- 1Department of Nuclear Medicine, The Affiliated Hospital of Southwest Medical University, Luzhou, Sichuan, China
- 2Nuclear Medicine and Molecular Imaging Key Laboratory of Sichuan Province, Luzhou, Sichuan, China
- 3Nuclear Medicine Institute of Southwest Medical University, Luzhou, Sichuan, China
Purpose: We aimed to compare the relative diagnostic efficacy of 68Ga-Labeled DOTA-ibandronic acid (68Ga-DOTA-IBA) to that of18F-NaF PET/CT as a mean of detecting bone metastases in patients with a range of cancer types
Methods: This study retrospectively enrolled patients with bone metastases associated with various underlying malignancies. All patients underwent both 68Ga-DOTA-IBA and 18F-NaF PET/CT scans. Histopathology and follow-up CT or MRI imaging results were used as reference criteria, with a minimum follow-up period of 3 months. The maximum Standardized Uptake Value (SUVmax) and number of bone metastases were recorded. The Target-Background Ratio (TBR) was calculated along with the detection rate, sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and accuracy of 68Ga-DOTA-IBA and 18F-NaF PET/CT imaging for overall and partial primary solid tumor bone metastases. Pearson chi-square test, McNemar test, and Kappa test was conducted to assess the correlation and consistency of diagnostic efficiency between the two imaging agents. Receiver Operating Characteristic curve (ROC curve) was performed to compare diagnostic performance and the area under the curve of the two imaging agents, determining optimal critical values for SUVmax and TBR in diagnosing bone metastasis. Differences in SUVmax and TBR values between the two imaging agents for detecting bone metastases were analyzed using the Wilcoxon signed rank test. The difference was statistically significant when P < 0.05
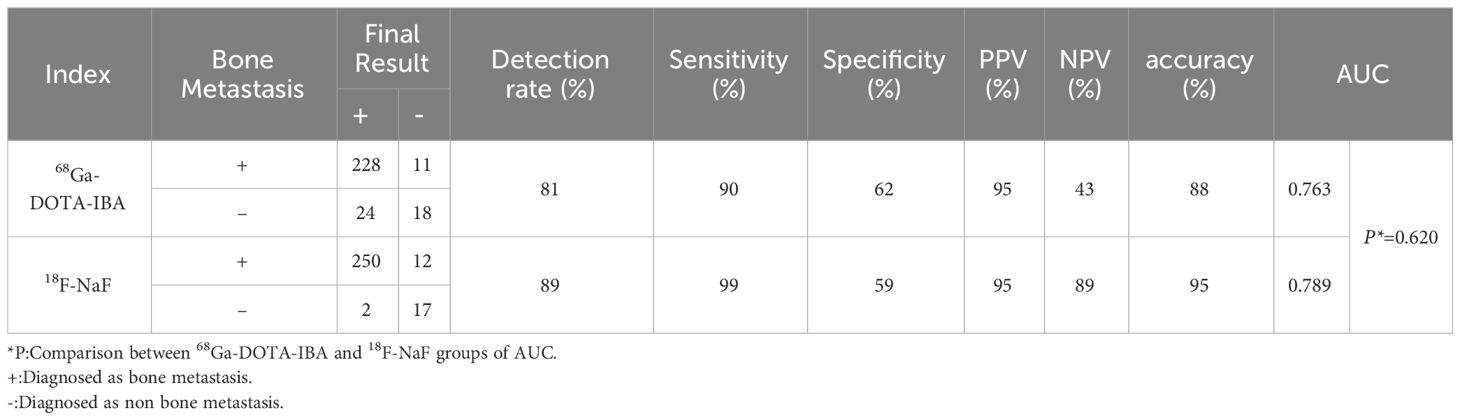
Results: A total of 24 patients (13 women and 11 men) were included in this study, with a mean age of 52 (interquartile range, 49-64 years). The detection rate, sensitivity, specificity, PPV, NPV, accuracy, and AUC of 68Ga-DOTA-IBA and 18F-NaF PET/CT for bone metastases were 81%, 90%, 62%, 95%, 43%, 88%, 0.763, and 89%, 99%, 59%, 95%, 89%, 95%, 0.789, respectively. There was no significant difference between the two imaging methods (P < 0.01), and there was a significant correlation (X2=168.43, P < 0.001) and a strong consistency (Kappa=0.774,P < 0.001) between the diagnostic results of the two imaging agents. The SUVmax values of lesions measured by 68Ga-DOTA-IBA and 18F-NaF imaging in 22 patients with bone metastasis were 5.1 ± 5.4 and 19.6 ± 15.1, respectively, with statistically significant differences (P<0.05). The TBR values of the two imaging methods were 5.0 ± 5.0 and 6.7 ± 6.4, respectively, with statistically significant differences (P<0.05). The AUC of the SUVmax of 68Ga-DOTA-IBA and 18F-NaF curves were 0.824 and 0.862, respectively, with no statistically significant difference (P=0.490). No significant difference was found in the AUC of the TBR of 68Ga-DOTA-IBA and 18F-NaF (0.832 vs 0.890; P=0.248). Subgroup analysis showed significant correlation between the two imaging agents in the diagnosis of bone metastases in lung cancer and breast cancer, with consistent diagnostic results. However, in the diagnosis of bone metastases in prostate cancer, there was a significant difference (P<0.001) and lack of consistency (P=0.109)
Conclusion: The diagnostic efficacy of 68Ga-DOTA-IBA for bone metastasis lesions is comparable to that of 18F-NaF. This finding holds significant clinical importance in terms of diagnosis of bone metastasis and selecting treatment plans for patients with malignant tumors
Introduction
Bone is the third common metastatic site of various solid tumors (including lung, breast, prostate, colon, thyroid, gynecology and melanoma), and notably arising from the breast (70%), prostate (85%) and lung (40%) (1). Unfortunately, once cancer spreads to the bones, it is difficult to cure and is associated with a range of complications, including pain, increased risk of fractures, and hypercalcemia, all of which significantly diminish the quality of life for affected patients (2, 3).
