” command was employed to perform heterogeneity analysis and compute the I2 value. Interpretation of I2 values is as follows: I2 less than 25% signifies low heterogeneity, between 25% and 50% suggests moderate heterogeneity, and exceeding 75% indicates high heterogeneity. For instances of low heterogeneity, a fixed-effects model was applied, while in situations of moderate or high heterogeneity, a random-effects model was employed (27).
3 Results
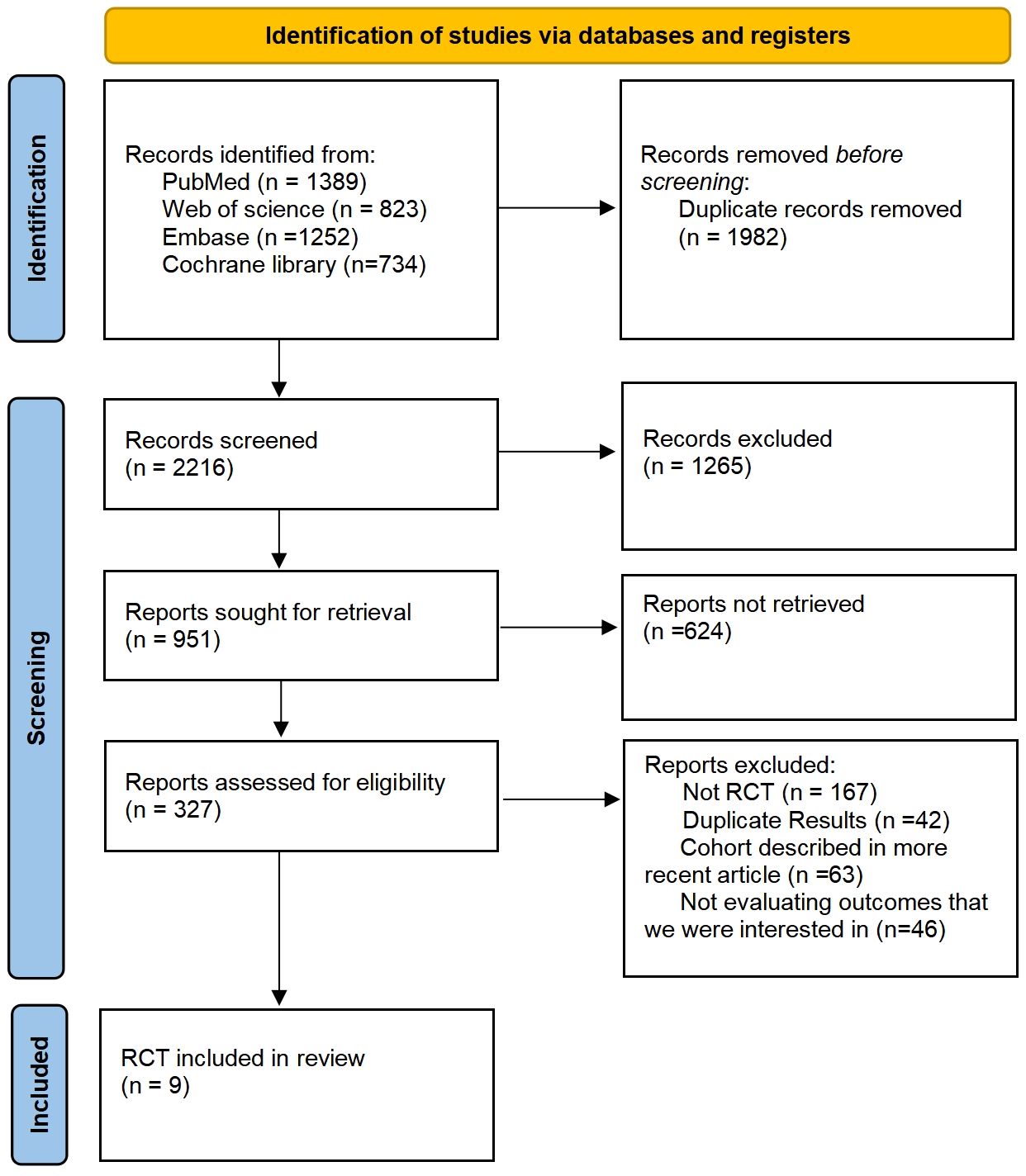
Following searches in the PubMed, Web of Science, Embase, and Cochrane Library databases, a total of 4198 articles were identified. Post removal of duplicates, 1982 articles were excluded from the analysis. The final selection process is depicted in Figure 1, with 9 RCTs being chosen for NMA. This study encompasses 9 RCTs, involving 2170 patients, and assesses 10 treatment regimens for Sq-NSCLC: pembrolizumab (pem), chemotherapy (chemo), pembrolizumab plus chemotherapy (pem-chemo), nivolumab plus ipilimumab (nivo-ipi), atezolizumab plus chemotherapy (atezo-chemo), cemiplimab (cemi), pembrolizumab plus ipilimumab (pem-ipi), camrelizumab plus chemotherapy (camre-chemo), tislelizumab plus chemotherapy (tisle-chemo), and sintilimab plus chemotherapy (sinti-chemo). Detailed results are outlined in Table 1.
3.1 Research characteristics
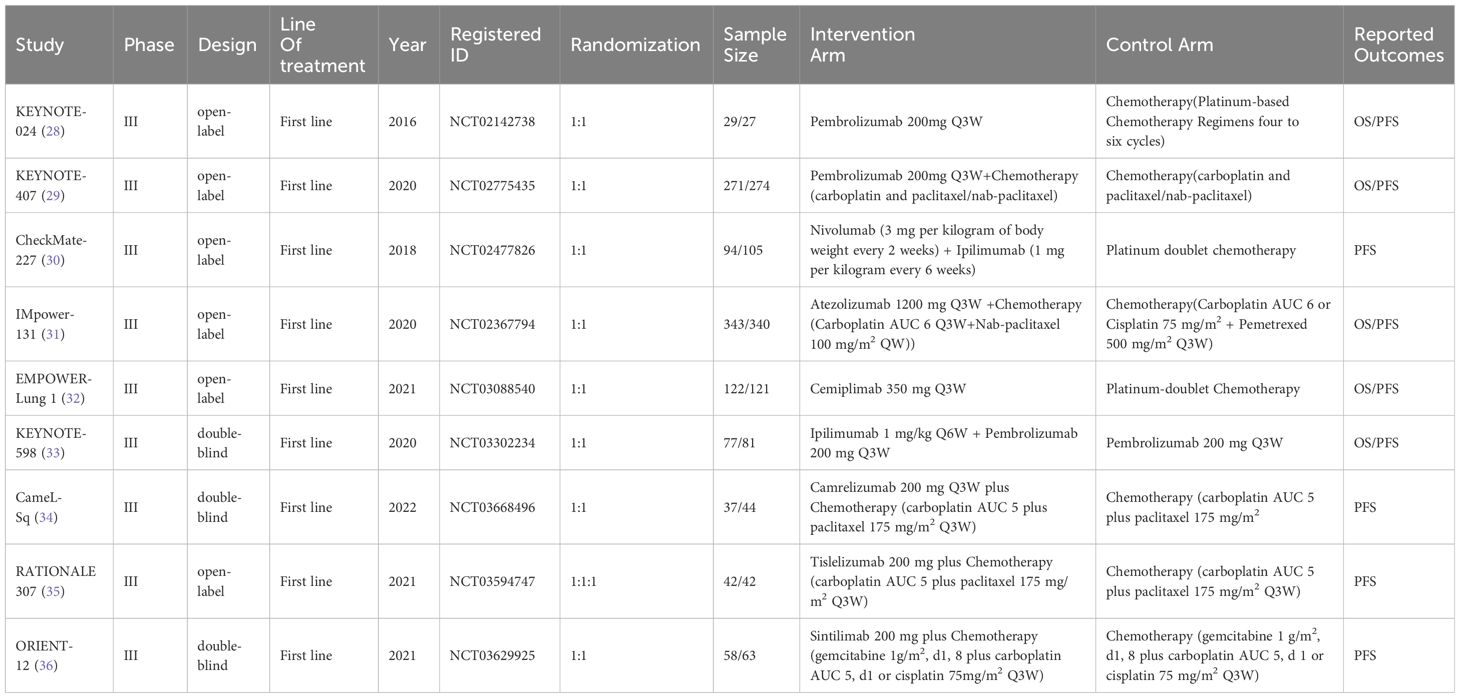
The experimental arms in two RCTs consisted of monotherapy with ICIs (KEYNOTE-024, EMPOWER-Lung 1), while the experimental arms in five other RCTs entailed a combination of ICIs and chemotherapy (KEYNOTE-407, IMpower-131, CameL-Sq, RATIONALE 307, ORIENT-12). Moreover, two additional RCTs featured experimental arms receiving a combination of ICIs (CheckMate-227, KEYNOTE-598). Figure 2 illustrates a comparative network plot for all observed outcomes. There are a total of 7 relevant first-line treatment regimens with overall survival as the outcome measure, and 10 relevant first-line treatment regimens with progression-free survival as the outcome measure. The network comparison diagram for the various treatment regimens in the nine randomized controlled trials is illustrated in Figure 2.
