
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Oncol. , 31 July 2024
Sec. Hematologic Malignancies
Volume 14 - 2024 | https://doi.org/10.3389/fonc.2024.1362367
Introduction: Extranodal NK/T-cell lymphoma (ENKTCL), a non-Hodgkin lymphoma, is known for its destructive local impact on nasal structures and systemic induction of inflammatory cytokines. Concurrent treatment with radiation and nonanthracycline- based chemotherapy has improved survival rates in patients with localized disease stages. However, survival outcomes vary significantly in advanced-stage and relapsed or refractory (R/R) cases.
Methods: Therefore, we conducted a meta-analysis using random effects models to assess prognostic factors in advanced or R/R ENKTCL, employing a digital extractor on Kaplan–Meier graphs owing to the scarcity of published prospective trials for these patients.
Results: We observed that patients with advanced ENKTCL treated with Lasparaginase had a median progression-free survival (PFS) of 14.3 months and an overall survival (OS) of 19 months. In R/R ENKTCL, PFS and OS were 11.7 and 15.6 months, respectively. Additionally, OS outcomes in advanced-stage ENKTCL were better in the asparaginase group than that in the non-asparaginase group, with PEG-asparaginase showing superior results compared with that using Lasparaginase. Epstein–Barr Virus (EBV)-DNA positivity in the bloodstream prior to treatment was associated with poor outcomes in advanced-stage ENKTCL, and similar trends were observed in patients with R/R ENKTCL and post-treatment EBV viremia.
Discussion: Collectively, these findings suggest that chemotherapy with Lasparaginase or PEG-asparaginase can enhance survival in advanced or R/R ENKTCL. However, future strategies must be developed to effectively suppress EBV viremia and achieve a deep response toward tumor eradication.
Patients with extranodal NK/T-cell lymphoma (ENKTCL) display distinctive characteristics compared with those with other non-Hodgkin cell lymphomas. ENKTCL primarily affects the nasal mucosa, leading to destruction of adjacent structures, including the nasopharynx, oropharynx, oral cavity, and hypopharynx. Notably, 72.4–75% of cases are diagnosed at stages I/II, with systemic spread being uncommon (1, 2).
Survival outcomes for patients with Ann Arbor stages I/II ENKTCL have improved substantially. Notably, the 5-year overall survival (OS) rates increased from 38% to 42% (3, 4) in the early 2000s, whereas 3-year OS rates have escalated to 85% in the last two decades (5, 6). These improvements are attributed to the administration of radiation therapy exceeding 50 Gy, complemented by non-anthracycline-based chemotherapy regimens. These regimens typically include agents such as etoposide, gemcitabine, ifosfamide, methotrexate, and platinum, employed as first-line therapies. The 5-year OS rates for patients with advanced-stage ENKTCL display significant variability, ranging from 30–74.3% (3, 7, 8). This variation is attributed to the use of L-asparaginase (L-Asp) or PEG-asparaginase (PEG-Asp), which typically result in improved outcomes. However, some studies report lower survival rates, specifically between 33.2–45.7%, when incorporating L-Asp (9, 10). Similarly, survival in relapsed or refractory (R/R) ENKTCL is inconsistent, with 5-year OS rates ranging from 24.8–55% (11, 12).
In this study, we integrated studies on advanced and R/R ENKTCL to estimate OS and progression-free survival (PFS) using random effects models for survival curve synthesis. Our meta-analysis, incorporating hazard ratios for OS, was conducted to discern the impact of various factors on survival outcomes, particularly comparing L-Asp and PEG-Asp treatments and the presence or absence of Epstein–Barr Virus (EBV) DNAemia.
Differences in results stem from heterogeneity in cohort subgroup analyses and the inclusion of relatively small patient groups; to address this, we utilized individual patient data (IPD) from published graphs and conducted an IPD meta-analysis to identify potential targets for future treatments of advanced or R/R ENKTCL.
Web-based retrieval was conducted manually through PubMed (January 2000 to November 2023) and Embase (January 2000 to November 2023), using the search term ENKTCL. Adult patients with newly diagnosed advanced-stage R/R ENKTCL were enrolled in phase I, II, and III clinical trials as well as retrospective studies. Two researchers (T.K. and G.M.) independently screened articles that met the eligibility criteria and extracted data from the literature.
Studies were analyzed using the following inclusion criteria: patients treated with chemotherapy regimens; reported survival data or curves; and published in English. The search included the keywords “extranodal natural killer T-cell lymphoma and chemotherapy.” The quality evaluation of the analyzed studies complied with the Cochrane Handbook for Systematic Reviews (Version 5.1.0) (13).
We selected studies that included data on OS and PFS for stages III and IV, or R/R ENKTCL. To analyze the factors that impact OS, we analyzed studies that presented data categorized by treatment with PEG-Asp and Asp. To evaluate the EBV status in the advanced-stage ENTKCL, we specifically focused on studies discussing EBV DNA before treatment and in cases of relapsed ENKTCL. Additionally, studies indicating post-treatment EBV positivity were included.
For the estimation of PFS and OS, we utilized IPD from the Kaplan–Meier (KM) package were used in patients with advanced-stage or R/R ENKTCL (14).. Unavailable or missing data were imputed using the graph and were reconstructed using ScanIt software (https://www.amsterchem.com/scanit.html). To ascertain factors affecting OS, we extracted results that included hazard ratios (HRs) and 95% confidence intervals (CIs). HRs were calculated to interpret prognostic factors, and a meta-analysis was performed using the meta and metafor packages (15, 16). A p-value < 0.05 indicated statistical significance. The heterogeneity test in these studies was considered statistically significant with a p-value <0.10. I2 was employed for quantitative analysis of heterogeneity: I2 < 25% indicated low heterogeneity, 25% ≤ I2 ≤ 50% suggested moderate heterogeneity, and I2 > 50% indicated high heterogeneity. The random effects model was employed to compare the HR and 95% CIs. Statistical analysis was performed using R software for statistical computing (R Foundation for Statistical Computing, Vienna, Austria, version 4.0.2).