Molecular imaging using bone targeted tracers has been applied in clinical practice for nearly 50 years and still plays an important role in the diagnosis and follow-up of bone metastases. It includes 99mTc-bisphosphonate for bone scanning and 18F-NaF and 18F-FDG for PET/CT (4–6). However, 18F-NaF requires a medical cyclotron for production, which limits its clinical application. Nevertheless, it generally exhibits higher sensitivity and specificity compared to 99mTc-MDP bone scanning and 18F-FDG (7–9). However, bisphosphonates have great value in the diagnosis and treatment of skeletal diseases, as they have a strong affinity for bones (10). Ibandronic acid (IBA) is a third-generation amino-bisphosphonate with antiresorptive and antihypercalcemic properties. We successfully synthesized chelate DOTA-containing ligand DOTA-IBA. Basic experimental articles on 68Ga-DOTA-IBA and 177Lu-DOTA-IBA have been published and proved to provide a set of potential theranostic radiopharmaceuticals and may have a good prospect for the management of bone metastasis (11–13). Gallium-68 (68Ga) is commonly used as a positron imaging agent in clinical practice and can be obtained using a germanium gallium generator (68Ge/68Ga). Furthermore, when assessing subsequent radionuclide therapy the imaging results of the 68Ga tracer outperform those of the 18F tracer (14). Similarly, The utilization of 68Ga/177Lu-DOTA-IBA can be beneficial in the diagnosis and assessment of bone metastases in malignant tumors. 68Ga-DOTA-IBA not only enables positron imaging but also capitalizes on IBA’s high adsorption rate on bones. As a novel bone-seeking positron radioactive drug, further research is needed to evaluate the diagnostic performance of 68Ga-DOTA-IBA. As such, we herein performed a comparative analysis of the relative performance of 68Ga-DOTA-IBA and 18F-NaF PET/CT for the detection of bone metastases in patients with a range of tumor types.
Patients and methods
Patients
This retrospective study was approved by the Ethics Committee of the Affiliated Hospital of Southwest Medical University (Ethics committee approval No.: KY2022114; Clinical trial registration No.: ChiCTR2200064487) and was conducted between September 2021 and May 2023. Patients with malignant tumors who underwent 68Ga-DOTA-IBA and 18F-NaF PET/CT imaging were enrolled in this study. The interval between the two examinations was less than 7 days. All of the above patients were recruited under written consent. The exclusion criteria were the skeletal metastatic lesions had received treatment before imaging analyses and patients who were lost to follow-up.
PET/CT imaging acquisition
The patients were weighed and no other special patient preparation was needed before the examination. 18F⁃NaF was produced by Siemens cyclotron. The 68Ga-DOTA-IBA labeling was carried out according to the method described previously (9). The radiochemical purity of 18F⁃NaF and 68Ga-DOTA-IBA were both exceeded 95%. Intravenous doses of 18F-NaF and 68Ga-DOTA-IBA were 3.7 MBq/kg(0.1mCi/kg) and 1.85MBq/kg(0.05mCi/kg), respectively. PET/CT scans (uMI780, United Imaging Healthcare) were performed approximately 45-60 minutes after intravenous administration. The CT scan was performed and acquired according to the following parameters: tube voltage, 120 kV; current, 120 mA; layer thickness 3.00mm, layer spacing 5 mm, pitch 0.813. The PET scan was then performed in 3D acquisition mode on the same bed as the CT. When the reconstruction was complete, the images were processed using PET/CT post-processing software.
Imaging interpretation
68Ga-DOTA-IBA and 18F-NaF PET/CT images were independently interpreted in a visual and semi-quantitative manner by two experienced nuclear medicine physicians. Based on the results of the 68Ga-DOTA-IBA and 18F-NaF imaging agents, the lesions were categorized into bone metastases, benign bone lesions, and normal bones. A lesion was defined as a region of abnormal radioactivity when the uptake value was significant higher than the surrounding normal bone background.
Reference standard
Bone metastases were confirmed by pathology. However, due to technical and ethical issues, pathological findings could not be performed on all suspected involved lesions. We used the results of imaging examination results (BS, CT, MRI, or PET/CT) and clinical follow-up (physical signs and follow-up imaging examination) as the reference standards. The follow-up time was at least 3 months. When pathological results are lacking, bone metastases were judged on the findings of typical performance (extensive bone metastases throughout the skeleton) on PET/CT and corresponding characteristic morphologic findings of metastasis on the CT component as well as the improvement or progression of bone metastatic lesions following treatment at follow-up imaging examination results. The lesions were categorized as benign lesions owing to typical appearance on imaging examination and no progressive performance was found during the follow-up period.
Statistical analysis
Statistical analysis was performed using SPSS 27.0 (IBM, Armonk, NY, USA). All quantitative information was expressed as the mean ± standard deviation. We compared the diagnostic performance and diagnostic efficacy of the two imaging modalities using Pearson chi-square test, McNemar test and the receiver operating characteristic curve (ROC curve). The Kappa test was utilized to assess the consistency of the diagnostic performance between the two imaging modes. A kappa value of ≥ 0.75 indicates good consistency, while a value between 0.4 and 0.75 suggests average consistency. A kappa value less than 0.4 indicates poor consistency. Comparison of SUVmax and TBR for 18F-NaF and 68Ga-DOTA-IBA for bone metastatic was performed using the Wilcoxon test. The ROC curve was used to obtain the optimal critical values for SUVmax and TBR diagnosis of bone metastases, and the differences in area under curve (AUC) were compared. P < 0.05 was considered to indicate a statistically significant difference.
Results
Patient characteristics
In total, 24 patients (13 women and 11 men) were included in this study, with a mean age of 52 (interquartile range, 49-64 years). There were 12 (50%) patients with lung cancer, 5 (21%) with breast cancer, 2 (8%) with prostate cancer, 2 (8%) with renal cancer, 1 (4%) with stomach cancer, 1 (4%) with pancreatic cancer, and 1 (4%) with rectal cancer. Patient characteristics are summarized in Table 1.
Comparison of 68Ga-DOTA-IBA versus 18F-NaF PET for detecting bone metastases
In our study, due to ethical and practical reasons (for example, patients with a history of pathologically diagnosed malignant tumors, multiple bone lesions, and they showed typical bone metastases in two or more imaging examinations), so there is no confirmation of bone metastasis based on biopsies. The final diagnosis of these metastases is based on comprehensive imaging findings (BS, CT, MRI or PET/CT) and clinical follow-up (physical signs and follow-up imaging examination). Bone metastases were both successfully detected by 68Ga-DOTA-IBA PET/CT and 18F-NaF PET/CT in 22 patients. A total of 252 lesions in 22 patients were diagnosed with bone metastasis. 29 lesions were categorized as benign lesions owing to typical appearance on imaging examination and no progressive performance was found during the follow-up period. 239 bone lesions were found to have increased uptake of 68Ga-DOTA-IBA. Among these 68Ga-DOTA-IBA-avid lesions, 228 bone lesions were considered to represent true positive lesions of bone metastasis, while 11 benign lesions with false positive uptake were identified. Benign lesions include degenerative osteophytes (2/11), arthritis (3/11), and fractures (6/11). In contrast, 18F-NaF detected 262 bone lesions with increased uptake, and 12 false positive lesions were found through 18F-NaF PET/CT imaging, including physiological uptake (1/12), arthritis (2/12), fractures (3/12), and degenerative osteophytes (6/12). Figure 1 showed that the physiological uptake of right sphenoid wing of a patient was false positive in 18F-NaF PET/CT imaging, while the uptake of 68Ga-DOTA-IBA was not significantly increased.