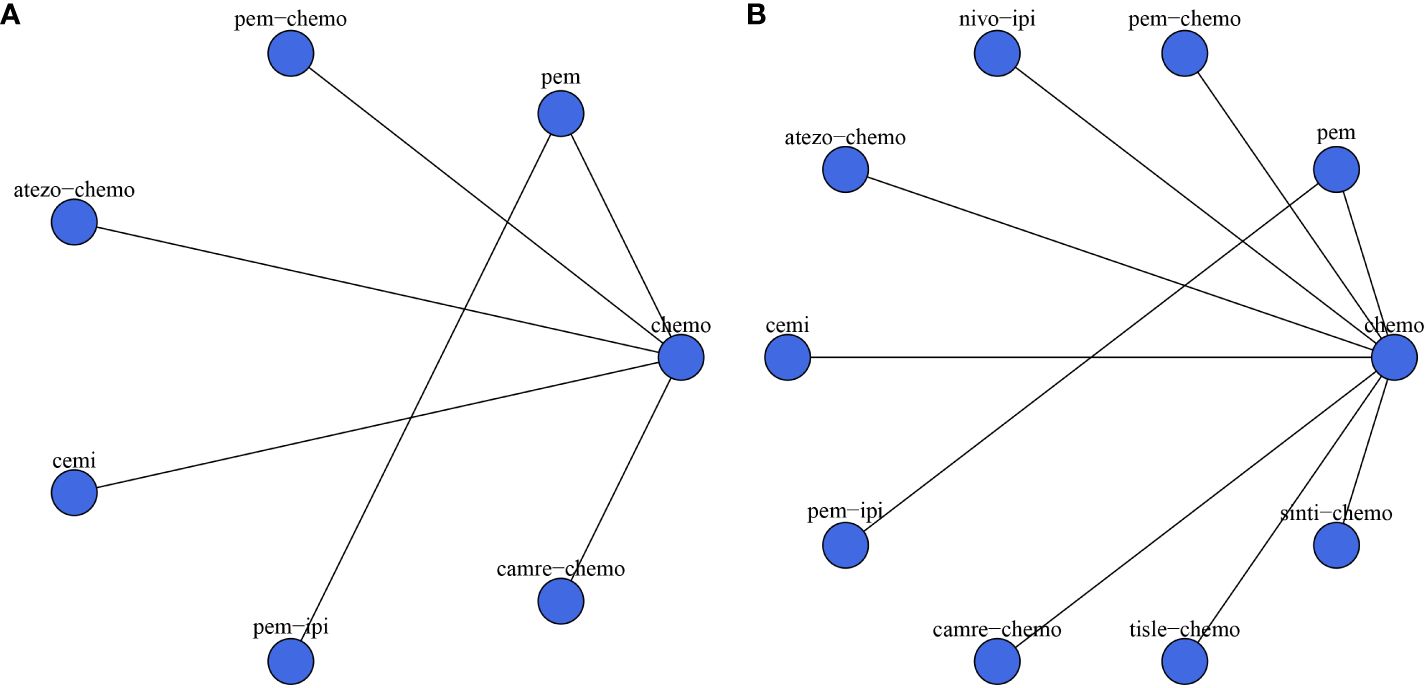
Figure 2 Network diagram. (A) overall survival (OS); (B) progression-free survival (PFS). In the figure, each point corresponds to a different treatment regimen, and the lines between points represent direct comparisons between two treatments. The thickness of the lines indicates the number of studies for each comparison.
3.2 Assessment of included trials
The results of the bias risk assessment for the 9 trials included in the study are illustrated in Figure 3. Overall, the bias risk across all studies is considered low, indicating a meticulous scientific approach in the design of these RCTs. Methodological details were validated through a thorough examination of the protocols for each RCT.
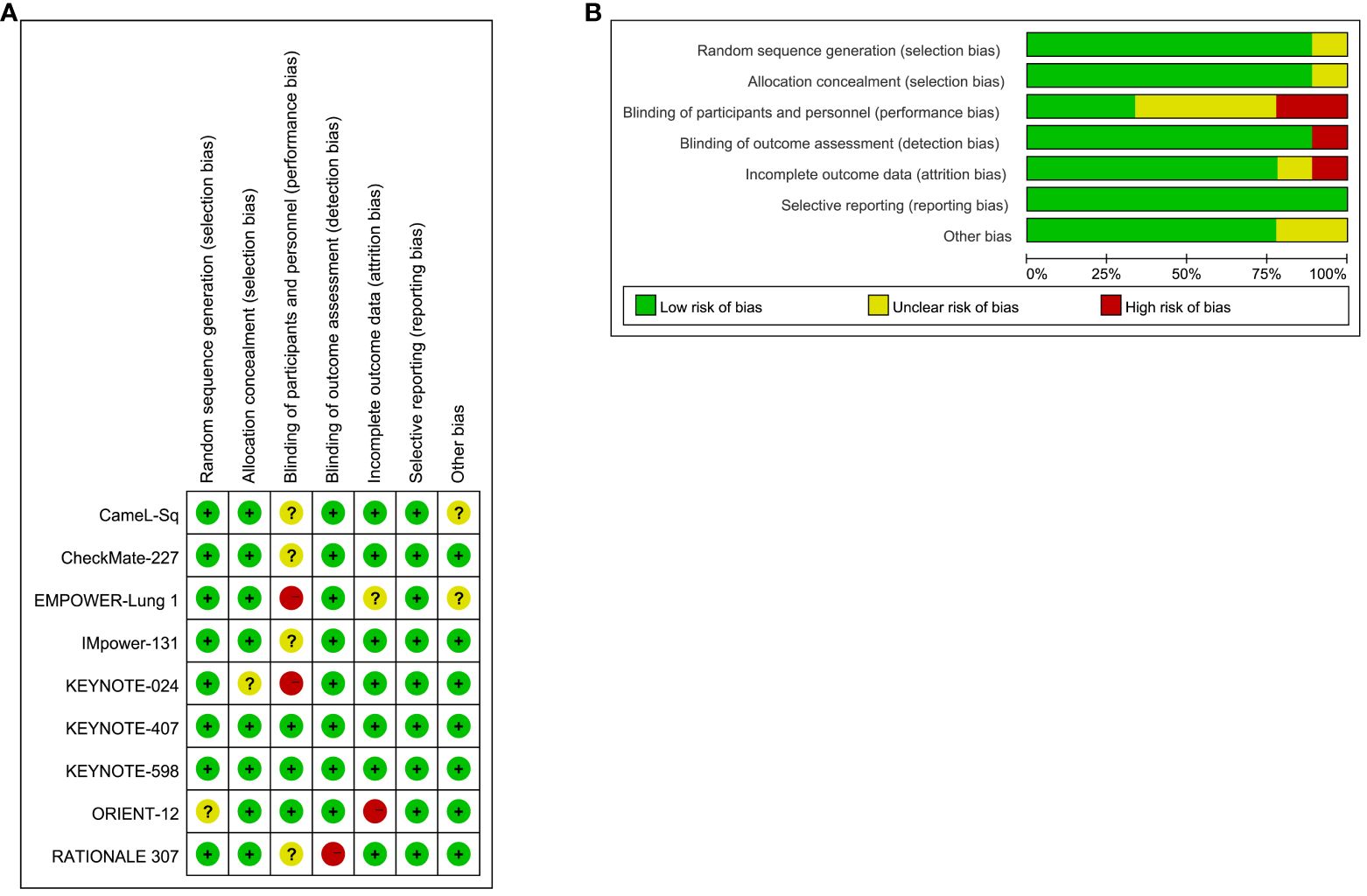
Figure 3 Risk of Bias Figure. (A) Summary of risk of bias: The authors rendered judgments for each methodological quality item for every study encompassed in the analysis; (B) Graphical representation of methodological quality: The authors’ assessments for each methodological quality item are depicted as percentages across all included studies.