According to the retrieval strategy, 3164 related references were checked. Among these, 548 were preliminarily screened after reading the title and abstract, excluding repetitive nonclinical studies and literature unrelated to treatments. Eventually, 19 studies were selected after reading the full articles (Figure 1).
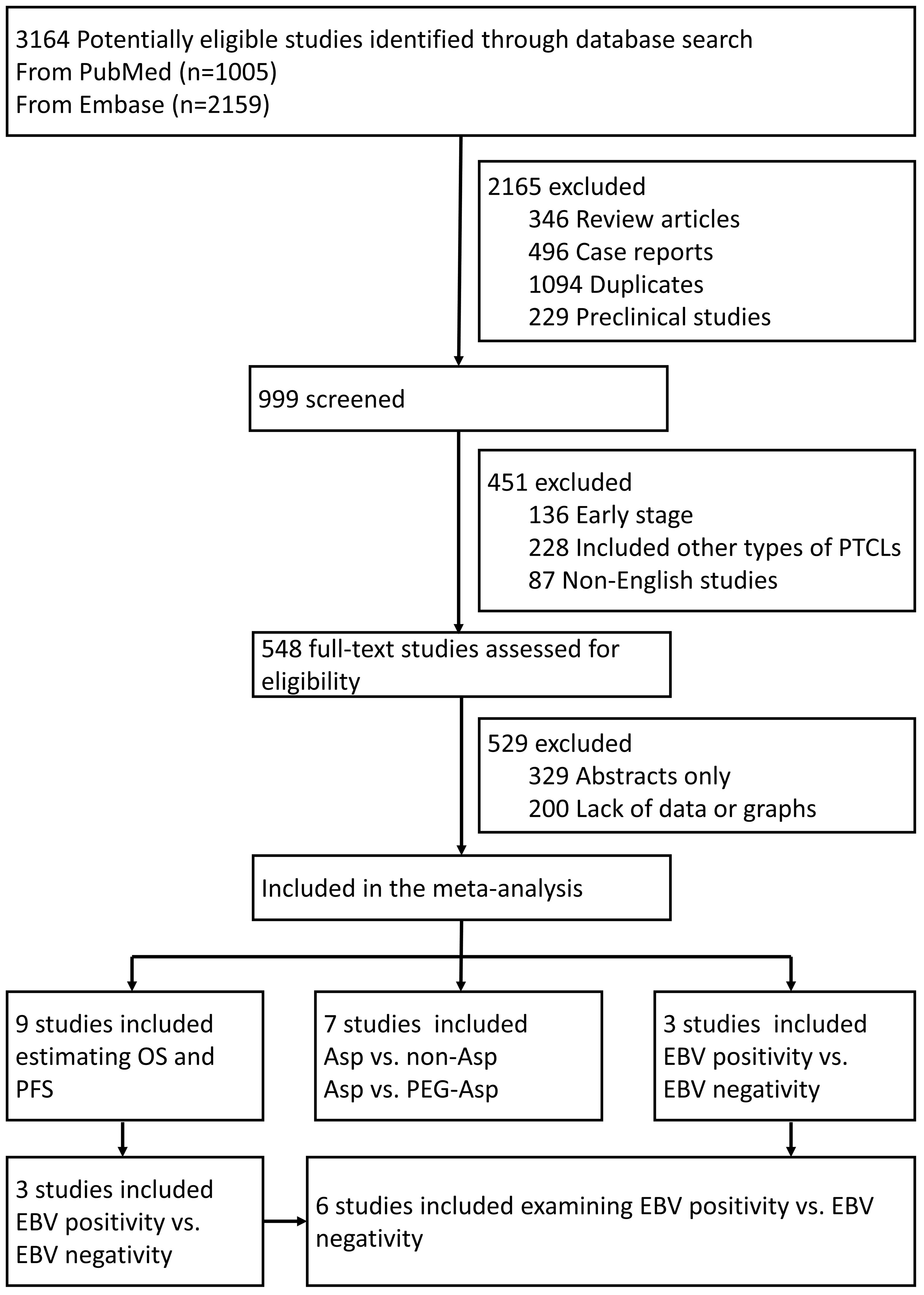
Figure 1 Flowchart of the trial selection process. PTCLs, Peripheral T-Cell Lymphoma; OS, overall survival; PFS, progression-free survival; Asp, L-asparaginases; EBV, Epstein–Barr Virus.
All included studies provided comprehensive details on patient conditions and complete result data but lacked detailed descriptions of randomization, blinding methods, follow-up losses, and allocation concealment. Consequently, the overall evaluation rate was relatively low. No significant publication bias was observed in the comparison of L-Asp versus non-Asp-based chemotherapy and PEG-Asp versus Asp-based chemotherapy in patients with advanced-stage ENKTCL. The same applies to elevated versus normal EBV DNA in pretreatment blood in stages I–IV ENKTCL patients (Supplementary Figures S1A–C). However, the comparison between elevated (over 500 copies) and normal EBV DNA in end-of-treatment blood in patients with R/R ENKTCL deviated from the expected plot. This deviation is likely attributed to the limited number of studies and their heterogeneity (Supplementary Figure S1D).
Among the 19 studies, six (9, 10, 17–20) extracted the IPD using graphs (Table 1). In the random effects model, 188 patients with advanced-stage III–IV ENKTCL treated with L- or PEG-Asp-based therapy showed an estimated median PFS of 14.3 months (95% CI not applicable; in the fixed-effects model: 9 months, 95% CI, 5.26–6.07) and an OS of 19 months (95% CI, 11.26–27.36) (Figures 2A, B).