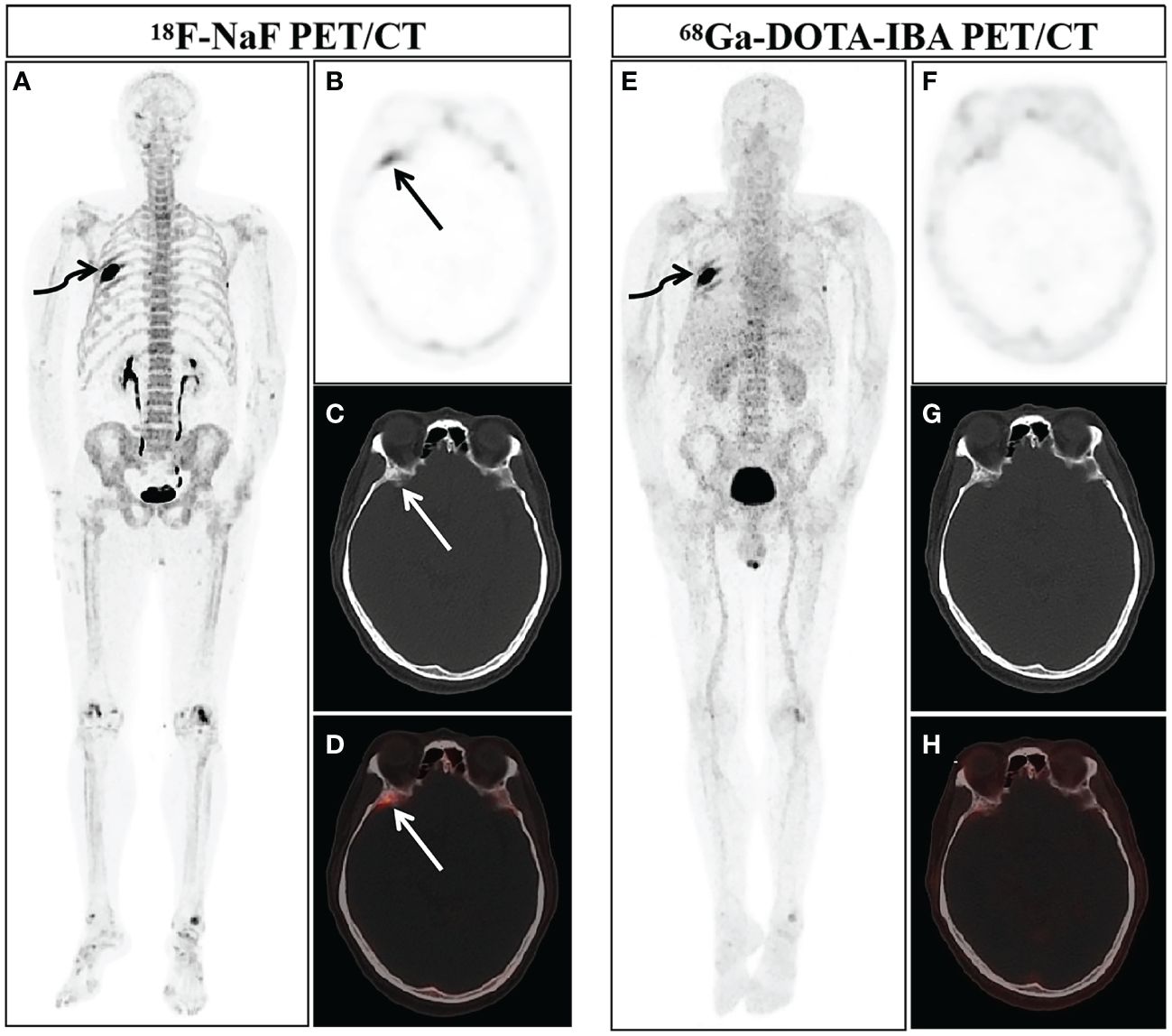
Figure 1 A 58-year-old man newly diagnosed with lung cancer was included in our study and underwent 18F-NaF and 68Ga-DOTA-IBA PET/CT. The MIP images of 18F-NaF (A) and 68Ga-DOTA-IBA (E) PET/CT both showed an increase in imaging agent uptake in the right rib (curved arrows). Combined with his history of malignant tumor and typical osteolytic bone destruction with soft tissue shadows, the diagnosis of bone metastasis was considered. The MIP image (A) and axial views (B: PET image; C: CT scan; D: PET/CT fused image) of 18F-NaF showed the increased imaging agent uptake of the right sphenoid wing (straight arrow, SUVmax 5.3), but no significant bone abnormalities were observed. 68Ga-DOTA-IBA PET/CT MIP (E) and axial views (F: PET image; G: CT scan; H: PET/CT fused image) revealed there was no significant abnormal increase in imaging agent uptake on the right sphenoid wing. After nearly three months of follow-up, according to the patient’s CT examination, there were no obvious abnormalities in the bone, and we believed that this was physiological uptake.
The detection rate, sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), accuracy, and the AUC of 68Ga-DOTA-IBA PET/CT and 18F-NaF PET/CT in the diagnosis of bone metastases were 81%, 90%, 62%, 95%, 43%, 88%, 0.763, and 89%, 99%, 59%, 95%, 89%, 95%, 0.789, respectively (Table 2). There was no statistically significant difference in diagnostic performance between the two imaging methods (P=1.00). Pearson chi-square test showed that there was a significant correlation between the diagnostic results of the two imaging agents (X2 = 168.43, P < 0.001). The diagnostic consistency of the two imaging methods for bone metastases was found to be good (Kappa=0.774, P<0.001). In addition, there was no statistically significant difference between the two imaging agents in diagnosing bone metastasis AUC (P=0.620) (Figure 2A).
