- 1Institute of Hematology, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China
- 2Key Lab of Molecular Biological Targeted Therapies of the Ministry of Education, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China
Osteolytic lesions are infrequently observed in adult patients with acute myeloid leukemia (AML). This report details the case of a 66-year-old male patient who presented with myeloid sarcoma (MS), osteolytic lesion and pancytopenia. Effective treatments were delayed due to diagnostic challenges and the rapid progression of the disease. It is essential to consider AML in the differential diagnosis when faced with a patient presenting osteolytic lesions and pancytopenia.
1 Introduction
Acute myeloid leukemia (AML) is a clonal, malignant disease of hematopoietic tissues characterized by accumulation of leukemic blast cells primarily in the marrow. It results in impaired production of normal blood cells, which leads to pancytopenia in blood routine test. AML always manifests with common symptoms such as pallor, fatigue, weakness, fever, bleeding (1).
Myeloid sarcoma(MS), also known as granulocytic sarcoma(GS), extramedullary myeloid tumor and chloroma, is defined by World Health Organization(WHO) as extramedullary tumor masses of myeloid blasts with tissue architecture effaced (2). MS can occur at any sites in the body, with the most common locations being the skin, lymph nodes, mediastinum, testis, intestine, bone and central nervous system(CNS) (3, 4). The incidence of MS in AML varies in different studies, ranging approximately from 0.8% to 10.4% (5–8).
Osteolytic lesions are a common feature in multiple myeloma (MM) and metastatic cancers, such as prostate cancer, thyroid cancer, lung cancer and others. However, osteolytic lesions are rarely reported in adult with AML (9–17).
This report details the case of a patient finally diagnosed with AML who presented primarily with MS, osteolytic lesion and pancytopenia.
2 Case report