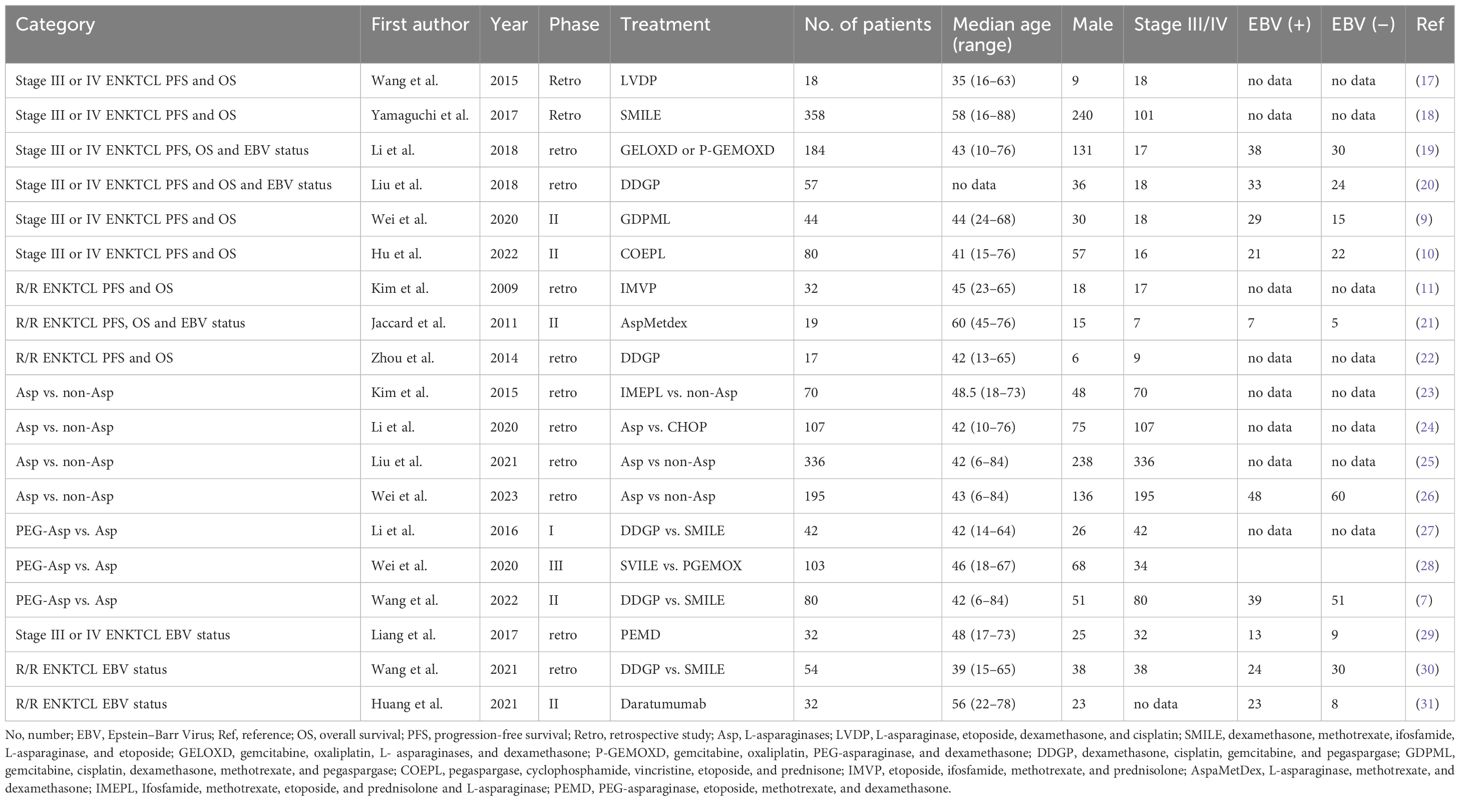
Table 1 Baseline characteristics of trials analyzing patients with advanced-stage or relapsed/refractory (R/R) ENKTCL.
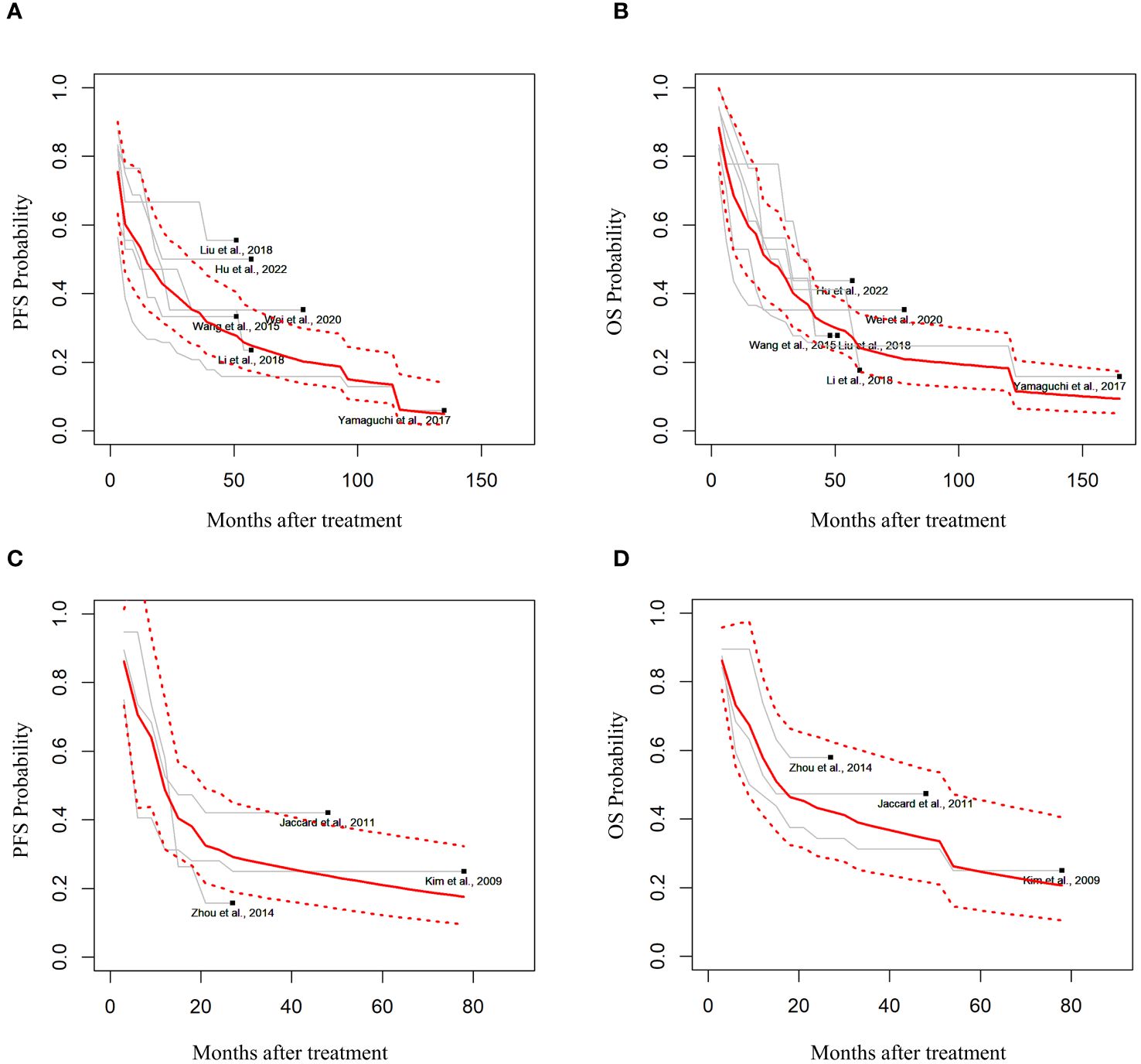
Figure 2 Estimated survival outcomes in advanced-stage and relapsed/refractory (R/R) ENKTCL. (A) Progression-free survival (PFS) and (B) overall survival (OS) in patients with stage III or IV ENKTCL. (C) PFS and (D) OS in patients with R/R ENKTCL.