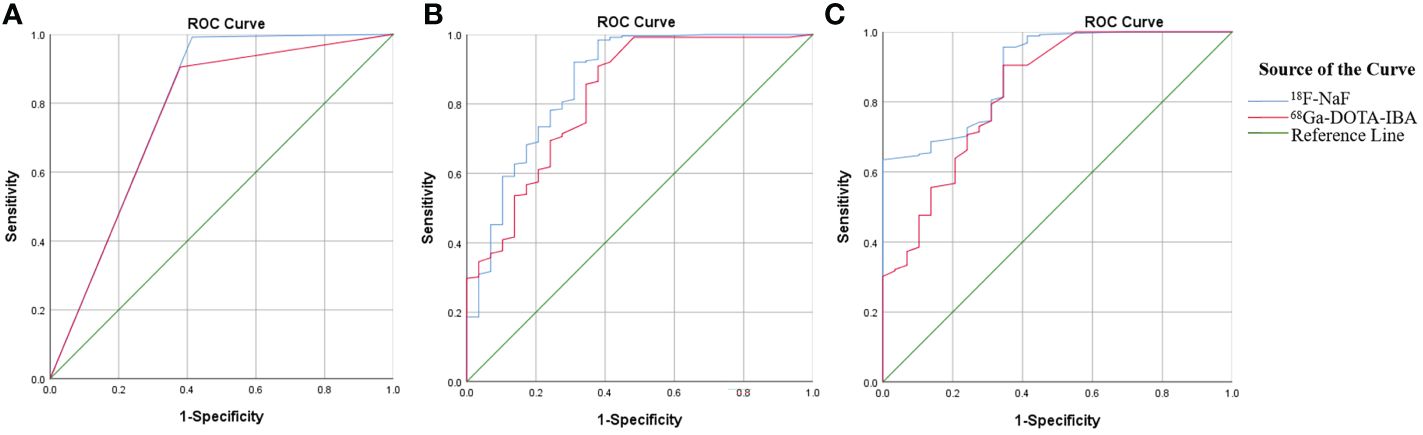
Figure 2 Comparison of ROC curves between 68Ga-DOTA-IBA and 18F-NaF PET/CT in diagnosing bone metastasis (A); Comparison of ROC curves for diagnosing bone metastasis using SUVmax in 68Ga-DOTA-IBA and 18F-NaF PET/CT imaging (B); Comparison of ROC curves for TBR diagnosis of bone metastasis in 68Ga-DOTA-IBA and 18F-NaF PET/CT imaging (C).
Comparative analysis of SUVmax of 68Ga-DOTA-IBA PET/CT and 18F-NaF PET/CT in bone metastasis
In our image analysis, physiological uptake areas in 68Ga-DOTA-IBA PET/CT is consistent with previous basic research (11). In general, 68Ga-DOTA-IBA is a radiopharmaceutical excreted through the kidney, with rapid soft tissue clearance and high bone uptake, and is retained for a long time in bone disease. The statistical analysis revealed that the SUVmax of 18F-NaF PET had a higher value for detecting bone metastasis compared to the SUVmax of 68Ga-DOTA-IBA PET (19.6 ± 15.1 vs 5.1 ± 5.4; P<0.05). To evaluate the diagnostic performance, ROC curve analysis was conducted using the SUVmax measurements from both 68Ga-DOTA-IBA and 18F-NaF imaging, along with the diagnostic results of all lesions (Figure 2B). The results indicated that the AUCs of SUVmax for diagnosing bone metastasis were 0.824 and 0.862 for 68Ga-DOTA-IBA and 18F-NaF, respectively. There was no statistically significant difference (P=0.490). Furthermore, the optimal diagnostic critical values were determined as 1.15 and 9.65 for 68Ga-DOTA-IBA and 18F-NaF, respectively (Table 3).
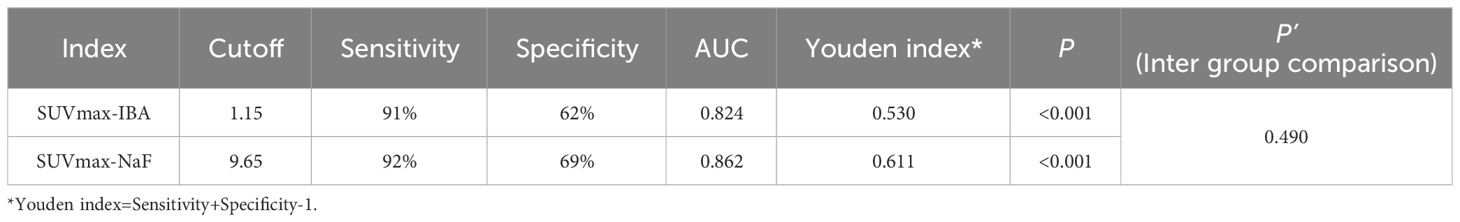
Table 3 Comparison Between 68Ga-DOTA-IBA and 18F-NaF using SUVmax in the Diagnosis of Bone Metastases.
Comparative analysis of TBR of 68Ga-DOTA-IBA PET/CT and 18F-NaF PET/CT PET/CT in bone metastasis
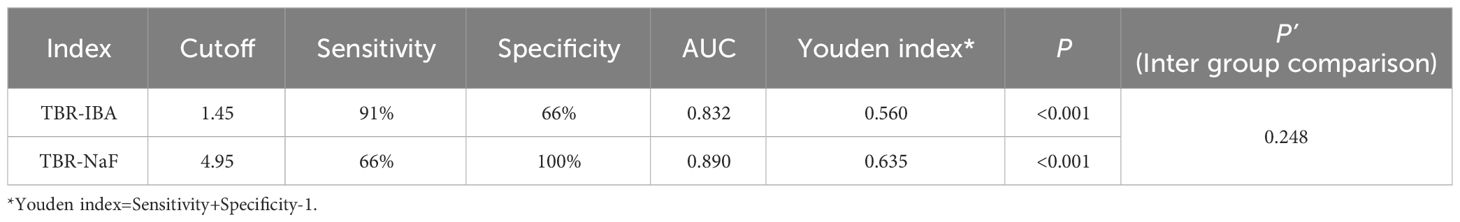
In the detection of bone metastasis, the TBR of 18F-NaF PET showed a slightly higher value compared to that of 68Ga-DOTA-IBA PET. This difference was found to be statistically significant (6.7 ± 6.4 vs 5.0 ± 5.0; P<0.05). ROC curve analysis was conducted to evaluate the diagnostic performance of TBR using both imaging agents, 68Ga-DOTA-IBA and 18F-NaF, for all lesions (Figure 2C). The AUC values for TBR in the diagnosis of bone metastases were 0.832 and 0.890, respectively, with corresponding optimal diagnostic cutoff values of 1.45 and 4.95 (Table 4). Notably, there was no significant difference in AUC between 68Ga-DOTA-IBA PET/CT and 18F-NaF PET/CT when using TBR for diagnosis (P=0.248).
Analysis of the efficacy of 68Ga-DOTA-IBA PET/CT and 18F-NaF PET/CT imaging in the diagnosis of bone metastases from lung, breast and prostate cancer
According to the pathological types of primary tumors, we analyzed lung cancer (n = 12), breast cancer (n = 5) and prostate cancer (n = 2) with bone metastases.
Among 12 lung cancer patients, 11 developed bone metastases, with a total of 133 lesions diagnosed as bone metastases. Based on the lesions, 117 positive lesions were correctly detected by 68Ga-DOTA-IBA PET/CT, with 5 true negatives. 18F-NaF PET/CT detected 124 positive lesions, including 3 false positives. No significant difference in the diagnostic efficacy of the two imaging agents (P=1.00), while Pearson chi-square test and Kappa test showed a significant association and a moderate consistency in diagnosing bone metastases between the two imaging agents (X2 = 35.104, P<0.001; Kappa=0.493, P<0.001).