Concerning selection bias, eight trials received a low-risk rating, while one trial (ORIENT-12) was classified as unclear. Regarding reporting bias, eight trials were evaluated as low risk, with one trial (KEYNOTE-024) designated as unclear. Implementation bias analysis revealed three trials at low risk, two trials (EMPOWER-Lung 1, KEYNOTE-024) at high risk, and four trials (CameL-Sq, CheckMate-227, IMpower-131, RATIONALE 307) with an unclear rating. Detection bias assessment resulted in eight trials with a low-risk designation, with only one trial (RATIONALE 307) considered high risk. Attrition bias evaluation categorized seven trials as low risk, one trial as high risk (ORIENT-12), and one trial (EMPOWER-Lung 1) as unclear.
All trials received a low-risk rating for reporting bias, primarily due to their analysis based on the intention-to-treat population and the comprehensive reporting of endpoints. However, it is noteworthy that two trials permitted crossover (CameL-Sq, EMPOWER-Lung 1), potentially introducing sources of bias.
3.3 Assessment of included trials Pairwise meta-analysis
Through a head-to-head meta-analysis, we evaluated the efficacy of ICIs compared to chemotherapy in patients with Sq-NSCLC), with OS and PFS as the outcome measures.
Five RCTs reported OS, with an I2 value of 0, indicating low heterogeneity. Employing a fixed-effects model, the results demonstrated an improvement in OS for S-NSCLC patients treated with ICIs compared to chemotherapy in first-line therapy (HR, 0.59; 95% CI, 0.47-0.75). Refer to Figure 4 for detailed results.
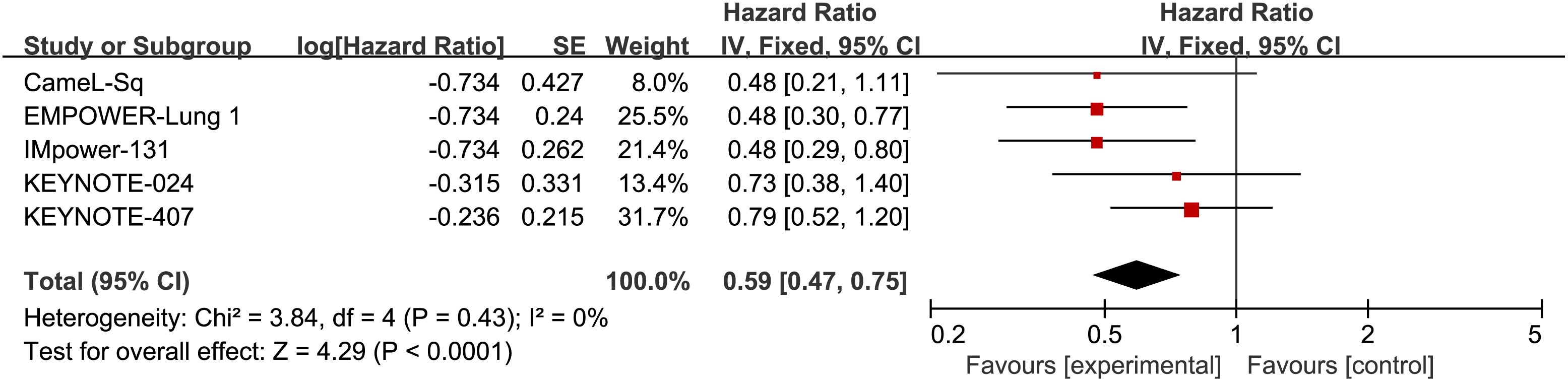
Figure 4 Forest plot illustrating the OS outcome measure. Comparing the Effectiveness of ICIs and Chemotherapy in Advanced Sq-NSCLC among Patients with PD-L1 Expression ≧̸50%.
Additionally, eight RCTs reported PFS, with an I2 value of 0, suggesting low heterogeneity. The application of a fixed-effects model revealed a noteworthy enhancement in PFS among patients with Sq-NSCLC undergoing ICI treatment in comparison to first-line chemotherapy (HR, 0.44; 95% CI, 0.37-0.52). Specific results can be found in Figure 5.
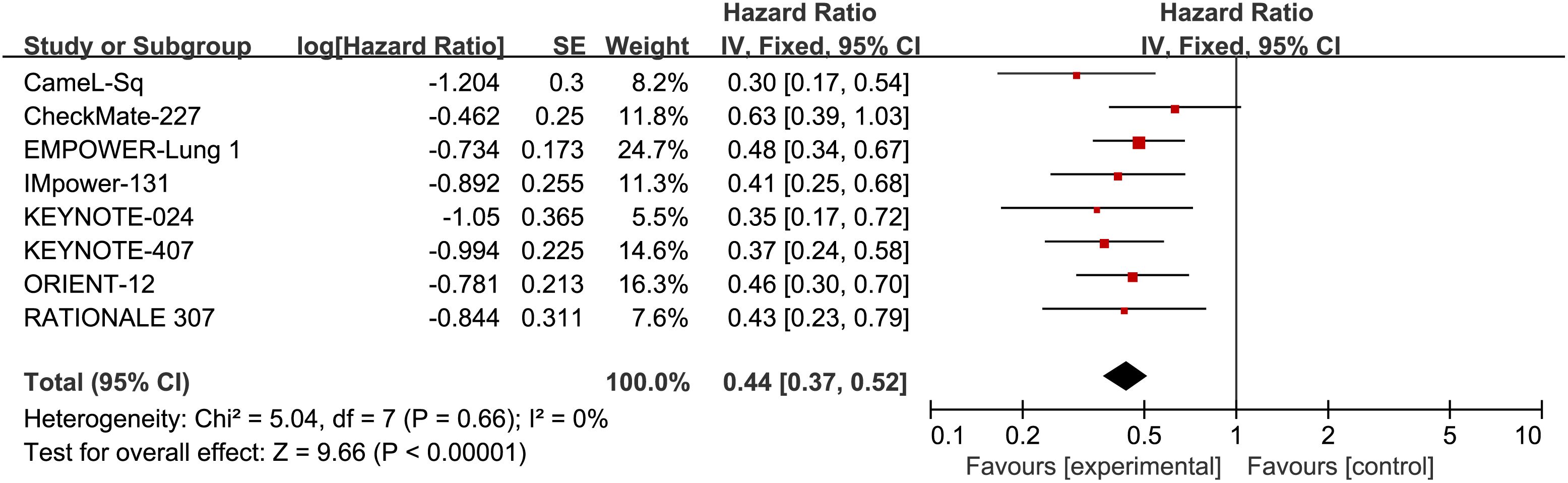
Figure 5 Forest plot illustrating the PFS outcome measure. Comparing the Effectiveness of ICIs and Chemotherapy in Advanced Sq-NSCLC among Patients with PD-L1 Expression ≥50%.