Three studies had estimated the survival outcomes in patients with R/R ENKTCL (11, 21, 22). Among 68 patients with R/R ENKTCL who were treated with L- or PEG-Asp-based treatment in random effects, the median PFS was estimated to be 11.7 months (95% CI, 4.76–16.43), and OS was 15.6 months (95% CI, 5.97–33.56) (Figures 2C, D).
Patients with stages III–IV or R/R ENKTCL were divided into Asp, non-Asp, EBV-negative, and EBV-positive groups. Table 1 summarizes the characteristics of the included studies. The HR evaluation of the 13 studies focused on four comparisons: Asp group versus non-Asp group, Asp group versus PEG-Asp group, and EBV DNA-negative group versus EBV DNA-positive group.
Among the 13 studies, four of them (23–26) compared the OS benefit between the Asp and non-Asp groups. A total of 481 patients, including 268 and 213 cases in the Asp and non-Asp group, respectively, were included in the analysis, and no statistical heterogeneity was observed (p = 0.529, I2 = 0%). Combined analysis results indicated a significant difference in HR between the groups [HR = 0.60, 95% CI (0.48–0.75), p < 0.001] using the random effects model for meta-analysis. Specifically, the Asp group demonstrated improved OS compared with that in the non-Asp group (Figure 3A).
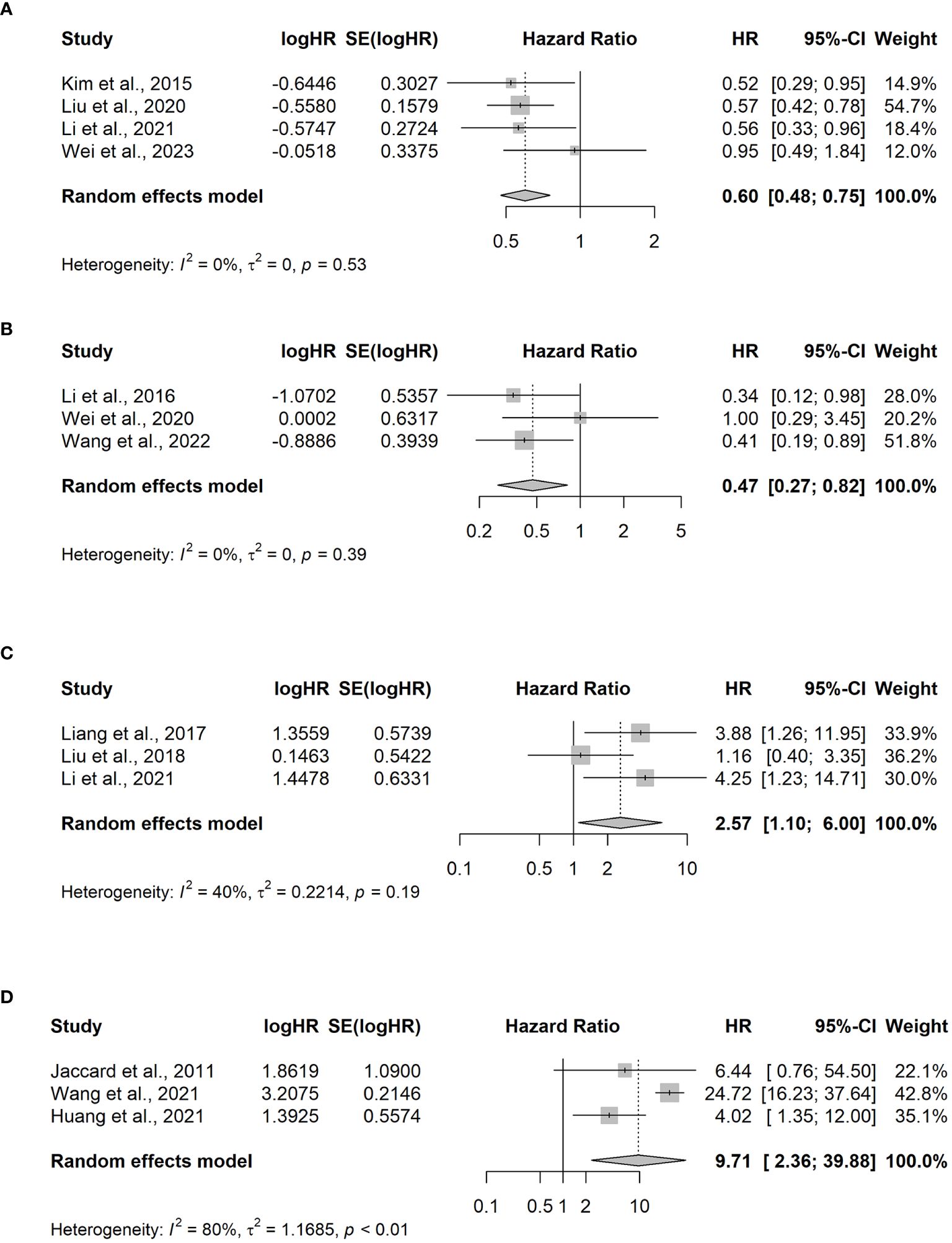
Figure 3 Meta-analysis of hazard ratios for factors impacting overall survival. (A) L-asparaginase (Asp) versus non-Asp-based chemotherapy; (B) PEG-asparaginase versus Asp-based chemotherapy in advanced-stage ENKTCL patients; (C) elevated versus normal EBV DNA levels in the blood pretreatment for III–V ENKTCL; (D) elevated versus normal EBV DNA levels in the blood at the end of treatment for relapsed/refractory ENKTCL. CI, confidence interval; HR, hazard ratio.