Among 21 lesions ultimately confirmed as bone metastases in 5 cases of breast cancer, both 68Ga-DOTA-IBA PET/CT and 18F-NaF PET/CT successfully detected 20 positive lesions. McNemar test revealed no significant difference in diagnostic efficacy between the two tracers (P=0.250). Pearson chi-square test and Kappa test indicated a significant association between the diagnostic results of the two tracers, with a strong consistency (X2 = 21.156, P<0.001; Kappa=0.767, P<0.001).
In 2 cases of prostate cancer patients, a total of 28 bone metastatic lesions were detected. 18F-NaF PET/CT correctly identified 28 positive lesions, while 68Ga-DOTA-IBA PET/CT successfully detected 13 positive lesions. Unlike lung cancer and breast cancer, McNemar test revealed a significant difference in the diagnostic efficacy of the two imaging agents (P<0.001). Pearson chi-square test and Kappa test indicated no significant association between the diagnostic results of the two imaging agents (X2 = 2.575, P=0.109), and there was no consistency in the diagnosis of bone metastasis between the two agents (Kappa=0.149, P=0.109). For detailed information on the detection rate, sensitivity, specificity, PPV, NPV, and accuracy of 68Ga-DOTA-IBA PET/CT and 18F-NaF PET/CT in diagnosing bone metastases in lung cancer, breast cancer, and prostate cancer, please refer to Table 5.
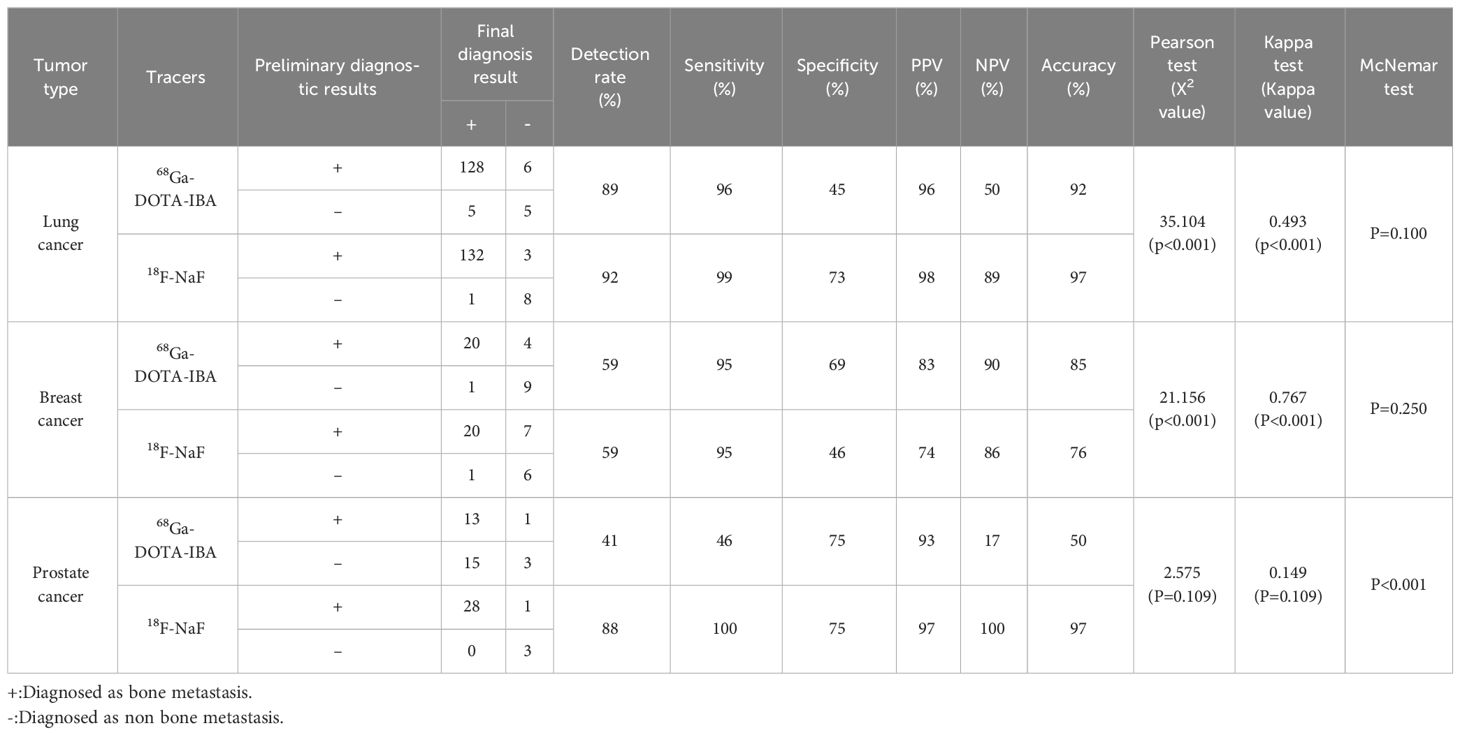
Table 5 Analysis of 68Ga-DOTA-IBA PET/CT and 18F-NaF PET/CT in the diagnosis of bone metastases of different solid tumors.
Discussion
Bone-targeting tracers are essential for diagnosing and monitoring bone metastases. The advancement of radioactive nuclides and molecular probes has resulted in their increased use in isotope therapy (15–19). This study aims to assess the effectiveness of 68Ga-DOTA-IBA PET/CT in detecting bone metastases and compare it with 18F-NaF PET/CT.
18F-NaF PET/CT is a well-established imaging tool for staging skeletal tumors, providing valuable information on disease location, extent, and treatment response. Its rapid extraction, minimal serum protein binding, and efficient clearance from soft tissues with high bone uptake result in superior image quality and consistency compared to traditional 99mTc-MDP (8, 9, 20). Ibandronic acid (IBA), a third-generation amino diphosphate, possesses anti-resorption and anti-hypercalcemia properties. The 68Ga-DOTA-IBA used in this study is a new precursor that has been shown to have advantages over 99mTc-MDP in detecting bone metastasis in previous studies (11, 13). Our preliminary research results indicated a significant association and strong consistency between 68Ga-DOTA-IBA and 18F-NaF PET/CT in diagnosing bone metastases. As a promising bone radiotracer, 68Ga-DOTA-IBA has the potential to offer diagnostic efficacy equivalent to that of 18F-NaF, suggesting it could become a routine diagnostic method or complementary tool for diagnosing bone metastases.