A 66-year-old male patient was admitted to the orthopedic department due to a lump on his left upper arm persisting for 2 months, accompanied by pressure pain and pathological fractures occurring 10 days prior. An FDG PET/CT (18F-fluorodeoxyglucose Positron Emission Tomography/Computed Tomography) scan revealed multiple osteolytic lesions, observed in the left mandible, right 7th costal rib, left 2nd anterior rib, right clavicle, right scapula, T12, L3, L5, S1 vertebral levels, bilateral proximal humeri, bilateral iliac bones, and femoral neck. Some lesions were accompanied with the formation of soft tissue masses with abnormal concentration of radioactive uptake (Figure 1), raising suspicion of metastatic malignancies. The physical examination indicated stable vital signs. The patient had a history of diabetes, managed with metformin, and untreated coronary heart disease. He had no history of previously diagnosed hematological neoplasm.
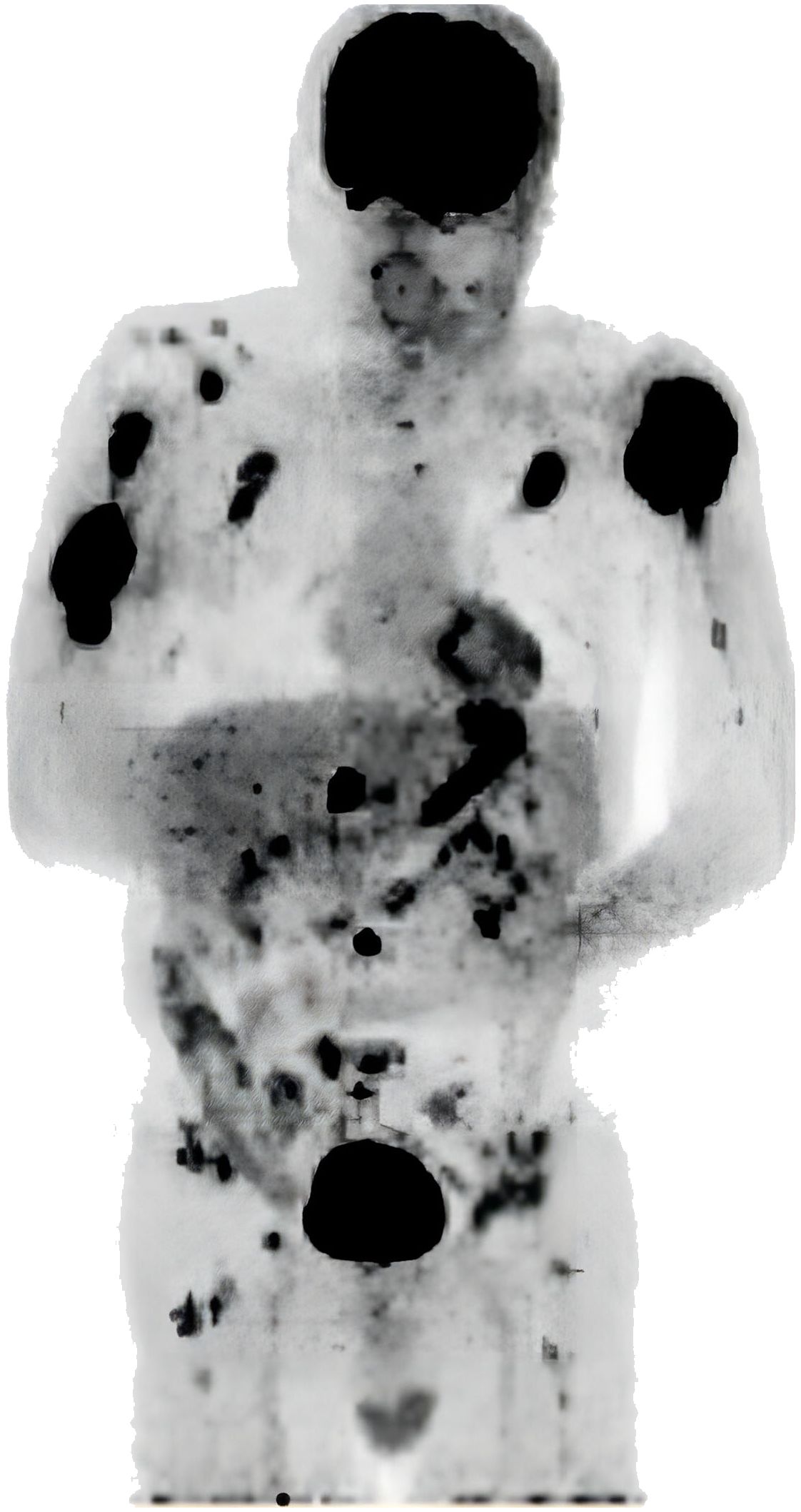
Figure 1 FDG PET/CT showing multiple osteolytic lesions, some lesions accompanied with the formation of soft tissue masses.
On the 2nd day of admission, Complete Blood Count (CBC) revealed a hemoglobin level of 78 g/L ↓ (130.0-175.0), erythrocyte count of 2.41 T/L↓(4.3-5.8), total leukocyte count of 3.23 G/L↓(3.5-9.5), and a platelet count of 35 G/L↓ (125–350). The patient’s inflammatory markers showed a high-sensitivity C-reactive protein (hs-CRP) level of 120.81mg/L↑ (<4) and an erythrocyte sedimentation rate (ESR) of 102mm/h↑ (0–15). The patient’s coagulation function, as well as liver and kidney functions, were within normal limits. A puncture of the left humerus was performed on the same day. On the 7th day, the patient developed a high fever up to 39°C, so that the antibiotic treatment had been upgraded.
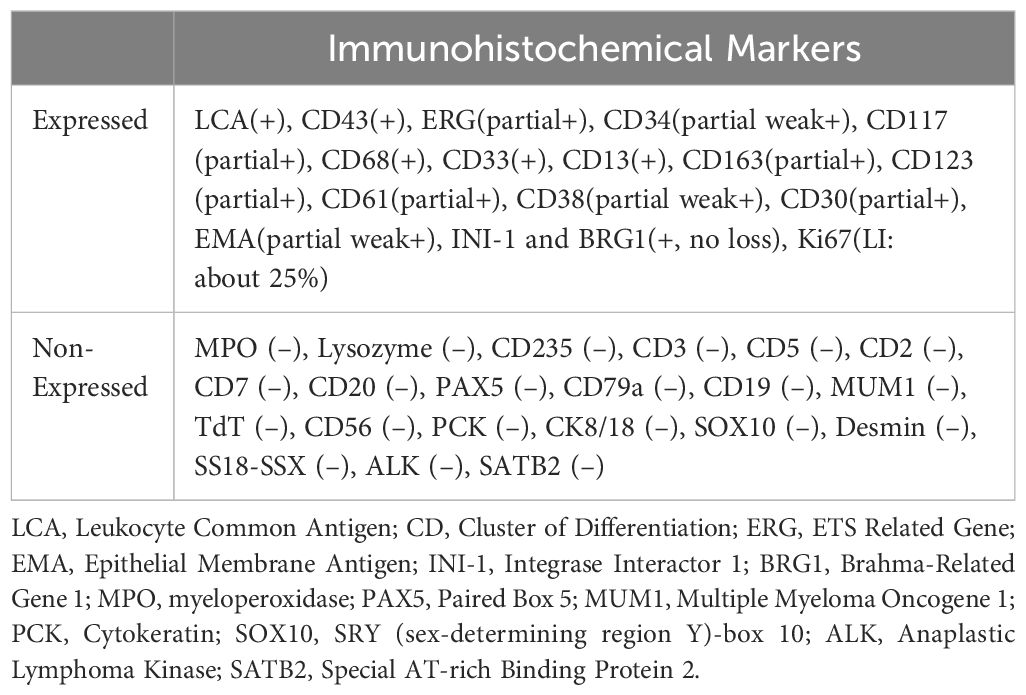
The puncture of the left humeral bone reported on the 10th day revealed that the principal substance within the bone trabeculae appeared as homogeneous, silty necrotic debris, with faint cellular remnants noted in specific regions. Immunohistochemical staining of cellular remnants was positive for LCA (Leukocyte Common Antigen) and negative for PCK (Cytokeratin) and S-100, indicating neoplastic necrosis with a high probability of lymphopoietic involvement. To elucidate the pathological nature of the tumor further, an incisional biopsy of the left humeral bone was conducted on the 13th day. Immunohistochemical staining of the biopsy specimen reported on the 20th day suggested myeloid sarcoma, with an immunophenotype showing co-expression of monocytic and megakaryocytic markers (Table 1).