To evaluate the survival benefit between the PEG-Asp and L-Asp groups, three studies were analyzed (7, 27, 28). Among the 174 patients included in the analysis, 97 and 56 were in the PEG-Asp and L-Asp groups, respectively. The random effects model revealed no statistical heterogeneity among studies (p = 0.39, I2 = 0%). The PEG-Asp group exhibited a lower HR than that of the L-Asp group [HR = 0.47, 95% CI (0.27–0.82), p = 0.007] (Figure 3B).
An analysis of three studies (19, 30, 31) revealed that among 147 patients newly diagnosed with ENKTCL, 84 and 63 belonged to the EBV DNA-positive and EBV DNA-negative groups, respectively. The EBV DNA-positive group before treatment exhibited poorer OS compared with that of the EBV DNA-negative group [HR = 2.57, 95% CI (1.10–6), p = 0.029] (Figure 3C).
Patients with R/R ENKTCL, who were possibly affected by EBV viremia, were included in the three studies (21, 31, 32). Among the 97 patients with R/R ENKTCL, 54 were EBV-positive at the end of the treatment, whereas 43 patients were EBV-negative. Significant differences were observed between the two groups [OR=9.71, 95% CI (2.36–39.88), p-value =0.002] (Figure 3D).
ENKTCL exhibits pathologic characteristics such as blood vessel destruction, non-caseous necrosis, and atypical lymphocyte proliferation, leading to fever and elevated inflammatory cytokine levels (32). Anthracycline-based chemotherapies, such as CHOP (cyclophosphamide, hydroxydaunorubicin, oncovin, and prednisone), are ineffective owing to p-glycoprotein/MDR1 gene expression (33, 34). To overcome this, combining radiation therapy (>50 Gy) with non-anthracycline chemotherapy has shown superior OS in patients with limited-stage ENKTCL (35–37).
In advanced-stage ENKTCL, radiation field setting is challenging, making L-Asp-based chemotherapy crucial for improving survival outcomes. Cancer cells lack asparagine synthetase and require extracellular asparagine for survival. L-Asp depletes plasma asparagine, halting intracellular protein biosynthesis and killing lymphoma cells. Asp resistance in cancer cells is an adverse prognostic factor for patient outcomes (38). Survival outcomes of Asp-based chemotherapy surpass non-Asp-based chemotherapies, such as SMILE (dexamethasone, methotrexate, ifosfamide, L-Asp, and etoposide) (12, 39) and DDGP (dexamethasone, cisplatin, gemcitabine, and PEG-asparaginase) (7). Meta-analyses also support this trend. Additionally, PEG-Asp, synthesized to decrease the immunogenicity of the enzyme and prolong its half-life, demonstrated greater efficacy than L-Asp. Although limited studies have confirmed this, maintaining stable plasma asparagine depletion remains essential for inducing tumor-suppressive conditions. PEG-Asp showed reduced toxicity in grade 3–4 leukopenia and allergic reactions (7, 27, 28). A discordance was observed concerning the elevation of alanine aminotransferase and thrombocytopenia, possibly owing to the combination of different cytotoxic drugs administered. No studies have compared the prognostic index for natural killer cell lymphoma plus EBV (PINK-E) score between Asp and PEG-Asp. However, Wang et al. reported that PFS was generally superior in the PEG-Asp group among individuals with EBV viremia within normal levels than in those with elevated viremia levels. They observed better PFS in individuals under 60 years of age compared with those over 60 years. These variables were included in the PINK-E score (7).
In patients with R/R ENKTCL who did not receive L-Asp-based chemotherapy, L-Asp showed a similar PFS to that of advanced ENKTCL. However, the OS was shorter than that of patients with stages III–IV ENKTCL. These data recommend the use of an L-Asp-containing regimen in newly diagnosed patients and transitioning to newer agents when relapse occurs.
OS varied among patients with advanced-stage ENKTCL. For example, Li et al. reported a 3-year PFS rate of 32.42% (19), whereas Hu et al. observed a rate of 48.1% (10). This discrepancy may stem from different proportions of EBV DNA elevation in each cohort (Table 1). Our findings indicate that EBV DNA viremia predicts a poor prognosis. In newly diagnosed ENKTCL, pretreatment EBV viremia was a significant factor. These studies included patients with both limited and advanced-stage disease; therefore, this interpretation should be approached with caution. Yan et al. demonstrated that limited-stage disease with plasma EBV positivity had outcomes similar to those of stages III–IV (40). For R/R ENKTCL, patients with sustained EBV viremia post-treatment exhibited worse OS compared with that in those without sustained viremia. Thus, sustained viremia can serve as a surrogate marker for predicting relapse. In summary, the presence of EBV viremia requires careful consideration, and further treatment plans are necessary for high-risk ENKTCL.
Targeted therapies, including those using brentuximab, pembrolizumab and daratumumab, do not ensure complete treatment, and their sustained responses are limited (41–43). To address EBV viremia and achieve long-term survival, targeting the EBV antibody (LMP1/LMP2) with cytotoxic T lymphocyte therapy (CTL) has shown complete remission (44, 45). While autologous transplantation offers limited survival benefits, allogeneic hematopoietic stem cell transplantation has shown efficacy in advanced-stage III/IV and R/R ENKTCL. Combinations of CTL with either autologous or allogeneic HSCT may serve as a curative approach (46, 47).