Furthermore, we made a subgroup analysis of lung cancer, breast cancer and prostate cancer with bone metastasis. However, because of the small sample size, we only made a preliminary analysis. The results revealed a significant correlation between the two imaging agents in diagnosing bone metastasis of lung cancer and breast cancer, with better consistency observed in breast cancer. However, there was no significant correlation or consistency in diagnosing bone metastasis of prostate cancer. Lung cancer, known for its osteophilic nature, can exhibit both osteolytic destruction and osteogenic metastasis. The specific pathological changes of bone metastasis of lung cancer can be affected by the degree of bone destruction, the scope and the speed of bone metastasis. Breast cancer, particularly invasive cases, commonly present with bone metastasis, predominantly showing osteolytic bone destruction accompanied by osteoblast reaction. Prostate cancer, typically slow-growing, may not be fatal in its primary form but can lead to severe complications and death when bone metastasis occurs, often displaying osteogenic changes (21). In our study, 68Ga-DOTA-IBA PET/CT were inconsistent with the higher sensitivity of 18F-NaF PET/CT in detecting bone metastases in prostate cancer. We believe this discrepancy may be due to the diffuse osteoblastic changes that often occur in the bones when prostate cancer metastasizes to the bone. Bisphosphonates primarily function to inhibit bone resorption and regulate bone mineralization. Therefore, when the bones are already undergoing active osteoblastic reactions, it may impact the uptake expression of 68Ga-DOTA-IBA PET/CT in osteoblastic metastatic lesions, thus affecting the diagnosis of bone metastasis.
We further analyzed semi-quantitative parameters of all lesions and found statistically significant differences in SUVmax and TBR between the two imaging agents. This difference is attributed to the higher uptake of 18F-NaF in bone and blood, possibly due to the faster clearance related to the closer resemblance of 18F-NaF to calcium salts compared to bisphosphonates (8). However, this higher uptake may also result in an increased risk of false positives for 18F-NaF. The radionuclide 68Ga was used to label DOTA-IBA. And, as mentioned above, the diagnostic efficacy of 68Ga-DOTA-IBA in prostate cancer bone metastasis has shown inconsistent similarity to that of 18F-NaF. This could be due to bisphosphonates’ mechanism of action, which enhances bone mineralization regulation (22, 23). Bisphosphonates exhibit non-uniform distribution in different bone tissue areas and can partially repair osteolysis lesions (24, 25). In our study, most patients had multiple bone metastases throughout the body, some of which were osteogenic. Thus, we propose that the factors affecting 68Ga-DOTA-IBA uptake are more likely to be related to the osteogenic reaction of the lesions, and then affect the detection of lesions. However, this view also needs to be further confirmed by larger sample size and more rigorous research.
On the other hand, The difference in SUVmax AUC between the two imaging agents was not statistically significant, as was the difference in AUC of TBR. This suggests that SUVmax and TBR may have little impact on the final diagnosis of bone metastasis in patients. Some studies have indicated that 18F-NaF PET/CT can detect more bone lesions than 99mTc-MDP, but some scholars argue that the number of lesions may not affect clinical treatment decisions when bone metastasis is diffuse (26). Therefore, 99mTc-MDP is considered sufficient for diagnosing bone metastases, supporting its diagnostic value. Similarly, although 18F-NaF PET/CT may detect more lesions and have a higher uptake value, the diagnostic performance of 68Ga-DOTA-IBA for bone metastasis is statistically consistent. Thus, when bone metastasis is diffuse, the number of lesions may not impact clinical treatment decisions.
In recent years, several radiopharmaceuticals have been utilized for the treatment of bone metastases, such as 32P, 89Sr, 223Ra, 188Re/186Re-HEDP, 153Sm-EDMTP, 177Lu-EDTMP, and 177Lu-BPAMD (27–30). While these therapeutic radiopharmaceuticals have shown promise in alleviating pain, they have not been ideal for theranostic purposes due to the lack of corresponding diagnostic analogs, with the exception of 68Ga/177Lu-BPAMD (30). The combination of 68Ga/177Lu-DOTA-IBA presents a promising set of theranostic radiopharmaceuticals and holds great potential for the management of bone metastases (13, 31, 32).
Although to some extent, 18F-NaF PET/CT is superior to 68Ga-DOTA-IBA. However, in the absence of fluorine-18, 68Ga-DOTA-IBA PET/CT offers an option for PET radiopharmaceuticals produced by generators. More importantly, 68Ga-DOTA-IBA PET/CT imaging can be used for dynamic diagnosis, staging and response evaluation of bone metastasis, making the corresponding radionuclide therapy (177Lu-DOTA-IBA) treatment personalized. In the case illustrated in Figure 3, a 52-year-old woman undergoing cancer surgery received 18F-NaF PET/CT for follow-up. The Maximum Intensity Projection (MIP) image (A) and sagittal view (B: PET image; C: CT scan; D: fusion image) of 18F-NaF PET/CT revealed bone destruction and increased uptake of the imaging agent in multiple skeletal areas (SUVmax 76.5). Additionally, abnormal uptake of the imaging agent throughout the body bones was observed on the 68Ga-DOTA-IBA PET/CT MIP. Although the SUVmax value on the tomographic image was lower than that of 18F-NaF (SUVmax 21.4), this discrepancy does not impact the diagnosis of bone metastasis. For patients undergoing corresponding radionuclide treatment, this information can help in evaluating the treatment’s effectiveness.