Due to the suspicion of hematological malignancies, the difficulty in controlling the fever and the persistent decrease in platelet count, the patient was transferred to the hematology department on the 17th day of admission. Immediate bone marrow aspiration and biopsy were conducted. Considering the patient’s age of 66, along with pancytopenia and multiple osteolytic lesions, multiple myeloma (MM) was the primary suspicion at that time. During the process of confirming the diagnosis and providing targeted treatment, the patient’s condition continued to deteriorate.
On the 19th day, the patient was transferred to the Hematology Care Unit (HCU) for the clouded consciousness, hypoxia and persistent high fever, prompting suspicion of hemophagocytic lymphohistiocytosis (HLH). The diagnostic criteria were met with a temperature exceeding 38.5°C for over 7 days, ferritin at a dilution of 1:20 measuring 15582.6 μg/L (21.8-275), and an sCD25 level of 10047.17 U/ml (39.26-265.5). However, since the remaining five criteria were either unknown or not satisfied, the diagnosis of HLH remained uncertain.
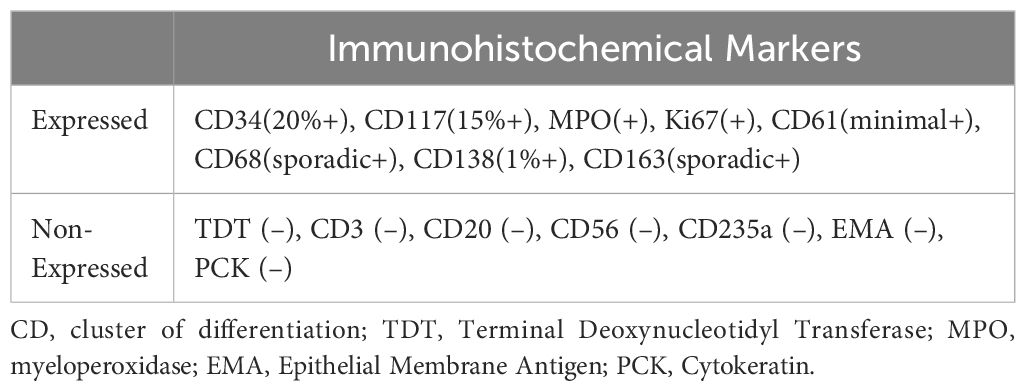
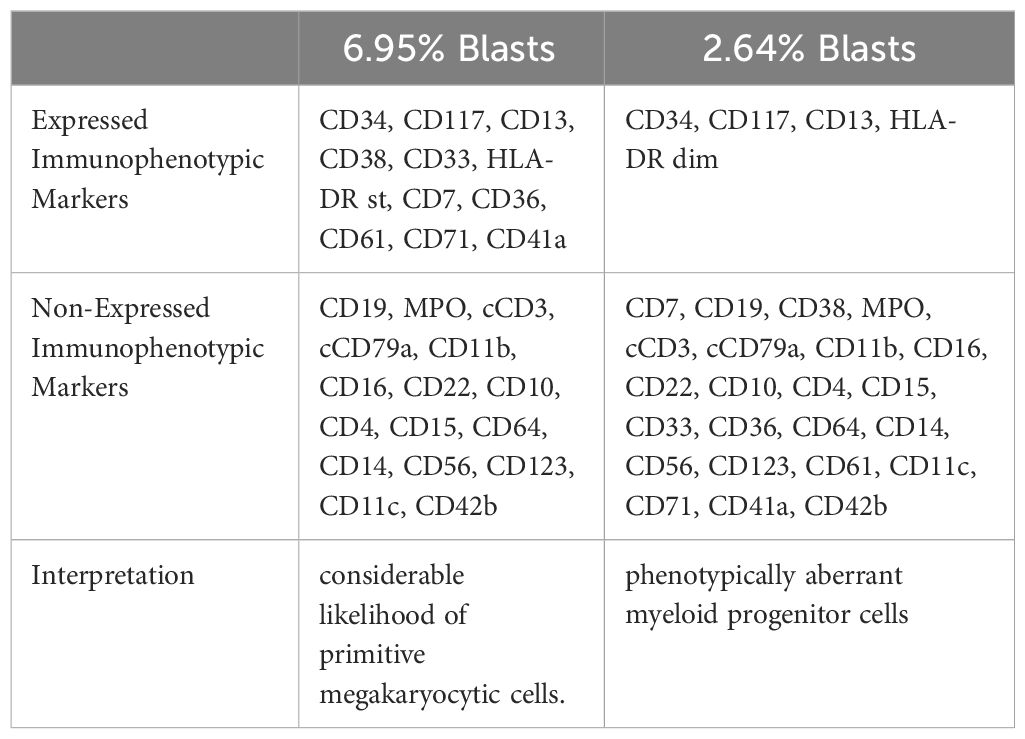