3.4 Network meta-analysis
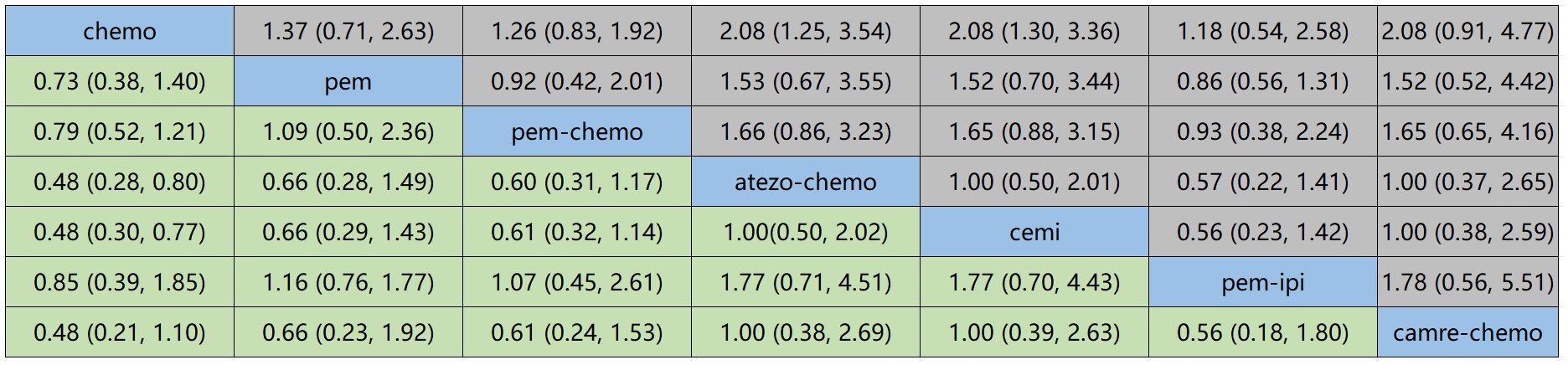
Figure 6 displays the indirect comparison results for OS, demonstrating that Ipilimumab plus Pembrolizumab, compared to chemotherapy, yielded a HR of 0.85 (95% CI, 0.21-1.10).
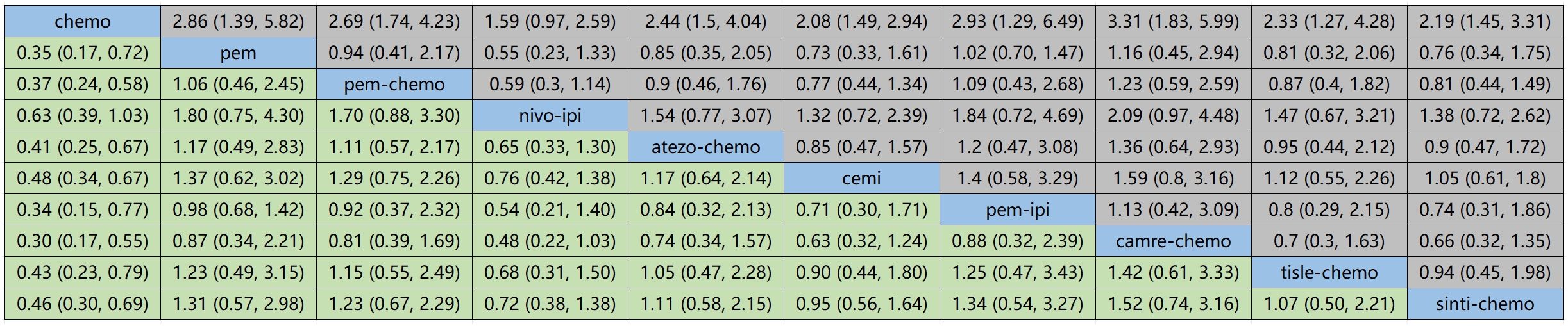
Figure 7 illustrates the indirect comparison results for progression-free survival (PFS), indicating that the combination of Ipilimumab and Pembrolizumab, as opposed to chemotherapy, yielded a HR of 0.34 (95% CI, 0.15-0.77).
3.5 Rankings
Figure 8 and Figure 9 represent ranking plots for patients with Sq-NSCLC undergoing various treatment regimens, with OS and PFS as the primary outcome measures.