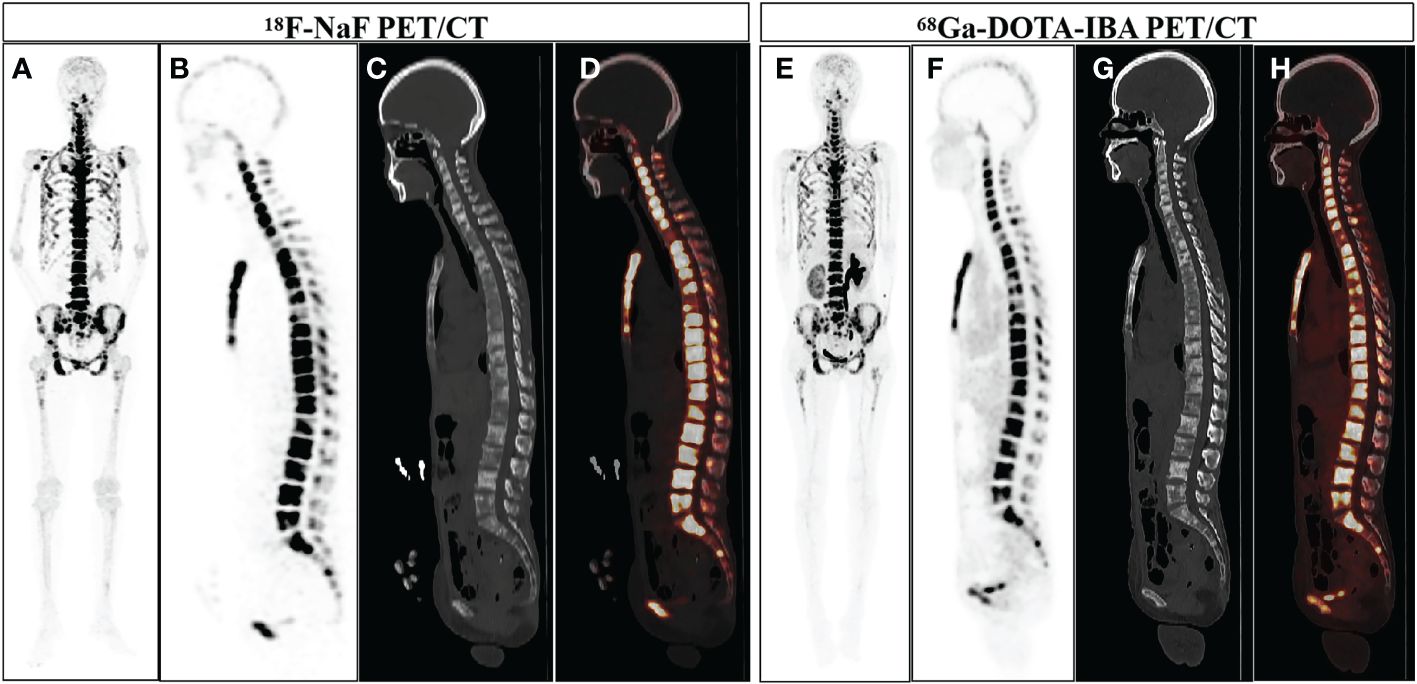
Figure 3 A 52-year-old woman who underwent gastric cancer surgery underwent 18F-NaF PET/CT examination for follow-up. The MIP image of 18F-NaF PET/CT (A) showed increased uptake of imaging agents in multiple skeletal areas throughout the body, and the sagittal views (B: PET image; C: CT scan; D: PET/CT fused image) more intuitively displayed bone destruction and increased uptake of imaging agents in multiple skeletal areas (SUVmax 76.5). 68Ga-DOTA-IBA PET/CT MIP (E) and the sagittal views (F: PET image; G: CT scan; H: PET/CT fused image) also showed increased uptake of bone imaging agents in multiple locations throughout the body (SUVmax 21.4).
This study has certain limitations. First, this is a retrospective analysis of a relatively small patient group (n=24), so these results were susceptible to selection bias. Secondly, due to technical and ethical issues, biopsy was not performed on all bone metastases regarding the biopsy of bone lesions in individuals with multiple suspected metastases, which limits the reference standards. Finally, the diversity of primary diagnosis of patients in our patient group may interrupt the homogeneity, the number of cases of different primary solid tumors in our study was relatively small, so we only made a preliminary analysis of the subgroup, and because of the complex mechanism of bone metastasis, we did not conduct a subgroup analysis of osteolytic and osteogenic metastasis.
Conclusion
Overall, while 68Ga-DOTA-IBA may not be as effective as 18F-NaF as a professional diagnostic bone imaging agent, statistically speaking, the final diagnosis results for all lesions are consistent. Its labeled nuclide is obtained from the generator, and the precursor retains the advantage of high bone adsorption rate of IBA, enabling the 68Ga/177Lu-DOTA-IBA theranostics pair to offer potential clinical benefits. These findings are preliminary, and further large-scale prospective trials are necessary to fully assess the value of 68Ga-DOTA-IBA in detecting bone metastases or different bone metastatic lesions in patients with various types of cancer.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving humans were approved by the Ethics Committee of the Affiliated Hospital of Southwest Medical University (Ethics committee approval No.: KY2022114; Clinical trial registration No.: ChiCTR2200064487). The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
JD: Conceptualization, Data curation, Formal analysis, Methodology, Writing – original draft. JY: Methodology, Resources, Writing – original draft. YW: Data curation, Resources, Writing – review & editing. GL: Methodology, Writing – review & editing. YC: Conceptualization, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was supported in part by research foundation projects from Luzhou Science & Technology Department (2021LZXNYD-C02) and (2021LZXNYD-P03).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Coleman RE, Croucher PI, Padhani AR, Clézardin P, Chow E, Fallon M, et al. Bone metastases. Nat Rev Dis Prime. (2020) 6:83. doi: 10.1038/s41572-020-00216-3
2. Fornetti J, Welm AL, Stewart SA. Understanding the bone in cancer metastasis. J Bone Miner Res. (2018) 33:2099–113. doi: 10.1002/jbmr.3618
3. Clézardin P, Coleman R, Puppo M, Ottewell P, Bonnelye E, Paycha F, et al. Bone metastasis: mechanisms, therapies, and biomarkers. Physiol Rev. (2021) 101:797–855. doi: 10.1152/physrev.00012.2019
4. Panagiotidis E, Zhang-Yin J. Current update and future applications of bone metastasis imaging: what's next? Q J Nucl Med Mol Imag. (2023) 67:247–8. doi: 10.23736/S1824-4785.23.03539-2
5. Zhang-Yin J. Indications et performances de la TEP/TDM au fluorure de sodium (FNA). Méd. Nucléaire. (2018) 42:285–92. doi: 10.1016/j.mednuc.2018.06.002
6. Vaz S, Usmani S, Gnanasegaran G, Van den Wyngaert T. Molecular imaging of bone metastases using bone targeted tracers. Q J Nucl Med Mol Imag. (2019) 63:112–28. doi: 10.23736/S1824-4785.19.03198-4
7. Zhang-Yin J, Panagiotidis E. Role of 18F-NaF PET/CT in bone metastases. Q J Nucl Med Mol Imag. (2023) 67:249–58. doi: 10.23736/S1824-4785.23.03534-3
8. Araz M, Aras G, Küçük ON. The role of 18F-NaF PET/CT in metastatic bone disease. J Bone Oncol. (2015) 4:92–7. doi: 10.1016/j.jbo.2015.08.002
9. Even-Sapir E, Metser U, Flusser G, Zuriel L, Kollender Y, Lerman H, et al. Assessment of Malignant skeletal disease: initial experience with 18F-fluoride PET/CT and comparison between 18F-fluoride PET and 18F-fluoride PET/CT. J Nucl Med. (2004) 45:272–8.