Serum electrophoresis reported on the 20th day revealed no evidence of monoclonal gammopathy. Immunohistochemical staining of bone marrow biopsy reported on the 20th day indicated features consistent with AML (Table 2). Bone marrow aspiration reported on the 21st day indicated that primitive blood cells accounted for 29% (Figure 2). Cytochemical staining revealed positivity for POX(Peroxidase), partial positivity for ANAE (Alpha-Naphthyl Acetate Esterase), and negativity for CE (Chloroacetate Esterase) and PAS(Periodic Acid-Schiff), indicative of myeloid differentiation. The peripheral blood smear showed 33% primitive blood cells. Immunophenotyping reported on the 23rd day revealed two populations of phenotypically aberrant myeloid progenitor cells within the nucleated cells (Table 3, Figure 3). MM-FISH (fluorescence in situ hybridization) reported on the 24th day revealed multiple abnormalities, including amplification of chromosome 1q21 (copy number = 3), 1p32, 4p16, 11q13, 14p32 and 17. Karyotype analysis showed an absence of the mitotic phase. The above results supported the diagnosis of AML.
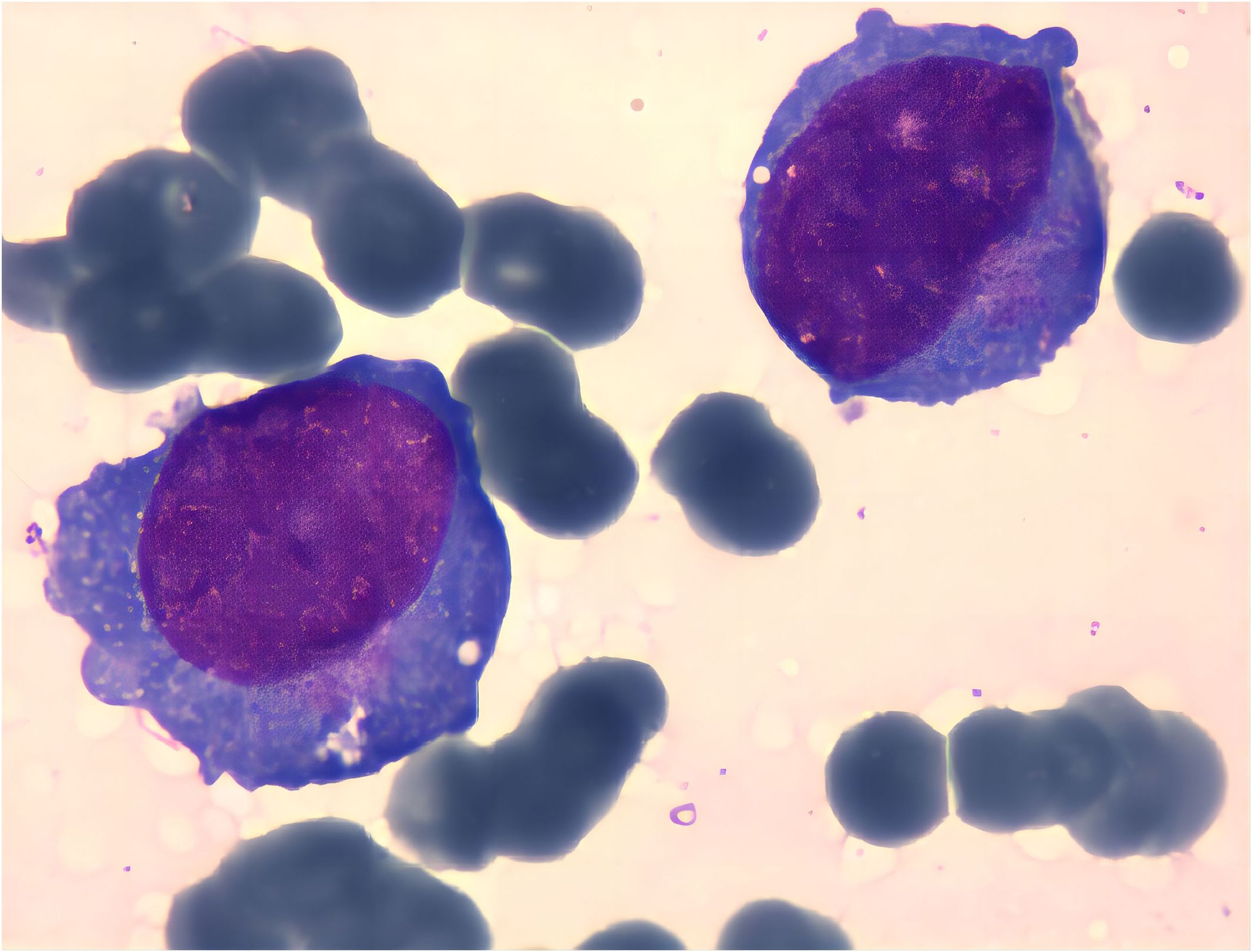
Figure 2 Cell bodies vary in size, with some notably larger. Cytoplasmic content ranges from scant to abundant, appearing grayish-blue upon staining. Some cells exhibit cytoplasmic extensions, containing fine pinkish granules. Nuclei display both regular and irregular shapes, with uniformly fine chromatin. Variability in nucleolar prominence is observed.