Studies on advanced-stage or R/R ENKTCL are relatively scarce compared with those on limited-stage ENKTCL, and survival outcomes vary across studies. Therefore, we gathered and integrated data using graphs for this analysis. To the best of our knowledge, this is the first attempt to conduct meta-analyses on individual ENKTCL patient data. We compared survival outcomes and identified factors affecting them. However, our study has some limitations. First, we performed a meta-analysis on both prospective trials and retrospective studies. Nonetheless, our data provide valuable reference points for future prospective randomized controlled trials. Additionally, most of our data were estimated from graphs rather than documented data, posing a risk of human error during the extraction process. However, by imputing missing data using established methods, we increased the reproducibility of our findings.
In conclusion, our data indicate that L-Asp significantly improves outcomes in Ann Arbor stages III–IV and R/R ENKTCL. Furthermore, EBV viremia is a crucial target and tracking marker for predicting survival.
The data analyzed in this study is subject to the following licenses/restrictions: The data presented in this study are available on request from the corresponding author. Requests to access these datasets should be directed to Tong Yoon Kim,dHlrQGNhdGhvbGljLmFjLmty.
TYK: Data curation, Formal Analysis, Methodology, Visualization, Writing – original draft, Writing – review & editing. TJK: Resources, Writing – review & editing. EH: Data curation, Writing – review & editing. GM: Data curation, Writing – review & editing. YJ: Formal analysis, Writing – review & editing. S-GC: Conceptualization, Formal Analysis, Resources, Writing – original draft, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This research was supported by a grant from the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI) and was funded by the Ministry of Health & Welfare, Republic of Korea (grant number HX23C008301).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2024.1362367/full#supplementary-material
1. Yang Y, Wang Y, Liu X, He X, Zhang LL, Wu G, et al. Progression-free survival at 24 months and subsequent survival of patients with extranodal NK/T-cell lymphoma: a China Lymphoma Collaborative Group (CLCG) study. Leukemia. (2021) 35:1671–82. doi: 10.1038/s41375-020-01042-y
2. Yoon SE, Song Y, Kim SJ, Yoon DH, Chen T-Y, Koh Y, et al. Comprehensive analysis of peripheral T-cell and natural killer/T-cell lymphoma in Asian patients: A multinational, multicenter, prospective registry study in Asia. Lancet Reg Health West Pac. (2021) 10:100126. doi: 10.1016/j.lanwpc.2021.100126
3. Li CC, Tien HF, Tang JL, Yao M, Chen YC, Su IJ, et al. Treatment outcome and pattern of failure in 77 patients with sinonasal natural killer/T-cell or T-cell lymphoma. Cancer. (2004) 100:366–75. doi: 10.1002/cncr.11908
4. Kim GE, Lee SW, Chang SK, Park HC, Pyo HR, Kim JH, et al. Combined chemotherapy and radiation versus radiation alone in the management of localized angiocentric lymphoma of the head and neck. Radiother Oncol. (2001) 61:261–9. doi: 10.1016/s0167-8140(01)00428-5
5. Kim SJ, Kim K, Kim BS, Kim CY, Suh C, Huh J, et al. Phase II trial of concurrent radiation and weekly cisplatin followed by VIPD chemotherapy in newly diagnosed, stage IE to IIE, nasal, extranodal NK/T-cell lymphoma: Consortium for Improving Survival of Lymphoma study. J Clin Oncol. (2009) 27:6027–32. doi: 10.1200/JCO.2009.23.8592
6. Zhang Y, Ma S, Cai J, Yang Y, Jing H, Shuang Y, et al. Sequential P-GEMOX and radiotherapy for early-stage extranodal natural killer/T-cell lymphoma: A multicenter study. Am J Hematol. (2021) 96:1481–90. doi: 10.1002/ajh.26335
7. Wang X, Zhang L, Liu X, Li X, Li L, Fu X, et al. Efficacy and safety of a Pegasparaginase-based chemotherapy regimen vs an L-asparaginase–based chemotherapy regimen for newly diagnosed advanced extranodal natural killer/T-cell lymphoma: A randomized clinical trial. JAMA Oncol. (2022) 8:1035–41. doi: 10.1001/jamaoncol.2022.1968
8. Lee K-W, Yun T, Kim D-W, Im S-A, Kim T-Y, Yoon S-S, et al. First-Line Ifosfamide Methotrexate Etoposide Prednisolone Chemother ± radiotherapy is active in stage I/II extranodal NK/T-cell lymphoma. Leukemia Lymphoma. (2006) 47:1274–82. doi: 10.1080/10428190600562823
9. Wei C, Cao X, Zhang W, Zhang Y, Wang W, Zhang L, et al. Combined gemcitabine, cisplatin, dexamethasone, methotrexate, and pegaspargase (GDP-ML) for patients with newly diagnosed extranodal natural killer/T cell lymphoma, nasal type: a single arm, single center, prospective phase 2 study. Ann Hematol. (2020) 99:2801–9. doi: 10.1007/s00277-020-04036-z
10. Hu S, Lin N, Liu J, Sun Y, Liu W, Wang X, et al. A prospective Phase II study of pegaspargase-COEP plus radiotherapy in patients with newly diagnosed extra-nodal NK/T-cell lymphoma. Front Oncol. (2022) 12:839252. doi: 10.3389/fonc.2022.839252