10. Luckman SP, Hughes DE, Coxon FP, Graham R, Russell G, Rogers MJ. Nitrogen-containing bisphosphonates inhibit the mevalonate pathway and prevent post-translational prenylation of GTP-binding proteins, including ras. J Bone Miner Res. (1998) 13:581–9. doi: 10.1359/jbmr.1998.13.4.581
11. Wang Y, Wang Q, Chen Z, Yang J, Liu H, Peng D, et al. Preparation, biological characterization and preliminary human imaging studies of 68Ga-DOTA-IBA. Front Oncol. (2022) 12:1027792. doi: 10.3389/fonc.2022.1027792
12. Wang Q, Yang J, Wang Y, Liu H, Feng Y, Qiu L, et al. Lutetium177-labeled DOTA-ibandronate: A novel radiopharmaceutical for targeted treatment of bone metastases. Mol Pharm. (2023) 20:1788–95. doi: 10.1021/acs.molpharmaceut.2c00978
13. Qiu L, Wang Y, Liu H, Wang Q, Chen L, Liu L, et al. Safety and efficacy of 68 ga- or 177 lu-labeled DOTA-IBA as a novel theranostic radiopharmaceutical for bone metastases : A phase 0/I study. Clin Nucl Med. (2023) 48:489–96. doi: 10.1097/RLU.0000000000004634
14. Nelson BJB, Andersson JD, Wuest F, Spreckelmeyer S. Good practices for 68Ga radiopharmaceutical production. EJNMMI Radiopharm. Chem. (2022) 7:27. doi: 10.1186/s41181-022-00180-1
15. Zidan L, Iravani A, Oleinikov K, Ben-Haim S, Gross DJ, Meirovitz A, et al. Efficacy and safety of (177)Lu-DOTATATE in lung neuroendocrine tumors: A bicenter study. J Nucl Med. (2022) 63:218–25. doi: 10.2967/jnumed.120.260760
16. Satapathy S, Bhattacharya A, Sood A, Kapoor R, Gupta R, Sood A, et al. Hematological markers as predictors of treatment outcomes with lutetium 177(177)Lu)-DOTATATE in patients with advanced neuroendocrine tumors. Cancer Biother Radiopharm. (2022) 37:23–9. doi: 10.1089/cbr.2021.0053
17. Makis W, McCann K, McEwan AJ. Extraventricular neurocytoma treated with 177Lu DOTATATE PRRT induction and maintenance therapies. Clin Nucl Med. (2015) 40:234–6. doi: 10.1097/RLU.0000000000000668
18. Umbricht CA, Benesova M, Schmid RM, Turler A, Schibli R, van der Meulen NP, et al. (44)Sc-PSMA-617 for radiotheragnostics in tandem with (177)Lu-PSMA-617-preclinical investigations in comparison with (68)Ga-PSMA-11 and (68)Ga-PSMA-617. EJNMMI Res. (2017) 7:9. doi: 10.1186/s13550-017-0257-4
19. Heinzel A, Boghos D, Mottaghy FM, Gaertner F, Essler M, von Mallek D, et al. (68)Ga-PSMA PET/CT for monitoring response to (177)Lu-PSMA-617 radioligand therapy in patients with metastatic castration-resistant prostate cancer. Eur J Nucl Med Mol Imag. (2019) 46:1054–62. doi: 10.1007/s00259-019-4258-6
20. Fan Z, Wang T, Zou L, Liu D. Comparison of the diagnostic value of 18F-NaF PET/CT and 99mTc-MDP SPECT for bone metastases: a systematic review and meta-analysis. Transl Cancer Res. (2023) 12:3166–78. doi: 10.21037/tcr
21. Yin JJ, Pollock CB, Kelly K. Mechanisms of cancer metastasis to the bone. Cell Res. (2005) 15:57–62. doi: 10.1038/sj.cr.7290266
22. Lin JH, Russell G, Gertz B. Pharmacokinetics of alendronate: an overview. Int J Clin Pract Suppl. (1999) 101:18–26.
23. Alvarez L, Peris P, Guanabens N, Vidal S, Ros I, Pons F, et al. Serum osteoprotegerin and its ligand in Paget's disease of bone: relationship to disease activity and effect of treatment with bisphosphonates. Arthritis Rheum. (2003) 48:824–8. doi: 10.1002/art.10834
24. Han J, Han L, Zhang L, Liu X, Zhang J, Zhang H, et al. Comparison of clinical effect in treatment of bone tumor between zoledronic acid needle and ibandronate needle. Pak J Pharm Sci. (2018) 31:1683–6.
25. Tzschentke TM. Pharmacology of bisphosphonates in pain. Br J Pharmacol. (2021) 178:1973–94. doi: 10.1111/bph.14799
26. Hillner BE, Siegel BA, Hanna L, Duan F, Quinn B, Shields AF. 18F-fluoride PET used for treatment monitoring of systemic cancer therapy: results from the National Oncologic PET Registry. J Nucl Med. (2015) 56:222–8. doi: 10.2967/jnumed.114.150391
27. Liepe K, Hliscs R, Kropp J, Runge R, Knapp FF Jr, Franke WG. Dosimetry of 188Re-hydroxyethylidenediphosphonate in human prostate cancer skeletal metastases. J Nucl Med. (2003) 44:953–60.
28. Parlak Y, Gumuser G, Sayit E. Samarium-153 therapy for prostate cancer:the evaluation of urine activity, staff exposure and dose rate from patients. Radiat Prot Dosimetry. (2015) 163:468–72. doi: 10.1093/rpd/ncu237
29. Chakraborty S, Das T, Banerjee S, Balogh L, Chaudhari PR, Sarma HD, et al. 177Lu-EDTMP: a viable bone pain palliative in skeletal metastasis. Cancer Biother Radiopharm. (2008) 23:202–13. doi: 10.1089/cbr.2007.374
30. Pfannkuchen N, Meckel M, Bergmann R, Bachmann M, Bal C, Sathekge M, et al. Novel radiolabeled bisphosphonates for PET diagnosis and endoradiotherapy of bone metastases. Pharm (Basel). (2017) 10:45. doi: 10.3390/ph10020045
31. Li H, Xu T, Hua Q, Wang L, Chen Y. 177Lu-DOTA-IBA therapy in prostate cancer with bone metastases. Clin Nucl Med. (2023) 48:740–2. doi: 10.1097/RLU.0000000000004717
Keywords: DOTA-IBA, 68Ga, PET/CT, 18F-NaF, bone metastases
Citation: Deng J, Yang J, Wang Y, Liu G and Chen Y (2024) Comparison of the relative diagnostic performance of 68Ga-DOTA-IBA and 18F-NaF for the detection of bone metastasis. Front. Oncol. 14:1364311. doi: 10.3389/fonc.2024.1364311
Received: 02 January 2024; Accepted: 11 March 2024;
Published: 22 March 2024.
Edited by:
Min-Ying Lydia Su, University of California, Irvine, United StatesReviewed by:
Jules Zhang-Yin, Clinique Sud Luxembourg, BelgiumJogeshwar Mukherjee, University of California, Irvine, United States
Copyright © 2024 Deng, Yang, Wang, Liu and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Guangfu Liu, MTE5MTAxNTc3OEBxcS5jb20=; Yue Chen, Y2hlbnl1ZTU1MjNAMTI2LmNvbQ==
†These authors have contributed equally to this work and share first authorship
 Jia Deng
Jia Deng Jian Yang
Jian Yang Yingwei Wang1,2,3†
Yingwei Wang1,2,3† Guangfu Liu
Guangfu Liu Yue Chen
Yue Chen