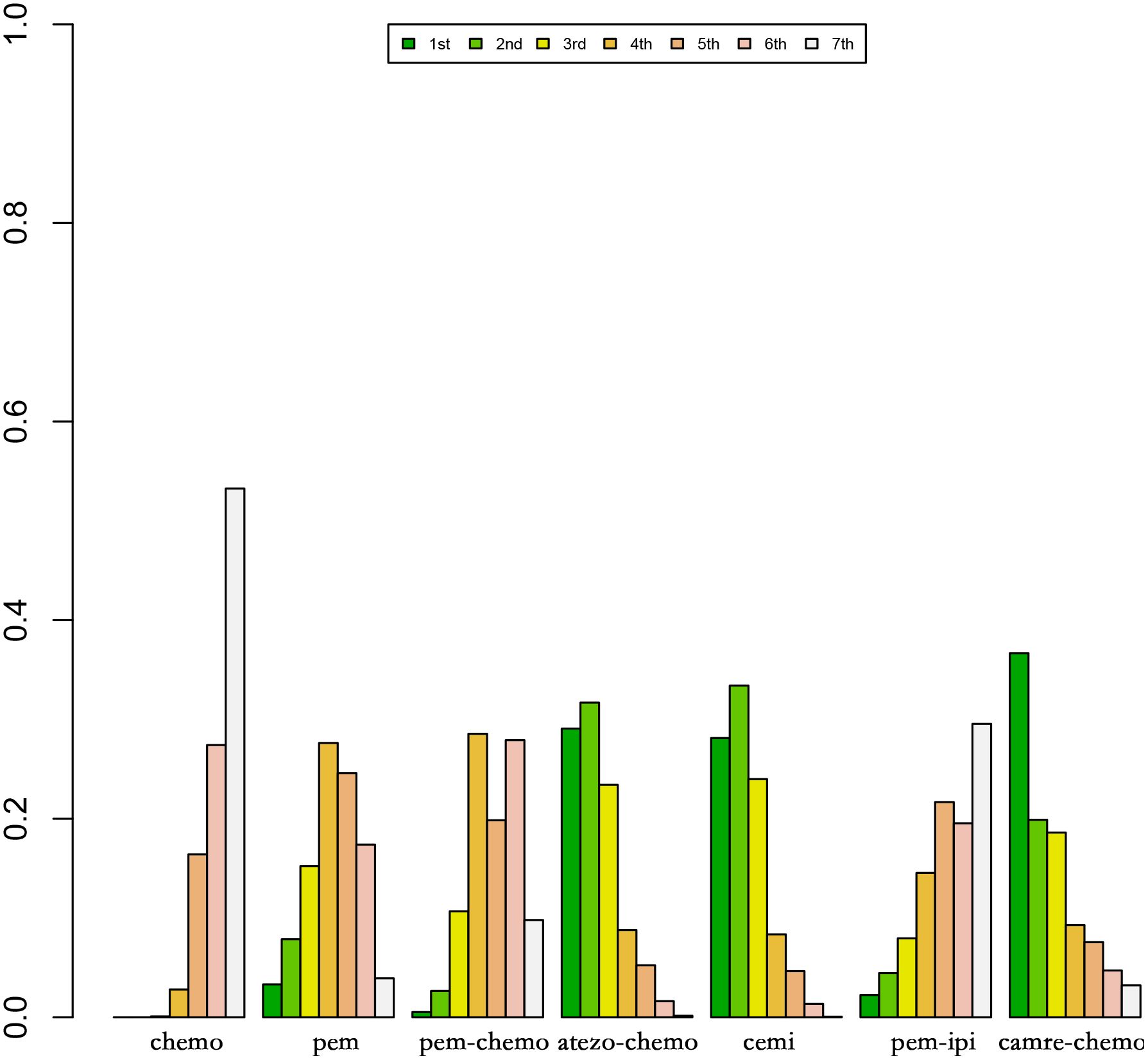
Figure 8 Ranking Plot of OS Treatment Effects. In the first-line treatment, various therapeutic approaches are employed for treating patients with PD-L1 expression ≥50% in Sq-NSCLC.
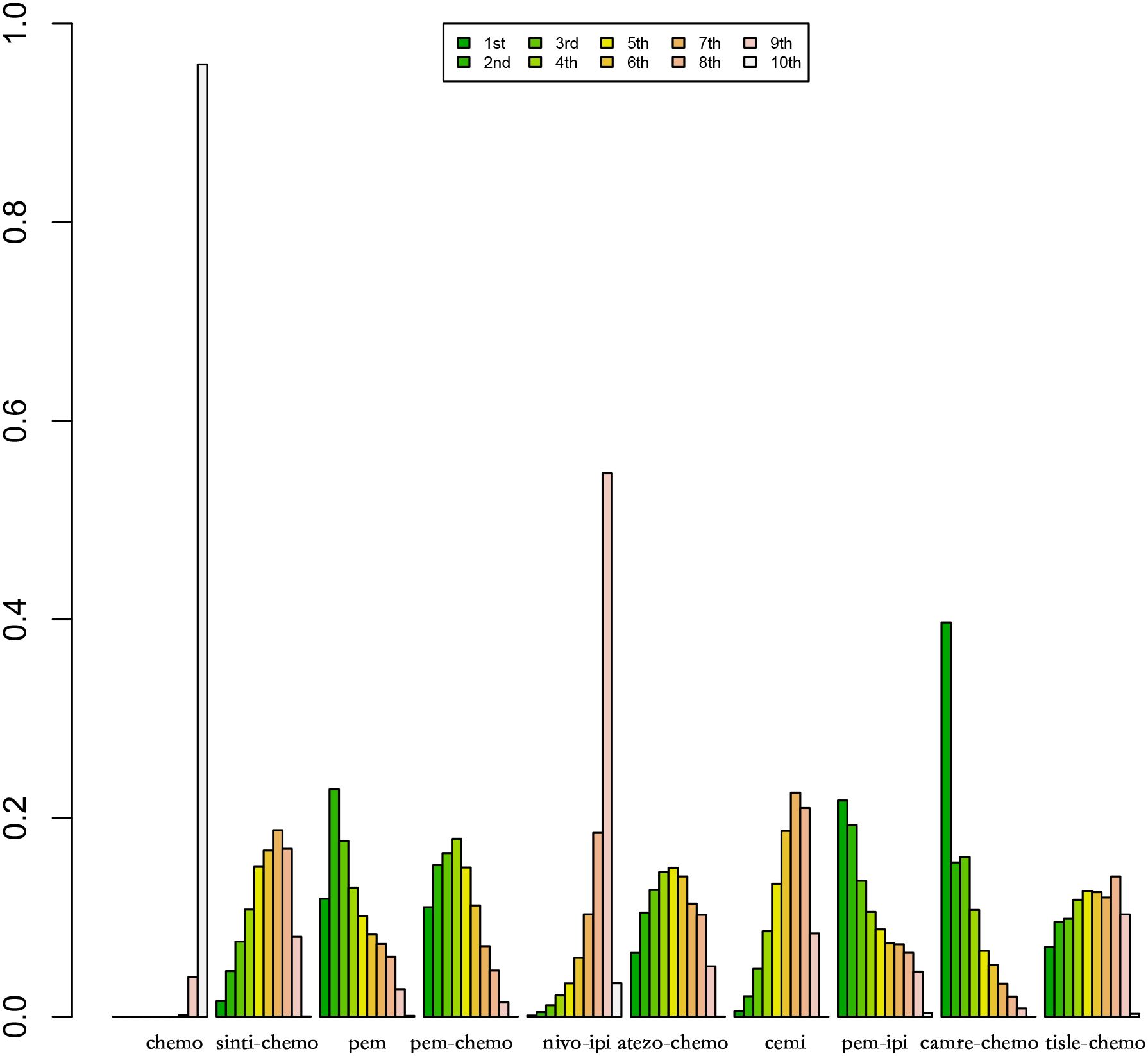
Figure 9 Ranking Plot of PFS Treatment Effects. In the first-line treatment, various therapeutic approaches are employed for treating patients with PD-L1 expression ≥50% in Sq-NSCLC.
In OS, the treatment combination of camrelizumab with chemotherapy stands out as the most probable to be the most effective (36.68%), followed by cemiplimab (33.86%) in second place and atezolizumab with chemotherapy (23.87%) ranking third.
Regarding PFS, the most likely most effective treatment is identified as camrelizumab with chemotherapy (39.70%), followed by pembrolizumab (22.88%) in the second position and pembrolizumab with chemotherapy (17.69%) in third place.
4 Discussion
This study conducts an extensive NMA to investigate first-line immunotherapy in advanced Sq-NSCLC patients with PD-L1 expression ≥50%. Sq-NSCLC, being more complex compared to NS-NSCLC, is influenced by various factors, including smoking, leading to a higher mutation rate (37). This serves as the justification for concentrating on this specific patient population.
Recognized as a robust predictive biomarker, PD-L1 expression has proven influential, with NSCLC patients showcasing high PD-L1 expression frequently demonstrating a more favorable response to immune checkpoint inhibitors. Therefore, this study explicitly focuses on patients with PD-L1 expression of 50% or higher (38). The goal of this research is to establish a more efficient and personalized first-line immunotherapy strategy for patients with squamous Sq-NSCLC featuring PD-L1 expression of 50% or higher. In the absence of direct head-to-head clinical trials comparing various ICIs, NMA serves as a complementary extension to traditional meta-analysis. NMA utilizes indirect comparisons of interventions from RCTs to rank the efficacy of different ICIs (39).