11. Kim BS, Kim DW, Im SA, Kim CW, Kim TY, Yoon SS, et al. Effective second-line chemotherapy for extranodal NK/T-cell lymphoma consisting of etoposide, ifosfamide, methotrexate, and prednisolone. Ann Oncol. (2009) 20:121–8. doi: 10.1093/annonc/mdn551
12. Yamaguchi M, Kwong YL, Kim WS, Maeda Y, Hashimoto C, Suh C, et al. Phase II study of SMILE chemotherapy for newly diagnosed Stage IV, relapsed, or refractory extranodal natural killer (NK)/T-cell lymphoma, nasal type: the NK-cell tumor study group study. J Clin Oncol. (2011) 29:4410–6. doi: 10.1200/JCO.2011.35.6287
13. Higgins J, Green S. Cochrane handbook for systematic reviews of interventions. version 5.1.0. Cochrane Collaboration. (2011). Available at: www.cochrane-handbook.org
14. Liu N, Zhou Y, Lee JJ. IPDfromKM: reconstruct individual patient data from published Kaplan-Meier survival curves. BMC Med Res Methodol. (2021) 21:111. doi: 10.1186/s12874-021-01308-8
15. Viechtbauer W. Conducting meta-analyses in R with the metafor package. J Stat Softw. (2010) 36:1–48. doi: 10.18637/jss.v036.i03
16. Balduzzi S, Rücker G, Schwarzer G. How to perform a meta-analysis with R: a practical tutorial. Evid Based Ment Health. (2019) 22:153–60. doi: 10.1136/ebmental-2019-300117
17. Wang YQ, Yang Y, Zhuo HY, Zou LQ, Jiang Y, Jiang M. Trial of LVDP regimen (l-asparaginase, etoposide, dexamethasone, and cisplatin, followed by radiotherapy) as first-line treatment for newly diagnosed, stage III/IV extranodal natural killer/T cell lymphoma. Med Oncol. (2015) 32:435. doi: 10.1007/s12032-014-0435-4
18. Yamaguchi M, Suzuki R, Oguchi M, Asano N, Amaki J, Akiba T, et al. Treatments and outcomes of patients with extranodal natural killer/T-cell lymphoma diagnosed between 2000 and 2013: A cooperative study in Japan. J Clin Oncol. (2017) 35:32–9. doi: 10.1200/JCO.2016.68.1619
19. Li JW, Li YJ, Zhong MZ, Liu XL, Li J, Li KL, et al. Efficacy and tolerance of GELOXD/P-GEMOXD in newly diagnosed nasal-type extranodal NK/T-cell lymphoma: A multicenter retrospective study. Eur J Haematol. (2018) 100:247–56. doi: 10.1111/ejh.13004
20. Liu T, Zhu F, Xiao Y, Li Q, Liu X, Yang K, et al. Pegaspargase, gemcitabine, dexamethasone, and cisplatin (P-GDP) combined chemotherapy is effective for newly diagnosed extranodal NK/T-cell lymphoma: a retrospective study. Cancer Manag Res. (2018) 10:5061–9. doi: 10.2147/CMAR.S179567
21. Jaccard A, Gachard N, Marin B, Rogez S, Audrain M, Suarez F, et al. Efficacy of L-asparaginase with methotrexate and dexamethasone (AspaMetDex regimen) in patients with refractory or relapsing extranodal NK/T-cell lymphoma, a phase 2 study. Blood. (2011) 117:1834–9. doi: 10.1182/blood-2010-09-307454
22. Zhou Z, Li X, Chen C, Li X, Zhang L, Li L, et al. Effectiveness of gemcitabine, pegaspargase, cisplatin, and dexamethasone (DDGP) combination chemotherapy in the treatment of relapsed/refractory extranodal NK/T cell lymphoma: a retrospective study of 17 patients. Ann Hematol. (2014) 93:1889–94. doi: 10.1007/s00277-014-2136-7
23. Kim M, Kim TM, Kim KH, Keam B, Lee SH, Kim DW, et al. Ifosfamide, methotrexate, etoposide, and prednisolone (IMEP) plus l-asparaginase as a first-line therapy improves outcomes in stage III/IV NK/T cell-lymphoma, nasal type (NTCL). Ann Hematol. (2015) 94:437–44. doi: 10.1007/s00277-014-2228-4
24. Li J, Li J, Zhong M, Zhou H, Yu B. The clinical features and survival outcome of 107 newly diagnosed advanced stage extranodal NK/T-cell lymphoma cases: A triple-center study. Cancer Manag Res. (2021) 13:1541–9. doi: 10.2147/CMAR.S292293
25. Liu W, Yang Y, Qi S, Wang Y, He X, Zhang L, et al. Treatment, survival, and prognosis of advanced-stage natural killer/T-cell lymphoma: an analysis from the China lymphoma collaborative group. Front Oncol. (2020) 10:583050. doi: 10.3389/fonc.2020.583050
26. Wei YC, Liu WX, Qi F, Zhang CG, Zheng BM, Xie Y, et al. Clinical features, prognostic stratification, and treatment of advanced-stage non-nasal type extranodal natural killer/T-cell lymphoma: a multi-institutional real-world study. Ann Hematol. (2023) 103:163–74. doi: 10.1007/s00277-023-05455-4
27. Li X, Cui Y, Sun Z, Zhang L, Li L, Wang X, et al. DDGP versus SMILE in newly diagnosed advanced natural killer/T-cell lymphoma: A randomized controlled, multicenter, open-label study in China. Clin Cancer Res. (2016) 22:5223–8. doi: 10.1158/1078-0432.CCR-16-0153
28. Wei L, Yang L, Ye J, Cong J, Li X, Yao N, et al. Outcomes of patients treated with SVILE vs. P-GemOx for extranodal natural killer/T-cell lymphoma, nasal type: a prospective, randomized controlled study. Cancer Biol Med. (2020) 17:795–804. doi: 10.20892/j.issn.2095-3941.2020.0160
29. Liang JH, Wang L, Peter Gale R, Wu W, Xia Y, Fan L, et al. Efficacy of pegaspargase, etoposide, methotrexate and dexamethasone in newly diagnosed advanced-stage extra-nodal natural killer/T-cell lymphoma with the analysis of the prognosis of whole blood EBV-DNA. Blood Cancer J. (2017) 7:e608. doi: 10.1038/bcj.2017.88