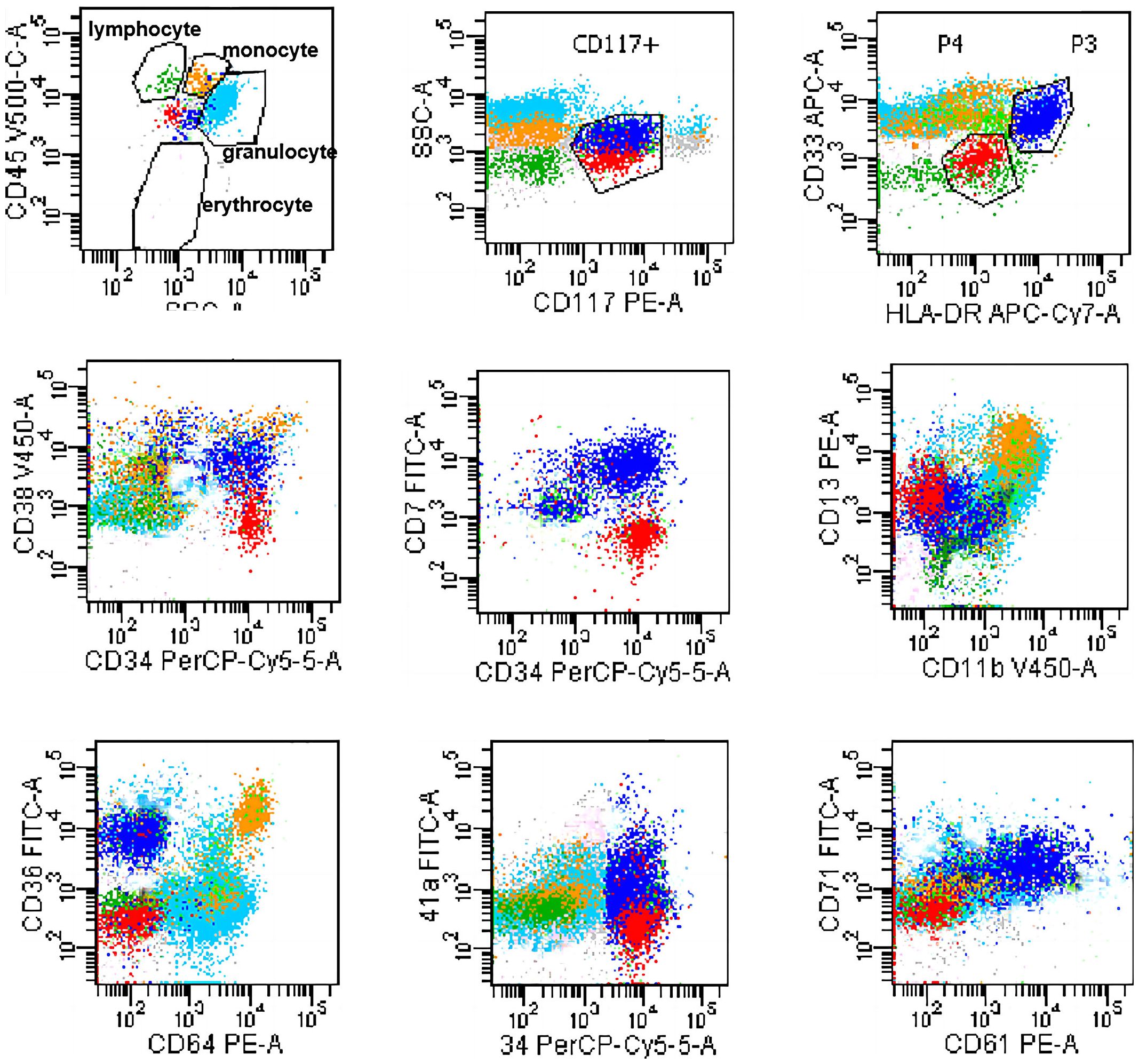
Figure 3 Flowcytometry dot plot showing two populations of phenotypically aberrant myeloid progenitor cells. Blue dots: 6.95% Blasts; Red dots: 2.64% Blasts.