This study demonstrates that, as a first-line immunotherapy regimen, the combination of camrelizumab and chemotherapy attains optimal values for OS and PFS in patients with advanced Sq-NSCLC exhibiting programmed death-ligand 1 expression of 50% or higher. This is consistent with findings from other NMA that suggest the combination of ICIs and chemotherapy provides the most effective outcomes in the first-line treatment of advanced NSCLC patients with positive PD-L1 expression (40). Pharmacologically, chemotherapy works by directly eliminating cancer cells and inhibiting their proliferation and division, while ICIs block the interaction between PD-1 and PD-L1/PD-L2, thereby inhibiting immune escape (41). These distinct mechanisms, acting synergistically through multiple pathways, enhance treatment effectiveness (41). In a comprehensive NMA study, pembrolizumab emerged as the preferred first-line immunotherapy for advanced NSCLC patients with positive PD-L1 expression, a result that contrasts with the outcomes observed in this study (42). After careful review, it is noted that the published NMA referenced above is from 2021, while the CameL-Sq randomized controlled trial featuring camrelizumab plus chemotherapy was published in 2022. Consequently, the camrelizumab plus Chemotherapy treatment modality was not included in this NMA. It is important to highlight that this NMA primarily focuses on NSCLC patients with PD-L1 expression ≥50%, whereas our study specifically centers on patients with Sq-NSCLC who also exhibit PD-L1 expression ≥50%. In a network meta-analysis, researchers posit that among first-line immunotherapy treatments for NSCLC patients with high PD-L1 expression (≥50%), the use of atezolizumab holds the highest probability of achieving the longest overall survival (OS) (43). Notably, this analysis, published in 2021, does not incorporate the CameL-Sq randomized controlled trial. This underscores the significance of our study, not only contributing to the advancement of personalized treatment approaches but also enhancing the reliability of conclusions by incorporating additional RCTs. Furthermore, this study notes that pembrolizumab, when employed as a first-line treatment for advanced Sq-NSCLC patients with positive PD-L1 expression, exhibits favorable PFS values (HR, 0.35; 95% CI, 0.17–0.72). This study demonstrates that atezolizumab with chemotherapy, when utilized as a first-line treatment for advanced Sq-NSCLC patients with PD-L1-positive expression, exhibits favorable overall survival outcomes (HR, 0.48; 95% CI, 0.28–0.80). In a network meta-analysis evaluating PD-(L)1 inhibitors as monotherapy for first-line treatment in NSCLC patients with high PD-L1 expression, researchers identified cemiplimab as the top-ranking agent for OS, followed by atezolizumab and pembrolizumab (42). In another network meta-analysis assessing immune monotherapy as a first-line treatment for advanced NSCLC patients with PD-L1 expression ≥50%, researchers found that cemiplimab and pembrolizumab exhibited favorable performance in terms of progression-free survival, overall survival, and overall response rate (ORR) (44). In our current study, cemiplimab also demonstrated favorable performance in OS (HR, 0.48; 95% CI, 0.30–0.77). These findings underscore the efficacy of cemiplimab as a monotherapy for the treatment of Sq-NSCLC patients with high PD-L1 expression.
The head-to-head meta-analysis conducted in this study reveals that, for squamous advanced NSCLC patients with PD-L1 expression ≥50%, first-line treatment with ICIs leads to better OS and PFS values compared to chemotherapy, consistent with previous meta-analysis findings (45). This further supports the notion that NSCLC patients with positive PD-L1 expression may experience certain advantages with ICIs over chemotherapy (46).This consistent trend across studies supports the growing understanding of the potential benefits associated with ICIs in this particular patient population, emphasizing the relevance of immunotherapy in the management of advanced NSCLC with elevated PD-L1 expression.
While this study provides valuable insights into personalized treatment strategies for first-line immunotherapy in NSCLC, it is imperative to recognize certain limitations. Firstly, all the RCTs included in this study reported PFS values, but only five reported OS values. This limited availability of OS data may restrict our ability to indirectly compare some treatment regimens based on OS, introducing a potential constraint to our conclusions. Secondly, diverse RCTs in this study utilized different approaches to detect PD-L1 expression in patients. Moreover, in certain trials, researchers did not explicitly specify the method employed to assess PD-L1 expression. The diverse methods for examining PD-L1 expression may lead to misclassification errors. As an example, the utilization of the SP142 assay to detect PD-L1 expression in tumor cells has demonstrated restricted sensitivity, which could potentially affect the precision of the study (47). Thirdly, safety data pertaining to PD-L1 expression of 50% or higher in advanced Sq-NSCLC, which were not documented in the incorporated RCTs, were not subject to assessment in this study. Fourthly, this study utilized OS and PFS values as outcome measures, but it is noteworthy that these may not fully encompass all treatment benefits for patients. When assessing the efficacy of immunotherapy, consideration of other crucial indicators such as quality of life and mental health is warranted. Unfortunately, these aspects are often overlooked by researchers due to the lack of standardized measurement criteria. Finally, due to the limited number of RCTs reporting the efficacy of ICIs in PD-L1 expression ≥50% squamous NSCLC in this study, caution is advised in interpreting the conclusions. This study provides substantial support for advancing personalized treatment strategies, furnishing a wealth of evidence-based guidance for making informed decisions regarding first-line immunotherapy in clinical practice for patients with squamous non-small cell lung cancer. The future direction of development not only includes PD-L1 expression, but also involves identifying predictive biomarkers for treatment response through tumor molecular analysis, such as EGFR mutations in NSCLC (48).
In future research, it is crucial to expand the sample size of RCTs, emphasize standardized PD-L1 expression detection methods, and comprehensively consider a broader array of clinical indicators. Additionally, a comprehensive consideration of a broader array of clinical indicators beyond traditional survival measures is warranted. This inclusive approach will enable a thorough and nuanced evaluation of the application of immunotherapy in patients with squamous NSCLC, shedding light on potential benefits beyond conventional endpoints and fostering a more comprehensive understanding of treatment outcomes.
5 Conclusions
This study suggests that in patients with Sq-NSCLC expressing PD-L1 at a level of 50% or higher, the initial immunotherapy selection of camrelizumab in combination with chemotherapy produces superior OS and PFS values. Additionally, for this patient subset, the use of ICIs demonstrates superior efficacy compared to chemotherapy.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author contributions
WC: Conceptualization, Data curation, Software, Writing – original draft, Writing – review & editing. HL: Conceptualization, Data curation, Supervision, Writing – original draft, Writing – review & editing. YWL: Methodology, Validation, Writing – review & editing. WX: Data curation, Software, Writing – review & editing. SF: Validation, Writing – review & editing. JS: Formal Analysis, Validation, Writing – review & editing. SL: Data curation, Supervision, Writing – review & editing. YL: Visualization, Writing – review & editing. LZ: Supervision, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
Thanks to LZ for her support and guidance on this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2024.1365255/full#supplementary-material
Abbreviations
NSCLC, Non-Small Cell Lung Cancer; Sq-NSCLC, Squamous Non-small Cell Lung Cancer; NS-NSCLC, Non-squamous NSCLC; NMAs, Network Meta-analyses; PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses; OS, Overall Survival; PFS, Progression-free Survival; ICIs, Immune Checkpoint Inhibitors; PROSPERO, Prospective Register of Systematic Reviews; HR, Hazard Ratios; Cis, Confidence Intervals; DIC, Deviance Information Criterion.