30. Wang X, Hu J, Dong M, Ding M, Zhu L, Wu J, et al. DDGP vs. SMILE in Relapsed/Refractory extranodal Natural Killer/T-cell Lymphoma, Nasal Type: A Retrospective Study of 54 Patients. Clin Transl Sci. (2021) 14:405–11. doi: 10.1111/cts.12893
31. Huang H, Zhu J, Yao M, Kim TM, Yoon DH, Cho SG, et al. Daratumumab monotherapy for patients with relapsed or refractory natural killer/T-cell lymphoma, nasal type: an open-label, single-arm, multicenter, phase 2 study. J Hematol Oncol. (2021) 14:25. doi: 10.1186/s13045-020-01020-y
32. McKelvie PA, Climent F, Krings G, Hasserjian RP, Abramson JS, Pilch BZ, et al. Small-cell predominant extranodal NK/T cell lymphoma, nasal type: clinicopathological analysis of a series of cases diagnosed in a Western population. Histopathology. (2016) 69:667–79. doi: 10.1111/his.12990
33. Yamaguchi M, Kita K, Miwa H, Nishii K, Oka K, Ohno T, et al. Frequent expression of P-glycoprotein/MDR1 by nasal T-cell lymphoma cells. Cancer. (1995) 76:2351–6. doi: 10.1002/1097-0142(19951201)76:11<2351::aid-cncr2820761125>3.0.co;2-1
34. Ando M, Sugimoto K, Kitoh T, Sasaki M, Mukai K, Ando J, et al. Selective apoptosis of natural killer-cell tumours by l-asparaginase. Br J Haematol. (2005) 130:860–8. doi: 10.1111/j.1365-2141.2005.05694.x
35. Li YX, Yao B, Jin J, Wang WH, Liu YP, Song YW, et al. Radiotherapy as primary treatment for stage IE and IIE nasal natural killer/T-cell lymphoma. J Clin Oncol. (2006) 24:181–9. doi: 10.1200/JCO.2005.03.2573
36. Moskowitz CH, Schöder H, Teruya-Feldstein J, Sima C, Iasonos A, Portlock CS, et al. Risk-adapted dose-dense immunochemotherapy determined by interim FDG-PET in advanced-stage diffuse large B-cell lymphoma. J Clin Oncol. (2010) 28:1896–903. doi: 10.1200/JCO.2009.26.5942
37. Vargo JA, Patel A, Glaser SM, Balasubramani GK, Farah RJ, Marks SM, et al. The impact of the omission or inadequate dosing of radiotherapy in extranodal natural killer T-cell lymphoma, nasal type, in the United States. Cancer. (2017) 123:3176–85. doi: 10.1002/cncr.30697
38. Akahane K, Kimura S, Miyake K, Watanabe A, Kagami K, Yoshimura K, et al. Association of allele-specific methylation of the ASNS gene with asparaginase sensitivity and prognosis in T-ALL. Blood Adv. (2022) 6:212–24. doi: 10.1182/bloodadvances.2021004271
39. Kwong YL, Kim WS, Lim ST, Kim SJ, Tang T, Tse E, et al. SMILE for natural killer/T-cell lymphoma: analysis of safety and efficacy from the Asia Lymphoma Study Group. Blood. (2012) 120:2973–80. doi: 10.1182/blood-2012-05-431460
40. Yan Z, Yao Z, Wang H, Yao S, Wang X, Gao Y, et al. Plasma EBV-DNA and peripheral blood mononuclear cell EBV-DNA have disparate clinical relevance in patients with extranodal NK/T-cell lymphoma. J Clin Virol. (2022) 157:105320. doi: 10.1016/j.jcv.2022.105320
41. Kim M, Lee JO, Koh J, Kim TM, Lee JY, Jeon YK, et al. A phase II study of Brentuximab vedotin in patients with relapsed or refractory Epstein-Barr virus-positive and CD30-positive lymphomas. Haematologica. (2021) 106:2277–80. doi: 10.3324/haematol.2021.278301
42. Kwong YL, Chan TSY, Tan D, Kim SJ, Poon LM, Mow B, et al. PD1 blockade with pembrolizumab is highly effective in relapsed or refractory NK/T-cell lymphoma failing l-asparaginase. Blood. (2017) 129:2437–42. doi: 10.1182/blood-2016-12-756841
43. Hari P, Raj RV, Olteanu H. Targeting CD38 in refractory extranodal natural killer cell–T-cell lymphoma. N Engl J Med. (2016) 375:1501–2. doi: 10.1056/NEJMc1605684
44. Bollard CM, Gottschalk S, Torrano V, Diouf O, Ku S, Hazrat Y, et al. Sustained complete responses in patients with lymphoma receiving autologous cytotoxic T lymphocytes targeting Epstein-Barr virus latent membrane proteins. J Clin Oncol. (2014) 32:798–808. doi: 10.1200/JCO.2013.51.5304
45. Cho SG, Kim N, Sohn HJ, Lee SK, Oh ST, Lee HJ, et al. Long-term outcome of extranodal NK/T cell lymphoma patients treated with postremission therapy using EBV LMP1 and LMP2a-specific CTLs. Mol Ther. (2015) 23:1401–9. doi: 10.1038/mt.2015.91
46. Jeong SH, Song HN, Park JS, Yang DH, Koh Y, Yoon SS, et al. Allogeneic stem cell transplantation for patients with natural killer/T cell lymphoid Malignancy: A multicenter analysis comparing upfront and salvage transplantation. Biol Blood Marrow Transplant. (2018) 24:2471–8. doi: 10.1016/j.bbmt.2018.07.034
Keywords: extranodal NK/T-cell lymphoma, advanced, relapsed/refractory, estimate individual patient data, meta-analysis, Epstein–Barr virus
Citation: Kim TY, Kim TJ, Han EJ, Min GJ, Jeon Y and Cho S-G (2024) Challenges in overcoming advanced-stage or relapsed refractory extranodal NK/T-cell lymphoma: meta-analysis of individual patient data. Front. Oncol. 14:1362367. doi: 10.3389/fonc.2024.1362367
Received: 28 December 2023; Accepted: 15 July 2024;
Published: 31 July 2024.
Edited by:
Onder Alpdogan, Thomas Jefferson University, United StatesReviewed by:
Sanjay De Mel, National University Cancer Institute, SingaporeCopyright © 2024 Kim, Kim, Han, Min, Jeon and Cho. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Seok-Goo Cho, Y2hvc2dAY2F0aG9saWMuYWMua3I=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.