Unfortunately, on the 21th day of admission, the patient suffered respiratory and cardiac arrest. Despite rescue efforts being ineffective, the patient was discharged at the request of the family. The exact diagnose was not confirmed until the day of discharge.
3 Discussion
This report details the case of a 66-year-old man presenting with a lump on his left upper arm and PET/CT findings indicating multiple osteolytic lesions. The diagnosis was obscured and delayed due to atypical bone symptoms. The obscured diagnosis and rapid disease progression resulted in inadequate treatment, significantly impacting the patient’s outcome.
The diagnosis of MM was ruled out based on bone marrow aspiration and biopsy results, along with a negative serum electrophoresis for monoclonal gammopathy. The diagnosis of AML was established through morphological and immunological evidence in the bone marrow, and the presence of myeloid sarcoma (MS). AML manifesting as MS and multiple osteolytic lesions causes significant diagnostic dilemma.
As previously reported, due to the lack of adequate immunohistochemical test, MS has often been misdiagnosed as lymphoproliferative disorder, particularly diffuse large B-cell lymphoma (DLBCL), Hodgkin lymphoma, small lymphocytic leukemia, and T-cell lymphoma. Additional common misdiagnoses encompass myeloma, thymoma, and extramedullary hematopoiesis (18). In our case, the immunohistochemical finding from the left humerus puncture with limited markers suggested neoplastic necrosis and a high likelihood of lymphopoietic neoplastic necrosis. The subsequent immunohistochemical test of incisional biopsy, which included sufficient molecular markers, confirmed the diagnosis of MS. This underscores the importance of adequate immunophenotyping in confirming MS.
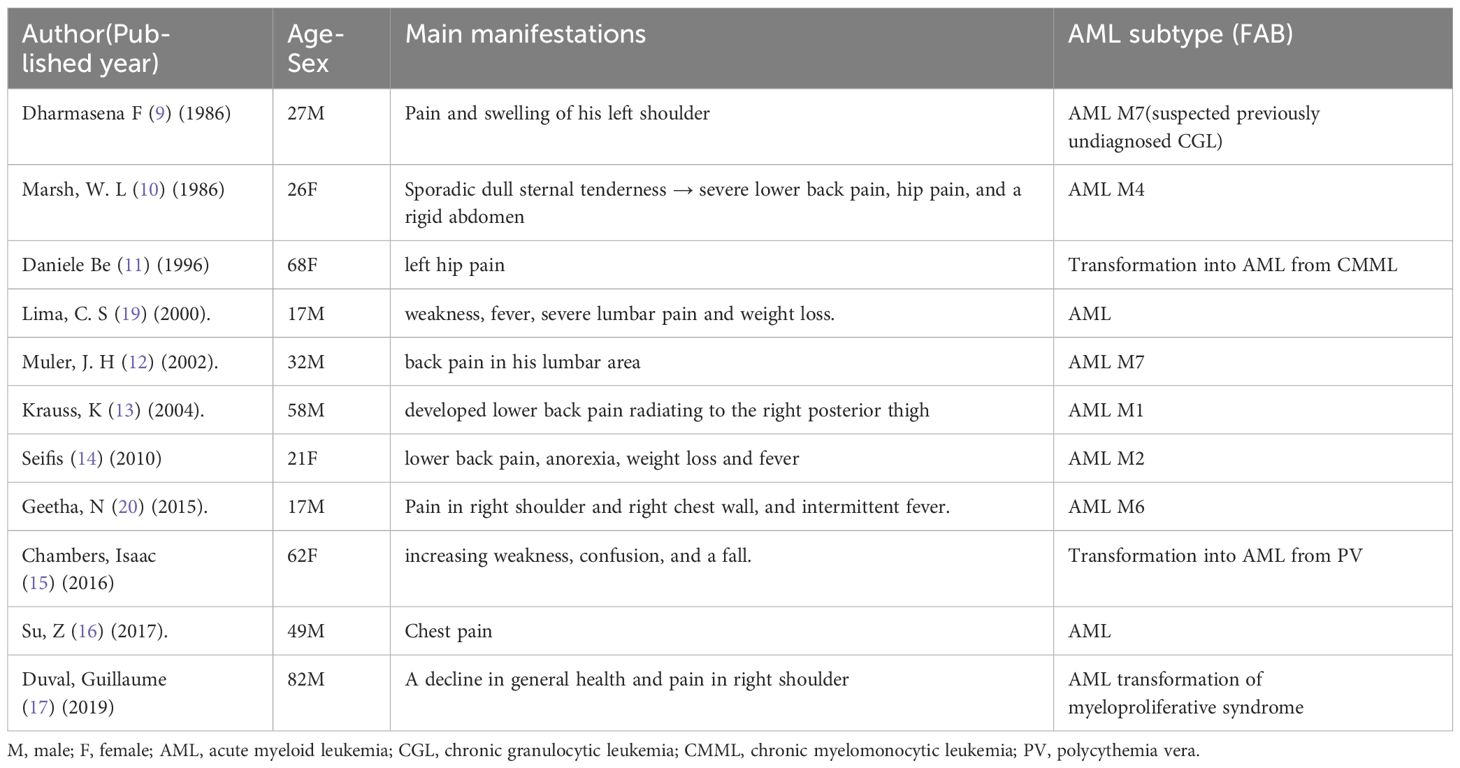
Osteolytic lesions are not a common feature of AML. The multiple osteolytic lesions are typically associated with metastatic cancers and MM. There have been only a few reported cases of AML presenting with osteolytic lesions in patients older than 14 years (Table 4). The osteolytic lesions were considered as a sarcomatous manifestation (19) or tumor-mediated bone erosion (13). A biopsy of an osteolytic lesion in an AML M7 patient revealed consistency with an extramedullary myeloid cell tumor with megakaryocyte differentiation (12). However, the specific pathophysiological mechanism of bone lytic lesions in AML remains elusive. Osteolytic lesions were considered as a poor prognosis in AML (19) or an advanced disease state at the course of AML transforming from MDS (11). Previous cases also reported relief of bone pain through local irradiation (9, 11, 19). Unfortunately, effective treatment options were limited by the rapid progress of the disease in this case.