References
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: Cancer J Clin. (2021) 71:209–49. doi: 10.3322/caac.21660
2. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA: Cancer J Clin. (2020) 70:7–30. doi: 10.3322/caac.21590
3. Goldstraw P, Chansky K, Crowley J, Rami-Porta R, Asamura H, Eberhardt WEE, et al. The IASLC lung cancer staging project: Proposals for revision of the TNM stage groupings in the forthcoming (Eighth) edition of the TNM classification for lung cancer. J Thorac Oncol Off Publ Int Assoc Study Lung Cancer. (2016) 11:39–51. doi: 10.1016/j.jtho.2015.09.009
4. Cortés ÁA, Urquizu LC, Cubero JH. Adjuvant chemotherapy in non-small cell lung cancer: state-of-the-art. Trans Lung Cancer Res. (2015) 4:191–7. doi: 10.3978/j.issn.2218-6751.2014.06.01
5. Malhotra J, Jabbour SK, Aisner J. Current state of immunotherapy for non-small cell lung cancer. Trans Lung Cancer Res. (2017) 6:196–211. doi: 10.21037/tlcr
6. Reck M, Rodríguez-Abreu D, Robinson AG, Hui R, Csőszi T, Fülöp A, et al. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. New Engl J Med. (2016) 375:1823–33. doi: 10.1056/NEJMoa1606774
7. Hellmann MD, Paz-Ares L, Caro RB, Zurawski B, Kim S-W, Costa EC, et al. Nivolumab plus ipilimumab in advanced non-small-cell lung cancer. New Engl J Med. (2019) 381:2020–31. doi: 10.1056/NEJMoa1910231
8. Hudson K, Cross N, Jordan-Mahy N, Leyland R. The extrinsic and intrinsic roles of PD-L1 and its receptor PD-1: implications for immunotherapy treatment. Front Immunol. (2020) 11:568931. doi: 10.3389/fimmu.2020.568931
9. Ippolito MR, Martis V, Martin S, Tijhuis AE, Hong C, Wardenaar R, et al. Gene copy-number changes and chromosomal instability induced by aneuploidy confer resistance to chemotherapy. Dev Cell. (2021) 56:2440–54.e6. doi: 10.1016/j.devcel.2021.07.006
10. Hargadon KM, Johnson CE, Williams CJ. Immune checkpoint blockade therapy for cancer: An overview of FDA-approved immune checkpoint inhibitors. Int immunopharmacology. (2018) 62:29–39. doi: 10.1016/j.intimp.2018.06.001
11. Topalian SL, Drake CG, Pardoll DM. Targeting the PD-1/B7-H1(PD-L1) pathway to activate anti-tumor immunity. Curr Opin Immunol. (2012) 24:207–12. doi: 10.1016/j.coi.2011.12.009
12. Wang B-Y, Huang J-Y, Chen H-C, Lin C-H, Lin S-H, Hung W-H, et al. The comparison between adenocarcinoma and squamous cell carcinoma in lung cancer patients. J Cancer Res Clin Oncol. (2020) 146:43–52. doi: 10.1007/s00432-019-03079-8
13. Papi A, Casoni G, Caramori G, Guzzinati I, Boschetto P, Ravenna F, et al. COPD increases the risk of squamous histological subtype in smokers who develop non-small cell lung carcinoma. Thorax. (2004) 59:679–81. doi: 10.1136/thx.2003.018291
14. Subramanian J, Morgensztern D, Goodgame B, Baggstrom MQ, Gao F, Piccirillo J, et al. Distinctive characteristics of non-small cell lung cancer (NSCLC) in the young: a surveillance, epidemiology, and end results (SEER) analysis. J Thorac Oncol Off Publ Int Assoc Study Lung Cancer. (2010) 5:23–8. doi: 10.1097/JTO.0b013e3181c41e8d
15. Socinski MA, Obasaju C, Gandara D, Hirsch FR, Bonomi P, Bunn P, et al. Clinicopathologic features of advanced squamous NSCLC. J Thorac Oncol Off Publ Int Assoc Study Lung Cancer. (2016) 11:1411–22. doi: 10.1016/j.jtho.2016.05.024
16. Cha YJ, Kim HR, Lee CY, Cho BC, Shim HS. Clinicopathological and prognostic significance of programmed cell death ligand-1 expression in lung adenocarcinoma and its relationship with p53 status. Lung Cancer (Amsterdam Netherlands). (2016) 97:73–80. doi: 10.1016/j.lungcan.2016.05.001
17. Chen X, Feng L, Huang Y, Wu Y, Xie N. Mechanisms and strategies to overcome PD-1/PD-L1 blockade resistance in triple-negative breast cancer. Cancers. (2022) 15: 104. doi: 10.3390/cancers15010104
18. Santarpia M, Aguilar A, Chaib I, Cardona AF, Fancelli S, Laguia F, et al. Non-small-cell lung cancer signaling pathways, metabolism, and PD-1/PD-L1 antibodies. Cancers. (2020) 12:1475. doi: 10.3390/cancers12061475
19. Herbst RS, Giaccone G, Fd M, Reinmuth N, Vergnenegre A, CH B, et al. Atezolizumab for first-line treatment of PD-L1-selected patients with NSCLC. New Engl J Med. (2020) 383:1328–39. doi: 10.1056/NEJMoa1917346
20. Ettinger DS, Wood DE, Aisner DL, Akerley W, Bauman JR, Bharat A, et al. Non-small cell lung cancer, version 3.2022, NCCN clinical practice guidelines in oncology. J Natl Compr Cancer Network JNCCN. (2022) 20:497–530. doi: 10.6004/jnccn.2022.0025
21. Hutton B, Salanti G, Caldwell DM, Chaimani A, Schmid CH, Cameron C, et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Internal Med. (2015) 162:777–84. doi: 10.7326/M14-2385
22. Rouse B, Chaimani A, Li T. Network meta-analysis: an introduction for clinicians. Internal Emergency Med. (2017) 12:103–11. doi: 10.1007/s11739-016-1583-7
23. Neupane B, Richer D, Bonner AJ, Kibret T, Beyene J. Network meta-analysis using R: a review of currently available automated packages. PLoS One. (2014) 9:e115065. doi: 10.1371/journal.pone.0115065
24. Shim SR, Kim SJ, Lee J, Rücker G. Network meta-analysis: application and practice using R software. Epidemiol Health. (2019) 41:e2019013. doi: 10.4178/epih.e2019013
25. Sterne JA, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. bmj. (2019) 366:4898. doi: 10.1136/bmj.l4898
26. Lin L, Chu H, Hodges JS. Sensitivity to excluding treatments in network meta-analysis. Epidemiol (Cambridge Mass). (2016) 27:562. doi: 10.1097/EDE.0000000000000482
27. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. Bmj. (2003) 327:557–60. doi: 10.1136/bmj.327.7414.557
28. Reck M, Rodríguez-Abreu D, Robinson AG, Hui R, Csőszi T, Fülöp A, et al. Updated analysis of KEYNOTE-024: pembrolizumab versus platinum-based chemotherapy for advanced non-small-cell lung cancer with PD-L1 tumor proportion score of 50% or greater. J Clin Oncol Off J Am Soc Clin Oncol. (2019) 37:537–46. doi: 10.1200/JCO.18.00149
29. Paz-Ares L, Vicente D, Tafreshi A, Robinson A, Parra HS, Mazières J, et al. A randomized, placebo-controlled trial of pembrolizumab plus chemotherapy in patients with metastatic squamous NSCLC: protocol-specified final analysis of KEYNOTE-407. J Thorac Oncol Off Publ Int Assoc Study Lung Cancer. (2020) 15:1657–69. doi: 10.1016/j.jtho.2020.06.015
30. Hellmann MD, Ciuleanu T-E, Pluzanski A, Lee JS, Otterson GA, Audigier-Valette C, et al. Nivolumab plus ipilimumab in lung cancer with a high tumor mutational burden. New Engl J Med. (2018) 378:2093–104. doi: 10.1056/NEJMoa1801946
31. Jotte R, Cappuzzo F, Vynnychenko I, Stroyakovskiy D, Rodríguez-Abreu D, Hussein M, et al. Atezolizumab in combination with carboplatin and nab-paclitaxel in advanced squamous NSCLC (IMpower131): results from a randomized phase III trial. J Thorac Oncol Off Publ Int Assoc Study Lung Cancer. (2020) 15:1351–60. doi: 10.1016/j.jtho.2020.03.028
32. Sezer A, Kilickap S, Gümüş M, Bondarenko I, Özgüroğlu M, Gogishvili M, et al. Cemiplimab monotherapy for first-line treatment of advanced non-small-cell lung cancer with PD-L1 of at least 50%: a multicentre, open-label, global, phase 3, randomised, controlled trial. Lancet (London England). (2021) 397:592–604. doi: 10.1016/S0140-6736(21)00228-2
33. Boyer M, Şendur MAN, Rodríguez-Abreu D, Park K, Lee DH, Çiçin I, et al. Pembrolizumab plus ipilimumab or placebo for metastatic non-small-cell lung cancer with PD-L1 tumor proportion score ≥ 50%: Randomized, double-blind phase III KEYNOTE-598 study. J Clin Oncol Off J Am Soc Clin Oncol. (2021) 39:2327–38. doi: 10.1200/JCO.20.03579
34. Ren S, Chen J, Xu X, Jiang T, Cheng Y, Chen G, et al. Camrelizumab plus carboplatin and paclitaxel as first-line treatment for advanced squamous NSCLC (CameL-sq): A phase 3 trial. J Thorac Oncol Off Publ Int Assoc Study Lung Cancer. (2022) 17:544–57. doi: 10.1016/j.jtho.2021.11.018
35. Wang J, Lu S, Yu X, Hu Y, Sun Y, Wang Z, et al. Tislelizumab plus chemotherapy vs chemotherapy alone as first-line treatment for advanced squamous non-small-cell lung cancer: A phase 3 randomized clinical trial. JAMA Oncol. (2021) 7:709–17. doi: 10.1001/jamaoncol.2021.0366
36. Zhou C, Wu L, Fan Y, Wang Z, Liu L, Chen G, et al. Sintilimab plus platinum and gemcitabine as first-line treatment for advanced or metastatic squamous NSCLC: results from a randomized, double-blind, phase 3 trial (ORIENT-12). J Thorac Oncol Off Publ Int Assoc Study Lung Cancer. (2021) 16:1501–11. doi: 10.1016/j.jtho.2021.04.011
37. Socinski MA, Obasaju C, Gandara D, Hirsch FR, Bonomi P, Bunn PA, et al. Current and emergent therapy options for advanced squamous cell lung cancer. J Thorac Oncol Off Publ Int Assoc Study Lung Cancer. (2018) 13:165–83. doi: 10.1016/j.jtho.2017.11.111
38. Voong KR, Feliciano J, Becker D, Levy B. Beyond PD-L1 testing-emerging biomarkers for immunotherapy in non-small cell lung cancer. Ann Trans Med. (2017) 5:376. doi: 10.21037/atm
39. Liu Y, Béliveau A, Wei Y, Chen MY, Record-Lemon R, Kuo P-L, et al. A gentle introduction to bayesian network meta-analysis using an automated R package. Multivariate Behav Res. (2023) 58:706–22. doi: 10.1080/00273171.2022.2115965
40. Kim JH, Jeong SY, Lee J-J, Park ST, Kim HS. A bayesian network meta-analysis of first-line treatments for non-small cell lung cancer with high programmed death ligand-1 expression. J Clin Med. (2022) 11:1492. doi: 10.3390/jcm11061492
41. Pu Y, Ji Q. Tumor-associated macrophages regulate PD-1/PD-L1 immunosuppression. Front Immunol. (2022) 13:874589. doi: 10.3389/fimmu.2022.874589
42. Majem M, Cobo M, Isla D, Marquez-Medina D, Rodriguez-Abreu D, Casal-Rubio J, et al. PD-(L)1 inhibitors as monotherapy for the first-line treatment of non-small-cell lung cancer patients with high PD-L1 expression: A network meta-analysis. J Clin Med. (2021) 10:1365. doi: 10.3390/jcm10071365
43. Wang D-D, Shaver LG, Shi F-Y, Wei J-J, Qin T-Z, Wang S-Z, et al. Comparative efficacy and safety of PD-1/PD-L1 immunotherapies for non-small cell lung cancer: a network meta-analysis. Eur Rev Med Pharmacol Sci. (2021) 25:2866–84. doi: 10.26355/eurrev_202104_25541
44. Freemantle N, Xu Y, Wilson FR, Guyot P, Chen C-I, Keeping S, et al. Network meta-analysis of immune-oncology monotherapy as first-line treatment for advanced non-small-cell lung cancer in patients with PD-L1 expression ≧̸50. Ther Adv Med Oncol. (2022) 14:17588359221105024. doi: 10.1177/17588359221105024
45. Herbst R, Jassem J, Abogunrin S, James D, McCool R, Belleli R, et al. A network meta-analysis of cancer immunotherapies versus chemotherapy for first-line treatment of patients with non-small cell lung cancer and high programmed death-ligand 1 expression. Front Oncol. (2021) 11:676732. doi: 10.3389/fonc.2021.676732
46. Planchard D, Popat S, Kerr K, Novello S, Smit EF, Faivre-Finn C, et al. Metastatic non-small cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol Off J Eur Soc Med Oncol. (2018) 29:iv192–237. doi: 10.1093/annonc/mdy275
47. Tsao MS, Kerr KM, Kockx M, Beasley M-B, Borczuk AC, Botling J, et al. PD-L1 immunohistochemistry comparability study in real-life clinical samples: results of blueprint phase 2 project. J Thorac Oncol Off Publ Int Assoc Study Lung Cancer. (2018) 13:1302–11. doi: 10.1016/j.jtho.2018.05.013
Keywords: squamous non-small cell lung cancer, chemotherapy, immune checkpoint inhibitors, network meta-analysis, randomized trials
Citation: Chen W, Liu H, Li Y, Xue W, Fan S, Sun J, Liu S, Liu Y and Zhang L (2024) First-line immunotherapy efficacy in advanced squamous non-small cell lung cancer with PD-L1 expression ≥50%: a network meta-analysis of randomized controlled trials. Front. Oncol. 14:1365255. doi: 10.3389/fonc.2024.1365255
Received: 04 January 2024; Accepted: 12 April 2024;
Published: 24 April 2024.
Edited by:
Alessandro Inno, Ospedale Sacro Cuore Don Calabria, ItalyReviewed by:
Carlos Cabrera-Gálvez, Clinica Mi Tres Torres, SpainValerio Gristina, University of Palermo, Italy
Copyright © 2024 Chen, Liu, Li, Xue, Fan, Sun, Liu, Liu and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lili Zhang, emhhbmctbGlseUAxNjMuY29t
†These authors have contributed equally to this work
 Wei Chen
Wei Chen Hangmei Liu†
Hangmei Liu† Shui Liu
Shui Liu