A peripheral blood smear was conducted performed concurrently with bone marrow aspiration in this case. The accessibility and efficiency of peripheral smear morphology render it a valuable routine diagnostic measure, especially in cases of pancytopenia. When conducted during the initial hospitalization, it can accelerate diagnosis and enable timely interventions.
Due to the patient’s passing and the discrepancy between the initial and definitive diagnoses, as well as the absence of the mitotic phase in cytogenetics, molecular analysis and AML-NGS data are not accessible. Nevertheless, molecular or cytogenetic studies would have been highly beneficial in similar cases to determine if a specific cytogenetic or molecular profile confers a higher risk for the manifestation of multiple osteolytic lesions or MS.
4 Conclusion
This case implies that AML must be considered in the differential diagnosis in face of a patient presenting with bone lesions and pancytopenia. Conducting a simple peripheral blood smear, ensuring adequate immunophenotyping of tissue sections, and performing timely bone marrow aspiration are crucial steps to ensure a correct diagnosis and prevent delays in initiating effective treatment.
Data availability statement
The original contributions presented in the study are included in the article. Further inquiries can be directed to the corresponding author. Requests to access these datasets should be directed to YWlsaXNoYUBodXN0LmVkdS5jbg==.
Ethics statement
The studies involving humans were approved by Institutional Review Board of Tongji Medical College, Huazhong University of Science and Technology. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
JZ: Writing – original draft, Writing – review & editing. SM: Writing – original draft, Writing – review & editing. LC: Resources, Writing – review & editing. LA: Funding acquisition, Writing – review & editing. YW: Funding acquisition, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the National Natural Science Foundation of China (No.81000211 and No.81500172) and Nature Science Foundation of Hubei Province (No.2020CFB790).
Acknowledgments
We are grateful to the affected family who consented to reporting the study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
2. Swerdlow S, Campo E, Harris NL. WHO classification of tumours of haematopoietic and lymphoid tissues. Bosman FT, Jaffe ES, Lakhani SR, Ohgaki H, editors. France: International Agency for Research on Cancer (2017). JWHOCoTRtEeL.
3. Pileri SA, Ascani S, Cox MC, Campidelli C, Bacci F, Piccioli M, et al. Myeloid sarcoma: clinico-pathologic, phenotypic and cytogenetic analysis of 92 adult patients. Leukemia. (2007) 21:340–50. doi: 10.1038/sj.leu.2404491
4. Kawamoto K, Miyoshi H, Yoshida N, Takizawa J, Sone H, Ohshima K. Clinicopathological, cytogenetic, and prognostic analysis of 131 myeloid sarcoma patients. Am J Surg pathol. (2016) 40:1473–83. doi: 10.1097/PAS.0000000000000727
5. Goyal G, Bartley AC, Patnaik MM, Litzow MR, Al-Kali A, Go RS. Clinical features and outcomes of extramedullary myeloid sarcoma in the United States: analysis using a national data set. Blood Cancer J. (2017) 7:e592. doi: 10.1038/bcj.2017.79
6. Shimizu H, Saitoh T, Hatsumi N, Takada S, Yokohama A, Handa H, et al. Clinical significance of granulocytic sarcoma in adult patients with acute myeloid leukemia. Cancer Sci. (2012) 103:1513–7. doi: 10.1111/j.1349-7006.2012.02324.x
7. Muss HB, Moloney WC. Chloroma and other myeloblastic tumors. Blood. (1973) 42:721–8. doi: 10.1182/blood.V42.5.721.721
8. Liu PI, Ishimaru T, McGregor DH, Okada H, Steer A. Autopsy study of granulocytic sarcoma (chloroma) in patients with myelogenous leukemia, Hiroshima-Nagasaki 1949-1969. Cancer. (1973) 31:948–55. doi: 10.1002/(ISSN)1097-0142
9. Dharmasena F, Wickham N, McHugh PJ, Catovsky D, Galton DA. Osteolytic tumors in acute megakaryoblastic leukemia. Cancer. (1986) 58:2273–7. doi: 10.1002/(ISSN)1097-0142
10. Marsh WL Jr., Bylund DJ, Heath VC, Anderson MJ. Osteoarticular and pulmonary manifestations of acute leukemia. Case report and review of the literature. Cancer. (1986) 57:385–90. doi: 10.1002/(ISSN)1097-0142
11. Ben-Dayan D, Zeidman A, Djaldetti M, Mittelman M. Osteolytic lesions as a presenting sign of acute nonlymphocytic leukemia. Acta haematologica. (1996) 96:249–50. doi: 10.1159/000203794
12. Muler JH, Valdez R, Hayes C, Kaminski MS. Acute megakaryocytic leukemia presenting as hypercalcemia with skeletal lytic lesions. Eur J haematol. (2002) 68:392–6. doi: 10.1034/j.1600-0609.2002.02715.x
13. Krauss K, Aydeniz B, Dohmen BM, Wehrmann M, Wallwiener D, Huober J. Value of positron emission tomography scan in staging cancers, and an unusual presentation of acute myeloid leukemia. CASE 3. Acute Myeloid Leukemia Presenting With Lytic Bone Lesions. J Clin Oncol. (2004) 22:2966–8. doi: 10.1200/JCO.2004.08.133
14. Seifi S AKA, Asvadi Kermani I, Dolatkhah R. An uncommon occurrence of acute myeloid leukemia in tabriz. J Clin Diagn Res. (2010) 4):3225–9.
15. Chambers I, Truong P, Kallail KJ, Palko W. Extensive bone marrow necrosis and osteolytic lesions in a case of acute myeloid leukemia transformed from polycythemia vera. Cureus. (2016) 8(6):e639. doi: 10.7759/cureus.639
16. Su Z, Wu F, Hu W, Liu X, Wu S, Feng X, et al. Philadelphia chromosome-positive acute myeloid leukemia with masses and osteolytic lesions: finding of 18F-FDG PET/CT. Front Med. (2017) 11:440–4. doi: 10.1007/s11684-017-0523-x
17. Duval G, Meytadier H, Bouvard B. Singular case of osteolytic lesions revealing transformation of myeloproliferative syndrome to acute leukemia. Joint Bone spine. (2019) 86:251–3. doi: 10.1016/j.jbspin.2018.11.004
18. Shallis RM, Gale RP, Lazarus HM, Roberts KB, Xu ML, Seropian SE, et al. Myeloid sarcoma, chloroma, or extramedullary acute myeloid leukemia tumor: A tale of misnomers, controversy and the unresolved. Blood Rev. (2021) 47:100773. doi: 10.1016/j.blre.2020.100773
19. Lima CS, Pinto Neto JV, da Cunha ML, Vassallo J, Cardinalli IA, De Souza CA. Osteolytic lesions as a presenting sign of acute myeloid leukemia. Haematologia. (2000) 30:325–31. doi: 10.1163/156855900300109576
Keywords: acute myeloid leukemia, osteolytic lesions, myeloid sarcoma, pancytopenia, diagnosis, case report
Citation: Zhang J, Mu S, Cai L, Ai L and Wu Y (2024) Osteolytic lesions as a presenting sign of acute myeloid leukemia: a case report. Front. Oncol. 14:1364266. doi: 10.3389/fonc.2024.1364266
Received: 02 January 2024; Accepted: 05 April 2024;
Published: 01 May 2024.
Edited by:
Mohamed A. Yassin, Qatar University, QatarReviewed by:
Osvaldo Padilla, Texas Tech University Health Sciences Center El Paso, United StatesSamah Kohla, Hamad Medical Corporation, Qatar
Copyright © 2024 Zhang, Mu, Cai, Ai and Wu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lisha Ai, YWlsaXNoYUBodXN0LmVkdS5jbg==; Yaohui Wu, d3loMTEwMzVAc2luYS5jb20=
†These authors have contributed equally to this work and share first authorship
 Jingqian Zhang1,2†
Jingqian Zhang1,2† Shidai Mu
Shidai Mu Lisha Ai
Lisha Ai